Abstract
The aim of this study was to evaluate the effect of acute and chronic physical and psychological stressors on the induction of oxidative stress in male rat liver. Male Wistar rats were randomly divided into 3 groups as following: control, physical and psychological stress groups. Stress was induced by communication box for one (acute), fifteen and thirty (chronic) days. Once stressor periods ended, rats were anesthetized and their liver dissected out for later assessments. Exposure to physical stress enhanced liver superoxide dismutase (SOD) (19.44 %) and glutathione S-transferase (GST) (21.84 %) activities and decreased glutathione (GSH) (30.03 %) level on the 1st day (p<0.05). SOD (24.13 and 18.43 %) and GST (27.77 and 21.27 %) activities were significantly increased, while catalase activity (29.74 and 24.41 %) and GSH level (35.05 and 31.05 %) were decreased in psychological stress group after 1 and 15 days (p<0.01 and p<0.05) compared to the 1st day value in control group, respectively. Psychological stress induced an increase in liver malondialdehyde (MDA) (46 %) and plasma corticosterone (36 %) levels on the 1st day (p<0.05). However, all parameters returned to their basal value after 30 days of stress.
The results suggest that exposure to acute physical and psychological stressors induce the production of reactive oxygen species and oxidative stress in rat liver due to GSH depletion and the decreased catalase activity. The elevation of lipid peroxidation and corticosterone level in acute psychological stress may lead to more profound oxidative damage than acute physical stress. Moreover, cell protection in hepatic tissue of chronically stressed rats is indicative of possible late adaptation of the animals to stress.
Keywords: Physical stress, psychological stress, oxidative stress, rat, liver
Introduction
Stress by any aversive stimulus, is a state of threatened homeostasis provoked by a psychological, environmental, or physiologic stressor and the ability to cope with such stressful stimuli is a crucial determinant of health and disease. Stress responses are regulated by interactions between physiological and neurochemical factors. Physical or psychological stress induce changes in hypothalamic–pituitary–adrenal axis which culminates with glucocorticoids and chemical mediators release including adrenocorticotropic hormone (ACTH), norepinephrine (NE), serotonin, dopamine, and acetylcholine (Nadeem et al., 2006[30]; Chakraborti et al., 2008[11]; Goncalves et al., 2008[18]). The metabolism of norepinephrine and dopamine leads to production of free radicals and reactive oxygen species (ROS). Glucocorticoids may increase the basal level of ROS in cells and also increase the toxicity of oxygen radical generators (Uysal et al., 2005[46]).
Understanding the molecular and cellular pathways activated in response to stress exposure is important for the development of pharmacological intervention to stress-induced diseases. One of the important mechanisms of action of stress is production of ROS, which can react with biological macromolecules such as DNA, proteins, carbohydrates and lipids. The ROS in the cells are neutralized by antioxidant defense system including superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST) and glutathione (GSH). Oxidative stress is the result of an imbalance between oxidants and antioxidants (Nadeem et al., 2006[30]; Kamper et al., 2009[22]). Several studies have shown that the alteration of antioxidant enzyme activities in different kinds of stress were associated with a depletion of GSH and an increase of lipid peroxidation, all of which can lead to oxidative stress and finally cell death (Pajovic et al., 2006[31]; Depke et al., 2009[12]; Lucca et al., 2009[25]; Ahmad et al., 2012[2]).
The ability to neutralize oxidant species differs in various tissues and stress models (Stojiljkovic et al., 2005[42]; Sahin and Gumuslu, 2007[36]; Kamper et al., 2009[22]). The oxidative injury appearing after stress exposure is not well understood. To the best of our knowledge, there is no data on time-dependent effect of acute and chronic physical and psychological stresses on antioxidant defense system in the liver. In the present study we tried to look at this effect with GSH concentration measurement, as well as assessing the activities of antioxidant enzymes (SOD, CAT and GST) and malondialdehyde (MDA) as an important index of lipid peroxidation in liver after two stress model exposure.
Materials and Methods
Chemicals
Reduced glutathione (GSH), Nitro-bluetetrazolium (NBT), 1-chloro-2,4-dinitrobenzene (CDNB) and 5,5'-dithiobis 2-nitrobenzoic acid (DTNB) were obtained from Sigma Chemical Company. All other chemicals used were of extra pure grade and obtained from Sigma and Merck.
Animals
Male Wistar rats (170-190 g, 2.5-3 months old) were obtained from Pasteur Institute (Tehran, Iran). Rats were housed 3 per cage and acclimated for at least 1 week prior to experimental use. All animals were fed a standard food (Pars Company of animal food producer, Iran) and water ad libitum and kept in a temperature-controlled room (20 to 22 °C) with a 12 h light/12 h dark cycle. All procedures were approved by the Animal Care and Use Committee of the Neuroscience Research Center, Shahid Beheshti University of Medical Sciences, Iran.
Stress protocol
Rats were randomly divided into 3 groups of 12 animals each as following: control group, physical and psychological stress groups. A communication box was used as stress stimulus device. This device is divided into nine compartments (16×16 cm) by transparent plastic sheets. In each session, five rats were exposed to physical stress by the electrical foot-shock (1 mA, 1 Hz) for a 10 s duration every 60 s (1 h/d) through the stainless steel grids. Four remaining animals in the other compartments were exposed to psychological stress by watching and hearing the struggle, jumping, and vocalization of the animals under foot-shock stress (Endo et al., 2001[14]). The animals of the stressed group were subdivided into acutely (1 day exposure to stress) and chronically stressed (15 and 30 days exposure to stress) groups. The animals of the control group had the same subgroups. These animals were placed in the box (1 h/d) without receiving any stress. The weight of the animals was recorded on the 1st, 15th, and 30th day of the experimental period.
Plasma and tissue preparation
At the end of the experimental periods, rats were anesthetized with diethyl ether, and blood samples were collected by cardiac puncture in tube containing 0.5 % heparin as the anticoagulant and immediately centrifuged at 1500×g for 10 minutes at 4 °C. Plasma were separated from erythrocytes and stored in 0.5 ml aliquots at –70 °C freezer for measurement of the corticosterone concentration. The liver was immediately removed, washed in ice-cold phosphate buffer saline (PBS). Washed tissues were immediately immersed in liquid nitrogen and stored at -70 °C until biochemical analysis.
On the day use, frozen tissue samples were quickly weighed and homogenized 1:10 in ice-cold 50 mM potassium phosphate buffer (pH 7.4) containing 1 mM EDTA. The homogenates were then centrifuged at 16000×g for 15 min at 4 °C. The supernatants were separated and used for enzyme activities assays and protein determination.
Measurement of plasma corticosterone level
Plasma corticosterone level was measured using the corticosterone Elisa kit (DRG, Germany). The plasma corticosterone was expressed as nmol/mL using corticosterone standards prepared.
SOD activity assay
The activity of SOD was determined using the method described by Winterbourn et al. (1975[47]) based on the ability of SOD to inhibit the reduction of NBT by superoxide. The absorbance of samples was read on a Genesys 10 UV spectrophotometer at 560 nm for 5 min. The amount of enzyme required to produce 50 % inhibition was taken as 1 U and results were expressed as U/mg protein.
CAT activity assay
CAT activity in tissue homogenates was measured spectrophotometrically at 240 nm by calculating the rate of degradation of H2O2 as the substrate of the enzyme using the method of Aebi, 1984[1]. A molar absorption of 43.6 Mcm-1 was used to determine CAT activity. Enzymatic activity was expressed as U/mg protein, one unit (U) of which was equal to 1 mole of H2O2 degraded/min/mg of protein.
GST activity assay
GST activity was assayed by monitoring the formation of the thioether product of the reaction between GSH and CDNB at 340 nm (Habig and Jakoby, 1981[19]). The enzyme activity was calculated using extinction coefficient 9.6 mM cm-1 and expressed as µmol CDNB utilized/min/mg protein.
Determination of GSH level
GSH level was measured using the method of Tietz (1969[45]). GSH in the supernatant was assayed at 412 nm by monitoring the absorbance of DTNB for 5 min. GSH levels were determined from a standard curve and expressed as nmol/mg protein.
Determination of MDA level
Liver MDA level as an indicator of lipid peroxidation was determined at 532 nm using 2-thiobarbituric acid according to the method of Satoh (1978[41]). MDA concentrations were determined using 1,1,3,3-tetraethoxypropane as standard and expressed as nmol/mg protein.
Protein level assay
The total protein contents of the samples were measured by Bradford’s method (1976[9]) using bovine serum albumin as standard.
Results
Effects of stress on the body weight
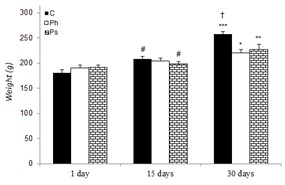
The changes in the body weight of control and stressed rats during various experimental periods are given in Figure 1(Fig. 1). The body weights of both control and stressed rats had a rising trend. The weights of control and stressed rats showed a significant increase on day 30 as compared to day 1 in the same group. However, on day 30, the weights of physical (p<0.05) and psychological (p<0.01) stress groups were significantly less than that of the control group.
Figure 1. Effect of acute and chronic physical and psychological stresses on the body weights in control and stressed rats. Control (C), physical (Ph) and psychological (Ps) stresses. Values are expressed as mean ± SEM of 12 rats. *p<0.05, **p<0.01 and ***p<0.01 vs. day 1 in the same group; †P<0.05 vs. day 30 in the stressed groups; #P<0.05 vs. day 30 in the same group.
Effects of stress on corticosterone level
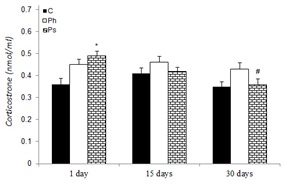
The effect of acute and chronic physical and psychological stresses on plasma corticosterone level is shown in Figure 2(Fig. 2). No significant difference was observed between corticosterone level of the control and physical stress groups at any time. The rats of psychological stress group showed significantly higher plasma corticosterone level (36 %) on the 1st day in comparison to the control rats at the same time. This stressed group showed a significant decrease in corticosterone level on the 30th (p<0.05) day of the experiment compared with the 1st day. In addition, no significant difference was observed between corticosterone levels of the acute and chronic physical and psychological stress groups throughout the trial.
Figure 2. Effect of acute and chronic physical and psychological stresses on plasma corticosterone level in control and stressed rats. Control (C), physical (Ph) and psychological (Ps) stresses. Values are expressed as mean ± SEM of 12 rats. *p<0.05 vs. day 1 in the control group; #P<0.05 vs. day 1 in the same group.
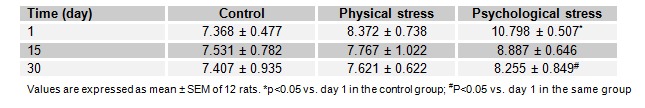
Effects of stress on GSH level
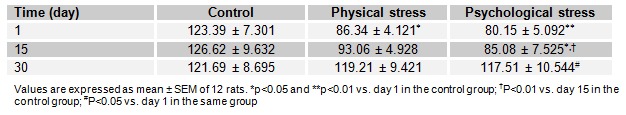
The effect of acute and chronic physical and psychological stresses on liver GSH level is depicted in Table 1(Tab. 1). GSH level was decreased as 30.03 and 24.58 % by physical stress and 35.05 and 31.05 % psychological stress on the 1st and 15th days of the experiment, as compared to the control rats, respectively. However, the recovery of the parameter was observed in rats of the stressed groups after 30 days.
Table 1. Table1: Effect of acute and chronic physical and psychological stresses on liver GSH level (nmol/mg protein) in control and experimental rats.
Effects of stress on antioxidant enzyme activities
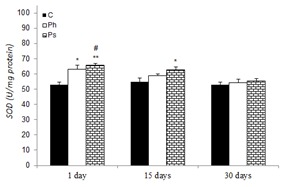
Mean liver SOD activity in control and stressed groups is shown in Figure 3(Fig. 3). SOD activity was increased in physical (19.44 and 15.30 %) and psychological (24.13 and 18.43 %) stress groups after 1 and 15 days compared to the control on the 1st day, respectively. However, the SOD activity after 30 days was not significant between control and stressed groups.
Figure 3. Effect of acute and chronic physical and psychological stresses on liver SOD activity in control and stressed rats. Control (C), physical (Ph) and psychological (Ps) stresses. Values are expressed as mean ± SEM of 12 rats. *p<0.05 and **p<0.01 vs. day 1 in the control group; #P<0.05 vs. day 30 in the same group.
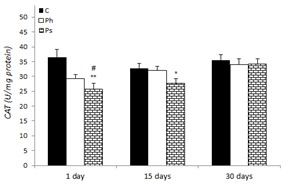
The alteration of CAT activity in liver rats of the control and stressed groups are presented in Figure 4(Fig. 4). CAT activity was significantly decreased as 29.74 and 24.41 % in psychological stress group after 1 and 15 days, as compared to the values of the control group on the 1st day. However, the recovery followed suit after 30 days in rats of this stress group. Exposure to physical stress did not change CAT activity at any time point.
Figure 4. Effect of acute and chronic physical and psychological stresses on liver CAT activity in control and stressed rats. Control (C), physical (Ph) and psychological (Ps) stresses. Values are expressed as mean ± SEM of 12 rats. *p<0.05 and **p<0.01 vs. day 1 in the control group; #P<0.05 vs. day 30 in the same group.
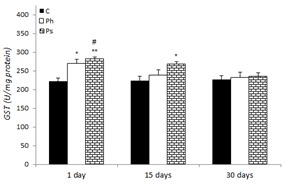
The changes of GST activity in the liver of control and stress-treated rats are shown in Figure 5(Fig. 5). An increase in GST activity was observed in physical (21.84 and 8 %) and psychological (27.77 and 21.27 %) stress groups after 1 and 15 days when compared to the control on the 1st day, respectively. However, there was no significant change observed in GST activity following 30 days of stress exposure in comparison to the control.
Figure 5. Effect of acute and chronic physical and psychological stresses on liver GST activity in control and stressed rats. Control (C), physical (Ph) and psychological (Ps) stresses. Values are expressed as mean ± SEM of 12 rats. *p<0.05 and **p<0.01 vs. day 1 in the control group; #P<0.01 vs. days 15 and 30 in the control group.
Effects of stress on MDA level
The effect of acute and chronic physical and psychological stresses on liver MDA level is depicted in Table 2(Tab. 2). Liver MDA level was significantly increased in the rats of psychological stress group (46 %) on the 1st day in comparison to the control rats at the same time. This stressed group showed a significant decrease in MDA level on the 30th day of the experiment compared with the 1st day. No significant difference was observed between liver MDA levels of the control and physical stress groups at any times. In addition, no significant difference was observed between MDA level of the acute and chronic physical and psychological stress groups throughout the trial.
Table 2. Effect of acute and chronic physical and psychological stresses on liver MDA level (nmol/mg protein) in control and experimental rats.
Discussion
Several studies have demonstrated that different kinds of stress cause reduction of body weight in experimental animals (Gamaro et al., 2003[16]; Bekris et al., 2005[5]; Zardooz et al., 2006[50]; Lucca et al., 2009[25]). In the present study, the weight of rats in physical and psychological stress groups were significantly less than the control group on the 30th day of the experiment. This may be due to the decreased sweet food consumption in stressed rats (Bekris et al., 2005[5]; Lucca et al., 2009[25]). Exposure to stress increased the activity of corticotropin releasing hormone (CRH), as an anorexigenic neuropeptide, which may lead to a reduction of food intake and consequently lesser weight gain in the stressed rats (Carrasco and Van de Kar, 2003[10]; Zardooz et al., 2006[50]). According to the previous studies, chronic exposure to psychological and social stresses increased both food intake and body weight in male rats and hamsters (Foster et al., 2006[15]; Rostamkhani et al., 2012[34]). A study showed that shaker stress for 7 days in male mice significantly decreased body weight, but had no effect on food intake (Bernatova et al., 2002[6]). Repeated social stress for 6 days increased food intake but decreased weight gain in male rats (Bhatnagar et al., 2006[7]).
Oxidative stress plays a crucial role in the initiation and progression of different diseases such as hepatocyte diseases (Depke et al., 2009[12]; Ahmad et al., 2012[2]). Different kinds of stress can promote the formation of ROS and oxidative stress conditions (Nadeem et al., 2006[30]; Kamper et al., 2009[22]). ROS are neutralized by the antioxidant defense mechanisms. SOD and CAT are the first line of cellular defense against oxidative injury. SOD catalyzes converts the superoxide anion into H2O2 and CAT degrades H2O2 to water (Sarumathi and Saravanan, 2012[40]). In the present study, the increased SOD activity and decreased CAT activity in the liver were significantly observed in psychological stress group after both 1 and 15 days. Physical stress significantly increased SOD activity without change in CAT activity on the 1st day of the experiment. However, the recovery of these parameters was observed after 30 days in rats of the stressed groups. The SOD elevation may provide mainly protection against stress induced liver injury. The high SOD activity may be attributed to increased superoxide anion production giving rise to an excess H2O2 in liver. The decreased or unaltered CAT activity leads to the accumulation of H2O2, which may be the cause of oxidative stress (Ahmad et al., 2012[2]; Sarumathi and Saravanan, 2012[40]). A study showed that chronic footshock stress for 21 days induced significant increases in brain SOD activity, with concomitant reduction in CAT activity (Bhattacharya et al., 2001[8]). In addition, several studies reported increased SOD and CAT activities (Sahin and Gumuslu, 2004[35]; Uysal et al., 2005[40]; Samson et al., 2007[39]; Ahmad et al., 2012[2]), others claimed decreases (Sarumathi and Saravanan, 2012[40]), while others found no significant differences in various tissues (Sahin et al., 2006[37]) in response to different stress models. We conclude that the response to stress would appear different according to the type, duraton, intensity, and history of the animal to stress (Papandreou et al., 2011[32]).
GST is present in various tissues and can conjugate ROS with GSH. It plays an important role in protecting tissue from oxidative stress and its levels can reflect the antioxidant capacity of the body (Habig and Jakoby, 1981[19]). In present study, the liver GST activity was significantly increased by both physical and psychological stressors as early as the first day, possibly due to the self-adjusting activity of the body to resist oxidative damage. These findings are in agreement with the results of the previous reports that cold and social stressors significantly increased the activity of GST in various tissues (Kaushik and Kaur, 2003[23]; Nadeem et al., 2006[30]; Yuksel et al., 2008[48]). Our results are inconsistent with those reported a decrease in GST activity in rats exposed to restraint stress for 1-6 h (Zaidi et al., 2005[49]; Atif et al., 2008[3]; Devaki et al., 2011[13]) and immobilization stress for 21 days (Sarumathi and Saravanan, 2012[40]).
GSH is an important non-enzymatic antioxidant that plays a crucial role in the detoxification of ROS (Sahin and Gumuslu, 2004[35]; Ghizoni et al., 2006[17]; Chakraborti et al., 2008[11]). GSH protects essential thiol groups from oxidation and serves as a substrate for glutathione peroxidase and GST. In addition, GSH is involved in maintenance of other antioxidants, such as ascorbate and α-tocopherol. GSH is synthesized in the cytoplasm of the liver cells and then distributed into different organs (Ghizoni et al., 2006[17]; Jafari et al., 2012[20]). In present study, GSH level was decreased in the liver of physical (after 1 day) and psychological (after 1 and 15 days) stress groups. The decreased GSH may be due to the presence of ROS produced by stress, the increased activity of GST enzyme (Figure 5) and limited GSH synthesis (Kaushik and Kaur, 2003[23]; Ghizoni et al., 2006[17]; Jafari et al., 2012[20]). Depletion of GSH leads to oxidized GSH (GSSG) production and finally decreased the GSH/GSSG ratio in different tissues in stressed rats, which is an index of tissue oxidative stress (Samson et al., 2007[39]). Our results are in agreement with the results that have found decreased levels of GSH in various tissues of rats exposed to different stressors (Samson et al., 2007[39]; Chakraborti et al., 2008[11]; Atif et al., 2008[3]; Sarumathi and Saravanan, 2012[40]; Ahmad et al., 2012[2]). However, Ghizoni and coworkers showed that acute restraint stress via the inhibition of nitric oxide synthase led to an increase in GSH levels in the cerebellum after 2 and 4 h of immobilization, but not in the cerebral cortex, striatum, and hippocampus (Ghizoni et al., 2006[17]). Evidences indicate that pretreatment with antioxidants, GSH, alpha-tocopherol and N-acetyl cysteine reduced the stress-induced oxidative stress in rats (Liu et al., 1994[24]; Chakraborti et al., 2008[11]).
Lipid peroxidation is an oxidative degeneration of polyunsaturated fatty acids, which causes impaired membrane structure and functions. MDA level, as an important indicator of LPO, indirectly reflects the extent of cellular injury in vivo (Chakraborti et al., 2008[11]; Jafari et al., 2012[20]). The present study showed that MDA content was significantly raised in rat liver following psychological stress exposure on the 1st day of the experiment. GSH depletion may lead to an increased lipid peroxidation, possibly due to the lowering of the cellular defense system against endogenous toxic intermediates (Chakraborti et al., 2008[11]). Numerous studies have shown that exposure to a variety of acute and chronic stress models (e. g., restraint, immobilization, cold and psychological stressors) significantly increased MDA content in various tissues of animals (Kaushik and Kaur, 2003[23]; Sahin and Gumuslu, 2004[35]; Zaidi et al., 2005[49]; Nadeem et al., 2006[30]; Samson et al., 2007[39]; Atif et al., 2008[3]; Devaki et al., 2011[13]; Ahmad et al., 2012[2]). However, acute footshock stress did not change MDA level in male and female rat brains (Uysal et al., 2005[46]). Chronic restraint stress for 21 days led to increased lipid peroxidation in the kidney but not in the liver and heart (Sahin et al., 2006[37]). In addition, psychological stress exposure for 2–16 h significantly increased the content of MDA in the mouse brain via an increase in neuronal nitric oxide synthase activity but had no effect on liver and serum MDA levels (Matsumoto et al., 1999[28]). These inconsistencies in results may be attributed to both the type of model and the duration of stress (Sahin et al., 2006[37]).
Glucocorticoid release in response to stress may play a critical role in activation of catecholamine transmission (Uysal et al., 2005[46]). The plasma corticosterone level is one of the most important indicators of stress (Sahin and Gumuslu, 2004[35]; Sahin and Gumuslu, 2007[36]). In this study, plasma corticosterone level was increased in psychological stress group on the 1st day. A return of corticosterone level to control values in the chronically stressed rats is indicative of possible late adaptation of the animals to stress (Ricart-Jane et al., 2002[33]; Teague et al., 2007[44]; Rostamkhani et al., 2012[34]; Zardooz et al., 2012[51]). The positive correlation between plasma corticosterone and liver MDA level (Figure 2(Fig. 2) and Table 2(Tab. 2)) in psychological stress suggests a probability that the elevation of corticosterone level in response to stress accelerates the generation of ROS leading to lipid peroxidation in the liver (Sahin and Gumuslu, 2007[36]; Tangaraj et al., 2007[43]). Our results are in accordance with the previous studies claiming that exposure to different types of acute stress, including immobilization, restraint and psychological stressors in rats significantly increased plasma corticosterone level (Sahin and Gumuslu, 2004[35]; Tangaraj et al., 2007[43]; Zardooz et al., 2006[50], 2012[51]; Rostamkhani et al., 2012[34]). No change in corticosterone level was reported following chronic stress exposure (Marin et al., 2007[27]; Moretti et al., 2012[29]).
The highest peak in MDA and corticosterone levels and the trough in the depleted GSH content were found in psychological stressed rats on the 1st day. Depletion of GSH and the decrease in the GSH/GSSG ratio may shift cells through apoptosis and necrosis (Jafari et al., 2012[20]). Several studies have shown that stress and high corticosterone levels induce cell death in rat brain (Behl et al., 1997[4]; Manoli et al., 2000[26]). In addition, psychological stress increased in vitro apoptosis of peripheral blood T lymphocytes (Sakami et al., 2003[38]; Jun et al., 2008[21]) and in BALB/c mice (Depke et al., 2009[12]). However, further studies are required to investigate the effects of physical and psychological stressors on cell death induction in a variety of tissues using an in vivo system.
In summary, exposure to physical and psychological stressors induce the production of ROS and oxidative stress in rat liver due to GSH depletion, increase SOD activity concomitant with decreased or unaltered catalase activity. The elevation of lipid peroxidation and corticosterone levels in situations of acute psychological stress may lead to even more profound oxidative damage than acute physical stress. Moreover, the induction of cell protection in hepatic tissue of chronically stressed rats give strong evidence that counter-regulatory mechanisms are activated to prevent further hepatocyte damage in these animals (Depke et al., 2009[12]).
Acknowledgements
The authors would like to thank Prof. Dr Alireza Asgari for careful review of the manuscript. This work was supported by a grant from Medical Faculty of Baqiyatallah University of Medical Sciences and Neuroscience Research Center, Shahid Beheshti University of Medical Sciences.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad A, Rasheed N, Chand K, Maury R, Banuc N, Palita G. Restraint stress-induced central monoaminergic and oxidative changes in rats and their prevention by novel Ocimum sanctum compounds. Indian J Med Res. 2012;135:548–554. [PMC free article] [PubMed] [Google Scholar]
- 3.Atif F, Yousuf S, Agrawal SK. Restraint stress-induced oxidative damage and its amelioration with selenium. Eur J Pharmacol. 2008;600:59–63. doi: 10.1016/j.ejphar.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 4.Behl C, Lezoualc'h F, Trapp T, Widmann M, Skutella T, Holsboer F. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinology. 1997;138:101–106. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- 5.Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Bernatova I, Key MP, Lucot JB, Morris M. Circadian differences in stress-induced pressor reactivity in mice. Hypertension. 2002;40:768–773. doi: 10.1161/01.hyp.0000036405.27562.02. [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya SK, Bhattacharya A, Das K, Muruganandam AV, Sairam1 K. Further investigations on the antioxidant activity of Ocimum sanctum using different paradigms of oxidative stress in rats. J Nat Remedies. 2001;1:6–16. [Google Scholar]
- 9.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborti A, Gulati K, Ray A. Age related differences in stress-induced neurobehavioral responses in rats: Modulation by antioxidants and nitrergic agents. Behav Brain Res. 2008;194:86–91. doi: 10.1016/j.bbr.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Depke M, Steil L, Domanska G, Volker U, Schutt C, Kiank C. Altered hepatic mRNA expression of immune response and apoptosis-associated genes after acute and chronic psychological stress in mice. Mol Immunol. 2009;46:3018–28. doi: 10.1016/j.molimm.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Devaki M, Nirupama R, Yajurvedi HN. Reduced antioxidant status for prolonged period due to repeated stress exposure in rat. J Stress Physiol Biochem. 2011;7:139–147. [Google Scholar]
- 14.Endo Y, Yamauchi K, Fueta Y, Lrie M. Changes of body temperature and plasma corticosterone level in rats during psychological stress induced by the communication box. Med Sci Monit. 2001;7:1161–1165. [PubMed] [Google Scholar]
- 15.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1284–R1293. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- 16.Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C. Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int. 2003;42:107–14. doi: 10.1016/s0197-0186(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 17.Ghizoni DM, Pavanati KC, Arent AM, Machado C, Faria MS, Pinto CM, et al. Alterations in glutathione levels of brain structures caused by acute restraint stress and by nitric oxide synthase inhibition but not by intraspecific agonistic interaction. Behav Brain Res. 2006;166:71–77. doi: 10.1016/j.bbr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Goncalves L, Dafre AL, Goncalves Carobrez S, Cezar Gasparotto O. A temporal analysis of the relationships between social stress, humoral immune response and glutathione-related antioxidant defenses. Behav Brain Res. 2008;192:226–31. doi: 10.1016/j.bbr.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Habig WT, Jakoby WB. Glutathion S-transferase (rat and human) Method Enzymol. 1981;77:218–31. doi: 10.1016/s0076-6879(81)77029-0. [DOI] [PubMed] [Google Scholar]
- 20.Jafari M, Salehi M, Ahmadi S, Asgari A, Abasnezhad M, Hajigholamali M. The role of oxidative stress in diazinon-induced tissues toxicity in Wistar and Norway rats. Toxicol Mech Methods. 2012;22:638–647. doi: 10.3109/15376516.2012.716090. [DOI] [PubMed] [Google Scholar]
- 21.Jun Y, Aiguo C, Maozi H. Effects of psychological stress on the hsp70 expression and apoptosis of the peripheral blood lymphocytes in rats. Acta Psychol Sin. 2008;40:717–22. [Google Scholar]
- 22.Kamper EF, Chatzigeorgiou A, Tsimpoukidi O, Kamper M, Dalla C, Pitychoutis PM, et al. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol Behav. 2009;98:215–22. doi: 10.1016/j.physbeh.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Kaushik S, Kaur J. Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin Chim Acta. 2003;333:69–77. doi: 10.1016/s0009-8981(03)00171-2. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Wang X, Mori A. Immobilization stress-induced antioxidant defense changes in rat plasma: effect of treatment with reduced glutathione. Int J Biochem. 1994;26:511–7. doi: 10.1016/0020-711x(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 25.Lucca G, Comim CM, Valvassori SS, Reus GZ, Vuolo F, Petronilho F, et al. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int. 2009;54:358–62. doi: 10.1016/j.neuint.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Manoli LP, Gamaro GD, Silveira PP, Dalmaz C. Effect of chronic variate stress on thiobarbituric-acid reactive species and on total radical-trapping potential in distinct regions of rat brain. Neurochem Res. 2000;25:915–921. doi: 10.1023/a:1007592022575. [DOI] [PubMed] [Google Scholar]
- 27.Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Yobimoto K, Huong NT, Abdel-Fattah M, Van Hien T, Watanabe H. Psychological stress-induced enhancement of brain lipid peroxidation via nitric oxide systems and its modulation by anxiolytic and anxiogenic drugs in mice. Brain Res. 1999;839:74–84. doi: 10.1016/s0006-8993(99)01715-1. [DOI] [PubMed] [Google Scholar]
- 29.Moretti M, Colla A, de Oliveira Balen G, dos Santos DB, Budni J, de Freitas AE, et al. Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J Psychiatr Res. 2012;46:331–340. doi: 10.1016/j.jpsychires.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Nadeem A, Masood A, Masood N, Afzal Gilani R, Ahmad Shah Z. Immobilization stress causes extra-cellular oxidant-antioxidant imbalance in rats: Restoration by L-NAME and vitamin E. Eur Neuropsychopharmacol. 2006;16:260–267. doi: 10.1016/j.euroneuro.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Pajovic SB, Pejic S, Stojiljkovic V, Gavrilovic Lj, Dronjak S, Kanazir DT. Alterations in hippocampal antioxidant enzyme activities and sympatho-adrenomedullary system of rats in response to different stress models. Physiol Res. 2006;55:453–460. doi: 10.33549/physiolres.930807. [DOI] [PubMed] [Google Scholar]
- 32.Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Ricart-Jane D, Rodriguez-Sureda V, Benavides A, Peinado-Onsurbe J, Lopez-Tejero MD, Llobera M. Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metabolism. 2002;51:925–931. doi: 10.1053/meta.2002.33353. [DOI] [PubMed] [Google Scholar]
- 34.Rostamkhani F, Zardooz H, Zahediasl S, Farrokhi B. Comparison of the effects of acute and chronic psychological stress on metabolic features in rats. J Zhejiang Univ Sci B. 2012;13:904–912. doi: 10.1631/jzus.B1100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahin E, Gumuslu S. Alterations in brain antioxidant status, protein oxidation and lipid peroxidation in response to different stress models. Behav Brain Res. 2004;155:241–8. doi: 10.1016/j.bbr.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 36.Sahin E, Gumuslu S. Stress-dependent induction of protein oxidation, lipid peroxidation and antioxidants in peripheral tissues of rats: comparison of three stress models (immobilization, cold and immobilization-cold) Clin Exp Pharmacol Physiol. 2007;34:425–31. doi: 10.1111/j.1440-1681.2007.04584.x. [DOI] [PubMed] [Google Scholar]
- 37.Sahin M, Sagdıc G, Elmas O, Akpınar D, Derin N, Aslan M, et al. Effect of chronic restraint stress and alpha-lipoic acid on lipid peroxidation and antioxidant enzyme activities in rat peripheral organs. Pharmacol Res. 2006;54:247–52. doi: 10.1016/j.phrs.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Sakami S, Nakata A, Yamamura T, Kawamura N. Psychological stress increases human T cell apoptosis in vitro. Neuroimmunomodulation. 2003;10:224–31. doi: 10.1159/000068326. [DOI] [PubMed] [Google Scholar]
- 39.Samson J, Sheeladevi R, Ravindran R. Oxidative stress in brain and antioxidant activity of Ocimum sanctum in noise exposure. Neurotoxicology. 2007;28:679–85. doi: 10.1016/j.neuro.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Sarumathi A, Saravanan N. Antioxidant status in kidney and liver of rats during immobilization stress and treated with Centella asiatica (Linn.) Int J Res Biol Sci. 2012;2:165–169. [Google Scholar]
- 41.Satoh K. Serum lipid peroxidation in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 42.Stojiljkovic V, Todorovic A, Kasapovic J, Pejic S, Pajovic SB. Antioxidant enzyme activity in rat hippocampus after chronic and acute stress exposure. Ann N Y Acad Sci. 2005;1048:373–6. doi: 10.1196/annals.1342.042. [DOI] [PubMed] [Google Scholar]
- 43.Tangaraj R, Rathakrishnan Ayyappan S, Manikandan P, Baskaran J. Antioxidant property of Emblica officinalis during experimentally induced restrain stress in rats. J Health Sci. 2007;53:496–499. [Google Scholar]
- 44.Teague CR, Dhabhar FS, Barton RH, Beckwith-Hall B, Powell J, Cobain M, et al. Metabonomic studies on the physiological effects of acute and chronic psychological stress in Sprague-Dawley rats. J Proteome Res. 2007;6:2080–2093. doi: 10.1021/pr060412s. [DOI] [PubMed] [Google Scholar]
- 45.Tietz F. Enzymic method for quantitative determination of nanogram amount of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 46.Uysal N, Acikgoz O, Gonenc S, Kayatekin BM, Kiray M, Sonmez A, et al. Effects of acute footshock stress on antioxidant enzyme activities in the adolescent rat brain. Effects Physiol Res. 2005;54:437–442. [PubMed] [Google Scholar]
- 47.Winterbourn C, Hawkins R, Brian M, Carrell R. The estimation of red cell superoxide dismutase activity. J Lab Clin Med. 1975;85:337–341. [PubMed] [Google Scholar]
- 48.Yuksel S, Asma D, Yesilada O. Antioxidative and metabolic responses to extended cold exposure in rats. Acta Biol Hung. 2008;59:57–66. doi: 10.1556/ABiol.59.2008.1.5. [DOI] [PubMed] [Google Scholar]
- 49.Zaidi SM, Al-Qirim TM, Banu N. Effect of antioxidant vitamins on glutathione depletion and lipid peroxidation induced by restraint stress in the rat liver. Drugs RD. 2005;6:157–165. doi: 10.2165/00126839-200506030-00004. [DOI] [PubMed] [Google Scholar]
- 50.Zardooz H, Zahedi A, Gharib Naseri MK. Effect of chronic psychological stress on insulin release from rat isolated pancreatic islets. Life Sci. 2006;79:57–62. doi: 10.1016/j.lfs.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 51.Zardooz H, Zahediasl S, Rostamkhani F, Farrokhi B, Nasiraei S, Kazeminezhad B, et al. Effects of acute and chronic psychological stress on isolated pancreatic islets’ insulin release. EXCLI J. 2012;11:163–175. [PMC free article] [PubMed] [Google Scholar]


