Abstract
Purpose.
In previous studies, conditional disruption of Klf4 in the developing mouse ocular surface from embryonic day 10 resulted in corneal epithelial fragility, stromal edema, and loss of conjunctival goblet cells, revealing the importance of Klf4 in ocular surface maturation. Here, we use spatiotemporally regulated ablation of Klf4 to investigate its functions in maintenance of adult corneal epithelial homeostasis.
Methods.
Expression of Cre was induced in ternary transgenic (Klf4LoxP/LoxP/Krt12rtTA/rtTA/Tet-O-Cre) mouse corneal epithelium by doxycycline administered through intraperitoneal injections and drinking water, to generate corneal epithelium–specific deletion of Klf4 (Klf4Δ/ΔCE). Corneal epithelial barrier function was tested by fluorescein staining. Expression of selected Klf4-target genes was determined by quantitative PCR (QPCR), immunoblotting, and immunofluorescent staining.
Results.
Klf4 was efficiently ablated within 5 days of doxycycline administration in adult Klf4Δ/ΔCE corneal epithelium. The Klf4Δ/ΔCE corneal epithelial barrier function was disrupted, and the basal cells were swollen and rounded after 15 days of doxycycline treatment. Increased numbers of cell layers and Ki67-positive proliferating cells suggested deregulated Klf4Δ/ΔCE corneal epithelial homeostasis. Expression of tight junction proteins ZO-1 and occludin, desmosomal Dsg and Dsp, basement membrane laminin-332, and corneal epithelial–specific keratin-12 was decreased, while that of matrix metalloproteinase Mmp9 and noncorneal keratin-17 increased, suggesting altered Klf4Δ/ΔCE corneal epithelial cell identity.
Conclusions.
Ablation of Klf4 in the adult mouse corneas resulted in the absence of characteristic corneal epithelial cell differentiation, disrupted barrier function, and squamous metaplasia, revealing that Klf4 is essential for maintenance of the adult corneal epithelial cell identity and homeostasis.
Keywords: Klf4, cornea, epithelial barrier, desmosome, basement membrane, squamous metaplasia
Spatiotemporally regulated ablation of Klf4 in adult mouse corneal epithelium led to deregulated proliferation, abnormal differentiation, disrupted barrier function, and squamous metaplasia, revealing that Klf4 is essential for maintenance of the adult corneal epithelial identity and homeostasis.
The cornea, comprised of an outer stratified squamous epithelium, central collagenous stroma, and inner endothelial monolayer, is essential for clear vision. The corneal epithelium, being the most anterior tissue of the eye, provides the first line of defense against noxious chemical, biological, and physical stimuli.1 Loss of corneal epithelial integrity is associated with debilitating ocular surface disorders, such as dry eye, ocular cicatricial pemphigoid, and Meesmann's dystrophy.1–3 The newborn mouse corneal epithelium consists of one to two cell layers of unstratified cells, which divide and differentiate in a highly coordinated process to form a three- to four-cell layered stratified epithelium by postnatal day 12 (PN12) concurrent with eyelid opening.4,5 The adult mouse corneal epithelium consists of six to eight stratified cell layers that express cornea-specific keratin-12 and adherens junctions in all layers, tight junction proteins at the superficial layers, desmosomes in the spinous cell layers, gap junctions, and hemidesmosomes in the basal cell layers.4–14 Mature corneal epithelial cells undergo recurring cycles of self-renewal, during which progenitor/stem cells residing in the limbus provide a source of transient amplifying cells that proliferate as they migrate centripetally, subsequently differentiating into mature epithelial cells as they move up from the basal layer to form wing cell and superficial cell layers to replace those lost through tissue turnover.15–17 Though several transcription factors, including Klf4, Klf5, Pax6, AP2α, HMGN1, Cited2, basonuclin, Sp1, AP1, NF1, EHF, and Oct1, are known to regulate corneal development, our understanding of the gene regulatory networks that control proper maintenance of the adult corneal epithelium remains incomplete.18–36
Krüppel-like factor-4 (Klf4), which regulates a diverse array of cellular events, including proliferation, differentiation, stem cell maintenance, development, and apoptosis, is one of the most highly expressed transcription factors in the mouse cornea.37–43 Our previous studies demonstrated that Klf4 has an integral role in the mouse ocular surface maturation.20,41,44–47 Though Klf4 conditional null (Klf4CN) mice, derived by mating Klf4LoxP/LoxP (Klf4f/f) with Klf4f/f/Le-Cre/- mice, wherein Klf4 was disrupted from embryonic day 10 (E10), were helpful for revealing the role of Klf4 in corneal maturation, they were not suitable for addressing the role of Klf4 in maintenance of the adult mouse cornea,48,49 As Le-Cre also is expressed in the eyelid epidermal epithelium, lacrimal glands, meibomian glands, and the conjunctival epithelium, it was impossible to parse out the direct effects of the absence of Klf4 in corneal epithelium from indirect effects of the defective surrounding tissues in Klf4CN mice.20,45,50,51 Moreover, hemizygous Le-Cre transgenic mice that we used to generate Klf4CN mice occasionally develop eye abnormalities on some genetic backgrounds even in the absence of LoxP sites, raising additional concerns about the validity of our findings related to corneal functions of Klf4.52 In this study, we have addressed those concerns by exploiting doxycycline-inducible expression of Cre recombinase in a ternary transgenic mouse line53,54 to selectively delete Klf4 within the corneal epithelium in a precisely spatiotemporally regulated manner. Based on the data obtained from this approach, we reported that Klf4 is essential for maintenance of the adult corneal epithelial cell identity and homeostasis.
Materials and Methods
Spatiotemporally Regulated Ablation of Klf4 and Measurement of Corneal Epithelial Permeability Barrier Function
Ternary transgenic (Klf4f/f/Krt12rtTA/rtTA/Tet-O-Cre) mice were generated on a mixed background by mating Klf4f/f with Krt12rtT/rtTAA/Tet-O-Cre mice as described earlier for other genes.20,53,54 Desired genotype of the ternary transgenic mice was confirmed by PCR with tail DNA. Expression of Cre was induced in ternary transgenic mice by doxycycline administered through intraperitoneal injections and drinking water to generate corneal epithelium-specific disruption of Klf4 (Klf4Δ/ΔCE). Ternary transgenic littermates injected with PBS and fed with sugar water served as the wild type (WT) controls in these experiments. The effect of loss of Klf4 on corneal epithelium was examined at two time points: after 5 days (Dox-5 Klf4Δ/ΔCE) and after 15 days (Dox-15 Klf4Δ/ΔCE) of doxycycline administration. Corneal epithelial permeability was tested by instilling 2 μL of 1% sodium fluorescein solution on the ocular surface, rinsing with PBS after 1 minute and photographing under blue light using a slit-lamp biomicroscope equipped with a digital camera. All studies reported here were performed with 8- to 10-week-old adult mice maintained in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee of the University of Pittsburgh, and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The data presented are representative of at least three independent experiments.
Histology
Eyes from carbon dioxide asphyxiated mice were fixed in freshly prepared 4% paraformaldehyde (Sigma-Aldrich Corp., St. Louis, MO, USA) in PBS (pH 7.4) for 24 hours at 4°C and embedded in paraffin. Central corneal 8 μm-thick sections were stained with hematoxylin and eosin (H&E), or PAS reagent following standard procedures. An Olympus BX60 microscope (Olympus America, Inc., Allentown, PA, USA) was used for light microscopy. Images were captured with a Spot digital camera (Spot Diagnostics Instruments, Inc., Sterling Heights, CA, USA) and processed in a similar manner using Adobe Photoshop (Adobe Systems, San Jose, CA, USA).
Isolation of Total RNA and Real-Time Quantitative PCR (QPCR)
The WT and the Klf4Δ/ΔCE corneal total RNA was isolated using RNeasy columns (Qiagen, Germantown, MD, USA), 100 ng of which was used for cDNA synthesis with mouse Maloney leukemia virus reverse transcriptase (Promega, Madison, WI, USA). TaqMan gene expression assays were performed in triplicate in an ABI StepOne Plus thermocycler with prestandardized gene-specific probes using 18S rRNA as endogenous control (Applied Biosystems, Foster City, CA, USA). The QPCR results were analyzed using StepOne software provided by the manufacturer (Applied Biosystems).
Antibodies
In this study, we used the following antibodies, which were previously tested and confirmed to cross-react with corresponding mouse antigens: goat anti-human actin, rabbit anti-human ZO-1, rabbit anti-human KRT17, goat anti-mouse Krt12, rabbit anti-human DSG, rabbit anti-human DSP, rabbit anti-human MMP9, and rabbit anti-human KLF4 from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rabbit anti-human laminin from Abcam (San Francisco, CA, USA); and rabbit anti-human Ki67 from Thermo-Fisher Scientific (Pittsburgh, PA, USA).
Immunoblots
Freshly dissected WT or Dox-15 Klf4Δ/ΔCE corneas were homogenized in urea buffer (8.0 M urea, 0.8% Triton X-100, 0.2% SDS, 3% β-mercaptoethanol, and proteinase inhibitors), insoluble material pelleted by centrifugation, the protein concentration in the supernatant quantified by the bicinchoninic acid (BCA) method, 10 μg of total protein was separated on 4% to 12% polyacrylamide gradient gels using Bis-Tris buffer system and electrophoretically blotted onto polyvinylidine fluoride (PVDF) membranes. The membranes were blocked with 4% milk in PBS containing 0.1% Tween-20 (PBST) for 1 hour at room temperature, washed twice with PBST for 5 minutes each, incubated with a 1:1000 dilution of the appropriate primary antibody overnight at 4°C, washed thrice with PBST for 5 minutes each, incubated with appropriate horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit IgG, or donkey anti-goat IgG; Life Technologies, Carlsbad, CA, USA) at a 1:2000 dilution for 1 hour at room temperature, washed thrice with PBST for 5 minutes each, and the immunoreactive bands detected by chemiluminescence using Super Signal West Pico solutions (Pierce, Rockford, IL, USA), and exposure of the processed blots to X-ray films, which were developed in a standard X-ray film developer. Developed X-ray films were scanned and densitometric measurements of the immunoreactive band intensities were performed using Image-J software (National Institutes of Health [NIH], Bethesda, MD, USA). Densitometric values obtained by cross-comparison of two each of the WT and Dox-15 Klf4Δ/ΔCE lysates were used for determining their relative levels, using actin band intensity as loading control.
Immunofluorescent Staining
Cryosections 8 μm thick from OCT-embedded eyeballs were fixed in freshly prepared buffered 4% paraformaldehyde for 30 minutes, washed thrice for 5 minutes each with PBS (pH 7.4), permeabilized with 0.1% Triton X-100 in PBS when necessary, followed by 3 washes of 5 minutes each with PBS, blocked with 10% goat or donkey serum in PBST for 1 hour at room temperature in a humidified chamber, washed twice with PBST for 5 minutes each, incubated with a 1:100 dilution of the primary antibody for 2 hours at room temperature, washed thrice with PBST for 5 minutes each, incubated with appropriate secondary antibody (Alexafluor 546-coupled goat anti-rabbit IgG, and Alexafluor 488-coupled donkey anti-goat IgG; Molecular Probes, Carlsbad, CA, USA) at a 1:200 dilution for 1 hour at room temperature, washed thrice with PBST for 5 minutes each, counterstained with 4′6-diamidino-2-phenylindole (DAPI), and mounted with Aqua-Poly/Mount (Polysciences, Warrington, PA, USA), and confocal images collected using an Olympus IX81 microscope (Olympus America, Inc.). The mean proliferative index (PI) in the WT and Klf4Δ/ΔCE corneas was determined by dividing the number of Ki67-positive cells by the total number of epithelial cells in multiple fields from different sections stained independently. Corneal epithelial tight junctions were visualized by whole-mount corneal immunofluorescent staining for ZO-1 as described previously,44 followed by confocal microscopy. Composite figures were assembled using images processed identically in Fluoview (Olympus America, Inc.), Adobe Photoshop, and/or Illustrator (Adobe Systems). The data presented here are representative of at least three independent experiments.
Results
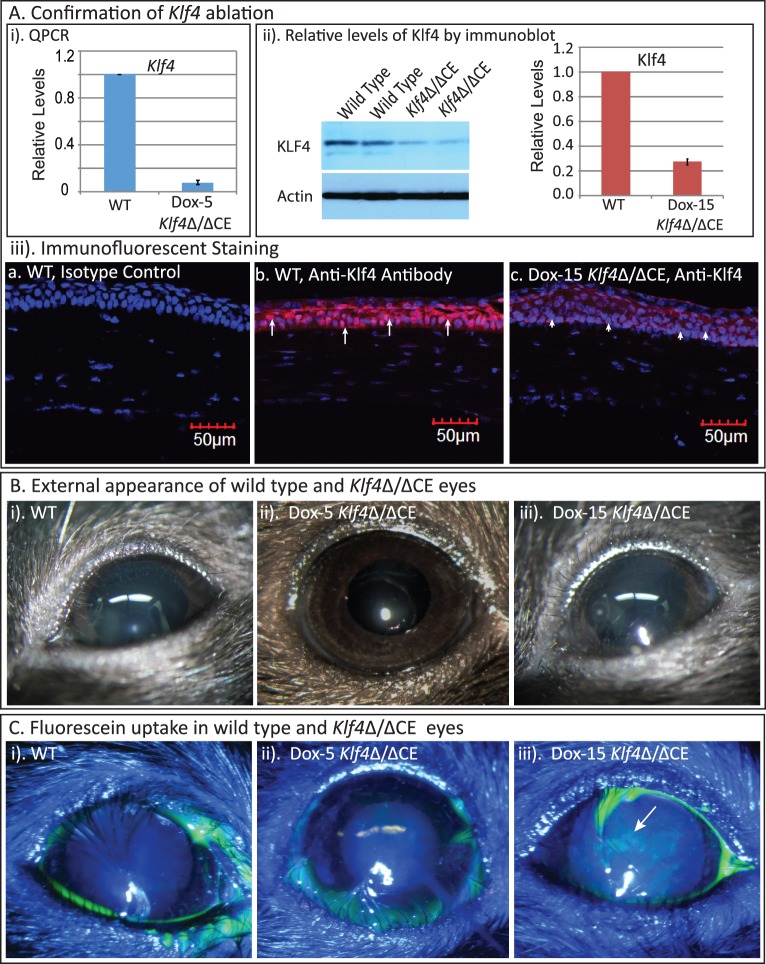
To determine the role of Klf4 in maintenance of the normally formed adult cornea, we disrupted Klf4 gene selectively in the 8- to 10-week-old mouse corneal epithelium using doxycycline-inducible ternary transgenic system.53,54 The QPCR indicated efficient ablation of Klf4 in the Klf4Δ/ΔCE mouse corneas after 5 days of doxycycline administration (Dox-5 Klf4Δ/ΔCE; Fig. 1Ai). Immunoblots revealed that the Klf4 levels within the Dox-15 Klf4Δ/ΔCE corneas decreased to approximately 25% of that in the WT (Fig. 1Aii), which was confirmed by immunofluorescent staining (Fig. 1Aiii). Visual examination did not reveal any apparent defects within the Dox-5 or Dox-15 Klf4Δ/ΔCE ocular surface (Fig. 1B). However, the Dox-15 Klf4Δ/ΔCE corneas were stained by fluorescein, unlike their WT and Dox-5 Klf4Δ/ΔCE counterparts, suggesting that the absence of Klf4 leads to defective epithelial barrier function in a normally formed cornea (Fig. 1C). Thus, Klf4 has an essential role in maintenance of adult corneal epithelial barrier function.
Figure 1.
Corneal epithelial-specific disruption of Klf4 in mature corneas affects epithelial barrier function. (A) Confirmation of Klf4 disruption. QPCR (A-i) and immunoblots (A-ii) revealed greater than 90% reduction in Klf4 transcripts and approximately 75% reduction in Klf4 protein, respectively, in the Klf4Δ/ΔCE corneas compared to the WT counterparts after 5 days of doxycycline treatment. Immunofluorescent staining (A-iii) detected nuclear presence of Klf4 in the WT ([A-iii-b] red, arrows), but not the Dox-15 Klf4Δ/ΔCE ([A-iii-c] arrowheads) corneal epithelial cells, confirming efficient disruption of Klf4. No background staining was observed in isotype antibody control ([A-iii-a]). (B) The WT (B-i), Dox-5 Klf4Δ/ΔCE (B-ii), and Dox-15 Klf4Δ/ΔCE (B-iii) eyes were indistinguishable by external examination. (C) Fluorescein stained the Dox-15 Klf4Δ/ΔCE ([C-iii], patches of green, indicated by arrow), but not the WT and Dox-5 Klf4Δ/ΔCE corneas (C-i, C-ii), indicating that the loss of Klf4 results in disrupted barrier function.
Histological examination revealed an increased number of epithelial cell layers and considerably enlarged basal epithelial cells in the Dox-5 Klf4Δ/ΔCE compared to the control WT corneas (Fig. 2i). Moreover, the characteristic flattened morphology of superficial cell layers was altered in the Dox-5 Klf4Δ/ΔCE corneal epithelium, where the superficial cells tended to remain spherical. Continued treatment with doxycycline for 15 days resulted in a more severe phenotype, with many loosely attached superficial epithelial cells and many more enlarged basal epithelial cells coupled with thinner and wavy basement membrane (Fig. 2ii). Examination of PAS-stained sections revealed an intact basement membrane in the WT, but not the Dox-15 Klf4Δ/ΔCE corneal epithelium (Fig. 2iii). Endothelial Descemet's membrane remained unaffected in the Dox-15 Klf4Δ/ΔCE corneas.
Figure 2.
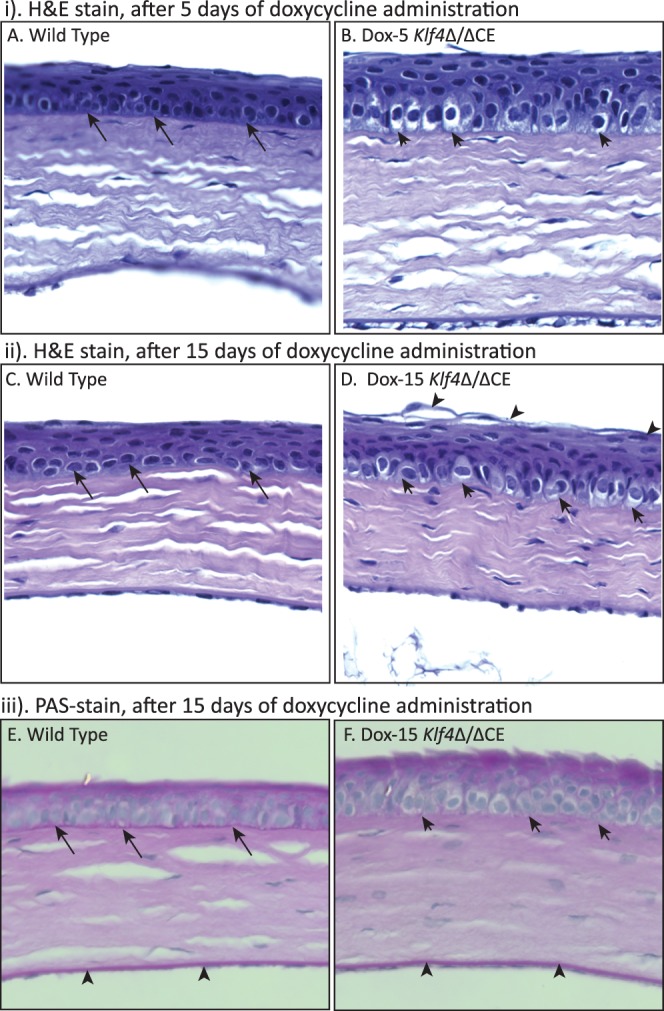
The Klf4Δ/ΔCE corneal epithelium displays signs of squamous metaplasia. Sections 8 μm thick from paraformaldehyde-fixed, paraffin-embedded WT (A, C, E) or the Klf4Δ/ΔCE (B, D, F) eyes were stained with H&E (A–D), or PAS (E, F). The Klf4Δ/ΔCE corneal epithelium contains more cell layers than the WT after 5 (i) or 15 (ii) days of doxycycline treatment. The basal epithelial cells are spherical in the Klf4Δ/ΔCE corneas ([B, D], short arrows) compared to the columnar ones in the WT ([A, C], long arrows). The most superficial cells are loosely attached in the Dox-15 Klf4Δ/ΔCE corneal epithelium ([D], arrowheads) compared to the WT (C). The PAS stain (iii) detected an intact basement membrane in the WT ([E], long arrows), but not the Dox-15 Klf4Δ/ΔCE ([F], short arrows) corneal epithelium. Endothelial Descemet's membrane remained unaffected in the Dox-15 Klf4Δ/ΔCE corneas ([E, F], arrowheads).
To examine if the elevated fluorescein uptake by Dox-15 Klf4Δ/ΔCE corneas is an outcome of altered tight junctions, we quantified the levels of tight junction proteins ZO-1 and occludin. Densitometry of immunoblots with actin as loading control quantified the amounts of ZO-1 and occludin within the Dox-15 Klf4Δ/ΔCE corneas to be approximately 30% and 45% of that in the WT, respectively (Fig. 3A). Consistently, immunofluorescent staining of whole-mount corneas detected ZO-1 as a continuous band in the WT superficial epithelial cell membranes, the intensity of which was sharply decreased in the Dox-15 Klf4Δ/ΔCE corneas (Fig. 3B). These results suggested that the defective tight junctions between the superficial epithelial cells account for the increased fluorescein uptake by Dox-15 Klf4Δ/ΔCE corneas.
Figure 3.
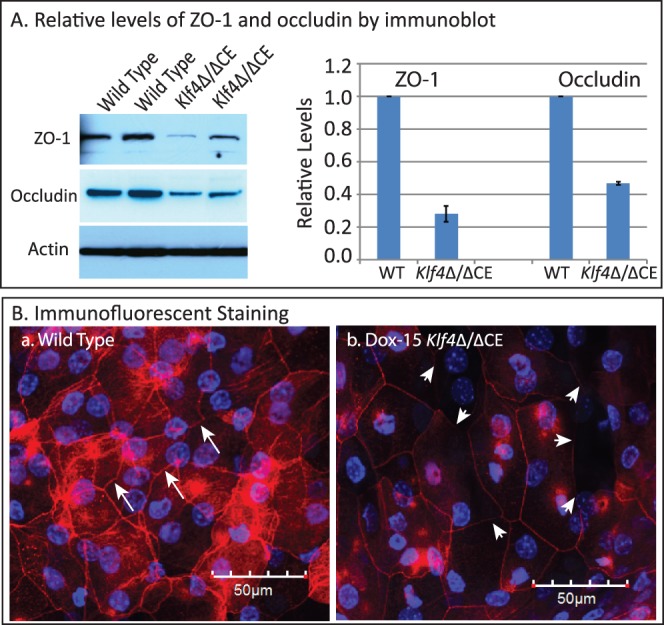
Decreased levels of epithelial tight junction proteins within the Dox-15 Klf4Δ/ΔCE corneas. Immunoblots (A) revealed decreased amounts of tight junction proteins ZO-1 and occludin in the Dox-15 Klf4Δ/ΔCE corneas. Densitometry with actin as loading control quantified the amounts of ZO-1 and occludin within the Dox-15 Klf4Δ/ΔCE corneas to be approximately 30% and 45% of that in the WT, respectively. Immunofluorescent staining of whole-mount corneas (B) detected ZO-1 as a continuous band in between the WT superficial epithelial cells ([B-a], long arrows), the intensity of which was sharply decreased in the Dox-15 Klf4Δ/ΔCE corneas ([B-b], short arrows).
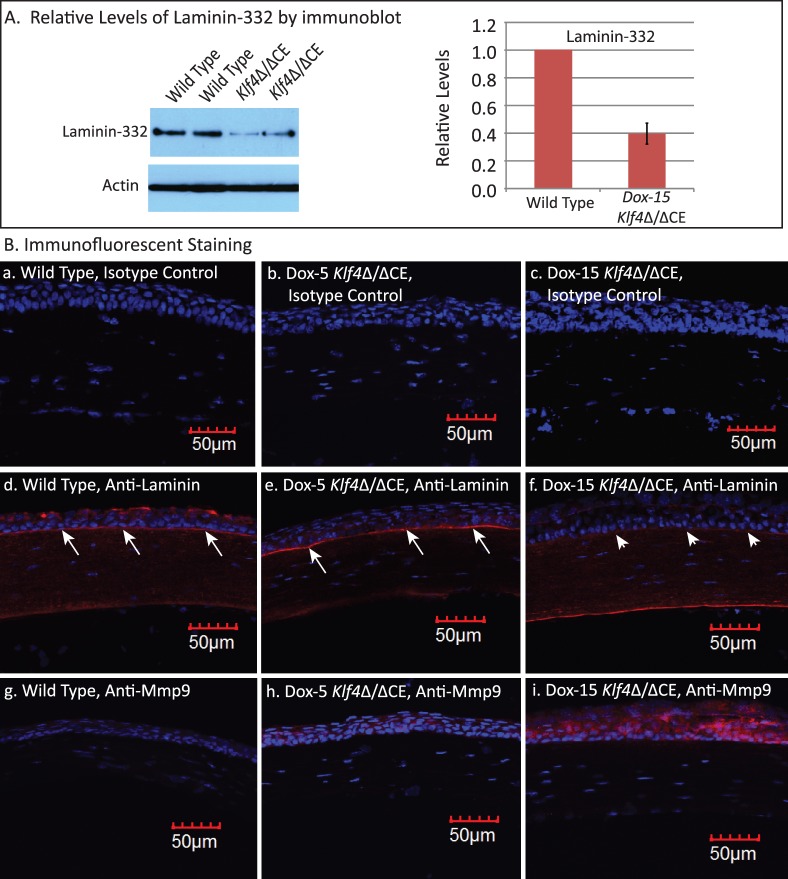
Consistent with the histological observations of Klf4Δ/ΔCE corneal epithelial basement membrane defects (Fig. 2), immunoblots revealed decreased expression of laminin-332 in the Dox-15 Klf4Δ/ΔCE corneas (Fig. 4A). Densitometry of immunoblots with actin as loading control quantified laminin-332 amounts within the Dox-15 Klf4Δ/ΔCE corneas to be approximately 40% of that in the WT (Fig. 4A). Immunofluorescent staining with anti-laminin antibody detected robust expression of laminin in the WT and Dox-5 Klf4Δ/ΔCE, but not the Dox-15 Klf4Δ/ΔCE corneal epithelial basement membrane (Fig. 4B). As basement membrane laminins are stable proteins with relatively long half-lives,55,56 we examined if their stability is compromised in Dox-15 Klf4Δ/ΔCE corneas due to elevated expression of matrix metalloproteinases (Mmps). Immunofluorescent staining revealed increased expression of Mmp9 in the Dox-15 Klf4Δ/ΔCE compared to the WT and Dox-5 Klf4Δ/ΔCE corneal epithelium, indicating that the elevated Mmp9 expression may have promoted the degradation of the Dox-15 Klf4Δ/ΔCE corneal epithelial basement membrane (Fig. 4B). Together, these results revealed that Klf4 has an important role in formation and maintenance of corneal epithelial basement membrane.
Figure 4.
The Dox-15 Klf4Δ/ΔCE corneal epithelial basement membrane is disrupted. (A) Immunoblots revealed decreased amount of laminin-332 within the Dox-15 Klf4Δ/ΔCE corneas. Densitometry with actin as loading control quantified the amount of laminin-332 within the Dox-15 Klf4Δ/ΔCE corneas to be approximately 40% of that in the WT. (B) Immunofluorescent staining detected a thick and continuous band of laminin corresponding to the epithelial basement membrane within the WT and the Dox-5 Klf4Δ/ΔCE corneas ([B-d, B-e], respectively; long arrows), but not the Dox-15 Klf4Δ/ΔCE corneas ([B-f], short arrows). In contrast, Mmp9 was more abundant in the Dox-15 Klf4Δ/ΔCE corneal epithelium (B-i), compared to the WT (B-g) and the Dox-5 Klf4Δ/ΔCE (B-h) corneas. No background staining was observed in isotype antibody control (B-a, B-b, B-c).
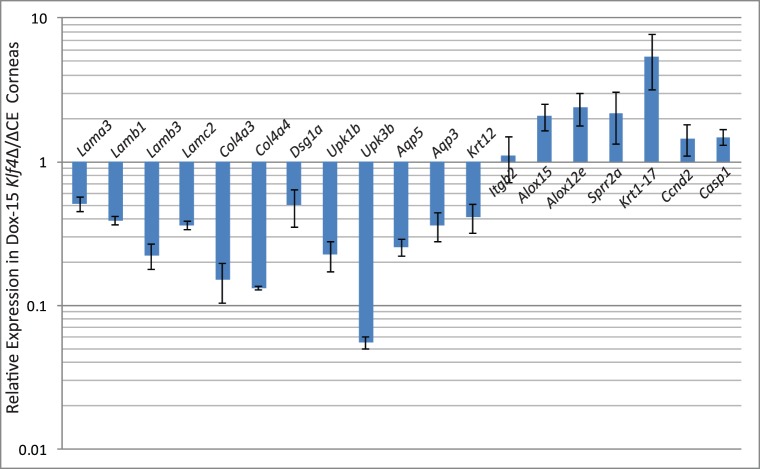
To decipher if the disruption of Klf4 in mature corneal epithelium alters the expression of selected corneal Klf4-target genes,46 we compared their levels by QPCR (Fig. 5). The expression of corneal epithelial basement membrane components Lama3, Lamb1, Lamb3, Lamc2, Col4a3, and Col4a457–62 was decreased in Dox-15 Klf4Δ/ΔCE corneas consistent with their defective basement membrane (Fig. 5). Expression of corneal epithelial markers Aqp5, Aqp3, and Krt12 was decreased, and that of markers of keratinized epithelia, such as Alox15, Alox12e, Sprr2a, and Krt17, increased, in Dox-15 Klf4Δ/ΔCE corneas (Fig. 5). Also, cell cycle regulator Ccnd2 expression was elevated, consistent with the increased number of cell layers in the Dox-15 Klf4Δ/ΔCE corneal epithelium (Fig. 5). Immunofluorescent staining with anti-Ki67 antibody revealed a mean of 3.50 Ki67-positive cells per 100 μm distance across central corneal epithelial cross-section in the WT (SD 0.005, N = 8), which increased to 4.52 Ki67-positive cells per 100 μm (SD 0.01, N = 8) within the Dox-15 Klf4Δ/ΔCE corneas (Fig. 6). The mean proliferative index determined by dividing the number of Ki67-positive cells by the total number of epithelial cells was significantly higher for the Dox-15 Klf4Δ/ΔCE (mean 0.144, SD 0.02, N = 8) compared to the WT (mean 0.107, SD 0.03, N = 8) corneal epithelial cells. Notably, some suprabasal cells also were Ki67-positive in the Dox-15 Klf4Δ/ΔCE corneal epithelium, suggesting that the loss of Klf4 disrupted the fine balance between epithelial cell proliferation and differentiation (Fig. 6). We then determined if the disruption of Klf4 in the Dox-15 Klf4Δ/ΔCE corneal epithelium is associated with an abnormal epithelial phenotype. Immunoblots revealed that the Dox-15 Klf4Δ/ΔCE corneal desmosomal components desmoglein (Dsg), and desmoplakins-I and -II (Dsp-I and Dsp-II) are downregulated to approximately 30%, 70%, and 70% of that in the WT, respectively (Fig. 7A), which was confirmed by immunofluorescent staining (Fig. 7B). Additional immunoblots and immunofluorescent staining revealed that the expression of corneal epithelial–specific keratin-12 is decreased by 65% and that of noncorneal keratin-17 increased by 4.6-fold in Dox-15 Klf4Δ/ΔCE corneas, consistent with their loss of corneal epithelial cell identity (Fig. 8). Together, these results demonstrated that Klf4 has an important role in maintenance of the corneal epithelial cell identity and structural integrity.
Figure 5.
Relative expression of selected Klf4-target genes in the Dox-15 Klf4Δ/ΔCE corneas. Total RNA was isolated from dissected WT and Dox-15 Klf4Δ/ΔCE corneas, converted into cDNA and the expression levels of selected Klf4-target genes compared by real-time QPCR. Expression levels of indicated genes are plotted relative to WT (equivalent to 1), on a logarithmic scale (n = 3). Error bars: standard error of means.
Figure 6.
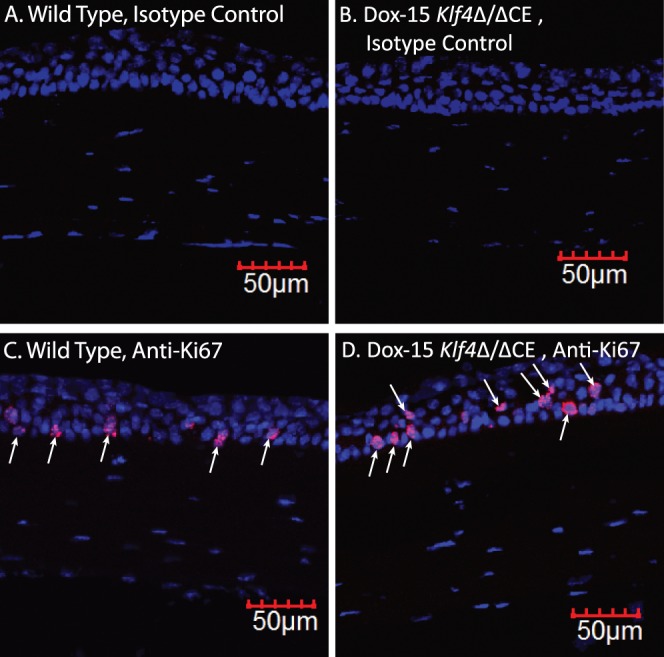
Increased proliferative index in the Dox-15 Klf4Δ/ΔCE corneal epithelium. Immunofluorescent staining detected a larger number of Ki67-positive cells (red; indicated by arrows) in the Dox-15 Klf4Δ/ΔCE corneal epithelium (D) compared to the WT (C). Some suprabasal cells also stained positive for Ki67 in the Dox-15 Klf4Δ/ΔCE corneal epithelium ([D], downward arrows). No background staining was observed in isotype antibody control (A, B).
Figure 7.
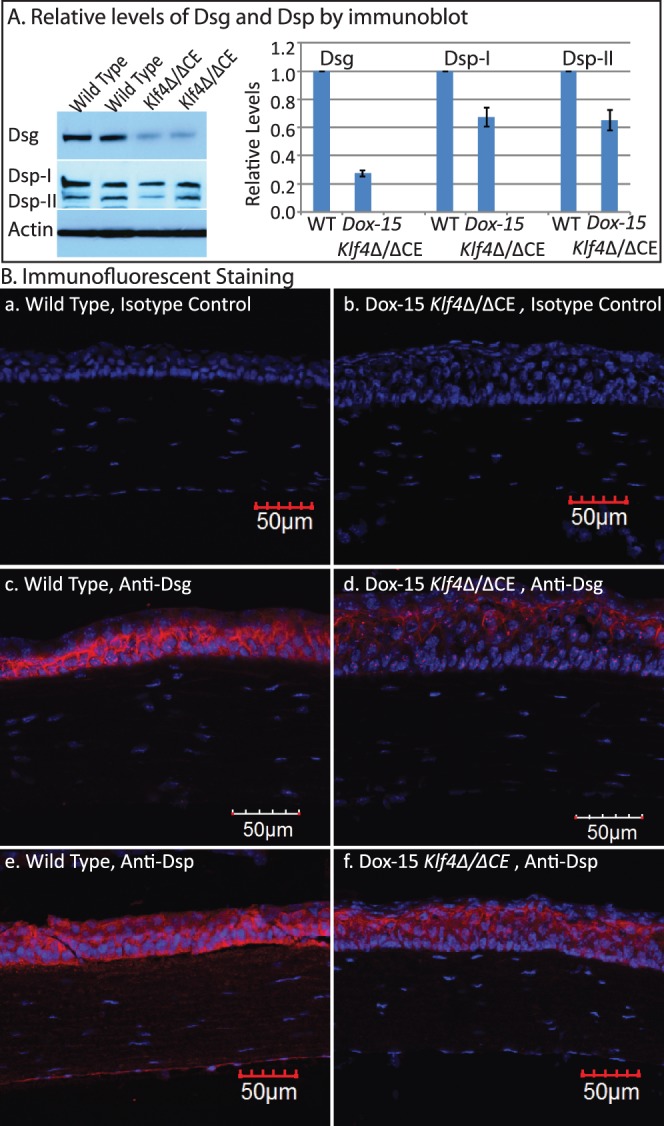
Decreased expression of desmosomal components in the Dox-15 Klf4Δ/ΔCE corneas. Immunoblots (A) revealed decreased amount of Dsg, Dsp-I, and Dsp-II in the Klf4Δ/ΔCE corneas after 15 days of doxycycline treatment. Densitometry with actin as loading control quantified the amounts of Dsg, Dsp-I, and Dsp-II within the Dox-15 Klf4Δ/ΔCE corneas to be approximately 30%, 70%, and 70% of that in the WT, respectively. Consistent with this, immunofluorescent staining (B) detected robust expression of Dsg, Dsp-I, and Dsp-II in the WT (red; [B-c, B-e], respectively), which was decreased in the Dox-15 Klf4Δ/ΔCE (B-d, B-f) corneal epithelium. No background staining was observed in isotype antibody control (B-a, B-b).
Figure 8.
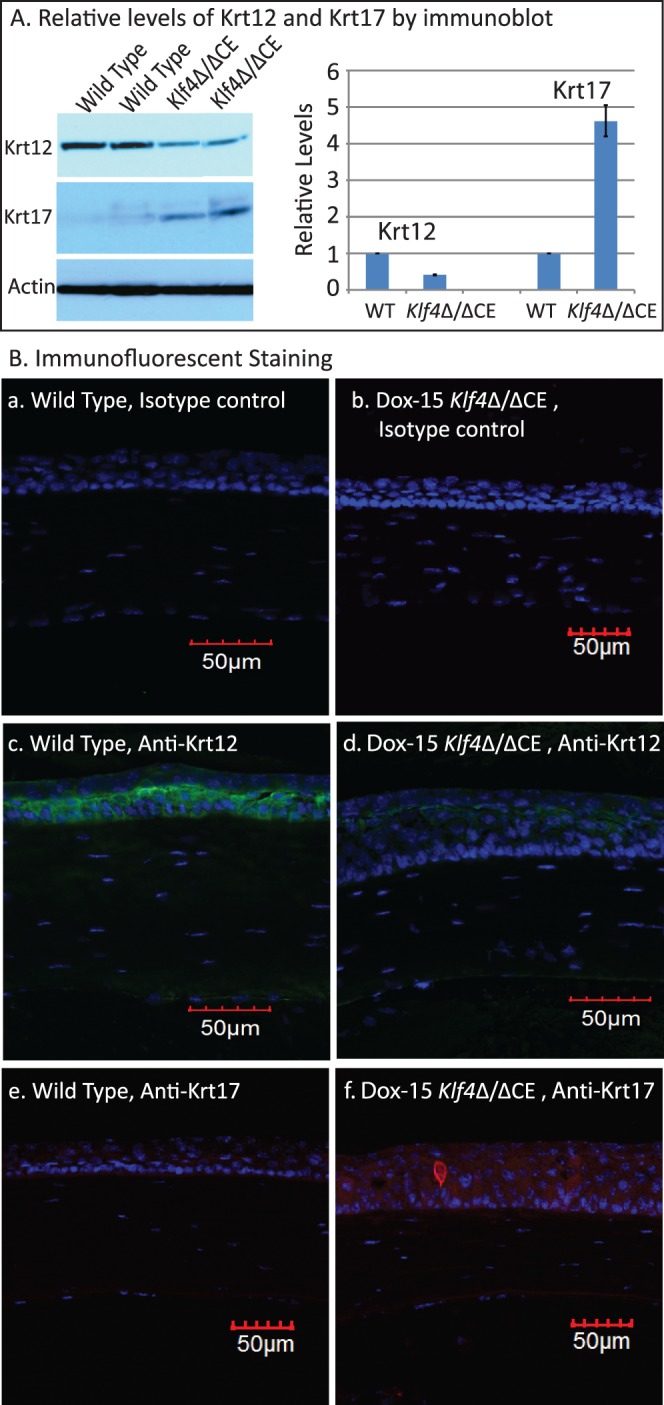
Dox-15 Klf4Δ/ΔCE corneal epithelial identity is altered. Immunoblots (A) revealed Krt12 to be decreased and Krt17 to be increased in the Dox-15 Klf4Δ/ΔCE corneas. Densitometry with actin as loading control quantified the amounts of Krt12 and Krt17 within the Dox-15 Klf4Δ/ΔCE corneas to be approximately 30% and 450% of that in the WT, respectively. Consistent with this, immunofluorescent staining detected robust expression of Krt12 in the WT (green; [B-c]), but not the Dox-15 Klf4Δ/ΔCE (B-d) corneal epithelium. In contrast, Krt17, undetectable in the WT corneas (red; [B-e]), was present in the Dox-15 Klf4Δ/ΔCE (red; [B-f]) corneal epithelium. No background staining was observed in isotype antibody control (B-a, B-b).
Discussion
The corneal epithelium is a constantly rejuvenating tissue at the forefront of our eyes, abnormalities in which lead to debilitating pain, corneal opacity, and blindness in severe cases. In spite of the important role of the epithelium in facilitating corneal transparence, our understanding of the molecular mechanisms regulating corneal epithelial homeostasis is incomplete. In this report, we demonstrated that spatiotemporally regulated disruption of Klf4 expression in normally-formed adult corneal epithelium results in multiple abnormalities, including loss of corneal epithelial barrier function, deformed epithelial basement membrane, increased cell proliferation, disrupted corneal epithelial structural integrity, and altered corneal epithelial identity. Together, these results provided convincing evidence that Klf4 has a prominent role in maintenance of mature corneal epithelial homeostasis.
Our previous studies used conditional disruption of Klf4 in the surface ectoderm-derived tissues of the eye with the help of Le-Cre transgene to uncover the functions of Klf4 in ocular surface maturation.20,44–46,49–51,63 In those studies, Klf4 was disrupted all over the ocular surface from approximately E-10, making it impossible to parse out the direct effects of the absence of Klf4 in the corneal epithelium from the indirect effects of the defects in ocular adnexae, and the functions of Klf4 in corneal maturation from those in maintenance of the mature cornea. Also, a recent report suggested that hemizygous Le-Cre transgenic mice occasionally develop eye abnormalities on some genetic backgrounds even in the absence of LoxP sites, raising concerns about the validity of our previous findings.52 In this study, we have overcome those limitations and dispelled any concerns by spatiotemporally regulated ablation of Klf4 within the adult corneal epithelium using Klf4f/f/Krt12rtTA/rtTA/Tet-O-Cre ternary transgenic mouse system.53,54 Taken together with our previous results,20,44–46,50,51,63 this report established that Klf4 is essential for maturation and maintenance of the mature corneal epithelial cells.
Diverse epithelial tissues in our body provide effective barriers against the external elements. The corneal epithelial barrier function is essential for defending the eye against chemical, biological, and physical insults. Loss of corneal epithelial barrier function is associated with painful ocular surface disorders, such as dry eye, ocular cicatricial pemphigoid, and Meesmann's dystrophy.1,2 The data presented here revealed a correlation between Dox-15 Klf4Δ/ΔCE corneal epithelial barrier disruption evidenced by fluorescein uptake, and abnormal tight junctions within the superficial cell layers evidenced by decreased ZO-1 expression. Our data also demonstrated that the absence of Klf4 results in imbalance in the rates of corneal epithelial cell proliferation, differentiation, and desquamation. The defective barrier and disrupted structural integrity in Klf4Δ/ΔCE corneas suggests that the previously described defects in Klf4CN corneal epithelial barrier function45 are a direct outcome of the absence of Klf4, and not an indirect result of the associated defects in ocular adnexae.20 Taken together with the data from other epithelial systems, this report cements the role of Klf4 as an important regulator of normal proliferation, differentiation, and desquamation of diverse types of epithelial tissues, including the corneal epithelium.39,42,48,50,64–66
The corneal epithelial basement membrane anchors epithelial cells to the stroma, provides scaffolding during embryonic development, migration, differentiation, and serves as a barrier for diffusion of cytokines between the epithelium and the stroma.62 Current data taken together with previous reports67,68 suggest that Klf4 contributes to the formation and maintenance of the epithelial basement membrane by promoting the expression of different components, including laminins and collagens. Comparison of the Dox-5 and Dox-15 Klf4Δ/ΔCE data suggests that the elevated expression of Mmp9 may have further promoted the degradation of Dox-15 Klf4Δ/ΔCE corneal epithelial basement membrane, akin to the degradation of basement membrane β4 integrin during corneal epithelial recurrent erosions.69
Though this report demonstrates the important contributions of Klf4 to maturation and maintenance of corneal epithelial homeostasis unequivocally, several interesting questions remain unanswered. For example, whether increased expression of Mmp9 in the Klf4Δ/ΔCE corneal epithelium represents de-repression in the absence of Klf4, or an indirect wound healing response to epithelial fragility70,71 remains to be determined. Similarly, whether any causality exists between disruption of the Klf4Δ/ΔCE corneal superficial epithelial barrier function and the basal lamina remains to be tested. It also would be interesting to withdraw doxycycline and test how long it takes for the defective Dox-15 Klf4Δ/ΔCE corneal epithelium to repair itself.
In summary, these results confirm that Klf4 has an essential role in establishment and maintenance of the corneal epithelial identity and structural integrity. The corneal epithelial characteristics, such as cell shape, polarity, cell-cell adhesion, and regenerative capacities, are directly dependent on its gene expression pattern, disruption of which results in the loss of epithelial cell identity, epithelial-to-mesenchymal transition (EMT), and squamous metaplasia.1,21,28,36,37 The Klf4Δ/ΔCE corneal epithelial deregulated proliferation, disrupted structural integrity, and loss of epithelial identity are consistent with the antiproliferative, antimetastatic, and EMT-suppressive nature of Klf4.38,40,42,72–78 How Klf4, a widely expressed transcription factor, exerts distinct regulatory influences on a uniquely specialized tissue, such as the corneal epithelium, remains to be established.
Acknowledgments
The authors thank Kate Davoli (Histology Core Facility) for help with histology and Kira Lathrop (Imaging Core Facility) for help with imaging. We dedicate this manuscript to the memory of our esteemed colleague David Beebe, who inspired many of us with his infectious enthusiasm for understanding eye development.
Supported by NIH Grant R01EY022898 (SKS), NEI Core Grant P30 EY08098, unrestricted grants from Research to Prevent Blindness and the Eye and Ear Foundation of Pittsburgh, and by startup funds from the Department of Ophthalmology, University of Pittsburgh (SKS). Supported in part by the Pennsylvania Lions Eye Research Sight Conservation Foundation, and a summer research fellowship from “Fight for Sight/The Eye Bank for Sight” (EED).
Disclosure: E.E. Delp, None; S. Swamynathan, None; W.W. Kao, None; S.K. Swamynathan, None
References
- 1. Kinoshita S,, Adachi W,, Sotozono C,, et al. Characteristics of the human ocular surface epithelium. Prog Retin Eye Res. 2001; 20: 639–673. [DOI] [PubMed] [Google Scholar]
- 2. Klintworth GK. The molecular genetics of the corneal dystrophies--current status. Front Biosci. 2003; 8: d687–713. [DOI] [PubMed] [Google Scholar]
- 3. Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1979; 63: 263–322. [DOI] [PubMed] [Google Scholar]
- 4. Zieske JD. Corneal development associated with eyelid opening. Int J Devel Biol. 2004; 48: 903–911. [DOI] [PubMed] [Google Scholar]
- 5. Wolosin JM,, Budak MT,, Akinci MA. Ocular surface epithelial and stem cell development. Int J Devel Biol. 2004; 48: 981–991. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki K,, Saito J,, Yanai R,, et al. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003; 22: 113–133. [DOI] [PubMed] [Google Scholar]
- 7. Balda MS,, Matter K. Tight junctions at a glance. J Cell Sci. 2008; 121: 3677–3682. [DOI] [PubMed] [Google Scholar]
- 8. Sugrue SP,, Zieske JD. ZO1 in corneal epithelium: association to the zonula occludens and adherens junctions. Exp Eye Res. 1997; 64: 11–20. [DOI] [PubMed] [Google Scholar]
- 9. Ban Y,, Dota A,, Cooper LJ,, et al. Tight junction-related protein expression and distribution in human corneal epithelium. Exp Eye Res. 2003; 76: 663–669. [DOI] [PubMed] [Google Scholar]
- 10. Kottke MD,, Delva E,, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006; 119: 797–806. [DOI] [PubMed] [Google Scholar]
- 11. Stepp MA,, Spurr-Michaud S,, Gipson IK. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Invest Ophthalmol Vis Sci. 1993; 34: 1829–1844. [PubMed] [Google Scholar]
- 12. Kumar NM,, Gilula NB. The gap junction communication channel. Cell. 1996; 84: 381–388. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi Y,, Call MK,, Liu CY,, et al. Monoallelic expression of Krt12 gene during corneal-type epithelium differentiation of limbal stem cells. Invest Ophthalmol Vis Sci. 2010; 51: 4562–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tanifuji-Terai N,, Terai K,, Hayashi Y,, Chikama T,, Kao WW. Expression of keratin 12 and maturation of corneal epithelium during development and postnatal growth. Invest Ophthalmol Vis Sci. 2006; 47: 545–551. [DOI] [PubMed] [Google Scholar]
- 15. Thoft RA. The role of the limbus in ocular surface maintenance and repair. Acta Ophthalmol Suppl. 1989; 192: 91–94. [DOI] [PubMed] [Google Scholar]
- 16. Thoft RA,, Wiley LA,, Sundarraj N. The multipotential cells of the limbus. Eye (Lond). 1989; 3: 109–113. [DOI] [PubMed] [Google Scholar]
- 17. Beebe DC,, Masters BR. Cell lineage and the differentiation of corneal epithelial cells. Invest Ophthalmol Vis Sci. 1996; 37: 1815–1825. [PubMed] [Google Scholar]
- 18. Chen Y,, Carlson EC,, Chen ZY,, et al. Conditional deletion of Cited2 results in defective corneal epithelial morphogenesis and maintenance. Dev Biol. 2009; 334: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kenchegowda D,, Swamynathan S,, Gupta D,, Wan H,, Whitsett J,, Swamynathan SK. Conditional disruption of mouse Klf5 results in defective eyelids with malformed meibomian glands abnormal cornea and loss of conjunctival goblet cells. Dev Biol. 2011; 356: 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Swamynathan SK,, Katz JP,, Kaestner KH,, Ashery-Padan R,, Crawford MA,, Piatigorsky J. Conditional deletion of the mouse Klf4 gene results in corneal epithelial fragility stromal edema, and loss of conjunctival goblet cells. Mol Cell Biol. 2007; 27: 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swamynathan SK. Ocular surface development and gene expression. J Ophthalmol. 2013; 2013: 103947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang X,, Tseng H. Basonuclin-null mutation impairs homeostasis and wound repair in mouse corneal epithelium. PLoS One. 2007; 2: e1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshida N,, Yoshida S,, Araie M,, Handa H,, Nabeshima Y. Ets family transcription factor ESE-1 is expressed in corneal epithelial cells and is involved in their differentiation. Mech Dev. 2000; 97: 27–34. [DOI] [PubMed] [Google Scholar]
- 24. West-Mays JA,, Sivak JM,, Papagiotas SS,, et al. Positive influence of AP-2alpha transcription factor on cadherin gene expression and differentiation of the ocular surface. Differentiation. 2003; 71: 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sivak JM,, West-Mays JA,, Yee A,, Williams T,, Fini ME. Transcription factors Pax6 and AP-2alpha interact to coordinate corneal epithelial repair by controlling expression of matrix metalloproteinase gelatinase B. Mol Cell Biol. 2004; 24: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okada Y,, Saika S,, Shirai K,, Ohnishi Y,, Senba E. Expression of AP-1 (c-fos/c-jun) in developing mouse corneal epithelium. Graefe's Arch Clin Exp Ophthalmol. 2003; 241: 330–333. [DOI] [PubMed] [Google Scholar]
- 27. Davis J,, Davis D,, Norman B,, Piatigorsky J. Gene expression of the mouse corneal crystallin Aldh3a1: activation by Pax6, Oct1, and p300. Invest Ophthalmol Vis Sci. 2008; 49: 1814–1826. [DOI] [PubMed] [Google Scholar]
- 28. Stephens DN,, Klein RH,, Salmans ML,, Gordon W,, Ho H,, Andersen B. The Ets transcription factor EHF as a regulator of cornea epithelial cell identity. J Biol Chem. 2013; 288: 34304–34324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adhikary G,, Crish JF,, Gopalakrishnan R,, Bone F,, Eckert RL. Involucrin expression in the corneal epithelium: an essential role for Sp1 transcription factors. Invest Ophthalmol Vis Sci. 2005; 46: 3109–3120. [DOI] [PubMed] [Google Scholar]
- 30. Gaudreault M,, Carrier P,, Larouche K,, et al. Influence of sp1/sp3 expression on corneal epithelial cells proliferation and differentiation properties in reconstructed tissues. Invest Ophthalmol Vis Sci. 2003; 44: 1447–1457. [DOI] [PubMed] [Google Scholar]
- 31. Birger Y,, Davis J,, Furusawa T,, Rand E,, Piatigorsky J,, Bustin M. A role for chromosomal protein HMGN1 in corneal maturation. Differentiation. 2006; 74: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collinson JM,, Chanas SA,, Hill RE,, West JD. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6(+/−) mouse. Invest Ophthalmol Vis Sci. 2004; 45: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 33. Davis J,, Duncan MK,, Robison WG,, Jr, Piatigorsky J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 2003; 116: 2157–2167. [DOI] [PubMed] [Google Scholar]
- 34. Ramaesh T,, Collinson JM,, Ramaesh K,, Kaufman MH,, West JD,, Dhillon B. Corneal abnormalities in Pax6+/− small eye mice mimic human aniridia-related keratopathy. Invest Ophthalmol Vis Sci. 2003; 44: 1871–1878. [DOI] [PubMed] [Google Scholar]
- 35. Ramaesh T,, Ramaesh K, Martin Collinson J, Chanas SA, Dhillon B, West JD. Developmental and cellular factors underlying corneal epithelial dysgenesis in the Pax6+/− mouse model of aniridia. Exp Eye Res. 2005; 81: 224–235. [DOI] [PubMed] [Google Scholar]
- 36. Li W,, Chen YT,, Hayashida Y,, et al. Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J Pathol. 2008; 214: 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Norman B,, Davis J,, Piatigorsky J. Postnatal gene expression in the normal mouse cornea by SAGE. Invest Ophthalmol Vis Sci. 2004; 45: 429–440. [DOI] [PubMed] [Google Scholar]
- 38. Rowland BD,, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006; 6: 11–23. [DOI] [PubMed] [Google Scholar]
- 39. Segre JA,, Bauer C,, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999; 22: 356–360. [DOI] [PubMed] [Google Scholar]
- 40. Yang Y,, Goldstein BG,, Chao HH,, Katz JP. KLF4 and KLF5 regulate proliferation, apoptosis and invasion in esophageal cancer cells. Cancer Biol Ther. 2005; 4: 1216–1221. [DOI] [PubMed] [Google Scholar]
- 41. Swamynathan SK. Kruppel-like factors: three fingers in control. Hum Genomics. 2010; 4: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghaleb AM,, Nandan MO,, Chanchevalap S,, Dalton WB,, Hisamuddin IM,, Yang VW. Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005; 15: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McConnell BB,, Ghaleb AM,, Nandan MO,, Yang VW. The diverse functions of Kruppel-like factors 4 and 5 in epithelial biology and pathobiology. Bioessays. 2007; 29: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swamynathan S,, Buela KA,, Kinchington P,, et al. Klf4 regulates the expression of Slurp1, which functions as an immunomodulatory peptide in the mouse cornea. Invest Ophthalmol Vis Sci. 2012; 53: 8433–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swamynathan S,, Kenchegowda D,, Piatigorsky J,, Swamynathan SK. Regulation of corneal epithelial barrier function by Kruppel-like transcription factor 4. Invest Ophthalmol Vis Sci. 2011; 52: 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Swamynathan SK,, Davis J,, Piatigorsky J. Identification of candidate Klf4 target genes reveals the molecular basis of the diverse regulatory roles of Klf4 in the mouse cornea. Invest Ophthalmol Vis Sci. 2008; 49: 3360–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young RD,, Swamynathan SK,, Boote C,, et al. Stromal edema in klf4 conditional null mouse cornea is associated with altered collagen fibril organization and reduced proteoglycans. Invest Ophthalmol Vis Sci. 2009; 50: 4155–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Katz JP,, Perreault N,, Goldstein BG,, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002; 129: 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ashery-Padan R,, Marquardt T,, Zhou X,, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000; 14: 2701–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gupta D,, Harvey SA,, Kaminski N,, Swamynathan SK. Mouse conjunctival forniceal gene expression during postnatal development and its regulation by Kruppel-like factor 4. Invest Ophthalmol Vis Sci. 2011; 52: 4951–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gupta D,, Harvey SA,, Kenchegowda D,, Swamynathan S,, Swamynathan SK. Regulation of mouse lens maturation and gene expression by Kruppel-like factor 4. Exp Eye Res. 2013; 116: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dora NJ,, Collinson JM,, Hill RE,, West JD. Hemizygous Le-Cre transgenic mice have severe eye abnormalities on some genetic backgrounds in the absence of LoxP sites. PLoS One. 2014; 9: e109193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chikama T,, Hayashi Y,, Liu CY,, et al. Characterization of tetracycline-inducible bitransgenic Krt12rtTA/+/tet-O-LacZ mice. Invest Ophthalmol Vis Sci. 2005; 46: 1966–1972. [DOI] [PubMed] [Google Scholar]
- 54. Chikama T,, Liu CY,, Meij JT,, et al. Excess FGF-7 in corneal epithelium causes corneal intraepithelial neoplasia in young mice and epithelium hyperplasia in adult mice. Am J Pathol. 2008; 172: 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Domogatskaya A,, Rodin S,, Tryggvason K. Functional diversity of laminins. Ann Rev Cell Dev Biol. 2012; 28: 523–553. [DOI] [PubMed] [Google Scholar]
- 56. Glentis A,, Gurchenkov V,, Vignjevic DM. Assembly heterogeneity, and breaching of the basement membranes. Cell Adh Migr. 2014; 8: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kuhn K. Basement membrane (type IV) collagen. Matrix Biol. 1995; 14: 439–445. [DOI] [PubMed] [Google Scholar]
- 58. Paulsson M. Basement membrane proteins: structure assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992; 27: 93–127. [DOI] [PubMed] [Google Scholar]
- 59. Ljubimov AV,, Burgeson RE,, Butkowski RJ,, Michael AF,, Sun TT,, Kenney MC. Human corneal basement membrane heterogeneity: topographical differences in the expression of type IV collagen and laminin isoforms. Lab Invest. 1995; 72: 461–473. [PubMed] [Google Scholar]
- 60. Ohji M,, SundarRaj N,, Hassell JR,, Thoft RA. Basement membrane synthesis by human corneal epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1994; 35: 479–485. [PubMed] [Google Scholar]
- 61. Fukuda K,, Chikama T,, Nakamura M,, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999; 18: 73–79. [PubMed] [Google Scholar]
- 62. Torricelli AA,, Singh V,, Santhiago MR,, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013; 54: 6390–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Swamynathan S,, Swamynathan SK. SLURP-1 modulates corneal homeostasis by serving as a soluble scavenger of urokinase-type plasminogen activator. Invest Ophthalmol Vis Sci. 2014; 55: 6251–6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McConnell BB,, Kim SS,, Yu K,, et al. Kruppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine. Gastroenterology. 2011; 141: 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang W,, Chen X,, Kato Y,, et al. Novel cross talk of Kruppel-like factor 4 and beta-catenin regulates normal intestinal homeostasis and tumor repression. Mol Cell Biol. 2006; 26: 2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ghaleb AM,, McConnell BB,, Kaestner KH,, Yang VW. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev Biol. 2011; 349: 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Piccinni SA,, Bolcato-Bellemin AL,, Klein A,, et al. Kruppel-like factors regulate the Lama1 gene encoding the laminin alpha1 chain. J Biol Chem. 2004; 279: 9103–9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miller KA,, Eklund EA,, Peddinghaus ML,, et al. Kruppel-like factor 4 regulates laminin alpha 3A expression in mammary epithelial cells. J Biol Chem. 2001; 276: 42863–42868. [DOI] [PubMed] [Google Scholar]
- 69. Pal-Ghosh S,, Blanco T,, Tadvalkar G,, et al. MMP9 cleavage of the beta4 integrin ectodomain leads to recurrent epithelial erosions in mice. J Cell Sci. 2011; 124: 2666–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mohan R,, Chintala SK,, Jung JC,, et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002; 277: 2065–2072. [DOI] [PubMed] [Google Scholar]
- 71. Fini ME,, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005; 24: S2–S11. [DOI] [PubMed] [Google Scholar]
- 72. Yori JL,, Johnson E,, Zhou G,, Jain MK,, Keri RA. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J Biol Chem. 2010; 285: 16854–16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cui J,, Shi M,, Quan M,, Xie K. Regulation of EMT by KLF4 in gastrointestinal cancer. Curr Cancer Drug Targets. 2013; 13: 986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tiwari N,, Meyer-Schaller N,, Arnold P,, et al. Klf4 is a transcriptional regulator of genes critical for EMT, including Jnk1 (Mapk8). PLoS One. 2013; 8: e57329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen Z,, Wang Y,, Liu W,, et al. Doxycycline inducible kruppel-like factor 4 lentiviral vector mediates mesenchymal to epithelial transition in ovarian cancer cells. PLoS One. 2014; 9: e105331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lin ZS,, Chu HC,, Yen YC,, Lewis BC,, Chen YW. Kruppel-like factor 4 a tumor suppressor in hepatocellular carcinoma cells reverts epithelial mesenchymal transition by suppressing slug expression. PLoS One. 2012; 7: e43593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu YN,, Abou-Kheir W,, Yin JJ,, et al. Critical and reciprocal regulation of KLF4 and SLUG in transforming growth factor beta-initiated prostate cancer epithelial-mesenchymal transition. Mol Cell Biol. 2012; 32: 941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li R,, Liang J,, Ni S,, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010; 7: 51–63. [DOI] [PubMed] [Google Scholar]