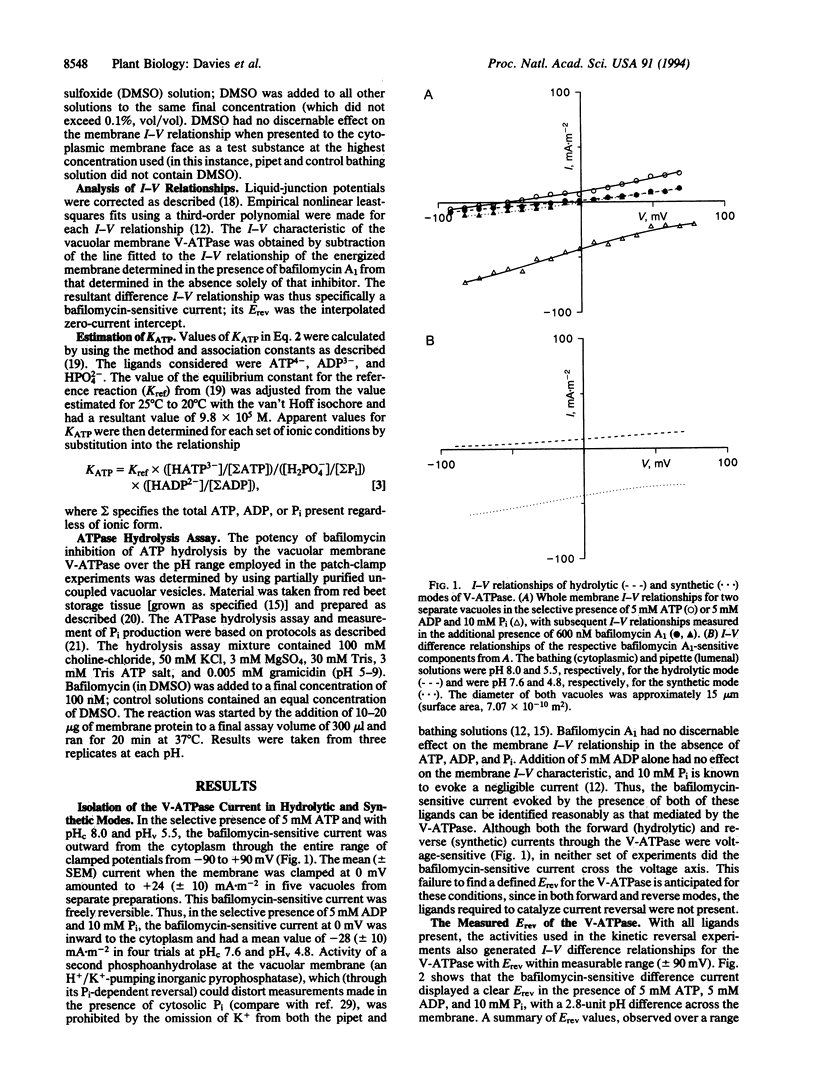
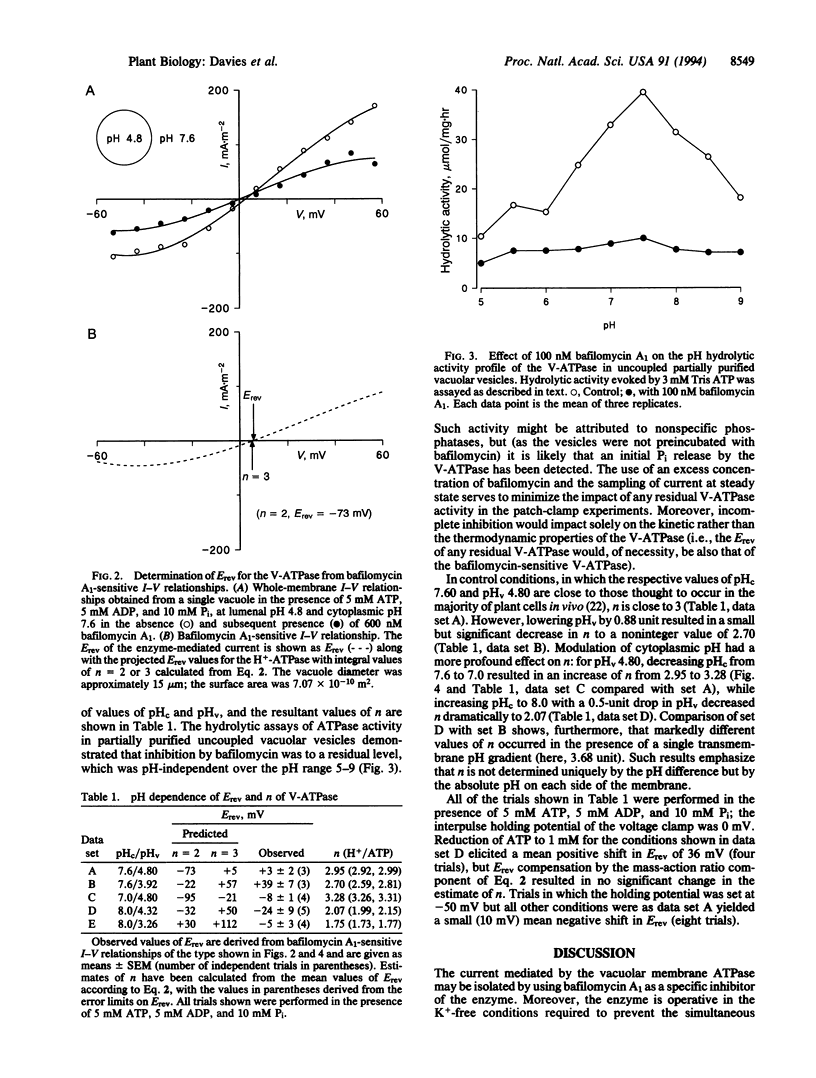
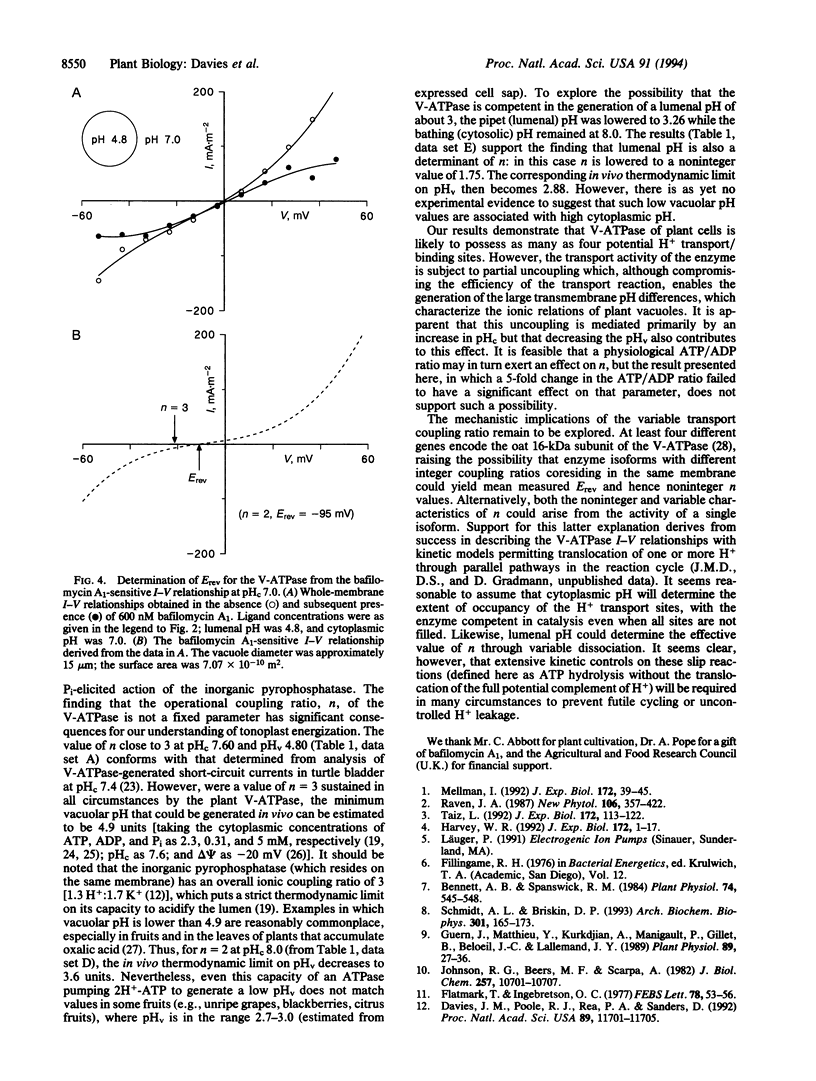
Abstract
The eukaryote endomembrane system contains a class of H(+)-pumping ATPase (H(+)-ATPases) of the vacuolar type (V-ATPases) that are responsible for the acidification of organelles. Their action is critical to numerous physiological processes, but the regulatory mechanisms that may control activity are not yet fully understood. The ratio of H+ transported per ATP hydrolyzed (n) has been determined thermodynamically for the red beet V-ATPase by using patch clamp. The value of n was found to range from 1.75 to 3.28 and was strictly dependent on cytoplasmic and lumenal pH. This suggests a mechanism by which V-ATPases are regulated by and might therefore control cytoplasmic and lumenal pH. Furthermore, the substantial capacity of plant vacuoles for H+ accumulation to pH 3 or lower can only be explained by the finding that n can adopt a value of < 2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: II. H/ATP Stoichiometry of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):545–548. doi: 10.1104/pp.74.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. M., Poole R. J., Rea P. A., Sanders D. Potassium transport into plant vacuoles energized directly by a proton-pumping inorganic pyrophosphatase. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11701–11705. doi: 10.1073/pnas.89.24.11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. M., Rea P. A., Sanders D. Vacuolar proton-pumping pyrophosphatase in Beta vulgaris shows vectorial activation by potassium. FEBS Lett. 1991 Jan 14;278(1):66–68. doi: 10.1016/0014-5793(91)80085-h. [DOI] [PubMed] [Google Scholar]
- Dixon T. E., Al-Awqati Q. H+/ATP stoichiometry of proton pump of turtle urinary bladder. J Biol Chem. 1980 Apr 25;255(8):3237–3239. [PubMed] [Google Scholar]
- Flatmark T., Ingebretsen O. C. ATP-dependent proton translocation in resealed chromaffin granule ghosts. FEBS Lett. 1977;78(1):53–56. doi: 10.1016/0014-5793(77)80271-8. [DOI] [PubMed] [Google Scholar]
- Guern J., Mathieu Y., Kurkdjian A., Manigault P., Manigault J., Gillet B., Beloeil J. C., Lallemand J. Y. Regulation of Vacuolar pH of Plant Cells: II. A P NMR Study of the Modifications of Vacuolar pH in Isolated Vacuoles Induced by Proton Pumping and Cation/H Exchanges. Plant Physiol. 1989 Jan;89(1):27–36. doi: 10.1104/pp.89.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hanada H., Moriyama Y., Maeda M., Futai M. Kinetic studies of chromaffin granule H+-ATPase and effects of bafilomycin A1. Biochem Biophys Res Commun. 1990 Jul 31;170(2):873–878. doi: 10.1016/0006-291x(90)92172-v. [DOI] [PubMed] [Google Scholar]
- Hanrahan J. W., Alles W. P., Lewis S. A. Single anion-selective channels in basolateral membrane of a mammalian tight epithelium. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7791–7795. doi: 10.1073/pnas.82.22.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W. R. Physiology of V-ATPases. J Exp Biol. 1992 Nov;172:1–17. [PubMed] [Google Scholar]
- Johnson R. G., Beers M. F., Scarpa A. H+ ATPase of chromaffin granules. Kinetics, regulation, and stoichiometry. J Biol Chem. 1982 Sep 25;257(18):10701–10707. [PubMed] [Google Scholar]
- Lai S. P., Watson J. C., Hansen J. N., Sze H. Molecular cloning and sequencing of cDNAs encoding the proteolipid subunit of the vacuolar H(+)-ATPase from a higher plant. J Biol Chem. 1991 Aug 25;266(24):16078–16084. [PubMed] [Google Scholar]
- Mellman I. The importance of being acid: the role of acidification in intracellular membrane traffic. J Exp Biol. 1992 Nov;172:39–45. doi: 10.1242/jeb.172.1.39. [DOI] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Chromatographic resolution of h-translocating pyrophosphatase from h-translocating ATPase of higher plant tonoplast. Plant Physiol. 1986 May;81(1):126–129. doi: 10.1104/pp.81.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeille F., Bligny R., Douce R. Is the cytosolic pi concentration a limiting factor for plant cell respiration? Plant Physiol. 1984 Feb;74(2):355–359. doi: 10.1104/pp.74.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. L., Briskin D. P. Energy transduction in tonoplast vesicles from red beet (Beta vulgaris L.) storage tissue: H+/substrate stoichiometries for the H(+)-ATPase and H(+)-PPase. Arch Biochem Biophys. 1993 Feb 15;301(1):165–173. doi: 10.1006/abbi.1993.1129. [DOI] [PubMed] [Google Scholar]
- Taiz L. THE PLANT VACUOLE. J Exp Biol. 1992 Nov 1;172(Pt 1):113–122. doi: 10.1242/jeb.172.1.113. [DOI] [PubMed] [Google Scholar]



