Abstract
Five new 2,9,9-trimethyl-6,7,8,9-tetrahydro-benzocyclohepten-5-ylidene-amine derivatives (16a-16e) were synthesized from α-dehydro-ar-himachalene (11) that was originally prepared from an isomeric mixture of α, β and γ himachalenes (10), the abundant sesquiterpenes of Cedrus deodara essential oil. In addition, different aryl himachalenes derivatives (9, 12, 14 and 15) were also formed from 11. The structures of the synthesized compounds were confirmed on the basis of their NMR, IR and mass spectral data. The prepared compounds were tested against a group of sixteen organisms including gram positive and gram negative bacterial and fungal strains. The introduction of a series of substituted imine groups into aryl himachalenes at 5th position (16a-16e) enhanced antimicrobial activity as compared to the aromatized derivatives (9, 12, 14 and 15) against gram-positive bacteria Bacillus subtilis, Micrococcus luteus and Staphylococcus aureus, and mycotoxigenic fungi Aspergillus parasiticus, A. ochraceous and A. sydowii. graphical Abstract, Figure 1(Fig. 1)
Keywords: himachalenes, Cedrus deodara, essential oil, antimicrobial activity
Introduction
The essential oil of the Himalayan Cedar (Cedrus deodara) plays an important role of starting material in the fragrance and pharmaceutical industries (Hossini et al., 2011[11]; Bhushan et al., 2006[2]). This oil contains three major sesquiterpenes named α-cis-, ß-, and γ-cis-himachalenes having hexahydrobenzocycloheptene as basic skeleton (Chaudhary et al., 2009[5]). Benzocycloheptene and their derivatives are the biologically potent class of bicyclic framework and are attractive synthetic targets for organic and medicinal chemists (Chaudhary et al., 2012[4]). These derivatives have been prepared by different routes, such as the enlargement of six-membered rings, cyclization (Lynch and Macdonald, 2009[14]; Hattori and Tanaka, 2002[10]) and coupling reactions or from benzocycloheptanone (Bohlmann et al., 2006[3]) involving either oxime formation, reductive amination, through azide formation, α-bromination or cyanoboration (Nedelec et al., 1979[16], Nakano et al., 2010[15]; Chow et al., 2009[6]; Tandon et al., 2004[24]). Nitrogen containing substituted benzocycloheptenes have been found to act as ORL-1 receptor agonists 1 (Figure 2(Fig. 2)) and ß3 adrenergic receptor agonists 2, α-sympathomimetic, anorexigenic 3, antidepressant 4, analgesic and antiarrhythmic agents 5. These were also reported for modulation of small-conductance calcium-activated potassium channels 6, for treatment of psoriasis 7, cardiovascular 8, and neurodegenerative diseases (Chaudhary et al., 2012[4]; Sorensen et al., 2008[23]; Zaratin et al., 2004[25]; Tandon et al., 2004[24]).
Figure 2. Structures of biologically potent benzocycloheptene analogues.
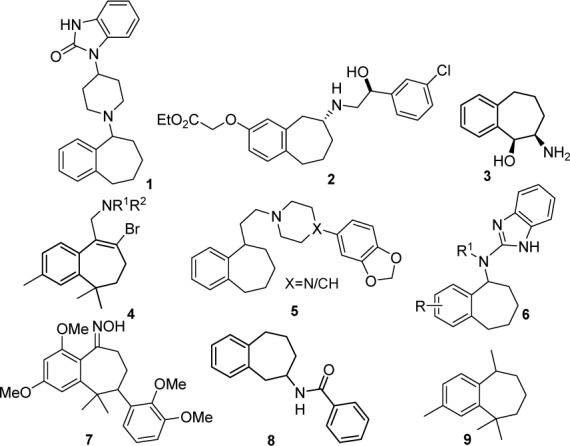
The reactivity of himachalenes has been studied extensively since their isolation by Dev and co-workers and subjected to various chemical modifications such as cyclopropanation, oxidation, hydroxylation and epoxidation and total syntheses to improve their biological activities (Chaudhary et al., 2012[4]; Daoubi et al., 2005[9]; Hossini et al., 2011[11]; Joseph and Dev, 1961[12]). On reaction with various aromatizing agents, mixture of products was yielded (Shankaranarayan et al., 1977[20]). Chemical analysis of the pheromone gland extract of sandfly and flea beetles revealed ar-himachalene 9, himachalene and their methyl derivatives as sex pheromones (Figure 2(Fig. 2)) (Zilkowski et al., 2006[27]). The insecticidal principles, himachalol and ß-himachalene showed potency against pulse beetle and housefly (Singh and Agarwal, 1988[22]). Hydroxylated derivatives of ß-himachalene possess promising antifungal potential against phytopathogen Botrytis cinerea (Daoubi et al., 2005[9]).
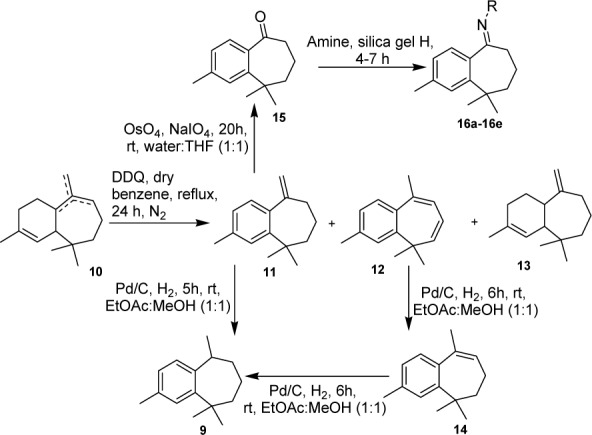
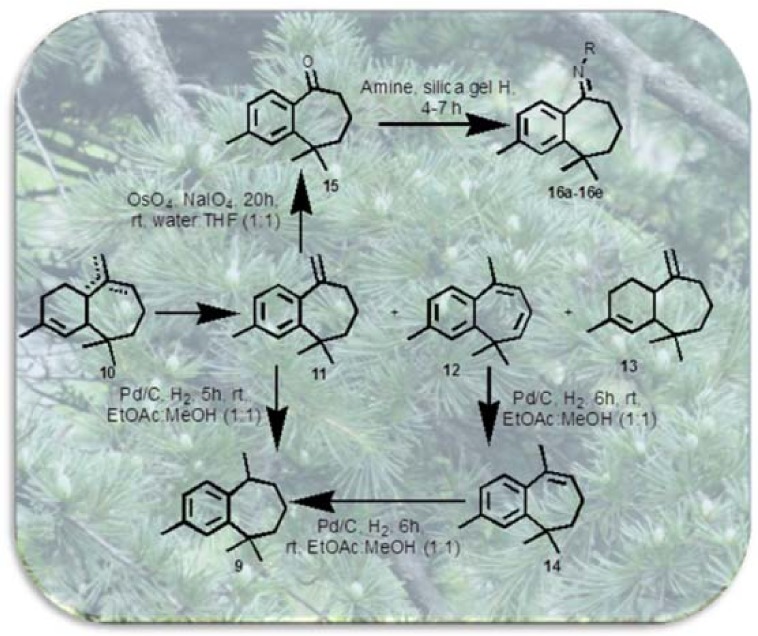
In continuation of our previous work (Chaudhary et al., 2012[4]) to synthesize nitrogen containing benzocycloheptene analogues, herein, we have developed a simple and practical pathway for the synthesis of important similar benzocycloheptene analogues from α-dehydro-ar-himachalene (11) (Figure 3(Fig. 3)) and evaluated them for antimicrobial activities against opportunistic human bacterial and fungal pathogens.
Figure 3. Figure 3: Synthesis of ar-himachalene derivatives from α-dehydro-ar-himachalene.
Materials and Methods
General
All NMR spectra were recorded on a Bruker Avance-300 instrument (300 MHz 1H, 75.46 MHz 13C) using CDCl3 and TMS as solvent and reference, respectively. Chemical shifts (δ) were given in parts per million. Silica gel (60-120 mesh) for column chromatography, TLC silica gel 60 F254 plates, silica gel (H) and all other chemicals and solvents used were purchased from Merck India Ltd. DMSO and ampicillin and nystatin was purchased from Sigma Aldrich Co., MO, USA and HiMedia Laboratories Pvt. Ltd., Mumbai, India respectively. GC-MS analysis was conducted on a GC-MS (QP2010) Shimadzu gas chromatograph mass spectrometer. A carbowax phase, BP-20 capillary column (30 m × 0.25 mm i.d. with film thickness 0.25 μm) was used with helium as a carrier gas at a flow rate of 1.1 ml/min on split mode (1:50). The injector temperature was programmed from 40-220 °C at 4 °C/min rise with 4 min hold at 40 °C and 15 min hold at 220 °C and interface temperatures were 250 °C. Ion source temperature was 200 °C. Sample (20 μl) was dissolved in 2 ml GC grade dichloromethane and sample injection volume was 2 μl. IR spectra were obtained on a Nicolet 5700 FTIR (Thermo, USA) spectrophotometer in the region 4000-400 cm-1 using KBr discs. Mass spectra were recorded on a Waters QTOF-MS with ESI using Waters Mass lynx 4.1 software.
General procedure for synthesis of 5H-benzocycloheptene 11-13
To a solution of himachalenes (10) (1g, 4.902 mmol) in dry benzene (30 ml) was added DDQ (1.1 g, 9.804 mmol) and the mixture was stirred at reflux for 24 h under N2. The solvent was removed under reduced pressure. The reaction was then quenched by adding 5% sodium bicarbonate solution and extracted with ethyl acetate. Organic layer was finally concentrated and chromatographed on silica gel (heptane 100%) to afford 11, 12 and 13 as colorless oil.
2,9,9-Trimethyl-5-methylene-6,7,8,9-tetrahydro-5H-benzocycloheptene 11
The spectroscopic data of compound 11 was reported earlier (Chaudhary et al., 2012[4]).
3,5,5,9-Tetramethyl-5H-benzocycloheptene 12
Colorless oil (yield 21 %). IR (KBr): λmax = 2955, 2924, 2873, 1721, 1283, 774 cm-1. 1H NMR (300 MHz, CDCl3) δ = 7.59 (1H, d, J = 7.6 Hz), 7.26 (1H, s), 7.16 (1H, d, J = 7.5 Hz), 6.40-6.42 (1H, m), 6.00-6.05 (1H, m), 5.61 (1H, d, J = 10.2 Hz), 2.47 (6H, s), 1.41 (6H, s). 13C NMR (75.4 MHz, CDCl3) δ = 144.9, 139.5, 138.4, 138.1, 134.0, 126.8, 125.6, 125.1, 124.2, 123.8, 37.9, 29.3, 25.5, 21.0. GC-MS (70 eV): tR = 35.704 min, m/z 200 [M+, C15H18], 198, 183, 168, 153, 141, 128, 115, 83.
3,5,5-Trimethyl-9-methylene-2,4a,5,6,7,8,9,9a-octahydro-1H-benzocycloheptene 13
Colorless oil (yield 15 %). IR (KBr): λmax = 3056, 1606, 1643, 1362, 720, 945 cm-1. 1H NMR (300 MHz, CDCl3) δ = 5.50 (1H, s), 4.79 (1H, s), 4.74 (1H, s), 2.83 (1H, m), 1.77-2.16 (7H, m), 1.69 (3H, s), 1.18-1.40 (4H, m), 1.02 (6H, s); 13C NMR (75.4 MHz, CDCl3) δ = 157.9, 133.9, 123.8, 111.3, 47.9, 40.0, 36.7, 38.4, 36.6, 32.2, 28.3, 26.7, 25.2, 24.2. GC-MS (70 eV): tR = 31.627 min, m/z 204 [M+, C15H24], 189, 175, 161, 147, 134, 119, 105, 93, 79, 69, 55.
2,5,9,9-Tetramethyl-6,7,8,9-tetrahydro-5H-benzocycloheptene 9
To a solution of 11 (45 mg, 0.225 mmol) in 2 ml of ethyl acetate and methanol (1:1, v/v) was added 40 mg of 10 % palladium on activated carbon. The mixture was stirred for 5 h under hydrogen, the reaction mixture was filtered, and the filtrate was evaporated. The crude product was purified by silica gel column chromatography (heptane 100 %) to give compound 9 as colorless oil (yield 80 %). IR (KBr): λmax = 3043, 2876, 2853, 1566, 1292, 905 cm-1. 1H NMR (300 MHz, CDCl3) δ = 7.29 (1H, s), 7.22 (1H, d, J = 7.8 Hz), 7.08 (1H, d, J = 7.7 Hz), 3.34-3.39 (1H, m), 2.41 (3H, s), 1.85-1.92 (2H, m), 1.57- 1.63 (2H, m), 1.52 (3H, s), 1.45 (2H, m) 1.43 (6H, s). 13C NMR (75.4 MHz, CDCl3) δ = 147.9, 141.4, 135.1, 128.4, 126.7, 125.6, 41.3, 39.7, 36.7, 34.7, 34.2, 24.3, 21.4, 21.2. GC-MS (70 eV): tR = 34.401 min, m/z 202 [M+, C15H22], 187, 159, 145, 131, 119, 105, 91, 77, 57.
3,5,5,9-Tetramethyl-6,7-dihydro-5H-benzocycloheptene 14
To a solution of 12 (46 mg, 0.232 mmol) in 2 ml of ethyl acetate and methanol (1:1, v/v) was added 40 mg, of 10 % palladium on activated carbon. The mixture was stirred for 6 h under hydrogen, the reaction mixture was filtered, and the filtrate was evaporated. The crude product was purified by silica gel column chromatography (heptane 100 %) to give compound 14 as colorless oil (yield 75 %). The compound 14 also yielded compound 9 by method as described above (yield 80%). IR (KBr): λmax = 3103, 2955, 2876, 1450, 1281, 872 cm-1. 1H NMR (300 MHz, CDCl3) δ = 7.19-7.29 (2H, m), 7.05-7.14 (1H, m), 5.94 (1H, m), 2.44 (3H, s), 2.40 (2H, m), 2.03 (3H, s), 1.84 (2H, m), 1.40 (6H, s). 13C NMR (75.4 MHz, CDCl3) δ = 146.6, 138.1, 137.3, 135.8, 127.7, 126.7, 126.5, 48.0, 38.2, 31.4, 26.3, 24.2, 21.6. GC-MS (70 eV): tR = 34.085 min, m/z 200 [M+, C15H20], 185, 171, 157, 143, 128, 115, 105, 91, 77.
2,9,9-Trimethyl-6,7,8,9-tetrahydro-benzocyclohepten-5-one 15
The compound 11 (95 mg, 0.475 mmol) and osmium tetroxide (1 mol %) in THF (0.5 ml) were added over a period of 30 min to a solution of sodium periodate (550.9 mg, 2.575 mmol) in water (0.5 ml). The mixture was stirred for further 20 h at room temperature. Extraction with ethyl acetate and diethyl ether followed by filtration through basic alumina and evaporation gave a yellow semisolid which on purification by silica gel column chromatography (hexane:EtOAc 97:3) gave 15 as light yellow semisolid (yield 73 %). IR (KBr): λmax = 2967, 2925, 2850, 1730, 800 cm-1. 1H NMR (300 MHz, CDCl3) δ = 7.60-7.67 (1H, m), 7.52-7.56 (1H, m), 7.38-7.41 (1H, m), 3.06 (2H, m), 2.69 (3H, s), 2.45-2.52 (2H, m), 2.18-2.24 (2H, m), 1.66 (6H, s). 13C NMR (75.4 MHz, CDCl3) δ = 208.5, 147.0, 140.7, 138.0, 128.5, 126.7, 126.3, 42.5, 40.1, 38.5, 31.6, 23.3, 21.4. HR-ESI-MS: 203.3000 ([M+H]+, C14H18O+; calc. m/z 203.3001).
General procedure for the synthesis of compounds 16a-16e
A mixture of 15 (56 mg, 0.277 mmol) and benzylamine (32.6 mg, 0.305 mmol) was uniformly adsorbed on the surface of activated silica gel (0.5 g) by dropwise addition under stirring, and the mixture was then stirred at 60 °C for 6 h to allow complete formation of the corresponding imine. The reaction was monitored by TLC and the reaction mixture was extracted with ethyl acetate. The organic layer finally concentrated and then chromatographed on silica gel (hexane:EtOAc 90:10) to afford 16a.
Benzyl-(2,9,9-trimethyl-6,7,8,9-tetrahydro-benzocyclohepten-5-ylidene)-amine 16a
Light brown semisolid (yield 74 %). IR (KBr): λmax = 3009, 2838, 2828, 1669, 1629, 1369, 843 cm-1. 1H NMR (300 MHz, CDCl3) δ = 6.86-7.50 (8H, m), 4.37-4.63 (1H, m), 2.38 (3H, s), 1.77-1.84 (2H, m), 1.45 (2H, m), 1.30 (6H, s), 1.24 (2H, m). 13C NMR (75.4 MHz, CDCl3) δ = 176.8, 146.5, 141.0, 138.3, 135.8, 128.8, 128.2, 127.4, 127.2, 126.9, 126.7, 57.9, 41.8, 38.9, 32.2, 30.2, 25.4, 22.0. HR-ESI-MS: 292.4380 ([M+H]+, C21H25N+; calc. 292.4378).
Cyclohexyl-(2,9,9-trimethyl-6,7,8,9-tetrahydro-benzocyclohepten-5-ylidene)-amine 16b
Prepared as described for 16a; starting from 15 (50 mg, 0.248 mmol) and cyclohexyl amine (27 mg, 0.272 mmol) and after purification with silica gel column chromatography (hexane:EtOAc, 90:10) to afford 16b as light brown semisolid (yield 78 %). IR (KBr): λmax = 3177, 2992, 2813, 1708, 1633, 1197, 920 cm-1. 1H NMR (300 MHz, CDCl3) δ = 7.15 (1H, m), 7.01 (1H, m), 6.83 (1H, m), 2.57 (1H, m), 2.38 (3H, s), 2.05-2.07 (2H, m), 1.70-1.76 (4H, m), 1.58-1.65 (7H, m), 1.40-1.42 (3H, m), 1.28 (6H, s). 13C NMR (75.4 MHz, CDCl3) δ = 174.0, 140.0, 138.1, 136.0, 129.2, 127.4, 126.6, 51.0, 41.4, 38.9, 32.3, 32.1, 31.4, 30.1, 25.3, 23.0, 21.9. HR-ESI-MS: 284.4588 ([M+H]+, C20H29N; calc. 284.4589).
Isobutyl-(2,9,9-trimethyl-6,7,8,9-tetrahydro-benzocyclohepten-5-ylidene)-amine 16c
Prepared as described for 16a; starting from 15 (99 mg, 0.491 mmol) and isobutyl amine (39.5 mg, 0.539 mmol) and after purification with silica gel column chromatography (hexane:EtOAc 90:10) to afford 16c as light brown semisolid (yield 71 %). IR (KBr): λmax = 3044, 2872, 2990, 1523, 1657, 1284, 791 cm-1. 1H NMR(300 MHz, CDCl3) δ = 7.03 (2H, m), 6.85 (1H, m), 2.38 (3H, s), 2.12 (1H, m), 1.92 (1H, m), 1.70(4H, m), 1.41 (2H, m), 1.27 (12H, s). 13C NMR (75.4 MHz, CDCl3) δ = 174.0, 145.8, 138.0, 136.1, 129.1, 127.3, 126.6, 61.8, 41.0, 38.9, 32.0, 31.7, 31.2, 30.3, 25.0, 21.8. IR. HR-ESI-MS: 258.4210 ([M+H]+, C21H25N+; calc. 258.4216).
Methyl-(2,9,9-trimethyl-6,7,8,9-tetrahydro-benzocyclohepten-5-ylidene)-amine 16d
Prepared as described for 16a; starting from 15 (100 mg, 0.495 mmol), methyl amine (17 mg, 0.545 mmol) and after purification with silica gel column chromatography (hexane:EtOAc 95:05) to afford 16d as light brown semisolid (yield 79 %). IR (KBr): λmax = 3020, 2845, 2880, 1683, 1697, 1254, 870 cm-1. 1H NMR (300 MHz, CDCl3) δ = 7.06-7.20 (3H, m), 2.36 (3H, s), 2.12 (3H, s), 1.96 (2H, m), 1.72 (4H, m), 1.34 (6H, s). 13C NMR (75.4 MHz, CDCl3) δ = 174.3, 147.8, 136.7, 130.4, 128.8, 126.9, 126.6, 41.0, 38.4, 37.8, 31.6, 30.1, 26.5, 21.9. HR-ESI-MS: 216.3414 ([M+H]+, C15H22N+; calc. 216.3419).
(2-Morpholin-4-yl-ethyl)-(2,9,9-trimethyl-6,7,8,9-tetrahydro-benzocyclohepten-5-ylidene)-amine 16e
Prepared as described for 16a; starting from 15 (106 mg, 0.525 mmol), methyl amine (75.2 mg, 0.578 mmol) and after purification with silica gel column chromatography (hexane:EtOAc 50:50) to afford 16e as light brown semisolid (yield 65 %). IR (KBr): λmax = 2817, 2791, 1650, 1629, 1486, 1397, 1107 cm-1. 1H NMR (300 MHz, CDCl3) δ = 6.90-7.27 (3H, m), 3.57 (6H, m), 2.65 (2H, m), 2.31 (4H, m), 2.23 (3H, s), 1.50-1.98 (6H, m), 1.20 (6H, s). 13C NMR (75.4 MHz, CDCl3) δ = 170.6, 147.1, 140.5, 136.8, 129.7, 127.3, 126.0, 66.7, 61.2, 53.5, 48.7, 40.8, 38.5, 36.2, 31.0, 25.9, 21.1. HR-ESI-MS: 315.4709 ([M+H]+, C20H31N2O+; calc. 315.4729).
Antimicrobial assays
Antimicrobial activity was tested against a panel of sixteen organisms: gram-positive bacteria- Bacillus subtilis (MTCC 121), Micrococcus luteus (MTCC 2470), Staphylococcus aureus (MTCC 96), Gram-negative bacteria- Burkholderia cepacea (MTCC 438), Escherichia coli (MTCC 43), Klebsiella pneumoniae (MTCC 109), Pseudomonas aeruginosa (MTCC 424), Enterobacter cloacae (MTCC 509) and fungal strains Candida albicans (MTCC 3017), Issatchenkia orientalis (MTCC 231), Aspergillus flavus (MTCC 277), A. niger (MTCC 404), A. parasiticus (MTCC 2797), A. sydowii (MTCC 4335) and A. ochraceous (MTCC 4893), Trichophyton rubrum (MTCC 296) procured from the Microbial Type Culture Collection at the Institute of Microbial Technology, India. Broth microdilution method was used for the determination of minimum inhibitory concentration (MIC) and minimum microcidal concentration (MMC) in triplicates (Cos et al., 2006[8]; Sharma et al., 2009[21]). Test compounds were dissolved in DMSO to prepare the stock solutions. Two-fold dilution series of test compounds were prepared for the dose range 3000-23.4 µg/ml in sterilized Mueller-Hinton broth (MHB) for bacteria and Sabouraud dextrose broth (SDB) for fungi in 96-well microtiter plates. Freshly grown bacterial and fungal cultures adjusted to 1.0×105 cfu/ml with sterile normal saline were used to inoculate the plates. The uninoculated sterilized medium with and without DMSO served as control. Ampicillin and nystatin served as positive controls for bacteria and fungi, respectively. The plates were incubated at 37 °C for 24 h for bacteria, 28 °C for 24 h for C. albicans, and 28 °C for 5 days for rest of the fungi. The plates were incubated for 12 h after addition of 5 µl resazurin indicator solution (5 mg/ml) to each well. The experiment included three replicates for each plate. The lowest concentration which prevented colour change from purple to pink was recorded as MIC, while the lowest concentration completely killing the inoculated microorganism was recorded as MMC.
Results and Discussion
To the best of our knowledge, the himachalenes have not yet been thoroughly investigated, and there are few methods reported involving synthesis of benzocycloheptene derivatives from himachalenes by simple pathway (Chaudhary et al., 2012[4]; Pandey and Dev, 1968[17]). The synthesis was carried out by the conversion of himachalenes 10 into benzocycloheptene derivatives namely α-dehydro-ar-himachalene 11, bisdehydro-ar-himachalene 12 and α-himachalene 13 by oxidative aromatization. In this study, α-dehydro-ar-himachalene 11 is used as precursor for the synthesis of substituted benzocycloheptenone. The benzocycloheptenone acts as an intermediate for synthesis of benzocycloheptene moiety and have been investigated for a wide spectrum of biological activities like cytotoxic, anticancer, antimicrobial and antagonistic activity (Bohlmann et al., 2006[3]). It was mostly synthesized by cyclization of phenyl pentanoic acid (Liu et al., 2008[13]). However, here, for the first time we conducted the synthesis of benzocycloheptenone starting from α-dehydro-ar-himachalene 11. Oxidation of terminal alkene of 11 was attempted with different oxidizing agents such as KMnO4, Hg(OAc)2, RuCl3/ NaIO4, Pd(OAc)2/O2 that led to the formation of mixture of products. Finally, the oxidation of exocyclic double bond of 11 was optimized with NaIO4 and OsO4 in water:THF (1:1, v/v) for 20 h at room temperature to produce corresponding benzocycloheptenone (15) in 73 % yield (Figure 3(Fig. 3)).
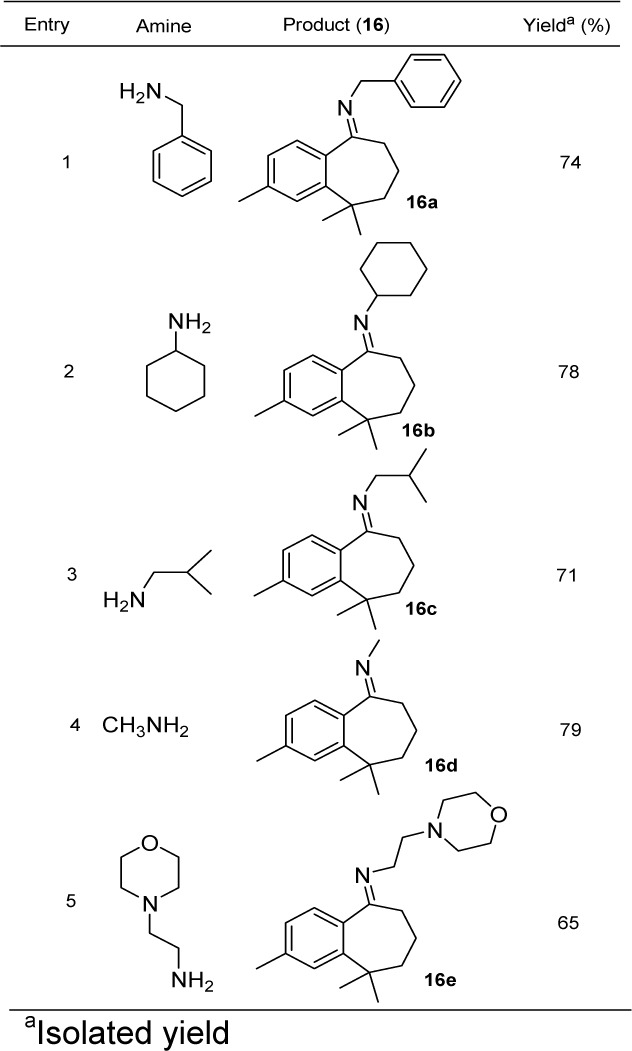
As nitrogen containing amino and imino benzocycloheptenes have been investigated to show diverse range of biological activities, the conversion of carbonyl moiety of benzocycloheptenone 15 into imino substituted benzocycloheptenes 16 was tried with reported conditions in several solvents such as toluene, benzene, DMF, THF but no successful results were obtained. Using dry silica gel (H) as a lewis acid and an appropriate amine gave good conversion of imine derivatives of benzocycloheptene (Figure 3(Fig. 3)) and no work up was required before purification through column chromatography. The mixture was directly transferred to column and purification was performed with hexane:EtOAc (90:10). With the optimized reaction conditions in hand, the scope of this reaction was then probed with a range of different amines (Table 1(Tab. 1)). Under similar conditions, the reactions of benzocyclohepten-5-one 15 with corresponding amines proceeded smoothly to form novel imine compounds 16a-16e in 65-79 % yields (Table 1(Tab. 1), entries 1-5).
Table 1. Synthesis of novel imine derivatives (16a-16e) of aryl himachalene.
The aryl himachalene was earlier prepared from mixture of himachalenes or 3-methylacetophenone (Abouhamza et al., 2001[1]). While in the present study aryl himachalene 9 was obtained by catalytic hydrogenation of α-dehydro-ar-himachalene 11 with Pd/C in ethyl acetate and methanol (1:1, v/v) in 80 % yield. Aryl himachalene 9 was also obtained by dual reduction of bisdehydro-ar-himachalene 12 with Pd/C in ethyl acetate and methanol via γ-dehydro-ar-himachalene 14 (Figure 3(Fig. 3)). The structures of synthesized compounds were established on the basis of IR, NMR and mass spectrometry.
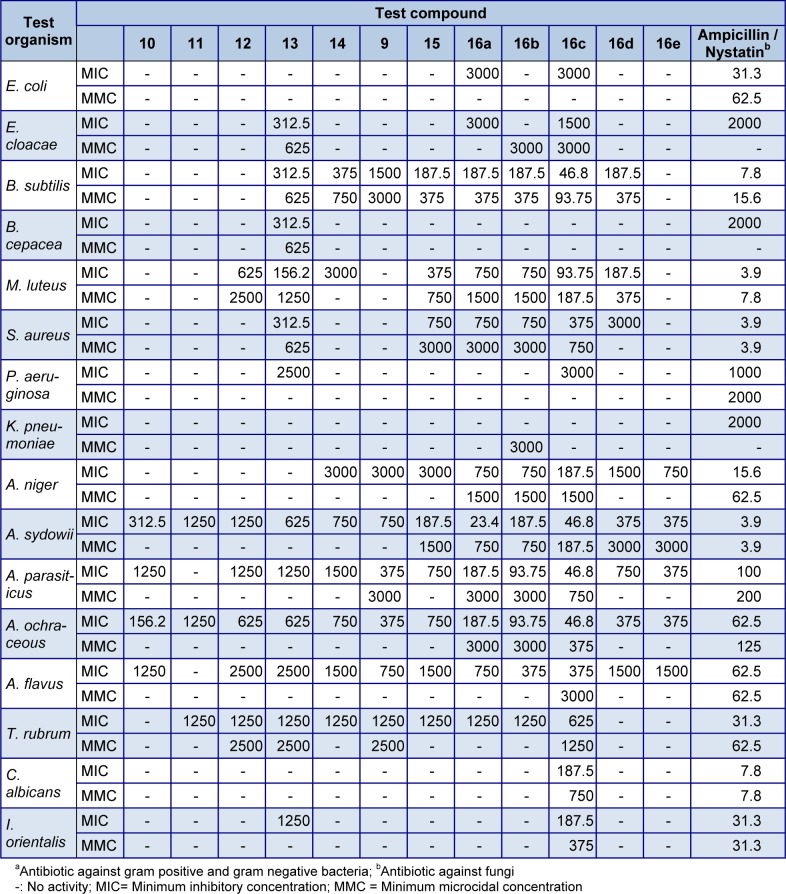
The antimicrobial activity of all the himachalene derivatives (9-15, 16a-16e) were tested against a panel of sixteen organisms: gram-positive bacteria- B. subtilis, M. luteus, S. aureus, gram-negative bacteria- B. cepacea, E. coli, K. pneumoniae, P. aeruginosa, E. cloacae and fungal strains C. albicans, I. orientalis, A. flavus, A. niger, A. parasiticus, A. sydowii, A. ochraceous and T. rubrum. It was observed that the aromatized himachalenes (ar-himachalene 9, α-dehydro-ar-himachalene 11, bisdehydro-ar-himachalene 12 and γ-dehydro-ar-himachalene 14) did not show antimicrobial activity against the gram-negative bacteria B. cepacea, E. coli, K. pneumoniae and P. aeruginosa even at a high concentration of 3000 µg/ml (Table 2(Tab. 2)). However, bis dehydro-ar-himachalene 12 exhibited antibacterial activity against the gram-positive bacteria M. luteus at MIC 625µg/ml, and ar-himachalene 9 and γ-dehydro-ar-himachalene 14 against B. subtilis at MIC 375 µg/ml and 1500 µg/ml, respectively. Non aromatic α-himachalene 13 was more active than aromatic himachalenes against both bacterial and fungal cultures. Insertion of carbonyl group at 5th position of α-dehydro-ar-himachalene 11 slightly enhanced the activity against B. subtilis, M. luteus, and S. aureus. Conversely, the substitution of carbonyl group of substituted benzocycoheptenone 15 by imine group (16a-16e) displayed a noticeable improvement in their antibacterial activity against B. subtilis, M. luteus and S. aureus with MIC ranging from 46.8-750 µg/ml. Antifungal activity of these compounds against A. sydowii, A. parasiticus and A. ochraceous was also high with MIC 23.4-187.5 µg/ml as reported against A. fumigatus for himachalol and other derivatives from Cedrus (Parveen et al., 2010[18]; Chowdhry et al., 1997[7]).
Table 2. Antimicrobial activity of derivatives of α-dehydro-ar-himachalene by broth microdilution .
method against standard microbial cultures (µg/ml)
The aromatized compounds, however, showed low activity against Aspergillus spp. High antimicrobial activity observed for compounds 16a-16e indicated that the nature of imine group at C-5 position markedly affects the activity profile of those compounds. The imine substituent 16c exhibited antimicrobial activity higher than 16a possibly due to its electron releasing effect. No activity was observed against C. albicans and I. orientalis except for the compound 16c which exhibited a broad spectrum antifungal activity. Antimicrobial activity of Cedrus extracts and essential oils has been previously reported particularly against S. aureus, B. subtilis, E. coli, P. aeruginosa and fungi of genus Aspergillus (Chowdhry et al., 1997[7]; Pawar et al., 2007[19]; Zeng et al., 2011[26]). However, there are no reports on the antimicrobial properties of mentioned aryl himachalene derivatives.·
Conclusion
In conclusion, analogues of aryl himachalenes were synthesized from readily available naturally occurring isomeric mixture of himachalenes via α-dehydro-ar-himachalene. Aromatization of himachalenes slightly increased the antibacterial activity which was further enhanced after insertion of imine moiety in novel imine compounds (16a-16e). The imine substituent containing isobutyl group 16c showed the highest activity amongst tested compounds. These derivatives could find potential in biological applications for drug design and development.
Notes
Pralay Das and Bikram Singh contributed equally as corresponding authors to this publication.
Corresponding authors:
Bikram Singh; Natural Product Chemistry and Process Development Division, CSIR-Institute of Himalayan Bioresource Technology, Palampur, H.P., 176 061, India; E-mail: bikramsingh@ihbt.res.in; Fax: +91-1894-230433;
Pralay Das; Natural Product Chemistry and Process Development Division, CSIR-Institute of Himalayan Bioresource Technology, Palampur-176 061, H.P., India; E-mail: pdas@ihbt.res.in; Phone: +91-1894-233339; Fax: +91-1894-230433
Acknowledgements
Authors are thankful to the Director, CSIR-Institute of Himalayan Bioresource Technology for providing facilities. Authors (A.C., S.S. and P.K.) acknowledge Council of Scientific and Industrial Research, Delhi for the awards of Senior Research Fellowships, and I.M. under the Council of Scientific and Industrial Research, Network Project NWP006.
Figure 1. Graphical abstract.
References
- 1.Abouhamza B, Allaoud, S, Karim A. ar-Himachalene. Molecules. 2001;6:M236. [Google Scholar]
- 2.Bhushan S, Singh J, Rao JM, Saxena AK, Qazi GN. A novel lignan composition from Cedrus deodara induces apoptosis and early nitric oxide generation in human leukemia Molt-4 and HL-60 cells. Nitric Oxide. 2006;14:72–88. doi: 10.1016/j.niox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Bohlmann R, Kroll J, Kuenzer H, Hegele-Hartung C, Lessl M, Lichtner R, et al. Benzocycloheptenes, process for their production, pharmaceutical preparations that contain the latter as well as their use for the production of pharmaceutical agents. US Patent 7145015 B2.2006. [Google Scholar]
- 4.Chaudhary A, Das P, Mishra A, Kaur P, Singh B, Goel RK. Naturally occurring himachalenes to benzocycloheptene amino vinyl bromide derivatives: as antidepressant molecules. Mol Divers. 2012;16:357–366. doi: 10.1007/s11030-012-9372-3. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary A, Kaur P, Singh B, Pathania V. Chemical composition of hydrodistilled and solvent volatiles extracted from woodchips of Himalayan Cedrus: Cedrus deodara (Roxb.) Loud. Nat Prod Commun. 2009;4:1257–1260. [PubMed] [Google Scholar]
- 6.Chow K, Fang, WK, Corpuz EG, Gil, DW, Garst ME. Substituted fluoroethyl ureas as alpha 2 adrenergic agents. US Patent 7598417 B2.2009. [Google Scholar]
- 7.Chowdhry L, Khan ZK, Kulshrestha DK. Comparative in vitro and in vivo evaluation of himachalol in murine invasive aspergillosis. Indian J Expt Biol. 1997;35:727–734. [PubMed] [Google Scholar]
- 8.Cos P, Vlietinck AJ, Berghe BV, Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro proof-of-concept. J Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Daoubi M, Hernaandez-Galaan R, Benharref A, Collado IG. Screening study of lead compounds for natural product-based fungicides: Antifungal activity and biotransformation of 6α,7α-dihydroxy-β-hima¬chalene by Botrytis cinerea. J Agri Food Chem. 2005;53:6673–6677. doi: 10.1021/jf050697d. [DOI] [PubMed] [Google Scholar]
- 10.Hattori K, Tanaka A. Benzocycloheptene derivatives. US Patent 6384072 B.2002. [Google Scholar]
- 11.Hossini I, Harrad MA, Ali MA, Firdoussi LE, Karim A, Valerga P, et al. Friedel-Craft acylation of ar-himachalene: Synthesis of acyl-ar-himachalene and a new acyl-hydroperoxide. Molecules. 2011;16:5886–5895. doi: 10.3390/molecules16075886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph TC, Dev S. Structure of himachalenes. Tetrahedron Lett. 1961;6:216–222. [Google Scholar]
- 13.Liu Y, Su J, Xiao JH, Jiang SB, Lu H, Zhong W, et al. Synthesis of benzocycloheptene derivatives as CCR5 antagonists with potent anti-HIV activity. Chinese Chem Lett. 2008;19:428–430. [Google Scholar]
- 14.Lynch KR, Macdonald TL. Benzocycloheptyl analogs having sphingosine 1-phosphate receptor activity. US Patent 0253761 A1.2009. [Google Scholar]
- 15.Nakano M, Minoguchi M, Hanano T, Ono S-I, Horiuchi H, Teshima K. Benzimidazole compound and pharmaceutical use thereof. US Patent 0120841 A1.2010. [Google Scholar]
- 16.Nedelec L, Pierdet A, Dumont C, Kannengiesser MH. 7-Amino-6,7-dihydro[5H]benzocycloheptene derivatives. US Patent 4148919, 1979. [Google Scholar]
- 17.Pandey RC, Dev S. Studies in sesquiterpenes-XXX. Synthesis of arhimachalene and himachalenes. Tetrahedron. 1968;24:3829–3839. [Google Scholar]
- 18.Parveen R, Azmi MA, Tariq RM, Mahmood SM, Hijazi M, Mahmud S, et al. Determination of antifungal activity of Cedrus deodara root oil and its compounds against Candida albicans and Aspergillus fumigatus. Pak J Bot. 2010;42:3645–3649. [Google Scholar]
- 19.Pawar VC, Thaker VS. Evaluation of the anti-Fusarium oxysporum f. sp cicer and anti-Alternaria porri effects of some essential oils. World J Microbiol Biotechnol. 2007;23:1099–1106. [Google Scholar]
- 20.Shankaranarayan R, Bisarya SC, Dev S. Studies in sesquiterpenes-LIV: Oxidohimachalene, a novel sesquiterpenoid from the wood of Cedrus deodara Loud. Tetrahedron. 1977;33:1207–1210. [Google Scholar]
- 21.Sharma N, Ghosh P, Sharma UK, Sood S, Sinha AK, Gulati A. Microwave-assisted efficient extraction and stability of juglone in different solvents from Juglans regia: Quantification of six phenolic constituents by validated RPHPLC and evaluation of antimicrobial activity. Anal Lett. 2009;42:2592–2609. [Google Scholar]
- 22.Singh D, Agarwal SK. Himachalol and himachalene: insecticidal principles of Himalayan cedarwood oil. J Chem Ecol. 1988;14:1145–1151. doi: 10.1007/BF01019342. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen US, Teuber L, Peters D, Strobaek D, Johansen TH, Nielsen KS, et a. 2-Aminobenzimidazole derivatives and their use as modulators of small-conductance calcium-activated potassium channels. European Patent EP1776348 B1.2008. [Google Scholar]
- 24.Tandon VK, Singh KA, Awasthi AK, Khanna JM, Lal B, Anand N. Chemo- and stereoselective synthesis of benzocycloheptene and 1-benzoxepin derivatives as α-sympathomimetic and anorexigenic agents. Bioorg Med Chem Lett. 2004;14:2867–2870. doi: 10.1016/j.bmcl.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 25.Zaratin PF, Petrone G, Sbacchi M, Garnier M, Fossati C, Petrillo P, et al. Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (-)-cis-1-methyl-7-[[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl]-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111) J Pharmacol Exp Ther. 2004;308:454–461. doi: 10.1124/jpet.103.055848. [DOI] [PubMed] [Google Scholar]
- 26.Zeng WC, Jia LR, Zhang Y, Cen JQ, Chen X, Gao H, et al. Antibrowning and antimicrobial activities of the water-soluble extract from pine needles of Cedrus deodara. J Food Sci. 2011;76:318–323. doi: 10.1111/j.1750-3841.2010.02023.x. [DOI] [PubMed] [Google Scholar]
- 27.Zilkowski BW, Bartelt RJ, Cosse AA, Petroski RJ. Male-produced aggregation pheromone compounds from the eggplant flea beetle (Epitrix fuscula): Identification, synthesis, and field biossays. J Chem Ecol. 2006;32:2543–2558. doi: 10.1007/s10886-006-9163-3. [DOI] [PubMed] [Google Scholar]