Abstract
In order to evaluate the role of ethyl acetate fraction (PB-EtAC) obtained from the Phyllostachys bambusoides leaves in the modulation of immune responses, detailed studies were carried out using a panel of in vivo assays. Oral administration of PB-EtAC (50–200 mg/Kg) stimulated the IgM and IgG titre expressed in the form of haemagglutination antibody (HA) titre. Further, it elicited a dose related increase in the delayed type hypersensitivity reaction (DTH) after 24 and 48 h in BALB/c mice. Besides augmenting the humoral and cell mediated immune response, the concentration of cytokines (IFN-γ, IL-2, and IL-4) in serum with respect to T cell interactions also increased significantly. It also induced macrophage phagocytosis, and nitric oxide (NO) production which resulted in a high degree of protection against Candida albicans and carbon clearance. Moreover, the enhancement in CD4 and CD8 cell populations as revealed by flow cytometry. Taken together this in vivo and ex vivo preclinical data, our results suggested that PB-EtAC acts as an effective immunostimulator eliciting both Th1 and Th2 immune responses. We are reporting first time the immunostimulatory potential of P. bambusoides and it might be regarded as a biological response modifier.
Keywords: haemagglutination antibody titre, delayed type hypersensitivity reaction, phagocytosis, nitric oxide, Candida albicans, flow cytometry
Introduction
Immunomodulation using medicinal plants can provide an alternative to conventional chemotherapy for a variety of diseases, especially when host defense mechanism has to be activated under the conditions of impaired immune response or when a selective immunosuppression is desired in situations like autoimmune disorders. In order to regulate the normal immunological functioning both immunostimulation and immunosuppression need to be tackled. Hence both immunosuppressing agents and immunostimulating agents have their own standing and search for better molecules exerting these activities is becoming the field of major interest all over the Earth (Patwardhan et al., 1990[22]).
Traditional Indian system of medicine like Siddha and Ayurveda have suggested means to increase the body’s natural resistance to disease. A large number of Indian medicinal plants and various ‘Rasayanas’ have been claimed to possess immunomodulatory activity (Atal et al., 1986[2]).
Phyllostachys bambusoides (bamboo) is a giant, woody grass distributed tropically and subtropically, and represents an important commodity. It is used as building material, food material, handicraft article and traditional medicine. It has mainly been used as a clinical Chinese traditional medicine to cure stomach-ache, diarrhoea or vomiting, chest diaphragm inflammation, restlessness and excessive thirst, and its efficacy had been recorded in the material medica of past dynasties in Chinese history (Zhang et al., 2004[34]). Recently, their potential health benefits and some biologically active components have been widely studied (Lu et al., 2005[19]). It is already reported to possess number of therapeutic uses including reduction in allergic response (Kim et al., 2012[17]), antioxidants (Mu et al., 2004[21]), antipyretic, analgesic as well as anticonvulsant (Kumar et al., 2011[18]). In recent time, focus on plant research has been intensified all over the world and a large number of evidence has been collected to show immense potential of medicinal plants used in various traditional system of medicine (Ponnuswamy and Devairrakam, 2011[23]). The principal nutrients thought to provide the protection afforded by leaves are antioxidants such as vitamin C, vitamin E, glycosides and flavonoids (including flavones, isoflavones, and anthocyanins). Convincing phytochemical research studies show that bamboo is a good source of flavonoids and glycosides that are a rich source of powerful antioxidants. The immune-stimulatory potential of P. bambusoides on immune system has not yet been explored. Therefore, the objective of the present study was evaluation of immunostimulatory potential of ethyl acetate fraction (PB-EtAC) from P. bambusoides against SRBC in BALB/c mice. In this attempt, the effects of PB-EtAC on humoral immunity keeping neutralizing antibodies in mind, cellular immune responses via delayed type hypersensitivity reaction, lymphocyte proliferation, macrophage phagocytosis, release of NO by the activated macrophages, cytokine profile and co-stimulatory molecules were investigated.
Materials and Methods
Materials
Ethyl acetate fraction (PB-EtAC) of alcoholic extract of the plant P. bambusoides was used in this study. The leaves of P. bambusoides were collected from the fields of University of Horticulture and Forestry, Nauni, Solan, India in July 2012. A voucher specimen (UHF/12530) has been deposited in the Herbarium Section of Department Forestry, Nauni University. Methanol was purchased from Qualigens, Mumbai, medium RPMI 1640 (Himedia, Bombay, India), 96 V wells microtitration plates and microtissue culture plates (96 U wells) from Tarson, trypan blue (Microlabs, Bombay), fetal calf serum (FCS) (Gibco, USA), Concanavalin-A (Con-A), lipopolysaccharide (LPS), gum acacia, dimethylsulphoxide (DMSO), penicillin, streptomycin, MTT (3-[(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide) and levamisole from Sigma were used.
Preparation of ethyl acetate fraction
The powdered plant material (750 g) was subjected to percolation process with 90 % methanol at room temperature. After exhaustive extraction, the methanolic extract (PB-EtAC) was concentrated under reduced pressure at 50–55 °C. Extract was adsorbed with silica and subjected to fractionation with various solvents like petroleum ether, chloroform and ethyl acetate. For pharmacological studies, a weighed amount of ethyl acetate fraction was suspended in a 1 % (w/v) aqueous acacia solution.
HPLC fingerprinting of the extract
HPLC fingerprinting of the PB-EtAC was developed as described earlier (Wang et al., 2012[30]). The separation was carried out on an Agilent 1200 series (USA), Eclips XBD C18 column, 4.6 × 150 mm, 5 µm particle sizes, and the temperature was maintained at 25 °C. Then, 25 µL of sample was injected into the column and eluted with a constant rate of 1.0 mL/min. HPLC-grade water with 0.5 % (v/v) glacial acetic acid and acetonitrile (85/15, v/v) were used as mobile phase in 85:15 ratio. The absorbance detector was operated at 345 nm.
Animals
The study was conducted on four to six week old male Balb/c mice (18-22 g). The ethical committee of the Indian Institute of Integrative Medicine (CSIR) instituted for animal handling approved all protocols. The animals were bred and maintained under standard laboratory conditions: temperature (25 ± 2 °C) and photoperiod of 12 h. Commercial pellet diet (Ashirwad Industries, Chandigarh, India) and water were given ad libitum. According to ethical regulations on animal research, all animals used in experimental work received human care.
Acute toxicity
Different doses (5-2000 mg/Kg, oral) of PB-EtAC were administered to groups of mice (6 in each group). The animals were continuously observed for 2 h to detect changes in the autonomic or behavioural responses. Mortality in each group was observed for 7 days. The doses of 50, 100 and 200 mg/Kg were selected based on the results of preliminary toxicity testing.
Immunization schedule
Sheep red blood cells (SRBC) were used as a source of T-dependent antigen. For this purpose, the blood was withdrawn from a healthy sheep in Alsever's solution (Alsever and Ainslie, 1941[1]). SRBC used for immunization were prepared in pyrogen-free normal saline. Mice were divided into five groups, each consisted of six animals. PB-EtAC at 50, 100 and 200 mg/Kg (in 200 µL of 1 % gum acacia) was administered orally by gavage for 14 days, daily. The dose volume was 0.2 mL. Control group received 1 % gum acacia. Levamisole, a known immune-stimulator reported to augment the antibody response (Tempero et al., 1995[26]), was given orally as positive control, at a dose of 2.5 mg/Kg body weight. All groups were immunized with 0.2 mL of SRBC (5×109) per mouse intra-peritoneal (i.p.) on day 0 of drug treatment.
Treatment
Animals were divided into five groups of six animals each: (Group I) normal control, received 1 % gum acacia; (Group II) positive control, received levamisole (2.5 mg/Kg body weight); (Group III) received PB-EtAC (50 mg/Kg body weight); (Group IV) received PB-EtAC (100 mg/Kg body weight); and (Group V) received PB-EtAC (200 mg/Kg body weight). Positive control, received levamisole and normal control mice received 1 % gum acacia administered per oral (p.o.). The PB-EtAC was dissolved in 1 % gum acacia and was administered per oral for 14 days and the dose volume was 0.2 mL.
Haemagglutination antibody (HA) titre
The animals were immunized by injecting 0.2 mL of 10 % of fresh SRBC suspension intraperitonially on day 0. Blood samples were collected in micro centrifuge tubes from individual animals by retro-orbital plexus on day 7 for primary antibody titre and day 14 for secondary antibody titre. Serum was separated and antibody levels were determined by the haemagglutination technique (Gupta et al., 2006[14]).
DTH reaction
PB-EtAC was administered 2 h after SRBC injection and once daily on consecutive days. Six days later, the thickness of the left hind footpad was measured with a spheromicrometer (pitch, 0.01 mm) and was considered as a control. The mice were then challenged by injecting 20 µL of 5×109 SRBC/mL intradermally into the left hind footpad. The foot thickness was measured again after 24 and 48 h (Bafna and Mishra, 2006[3]).
Splenocyte proliferation assay
Spleen collected under aseptic conditions in HBSS was minced using a pair of scissors and passed through a fine steel-mesh to obtain a homogeneous cell suspension and the erythrocytes were lysed with RBC lysis solution. After centrifugation (380 × g at 4 °C for 10 min), the pelleted cells were washed three times with PBS and re-suspended incomplete medium [RPMI 1640 supplemented with 12 mM HEPES (pH 7.1), 0.05 mM 2-mercaptoethanol, 100 IU/mL penicillin, 100 µg/mL streptomycin, and 10 % FCS]. Cell number was counted with a haemocytometer by the trypan blue dye exclusion technique. Cell viability exceeded 95 % (Wang and Li, 2002[31]).
To evaluate the effect of PB-EtAC on the proliferation of splenic lymphocytes, spleen cell suspension (2×106 cell/mL) was pipetted into 96 well plates (200 µL/well) and cultured at 37 °C for 72 h in a humid saturated atmosphere containing 5 % CO2 in the presence of Con-A (5 µg/mL) and LPS 10 µg/mL). After 72 h, 20 µL of MTT solution (5 mg/mL) were added to each well and incubated for 4 h. The plates were centrifuged (1400 × g, 5 min) and the untransformed MTT was removed carefully by pipetting. To each well, 100 µL of a DMSO working solution (192 µL DMSO with 8 µL 1 M HCl) was added, and the absorbance was evaluated in an ELISA reader at 570 nm after 15 min (Khajuria et al., 2008[16]).
Macrophage function assay (NO production)
Immunized mice were administered with plant extract for 14 days. On day 14 the mice were sacrificed and injected with 10 mL of ice cold IRPMI medium (i.p.). Macrophages were collected peritonially after lavaging the mice using the same syringe in centrifuge tubes. The tube was centrifuged at 1800 rpm for 10 min at 4 °C. The supernatant was discarded and the pellet was dissolved in RBC lysis buffer (2 mL), incubating the tube in ice for 5 min. The tube was centrifuged again for 10 min and the pellet was dissolved in 1 mL of RPMI media. The viaibility of cells was checked in trypan blue. The cells were cultured in the U-bottomed 96 well plates. The plate was incubated in CO2 incubator for 24 h. After 24 h the plate was centrifuged for 10 min at 2000 rpm. 100 µL of supernatant was collected from the plates and poured it in another plate in the same fashion. 100 µL of Griss reagent III (Griss reagent I & Griss II in 1:1 ratio) was added to the supernatant of each well. Thereafter 100 µL of DMSO was added in each well of the standard plate containing the pellet and was read at 540 nm (Green et al., 1982[13]; Ganju et al., 2007[10]).
In vivo carbon clearance and Candida albicans clearance
Phagocytic function of the RES (reticulo-endothelial system) was assayed in groups of 5 mice each by injecting i.v. 160 mg/Kg body weight of 1.6 % suspension of gelatin stabilized carbon particles of 20-25 µm size (Hudson and Hay, 1980[15]). Blood samples were collected from the retro-orbital plexus immediately before and at various intervals between 0 and 60 min after carbon injection. An aliquot (10 µL) of blood samples was lysed with 2 mL of 0.1 % acetic acid and transparency determined spectrophotometrically at a wavelength of 675 nm (Uvikon 810, spectrophotometer, Kontron Ltd., Switzerland) until transparency equivalent to standard (original pre-injection blood sample) was obtained as per method described by Atal et al. (1986[2]).
The peritoneal macrophages of PB-EtAC (50, 100 and 200 mg/Kg) treated mice were harvested by flushing the cavity with 5 mL of RPMI-1640 medium, pelleted by centrifugation at 1100 rpm for 10 min, re-suspended in RPMI-1640 containing 10 % heat activated fetal bovine serum, 4 mM glutamine, 100 units/mL penicillin, 100 µg/mL streptomycin and 100 mM sodium pyruvate. The viable cell suspension (2×106 cells/mL) was allowed to adhere to glass slides for 2 h at 37 °C in a humidified CO2 incubator. The glass slides were washed thoroughly to remove non-adherent cells. 100 µL of opsonized Candida cells (100 °, 30 min) was then spread over the adherent cells. The slides were incubated for 15 min in a humidified CO2 incubator and stained with trypan–eosin after thorough washing with PBS to determine the adherent cells containing yeast cells microscopically. The number of yeast cells ingested per macrophage was taken as the phagocytic index (Freidlin et al., 1988[9]).
Determination of IFN-γ, IL-2 and IL-4 by the ELISA method
Serum was collected 4 h after final oral administration of the alcoholic extract of PB-EtAC. The IFN-γ, IL-2 and IL-4 were measured with an enzyme linked immuno-sorbent assay (ELISA kit, R&D Systems Quantikine) according to the instructions of the manufacturer (Farrar and Schreiber, 1983[8]; Beutler and Cerami, 1989[5]).
Lymphocyte phenotyping in spleen
The spleen (1/3 of the organ) was placed in PBS buffer (without Mg2+ and Ca2+) and stored on ice prior to preparation of single cell suspension. Splenic erythrocytes were lysed with red blood cell lysis buffer (BD Pharmingen). Cell suspensions were refrigerated (4 °C) pending staining with antibodies. All reagents were purchased from BD Pharmingen. For each sample, 2×106 cells were stained with conjugated anti-CD8 FITC and anti-CD4 PE antibodies. After staining with antibodies, cells were washed and re-suspended in PBS for flow cytometric analysis which was performed on a FACS Calibur flow cytometer equipped with Cell Quest software (Becton Dickinson) (Gupta et al., 2006[14]).
Statistics
Data were expressed as mean ± SEM, and statistical analysis was carried out using one-way ANOVA (Bonferroni correction multiple comparison test). All in ex vivo experiments were carried out in triplicates and represented as mean ± SEM. Dunnett's test was used to analyze the different variables in the same subject, and p values <0.05 have been taken as statistically significant.
Results
HPLC fingerprinting of PB-EtAC
The HPLC chromatogram of PB-EtAC is shown in Figure (Fig. 1)1. It showed 10 peaks with varying retention times (2.44, 3.63, 4.27, 6.27, 9.60, 11.92, 13.63, 18.03, 27.08 and 34.43) including the presence of isoorientin (11.92); orientin (13.63) and isovitexin (27.08).
Figure 1. HPLC fingerprinting of PB-EtAC showing presence of isoorientin (11.92); orientin (13.63) and isovitexin (27.08) in different Rt. The separation was carried out Eclips XBD C18 column, 4.6 × 150 mm, 5 µm particle size, and the temperature was maintained at 25 °C.

Effect of PB-EtAC on anti-SRBC antibody titre
Anti-SRBC antibody (IgM and IgG) titres were measured in mice sera of different groups, collected retro-orbitally on 7 and 14 days after immunization and treatment. Anti-SRBC antibody titres increased in mice treated with three doses of PB-EtAC (50, 100 and 200 mg/Kg) after seven days when compared with control. A similar profile was obtained after 14 days, with IgG predominating over IgM (Figure 2(Fig. 2)). The maximum effect was observed at 200 mg/Kg in both primary and secondary antibody titre (p<0.01). Further decrease in dose (50 mg/Kg) showed a decreased response. Administration of levamisole (2.5 mg/Kg, p.o.), was used as a positive control resulted in a significant increase in the humoral antibody titre compared with the control animals.
Figure 2. Antibody titres (IgM and IgG) in mice sera were measured on day 7 and day 14 after immunization. Data are mean ± S.E. of six animals. *P<0.05 and **P<0.01 compared with control group determined by one-way ANOVA (Bonferroni correction multiple comparison test). For experimental details refer to Materials and methods section.
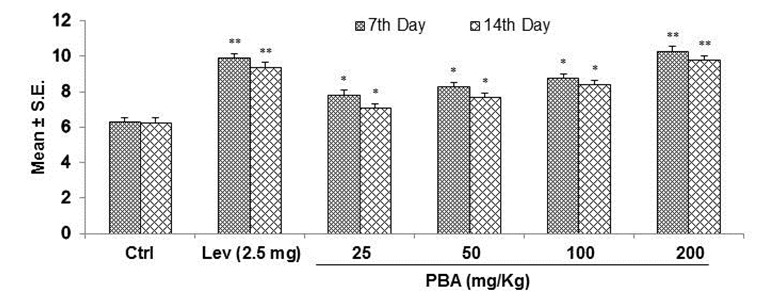
Effect of PB-EtAC on delayed type hypersensitivity (DTH) reaction in mice
In order to assess the cell-mediated immune response, DTH reaction to SRBC was measured as given in Figure 3(Fig. 3), in which data are expressed in terms of the swelling of the footpad. After administration of the PB-EtAC (50–200 mg/Kg, p.o.), a significant increase (p<0.01) in footpad thickness was found at 24 and 48 h as compared with the control group: maximum increase being observed at 200 mg/Kg. Further decrease in dose (50 mg/Kg) showed a decreased response.
Figure 3. DTH response was determined in SRBC immunized, PB-EtAC treated mice at 24 and 48 h after antigen challenge. Data are mean ± S.E. of six animals. *P<0.05 and **P<0.01 compared with control group determined by one-way ANOVA (Bonferroni correction multiple comparison test). For experimental details refer to Materials and methods section.
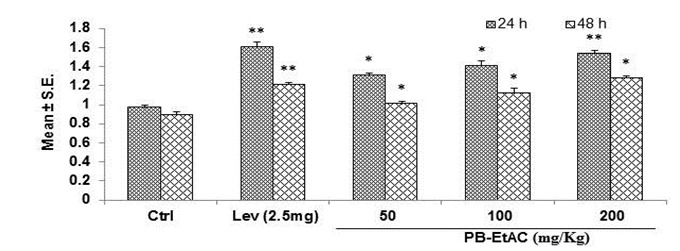
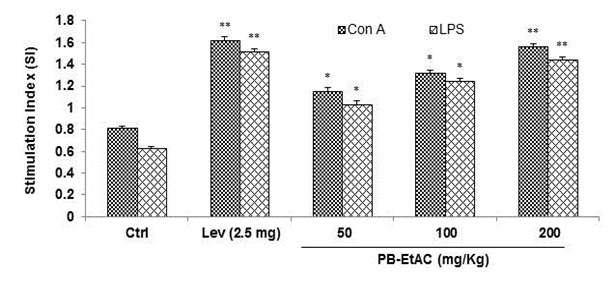
Effect of PB-EtAC on splenocyte proliferation assay
The effect of PB-EtAC on Con-A and LPS-stimulated splenocyte proliferation in immunized mice is shown in Figure 4(Fig. 4). Lymphocyte proliferation was studied by MTT assay. PB-EtAC caused profound lymphocyte activation which triggered significant (P<0.01) and concentration-dependent proliferation of naive murine splenocytes. Con-A (5 µg/mL) and LPS (10 µg/ mL) stimulated splenocyte proliferation was significantly enhanced by PB-EtAC with the maximum effect at 200 mg/Kg dose and this cellular proliferation was increased up to two fold in Con-A and LPS treated cells respectively, compared to the control. Further decrease in dose (50 mg/Kg) showed a decreased response.
Figure 4. Influence of PB-EtAC (50–200 mg/Kg) on proliferation of T and B lymphocytes ex vivo. Mice were exposed to graded doses of PB-EtAC p.o. daily for 14 days. Control mice received the vehicle only. Splenocytes were isolated and stimulated with sub-optimal amounts of mitogens; Con A (5 µg/mL), LPS (10 µg/mL) for T and B cell proliferation, respectively. Cells were incubated for 72 h and proliferation was measured by MTT reduction assay. Splenocyte proliferation is expressed as the stimulation index (SI). Data are mean ± S.E. of six animals. *P<0.05 and **P<0.01 compared with control group determined by one-way ANOVA (Bonferroni correction multiple comparison test). For experimental details refer to Materials and methods section.
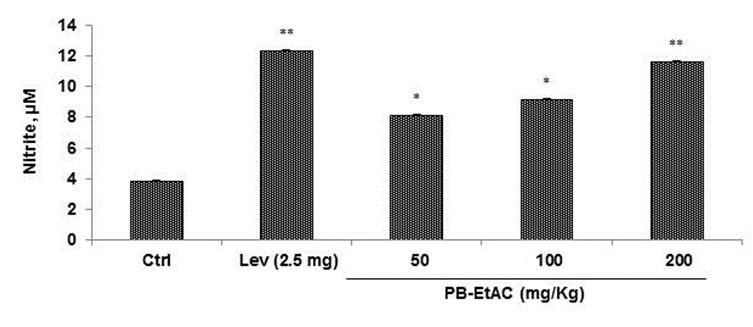
Effect of PB-EtAC on macrophage function assay (NO production)
The effect of PB-EtAC on macrophage function was assessed by measuring the amount of NO produced from peritoneal macrophages of PB-EtAC treated mice. Griess reagent was used to measure the nitrite levels, the stable end-product of NO metabolism. Macrophages were cultured in RPMI-FBS (10 %) with LPS (1 µg/mL) and contents of nitrite was measured in the supernatants. The nitrite concentration was determined by extrapolation from a sodium nitrite standard curve and the results are expressed in µM. The effect of PB-EtAC (50, 100 and 200 mg/Kg) on nitric oxide production is shown in Figure 5(Fig. 5). Increasing doses of PB-EtAC (50–200 mg/Kg) significantly enhanced the nitrite content in peritoneal macrophages. The maximum effect was observed at 200 mg/Kg dose compared with the control group.
Figure 5. Effect of PB-EtAC (50-200 mg/Kg) on nitrite content in macrophages. Peritoneal macrophages were isolated from mice given graded doses of PB-EtAC for 14 days. Macrophages (3×106 cells/well) were cultured and stimulated with LPS (1 µg/mL) for 48 h. The supernatants were used for nitrite assay using Griess reagent. Results are expressed in µM. Data are mean ± S.E. of six animals. *P<0.05 and **P<0.01 compared with control group determined by one-way ANOVA (Bonferroni correction multiple comparison test). For experimental details refer to Materials and methods section.
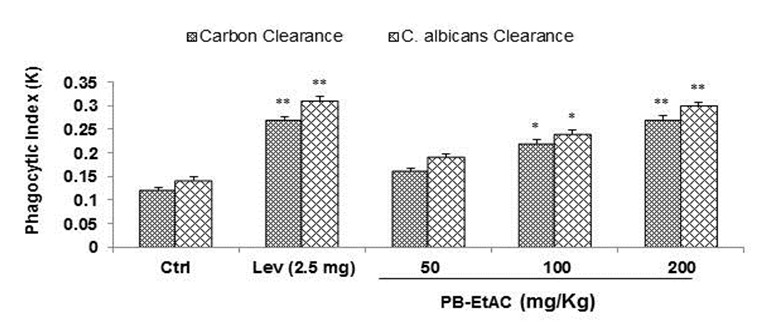
Effect of PB-EtAC on carbon clearance and C. albicans clearance test
Oral administration of PB-EtAC (50–200 mg/Kg) for 5 days and 30 min prior to injection of colloidal carbon increased the clearance rate of carbon particles from mouse reticulo-endothelial system. A sig-nificant increase in phagocytic index was obtained at all dose levels (Figure 6(Fig. 6)). Candida cells were eliminated within 0.5 h from the peritoneal cavity of mice treated with PB-EtAC against 4 h in control mice. There was a significant clearance of C. albicans in PB-EtAC treated mice as compared with control mice.
Figure 6. Effect of PB-EtAC on carbon clearance and Candida albicans clearance in mice. Phagocytic function of the PB-EtAC-treated macrophages was assayed in groups of 5 mice. The rate of carbon clearance and Candida albicans clearance, termed hereafter in the text as phagocytic index (K), was calculated from the slope of each time concentration curve drawn by plotting 100-mean transmittance values as ordinate in a semi logarithmic paper against time as abscissa. Values are means ± SE (n=4); *P<0.05 and **P<0.01 (control vs. PB-EtAC-treated groups; one-way ANOVA followed by Bonferroni multiple comparison test). For experimental details refer to Materials and methods section.
Effect of PB-EtAC on IFN-γ, IL-2 and IL-4 in mice sera by ELISA
In order to establish that Th1 and Th2 cytokines were involved in the immunostimulatory activity of PB-EtAC, cytokine secretion patterns were analyzed in the sera of immunized mice. PB-EtAC caused a significant (P<0.01) dose dependent up-regulation of the Th1 (IFN-γ, IL-2) and Th2 (IL-4) cytokines at the doses of 50, 100 and 200 mg/Kg: maximum response being at 200 mg/Kg dose compared with the control group (Figure 7a-c(Fig. 7)). Further decrease in the dose (50 mg/Kg) showed a decreased response.
Figure 7. (a) Effect of PB-EtAC on IFN-γ production in mice sera. Serum was collected 4 h after the final oral administration of PB-EtAC (50, 100 and 200 mg/Kg). IFN-γ concentration was measured by enzyme-linked immunosorbent assay (ELISA kit, BD Opt EIA set) according to the instructions of the manufacturer. (b) Effect of PB-EtAC on IL-2 production in mice sera. Serum was collected 4 h after the final oral administration of PB-EtAC (50, 100 and 200 mg/Kg). IL-2 concentration was measured by enzyme-linked immunosorbent assay (ELISA kit, BD Opt EIA set) according to the instructions of the manufacturer. (c) Effect of PB-EtAC on IL-4 production in mice sera. Serum was collected 4 h after the final oral administration of PB-EtAC (50, 100 and 200 mg/Kg). IL-4 concentration was measured by enzyme-linked immunosorbent assay (ELISA kit, BD Opt EIA set) according to the instructions of the manufacturer.
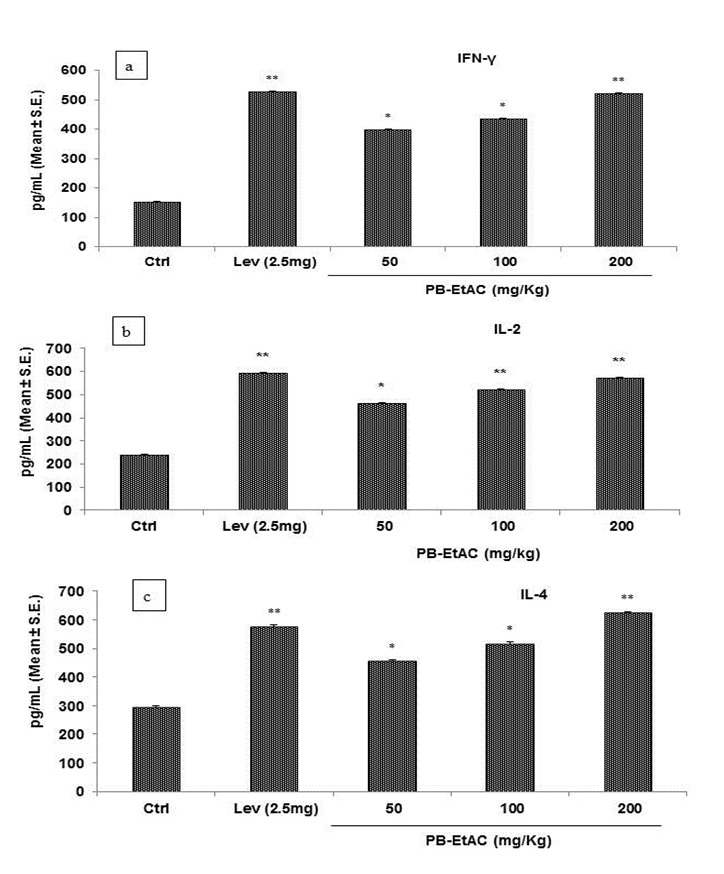
Data are mean ± S.E. of six animals. *P<0.05 and **P<0.01 compared with control group determined by one-way ANOVA (Bonferroni correction multiple comparison test). For experimental details refer to Materials and methods section.
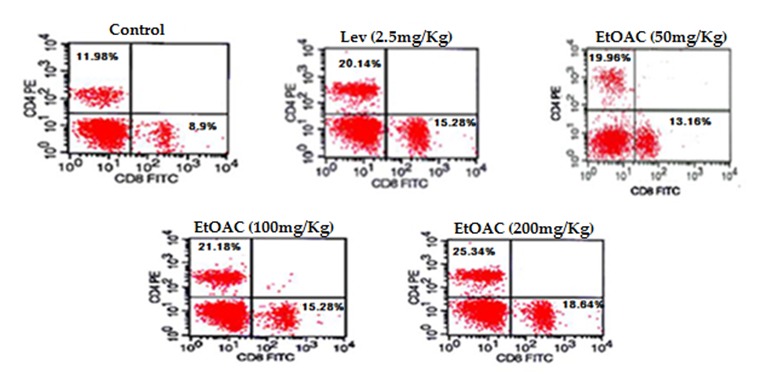
Effect of PB-EtAC on lymphocyte phenotyping in spleen
The effect of PB-EtAC on the population of cell surface markers like CD4 and CD8 populations was determined in splenocytes prepared from spleen of mice collected after 14 days of oral treatment of PB-EtAC. Results are presented in Figure 8(Fig. 8). PB-EtAC caused a significant (p<0.01) dose dependent increase in both CD4 and CD8 population at the doses of 100 and 200 mg/Kg: maximum response being at 200 mg/Kg dose compared with the control group.
Figure 8. Flow cytometric analysis of the expression of co-stimulatory signal molecules in spleen-derived macrophages. To quantify the expression of co-stimulatory molecules, 2×106 macrophages were stained with FITC-labeled anti-CD8 and PE-labelled anti-CD4 mAbs. Other conditions were the same as described in Materials and methods section. Data represented by percent CD8/CD4 positive cell populations of six animals.
Discussion
Bamboo has been used as a food material and the primary role of food is to provide sufficient nutrients to meet metabolic requirements or to modulate various physiological functions that may play beneficial roles in some diseases (Granato et al., 2010[12]). To the best of our knowledge, this study is the first to show the immunostimulatory potential of bamboo. Traditionally a large number of plants used in medicine have been shown to possess immunomodulating activities (Choi et al., 2004[6]) and are being extensively explored for their potential in the treatment and prevention of chronic disease. Immunomodulatory agents of animal and plant origin increase the immune responsiveness of the body against pathogen by activating immune competent cells (Sharma et al., 2012[24]). There is strong requirement of the drugs which can boost immune system to combat the immunosuppressive consequences caused by stress, chronic diseases like tuberculosis, conditions of impaired immune responsiveness (e.g. AIDS), etc. (Wagner, 1999[29]). One of the most well-studied plant species in terms of medicinal plant is P. bambusoides, found widely in Asian countries including India, Korea, China and Japan from which the largest number of compounds (flavonoids, glycosides, saponins, tannins, proteins, carbohydrates, etc.) has been identified (Tomar et al., 2012[27]).
In the present study, immunomodulatory potential of PB-EtAC was explored extensively on the modulation of both T and B cells in relation to serum immunoglobulins IgM and IgG to T-dependent antigen SRBC. Primarily, the antibody response to SRBC was observed by the haemagglutination titre. The augmentation of humoral antibody response to T-dependent antigen (SRBC) reveals the increased responsiveness of macrophages since the antibody production was closely associated with the co-operation of macrophages, T and B lymphocyte responsiveness (Benacerraf, 1978[4]). The T cells in turn participate in the expression of cell mediated immunity contributing to DTH. A DTH reaction is an expression of cell-mediated immunity and plays a role in many inflammatory disorders (Gongora et al., 2000)[11]. Treatment with PB-EtAC enhanced the DTH reaction, as reflected by the increased footpad thickness compared to the control group, suggesting heightened infiltration of macrophages to the inflammatory site. Moreover, PB-EtAC stimulated phagocytosis and augmented Con-A and LPS induced splenocyte proliferation.
Cytokines are major factors involved in regulation of the immune response to antigens and infectious agents. Th1 cells are able to produce IL-2 and IFN-γ whereas Th2 cells can produce IL-4 (Villard et al., 1999[28]). The augmentation of T and B cells with PB-EtAC may be due to a cytokine-mediated mechanism. Since IFN-γ is up regulated in PB-EtAC treated groups, it is an important immunoregulatory molecule which protects against viral infections, induces the generation of T cells, activates macrophages and regulates crossly Th1 and Th2 cells. IFN-γ can enhance immunoregulatory ability. IL-4, known to activate monocytes or macrophages, is produced by T helper (Th2) cells or mast cells. Investigation of the balance of Th1 and Th2 cytokine production should be helpful to understanding the outcomes of different immune responses and are clinically useful in treating immunologically deregulated states (Fang et al., 2005[7]). PB-EtAC upregulated the production of IL-4 with a significant release of IFN-γ and IL-2, thus regulating the Th1 and Th2 balance.
Macrophages are important cells for the immune system which play an important role in host defense mechanisms for protection from microbial invaders. When macrophages are stimulated with foreign substances, a variety of cytokines and chemicals are released to induce fundamental defense systems. Among them, TNF-α is a representative cytokine secreted by macrophages that plays a key role in the cytokine network, e.g. T cell and NK cell activation (Tanaka et al., 1999[25]). Also, TNF-α is a major pro-inflammatory cytokine characteristically produced at sites of inflammation by macrophages and is considered to help in eliminating certain invaders. Its levels in plasma are directly correlated with the ability of phagocytes to generate superoxide and to increment the activity of iNOS and, thus, NO levels (Miesel et al., 1996[20]). Furthermore, TNF-α produced by activated macrophages, enhances the cytotoxic action of macrophages. We observed that PB-EtAC strongly augmented the release of nitric oxide from macrophages. Our results thus suggested that PB-EtAC is capable of stimulating immune functions of macrophages through increase in NO production. The exact mechanism by which PB-EtAC stimulates the macrophages and their subsequent NO production could be due to macrophage specific TNF-α release, which plays a dominant role in the augmentation of macrophage function. Moreover, PB-EtAC showed a significant increase in the phagocytic activity of macrophages against Candida.
To further elucidate the mechanism of PB-EtAC as an immunomodulator, the effects of PB-EtAC on both CD4+ and CD8+ spleen T lymphocytes populations in SRBC immunized BALB/c mice were analyzed by flow cytometric assay. T cells differentiate into two different subsets according to their specific membrane molecule, that is CD4+ and CD8+ T lymphocytes, which play different roles in immunomodulation. It is well known that Th cells and cytotoxic T lymphocytes (CTLs) responses are associated with the enhancement of CD4+ and CD8+ T-lymphocytes respectively. Th cells can promote proliferation, maturation, and immunologic function of other cell types, and specific lymphokines secreted by Th cells are very important for the activities of B cells, macrophages, and CTLs (Zhang et al., 2009[33]). In contrast, CTLs are extremely important in the defense against viral infections. The results of present investigation revealed that the percentage of CD4+ and CD8+ T lymphocytes in SRBC-immunized mice were greatly augmented by PB-EtAC. The enhanced percentages of both CD4+ and CD8+ T lymphocytes in whole blood indicated that both Th and CTLs were activated greatly by PB-EtAC. This report is the first to show the immunostimulatory potential of P. bambusoides. It provides the baseline information regarding the potential use of this plant species as an immune-nutrition supplement. Therefore, it could be a drug of choice, effective in treating the diseases where the underlying defect is a T-cell and B-cell deficiency or phagocytic dysfunction.
References
- 1.Alsever JB, Ainslie RB. A new method for the preparation of dilute blood plasma and the operation of a complete transfusion service. NY Stat J Med. 1941;41:126–131. [Google Scholar]
- 2.Atal CK, Sharma ML, Kaul A, Khajuria A. Immunomodulating agents of plant origin. I: Preliminary screening. J Ethnopharmacol. 1986;18:133–141. doi: 10.1016/0378-8741(86)90025-5. [DOI] [PubMed] [Google Scholar]
- 3.Bafna AR, Mishra SH. Protective effect of bioactive fraction of Sphaeranthus indicus Linn against cyclophosphamide induced suppression of humoral immunity in mice. J Ethnopharmacol. 2006;104:426–429. doi: 10.1016/j.jep.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 4.Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978;120:1809–1812. [PubMed] [Google Scholar]
- 5.Beutler B, Cerami A. The biology of cachectin/TNF - a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 6.Choi EM, Koo JK, Hwang JK. Immune cell stimulating activity of mucopolysaccharide isolated from yam (Dioscoria batatasi) J Ethnopharmacol. 2004;91:1–6. doi: 10.1016/j.jep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Fang SP, Tanaka T, Tago F, Okamoto T, Kojima S. Immunomodulatory effects of Gyokuheifusan on IFN-γ/IL-4 (Th1/Th2) balance in ovalbumin (ova) induced asthma model mice. Biol Pharm Bull. 2005;28:829–833. doi: 10.1248/bpb.28.829. [DOI] [PubMed] [Google Scholar]
- 8.Farrar MA, Schreiber RD. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 9.Freidlin IS, Artemenko NK, Freidlin TS, Shcherbak IG, Danilevskii YO, Kuzmin VI, et al. Activation of macrophages by a synthetic antioxidant. Bull Exp Biol Med. 1988;105:72–75. [PubMed] [Google Scholar]
- 10.Ganju L, Karan D, Chanda S, Srivastava KK, Sawhney RC, Selvamurthy W. Immunomodulatory effects of agents of plant origin. Biomed Pharmacother. 2007;57:296–300. doi: 10.1016/s0753-3322(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 11.Gongora L, Manez S, Rosa M, Giner RM, Recio MC, Rios JL. On the activity of trifluoperazine and palmitoylcarnitine in mice: delayed hypersensitivity models. Life Sci. 2000;66:183–188. doi: 10.1016/s0024-3205(00)00447-1. [DOI] [PubMed] [Google Scholar]
- 12.Granato D, Branco GF, Nazzaro F, Cruz AG, Faria JAF. Functional foods and non dairy probiotic food development: trends, concepts and products. Compr Rev Food Sci Food Saf. 2010;9:292–302. doi: 10.1111/j.1541-4337.2010.00110.x. [DOI] [PubMed] [Google Scholar]
- 13.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Khajuria A, Singh J, Bedi KL, Satti NK, Dutt P, et al. Immunomodulatory activity of biopolymeric fraction RLJ-NE-205 from Picrorhiza kurroa. Int Immunopharmacol. 2006;6:1543–1549. doi: 10.1016/j.intimp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Hudson L, Hay FC, editors. Practical immunology. 2nd ed. London: Blackwell; 1980. [Google Scholar]
- 16.Khajuria A, Gupta A, Suden P, Singh S, Malik F, Singh J, et al. Immunomodulatory activity of biopolymeric fraction BOS. 2000 from Boswellia serrata. Phytother Res. 2008;22:340–348. doi: 10.1002/ptr.2320. [DOI] [PubMed] [Google Scholar]
- 17.Kim L, Potts R, Eeuwes C, Dominic A, Nevis I, Kim HL. Measured depth of subcutaneous tissue on posterolateral arm of aeroallergen immunotherapy patients. Allergy Asthma Clin Immunol. 2012;8:A7. [Google Scholar]
- 18.Kumar S, Sharma G, Sharma A, George M, Joseph L. Anticonvulsant activity of alcoholic extract of Phyllostachys bambusoide. Int J Pharm Pharm Sci. 2011;3:125–127. [Google Scholar]
- 19.Lu B, Wu X, Tie X, Zhang Y, Zhang Y. Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem Toxicol. 2005;43:783–92. doi: 10.1016/j.fct.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Miesel R, Kurpisz M, Kroeger H. Suppression of inflammatory arthritis by simultaneous inhibition of nitric oxide synthase and NADPH oxidase. Free Radic Biol Med. 1996;20:75–81. doi: 10.1016/0891-5849(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 21.Mu J, Uehara T, Li J, Furuno T. Identification and evaluation of antioxidant activities of bamboo extracts. Forest Stud China. 2004;6:1–5. [Google Scholar]
- 22.Patwardhan B, Kalbag D, Patki PS, Nagsampagi BA. Search of immunomodulatory agents: a review. Ind Drugs. 1990;28:348–358. [Google Scholar]
- 23.Ponnuswamy S, Devairrakam WJEG. Comparative study of primary metabolites in different plant parts of Clitoria ternatea Linn. J Chem Pharm Res. 2011;3:614–617. [Google Scholar]
- 24.Sharma U, Bala M, Kumar N, Singh B, Munish RK, Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J Ethnopharmacol. 2012;141:918–926. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka T, Sugiura H, Inaba R, Nishikawa A, Murakami A, Koshimizu K, et al. Immunomodulatory action of citrus auraptene on macrophage functions and cytokine production of lymphocytes in female BALB/mice. Carcinogenesis. 1999;20:1471–1476. doi: 10.1093/carcin/20.8.1471. [DOI] [PubMed] [Google Scholar]
- 26.Tempero MA, Haga Y, Sivinsk C, Birt D, Klassen L, Thiele G. Immunologic effects of levamisole in mice and humans: evidence for augmented antibody response without modulation of cellular cytotoxicity. J Immunother. 1995;17:47–57. [PubMed] [Google Scholar]
- 27.Tomar SK, Sharma G, George M. Skeletal muscle relaxant activity of alcoholic extract of Phyllostachys bambusoides in Wistar rats. Int J Pharm Pharm Sci. 2012;4:370–373. [Google Scholar]
- 28.Villard JF, Pellegrin JL, Ranchin V, Schaeverbeke T, Dehais J, Longy-Boursier M, et al. Th1 (IL-2, interferon-gamma (IFN-γ)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1999;115:189–195. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner H. Immunomodulatory agents from plants. 1st ed. Basel: Birkhäuser; 1999. [Google Scholar]
- 30.Wang J, Yue Y, Jiang H, Tang F. Rapid screening for flavone C-glycosides in the leaves of different species of bamboo and simultaneous quantitation of four marker compounds by HPLC-UV/DAD. Int J Anal Chem. 2012;2012:205101. doi: 10.1155/2012/205101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YP, Li XY. Effect of astragaloside IV on T, B lymphocyte proliferation and peritoneal macrophage function in mice. Acta Pharmacol Sin. 2002;3:263–266. [PubMed] [Google Scholar]
- 32.Wu D, Chen J, Lu B, Xiong L, He Y, Zhang Y. Application of near infrared spectroscopy for the rapid determination of antioxidant activity of bamboo leaf extract. Food Chem. 2012;135:2147–2156. doi: 10.1016/j.foodchem.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Zhang H, Zhao J. The role of CD4 T cell help for CD8 CTL activation. Biochem Biophys Res Commun. 2009;384:405–408. doi: 10.1016/j.bbrc.2009.04.134. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Wu X, Ren Y, Fu J, Zhang Y. Safety evaluation of a triterpenoid-rich extract from bamboo shavings. Food Chem Toxicol. 2004;42:1867–1875. doi: 10.1016/j.fct.2004.07.005. [DOI] [PubMed] [Google Scholar]


