Abstract
The role of neuronal nitric oxide synthase (nNOS) in the central mechanism of neuropathic pain and long-term potentiation (LTP) of peripheral afferents remains obscure. The current study investigated the effect of intrathecal application of 7-nitroindazole (7-NI), a selective nNOS inhibitor (8.15 µg/5µl), on mechanical allodynia on day 14 after L5 spinal nerve transection. Furthermore, using in vivo single unit extracellular recording, we examined the effect of 7-NI on the induction of LTP of Aδ- and C-fiber-evoked responses. We have demonstrated that 7-NI attenuates nerve-injury-evoked mechanical allodynia. Additionally, our electrophysiological study has shown that the spinal administration of 7-NI significantly inhibits the induction of the LTP of Aδ- and C-fiber-evoked responses on day 14 after neuropathy. These data suggest that activation of nNOS may be crucial for the induction of the spinal LTP of Aδ- and C-fiber-evoked responses following peripheral nerve damage.
Keywords: neuropathic pain, nNOS, LTP, dorsal horn
Introduction
Neuropathic pain is a chronic disease with drastic impairments in quality of life, and has a high economic impact on society. Neuropathic pain is characterized by the presence of increased duration and amplitude of response to noxious stimuli (hyperalgesia), pain in response to normally innocuous stimuli (allodynia), and spontaneous pain (Hama and Borsook, 2005[11]; Mirzaei et al., 2010[23]; Nazemi et al., 2012[25]). The central mechanisms of neuropathic pain are not clear, and it is not established how pain hypersensitivity after nerve injury may persist for years despite the perfect healing of the nerve (Kumazawa, 1998[16]; Schmidtko et al., 2009[31]). It is conceivable, however, that some forms of learning processes such as LTP in the spinal cord may underlie this phenomenon (Ji et al., 2003[13]; Ikeda et al., 2009[12]). Noxious stimuli or nerve damage may lead to LTP in the spinal cord pathways (Svendsen et al., 1997[33]; Sandkuhler and Liu, 1998[30]; Wallin et al., 2003[39]). It seems that nerve injury-evoked LTP amplifies pain input in the spinal cord and contributes to the development of pain-related behaviors (Sandkuhler and Liu, 1998[30]; Svendsen et al., 2000[33]). However, the potential factors that may contribute to LTP are far from clear. Peripheral nerve damage may result in central hypersensitivity by activation of NMDA receptors and subsequent activation of nNOS (Bredt and Synder, 1992[2]; Luo and Vincent, 1994[18]; Montague et al., 1994[24]; Cury et al., 2011[7]). However, the role of nNOS and its correlation with the development of neuropathic behaviors and spinal LTP remains unknown, and targeting nNOS may provide a novel treatment avenue. Pharmacological studies regarding the role of nNOS in the development of nerve-injury-evoked hypersensitivity have been controversial. In some studies, the administration of nNOS inhibitors has reduced pain-related behaviors (Guan et al., 2007[10]; Tanabe et al., 2009[37]; Ding et al., 2010[8]; Choi et al., 2012[6]). On the contrary, some behavioral studies have shown that nNOS inhibitors had no effect on diabetic or nerve-injury-induced allodynia (Calcutt and Chaplan, 1997[3]; Luo et al., 1999[19]; Lee et al., 2005)[17]. Thus, in a series of behavioral studies have been reported both pro-nociceptive and anti-nociceptive effect of nNOS in different types of neuropathy models. The first aim of our study was therefore to evaluate nNOS inhibition on mechanical allodynia on day 14 after L5 spinal nerve transection. To our knowledge, there is no direct electrophysiological evidence to identify the effect of nNOS inhibition on the induction of LTP of Aδ- and C-fiber-evoked responses in single WDR dorsal horn neurons in neuropathic rats, using single-unit extracellular recording. Therefore, our main aim was to evaluate the contribution of spinal nNOS in the development of neuropathic pain by assessing the intrathecal effects of 7-NI (nNOS selective inhibitor) on the induction of LTP of Aδ- and C-fiber-evoked responses in the dorsal horn in L5 spinal nerve transected rats.
Material and Methods
Animals
Male Wistar rats (Pasture Institute, Tehran, Iran), weighing 180-200 g at the beginning of the study were used. The rats were housed in separate cages and maintained in a temperature controlled colony room under 12 hr light-dark cycle with free access to food and water. Animal experiments were performed in accordance with the National Institute of Health (NIH) Guidelines for the Care and Use of Laboratory Animals, and were approved by the Animal Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (194/-90/3/18; 2011). All experiments started at 08:00 A.M.
Induction of neuropathic pain
Neuropathic pain was induced according to the method described by Kim and Chung (1992[15]). Animals were anesthetized by pentobarbital sodium (60 mg/kg, intraperitoneal). The left paraspinal muscles were separated at the L4-S2 levels, and the left transverse process of the L6 vertebra was removed. The L5 spinal nerve was isolated and tightly ligated using silk thread (6-0), and then the distal part to the ligature was transected just to make sure all fibers were interrupted. The wound was closed afterwards with 3-0 silk thread. Great care was taken to avoid any damage to the L4 nerve. In a control sham group, the surgical procedure was performed identical to that described above, except that the left L5 spinal nerve was not ligated and transected. Only animals showing no sign of motor deficiency were considered for further experimentation.
Behavioral study
We used the Von Frey test to confirm the successful induction of neuropathy prior to the electrophysiological study. Sham-operated (n=8) and nerve-injury rats (n=8) were examined for the development of mechanical allodynia 1 day before neuropathy and 2, 5, 7, 14, 21, and 28 days after neuropathy.
Mechanical allodynia
To measure paw withdrawal threshold (PWT) to mechanical stimuli, each animal was placed in a Plexiglas chamber on a metal mesh floor. Mechanical stimuli were applied perpendicular to the plantar surface at the proximal half of the third and fourth toes of the injured hind paw using a set of Von Frey monofilaments (Stoelting Co., Wood Dale, IL, USA). Thresholds were determined by the up-down method of Dixon (1980[9]). The evoked-hind paw (left hind paw) was stimulated using one of a series of 7 Von Frey filaments with logarithmically incremental stiffness (2, 4, 6, 8, 15, 26 and 60 g). Each monofilament was applied to the left hind paw for approximately 2-3 s, with sufficient force causing the filament to buckle. Each particular filament was applied 3 times at approximately 5 min intervals. Each application was started using an 8 g stimulus and either increased or decreased in intensity until an obvious paw withdrawal was observed. Quick withdrawal or licking of the paw in response to the stimulation by a particular filament was considered as a positive response. In the presence of a response, the filament of the next feeble force was applied. In the absence of a paw withdrawal reaction, the filament of the next intense force was applied. Paw withdrawal reaction in at least two out of the three trials was considered as a positive response.
Implantation of intrathecal catheter
Under pentobarbital sodium (60 mg/Kg, intraperitoneal) anesthesia, implantation of intrathecal cannula was performed according to the method described by Storkson et al. (1996[32]). A PE-10 polyethylene tube was inserted between the L5 and L6 vertebrae and carefully inserted in to the subarachnoid space in the cranial direction to reach the lumbar enlargement. The outer part of the catheter was plugged and fixed onto the skin. The correct subarachnoid positioning was determined by a typical tail or hind paw flick. The surgical procedure was performed under sterile conditions. Motor dysfunction was determined by evaluating the ability of the rat to stand and ambulate in a normal posture and make use of the hind paw. Only animals without evidence of motor deficits following the spinal nerve transection and catheter insertion were included in the study.
Recording of mechanical allodynia after intrathecal administration of nNOS inhibitor
To examine the effect of intrathecal injection of 7-NI (an nNOS selective inhibitor) on nerve-injury-induced mechanical allodynia, the neuropathic rats (n=8) received an intrathecal injection of 7-NI (8.15 µg/5 µl) and the sham-operated rats (n=8) received an equal volume of 0.9 % physiological saline. The drug was injected over a period of 60 sec, followed by 5 µl of 0.9 % physiological saline to flush the catheter. Mechanical allodynia was evaluated, just before and 30, 60, 90, 120, 150, and 180 min after the administration of 7-NI on day 14 after neuropathy. Rats were completely recovered (i.e., became spontaneously active) within 5 min after the administration of the drug. The dose of drug administration was based on prior studies (Guan et al., 2007[10]).
In vivo extracellular single unit recording
Extracellular single unit recording was performed in sham-operated (n=5) and neuropathic rats (n=5) on day 14 after neuropathy, according to the established methods (Svendsen et al., 1999[34]). Animals were anesthetized with 2.0-2.5 % isoflurane (66 % N2O and 33 % O2). Rats were then mounted onto a homemade spinal stereotaxic instrument (Borge Sanat, Tehran, Iran). Body temperature was maintained at 36 °C to 37 °C by a heating blanket. The vertebral column was rigidly fixed by two clamps (rostral and caudal) to the exposed spinal cord segments to ensure stability during electrophysiological recordings. A laminectomy was performed at vertebrae T13-L1 to expose the lumbar enlargement, where the sciatic nerve roots enter the spinal cord. The left sciatic nerve was dissected free for a length of at least 1 cm at the mid-thigh level. A bipolar silver hook electrode (2 mm distance between hooks) was placed immediately proximal to the division of the main branches of the nerve for electrical stimulation. Both the electrode and the nerve were isolated from the surrounding tissues by a plastic film. Parylene-coated tungsten microelectrode with impedance 2-5 MΩ (Friedrick Hear & CO., Bowdoinham, ME, USA) was derived vertically into the dorsal horn using a micromanipulator in 10 µm steps. Recorded signals were amplified by a data acquisition system (D3111; Science Beam CO., Tehran, Iran), and were continuously captured on a Pentium 4 computer using the Neurocomet software (Science Beam CO., Tehran, Iran). The signals were filtered using a bandwidth of 300-3000 Hz. The number of stored digital spikes for each stimulus was counted in 1 ms bin sizes using eProb spike software (Science Beam CO., Tehran, Iran) to construct peri-stimulus time histograms (PSTHs). Extracellular single unit activity was recorded from neurons at the depths of 500-1000 µm from the surface of the dorsal horn, according to the definition by Urch and Dickenson (2003[38]). Only one cell was studied in each animal. All neurons were characterized by the response to natural stimuli (touch, pressure, and pinch) before the experiments. Only WDR neurons, which respond to both non noxious tactile stimuli and noxious pinch in a graded manner, were studied. All electrical stimuli were delivered to the sciatic nerve by a silver hook electrode. The Aδ- and C-fiber thresholds were defined at the beginning of the experiment by test stimulus of a single square pulse (0.5 ms, every 5 min, started from 100 µA) in sham and neuropathic rats. For the baseline recording, a test stimulus was delivered every 4 min (12 test stimuli, 2 ms rectangular pulses, 1.5×C-fibers response threshold). After baseline recording, induction of LTP was obtained by conditioning high-frequency stimulation of sciatic nerve (HFS) (0.5 ms rectangular pulses, 6×C-fiber response threshold, 20 trains of 2 sec duration, 100 Hz, 10 sec intervals between the trains). After 15 min, the Aδ- and C-fiber-evoked responses to single electrical test stimuli (24 stimuli) were recorded, in the same way as for the baseline recording. To examine the spinal effect of nNOS inhibition on the induction of LTP of the Aδ- and C-fiber-evoked responses, 20 min after baseline recording (after the sixth test stimulus), 7-NI (8.15 µg/5 µl) was applied topically to the dorsal surface of the spinal cord. Then, post-drug-evoked responses were recorded up to 2 hrs. Aδ-and C-fiber-evoked responses were characterized by the latency of evoked responses. The number of evoked spikes in 20-90 ms after a test stimulus was defined as Aδ-evoked responses and in 90-300 ms was defined as C-fiber-evoked responses.
Statistics
All data were presented as mean ± standard error of the mean (SEM). In the behavioral study, the data was analyzed using one-way repeated measures and two-way ANOVA, followed by Bonferroni post-hoc test. In the electrophysiological study, extracellular recordings of WDR neurons were performed by 12 test stimuli before the HFS and 24 test stimuli after the HFS. The averages of three consecutively evoked responses were converted to one value, producing 4 values before HFS (12/3) and 8 values after HFS (24/3). The effect of the 7-NI on the evoked-responses was analyzed using one-way ANOVA followed by Tukey post-hoc test. Graphs were drawn and statistical analyses were performed using Graphpad Prism version 5.0 (Graphpad Prism software, San Diego, CA, USA).
Results
Effect of intrathecal injection of nNOS selective inhibitor on nerve injury-induced mechanical allodynia
The L5 nerve transection elicited long-term mechanical allodynia on the evoked hind paw. This mechanical allodynia appeared on day 2, reached a peak level on day 7, and persisted up to 28 days after nerve transection (Figure 1A(Fig. 1)). The mean of mechanical threshold (before neuropathy) was 27.50±4.96 g and L5 nerve transection significantly decreased mechanical threshold to 4.75±0.52 g on day 14 after neuropathy. Furthermore, we examined the effect of intrathecal injection of 7-NI (a selective nNOS inhibitor) on nerve injury-induced mechanical allodynia on day 14 after neuropathy. In the 7-NI-treated group (n=8, 8.15 µg/5 µl), a significant decrease in mechanical allodynia appeared 60 min after the administration of 7-NI (15.13±2.63 g), and disappeared 180 min (7.37±1.29 g) after the administration of 7-NI (Figure 1B(Fig. 1)).
Figure 1. Mechanical allodynia-induced by L5 nerve transection. (A) Time course of mechanical allodynia after neuropathy. (B) Effect of 7-NI (8.15 µg/5 µl) on the mechanical allodynia. 7-NI administrated intrathecally at time zero. Paw withdrawal threshold (PWT) measured by Von Frey stimulation and expressed in grams of pressure on day 0 (one day before nerve transection) up to 28 day. Each point represents the mean±SEM (8 rats per group). *p<0.05, **p<0.01 and ***p<0.001 indicate a significant difference between neuropathy and sham-operated animals.

Electrophysiological study
The effect of nNOS inhibition on Aδ- and C-fibers threshold in neuropathic rats
To study the role of nNOS in hyperexcitability of pain-related afferent fibers in the spinal cord, the thresholds for the activation of Aδ- and C-fibers were examined in sham and neuropathic rats. In sham rats, the activation thresholds of Aδ- and C-fibers were 466±14.54 µA and 1188±27.09 µA, respectively. In neuropathic rats, the activation thresholds of Aδ- and C-fibers were 340±18.70 µA and 924±18.65 µA, respectively, which were significantly lower than sham rats. Spinal application of 7-NI in neuropathic rats significantly increased the activation threshold of Aδ- and C-fibers (548±24.65; 1418±19.90 µA, respectively) (Figure 2(Fig. 2)).
Figure 2. Threshold for the activation of Aδ- and C-fibers in sham-operated and neuropathic rats. Each value represents the mean±SEM (5 rats per group). *p<0.05 indicates a significant difference with sham animals. #p<0.05, indicates a significant difference with neuropathic rats.

The effect of nNOS inhibition on the induction of LTP in the spinal dorsal horn in neuropathic rats
HFS induced significant LTP of Aδ- and C-fiber-evoked responses in both sham and neuropathic rats up to 2 hrs. LTP of Aδ-fiber-evoked responses in neuropathic rats was smaller than sham rats (159.45±5.95 vs. 244.02±14.41 % of baseline). Also, LTP of C-fiber-evoked responses in neuropathic rats was smaller than sham rats (171.40±4.25 vs. 226.18±5.54 % of baseline) (Figures 3-5(Fig. 3)(Fig. 4)(Fig. 5)).
Figure 3. LTP of Aδ-fiber-evoked responses in single WDR dorsal horn neurons in % of baseline (mean±SEM). (A) The effect of intrathecal injection of 7-NI (8.15 µg/5 µl) on Aδ-fiber-evoked responses in neuropathic rats. HFS was applied to the sciatic nerve at time zero. Post-HFS responses were recorded every 4 min for up to 2 h. (B) Statistical comparison of the % of potentiation of Aδ-fiber-evoked responses at time 0-2 h after HFS. The values are shown based on the average of three consecutive responses. *p<0.05 indicates a significant difference with sham animals. #p<0.05, indicates a significant difference with neuropathic rats.

Figure 4. LTP of C-fiber-evoked responses in single WDR dorsal horn neurons in % of baseline (mean±SEM). (A) C-fiber evoked-responses between experimental groups. HFS was applied to sciatic nerve at time zero. Post-HFS responses were recorded every 4 min up to 2 h. The effect of intrathecal injection of 7-NI (8.15 µg/5 µl) on C-fiber-evoked responses in neuropathic rats is demonstrated. (B) Statistical comparison of the % of potentiation of C-fiber-evoked responses at time 0-2 h after HFS. The values are based on the average of three consecutive responses. *p<0.05, indicates a significant difference with sham animals. #p<0.05, indicates a significant difference with neuropathic rats.

Figure 5. Examples of the raw C-fiber-evoked responses and pre-stimulus diagram of a characteristic WDR neuron. (A1, A2) C-fiber-evoked responses before and after HFS in the sham rats following the first test stimulus. (B1, B2) C-fiber-evoked responses before and after HFS in neuropathic rats following the first test stimulus, (C1) C-fiber-evoked responses before HFS in the neuropathic rats following the first test stimulus, (C2) C-fiber-evoked responses 8 min after spinal application of 7-NI (8.15 µg/5 µl) in the baseline recording, (C3, C4) C-fiber-evoked-responses 60 min and 120 min after the spinal application of 7-NI in the neuropathic rats.
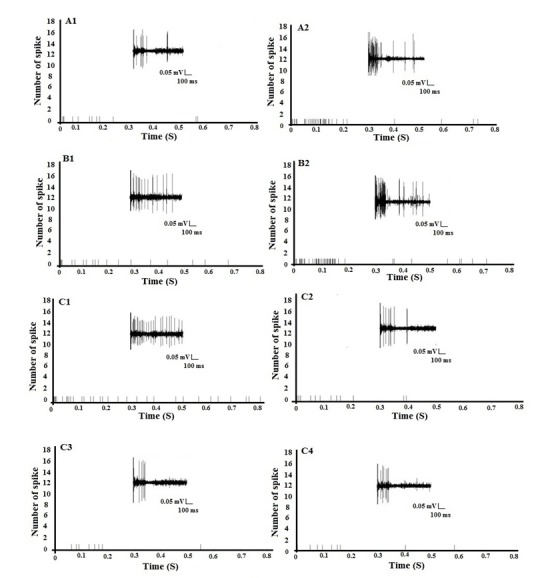
Additionally, we examined whether spinal cord nNOS contributes to the induction of LTP of Aδ- and C-fiber-evoked responses in dorsal horn WDR neurons on day 14 after neuropathy. 7-NI (n=5, 8.15 µg/5 µl) was applied directly onto the recording segment after the sixth test stimulus of the baseline recording (before HFS). We found that the spinal application of 7-NI completely blocked the induction of LTP of Aδ- and C-fiber-evoked responses in neuropathic rats (54.83±2.35 and 74.39±3.48 % of baseline, respectively) (Figures 3-5(Fig. 3)(Fig. 4)(Fig. 5)).
Discussion
In the present study, we demonstrated that spinal application of the selective nNOS inhibitor (7-NI) suppresses the induction of LTP of Aδ- and C-fiber-evoked responses in single WDR spinal neurons up to 2 hrs on day 14 after neuropathy. Also, interathecal application of 7-NI decreased mechanical allodynia on day 14 after neuropathy. It has been shown that nNOS has an important pro-nociceptive role in the development of pain-related behaviors (Meller et al., 1992[21]; Meller and Gebhart, 1993[20]; Wu et al., 2001[40]; Keilhoff et al., 2013[14]). Chacur et al. (2010[5]) have also shown 7-NI blocks hyperalgesia and allodynia on days 7, 15 and 30 after sciatic nerve transection. Also, administration of L-NAME, a non-selective NOS inhibitor, and Nω-propyl-l-arginine, a highly selective of nNOS inhibitor, exhibit analgesic effects on thermal and mechanical sensitivity 7 days after partial sciatic nerve ligation (Takasu et al., 2006[36]; Tanabe et al., 2009[37]). In the current study, intrathecal administration of 7-NI decreased the mechanical allodynia-induced by L5 spinal nerve transection 60 min after injection, and the effect persisted up to 150 min on day 14 after neuropathy. Our results support the hypothesis that nNOS may be important for the development of neuropathic pain. However, there are some controversial pieces of evidence about pro-nociceptive effects of nNOS. For example, Lee et al. (2005[17]) have reported that intraperitoneal administration of LY457963, a potent nNOS inhibitor does not reduce mechanical sensitivity in the neuropathic rats. Also, Luo et al. (1999[19]) have demonstrated that subcutaneous administration of the L-NAME and 7-NI does not change the mechanical allodynia in neuropathic rats. It seems that the nNOS contribution in the development of neuropathic pain is dependent on the genetic composition, age, environment and type of nerve damage. Furthermore, to obtain more information about the nNOS contribution in neuropathic pain and its correlation with the development of neuropathic behaviors we examined the spinal inhibition of nNOS on the induction of LTP of Aδ- and C-fiber-evoked responses in single WDR spinal neurons up to 2 hrs on day 14 after neuropathy. LTP was first described at synapses in the hippocampus as a primary model to study the synaptic basis of learning and memory (Bliss and Lomo, 1973[1]). Subsequently, several studies showed that painful electrical or natural sensory stimulation could induce synaptic plasticity such as LTP of C-fiber in spinal dorsal horn neurons (Randic et al., 1993[29]; Pockett, 1995[28]; Sandkuhler and Liu, 1998[30]). Induction of LTP critically depends on the stimulation parameters, type of stimulation, and the type of afferent peripheral fibers. Sandkuhler and Liu (1998[30]) have shown that supra-maximal electrical stimulation of sural nerve (20-30 V, 0.5 ms) elicited LTP of spinal C-fiber-evoked field potentials in the superficial lumbar dorsal horn (substantia gelatinosa) of intact rats. They have also shown that noxious skin heating (70 °C) or noxious pinching of skin induced LTP in spinalized rats but not in intact rats. In our electrophysiological study, we have demonstrated that the baseline of Aδ- and C-fiber-evoked responses (before HFS) in neuropathic rats is higher than sham rats (Aδ-fiber: 4.73 vs. 3.23 spikes/stimulus; C-fiber: 9.2 vs. 7.4 spikes/stimulus, data not shown). However, interestingly the HFS induced significantly smaller LTP of the Aδ- and C-fiber-evoked response in neuropathic rats compared to sham-operated rats (Figures 3-5(Fig. 3)(Fig. 4)(Fig. 5)). These observations could be explained by either dorsal horn hypersensitivity induced by neuropathy or decreased input to the dorsal horn by the HFS in neuropathic rats. Campbell and Meyer (2006[4]) have reported that L5 nerve transection induced abnormal ectopic discharge in injured (L5) and uninjured (L4-L6) nerves, resulting in spinal hypersensitivity and LTP. Therefore, spinal excitability evoked by our neuropathy model may explain why the HFS was less effective in neuropathic rats. In our model of neuropathy, afferent input was lost as a consequence of the L5 spinal nerve transection. However, despite this loss of afferent input, we have shown that the baseline Aδ- and C-fiber-evoked responses in neuropathic rats were higher than in sham rats, and the activation threshold of Aδ- and C-fibers was significantly lower in neuropathic rats (Figure 2(Fig. 2)). Accordingly, the present results show that L5 nerve transection increases dorsal horn sensitivity and this spinal hypersensitivity may compensate for the loss of afferent input. Consistent with our results, Palecek et al. (1992[27]) have shown that spinal nerve ligation decreases the evoked-responses in the damaged L7 segment on day 14 after neuropathy in primates, but in neurons rostral to the L7 segment, the evoked responses increased and the threshold decreased. However, Xing et al. (2007[41]) have reported that high frequency, low intensity stimulation of sciatic nerve induces LTP of C-fiber-evoked field potentials in dorsal horn neurons only in nerve ligated rats, and not in sham rats. These different observations in the electrophysiological studies may be due to the fact that both injured and uninjured spinal segments have been studied in neuropathic animals. Moreover, we have shown that spinal application of nNOS inhibitor (7-NI) attenuates baseline Aδ- and C-fiber-evoked responses (before HFS) and blocks the induction of LTP in both fibers (after HFS) on day 14 after neuropathy. There is little knowledge on how NO may act in the spinal plasticity. nNOS activity in the central nervous system requires the influx of Ca2+ (Oess et al., 2006[26]). It is well accepted that ectopic action potential in injured spinal nerves elicited high level of Ca2+ in the dorsal horn neurons by activation of NMDA receptors, and by triggering a cascade of events that include the activation of nNOS and subsequent generation of NO. Additionally, NO may act as a retrograde transmitter and enhance the release of substance P and glutamate from Aδ- and C-fiber terminals, contributing to the development of LTP (Miclescu and Gordh, 2009[22]). In conclusion, the present results demonstrate that nNOS inhibition suppresses the induction of LTP of Aδ- and C-fiber-evoked responses in single WDR dorsal horn neurons. We propose that nNOS is involved in the development of pain behaviors by inducing LTP of pain-related afferents in the spinal dorsal horn. Therefore, nNOS inhibition may represent a potential new therapeutic strategy for chronic neuropathic pain relief.
Acknowledgements
This study was conducted as part of a Ph.D. student thesis project in the Department of Neurophysiology, Fculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredt D, Synder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 3.Calcutt NA, Chaplan SR. Spinal pharmacology of tactile allodynia in diabetic rats. Br J Pharmacol. 1997;122:1478–1482. doi: 10.1038/sj.bjp.0701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chacur M, Matos JB, Alves AS, Rodrigues AC, Gutierrez V, Cury Y, et al. Participation of neuronal nitric oxide synthase in experimental neuropathic pain induced by sciatic nerve transection. Braz J Med Biol Res. 2010;43:367–376. doi: 10.1590/S0100-879X2010007500019. [DOI] [PubMed] [Google Scholar]
- 6.Choi JI, Kim WM, Lee HG, Kim YO, Yoon MH. Role of nitric oxide synthase in the antiallodynic effects of intrathecal EGCG in a neuropathic pain rat model. Neurosci Lett. 2012;510:53–57. doi: 10.1016/j.neulet.2011.12.070. [DOI] [PubMed] [Google Scholar]
- 7.Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: the dual effect of nitric oxide in the nociceptive system. Nitric Oxide. 2011;25:243–254. doi: 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Ding XL, Wang YH, Ning LP, Zhang Y, Ge HY, Jiang H, et al. Involvement of TRPV4-NO-cGMP-PKG pathways in the development of thermal hyperalgesia following chronic compression of the dorsal root ganglion in rats. Behav Brain Res. 2010;208:194–201. doi: 10.1016/j.bbr.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 10.Guan Y, Yaster M, Raja SN, Tao YX. Genetic knockout and pharmacologic inhibition on neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain. 2007;3:29. doi: 10.1186/1744-8069-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hama AT, Borsook D. Behavioral and pharmacological characterization of a distal peripheral nerve injury in the rat. Pharmacol Biochem Behav. 2005;8:170–181. doi: 10.1016/j.pbb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda H, Kiritoshi T, Kazuyuki M. Synaptic plasticity in the spinal dorsal horn. Neurosci Res. 2009;64:133–136. doi: 10.1016/j.neures.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Keilhoff G, Schroder H, Peters B, Becker A. Time-course of neuropathic pain in mice deficient in neuronal or inducible nitric oxide synthase. Neurosci Res. 2013;77:215–221. doi: 10.1016/j.neures.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 16.Kumazawa T. Primitivism and plasticity of pain-implication of polymodal receptors. Neurosci Res. 1998;32:9–31. doi: 10.1016/s0168-0102(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Singh JP, Lodge D. Experiments with nitric oxide synthase inhibitors in spinal nerve ligated rats provides no evidence of a role for nitric oxide in neuropathic mechanical allodynia. Neurosci Lett. 2005;385:179–183. doi: 10.1016/j.neulet.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 18.Luo D, Vincent SR. NMDA-dependent nitric oxide release in the hippocampus in vivo: interactions with noradrenaline. Neuropharmacology. 1994;33:1345–1350. doi: 10.1016/0028-3908(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 19.Luo ZD, Chaplan SR, Scott BP, Cizkova D, Calcutt NA, Yaksh TL. Neuronal nitric oxide synthase mRNA upregulation in rat sensory neurons after spinal nerve ligation: lack of a role in allodynia development. J Neurosci. 1999;19:9201–9208. doi: 10.1523/JNEUROSCI.19-21-09201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 21.Meller ST, Pechman PS, Gebhart GF, Maves TJ. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience. 1992;50:7–10. doi: 10.1016/0306-4522(92)90377-e. [DOI] [PubMed] [Google Scholar]
- 22.Miclescu A, Gordh T. Nitric oxide and pain: ‘Something old, something new’. Acta Anaesthesiol Scand. 2009;53:1107–1120. doi: 10.1111/j.1399-6576.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- 23.Mirzaei V, Manaheji H, Maghsoudi N, Zarringhalam J. Comparison of changes in mRNA expression of spinal glutamate transporters following induction of two neuropathic pain models. Spinal Cord. 2010;48:791–797. doi: 10.1038/sc.2010.21. [DOI] [PubMed] [Google Scholar]
- 24.Montague PR, Gancyco CD, Winn MJ, Marchase RB, Friedlander MJ. Role of NO production in NMDA receptor-mediated neurotransmitter release in cerebral cortex. Science. 1994;263:973–977. doi: 10.1126/science.7508638. [DOI] [PubMed] [Google Scholar]
- 25.Nazemi S, Manaheji H, Zaringhalam J, Sadeghi M, Haghparast A. Post-injury repeated administrations of minocycline improve the anti-nociceptive effect of morphine in chronic constriction injury model of neuropathic pain in rats. Pharmacol Biochem Behav. 2012;102:520–525. doi: 10.1016/j.pbb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Oess S, Icking A, Fulton D, Govers R, Muller W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J. 2006;396:401–409. doi: 10.1042/BJ20060321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palecek J, Dougherty PM, Kim SH, Paleckova V, Lekan H, Chung JM, et al. Responses of spinothalamic tract neurons to mechanical and thermal stimuli in an experimental model of peripheral neuropathy. J Neurophysiol. 1992;67:1562–1573. doi: 10.1152/jn.1992.68.6.1951. [DOI] [PubMed] [Google Scholar]
- 28.Pockett S. Long-term potentiation and depression in the intermediate gray matter of rat spinal cord in vitro. Neuroscience. 1995;67:791–798. doi: 10.1016/0306-4522(95)00077-v. [DOI] [PubMed] [Google Scholar]
- 29.Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;12:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandkuhler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci. 1998;10:2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- 31.Schmidtko A, Gao W, König P, Heine S, Sausbier M, Ruth P, et al. cGMP-dependent signaling pathways in spinal pain processing. BMC Pharmacology. 2009;9(Suppl 1):S37. [Google Scholar]
- 32.Storkson RV, Kjorsvik A, Tjolsen A, Hole K. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Meth. 1996;65:167–172. doi: 10.1016/0165-0270(95)00164-6. [DOI] [PubMed] [Google Scholar]
- 33.Svendsen F, Hole K, Tjolsen A. Long-term potentiation in single wide dynamic range neurons induced by noxious stimulation in intact and spinalized rats. Prog Brain Res. 2000;129:153–161. doi: 10.1016/S0079-6123(00)29011-0. [DOI] [PubMed] [Google Scholar]
- 34.Svendsen F, Rygh LG, Gjerstad J, Fiska A, Hole K, Tjolsen A. Recording of long term potentiation in single dorsal horn neurons in vivo in the rat. Brain Res Prot. 1999;4:165–172. doi: 10.1016/s1385-299x(99)00018-5. [DOI] [PubMed] [Google Scholar]
- 35.Svendsen F, Tjolsen A, Hole K. LTP of spinal A beta and C-fiber evoked responses after electrical sciatic nerve stimulation. Neuroreport. 1997;8:3427–3430. doi: 10.1097/00001756-199711100-00002. [DOI] [PubMed] [Google Scholar]
- 36.Takasu K, Honda M, Ono H, Tanabe M. Spinal alpha2-adrenergic and muscarinic receptors and the NO release cascade mediate supraspinally produced effectiveness of gabapentine at decreasing mechanical hypersensitivity in mice after partial nerve injury. Br J Pharmacol. 2006;148:233–244. doi: 10.1038/sj.bjp.0706731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanabe M, Nagatani Y, Saitoh K, Takasu K, Ono H. Pharmacological assessments of nitric oxide synthase isoforms and downstream diversity of NO signaling in the maintenance of thermal and mechanical hypersensitivity after peripheral nerve injury in mice. Neuropharmacology. 2009;56:702–708. doi: 10.1016/j.neuropharm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Urch CE, Dickenson AH. In vivo single unit extracellular recordings from spinal cord neurons of rats. Brain Res Brain Res Protoc. 2003;12(1):26–34. doi: 10.1016/s1385-299x(03)00068-0. [DOI] [PubMed] [Google Scholar]
- 39.Wallin J, Fiskå A, Tjølsen A, Linderoth B, Hole K. Spinal cord stimulation inhibits long-term potentiation of spinal wide dynamic range neurons. Brain Res. 2003;973:39–43. doi: 10.1016/s0006-8993(03)02530-7. [DOI] [PubMed] [Google Scholar]
- 40.Wu J, Fang L, Lin Q, Willis WD. Nitric oxide synthase in spinal cord central sensitization following interadermal injection of capsaicin. Pain. 2001;94:47–58. doi: 10.1016/S0304-3959(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 41.Xing GG, Liu FY, Qu XX, Han JS, Wan Y. Long term synaptic plasticity in the spinal dorsal horn and its modulation by electroacupuncture in rats with neuropathic pain. Exp Neurol. 2007;208:323–332. doi: 10.1016/j.expneurol.2007.09.004. [DOI] [PubMed] [Google Scholar]


