Abstract
Synthetic 3-hydroxyflavone analogues (JY-1, JY-2, JY-3, JY-4), were tested for antidiabetic activity in high-fructose-diet-fed (66 %, for 6 weeks) insulin-resistant Wistar rats (FD-fed rats). The fasting blood glucose, insulin, creatinine and AGEs were decreased to near normal upon treatment with test compounds. Insulin resistance markers such as HOMA-IR, K-ITT, plasma triglycerides, lipids, endogenous antioxidant defense and glycogen were restored in FD-fed rats after treatment with 3-hydroxyflavones. It is known that insulin resistance is partly because of oxidative stress and hence antioxidant activity was determined. They exhibited significant in vitro DPPH and ABTS radical scavenging activity (IC50: 10.66-66.63 µM). Test compounds inhibited ROS and NO production in RAW 264.7 cells (IC50: 10.39–42.63 µM) and they were found as potent as quercetin. Further, the test compounds inhibited lipid peroxidation at low concentrations (IC50: 99.61-217.47 µM). All test compounds at concentrations 100-200 µM protected calf thymus DNA-damage by Fenton reaction. In addition, test compounds inhibited protein glycation in different in vitro antiglycation assays. JY-2 showed maximum potency in all the stages of glycation which was comparable to the standard quercetin and aminoguanidine. Test compounds also enhanced the glucose uptake by L6 myotubes at an EC50 much lower than that of quercetin. Thus the synthetic 3-hydroxyflavones were found to have good antidiabetic activity by pleotropic and multimodal suppression of insulin resistance and enhancement of glucose uptake by skeletal muscles. These compounds are non-toxic at the doses tested. Further, the combined antioxidant and antiglycation activities of these molecules have complementary benefits in management of diabetes.
Keywords: 3-hydroxyflavone, high-fructose-fed rats, insulin resistance, antioxidant, antiglycation, glucose uptake
Introduction
Flavonoids are widely distributed in plants and identified as potential leads in antidiabetic drug discovery (Mukherjee et al., 2006[24]). Flavonoids exhibit antidiabetic action by multimodal mechanisms and also influence the pleiotropic mechanisms involved in insulin signaling (Cazarolli et al., 2008[7]). Flavonoids, owing to their potent antioxidant activity, can strongly inhibit the formation of glycosylation end products (AGEs) and also counteract the contribution of oxidative stress to disease progression (Wu and Yen, 2005[41]).
Oxidative stress is one of the major causes for disease burden in diabetes and related metabolic disorders and antioxidant supplementation is proven to be beneficial (Houstis et al., 2006[14]; Rahimi et al., 2005[27]). Reactive oxygen species (ROS) and reactive nitrogen species (RNS) cause DNA damage and peroxidation of membrane lipids. Superoxide (O2•-) radicals generated during glucose-oxidation reacts with nitric oxide (NO) released by endothelium and macrophages forming highly reactive peroxynitrite radical (ONOO-), and eventually aggravating the inflammatory responses leading to diabetic micro and macrovascular complications (Son, 2012[33]).
In diabetes, increased free radical generation leads to protein cross-linking, protein glycosylation and generation of AGEs. Increased ROS and reactive carbonyl species (RCS) such as 3-deoxyglucosone, glyoxal, and methylglyoxal (MGO), are critical intermediates formed during protein glycation. The end product of Amadori rearrangement in aldemine linkage between glucose and protein is called fructosamine (1-amino-1-deoxyfructose/isoglucosamine). All the above mentioned intermediates are identified as the important precursors of AGEs in diabetes (Goldin et al., 2006[12]). Further, AGEs can bind to cell membrane receptor called RAGE (receptor for AGEs) and mediate the release of a series of inflammatory mediators and growth factors. Reports suggest that AGE-RAGE is the predominant mechanism in pathogenesis of nephropathy and retinopathy (Ramasamy et al., 2011[28]). Hence, antidiabetic molecules with antioxidant and antiglycation activity will have additional benefits over currently available antidiabetic drugs.
Quercetin, one among the most potent flavonoids from natural sources studied for antidiabetic activity, acts by multiple mechanisms and has a potential to emerge as a nutraceutical (Boots et al., 2008[5]). Our lab previously reported that the plant extract Dodonaea viscosa (Family: Sapindaceae) areolar parts showed antidiabetic activity in high-fat low-dose streptozotocin (STZ) rats (Veerapur et al., 2010[39]) and in high-fructose induced insulin resistance in rats (Veerapur et al., 2010[40]). The major constituent responsible for the antidiabetic activity of the plant extract, Dodonaea viscosa was a flavonoid, quercetin.
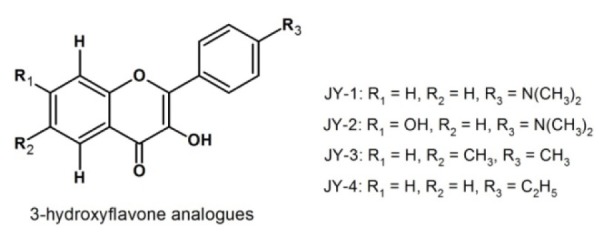
Though, plant flavonoids are available in plenty, isolation and purification is a major hurdle (Havsteen, 2002[13]). Further, structural diversity of the flavonoid nucleus offers scope to generate synthetic derivatives with enhanced biological activity and bioavailability. In view of these, we synthesized few 3-hydroxflavone analogues by standard procedure (Jayashree et al., 2010[17]) and selected four promising analogues (namely, JY-1, JY-2, JY-3 and JY-4; Figure 1(Fig. 1)), for evaluation of their antidiabetic activity.
Figure 1. Structures of 3-hydroxyflavone analogues.
Materials and Methods
Drugs and chemicals
2,2'-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethylbenzothiazoline-6-sul-phonic acid) (ABTS), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), calf thymus DNA, quercetin, deoxy-D-ribose, thiobarbituric acid (TBA), Tris-Acetate-EDTA (TAE) buffer, agarose gel, MGO, aminoguanidine hemisulfate (AG), Trypan blue, lipopolysaccharide (LPS) from E. coli serotype 0111:B4, 2',7'-dichlorodihydrofluorescin diacetate (DCFH-DA), NG-nitro-L-arginine methyl ester (L-NAME), diphenylene iodonium (DPI), Dulbeccos’ Modified Eagle Medium (DMEM) for cell culture, bovine serum albumin (BSA) 5,5'-dithio-bis-2-nitrobenzoic acid (DTNB, Ellman’s reagent), 1-chloro-2,4-dinitrobenzene (CDNB), reduced glutathione (GSH) and superoxide dismutase (SOD) were purchased from Sigma-Aldrich, USA. Fetal Calf Serum was purchased from Invitrogen, USA. Gentamicin was purchased from Abbott Healthcare Pvt. Ltd, Mumbai, India. Carboxymethyl cellulose (CMC), haemoglobin, nitroblue tetrazolium (NBT), fructose, anthrone and oyster-glycogen were purchased from Himedia, Mumbai. Pioglitazone was a gift from Hetero Drugs, Hyderabad, India. Other reagents were purchased from standard chemical suppliers. The test compounds, quercetin and pioglitazone were suspended in 0.5 % CMC before administration to the animals.
Animals
Male Wistar rats (7 to 8 week; 150–180 g), maintained in sanitized polypropylene cages (3 per cage) in air conditioned rooms (23±2 °C, 35–60 % humidity with 12 light–dark cycle) were obtained from the central animal facilities of Manipal University. The rats were fed with standard rat pellet diet (Hindustan Lever Ltd., Mumbai, India) and water ad libitum. Prior approval was obtained from the Institutional Animal Ethical Committee (IAEC) (letter No. IAEC/KMC/ 06/2006–2007) and the experiments were conducted according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India.
Acute oral toxicity and dose optimization study of 3-hydroxyflavone analogues
Acute oral toxicity study was carried out as per the OECD guidelines TG-425. As flavonoids are known antioxidants with biological activity, we chose test compounds for a detailed antioxidant activity using the antioxidant biomarker ferric reducing ability of plasma (FRAP). Results were used to calibrate and optimize the dose (Benzie and Strain, 1996[3]). Five male Wistar rats weighing 150-180 g, in each group were administered 10, 20, 40, 80 and 160 mg/kg/day, p.o., doses of synthesized test compounds for seven days. On the last day, 2 h after ingestion of test compounds, blood was collected by retro-orbital veni-puncture and the plasma separated to determine FRAP by the standard procedure using TPTZ-reagent. The FRAP values were obtained in triplicate and expressed as millimoles of ferric ions reduced to ferrous ions per ml of plasma. Control (treated with vehicle) and quercetin-treated (10 mg/kg/p.o./day) animals were used for comparison. In continuation of the toxicity studies, a sub-acute tolerance test for 28 days was carried with the optimum dose in order to examine untoward effects of test compounds.
Antidiabetic activity of 3-hydroxyflavone analogues
Induction of insulin resistance by high fructose diet (FD) and treatment protocol
Wistar rats were made insulin resistant by feeding high-fructose diet (FD) for 6 weeks (42 days). The FD was prepared by mixing 66 % fructose with the normal pellet powder (Veerapur et al., 2010[40]). The experimental rats were divided into 8 groups (n=6) and they were treated orally as follows
G1: Normal pellet diet (NPD) + 0.5 % CMC (2 ml/kg)
G2: FD + 0.5 % CMC (2 ml/kg)
G3: FD + Piolglitazone (10 mg/kg)
G4: FD + Quercetin (10 mg/kg)
G5: FD + JY-1 (80 mg/kg)
G6: FD + JY-2 (80 mg/kg)
G7: FD + JY-3 (80 mg/kg)
G8: FD + JY-4 (80 mg/kg)
Male rats were used because females have been shown to be protected from changes in fructose-induced hyperinsulinemia and insulin resistance (Galipeau et al., 2002[11]).
Effects of test compounds on FD-fed insulin resistant rats
Food intake and body weight monitoring
High-fructose diet (FD) was freshly prepared daily and rats (3 per cage) were provided with approximately 80 g fresh feed per cage, between 9.00–11.00 am daily. Approximately 20–25 g feed remained on the next day. The same quantity of feed was provided at the same time throughout the study period. In order to reduce the amount of left-over feed and to eliminate spillage, the quantity of feed provided daily was only slightly more than what was consumed each day. Total consumption was estimated from the difference in the weight of feed added each day and then subtracting the weight of the feed left-over after 24 h consumption on Mondays, Wednesdays, and Fridays. Three values obtained for each week were averaged to provide an index of daily consumption for the week. Data were not included whenever excessive spillage was observed. Cumulative food intake was calculated by multiplying average daily food intake by 7, then summing the calculated weekly averages throughout the treatment period. The cumulative food intake was calculated per 100 g body weight of animal.
The body weights of each rat in respective groups were recorded each week during the treatment period. The initial body weight before start of FD-feeding was considered as the basal weight of the rat. At the end of the study, body weights of the treated groups (G3 to G8) were compared with induced group (G2) and the normal control group (G1).
Fasting blood glucose and oral glucose tolerance test
On 42nd day, the 12 fasted rats were administered glucose (2 g/kg, p.o.) and blood samples were collected from the caudal vein by means of a small incision at the end of the tail at 0 min (immediately after glucose load), 15, 30, 60, 90 and 120 min after glucose administration. Blood glucose was estimated by glucose oxidase strips using a glucometer (Accu-chek® Active, Roche diagnostics). The results are expressed as integrated area under curve for glucose, using GraphPad Prism software.
Estimation of serum lipid profiles
Serum triglyceride (TG), total cholesterol (TC) and high density lipoprotein (HDL) were estimated by semi-autoanalyser, Star 21 (Seac Radim group) using diagnostic reagent kit (Aspen Laboratories, Delhi). Very low density lipoprotein (VLDL) and low density lipoprotein (LDL) were calculated by the following formulae (Veerapur et al., 2010[40]).
VLDL = (TG) /5; LDL = TC – VLDL – HDL
TC/HDL ratio was calculated as a marker of dyslipidemia
Estimation of serum insulin and homeostatic model assessment (HOMA-IR)
Serum insulin was estimated by radioimmunoassay using an immunoassay kit. The insulin resistance index was assessed by HOMA-IR, calculated by the following formula (Veerapur et al., 2010[40]):
HOMA-IR = [fasting plasma glucose (mg/dl) x fasting plasma insulin (µ IU/ml)] /405
Determination of insulin tolerance test
Insulin tolerance test was carried out by intraperitoneal injection of insulin bolus (4 IU/kg; Actrapid®, Novo-Nordisk). Blood glucose was determined immediately before and 5, 10, 15, 30, 60, 90, and 120 min after the injection. The first-order rate constant for the disappearance rate of glucose from plasma (K-ITT) was estimated from the slope of the regression line of log plot of the blood glucose against time (Akinmokun et al., 1992[2]).
Estimation of serum AGEs and creatinine
The serum AGEs was determined by previously reported specific fluorescence assay (Yanagisawa et al., 1998[42]). Serum creatinine was determined by the modified Jaffe’s method using commercially available kit (Aspen Laboratories, Delhi) with an auto-analyzer (Star 21, Seac Radim Group).
Biochemical parameters from rat liver homogenate
After OGTT, the rats were sacrificed by cervical dislocation and liver was perfused with saline, dissected out, and a 10 % liver homogenate was prepared with ice-cold saline-EDTA using Teflon-glass homogenizer (Yamato LSG LH-21, Japan). The homogenate was centrifuged at 10,000 rpm for 10 min (at 4-8 °C) using cooling centrifuge (MIKRO 220R, Hettick Lab, Germany). The pellet was discarded and supernatant obtained was used for the estimation of oxidative stress markers in the homogenate. The enzymatic antioxidants such as glutathione-S-transferase (GST), catalase and SOD were determined by standard methods. Similarly non-enzymatic antioxidants such as TBA-reactive substances (TBARS), GSH and total thiols were estimated by standard colourimetric assays (Veerapur et al., 2010[40]). The total protein was determined by Lowry’s method. The concentration of antioxidant enzymes was expressed in terms of protein content in the liver homogenate. Liver glycogen was estimated by the standard method using anthrone reagent (van der Vies, 1954[38]). The glycogen content in liver was expressed in mg/g wet weight.
Glucose uptake from isolated hemi-diaphragm of insulin resistant rats
The hemi-diaphragms were isolated from the FD-rats and glucose uptake was studied in the presence and absence of insulin (Veerapur et al., 2010[39]).
Antioxidant and antiglycation activity of 3-hydroxyflavone analogues
Free radical-scavenging activities of test compounds were measured in terms of hydrogen donating or radical-scavenging ability using the stable radicals such as, DPPH and ABTS. The hydroxyl radical (•OH) scavenging, inhibition of lipid peroxidation and calf thymus DNA protection by test compounds were carried out as per the standard methods (Prabhakar et al., 2006[26]). Quercetin was used as standard. The antioxidant activity of the test compounds was also assessed by their ability to inhibit LPS-induced ROS and NO-production in RAW 264.7 cells (Mathew et al., 2013[21]). L-NAME was used as standard for NO-inhibition assay and DPI in intracellular ROS inhibition assay.
The compounds were tested for their antiglycation properties by hemoglobin-glucose assay (Somani et al., 1989[32]), BSA-MGO assay and BSA-glucose assay (Bhavesh et al., 2013[4]). Further, antiglycation activity was also determined by fructosamine assay (Sharma et al., 2002[30]).
Glucose uptake from L6 myotubes
L6 rat skeletal muscle cell-line was purchased from National Centre for Cell Sciences (NCCS), Pune. 2500 cells/well in complete DMEM media were seeded in a 96-well black plate and incubated at 37 °C, 5 % CO2. After 24 h, the wells were replaced with sterile HBSS without glucose for 5 h, followed by incubation with test compounds (10-200 µM)/vehicle (untreated control) in sterile PBS for 1 h. Insulin (100 nM), used as the standard, was incubated for 10 min. The cells were exposed to 2-NBDG (100 µM) for 5 min and the fluorescence intensity was measured, after replacing the wells with PBS, using a spectrofluorimeter. 2-NBDG is a fluorescent D-glucose derivative chemically, 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose with λex/em = 465/540 nm (Zou et al., 2005[43]). The percentage increase in basal glucose uptake with respect to untreated control was calculated and 50 % effective concentration (EC50) was determined graphically.
Statistical analysis
Comparison of mean ± SEM values between various groups was performed by one-way ANOVA followed by Tukey post-test. Data analysis was carried out with the help of GraphPad prism Software, version 5.00 (San Diego, CA, USA). Treatment groups were compared to induced control (FD-rats) and normal control (NPD-rats). P < 0.05 was considered as statistically significant.
Results
Acute oral toxicity and dose optimization of 3-hydroxyflavone analogues
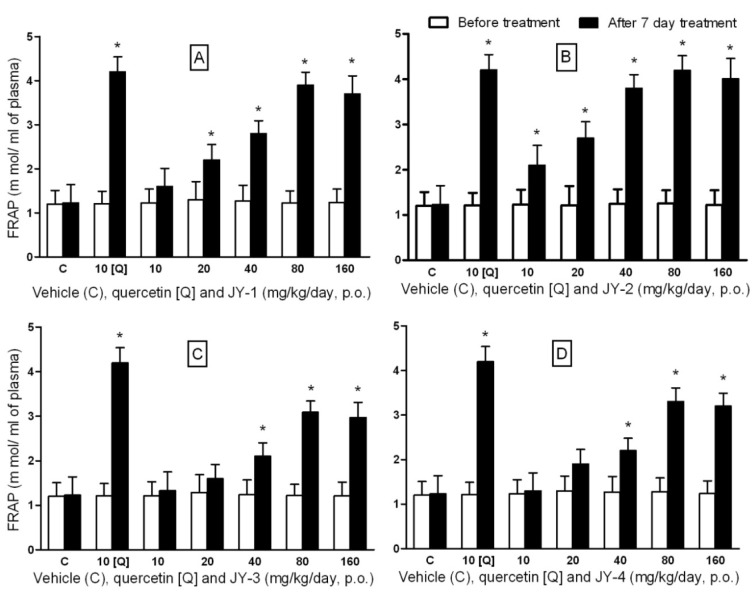
The test compounds at doses 2 g/kg, p.o., did not produce any mortality in rats within 72 h. No detectable symptoms of toxicity to central nervous system and autonomic nervous system were noted. FRAP was reported to be a reliable biomarker of antioxidant activity for flavonoids and flavonoid-rich fruits (Lotito and Frei, 2006[20]). We applied this assay for optimizing the dose for antidiabetic activity. There was a graded increase in FRAP upon treatment with the test compounds, which peaked at 80 mg/kg/day, p.o., for all the test compounds (Figure 2(Fig. 2)). After seven days of treatment, test compounds at 80 mg/kg, were found to be comparable in efficacy to quercetin at 10 mg/kg/day. Above 80 mg/kg, the activity did not increase with higher doses. Hence the dose of all test compounds was optimized to 80 mg/kg/day, p.o., for the rest of the study.
Figure 2. FRAP values for dose optimization of test compounds in Wistar rats.
Ferric reducing power of plasma (FRAP) was expressed in mmol of ferric reduced to ferrous form per ml of plasma (Values are expressed in mean ± SEM; n=5). [A], [B], [C] and [D] represents compounds JY-1, JY-2, JY-3 and JY-4 respectively; (*: p < 0.05 compared to day 0, in each group; values are mean ± SEM; n=5).
The test compounds upon treatment for 28 days with the optimized dose did not show any untoward effects, when observed both for CNS and ANS related symptoms. There were no observable changes in the hematological parameters and none of the compounds had any significant effects on fasting blood glucose and lipid profile.
High-fructose diet induced diabetes and insulin resistance in Wistar rats
Food intake and body weight
The high-fructose (66 % w/w x 42 days) increased the body weight significantly (1.25 fold) compared to NPD-rats (Figure 3A(Fig. 3)). Increase in body weight was correlated to cumulative increase in food intake (Figure 3B(Fig. 3)). Body weights were significantly lower in treated group compared to the FD-rats. In multiple comparisons with pioglitazone, quercetin, JY-2 and JY-1, there was no significant difference in body weights and food intake, indicating JY-2 has highest activity in lowering the body weight and food intake, among the four compounds selected followed by JY-1.
Figure 3. 3-Hydroxyflavones on body weight and cumulative food intake in FD-fed rats.
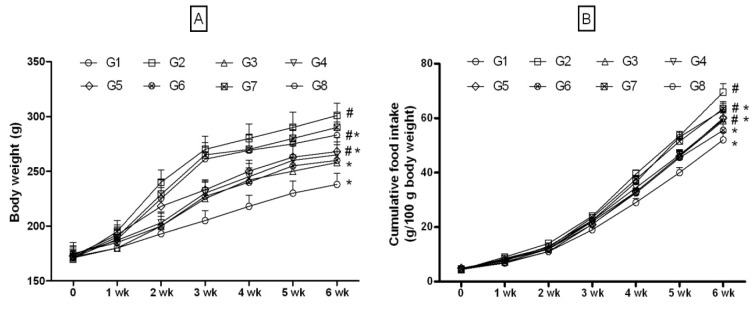
A: Body weight (g); B: Cumulative food intake; G1: Normal control; G2: FD-fed diabetic control; G3: Diabetic + pioglitazone (10 mg/kg/day, p.o.); G4: Diabetic + Quercetin (10 mg/kg/day, p.o.); G5: Diabetic + JY-1; G6: Diabetic + JY-2; G7: Diabetic + JY-3; G8: Diabetic + JY-4; (test compounds at optimized dose 80 mg/kg/day, p.o.);
#: p < 0.05 treatment groups are compared to normal rats (G1); *: p < 0.05 treatment groups are compared to induced group (G2).
Induction of insulin resistance in rats
In FD-rats there was significant increase in fasting glucose levels compared to NPD-rats (142.3 ± 11.4 vs 93.2 ± 9.4 mg/dl of NPD-rats). NPD-fed rats (G1) did not produce significant changes in blood glucose upon challenge with glucose (2 g/kg). But, there was significant impairment in tolerance to exogenously administered glucose in FD-fed rats compared to NPD-rats (Figure 4(Fig. 4)). The AUC was significantly increased for FD-rats compared to NPD-rats (Figure 4(Fig. 4)). Also, HOMA-IR (33.87 ± 5.1 in FD vs 7.35 ± 3.08 in NPD) calculated from the serum insulin levels (97.24 ± 10.4 vs 31.9 ±7.9 µIU/ml) were increased significantly (Figure 5(Fig. 5)). These parameters confirmed the induction of insulin resistance in FD-fed rats.
Figure 4. Effect of 3-hydroxyflavones on fasting blood glucose and OGTT in FD-fed rats.
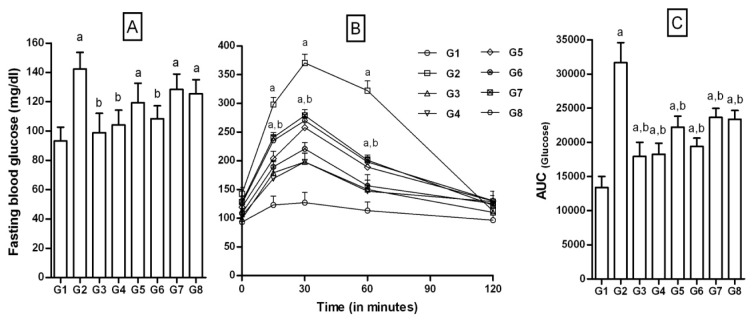
A: Fasting blood glucose (FBG) mg/dl; B: OGTT; C: Area under the curve [AUC]; G1: Normal control; G2: FD-fed diabetic control; G3: Diabetic + pioglitazone (10 mg/kg/day, p.o.); G4: Diabetic + Quercetin (10 mg/kg/day, p.o.); G5: Diabetic + JY-1; G6: Diabetic + JY-2; G7: Diabetic + JY-3; G8: Diabetic + JY-4; (test compounds at optimized dose 80 mg/kg/day, p.o.); a: p < 0.05 treatment groups are compared to normal control (G1); b: p < 0.05 treatment groups are compared to FD-fed diabetic control group (G2).
Figure 5. Effect of 3-hydroxyflavones on serum insulin, HOMA-IR and insulin tolerance in FD-fed rats.
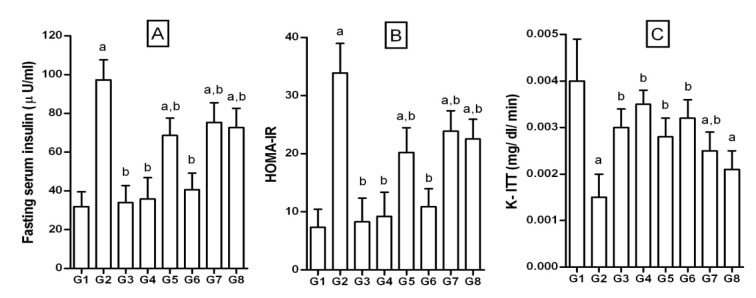
A: Fasting serum insulin (µIU/ml); B: HOMA-IR: Defined as homeostatic model assessment of insulin resistance and calculated by the formula HOMA-IR = [fasting plasma glucose (mg/dl) x fasting plasma insulin (µIU/ml)]/405; C: K-ITT: Defined as first-order rate constant for the disappearance rate of glucose from plasma was estimated from the slope of the regression line of the log plot of blood glucose against time; G1: Normal control; G2: FD-fed diabetic control; G3: Diabetic + pioglitazone (10 mg/kg/day, p.o.); G4: Diabetic + Quercetin (10 mg/kg/day, p.o.); G5: Diabetic + JY-1; G6: Diabetic + JY-2; G7: Diabetic + JY-3; G8: Diabetic + JY-4; (test compounds at optimized dose 80 mg/kg/day, p.o.); a: p < 0.05 treatment groups are compared to normal control (G1); b: p < 0.05 treatment groups are compared to FD-fed diabetic control group (G2).
Effect of 3-hydroxyflavone analogues in FD-rats
Fasting blood glucose and OGTT
Upon treatment with test compounds, FBG were significantly lowered. Reduction was most significant with JY-2 and JY-1. AUC for the 120 min interval was not altered significantly for all test compounds selected. The AUC for JY-2 was significantly lower than that of FD-rats and was most potent among the selected compounds (p > 0.05) and efficacy was comparable to that of quercetin and pioglitazone (Figure 4(Fig. 4)).
Fasting insulin, HOMA-IR, K-ITT values
Fasting insulin level of FD-rats was significantly higher than that of NPD-rats (97.24 ± 10.41 vs 31.93 ± 7.58 µIU/ml in NPD; Figure 5A(Fig. 5)). The degree of insulin resistance calculated by HOMA-IR was significantly higher in FD-rats than controls (33.87 ± 5.10 vs 7.35 ± 3.08 of NPD; Figure 5B(Fig. 5)). The test compounds, pioglitazone and quercetin when administered to FD-rats, resulted in significantly reduced serum insulin levels. Similarly the HOMA-IR values decreased significantly. JY-2 was found to be the most effective in reducing FD-induced insulin resistance.
Glucose clearance on administration of insulin (4 IU/kg, i.p.) was rapid in the NPD-rats compared to FD-rats, confirming diet-induced insulin resistance (Figure 5C(Fig. 5)). Insulin-induced plasma glucose decay rate constant (K-ITT) was significantly lower for the NPD-rats compared to FD-rats (0.004 ± 0.0009 vs 0.0015 ± 0.0005 mg/dl/min). JY-2, JY-1 and quercetin were apparently more beneficial than pioglitazone in insulin-induced plasma glucose decay.
Serum AGEs and creatinine
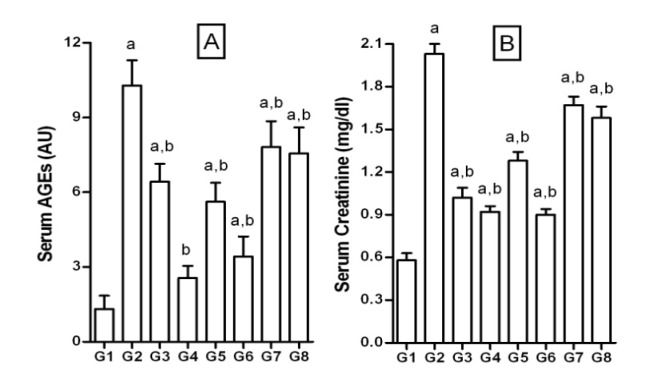
The serum AGEs levels were determined by measuring the relative fluorescence in arbitrary units (AU). There was significant increase in AGEs (≈8 folds) in FD-rats compared to the NPD-rats (10.22 ± 1.02 vs 1.32 ± 0.53 AU; Figure 6A(Fig. 6)). Serum AGEs levels were significantly brought down by treatment with test compounds, quercetin and pioglitazone. Further, the increase in AGEs was associated with a proportionate increase in the serum creatinine levels (Figure 6B(Fig. 6)). FD feeding increased serum creatinine significantly when compared to the NPD-rats (2.03 ± 0.07 vs 0.58 ± 0.05 mg/dl in NPD-rats). Likewise, there was a significant decrease in the elevated serum creatinine levels upon treatment with test compounds. JY-2 treatment was most effective in lowering creatinine levels among the selected compounds.
Figure 6. Effect of 3-hydroxyflavones on serum AGEs and creatinine in FD-fed rats.
A: Serum advanced glycosylation end products (AGEs) measured as arbitrary units (AU); B: Serum creatinine (mg/dl); G1: Normal control; G2: FD-fed diabetic control; G3: Diabetic + pioglitazone (10 mg/kg/day, p.o.); G4: Diabetic + Quercetin (10 mg/kg/day, p.o.); G5: Diabetic + JY-1; G6: Diabetic + JY-2; G7: Diabetic + JY-3; G8: Diabetic + JY-4; (test compounds at optimized dose 80 mg/kg/day, p.o.); a: p < 0.05 treatment groups are compared to normal control (G1); b: p < 0.05 treatment groups are compared to FD-fed diabetic control group (G2).
Lipid parameters
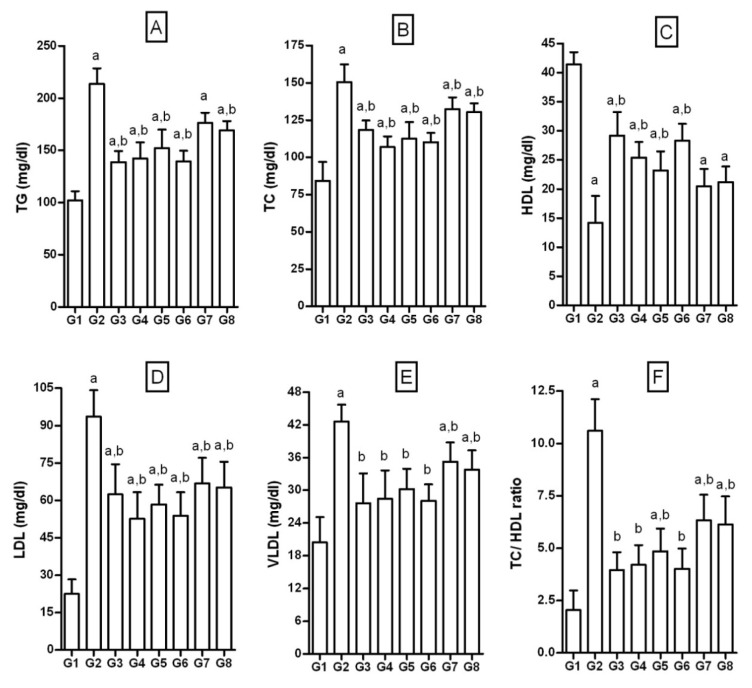
The serum TG, TC, LDL and VLDL values were significantly increased and HDL, was significantly decreased in FD-rats, when compared to NPD-rats (TG = 214.6 ± 15.1 vs 102.25 ± 8.6; TC = 150.68 ± 11.8 vs 84.23 ± 12.7; LDL= 93.57 ± 10.70 vs 22.55 ± 5.8; VLDL = 42.60 ± 3.2 vs 20.42 ± 4.5; HDL = 14.2 ± 4.62 vs 41.42 ± 2.1; Figure 7(Fig. 7)). Thus, there was significant dyslipidemia in FD-rats. The TC/ HDL ratio, a marker of dyslipidemia, was significantly increased in FD-rats compared to the NPD-rats (10.65 ± 1.6 vs 2.04 ± 0.94). Treatment with test compounds, pioglitazone and quercetin significantly normalized both serum lipid levels and TC/HDL ratio. JY-1 and JY-2 treatment significantly lowered dyslipidemia marker TC/HDL to 4.85 ± 1.16 and 4.02 ± 0.97, as opposed to 10.60 ± 1.52 in FD). Correction of dyslipidemia is the most significant outcome of JY-2 and JY-1 treatment, which was found to be comparable to quercetin and pioglitazone.
Figure 7. Effect of 3-hydroxyflavones on serum lipid profile in FD-fed rats.
A: TG: Triglycerides; B: TC: Total cholesterol; C: HDL: High density lipoprotein; D: LDL: Low density lipoprotein; E: VLDL: Very low density lipoprotein; F: TC/ DHL–ratio; G1: Normal control; G2: FD-fed diabetic control; G3: Diabetic + pioglitazone (10 mg/kg/day, p.o.); G4: Diabetic + Quercetin (10 mg/kg/day, p.o.); G5: Diabetic + JY-1; G6: Diabetic + JY-2; G7: Diabetic + JY-3; G8: Diabetic + JY-4; (test compounds at optimized dose 80 mg/kg/day, p.o.); All values mean ± SEM (n=6); a: p < 0.05 treatment groups are compared to normal control (G1); b: p < 0.05 when treatment groups are compared to induced group (G2).
Endogenous antioxidant and glycogen in liver
GSH
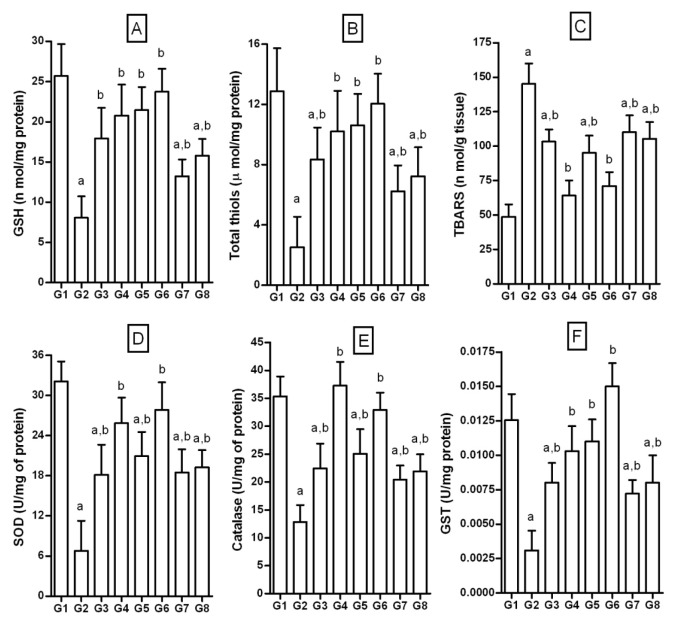
NPD-rats had basal GSH level of 25.68±3.99 nmol/mg of protein. Fructose feeding for 6 weeks significantly reduced GSH levels (8.1±3.69 nmol/mg of protein). Treatment with test compounds was effective in increasing GSH levels in FD-rats. Similarly, quercetin and pioglitazone significantly restored GSH levels (Figure 8A(Fig. 8)).
Figure 8. Effect of 3-hydroxyflavones on enzymatic, non-enzymatic antioxidants and TBARS in liver of FD-fed rats.
A: GSH (nmol/ mg proteins); B: Total thiols (µmol/ mg protein); C: TBARS nmol/ g of tissue; D: SOD U/mg protein; E: Catalase (U/mg proteins); F: GST U/mg proteins; G1: Normal control; G2: FD-fed diabetic control; G3: Diabetic + pioglitazone (10 mg/kg/day, p.o.); G4: Diabetic + Quercetin (10 mg/kg/day, p.o.); G5: Diabetic + JY-1; G6: Diabetic + JY-2; G7: Diabetic + JY-3; G8: Diabetic + JY-4; (test compounds at optimized dose 80 mg/kg/day, p.o.); All values mean ± SEM (n=6); a: p < 0.05 treatment groups are compared to normal control (G1); b: p < 0.05 when treatment groups are compared to induced group (G2).
Total thiols
FD-rats showed significantly decreased total thiol levels compared to NPD-rats (2.52 ± 2.03 vs 12.87 ± 2.9 µmol/ mg of protein). Treatment with test compounds restored thiols significantly compared to the FD-rats. The standard flavonoid, quercetin and pioglitazone also restored the total thiols significantly compared to FD-rats (Figure 8B(Fig. 8)).
TBARS
FD-feeding increased TBARS levels by approximately three folds (48.6 ± 9.1 nmol/g in NPD vs 145.2 ± 14.7 nmol/g in FD (Figure 8C(Fig. 8)). Treatment with test compounds significantly lowered the TBARS levels compared to FD-rats. Standard flavonoid quercetin and pioglitazone significantly normalized the levels of TBARS indicating significant protection from lipid peroxidation in liver.
SOD
FD-rats significantly reduced SOD by about 4.5 folds (6.75 ± 4.49 vs 32.07 ± 2.39 U/mg of protein). Treatment with compounds significantly restored the SOD levels. Similarly, standards quercetin and pioglitazone significantly increased SOD levels to near normal values (Figure 8D(Fig. 8)).
Catalase: FD significantly decreased liver catalase levels by about 3 folds (12.83 ± 3.6 U/mg vs 35.35 ± 3.6 U/mg of protein). However, treatment with test compounds increased the catalase levels compared to the FD-rats. Standards quercetin and pioglitazone significantly increased catalase levels to near normal values (Figure 8E(Fig. 8)).
GST
The normal basal level of GST in NPD-rats was 0.013 ± 0.002 U/mg of protein in liver. Fructose feeding showed significantly decreased levels of GST by about 4 folds (0.0031 ± 0.0014 U/mg). However, treatment with test compounds restored the GST activity significantly compared to FD-rats. The standards quercetin and pioglitazone significantly increased GST levels to the normal values (Figure 8F(Fig. 8)).
Liver glycogen
FD-rats showed significantly decreased glycogen in liver (4.32 ± 1.09 mg/g vs 13.21±1.41 mg/g of wet liver). However, treatment with test compounds significantly increased the liver glycogen content compared to that of FD-rats. Quercetin and pioglitazone showed significantly increased levels of liver glycogen to near normal values (Figure 9(Fig. 9)).
Figure 9. Effect of 3-hydroxyflavones on liver glycogen in FD-fed rats.
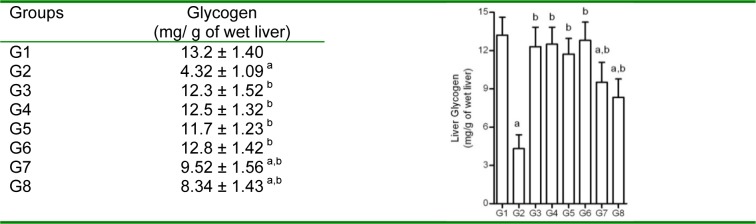
Values are in mg/g of wet liver; G1: Normal control; G2: FD-fed diabetic control; G3: Diabetic + pioglitazone (10 mg/kg/day, p.o.); G4: Diabetic + Quercetin (10 mg/kg/day, p.o.); G5: Diabetic + JY-1; G6: Diabetic + JY-2; G7: Diabetic + JY-3; G8: Diabetic + JY-4; (test compounds at optimized dose 80 mg/kg/day, p.o.); All values mean ± SEM (n=6); a: p < 0.05 treatment groups are compared to normal control (G1); b: p < 0.05 when treatment groups are compared to induced group (G2).
Glucose uptake from hemi-diaphragm muscle
The hemi-diaphragms from rats fed with FD showed significant reduction in glucose uptake in vitro (4.21 ± 1.25 vs 10.28 ± 2.51 mg/g of wet tissue/ 30 min, in NPD-rat (Figure 10(Fig. 10)). In the presence of insulin (35 µIU/ml), hemi-diaphragms from FD-rats did not show significant increase in glucose uptake, indicating insulin resistance in skeletal muscles. The treatment with test compounds, quercetin and pioglitazone significantly improved in vitro glucose uptake by skeletal muscles both in the presence and absence of insulin. JY-2 and JY-1 treatment significantly increased in vitro skeletal muscle glucose uptake in the presence of insulin (22.12 ± 2.51 mg/g of wet tissue/30 min) indicating reversal of insulin resistance in FD-rats.
Figure 10. Effect of 3-hydroxyflavones on glucose uptake from isolated hemi-diaphragms of FD-fed rats.
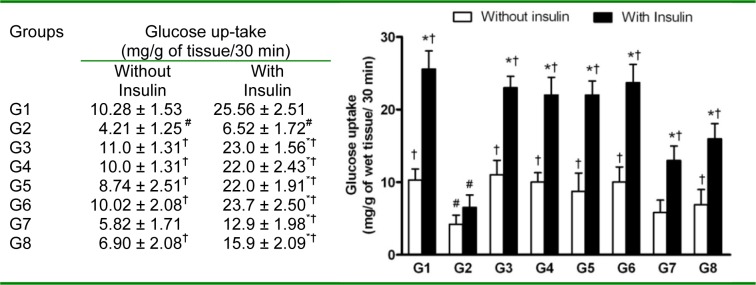
The experiment was conducted on the hemi-diaphragm isolated from the respective groups and compared with insulin and without insulin (35 µIU/ml); G1: Normal control; G2: FD-fed diabetic control; G3: Diabetic + pioglitazone (10 mg/kg/day, p.o.); G4: Diabetic + Quercetin (10 mg/kg/day, p.o.); G5: Diabetic + JY-1; G6: Diabetic + JY-2; G7: Diabetic + JY-3; G8: Diabetic + JY-4; (test compounds at optimized dose 80 mg/kg/day, p.o.); Values are mean ± SEM; All values mean ± SEM (n=6); (*: p < 0.05 compared to same group in presence of insulin;
#: p < 0.05 compared to G1 in presence/absence of insulin; †: p < 0.05 compared to G2 in presence/absence of insulin).
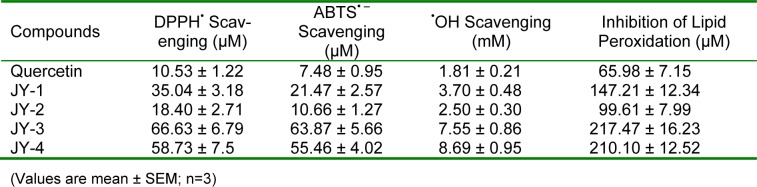
Antioxidant activity of 3-hydroxyflavone analogues
The results of antioxidant activities, expressed as IC50, with different free radicals are shown in Table 1(Tab. 1). Among the 4 molecules selected, JY-2 showed the greatest antioxidant activity comparable to quercetin followed by JY-1, JY-4 and JY-3 in DPPH, ABTS and hydroxyl radical scavenging assay (Table 1(Tab. 1)). The Fe3+ induced lipid peroxidation was inhibited by the test compounds at low concentrations and expressed as IC50. The decreasing order of inhibition of lipid peroxidation is JY2>JY1>JY4>JY3 (Table 1(Tab. 1)).
Table 1. Comparison of the IC50 of 3-hydroxyflavones against various free radicals.
Transition metals such as iron and copper can participate in FR to produce free radicals such as •OH, which cause oxidative DNA damage. •OH formed from FR attacks guanosine residues, resulting in DNA strand break. Hydroxyl radical scavengers can protect DNA from such damages. All four compounds selected have inhibited calf thymus DNA damage by FR at 100-200 µM (Figure 11(Fig. 11)). Calf thymus DNA protection study is a qualitative assay for the antioxidant efficacy of the compounds, and this assay has more relevance for in vivo correlations.
Figure 11. Calf thymus DNA protection by 3-hydro-xyflavones.
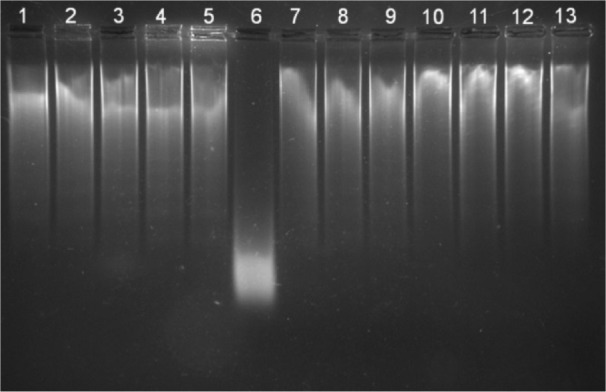
Lane 1: Control DNA; Lane 2: JY-1 (200 μM) and DNA; Lane 3: JY-2 (200 μM) and DNA; Lane 4: JY-3 (400 μM) and DNA; Lane 5: JY-4 (400 μM) and DNA; Lane 6: Fenton reaction (FR) induced DNA damage; Lane 7: JY-1 (100 μM) + FR with DNA; Lane 8: JY-1 (200 μM) + FR with DNA; Lane 9: JY-2 (100 μM) + FR with DNA; Lane 10: JY-2 (200 μM) + FR with DNA; Lane 11: JY-3 (200 μM) + FR with DNA; Lane 12: JY-3 (400 μM) + FR with DNA; Lane 13: JY-4 (200 μM) + FR with DNA.
LPS-stimulation of RAW 264.7 cells generates intracellular NO and ROS. In the presence of test compounds (3-200 µM) the NO release is scavenged significantly (Table 2(Tab. 2)). The 50 % inhibitory concentration (IC50) was comparable to the quercetin. L-NAME (a standard NOS inhibitor) at 50 µM inhibited NO by 15.46 ± 0.048 %. ROS can be detected based on the oxidation of the cell-permeable non-fluorescent probe DCFH-DA to yield the highly fluorescent DCF (Chen et al., 2010[9]). DPI, inhibitor of NADPH-oxidase, is a known standard for ROS-inhibition. Test compounds inhibited ROS with an efficacy comparable to that of quercetin. DPI (5 µM) showed 56.06 ± 19.75 % ROS-inhibition (Table 2(Tab. 2)).
Table 2. Inhibition of NO and ROS by 3-hydroxyflavones in LPS activated RAW cells.
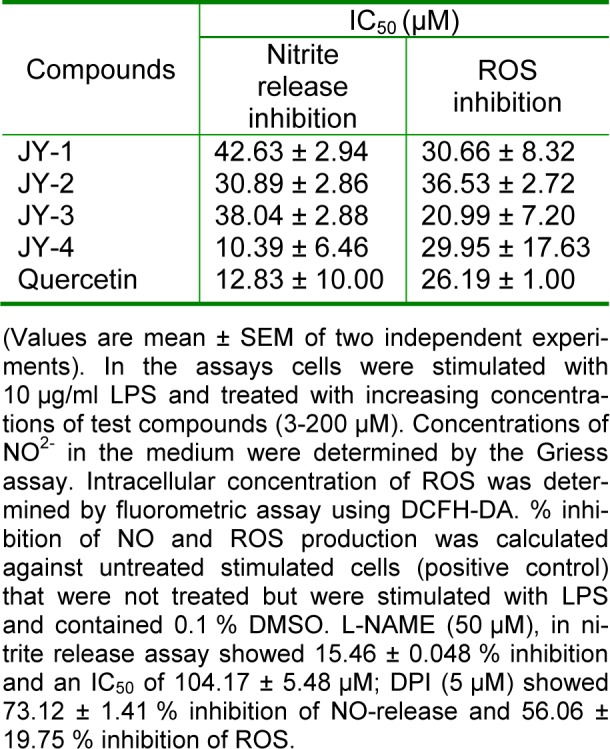
Antiglycation activity of 3-hydroxyflavone analogues
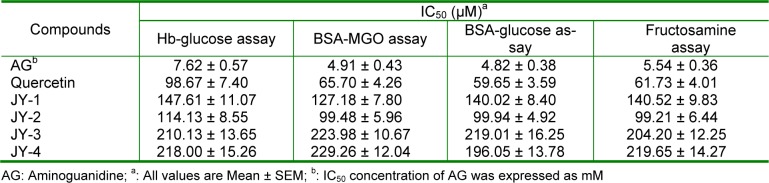
The antiglycation activity is expressed as IC50 as shown in Table 3(Tab. 3). In the early stage glycosylation (Hb-glucose assay), test compounds showed higher IC50 compared to the middle stage and late stage AGEs formation (BSA-MGO and BSA-Glucose assays respectively). Results of the antiglycation potency, as measured by Hb-glucose assay, is in the decreasing order of JY-2>JY-1>JY-3>JY-4. Test compounds showed equal potencies in the middle stage and late stage glycation assays. Overall, the order of potency of newly synthesized compounds is JY-2>JY-1>JY-3>JY-4. JY-2 showed the maximum potency in all the stages of glycation assays which was comparable to the standards quercetin and aminoguanidine (Table 3(Tab. 3)).
Table 3. In vitro antiglycation activity of 3-hydroxyflavones using quercetin and aminoguanidine as positive control.
Glucose uptake from L6 myotubes by 3-hydroxyflavone analogues
In the presence of test compounds the glucose uptake by L6 myotubes was enhanced significantly. There was no significant difference between glucose uptake induced by test compounds and standard quercetin (p>0.05) indicating that the test compounds were comparable to quercetin in enhancing the glucose uptake in vitro. The test compounds showed a dose-dependent increase in glucose uptake and were relatively more potent than quercetin with reference to the individual EC50 values. The test compound JY-4 showed highest activity followed by JY-3, JY-2 and JY-1 (Figure 12(Fig. 12)).
Figure 12. Effect of 3-hydroxyflavones on glucose uptake by L6 myotube.
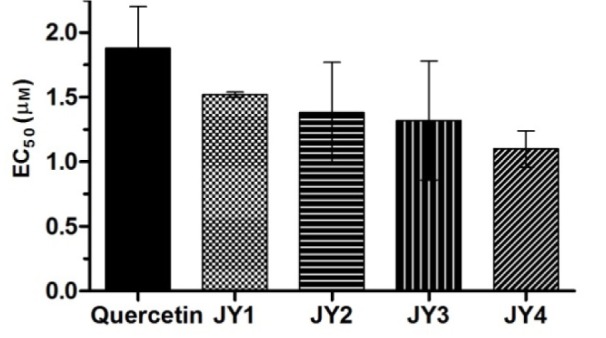
Graph represents effective concentration (EC50) of 3-hydroxyflavone analogues for percentage increase in glucose uptake by L6 myotube with respect to untreated control; Quercetin was used as standard flavonoid for comparison; Insulin (100 nM) increased basal glucose uptake by 222.40 ± 4.20 % was used as standard; Data expressed as mean ± SEM.
Discussion
The increased consumption of fructose has been proposed as the cause of increased incidence of diabetes and metabolic syndrome in human population (Johnson et al., 2007[18]). In humans, high fructose diets induce dyslipidemia and modulate intracellular lipid deposition (ectopic lipids) which is nothing but the deposition of triglyceride in cytoplasm of non-adipose cells, such as hepatocytes, muscle fibers, or endocrine cells. Such ectopic lipid deposition in the liver and skeletal muscle is closely linked to tissue-specific insulin resistance (Hyakukoku et al., 2003[16]). In addition, fructose may impair endothelial function through increased creatinine and uric acid production (Le et al., 2009[19]; Tappy and Le, 2010[35]).
It is a well-known fact that fructose feeding in rats increases TG levels, leading to insulin resistance as well as obesity (Patel et al., 2009[25]). In our study we found that body weight of rats increased significantly after 6 weeks of high fructose feeding, while basal body weights correlated with total food intake (Figure 3(Fig. 3)). However, test compounds did not increase the body weight of FD-rats when compared to controls. JY-2 and JY-1 significantly inhibited body weight gain with an efficacy comparable to that of pioglitazone and quercetin. The observation made here was that the decrease in body weight is mainly because of decrease in food intake, which may be due to combined effect of appetite suppression, hypoglycemic and hypolipidemic activity.
It was also observed that TGs were elevated in FD-rats. Test compounds, particularly JY-2, restored dyslipidemic markers to near-normal values in FD-rats. Dietary fructose has been shown to increase ectopic fat deposition in tissues and its normalization in FD-rats could have been at least partly mediated by the anti-lipodystropic action of test compounds. Further, test compounds could bring down elevated insulin levels and HOMA-IR, a biomarker for insulin resistance (Matthews et al., 1985[23]). The increased lipid levels in FD-rats possibly could have resulted in the increase of serum insulin, HOMA-IR and FBG (Figure 4(Fig. 4) and 5(Fig. 5)). In other words, FD-rat model combines the symptoms of hyperglycemia, dyslipidemia and hyperinsulinemia in the same animal. JY-2 and JY-1 were found to normalize all three parameters in fructose-fed rats. Further, JY-2 and JY-1 significantly increased the insulin induced glucose clearance (K-ITT) in the same experimental model.
High fructose feeding in rats mimics many of the symptoms of type 2 diabetes (T2D) in humans, especially insulin resistance, glucose intolerance, dyslipidemia and renal impairment. Despite several limitations, the FD-rat model may be useful to define potential treatments for T2D or the metabolic syndrome (Patel et al., 2009[25]). Similarly, high fructose consumption induces oxidative stress, which is a cause for the metabolic complications of diabetes (Delbosc et al., 2005[10]). In our study, we found that the antioxidant defense in liver was significantly impaired in FD-rats. The levels of TBARS increased and that of total thiols, GSH, catalase, SOD and GST decreased in FD-rats. Treatment with JY-2, JY-1 quercetin and pioglitazone significantly restored the antioxidant defense (Figure 8(Fig. 8)).
High fructose feeding decreases insulin-induced insulin receptor substrates (IRS-1/2) phosphorylation in both the liver and muscle in rats (Tappy and Le, 2010[35]). This, in turn, will lead to polyol formation and AGEs, which can contribute to the numerous complications seen in diabetes. Further, oxidative stress due to chronic hyperglycemia is a well-known cause for plasma and tissue protein glycation. Glycation-induced hemoglobin modifications (HbA1c) due to hyperglycemia are known to cause further oxidative stress via iron-dependent free radical reactions. Furthermore, the increased AGEs in the plasma are associated with increased serum creatinine. Our findings were in accordance with earlier findings in the literature (Yanagisawa et al., 1998[42]). The serum AGEs and creatinine increased significantly in FD-rats and treatment with the antioxidant molecule JY-1 and JY-2 normalized these elevated levels supporting the alleviation of oxidative stress. JY-1 and JY-2 were almost as efficacious as quercetin and pioglitazone in lowering elevated serum AGEs and creatinine.
Fructose diet induces depletion of glycogen, chiefly in liver, resulting from decreased activity of glycogen synthase, glucokinase activity or down-regulation of gene expression for these enzymes owing to insulin resistance (Tappy and Le, 2010[35]). Thus, chronic fructose diet leads to increased efflux of glucose. Decreased liver glycogen in FD-rats supports earlier reports from the literature. Treatment with JY-1 and JY-2 significantly normalized glycogen levels in the liver. JY-2 and JY-1 treatment also increased liver glycogen by three-fold compared to FD-rats, which is comparable to that of quercetin and pioglitazone.
ROS are believed to play a causal role in multiple forms of insulin resistance (Houstis et al., 2006[14]). Oxidative stress is one among the major factors that leads to several diabetes-related metabolic complications such as nephropathy, retinopathy and all types of neuropathies, and antioxidants are found to be beneficial (Calcutt et al., 2009[6]). The test compounds selected for the study have shown good in vitro antioxidant activity. In these compounds 3-OH group is contributing to antioxidant activity. JY-2 has an additional 7-OH group which might be contributing to the higher antioxidant activity. Also, the C-ring, rather than B-ring of flavone is contributing more to its’ DPPH radical scavenging activity (Tsimogiannis and Oreopoulou, 2006[37]). Hence, modification of γ-benzopyrone ring in flavones could yield better antioxidant molecules. JY-1 and JY-2 possess -N,N-dimethylamino group, which could have enhanced the antioxidant activity of these flavones.
Hydroxyl radical is the most deleterious oxidizing radical among those present in disease pathophysiology and reacts instantaneously with important molecules such as DNA and membrane lipids. In the assay system, •OH radical is generated by a mixture of ascorbic acid, H2O2 and ferric-EDTA. The •OH attacks deoxyribose, and initiates a cascade of reactions leading to fragmentation. One of the fragments generated is malondialdehyde (MDA). MDA reacts with thiobarbituric acid (TBA) at low pH, which upon heating will give a pink chromogen (adduct of TBA and MDA) (Cheeseman et al., 1988[8]). In the presence of test compounds, low absorption values indicate the protection of deoxyribose from •OH.
Though the test compounds have good in vitro radical scavenging activity, this cannot be concluded for their therapeutic applications, because these in vitro assays are done in non-physiological pH values and hence, it is impossible to extrapolate the results to physiological environment. The proof of bio-efficacy must emanate from application of reliable in vivo models where markers of baseline oxidative damage are examined from the standpoint of how they are affected by changes in diet or endogenous antioxidant defense. In this view the inhibition of lipid peroxidation and DNA protection assays will have greater impetus and greater biological relevance than DPPH, ABTS•- and •OH scavenging activity (Huang et al., 2005[15]).
Inhibition of lipid peroxidation is also an indicator of O2•- and lipid radical inhibition activity. Lipid peroxidation is initiated by abstraction of a hydrogen atom from bis-allylic methylene carbon positioned on a polyunsaturated side chain of a lipid molecule. These carbons are attacked preferentially over the secondary -C-H bonds because of the production of a resonance-stabilized carbon-centered radical. As the name peroxidation indicates, the auto-reaction initiation follows the carbon radical to be stabilized by a molecular rearrangement to form a conjugated diene. Further, a variety of propagation reactions may take place. The most likely reaction that takes place is between lipid radical and oxygen. After the addition of molecular oxygen to the lipid radicals, which is very fast in aerobic environment, the resulting peroxyl radical can attack neighboring polyunsaturated fatty acids or undergo intramolecular cyclization followed by breakdown to compounds such as, ethane, n-pentane and MDA (Huang et al., 2005[15]). We followed ferrous sulfate stimulated lipid peroxidation in the rat brain homogenate resulting in the formation of MDA. In the presence of antioxidants, formation of this MDA-TBA chromogen is reduced, which is expressed as antioxidant activity.
Natural flavonoids, at low concentrations have shown prevention of DNA damage whereas high concentrations (above 100 µM) have shown to induce DNA-damage and this was attributed to increase in the number of OH groups present in flavonoids. Our investigational compounds have minimum number of OH group and could have higher safety in preventing DNA damage.
RAW 264.7 cells are murine macrophages generally used for determination of intracellular ROS which are obligatory mediators, generated via the activation of NADPH-oxidase and other LPS signaling. NO is a highly reactive molecule and inducible nitric oxide synthase (iNOS) catalyses the reaction leading to increased RNS in inflammatory conditions. LPS induces vascular contractile and energetic failure by both exogenous and endogenous NO and ONOO- in pathogenesis of endotoxic shock (Szabo et al., 1996[34]). In diabetes the chronic increase-ROS associated ONOO- production has similar consequences of micro- and macrovascular complications (Son, 2012[33]). Many natural flavonoids studied for inhibition of NO in RAW 264.7 cells, is reported to down-regulate gene transcription rather than directly inhibiting iNOS. Some flavonoids target nuclear factor kappa B (NFϰB) gene expression and interferon (IFN-γ) induced NFϰB transactivation. The investigational molecules were potent NO release inhibitors and the IC50 values were comparable to the standard natural flavones.
The antiglycation activities of flavonoids have been reviewed by experts elsewhere (Matsuda et al., 2003[22]). However, this theory has several limitations because (1) only one type of assay (BSA-glucose method) was used to compare flavonoids (2) only natural flavonoids are considered. In the present work, we have determined the early stage (Amadori products) middle stage (RCS) and last stage (AGEs formation and cross-linking) glycoxidation and its inhibition by test compounds.
The phenomenon of protein glycation was demonstrated in the reaction mixtures of protein with glucose by several model systems in vitro. Glucose and dicarbonyl compound (such as MGO) are used as glycation agents, which are commonly adopted for the antiglycation studies. Hemoglobin and BSA, used as amine sources, serve as targets for glycation agent (Wu and Yen, 2005[41]). In the initial stage, glucose reacts with an amine group to form a labile Schiff base that rearranges to the Amadori product. The Schiff base is highly prone to oxidation and free radical generation, which leads to the formation of RCS such as glyoxal. BSA-glucose assay is the last stage glycosylation assay, where some amount of intermediate Amadori products remains unreacted are determined by fructosamine assay (Sharma et al., 2002[30]).
Increased glycation and build-up of tissue AGEs have been implicated in diabetic complications because they can alter enzymatic activity, decrease ligand binding, modify protein half-life and alter immunogenicity, which in turn play a role in atherogenesis. Glycation-derived free radicals can cause protein fragmentation and oxidation of nucleic acids and lipids. The oxidized lipids enhance the inflammatory condition and are called as advanced lipoxidation end-products (ALEs) (Thorpe and Baynes, 2003[36]). The amino groups of adenine and guanine bases in DNA are also susceptible to glycation and AGEs formation, probably by reactive intracellular sugars. AGEs could also form on phospholipids and induce lipid peroxidation by a direct reaction between glucose and amino groups on phospholipids such as phosphatidyl ethanolamine and phosphatidylserine residues (Ahmed, 2005[1]). Given the link mentioned above between glycation and oxidation, we hypothesized that antioxidant flavones might possess antiglycoxidative activities. Many data have shown that typical antioxidants/nutrients such as vitamin B1 (thiamine pyrophosphate), B6 (pyridoxamine), C, E, niacinamide, carnosine, and sodium selenite inhibited the in vivo and in vitro AGEs formation (Ahmed, 2005[1]; Reddy and Beyaz, 2006[29]). Similarly, compounds such as aminoguanidine, carnosine, homocarnosine, aspirin, metformin OPB-9195, phenacylthiazolium bromide derivatives are found to be effective antiglycation molecules (Reddy and Beyaz, 2006[29]). Our studies demonstrated that the synthetic 3-hydroxyflavones having antioxidant activity will inhibit the protein glycation effectively and significantly both in vitro and in vivo and are comparable to that of standard flavonoid quercetin.
Test compounds enhanced glucose uptake in L6 myotubes indicating that they have an insulinomimetic activity on muscle cells. Such type of observation has also been reported for natural flavonoids (Zygmunt et al., 2010[44]). Hence the test compounds might have the ability to modulate glucose transporter (GLUT-4) in skeletal muscle cells. It was noted that the test compounds are more potent than quercetin and among the test compounds JY-4 was most potent in enhancing the glucose uptake. These finding were also supported by glucose uptake by insulin-resistant isolated hemi-diaphragm of FD-fed rats. These findings are in accordance with published reports which say that fructose intake reduces GLUT4 translocation in the skeletal muscle, leading to a drastic decrease in glucose uptake (Shih et al., 2009[31]). Insulin-mediated (35 µIU/ml) glucose uptake in hemi-diaphragm was significantly increased in the treated groups, but not in untreated FD-rats.
Conclusion
The test molecules are novel synthetic leads (JY-1, JY-2, JY-3 and JY-4) that could potentially alleviate insulin resistance and reduce hyperglycemia, dyslipidemia and increase in vivo antioxidant defense in high-fructose fed insulin resistant rats. These compounds enhanced glucose uptake by L6 myotubes to a greater extent than quercetin. Although we have not been able to conclusively establish the precise mechanism of action of this molecule, it is possibly related to the potent in vivo antioxidant activity of this molecule. Free radical scavenging activity of JY-1, JY-2 JY-3 and JY-4 can be attributed to their ability to donate hydrogen, donate/accept electron, quenching of •OH, protection of DNA from Fenton-reagent induced damage and inhibition of lipid peroxidation. Further, NO-scavenging and ROS-inhibition in RAW 264.7 cells is suggestive of a preventive mechanism in diabetic vascular complications. The good antiglycation activity of test compounds (both in vitro and in vivo) can be attributed to their high antioxidant activity. Thus, results suggest that these synthetic flavones could be playing a role either in modulating glucose uptake in liver and skeletal muscles or in stimulating insulin action. Above conjectures warrants further study on mechanism of action of test compounds.
Acknowledgements
Authors acknowledge All India Council for Technical Education (AICTE) for providing the financial support (Scheme No. 8023/BROR/RID/RPS-171/2008-2009). Authors also acknowledge Dr. Rajesh Kumar and Dr. Nandini Pandit, Dept of Nuclear Medicine, Kasturba Medical College, Manipal for providing the facilities for radioimmunoassay of insulin, and the Indian Institute of Sciences for providing the NMR spectral data of test compounds.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Ahmed N. Advanced glycation endproducts - role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Akinmokun A, Selby PL, Ramaiya K, Alberti KGMM. The short insulin tolerance test for determination of insulin sensitivity: A comparison with the euglycaemic clamp. Diabetic Med. 1992;9:432–437. doi: 10.1111/j.1464-5491.1992.tb01813.x. [DOI] [PubMed] [Google Scholar]
- 3.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 4.Bhavesh VD, Nayak Y, Jayashree BS. In vitro antioxidant and antiglycation activity of zingiber zerumbet (wild zinger) rhizome extract. Int J Res Pharm Sci. 2013;4:482–489. [Google Scholar]
- 5.Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Calcutt NA, Cooper ME, Kern TS, Schmidt AM. Therapies for hyperglycaemia-induced diabetic complications: from animal models to clinical trials. Nat Rev Drug Discov. 2009;8:417–429. doi: 10.1038/nrd2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazarolli LH, Zanatta L, Alberton EH, Figueiredo MS, Folador P, Damazio RG, et al. Flavonoids: cellular and molecular mechanism of action in glucose homeostasis. Mini Rev Med Chem. 2008;8:1032–1038. doi: 10.2174/138955708785740580. [DOI] [PubMed] [Google Scholar]
- 8.Cheeseman KH, Beavis A, Esterbauer H. Hydroxyl-radical-induced iron-catalysed degradation of 2-deoxyribose. Quantitative determination of malondialdehyde. Biochem J. 1988;252:649–653. doi: 10.1042/bj2520649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Zhong Z, Xu Z, Chen L, Wang Y. 2',7'-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic Res. 2010;44:587–604. doi: 10.3109/10715761003709802. [DOI] [PubMed] [Google Scholar]
- 10.Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, et al. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. 2005;179:43–49. doi: 10.1016/j.atherosclerosis.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Galipeau D, Verma S, McNeill JH. Female rats are protected against fructose-induced changes in metabolism and blood pressure. Am J Physiol Heart Circ Physiol. 2002;283:H2478–H2484. doi: 10.1152/ajpheart.00243.2002. [DOI] [PubMed] [Google Scholar]
- 12.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 13.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 14.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 15.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 16.Hyakukoku M, Higashiura K, Ura N, Murakami H, Yamaguchi K, Wang L, et al. Tissue-specific impairment of insulin signaling in vasculature and skeletal muscle of fructose-fed rats. Hypertens Res. 2003;26:169–176. doi: 10.1291/hypres.26.169. [DOI] [PubMed] [Google Scholar]
- 17.Jayashree BS, Thejaswini JC, Nayak Y, Kumar DV. Synthesis of novel flavone acyl esters and correlation of log P value with antioxidant and antimicrobial activity. Asian J Chem. 2010;22:1055–1066. [Google Scholar]
- 18.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 19.Le KA, Ith M, Kreis R, Faeh D, Bortolotti M, Tran C, et al. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr. 2009;89:1760–1765. doi: 10.3945/ajcn.2008.27336. [DOI] [PubMed] [Google Scholar]
- 20.Lotito SB, Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41:1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Mathew G, Jacob A, Durgashivaprasad E, Reddy ND, Unnikrishnan MK. 6b,11b-Dihydroxy-6b,11b-dihydro-7H-indeno1,2-bnaphtho[2,1-d]furan-7-one (DHFO), a small molecule targeting NF-kappaB, demonstrates therapeutic potential in immunopathogenic chronic inflammatory conditions. Int Immunopharmacol. 2013;15:182–189. doi: 10.1016/j.intimp.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda H, Wang T, Managi H, Yoshikawa M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg Med Chem. 2003;11:5317–5323. doi: 10.1016/j.bmc.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assess-ment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee PK, Maiti K, Mukherjee K, Houghton PJ. Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol. 2006;106:1–28. doi: 10.1016/j.jep.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Patel J, Iyer A, Brown L. Evaluation of the chronic complications of diabetes in a high fructose diet in rats. Indian J Biochem Biophys. 2009;46:66–72. [PubMed] [Google Scholar]
- 26.Prabhakar KR, Veeresh VP, Vipan K, Sudheer M, Priyadarsini KI, Satish RB, et al. Bioactivity-guided fractionation of Coronopus didymus: A free radical scavenging perspective. Phytomedicine. 2006;13:591–595. doi: 10.1016/j.phymed.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reddy VP, Beyaz A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug Discov Today. 2006;11:646–654. doi: 10.1016/j.drudis.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Sharma SD, Pandey BN, Mishra KP, Sivakami S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J Biochem Mol Biol Biophys. 2002;6:233–242. doi: 10.1080/10258140290031554. [DOI] [PubMed] [Google Scholar]
- 31.Shih CC, Lin CH, Lin WL, Wu JB. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol. 2009;123:82–90. doi: 10.1016/j.jep.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 32.Somani BL, Sinha R, Gupta MM. Fructosamine assay modified for the estimation of glycated hemoglobin. Clin Chem. 1989;35:497. [PubMed] [Google Scholar]
- 33.Son SM. Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes. Diabetes Metab J. 2012;36:190–198. doi: 10.4093/dmj.2012.36.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo C, Zingarelli B, Salzman AL. Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous nitric oxide and peroxynitrite. Circ Res. 1996;78:1051–1063. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- 35.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 36.Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;253:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 37.Tsimogiannis DI, Oreopoulou V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′,4′-hydroxy substituted members. Innov Food Sci Emerg Tech. 2006;7:140–146. [Google Scholar]
- 38.van der Vies J. Two methods for the determination of glycogen in liver. Biochem J. 1954;57:410–416. doi: 10.1042/bj0570410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veerapur VP, Prabhakar KR, Kandadi MR, Srinivasan KK, Unnikrishnan MK. Antidiabetic effect of Dodonaea viscosa aerial parts in high fat diet and low dose streptozotocin-induced type 2 diabetic rats: a mechanistic approach. Pharm Biol. 2010;48:1137–1148. doi: 10.3109/13880200903527736. [DOI] [PubMed] [Google Scholar]
- 40.Veerapur VP, Prabhakar KR, Thippeswamy BS, Bansal P, Srinivasan KK, Unnikrishnan MK. Antidiabetic effect of Dodonaea viscosa (L). Lacq. aerial parts in high fructose-fed insulin resistant rats: a mechanism based study. Indian J Exp Biol. 2010;48:800–810. [PubMed] [Google Scholar]
- 41.Wu CH, Yen GC. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem. 2005;53:3167–3173. doi: 10.1021/jf048550u. [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa K, Makita Z, Shiroshita K, Ueda T, Fusegawa T, Kuwajima S, et al. Specific fluorescence assay for advanced glycation end products in blood and urine of diabetic patients. Metabolism. 1998;47:1348–1353. doi: 10.1016/s0026-0495(98)90303-1. [DOI] [PubMed] [Google Scholar]
- 43.Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods. 2005;64:207–215. doi: 10.1016/j.jbbm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Zygmunt K, Faubert B, MacNeil J, Tsiani E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem Biophys Res Commun. 2010;398:178–183. doi: 10.1016/j.bbrc.2010.06.048. [DOI] [PubMed] [Google Scholar]