Abstract
Time-dependent changes in blood-based protein biomarkers can help identify the pathological processes in blast-induced traumatic brain injury (bTBI), assess injury severity, and monitor disease progression. We obtained blood from control and injured mice (exposed to a single, low-intensity blast) at 2-h, 1-day, 1–week, and 1-month post-injury. We then determined the serum levels of biomarkers related to metabolism (4-HNE, HIF-1α, ceruloplasmin), vascular function (AQP1, AQP4, VEGF, vWF, Flk-1), inflammation (OPN, CINC1, fibrinogen, MIP-1a, OX-44, p38, MMP-8, MCP-1 CCR5, CRP, galectin-1), cell adhesion and the extracellular matrix (integrin α6, TIMP1, TIMP4, Ncad, connexin-43), and axonal (NF-H, Tau), neuronal (NSE, CK-BB) and glial damage (GFAP, S100β, MBP) at various post-injury time points. Our findings indicate that the exposure to a single, low-intensity blast results in metabolic and vascular changes, altered cell adhesion, and axonal and neuronal injury in the mouse model of bTBI. Interestingly, serum levels of several inflammatory and astroglial markers were either unchanged or elevated only during the acute and subacute phases of injury. Conversely, serum levels of the majority of biomarkers related to metabolic and vascular functions, cell adhesion, as well as neuronal and axonal damage remained elevated at the termination of the experiment (1 month), indicating long-term systemic and cerebral alterations due to blast. Our findings show that the exposure to a single, low-intensity blast induces complex pathological processes with distinct temporal profiles. Hence, monitoring serum biomarker levels at various post-injury time points may provide enhanced diagnostics in blast-related neurological and multi-system deficits.
Keywords: blast, traumatic brain injury, mouse, serum, biomarkers
Introduction
The mild form of traumatic brain injury (mTBI, also called concussion) accounts for ~75% of all head injuries among civilians and military personnel (1, 2). In the U.S. military, mTBI, mostly caused by explosive weaponry, has affected ~300,000–500,000 military personnel during the last 15 years (3–5). Numerous terms, including blast-induced neurotrauma (BINT) (3, 4), blast-induced TBI, or just blast TBI (bTBI), have been coined to describe the unique clinical entity of blast-induced neurological deficits caused by interwoven mechanisms of systemic, local, and cerebral responses to blast exposure(s) (4, 5).
Similar to civilian concussion, the acute symptoms of mild, blast-induced TBI (mbTBI) are usually mild and transient (6). However, in addition to the large number of affected individuals, mbTBI is a significant health issue for several reasons. Repetitive mild injuries – if they occur within the period of increased cerebral vulnerability – can result in a disproportionally severe acute outcome (7, 8). Moreover, mbTBI, especially when repeated, could significantly increase the probability of late onset neurodegenerative conditions, such as chronic traumatic encephalopathy (CTE) (9, 10). Finally, blast interacting with the entire body and not only with the head, induces complex co-occurring systemic, local, and cerebral responses to injury (11–14). Thus, the neurological deficits that develop after blast exposure(s) are resulted not only from the brain damage caused via head-blast interaction but also from multiple, synergistically acting injury mechanisms. Because of such complexity, the diagnosis of blast-induced neurological consequences, both during the acute and chronic injury phases, is extremely challenging: the manifestations of mbTBI are often masked by symptoms of systemic changes.
It is noteworthy that many of the acute and subacute neurobehavioral symptoms of mbTBI, such as impaired cognition and emotional instability, are shared with post-traumatic stress disorder (PTSD), a complex clinical syndrome triggered by extreme psychological stress without physical injury (15). Since the diagnosis of mbTBI and PTSD are often solely based on neuropsychological tests and self-reported information, the differential diagnosis is challenging due to the absence of objective outcome measures. While earlier studies using rodent models have suggested that the pathobiology of PTSD and mbTBI may involve distinct mechanisms (15–18), the precise identities of the molecular events leading to the observed functional changes are still unclear (19).
The increasing numbers of research studies using various animal models, in combination with analytical techniques such as histopathology and proteomics, have identified some of the mechanisms underlying the pathology of bTBI. These findings include metabolic and vascular changes; axonal, neuronal and glial damage; inflammation; and altered cell adhesion, among others (13, 20–29). In this study, we used the previously developed, well-standardized, and validated mouse model of primary blast injuries (30), which reproduces the main consequences of blast exposures: changes in general physiology, multi-organ damages, systemic compensatory mechanisms, as well as neurological deficits seen in individuals exposed to differing blast intensity levels. Our intention was to reproduce the full spectrum of injury response mechanisms induced by primary blast and not only head trauma as an isolated entity (30, 31). Previous works with this model has determined that the vital functions, memory and cognitive performance, and behavioral impairments are comparable with the symptoms of mild and moderate TBI in war fighters exposed to blast (30, 31). Those findings also implicated inflammation as a potential mechanistic explanation for the long-term neurological deficits after blast exposure.
Using the same mouse model of bTBI (30, 31), we determined changes in the serum levels of protein biomarkers at 2 h, 1 day, 1 week, and 1 month after exposure. We selected 31 biomarkers indicative of changes in metabolic and vascular functions; cell adhesion; axonal, neuronal, and glial integrities; and inflammation to determine the temporal pattern of changes from early/acute (2 h) to late/chronic (1 month) post-injury time points. We found that the exposure to low-intensity blast triggers complex changes in serum levels of biomarkers, many of them remaining highly elevated at 1 month after the injury suggesting that a single exposure to low-intensity blast can induce lasting molecular pathologies. Our findings have implications for determining the period of ICV after mbTBI, and identifying the distinct pathologies that involve a widespread systemic response in the pathobiology of blast-induced neurological deficits. Moreover, our results underline the role of long-term monitoring of serum protein biomarkers in the differential diagnosis between mbTBI and PTSD as well as mbTBI and impact TBI. As such, our findings imply that continuous assessments of serum protein biomarkers could help in predicting the outcome of mbTBI and preempting multi-organ complications of blast exposure.
Materials and Methods
All protocols involving the use of animals complied with the Guide and Care and Use of laboratory Animals published by the NIH (DHEW publication NIH 85-23-2985) and were approved by the Johns Hopkins University Animal Use Committee. Exposures were performed at the Johns Hopkins University Applied Physics Laboratory (JHU/APL) using a modular, multi-chamber shock tube capable of reproducing complex shock wave signatures seen in theater (30, 31).
Blast injury
Twenty-five 3–4-month-old C57/B16 mice (Jackson Laboratories) were used. Animals (weighing 22–24 g at the beginning of the study) were divided into three experimental groups: naïve (n = 5), sham (n = 5), and injured (n = 20). Animals were housed in pairs in standard mouse cages in a reverse 12 h light and 12 h dark cycle with access to food and water ad libitum. Animals in the sham and in the injured groups were anesthetized with 4% isoflurane evaporated in a gas mixture containing 30% oxygen/70% nitrous oxide and applied through a nose mask. The animals were allowed to breathe spontaneously without tracheal intubation. Mice were then mounted in a supine position to the animal holder and placed in the shock tube as described (30). The animal holder positioned the specimen at 53 cm (20.87˝) upstream from the driven section opening, so that only a well-formed incident shock wave loaded the animal and potential rarefactions from the tube opening were minimized. The neck, head, torso, and abdomen of the animal were fixed to the animal holder to avoid any movement, thus eliminating tertiary blast effects. The loading conditions (mean ± SD) were the following: measured membrane rupture pressure 19.2 ± 2.7 psig (132.38 ± 18.62 kPag); measured static pressure 7.5 ± 1.1 psig (51.71 ± 7.58 kPag); time pressure rise 0.010284 ± 0.000964 s; time pressure fall 0.0048 ± 0.0027; and pulse width 15.3 ± 2.9 ms. Sham animals were handled identically except exposure to the pressure wave.
Body weight
Measurements were performed at baseline, and at 1-day, 3-day, 5-day, 1-week, and 1-month post-injury.
Acute neurological outcome
Neurological outcome was evaluated immediately after exposure by observing the spontaneous behavior of the animal and by assessing the tail pinch reflex (time of response to pain stimulus), corneal reflex (time of response to corneal touch), and righting reflex (time of self-correction in an animal placed on its back to return to its normal upright position).
Blast injury severity
The mortality rate was assessed at 24 h post-trauma. Injured mice were sacrificed at 2-h (n = 5), 1-day (n = 5), 1-week (n = 5), and 1-month (n = 5) post-injury. Blast injury severity in animals with lethal outcome and in survival animals at the end of the 30-day post-exposure period was performed using the Pathology Scoring System (PSS) for Blast Injuries (32) modified as previously described (12, 30, 33).
Biosamples
Animals were anesthetized with 4% isoflurane evaporated in a gas mixture containing 30% oxygen/70% nitrous oxide and applied through a nose mask. After a midline thoracic incision, the heart was carefully exposed and blood samples were obtained via cardiac puncture and collected into BD Vacutainer SST plastic serum tube with clot activator and gel for serum separation (Ref. 367988). The blood was allowed to clot at room temperature for 40 min followed by centrifugation at 2000 g for 15 min. Collected supernatants were then divided into 200 μl aliquots, flash-frozen, and stored at −80°C until use.
Sample preparation for reverse phase protein microarray assay
Serum samples were manually diluted 1:10 with 3× print buffer; samples were further diluted using a JANUS Varispan Integrator and Expanded Platform Workstation (PerkinElmer, Waltham, MA, USA) resulting in a 11-point serial dilution in triplicates in 384-well microarray plates (X7022; Fisher Scientific, Pittsburgh, PA, USA) (14, 18, 23, 34). The plates were then transferred into an Aushon 2470 Arrayer (Aushon Biosystems, Billerica, MA, USA) and printed on ONCYTE Nova single-pad nitrocellulose coated glass slides (Grace Bio-Labs, Bend, OR, USA). The Aushon 2470 Arrayer was set up with 16 pins and programed for single deposit. The spot diameter was set to 250 nm with a spacing of 500 nm between dots on the x-axis and 375 nm on the y-axis. Wash time was set at 2 s without delays. Following desiccation at 4°C overnight, slides were blocked with 5% non-fat dry milk in 1× TBS with 0.1% Tween 20 (TBST). Primary antibodies (see Table S1 in Supplementary Material) were diluted to 10× the optimal Western analysis concentration in antibody incubation buffer [0.1% BSA, protease inhibitors (EDTA-free Halt protease and phosphatase inhibitor cocktail, Thermo Fisher, Waltham, MA, USA), 1× TBS, 0.5% Tween 20] (14, 18, 23, 34). Slides were incubated with the primary antibody solution overnight at 4°C covered by a cover slip (Nunc* mSeries LifterSlips, Fisher Scientific, Pittsburg, PA, USA). The following day slides were thoroughly washed three times with TBST (1× TBS in 0.1% Tween 20) and incubated with the secondary antibodies [Alexa Fluor® 635 goat anti-mouse (A-31574), Alexa Fluor® 647 goat anti-rabbit (A-21245), 647 rabbit anti-goat (A-21446) or Alexa Fluor® 633 donkey anti-sheep IgG (H + L) (A-21100)] (all from Invitrogen) diluted at 1:6000 in antibody incubation buffer for 1 h at room temperature. After three thorough washes in TBST followed by a single wash with 1× TBS slides were air dried and the intensity of fluorescent signals were determined in a Scan Array Express HT microarray scanner (Perkin Elmer, Waltham, MA, USA) using a 633 nm wavelength laser and a 647 nm filter.
Data analysis
Data were imported into a Microsoft Excel-based bioinformatics program for analysis (14, 18, 23, 34). The program imports intensity data from the scanner and calculates total net intensity after local background subtraction (i.e., the intensities of secondary antibody alone and for each corresponding primary antibody) for each spot. Intensity data from the dilution series of each sample were then plotted against dilution on a log–log graph. Primary data were manually curated by linear regression of the log–log data and were plotted after the removal of flagged data. Flagged data include spot intensities in the saturation range or noise range, signal to noise ratios <2, or high variability between duplicate spots (>10–15%). The total amount of antigen is determined by the y-axis intercept or y-cept (i.e., by extrapolating the regression line to zero); reported values are log 10.
Differences in protein biomarker levels at each of the post-injury time points relative to naïve levels were analyzed with independent t-tests. A Bonferroni-adjusted significance level of 0.0125 was calculated to account for the increased possibility of type I error. Relative intensity data (y-cept values) were presented as the mean ± SEM. Statistical significance is reported for naïve vs. injured mice at each time point where applicable (Figures 1–4).
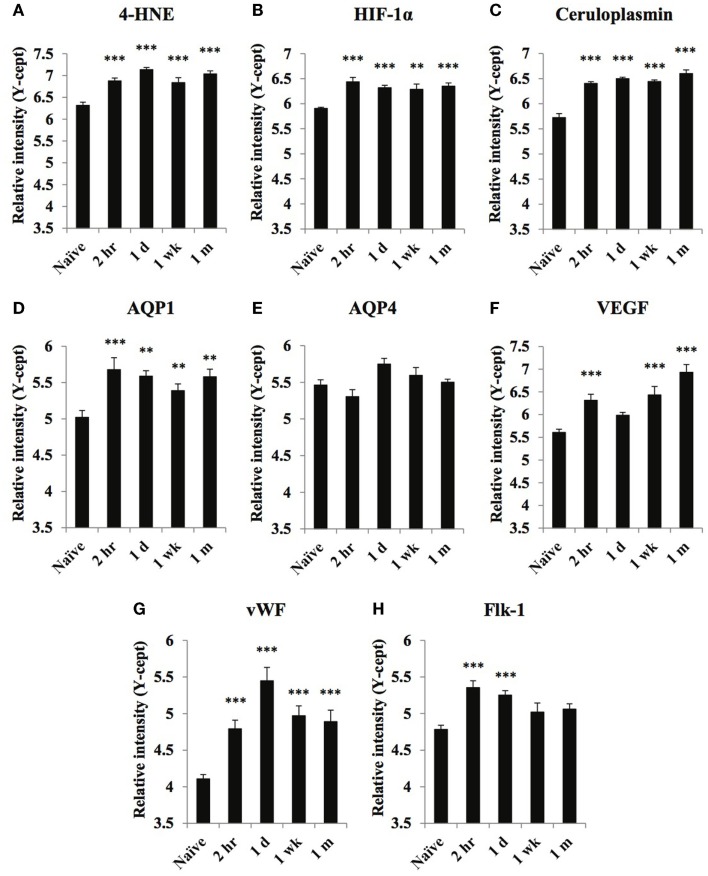
Figure 1.
Temporal changes of select oxidative stress and vascular markers in serum obtained from naïve and at 2 h, 1 day, 1 week, and 1 month of post-blast over pressure exposed animals. Protein levels of oxidative stress markers 4-HNE (A), HIF-1α (B), ceruloplasmin (C), vascular related biomarkers AQP1 (D), AQP4 (E), VEGF (F), vWF (G), and FLK-1 (H) were assayed using reverse phase protein microarray (RPPM) and are expressed as y-cept values (log 10). Data are presented as the mean ± SEM; **p < 0.01, ***p < 0.001.
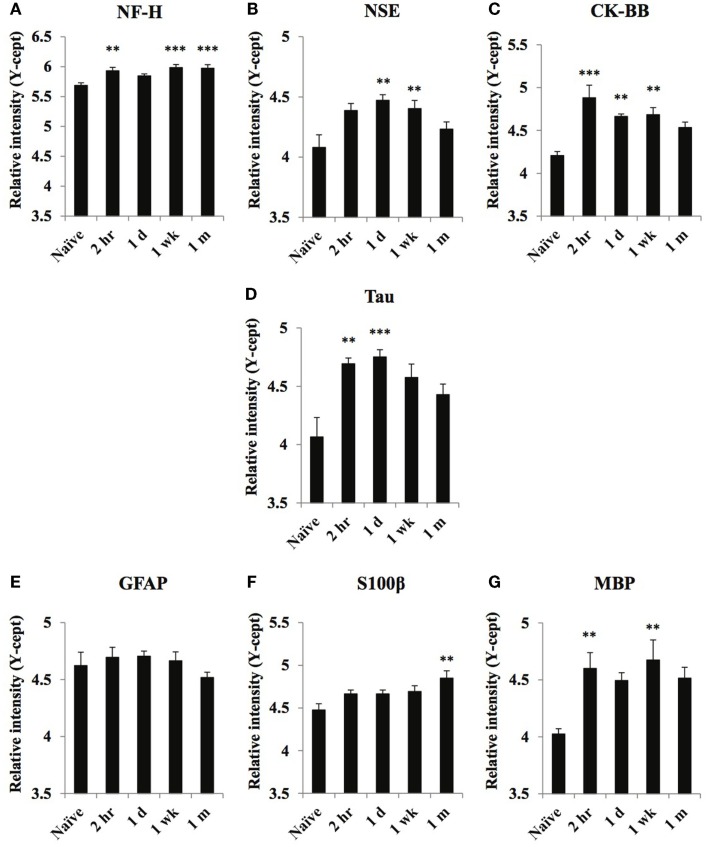
Figure 4.
Temporal changes of select neuronal and glial damage markers in serum obtained from naïve and at 2 h, 1 day, 1 week, and 1 month of post-blast over pressure exposed animals. Protein levels of neuronal markers NF-H (A), NSE (B), CK-BB (C), Tau (D), glial markers GFAP (E), S100β (F), and MBP (G) were assayed using reverse phase protein microarray (RPPM) and are expressed as y-cept values (log 10). Data are presented as the mean ± SEM; **p < 0.01, ***p < 0.001.
Results
Body weight
Blast exposure caused significant weight loss at days 3 and 5 after injury: 27.5 ± 0.65 g and 27.7 ± 0.53 g, respectively, compared to the pre-injury value of 29.3 ± 0.35 g. The body weight (BW) of injured mice was normalized 1 week after the exposure.
Acute neurological outcome
Following the cessation of anesthesia, control mice recovered the tail pinch reflex after a mean 1.65 ± 0.15 min, corneal reflex after 1.86 ± 0.17 min, and righting reflex after 2.73 ± 0.25 min. The blast exposure somewhat extended the time needed for reflex recovery (tail pinch reflex: 2.43 ± 1.37 min; corneal reflex: 3.37 ± 2.13 min; and righting reflex: 3.42 ± 2.02 min). Nevertheless, these changes were not statistically significant due to relatively large variations between animals.
Blast injury severity
The mortality rate at 24 h after exposure was 4.5%, which is comparable to the 5% mortality rate in animals exposed to mild intensity blast reported in our previous study (30). Death occurred immediately, mostly due to cardiorespiratory depression and did not correspond to any organ damage. In our current study, the total blast injury severity score was 22.25 ± 3.11 PSS compared to 24.44 ± 0.50 PSS in our original paper (30). Morphological damage in the form of slight petechiae and rare ecchymosis were mainly found in the lungs of exposed mice. Gross pathological observation of the brains following mild blast injury showed an absence of subarachnoid hemorrhage, focal lesions, or contusions.
General biomarker trends
We found no significant differences in serum biomarker levels between naïve and sham animals. However, the exposure to a single, low-intensity blast resulted in substantial changes in the serum levels of a number of biomarkers indicating changes in metabolic and vascular functions, altered cell adhesion, as well as neuronal, axonal, and glial damage. Interestingly, serum levels of the majority of inflammatory and astroglial markers did not change, while other biomarkers showed distinct temporal patterns (summarized in Table 1).
Table 1.
Summary of injury-induced changes in serum biomarker levels relative to control levels.
| Markers | Post-injury time points | |||
|---|---|---|---|---|
| 2 h | 1 day | 1 week | 1 month | |
| Metabolism | ||||
| 4-HNE | ↑ | ↑ | ↑ | ↑ |
| HIF-lα | ↑ | ↑ | ↑ | ↑ |
| Ceruloplasmin | ↑ | ↑ | ↑ | ↑ |
| Vascular function | ||||
| AQP1 | ↑ | ↑ | ↑ | ↑ |
| AQP4 | Ø | Ø | Ø | Ø |
| VEGF | ↑ | Ø | ↑ | ↑ |
| vWF | ↑ | ↑ | ↑ | ↑ |
| Flk-1 | ↑ | ↑ | Ø | Ø |
| Inflammation | ||||
| OPN | ↑ | ↑ | Ø | ↑ |
| CINC1 | Ø | ↑ | ↑ | Ø |
| Fibrinogen | ↑ | Ø | Ø | Ø |
| MlP-lα | Ø | ↑ | Ø | Ø |
| OX-44 | ↑ | ↑ | ↑ | Ø |
| p38 | ↑ | ↑ | Ø | Ø |
| MMP-8 | Ø | Ø | Ø | Ø |
| MCP-1 | Ø | Ø | Ø | Ø |
| CCR5 | Ø | Ø | Ø | Ø |
| CRP | Ø | Ø | Ø | Ø |
| Galectin-1 | Ø | Ø | Ø | Ø |
| Cell adhesion and extracellular matrix | ||||
| Integrin α6 | ↑ | ↑ | ↑ | ↑ |
| TIMP1 | ↑ | ↑ | ↑ | ↑ |
| TIMP4 | ↑ | ↑ | Ø | Ø |
| Ncad | Ø | Ø | Ø | Ø |
| Connexin-43 | ↑ | ↑ | ↑ | ↑ |
| Axonal damage | ||||
| NF-H | ↑ | Ø | ↑ | ↑ |
| Tau | ↑ | ↑ | Ø | Ø |
| Neuronal damage | ||||
| NSE | Ø | ↑ | ↑ | Ø |
| CK-BB | ↑ | ↑ | ↑ | Ø |
| Glial damage | ||||
| GFAP | Ø | Ø | Ø | Ø |
| S100β | Ø | Ø | Ø | ↑ |
| MBP | ↑ | Ø | ↑ | Ø |
Metabolic and vascular markers
Of the three metabolic and oxidative stress markers, ceruloplasmin showed the highest initial increase at 2 h compared to naïve levels; serum levels continued to increase reaching a maximum at 1-month post-injury (Figure 1C). HIF-1α levels were highest at 2 h post-injury, remaining elevated up to 1 month after injury (Figure 1B). 4-HNE serum levels were highest at 1 day and 1-month post-injury (Figure 1A).
Of the markers related to vascular function, serum levels of AQP1, VEGF, and vWF showed the most substantial increases in response to injury, each displaying a distinct temporal pattern (Figures 1D,F,G). The changes in VEGF levels showed a bi-phasic profile. After an initial peak at 2 h, VEGF levels returned close to normal before peaking again at 1-month post-injury (Figure 1F). vWF levels were highest 1 day post-exposure and while serum levels declined over time, they were still significantly higher than naïve values 1 month after the injury. Serum concentrations of the other vascular marker, Flk-1, showed an early increase at 2 h that was somewhat sustained at 1 day; by 1 wk levels returned to normal, control values. We found no significant increase in serum AQP4 levels at any post-injury time points (Figure 1E).
Inflammatory markers
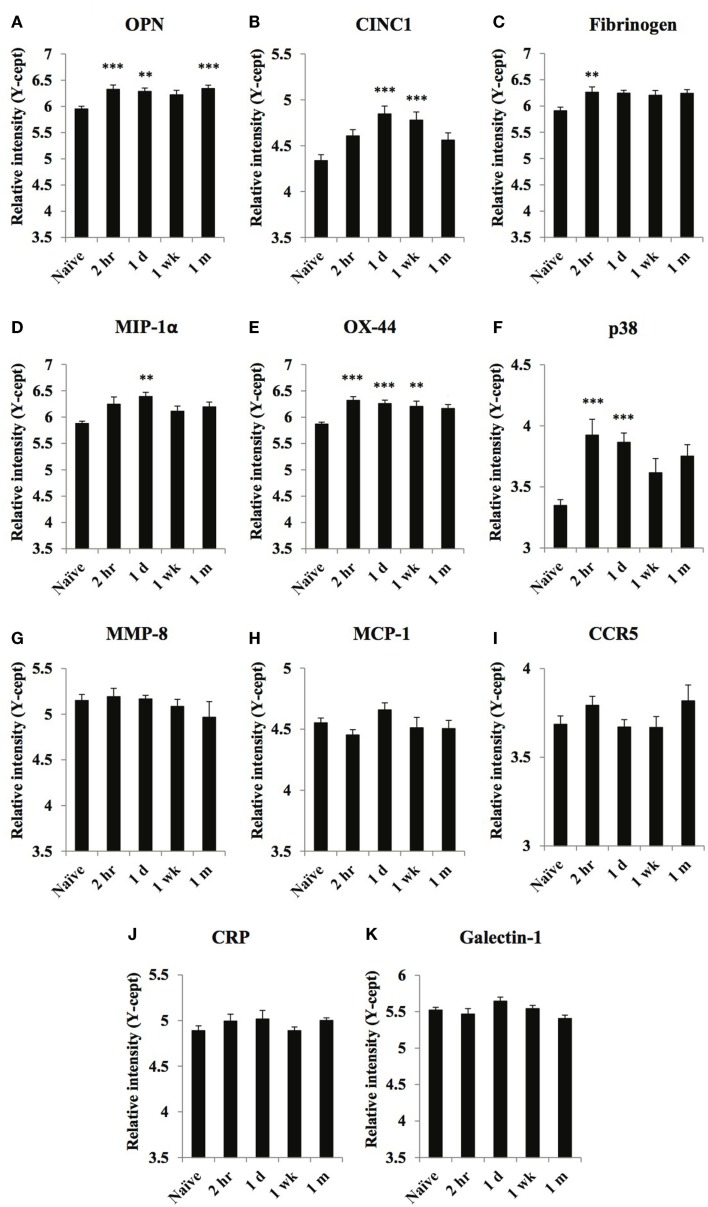
Serum levels of a few inflammatory markers, OPN, fibrinogen, OX-44, and p38, increased rapidly after injury; marker levels (except OPN) returned to control values at or close to their peak level at 1-month post-injury (Figures 2A,C,E,F). The serum levels of CINC1 and MIP-1α (Figures 2B,D) were increased only at 1 day or 1 week after injury then decreased at 1 month.
Figure 2.
Temporal changes of select inflammatory markers in serum obtained from naïve and at 2 h, 1 day, 1 week, and 1 month of post-blast over pressure exposed animals. Protein levels of OPN (A), CINC1 (B), fibrinogen (C), MIP-1α (D), OX-44 (E), p38 (F), MMP-8 (G), MCP-1 (H), CCR5 (I), CRP (J), and galectin-1 (K) were assayed using reverse phase protein microarray (RPPM) and are expressed as y-cept values (log 10). Data are presented as the mean ± SEM; **p < 0.01, ***p < 0.001.
Serum levels of MMP-8, MCP-1, CCR5, CRP, and galectin-1 (Figures 2G–K) did not change significantly compared to control values.
Cell adhesion and extracellular matrix markers
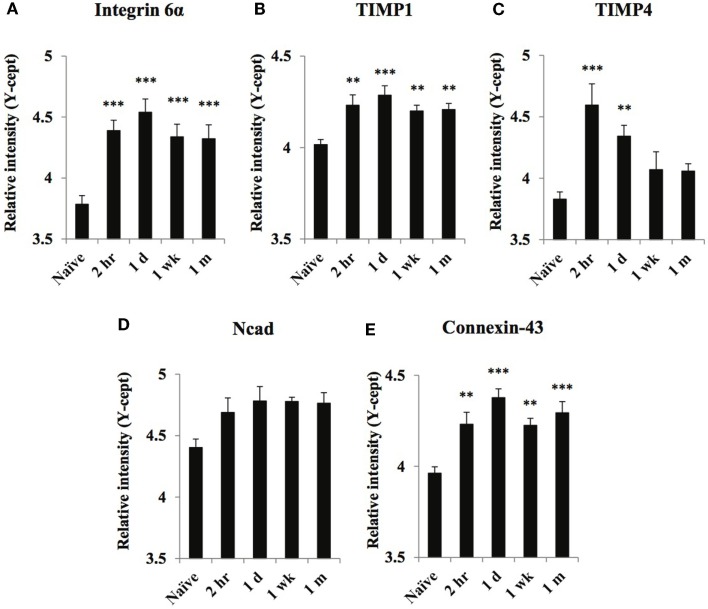
Serum concentrations of all cell adhesion proteins but Ncad increased in response to the injury, although the temporal profiles were different (Figure 3). Serum levels of all markers but TIMP4 reached their maximum at 1-day post-injury and remained elevated until the end of the study. TIMP4 levels were highest at 2 h after injury, decreased by 1 week, and returned to control levels by 1 month (Figure 3C).
Figure 3.
Temporal changes of select cell surface markers in serum obtained from naïve and at 2 h, 1 day, 1 week, and 1 month of post-blast over pressure exposed animals. Protein levels of integrin 6α (A), TIMP1 (B), TIMP4 (C), Ncad (D), and Connexin-43 (E) were assayed using reverse phase protein microarray (RPPM) and are expressed as y-cept values (log 10). Data are presented as the mean ± SEM; **p < 0.01, ***p < 0.001.
Axonal, neuronal, and glial damage markers
Serum levels of the axon- and neuron-specific markers, NF-H, Tau, NSE, and CK-BB, increased moderately in response to blast injury (Figures 4A–D). At 1 month after injury, NSE, CK-BB, and tau levels returned to control levels, whereas NF-H remained significantly elevated. Serum levels of the astroglia markers GFAP and S100β were not significantly increased at any of the post-injury time points (except for a moderate elevation in S100β at the 1-month time point) (Figures 4E,F). Conversely, MBP levels showed robust increases at 2-h and 1-week post-injury (Figure 4G).
Discussion
Cognitive and emotional symptoms have been consistently observed in soldiers exposed to blast(s). It has been reported that blast forces alone can cause deleterious behavioral changes when evaluated with selected measures of personality (35). Blast exposure has also been linked to self-perceived cognitive decline in soldiers with mbTBI (36). Recent experimental data support the notion that the exposure to blast (as an emotionally traumatizing event) can alter memory function and cause anxiety (37). Furthermore, it has been recognized that individuals with bTBI also suffer from PTSD (38, 39) and that these two clinical conditions likely develop in parallel leading to a unique and complex clinical entity. Hence, the differential diagnosis between PTSD and bTBI only has merit if it determines distinct therapeutic approaches or if it serves to pre-empt bTBI complications.
The diagnosis of mild blast TBI is hugely misleading; it only indicates the brain’s immediate response to blast and does not take into account the severity of long-term complications. Nevertheless, without better diagnostic nomenclature, we continue to use this term (i.e., mbTBI) both in clinical practice and basic science research. Bearing in mind that there are over 300,000 veterans diagnosed with mbTBI, the timely diagnosis of mbTBI still remains an unsolved problem. Our main goal in this study was to use our previously standardized model and experimental set-up of low-intensity blast exposure generated in laboratory conditions to identify a set of biomarkers that would provide insight into the complex pathobiology of blast-induced neurological deficits (in this paper termed mbTBI to annotate that it was induced by low-intensity primary blast). Chronic elevation in serum levels of vascular and inflammatory biomarkers has been previously found (14, 34), suggesting a profound systemic response to blast exposure; such distinctive biomarker patterns could be useful in differentiating mbTBI from impact TBI and TBI from PTSD.
In our previous study that outlined the main features of our experimental model (30), we compared several shockwave forms: (1) the Friedländer waveform, a simplified qualitative depiction of an explosion-generated shockwave showing positive and negative phases of a pressure wave; (2) an open field shockwave generated by detonating 816.47 g (1.8 lbs) of a TNT-equivalent explosive charge in open field (even terrain, no surrounding objects) conditions. The static pressure was recorded 3.6 m from the source; (3) a complex shockwave form generated by detonating 725.75 g (1.6 lbs) of a TNT-equivalent explosive charge in an urban-environment. The static pressure was recorded 2.3 m from the source; and (4) a moderate intensity shockwave form generated in our shock tube. The static pressure was recorded 4.5 m downstream from the shock tube diaphragm. The blast signatures showed a similarity between the shapes of the shock tube output and the open field shockwave; the intensity of the shock tube-generated overpressure has been modified based on the Bowen curve calculations for small experimental animals.
In this study, we used our previously standardized mouse model of primary blast injuries, generated using a modular, multi-chamber shock tube capable of reproducing complex shock wave signatures seen in theater (30, 31), to elucidate the molecular mechanisms that underlie the neurobehavioral and functional deficits observed clinically and modeled experimentally (20, 28, 40, 41). The experimental set-up in our current study is comparable, albeit not identical to the previous design (30). Nevertheless, the general response to the blast exposure including BW changes, acute neurological recovery, and tissue damage in multiple organs (PSS score) is very similar in both studies. This implies that all other outcome measures (i.e., behavior, motor, and cognitive function) are also comparable in the two studies.
Using antibody-based proteomic analyses, we monitored changes in the serum levels of 31 protein biomarkers up to 1-month post-injury and identified a number of leading pathologies that include metabolic and vascular changes with minimal neuronal and glial damage. More specifically, we collected blood samples from injured mice at 2-h, 1-day, 1-week, and 1-month post-blast to determine the short- and long-term changes in protein markers related to metabolism (4-HNE, HIF-1α, ceruloplasmin), vascular function (AQP1, AQP4, VEGF, vWF, Flk-1), inflammation (OPN, CINC1, fibrinogen, MIP-1α, OX-44, p38, MMP-8, MCP-1 CCR5, CRP, galectin-1), cell adhesion and the extracellular matrix (integrin α6, TIMP1, TIMP4, Ncad, connexin-43), axonal (NF-H, Tau), neuronal (NSE, CK-BB), and glial damage (GFAP, S100β, MBP).
Clinical observations and experimental studies using different TBI models have shown that mTBI is followed by metabolic changes, including oxidative stress (8, 42, 43). In this study, we found that all three metabolic markers responded to mbTBI with rapid and lasting increases. Serum levels of 4-HNE, a byproduct of lipid peroxidation, was significantly increased 2 h after injury and its levels remained elevated at 1-month post-injury. Previous studies of chronic otitis, bipolar disorder, and experimental TBI have found associations between elevated serum 4-HNE levels and oxidative stress (44–47). HIF1α, a transcription factor, is part of organisms’ adaptive response to noxious insults involving brain hypoxia (e.g., TBI and stroke); blocking HIF-1α has been found to ameliorate neuronal and vascular damage (48–51). Ceruloplasmin is involved in facilitating the export of un-bound iron and iron metabolism in the brain (52, 53). Iron metabolism in the CNS must be tightly regulated as excess iron can lead to metabolic stress. TBI causes an elevation of non-heme bound iron independent of hemorrhage or microbleeding; excess iron can trigger the production of reactive oxygen species leading to oxidative stress and inflammation. Therefore, increased ceruloplasmin levels after TBI may reflect a compensatory response to injury-induced increases in free iron (52).
Previous data indicated that the exposure to low-intensity blast can increase serum levels of biomarkers related to various vascular functions (14, 34). The vascular markers we tested in this study, especially VEGF and vWF, showed robust changes of differing temporal patterns in response to blast. VEGF, a trophic factor of endothelial origin, is a key regulator of numerous vascular functions such as the regulation of endothelial permeability, microvascular density, and angiogenesis among others (54–56). Hypoxia is one of the inducers of VEGF expression, and elevated serum levels of VEGF have been associated with various tumors and inflammatory processes. We previously found similarly elevated serum VEGF levels in other animal models of TBI (14, 34). The temporal profile of serum VEGF levels showing a robust and steady increase that peaks at 1-month post-injury suggests continuing vascular changes with a tendency for chronicity. Such a temporal profile may implicate a lasting peripheral/systemic pathological response to low-intensity blast in mice.
The importance of the blast-induced hydrodynamic pulse through venous vasculature has been demonstrated in recently published experimental work by Simard et al. (57). In rats exposed to thorax only blast injury, the authors found perivascular, mainly perivenular, changes in the brain consistent with neuroinflammation: upregulation of TNF-α accompanied by the finding of ED-1-positive cells (macrophages or activated microglia). It has been suggested that the hydrodynamic pulse radiates through vasculature away from its site of origin, ascending easily into the vasculature of the brain via veins since there are no valves to impede pressure transmission. The passage of the pressure throughout the body, besides its direct effects on the vasculature, also activates the autonomous system triggering parasympathetic reflexes causing a sudden drop in blood pressure and heart rate as well as intermittent periods of tachypnea and bradypnea. The ensuing hypoxia caused either by the vagal reflex loop, lung parenchyma damage, or both, further contributes to the changes in systemic and cerebral metabolism as well as in vascular tone.
AQP1 serum levels exhibited a temporal profile similar to that of VEGF. It was significantly increased at 2 h after injury and remained elevated at 1 month. In the CNS, AQP1 is mostly expressed by epithelial cells of the choroid plexus; as the major water-transporting protein in choroid plexus, it is involved in CSF production (58). However, AQP1 is also expressed in several non-neural cells including epithelial cells of the lung and peritoneum among others. (59). Chronically elevated serum levels of AQP1 can indicate injury to peripheral organs, such as the lungs or the GI tract; indeed, their roles have been suggested in the peripheral pathomechanism of bTBI (13, 30).
Serum levels of vWF, another marker of vascular/endothelial stress/damage, also showed a robust response to injury. vWF, an endothelium-specific glycoprotein, is primarily involved in restoring homeostasis in response to endothelial stress, recruiting platelets, and regulating blood coagulation (60, 61). Similar to VEGF, vWF is also involved in the regulation of angiogenesis, cell proliferation, and inflammation (61). Elevated vWF serum level is an indicator of increased vascular permeability in various disease conditions and of unfavorable outcome in TBI (62). Increased vWF serum levels have also been found in systemic inflammation (63, 64).
Consistent with the increase in vWF, we also found significant increases in the serum levels of several inflammatory markers in response to low-intensity blast. The levels of OPN remained elevated at 1 month after injury indicating that low-intensity blast might trigger a chronic inflammatory process. This finding is consistent with our previous observation in the same model of bTBI, which demonstrated a substantial ongoing systemic inflammatory process that included the CNS (30). Elevated OPN levels can indicate an autoimmune process as seen in multiple sclerosis (65–67).
CINC1 is produced by astrocytes in response to various noxious stimuli, including oxidative stress (68). Elevated serum levels of fibrinogen, a plasma glycoprotein, are another indication of altered microcirculation and inflammation; increased levels of fibrinogen can increase blood viscosity as well as vascular permeability (69, 70). According to studies showing correlation between elevated fibrinogen precursor protein and Alzheimer’s disease, elevated fibrinogen levels can link inflammation and vascular damage to neurodegeneration. MIP1α is a proinflammatory chemokine, and its elevated serum levels are yet another indicator of inflammation (71).
Inflammation can also be triggered by injury-induced changes in cellular adhesion (72, 73). The transient increase in serum levels of cell adhesion/cell surface markers can be an indication of low-intensity blast’s ability to interrupt cellular connectivity, which may in turn contribute to the initiation and/or potentiation of the inflammatory response. The blast-induced cell surface disruption seems to be transient as shown by the early peaks in integrin α6, TIMP1, TIMP4, Ncad, and connexin-43 serum levels. At 1 month after injury, levels of TIMP4 and Ncad were back or close to control values. TIMP4 is an inhibitor of matrix metalloproteinases involved in the degradation of the extracellular matrix, thus, cell surface remodeling can be part of the response to low-intensity blast exposure (74–76). N-cadherin, a multifunctional molecule with important roles in cell adhesion, is also involved in mediating astrogliosis (77, 78).
The changes in serum protein biomarkers discussed above can reflect a systemic or at least partly systemic molecular response to blast. Indeed, previous studies have shown that the exposure to blast triggers complex systemic responses, including a systemic inflammatory response and altered physiological functions (13, 14, 30). These – and other systemic changes – can contribute significantly to the pathomechanisms of mbTBI. In addition to the systemic response to blast, we found indications of axonal, neuronal, and glial damage. Our previous works have demonstrated that the exposure to a single low-intensity blast can trigger changes in the serum levels of markers indicating neuronal and astroglial damage (17, 20, 23, 79, 80). A recent clinical study found elevated serum levels of ubiquitin C-terminal hydrolase-L1 (UCH-L1), αII-spectrin breakdown products (SBDPs), and GFAP in soldiers repeatedly exposed to low-intensity explosive blast indicating damage to neuronal and glial structural integrity (81).
Serum levels of the neuronal markers NSE and CK-BB were only increased during the acute and subacute post-injury period, and returned to normal levels at 1 month. Of the two axonal damage markers, the serum levels of NF-H showed rapid and sustained increases, whereas Tau levels were increased only during the acute phase of injury (2 h and 1 day). Elevated serum levels of various neurofilament proteins have been found to clinically correlate with injury severity; as such, they could be useful in predicting injury outcome (82). In a large animal model of bTBI, rapid rise of serum NF-H levels has been shown to correlate with unfavorable outcome (79). Clinically, elevated serum levels of total tau have been found to be good and bad predictors of outcome after mTBI (83–86). It should be noted that clinical studies typically analyze serum levels of protein biomarkers (e.g., tau) at a single – and not always at the same – post-injury time point(s). As our current study illustrates, serum levels of protein biomarkers change as a function of time elapsed after injury. Therefore, a negative finding based on a single measurement has little diagnostic (or scientific) value. An elegant experimental study has shown that tissue levels of various tau proteins, including cleaved and phosphorylated forms, were increased at the 24-h post-injury time point and remained increased at 30 days in the brains of mice exposed to mild blast (87). These finding implicate tau pathology as one of the long-term consequences of a single mild bTBI, but in the absence of serum data it is unclear how intracerebral findings translate into changes detectable in the serum. Furthermore, dysfunction of the glymphatic pathway (triggered by TBI) can substantially affect the intracerebral accumulation of tau and modify its serum levels as discussed later (88).
The sustained increase in serum NF-H levels, especially in the light of the similarly elevated MBP levels, indicates ongoing white matter damage. White matter injury detected by diffusion-tensor imaging (DTI) has been identified as a hallmark of mTBI (21, 89, 90). Indeed, in our current study as well as previous experiments, we have found indications of myelin damage reflected by elevated serum MBP levels (79). Both experimental and clinical imaging studies have found white matter tract damages following blast exposure(s) (89, 91, 92). The exact pathology that can cause and or sustain long-term axonal damage is currently not fully understood, but inflammatory responses have been implicated in the long-term effect of bTBI (93).
Of the astroglial markers, only S100β levels increased significantly and only at the 1-month time point. Astrogliosis, one of the hallmarks of TBI, represents a neuroprotective response to injury (94). Intriguingly, GFAP levels did not increase significantly at any of the measured time points. These somewhat conflicting findings can be explained by the fact that S100β is a cytoplasmic and partially membrane-bound protein, whereas GFAP is a cytoskeletal protein. Although the exact relationship between physical forces and structural damage at the molecular level is not known, one can hypothesize that cytoplasmic proteins can likely be released more easily from mildly damaged cells upon physical impact than structural proteins such as GFAP. Cytoskeletal proteins may require larger physical/kinetic forces to damage the cytoskeletal network as well as the cellular membrane to enable the release of GFAP into the extracellular space. It should be noted that S100β is not entirely astroglia-specific, so the detected increases can be from other non-neural cells (95, 96). Nevertheless, clinical observations have shown that increased serum S100β levels of non-neural origin are typically observed at the earliest post-injury time points, whereas the delayed increases in serum S100β are usually of neural origin and have been associated with unfavorable outcome in severe TBI (97). In contrast to GFAP, MBP levels increased substantially at all post-injury time points, and remained close to its peak level at 1 month after injury. Thus, our findings implicate lasting myelin as well as axonal damage in the pathobiology of mbTBI as discussed above. These findings are consistent with previous clinical imaging studies showing chronic white matter damage in military personnel exposed to blast (90, 92).
It has been assumed that the elevated serum levels of neural-specific protein biomarkers also reflect damage and/or at least a temporary opening of the blood–brain barrier (BBB) in TBI. BBB opening, however, is associated with moderate or severe TBI, and mild TBI typically does not increase BBB permeability. This paradox has been solved with the recent discovery of the brain glymphatic system (98). In this model, the CSF moving through the glymphatic pathway transports biomarkers to the blood via the cervical lymphatic system, subsequently ending up in the systemic circulation. According to this model, no BBB disruption is needed for proteins released from damaged neural cells to reach the systemic circulation. However, the brain-to-blood transport of protein biomarkers depends on the activity of glymphatic system and this activity is regulated by multiple clinically relevant factors such as sleep deprivation (99). Future experiments analyzing injury-induced changes in both the serum and the CSF can provide critical information about the relationship between intra- and extra-cranial pathologies.
Conclusion
The extremely complex nature of blast injuries requires full understanding of blast physics and effects, and a model reproducing multiple aspects of blast injuries should be defined with particular scientific fidelity to conditions observed in theater. Otherwise, a model will lack military and clinical relevance and the obtained results might be dangerously misleading (11, 100). Thus, we strongly believe that if we aspire to understand the origin of blast-induced acute and chronic neurological deficits, we should reproduce the injurious environment our soldiers are exposed to as close as possible: this also requires reproducing the main response mechanisms to blast exposure(s) – systemic, local, and cerebral – as reported in clinical studies.
There is a continuing discussion about adequate scaling laws that would reliably estimate the exposure conditions causing comparable injuries in humans and animals. Many of the new suggestions about scaling laws focus on the physical mechanisms that determine brain response to blast or differences in properties of soft tissues of the head or the major anatomical differences, particularly of the skull (101–103). Nevertheless, these approaches fail to take into account the specificity of the animals’ physiological tolerance toward blast exposure, which often differs from the human tolerance. Indeed, the human body can survive relatively high blast overpressure without experiencing barotrauma. A 5-psi blast overpressure would rupture eardrums in about 1% of subjects, whereas the threshold for lung damage occurs at about 15-psi blast overpressure and a 35–45 psi overpressure may cause 1% fatalities (104). Hence, in our model, we used the Bowen animal-to-human scaling laws for blast effects on the animals, which is based on mass scaling, to set the basic parameters for shockwave exposure; then, we fine tuned to conditions to reach the clinical features of blast injuries and blast-induced traumatic brain injuries described in humans exposed to blast (6, 24, 25, 105–107).
Although the research community’s interest is almost entirely focused on neurological deficits due to blast, which grouped together often called bTBI and a “signature wound” of recent wars, accumulating evidence provides more and more support to the importance of systemic, multi-organ mechanisms in the pathobiology of blast-induced neurological deficits. Bearing this in mind, we used the previously developed, well-standardized, and validated mouse model of primary blast injuries (30), which reproduces the main consequences of blast exposures: changes in general physiology, multi-organ damages, systemic compensatory mechanisms, as well as neurological deficits seen in individuals exposed to differing blast intensity levels. Thus, our intention was never to reproduce only a head injury (i.e., isolated TBI) in classical terms, but the full spectrum of injury response mechanisms induced by primary blast. These include the autonomous system activation including the parasympathic reflexes; hypoxia caused either by the vagal reflex loop, lung parenchyma damage or both; changes in vascular tonus or systemic and/or cerebral metabolism – all these, rather than direct brain parenchyma damage, might underlie the blast-induced neurological deficits. Indeed, our current data provide further support to the importance of systemic changes in the pathobiology of blast-induced neurological deficits.
In summary, this is the first study to determine the temporal pattern of changes in the serum levels of protein biomarkers after a single exposure to low-intensity blast in the high-fidelity mouse model of bTBI. Our findings implicate oxidative stress, vascular changes, inflammation, altered cell adhesion, neuronal, and glial damage/loss in the pathobiology of bTBI. The observed temporal profiles of markers illustrate the dynamic nature of numerous molecular mechanisms related to the systemic and cerebral response to blast. Our work is limited by the incomplete understanding of the physics of explosive blast, how the physical event translates into biological response(s) and importantly, how well rodent models replicate the human condition. Proteolytic cleavage of protein biomarkers is also a potential issue for Reverse Phase Protein Microarray (RPPM) like all other antibody-based systems, such as the ELISA, Luminex, or Western blots. Nonetheless, our findings underline the importance of monitoring serum biomarker levels over a prolonged period of time. Determining the temporal pattern of molecular changes can provide critical information about the progression or regression of the individual pathomechanisms and help in the development of targeted and timely therapeutic interventions for improved prognosis.
Author Contribution
FA performed the animal experiments, collected and processed the biosamples, performed the proteomic analysis of the samples, analyzed the data, generated the figures, and wrote the first draft. IC designed and supervised the blast experiments, co-wrote, and edited the manuscript. SP performed part of the animal experiments, assisted FA with collecting the biosamples, and reviewed the manuscript. DA designed the proteomics analysis, analyzed and interpreted the data, and wrote the manuscript.
Conflict of Interest Statement
No conflicting financial interests exist. The views, opinions, and/or findings contained in this article are those of the authors and should not be interpreted as representing the official views or policies, either expressed or implied, of the Department of Defense (DoD). Approved for public release, distribution unlimited.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fneur.2015.00114/abstract
Acknowledgments
This work was supported by the Center for Neuroscience and Regenerative Medicine, grant G170AZ. We thank Ms. Alaa Kamnaksh and Mr. John Olson for their editorial help.
References
- 1.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia (2003) 44(Suppl 10):2–10. 10.1046/j.1528-1157.44.s10.3.x [DOI] [PubMed] [Google Scholar]
- 2.Laker SR. Epidemiology of concussion and mild traumatic brain injury. PM R (2011) 3:S354–8. 10.1016/j.pmrj.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 3.Appelboom G, Han J, Bruce S, Szpalski C, Connolly ES., Jr Clinical relevance of blast-related traumatic brain injury. Acta Neurochir (Wien) (2012) 154:131–4. 10.1007/s00701-011-1210-3 [DOI] [PubMed] [Google Scholar]
- 4.Elder GA, Mitsis EM, Ahlers ST, Cristian A. Blast-induced mild traumatic brain injury. Psychiatr Clin North Am (2010) 33:757–81. 10.1016/j.psc.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Vanderploeg RD, Belanger HG, Horner RD, Spehar AM, Powell-Cope G, Luther SL, et al. Health outcomes associated with military deployment: mild traumatic brain injury, blast, trauma, and combat associations in the Florida National Guard. Arch Phys Med Rehabil (2012) 93:1887–95. 10.1016/j.apmr.2012.05.024 [DOI] [PubMed] [Google Scholar]
- 6.Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, et al. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil (2009) 24:14–23. 10.1097/HTR.0b013e31819581d8 [DOI] [PubMed] [Google Scholar]
- 7.Povlishock JT. The window of risk in repeated head injury. J Neurotrauma (2013) 30:1. 10.1089/neu.2013.9942 [DOI] [PubMed] [Google Scholar]
- 8.Prins MPD, Alexander D, Giza CC, Hovda D. Repeat mild traumatic brain injury: mechanisms of cerebral vulnerability. J Neurotrauma (2012) 30:30–8. 10.1089/neu.2012.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav (2012) 6:244–54. 10.1007/s11682-012-9164-5 [DOI] [PubMed] [Google Scholar]
- 10.Lakis N, Corona RJ, Toshkezi G, Chin LS. Chronic traumatic encephalopathy – neuropathology in athletes and war veterans. Neurol Res (2013) 35:290–9. 10.1179/1743132813Y.0000000177 [DOI] [PubMed] [Google Scholar]
- 11.Cernak I. Blast injuries and blast-induced neurotrauma – overview of pathophysiology and experimental knowledge: models and findings. In: Kobeissy F, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press; (2015). p. 629–42. [PubMed] [Google Scholar]
- 12.Cernak I, Wang ZG, Jiang JX, Bian XW, Savic J. Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J Trauma (2001) 50:695–706. 10.1097/00005373-200104000-00017 [DOI] [PubMed] [Google Scholar]
- 13.Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front Neurol (2010) 1:151. 10.3389/fneur.2010.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed FA, Kamnaksh A, Kovesdi E, Long JB, Agoston DV. Long-term consequences of single and multiple mild blast exposure on select physiological parameters and blood-based biomarkers. Electrophoresis (2013) 34:2229–33. 10.1002/elps.201300077 [DOI] [PubMed] [Google Scholar]
- 15.Jaffee MS, Meyer KS. A brief overview of traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD) within the Department of Defense. Clin Neuropsychol (2009) 23:1291–8. 10.1080/13854040903307250 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Huang W. Non-impact, blast-induced mild TBI and PTSD: concepts and caveats. Brain Inj (2011) 25:641–50. 10.3109/02699052.2011.580313 [DOI] [PubMed] [Google Scholar]
- 17.Kamnaksh A, Kovesdi E, Kwon SK, Wingo D, Ahmed F, Grunberg NE, et al. Factors affecting blast traumatic brain injury. J Neurotrauma (2011) 28:2145–53. 10.1089/neu.2011.1983 [DOI] [PubMed] [Google Scholar]
- 18.Kwon SK, Kovesdi E, Gyorgy AB, Wingo D, Kamnaksh A, Walker J, et al. Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Front Neurol (2011) 2:12. 10.3389/fneur.2011.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawa L, Arborelius U, Yoshitake T, Kehr J, Hokfelt T, Risling M, et al. Neurotransmitter systems in a mild blast traumatic brain injury model: catecholamines and serotonin. J Neurotrauma (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agoston DV, Elsayed M. Serum-based protein biomarkers in blast-induced traumatic brain injury spectrum disorder. Front Neurol (2012) 3:107. 10.3389/fneur.2012.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamnaksh A, Budde MD, Kovesdi E, Long JB, Frank JA, Agoston D. Diffusion tensor imaging reveals acute subcortical changes after mild blast-induced traumatic brain injury. Sci Rep (2014) 4:4809. 10.1038/srep04809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochanek PM, Dixon CE, Shellington DK, Shin SS, Bayir H, Jackson E, et al. Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J Neurotrauma (2013) 30(11):920–37. 10.1089/neu.2013.2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovesdi E, Kamnaksh A, Wingo D, Ahmed F, Grunberg NE, Long JB, et al. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front Neurol (2012) 3:111. 10.3389/fneur.2012.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war veterans with persistent post-concussive symptoms. Neuroimage (2011) 54(Suppl 1):S76–82. 10.1016/j.neuroimage.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K, et al. Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J Neurotrauma (2014) 31(5):425–36. 10.1089/neu.2013.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenfeld JV, McFarlane AC, Bragge P, Armonda RA, Grimes JB, Ling GS. Blast-related traumatic brain injury. Lancet Neurol (2013) 12:882–93. 10.1016/S1474-4422(13)70161-3 [DOI] [PubMed] [Google Scholar]
- 27.Gyorgy AB, Walker J, Wingo D, Eidelman O, Pollard HB, Molnar A, et al. Reverse phase protein microarray technology in traumatic brain injury. J Neurosci Methods (2010) 192:96–101. 10.1016/j.jneumeth.2010.07.029 [DOI] [PubMed] [Google Scholar]
- 28.Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab (2010) 30:255–66. 10.1038/jcbfm.2009.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morey RA, Haswell CC, Selgrade ES, Massoglia D, Liu C, Weiner J, et al. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum Brain Mapp (2013) 34:2986–99. 10.1002/hbm.22117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, et al. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis (2011) 41:538–51. 10.1016/j.nbd.2010.10.025 [DOI] [PubMed] [Google Scholar]
- 31.Koliatsos VE, Cernak I, Xu L, Song Y, Savonenko A, Crain BJ, et al. A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J Neuropathol Exp Neurol (2011) 70:399–416. 10.1097/NEN.0b013e3182189f06 [DOI] [PubMed] [Google Scholar]
- 32.Yelverton JT. Pathology scoring system for blast injuries. J Trauma (1996) 40:S111–5. 10.1097/00005373-199603001-00025 [DOI] [PubMed] [Google Scholar]
- 33.Cernak I, Savic J, Zunic G, Pejnovic N, Jovanikic O, Stepic V. Recognizing, scoring, and predicting blast injuries. World J Surg (1999) 23:44–53. 10.1007/s002689900563 [DOI] [PubMed] [Google Scholar]
- 34.Kamnaksh A, Kwon SK, Kovesdi E, Ahmed F, Barry ES, Grunberg NE, et al. Neurobehavioral, cellular, and molecular consequences of single and multiple mild blast exposure. Electrophoresis (2012) 33:3680–92. 10.1002/elps.201200319 [DOI] [PubMed] [Google Scholar]
- 35.Mendez MF, Owens EM, Jimenez EE, Peppers D, Licht EA. Changes in personality after mild traumatic brain injury from primary blast vs. blunt forces. Brain Inj (2013) 27:10–8. 10.3109/02699052.2012.722252 [DOI] [PubMed] [Google Scholar]
- 36.Spencer RJ, Drag LL, Walker SJ, Bieliauskas LA. Self-reported cognitive symptoms following mild traumatic brain injury are poorly associated with neuropsychological performance in OIF/OEF veterans. J Rehabil Res Dev (2010) 47:521–30. 10.1682/JRRD.2009.11.0181 [DOI] [PubMed] [Google Scholar]
- 37.Xie K, Kuang H, Tsien JZ. Mild blast events alter anxiety, memory, and neural activity patterns in the anterior cingulate cortex. PLoS One (2013) 8:e64907. 10.1371/journal.pone.0064907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner LA, Ivins BJ, Schwab K, Warden D, Nelson LA, Jaffee M, et al. Traumatic brain injury, posttraumatic stress disorder, and postconcussive symptom reporting among troops returning from Iraq. J Head Trauma Rehabil (2010) 25:307–12. 10.1097/HTR.0b013e3181cada03 [DOI] [PubMed] [Google Scholar]
- 39.MacDonald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, et al. Prospectively assessed clinical outcomes in concussive blast vs nonblast traumatic brain injury among evacuated US military personnel. JAMA Neurol (2014) 71:994–1002. 10.1001/jamaneurol.2014.1114 [DOI] [PubMed] [Google Scholar]
- 40.Elder GA, Cristian A. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt Sinai J Med (2009) 76:111–8. 10.1002/msj.20098 [DOI] [PubMed] [Google Scholar]
- 41.Magnuson J, Leonessa F, Ling GS. Neuropathology of explosive blast traumatic brain injury. Curr Neurol Neurosci Rep (2012) 12(5):570–9. 10.1007/s11910-012-0303-6 [DOI] [PubMed] [Google Scholar]
- 42.Tavazzi B, Signoretti S, Lazzarino G, Amorini AM, Delfini R, Cimatti M, et al. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery (2005) 56:582–9. 10.1227/01.NEU.0000156715.04900.E6 [DOI] [PubMed] [Google Scholar]
- 43.Tavazzi B, Vagnozzi R, Signoretti S, Amorini AM, Belli A, Cimatti M, et al. Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses – part II. Neurosurgery (2007) 61:390–5. 10.1227/01.NEU.0000255525.34956.3F [DOI] [PubMed] [Google Scholar]
- 44.Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma (2004) 21:9–20. 10.1089/089771504772695904 [DOI] [PubMed] [Google Scholar]
- 45.Niki E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors (2008) 34:171–80. 10.1002/biof.5520340208 [DOI] [PubMed] [Google Scholar]
- 46.Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, et al. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci (2002) 22:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Versace A, Andreazza AC, Young LT, Fournier JC, Almeida JR, Stiffler RS, et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry (2013) 19(2):200–8. 10.1038/mp.2012.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired expression of neuroprotective molecules in the HIF-1alpha pathway following traumatic brain injury in aged mice. J Neurotrauma (2009) 26:1557–66. 10.1089/neu.2008-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W, Jadhav V, Tang J, Zhang JH. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxic-ischemic model. Neurobiol Dis (2008) 31:433–41. 10.1016/j.nbd.2008.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li A, Sun X, Ni Y, Chen X, Guo A. HIF-1alpha involves in neuronal apoptosis after traumatic brain injury in adult rats. J Mol Neurosci (2013) 51:1052–62. 10.1007/s12031-013-0084-7 [DOI] [PubMed] [Google Scholar]
- 51.Shenaq M, Kassem H, Peng C, Schafer S, Ding JY, Fredrickson V, et al. Neuronal damage and functional deficits are ameliorated by inhibition of aquaporin and HIF1alpha after traumatic brain injury (TBI). J Neurol Sci (2012) 323:134–40. 10.1016/j.jns.2012.08.036 [DOI] [PubMed] [Google Scholar]
- 52.Ayton S, Zhang M, Roberts BR, Lam LQ, Lind M, McLean C, et al. Ceruloplasmin and beta-amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radic Biol Med (2014) 69:331–7. 10.1016/j.freeradbiomed.2014.01.041 [DOI] [PubMed] [Google Scholar]
- 53.Dash PK, Redell JB, Hergenroeder G, Zhao J, Clifton GL, Moore A. Serum ceruloplasmin and copper are early biomarkers for traumatic brain injury-associated elevated intracranial pressure. J Neurosci Res (2010) 88:1719–26. 10.1002/jnr.22336 [DOI] [PubMed] [Google Scholar]
- 54.Croll SD, Goodman JH, Scharfman HE. Vascular endothelial growth factor (VEGF) in seizures: a double-edged sword. Adv Exp Med Biol (2004) 548:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerstner ER, Duda DG, di Tomaso E, Ryg PA, Loeffler JS, Sorensen AG, et al. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Oncol (2009) 6:229–36. 10.1038/nrclinonc.2009.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue – beyond blood vessels. Exp Neurol (2004) 187:246–53. 10.1016/j.expneurol.2004.01.022 [DOI] [PubMed] [Google Scholar]
- 57.Simard JM, Pampori A, Keledjian K, Tosun C, Schwartzbauer G, Ivanova S, et al. Exposure of the thorax to a sublethal blast wave causes a hydrodynamic pulse that leads to perivenular inflammation in the brain. J Neurotrauma (2014) 31(14):1292–304. 10.1089/neu.2013.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Badaut J, Fukuda AM, Jullienne A, Petry KG. Aquaporin and brain diseases. Biochim Biophys Acta (2014) 1840:1554–65. 10.1016/j.bbagen.2013.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett (2003) 555:72–8. 10.1016/S0014-5793(03)01083-4 [DOI] [PubMed] [Google Scholar]
- 60.Lenting PJ, Casari C, Christophe OD, Denis CV. von Willebrand factor: the old, the new and the unknown. J Thromb Haemost (2012) 10:2428–37. 10.1111/jth.12008 [DOI] [PubMed] [Google Scholar]
- 61.Rauch A, Wohner N, Christophe OD, Denis CV, Susen S, Lenting PJ. On the versatility of von Willebrand factor. Mediterr J Hematol Infect Dis (2013) 5:e2013046. 10.4084/MJHID.2013.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Oliveira CO, Reimer AG, Da Rocha AB, Grivicich I, Schneider RF, Roisenberg I, et al. Plasma von Willebrand factor levels correlate with clinical outcome of severe traumatic brain injury. J Neurotrauma (2007) 24:1331–8. 10.1089/neu.2006.0159 [DOI] [PubMed] [Google Scholar]
- 63.Bickel C, Rupprecht HJ, Blankenberg S, Espiniola-Klein C, Schlitt A, Rippin G, et al. Relation of markers of inflammation (C-reactive protein, fibrinogen, von Willebrand factor, and leukocyte count) and statin therapy to long-term mortality in patients with angiographically proven coronary artery disease. Am J Cardiol (2002) 89:901–8. 10.1016/S0002-9149(02)02236-1 [DOI] [PubMed] [Google Scholar]
- 64.Brill A. Stroke-associated inflammation: is von Willebrand factor a ‘bad guy’? J Thromb Haemost (2012) 10:1662–4. 10.1111/j.1538-7836.2012.04820.x [DOI] [PubMed] [Google Scholar]
- 65.Braitch M, Constantinescu CS. The role of osteopontin in experimental autoimmune encephalomyelitis (EAE) and multiple sclerosis (MS). Inflamm Allergy Drug Targets (2010) 9:249–56. 10.2174/187152810793358778 [DOI] [PubMed] [Google Scholar]
- 66.Butler WT. The nature and significance of osteopontin. Connect Tissue Res (1989) 23:123–36. 10.3109/03008208909002412 [DOI] [PubMed] [Google Scholar]
- 67.Shin T. Osteopontin as a two-sided mediator in acute neuroinflammation in rat models. Acta Histochem (2012) 114:749–54. 10.1016/j.acthis.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 68.Katayama T, Tanaka H, Yoshida T, Uehara T, Minami M. Neuronal injury induces cytokine-induced neutrophil chemoattractant-1 (CINC-1) production in astrocytes. J Pharmacol Sci (2009) 109:88–93. 10.1254/jphs.08298FP [DOI] [PubMed] [Google Scholar]
- 69.Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol (2012) 34:43–62. 10.1007/s00281-011-0290-8 [DOI] [PubMed] [Google Scholar]
- 70.Muradashvili N, Lominadze D. Role of fibrinogen in cerebrovascular dysfunction after traumatic brain injury. Brain Inj (2013) 27(13–14):1508–15. 10.3109/02699052.2013.823562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hsieh CH, Frink M, Hsieh YC, Kan WH, Hsu JT, Schwacha MG, et al. The role of MIP-1 alpha in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol (2008) 181:2806–12. 10.4049/jimmunol.181.4.2806 [DOI] [PubMed] [Google Scholar]
- 72.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol (2006) 147(Suppl 1):S232–40. 10.1038/sj.bjp.0706400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai YD, Liliang PC, Cho CL, Chen JS, Lu K, Liang CL, et al. Delayed neurovascular inflammation after mild traumatic brain injury in rats. Brain Inj (2013) 27:361–5. 10.3109/02699052.2012.750738 [DOI] [PubMed] [Google Scholar]
- 74.Borkakoti N. Matrix metalloproteases: variations on a theme. Prog Biophys Mol Biol (1998) 70:73–94. 10.1016/S0079-6107(98)00003-0 [DOI] [PubMed] [Google Scholar]
- 75.Fujimoto M, Takagi Y, Aoki T, Hayase M, Marumo T, Gomi M, et al. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J Cereb Blood Flow Metab (2008) 28:1674–85. 10.1038/jcbfm.2008.59 [DOI] [PubMed] [Google Scholar]
- 76.Gardner J, Borgmann K, Deshpande MS, Dhar A, Wu L, Persidsky R, et al. Potential mechanisms for astrocyte-TIMP-1 downregulation in chronic inflammatory diseases. J Neurosci Res (2006) 83:1281–92. 10.1002/jnr.20823 [DOI] [PubMed] [Google Scholar]
- 77.Jang YN, Jung YS, Lee SH, Moon CH, Kim CH, Baik EJ. Calpain-mediated N-cadherin proteolytic processing in brain injury. J Neurosci (2009) 29:5974–84. 10.1523/JNEUROSCI.6178-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc Natl Acad Sci U S A (2013) 110:11612–7. 10.1073/pnas.1300378110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gyorgy A, Ling G, Wingo D, Walker J, Tong L, Parks S, et al. Time-dependent changes in serum biomarker levels after blast traumatic brain injury. J Neurotrauma (2011) 28:1121–6. 10.1089/neu.2010.1561 [DOI] [PubMed] [Google Scholar]
- 80.Ahmed F, Gyorgy A, Kamnaksh A, Ling G, Tong L, Parks S, et al. Time-dependent changes of protein biomarker levels in the cerebrospinal fluid after blast traumatic brain injury. Electrophoresis (2012) 33:3705–11. 10.1002/elps.201200299 [DOI] [PubMed] [Google Scholar]
- 81.Tate CM, Wang KK, Eonta S, Zhang Y, Carr W, Tortella FC, et al. Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: a breacher pilot study. J Neurotrauma (2013) 30:1620–30. 10.1089/neu.2012.2683 [DOI] [PubMed] [Google Scholar]
- 82.Anderson KJ, Scheff SW, Miller KM, Roberts KN, Gilmer LK, Yang C, et al. The phosphorylated axonal form of the neurofilament subunit NF-H (pNF-H) as a blood biomarker of traumatic brain injury. J Neurotrauma (2008) 25:1079–85. 10.1089/neu.2007.0488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bazarian JJ, Zemlan FP, Mookerjee S, Stigbrand T. Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj (2006) 20:759–65. 10.1080/02699050500488207 [DOI] [PubMed] [Google Scholar]
- 84.Gatson J, Diaz-Arrastia R. Tau as a biomarker of concussion. JAMA Neurol (2014) 71(6):677–8. 10.1001/jamaneurol.2014.443 [DOI] [PubMed] [Google Scholar]
- 85.Liliang PC, Liang CL, Weng HC, Lu K, Wang KW, Chen HJ, et al. Tau proteins in serum predict outcome after severe traumatic brain injury. J Surg Res (2010) 160:302–7. 10.1016/j.jss.2008.12.022 [DOI] [PubMed] [Google Scholar]
- 86.Ma M, Lindsell CJ, Rosenberry CM, Shaw GJ, Zemlan FP. Serum cleaved tau does not predict postconcussion syndrome after mild traumatic brain injury. Am J Emerg Med (2008) 26:763–8. 10.1016/j.ajem.2007.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huber BR, Meabon JS, Martin TJ, Mourad PD, Bennett R, Kraemer BC, et al. Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J Alzheimers Dis (2013) 37:309–23. 10.3233/JAD-130182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci (2014) 34:16180–93. 10.1523/JNEUROSCI.3020-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calabrese E, Du F, Garman RH, Johnson GA, Riccio C, Tong LC, et al. Diffusion tensor imaging reveals white matter injury in a rat model of repetitive blast-induced traumatic brain injury. J Neurotrauma (2014) 31(10):938–50. 10.1089/neu.2013.3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davenport ND, Lim KO, Armstrong MT, Sponheim SR. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage (2012) 59:2017–24. 10.1016/j.neuroimage.2011.10.050 [DOI] [PubMed] [Google Scholar]
- 91.Jorge RE, Acion L, White T, Tordesillas-Gutierrez D, Pierson R, Crespo-Facorro B, et al. White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry (2012) 169:1284–91. 10.1176/appi.ajp.2012.12050600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mac Donald C, Johnson A, Cooper D, Malone T, Sorrell J, Shimony J, et al. Cerebellar white matter abnormalities following primary blast injury in US military personnel. PLoS One (2013) 8:e55823. 10.1371/journal.pone.0055823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med (2012) 4:134ra60. 10.1126/scitranslmed.3004862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol (2008) 209:294–301. 10.1016/j.expneurol.2007.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderson RE, Hansson LO, Nilsson O, Dijlai-Merzoug R, Settergren G. High serum S100B levels for trauma patients without head injuries. Neurosurgery (2001) 48:1255–8. 10.1227/00006123-200106000-00012 [DOI] [PubMed] [Google Scholar]
- 96.Romner B, Ingebrigtsen T. High serum S100B levels for trauma patients without head injuries. Neurosurgery (2001) 49:1490; author reply 1492–3. 10.1097/00006123-200112000-00054 [DOI] [PubMed] [Google Scholar]
- 97.Thelin EP, Johannesson L, Nelson D, Bellander BM. S100B is an important outcome predictor in traumatic brain injury. J Neurotrauma (2013) 30:519–28. 10.1089/neu.2012.2553 [DOI] [PubMed] [Google Scholar]
- 98.Nedergaard M. Neuroscience. Garbage truck of the brain. Science (2013) 340:1529–30. 10.1126/science.1240514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci (2015) 35:518–26. 10.1523/JNEUROSCI.3742-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cernak I. Blast-induced neurotrauma models and their requirements. Front Neurol (2014) 5:128. 10.3389/fneur.2014.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rafaels K, Bass CR, Salzar RS, Panzer MB, Woods W, Feldman S, et al. Survival risk assessment for primary blast exposures to the head. J Neurotrauma (2011) 28:2319–28. 10.1089/neu.2009.1207 [DOI] [PubMed] [Google Scholar]
- 102.Rafaels KA, Bass CR, Panzer MB, Salzar RS, Woods WA, Feldman SH, et al. Brain injury risk from primary blast. J Trauma Acute Care Surg (2012) 73:895–901. 10.1097/TA.0b013e31825a760e [DOI] [PubMed] [Google Scholar]
- 103.Jean A, Nyein MK, Zheng JQ, Moore DF, Joannopoulos JD, Radovitzky R. An animal-to-human scaling law for blast-induced traumatic brain injury risk assessment. Proc Natl Acad Sci U S A (2014) 111:15310–5. 10.1073/pnas.1415743111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bowen IG, Fletcher ER, Richmond DR. Estimate of Man’s Tolerance to the Direct Effects of Air Blast. Washington, DC: Defense Atomic Support Agency; (1968). p. 1–44. [Google Scholar]
- 105.Carayon A. [Cerebral lesions caused by blast injury. Apropos of 57 cases]. Mem Acad Chir (Paris) (1963) 89:509–13. [PubMed] [Google Scholar]
- 106.Garai O. Blast injury: non-fatal case with neurological signs. Lancet (1944) 17:788–9. [Google Scholar]
- 107.Warden DL, French LM, Shupenko L, Fargus J, Riedy G, Erickson ME, et al. Case report of a soldier with primary blast brain injury. Neuroimage (2009) 47(Suppl 2):T152–3. 10.1016/j.neuroimage.2009.01.060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.