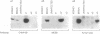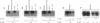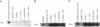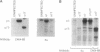Abstract
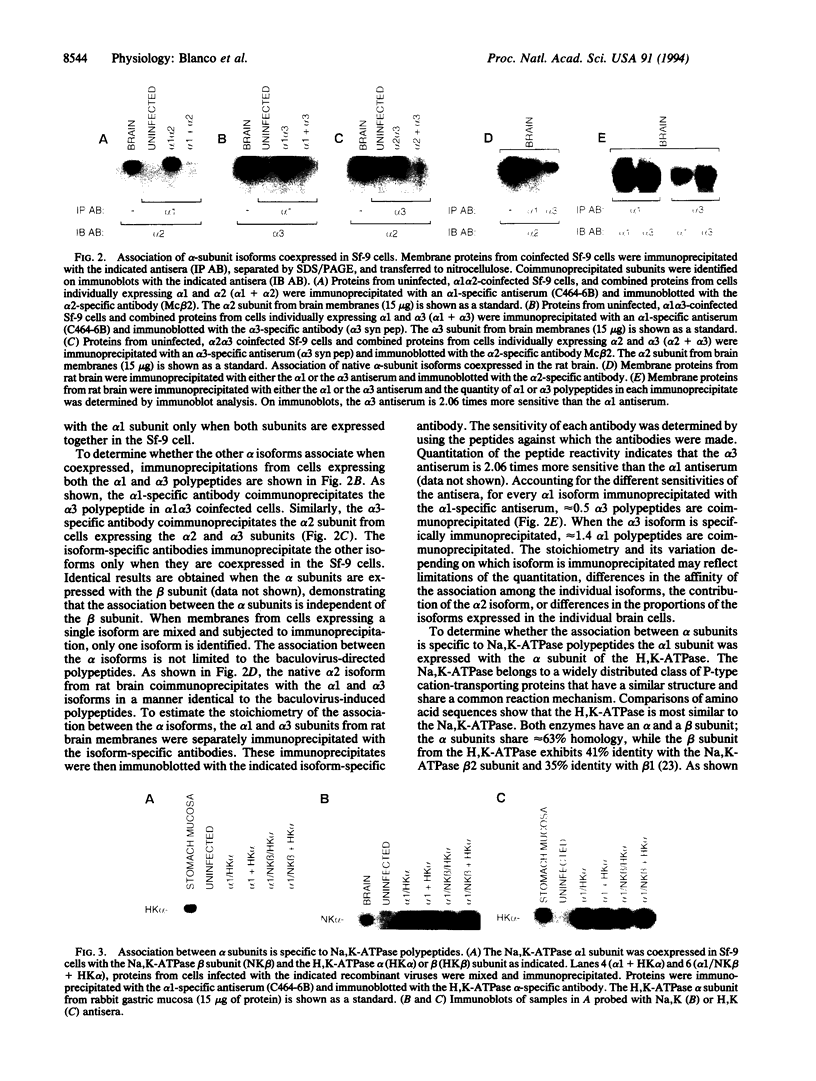
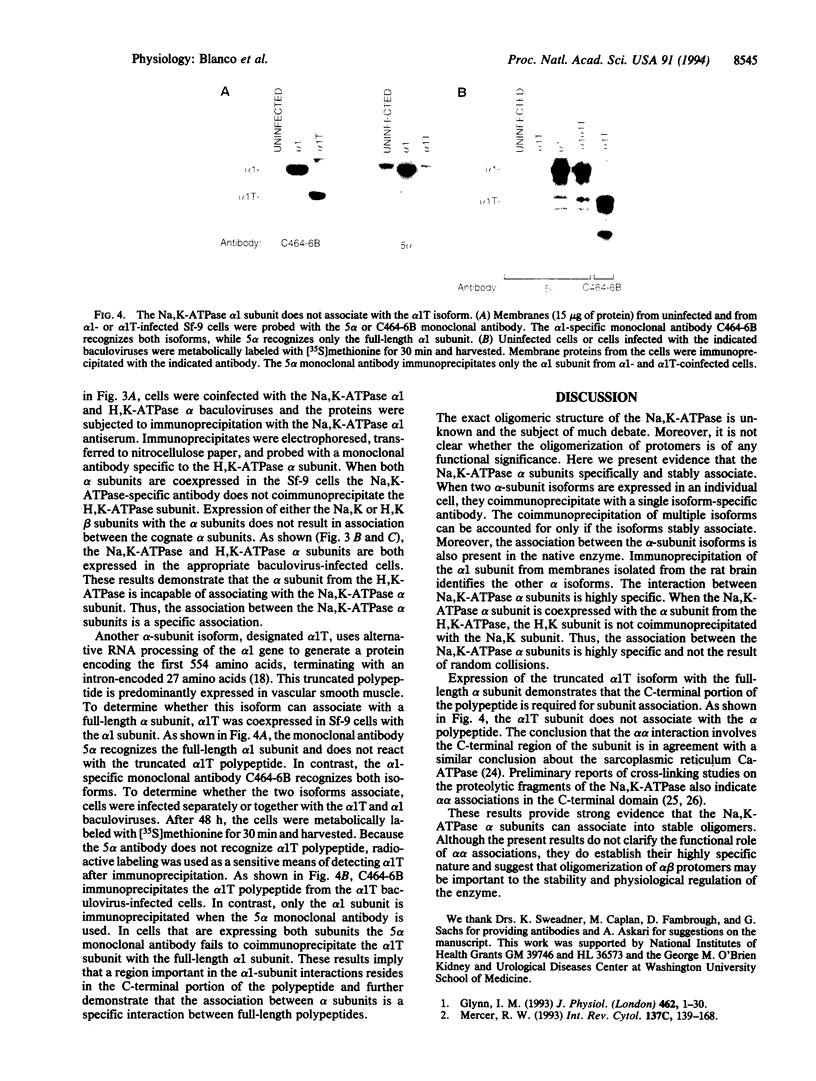
The Na,K-ATPase is a heterodimer consisting of an alpha and a beta subunit. Both Na,K-ATPase subunits are encoded by multigene families. Several isoforms for the alpha (alpha 1, alpha 2, and alpha 3) and beta (beta 1, beta 2, and beta 3) subunits have been identified. All these isoforms are capable of forming functionally active enzyme. Although there is general agreement that the Na,K-ATPase consists of alpha and beta subunits in equimolar amounts, the quaternary structure of the Na,K-ATPase and its functional significance is unknown. Several studies have demonstrated that the enzyme exists within the plasma membrane as an oligomer of alpha beta dimers. However, because the alpha beta protomer seems to be catalytically competent, the possibility exists that higher oligomers are irrelevant to function. The ability to express different alpha isoforms in insect cells and the availability of isoform-specific antibodies has provided the opportunity to test for the existence of stable and specific associations among alpha subunits. By coexpressing different alpha-subunit isoforms in cultured cells, we demonstrate that the Na,K-ATPase alpha subunits specifically and stably associate into oligomeric complexes. This same association among alpha-subunit isoforms was demonstrated in the native enzyme from rat brain. The interaction between Na,K-ATPase alpha subunits is highly specific. When the Na,K-ATPase alpha subunit is coexpressed with the alpha subunit from the H,K-ATPase, the H,K subunit does not associate with the Na,K subunit. Moreover, expression of the truncated alpha 1T isoform with the full-length alpha subunit demonstrates that the C-terminal portion of the polypeptide is important in the alpha-subunit association. Although these results do not clarify the functional role of alpha alpha associations, they do establish their highly specific nature and suggest that oligomerization of alpha beta protomers may be important to the stability and physiological regulation of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amler E., Abbott A., Ball W. J., Jr Structural dynamics and oligomeric interactions of Na+,K(+)-ATPase as monitored using fluorescence energy transfer. Biophys J. 1992 Feb;61(2):553–568. doi: 10.1016/S0006-3495(92)81859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari A. (Na+ + K+)-ATPase: on the number of the ATP sites of the functional unit. J Bioenerg Biomembr. 1987 Aug;19(4):359–374. doi: 10.1007/BF00768539. [DOI] [PubMed] [Google Scholar]
- Askari A. Overview: ligand binding sites of (Na+ + K+)-ATPase: nucleotides and cations. Prog Clin Biol Res. 1988;268A:149–165. [PubMed] [Google Scholar]
- Blanco G., Beaugé L. Pools of (Na+,K+)-ATPase isoforms in unborn and adult rats can be detected by their sensitivity to strophanthidin, but have similar reactivity to Na, K and ATP. Prog Clin Biol Res. 1988;268A:271–278. [PubMed] [Google Scholar]
- Blanco G., Xie Z. J., Mercer R. W. Functional expression of the alpha 2 and alpha 3 isoforms of the Na,K-ATPase in baculovirus-infected insect cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1824–1828. doi: 10.1073/pnas.90.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum E., Schoner W. Phosphate binding and ATP-binding sites coexist in Na+/K(+)-transporting ATPase, as demonstrated by the inactivating MgPO4 complex analogue Co(NH3)4PO4. Eur J Biochem. 1991 Jan 30;195(2):407–419. doi: 10.1111/j.1432-1033.1991.tb15720.x. [DOI] [PubMed] [Google Scholar]
- Chow D. C., Browning C. M., Forte J. G. Gastric H(+)-K(+)-ATPase activity is inhibited by reduction of disulfide bonds in beta-subunit. Am J Physiol. 1992 Jul;263(1 Pt 1):C39–C46. doi: 10.1152/ajpcell.1992.263.1.C39. [DOI] [PubMed] [Google Scholar]
- Craig W. S. Monomer of sodium and potassium ion activated adenosinetriphosphatase displays complete enzymatic function. Biochemistry. 1982 Oct 26;21(22):5707–5717. doi: 10.1021/bi00265a049. [DOI] [PubMed] [Google Scholar]
- DeTomaso A. W., Xie Z. J., Liu G., Mercer R. W. Expression, targeting, and assembly of functional Na,K-ATPase polypeptides in baculovirus-infected insect cells. J Biol Chem. 1993 Jan 15;268(2):1470–1478. [PubMed] [Google Scholar]
- Glynn I. M. Annual review prize lecture. 'All hands to the sodium pump'. J Physiol. 1993 Mar;462:1–30. doi: 10.1113/jphysiol.1993.sp019540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Mimura K., Matsui H., Takagi T. Minimum enzyme unit for Na+/K+-ATPase is the alpha beta-protomer. Determination by low-angle laser light scattering photometry coupled with high-performance gel chromatography for substantially simultaneous measurement of ATPase activity and molecular weight. Biochim Biophys Acta. 1989 Aug 7;983(2):217–229. doi: 10.1016/0005-2736(89)90237-x. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L., Andersen J. P. Thermoinactivation and aggregation of alpha beta units in soluble and membrane-bound (Na,K)-ATPase. Biochemistry. 1986 May 20;25(10):2889–2897. doi: 10.1021/bi00358a023. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lingrel J. B. Na,K-ATPase: isoform structure, function, and expression. J Bioenerg Biomembr. 1992 Jun;24(3):263–270. doi: 10.1007/BF00768847. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Hyman R., Ahmad M., Allen J. C., Pressley T. A., Allen P. D., Nadal-Ginard B. Vascular smooth muscle expresses a truncated Na+, K(+)-ATPase alpha-1 subunit isoform. J Biol Chem. 1991 Sep 25;266(27):18308–18312. [PubMed] [Google Scholar]
- Mercer R. W. Structure of the Na,K-ATPase. Int Rev Cytol. 1993;137C:139–168. [PubMed] [Google Scholar]
- Molnar E., Seidler N. W., Jona I., Martonosi A. N. The binding of monoclonal and polyclonal antibodies to the Ca2(+)-ATPase of sarcoplasmic reticulum: effects on interactions between ATPase molecules. Biochim Biophys Acta. 1990 Apr 13;1023(2):147–167. doi: 10.1016/0005-2736(90)90410-p. [DOI] [PubMed] [Google Scholar]
- Nørby J. G., Jensen J. Functional significance of the oligomeric structure of the Na,K-pump from radiation inactivation and ligand binding. Soc Gen Physiol Ser. 1991;46:173–188. [PubMed] [Google Scholar]
- Reynolds J. A. Overview: the oligomeric structure of the Na,K pump protein. Prog Clin Biol Res. 1988;268A:137–148. [PubMed] [Google Scholar]
- Sachs G., Besancon M., Shin J. M., Mercier F., Munson K., Hersey S. Structural aspects of the gastric H,K-ATPase. J Bioenerg Biomembr. 1992 Jun;24(3):301–308. doi: 10.1007/BF00768850. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989 May 9;988(2):185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Ward D. G., Cavieres J. D. Solubilized alpha beta Na,K-ATPase remains protomeric during turnover yet shows apparent negative cooperativity toward ATP. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5332–5336. doi: 10.1073/pnas.90.11.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]