Abstract
Bisphenol A (BPA) is widely used in manufacturing industries. It is commonly detected in the environment and was reported to exert oestrogenic effects which may be harmful to the reproductive system. The present study was carried out to observe the effects of oral administration of BPA on the development of the reproductive organs and plasma sex hormone levels in prepubertal male Sprague-Dawley (SD) rats. Prepubertal male SD rats (n=8 in each group) were administered BPA in the doses of 1, 5, 10 and 100 mg/kg BW (body weight) via oral gavage for a period of 6 weeks. The control animals received the vehicle for BPA (Tween 80 in distilled water). Following 6 weeks of BPA exposure, the rats exhibited less evidence of spermatogenesis. There was seminiferous epithelial damage which included disruption of intercellular junctions and sloughing of germ cells into the seminiferous tubular lumen. Furthermore, the lumina of the seminiferous tubules and the epididymis of these animals were filled with immature germ cells and cellular debris. This damage may lead to the significant reduction in the seminiferous tubular diameter in BPA-treated animals. These findings were associated with the significant lower plasma testosterone and 17β-oestradiol levels. There was no significant difference between the body weight gain, the absolute as well as relative testis weight or epididymal weight of BPA-treated animals when compared to the control animals. The findings provided further evidence of the detrimental effects of BPA on the male reproductive system.
Keywords: bisphenol A, testis, anatomy, spermatogenesis, testosterone, 17ß-oestradiol
Introduction
Various endocrine disrupting chemicals (EDCs) are commonly used in daily life. These chemicals are also present in the environment and prove to be harmful to human and animals. The environmental contaminants affect normal functions of the endocrine and reproductive systems either by mimicking or inhibiting endogenous hormone actions, or modulating the synthesis of hormones (Sonnenschein and Soto, 1998[26]). The developmental stage (embryonic, fetal and juvenile) is sensitive to EDC (Kavlock et al., 1996[12]). Exposure of the fetus to some oestrogenic compounds alters the growth and development of the reproductive organs that may lead to infertility or cancer (Naciff et al., 2002[14]; Timms et al., 2005[28]). An oestrogenic EDC can cause alteration in the hypothalamus-pituitary-testicular axis leading to dysfunctional reproductive system later in adulthood (Ramos et al., 2003[21]; Timms et al., 2005[28]).
A common EDC found in the environment is bisphenol A (BPA), which is widely used in manufacturing polycarbonate and plastic materials. Human exposure to BPA may originate from various sources and routes, including leaching from plastic lining of food cans, polycarbonate baby bottles, white dental fillings and sealants (Brotons et al., 1995[4]; Chung et al., 2012[7]; Geens et al., 2012[10]). This is evident from reports of BPA being detected in canned food (4-23 µg/can), canned beverages (7-58 µg/g) and saliva (90-913 µg/saliva one hour following application dental sealant) (Brotons et al., 1995[4]; Olea et al., 1996[17]). BPA exposure does not always occur only in the adult period, rather it even occurs in the foetal and neonatal period and the toxicokinematics is different in foetuses and neonates (Viberg and Lee, 2012[31]).
A European study described that migration of bisphenol A diglycidylether (BADGE) occurs in all species with relation to storage time-dependent manner, content of fat and the migration is not affected by sterilization conditions. It is also interesting to note that European legislation does not allow the use and presence of BADGE (Cabado et al., 2008[5]). Studies reported that these products leach BPA, which causes contamination of the environment thereby resulting human exposure and uptake in day-to-day life (Viberg and Lee, 2012[31]). Interestingly, BPA has also been detected in the body fluids and tissues such as saliva, breast milk, placenta, amniotic fluid, blood and serum (Vandenberg et al., 2010[30]). BPA has also been documented as a potential endocrine disruptor and testicular toxicant (D'Cruz et al., 2012[8]).
This clearly indicates that human exposure to this endocrine disruptor is inevitable and warrants an assessment for the risks involved. Keeping the above facts in mind, the present study was undertaken to observe the effect of BPA on the development and hormonal status in experimental animals.
An earlier study showed that mitochondrial enzymes in the testis such as succinate dehydrogenase, malate dehydrogenase, isocitrate dehydrogenase, monoamine oxidase and NAD dehydrogenase decreased in mice exposed to BPA (Anjum et al., 2011[2]). BPA has been described to induce the conversion of xanthine dehydrogenase into xanthine oxidase in the rat liver thereby increasing the reactive oxygen species (ROS) (Sakuma et al., 2010[25]). ROS generation and oxidative DNA may be responsible for the detrimental side effects of BPA on the human body.
The risk assessment studies for BPA invariably used animal models such as rats and mice. Previous studies on BPA were mostly performed on prenatal animals. The rate at which BPA is cleared from the body system was reported to be dependent on the age and gender of the animal (Matsumoto et al., 2002[13]; Inoue et al., 2005[11]). The free level of BPA remains much longer in the body system of neonatal rats compared to the adults (Matsumoto et al., 2002[13]). Their capacity to metabolize BPA was found to be increased in relation to the age of the animals (Domoradzki et al., 2004[9]).
The effects of BPA on pubertal development of the reproductive system still remain unclear. Many of the past studies focused on the reproductive system but there is paucity of literature on the histological studies with concomitant observation on the hormonal status. Majority of the population in the developing countries may not be aware of the harmful effects of BPA on the human body. Therefore, the present study was aimed to observe the effects of exogenous BPA on pubertal development of pre-pubertal male Sprague-Dawley (SD) rats.
Materials and Methods
Animals
Thirty two prepubertal male SD rats [postnatal day (PND) 21] were obtained from the Laboratory Animal Center, Faculty of Medicine, University of Malaya. The animals were maintained under controlled temperature of 23-25 °C and relative humidity of 57–60 % with 12 hr photo period. They were fed with rodent feed (Gold Coin Feedmills Pte. Ltd., Malaysia) and water ad libitum supplied in glass bottles. The study design and methodology was approved by the Animal Care and Use Committee of Faculty of Medicine, University of Malaya.
Animals were allowed to acclimatise for one week prior to treatment on PND 28. They were randomly divided into 4 groups (n=8 in each group): (i) vehicle control (Tween-80 in distilled water), (ii) BPA 1 (1 mg/kg BW of BPA, low), (iii) BPA 5 (5 mg/kg BW of BPA, medium) and (iv) BPA 100 (100 mg/kg BW of BPA, high). Tween-80 (Sigma Aldrich, USA) in distilled water (1:9 v/v) was used as vehicle for BPA (Sigma Aldrich, USA). Since the ‘no-observed-adverse-effect levels’ (NOAEL) for BPA was 5 mg/kg BW/day (Poole, van Herwijnen et al. 2004[20]), we utilized BPA at doses 1, 5 and 100 mg/kg BW which were below, at and above NOAEL. BPA was administered by oral gavage route from PND 28-70 at 10:00-12:00 h, daily. Body weights of the rats were recorded weekly.
Hormone analysis
At the end of the experimental period, animals were anesthetized under diethyl ether followed by an intraperitoneal injection of chloral hydrate (BDH, England) on PND 70 and sacrificed. Blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA) (BD Vacutainer, USA) as the anticoagulant, centrifuged and plasma obtained was stored at -80 °C for testosterone and 17β-oestradiol measurement. Plasma levels of testosterone and 17β-oestradiol were determined using enzyme immunoassay (EIA) kits (Cayman Chemical, Ann Arbor, USA).
Morphological analysis
Selected reproductive organs were harvested and weighed. The weight of these organs was recorded and then standardised against the body weight to obtain relative organ weights. These organs were subsequently fixed in Bouin’s solution and processed for histological evaluation. Paraffin sections (5 µm) of these organs were stained with Hematoxylin and eosin. Testes were examined and the seminiferous tubule diameter was measured using an image analyzer (Wetzlar, Germany).
Statistical analysis
Data was analysed using a one-way ANOVA (Prism 2.0, Graph Pad software, USA) and Dunnett’s test was used to compare between the control and treatment groups. Statistical significance was accepted at P < 0.05.
Results
Body and organ weight
All animals in the control group as well as BPA-treated groups, showed similar progressive increase in body weight throughout the six weeks’ period of study (Table 1(Tab. 1)). No difference in the absolute as well as relative weights of the testis, epididymis, seminal vesicle and prostate between the control and the BPA-treated groups was observed.
Table 1. Mean body weight of rats, mean seminiferous tubule diameters, and mean absolute and relative organ weights of control and BPA-treated rats.
Seminiferous tubule diameter
The rats treated with the highest dose of BPA (100 mg/kg bw per day) had significantly reduced seminiferous tubule diameter (p < 0.05) compared to control while the lower doses used in this study did not cause significant changes in the tubule diameter (Table 1(Tab. 1)).
Hormonal status
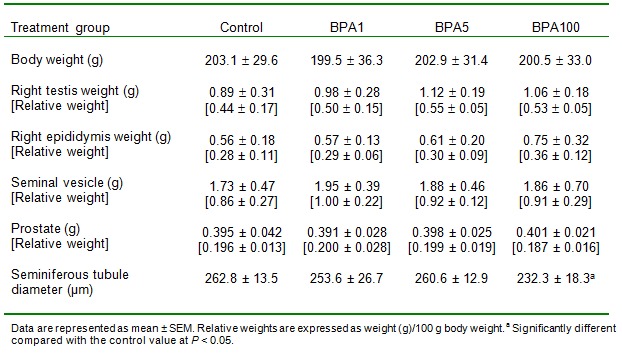
Plasma testosterone and 17β-oestradiol declined significantly following 42 days of BPA treatment in all treated groups when compared to the control (Figures 1(Fig. 1) and 2(Fig. 2)). BPA1-treated group had the lowest testosterone level with the mean concentration of 96 ± 21 pg/mL (p<0.01; versus the control group 227 ± 21 pg/mL), whereas the BPA100 treated group had markedly suppressed 17β-oestradiol with the mean concentration of 12 ± 2 pg/mL (p<0.01; versus the control group 35 ± 4 pg/mL).
Figure 1. Effects of varying doses of BPA on plasma free testosterone levels (mean ± SEM). Differences were statistically significant compared with the control value at a P < 0.01 and b P < 0.05.
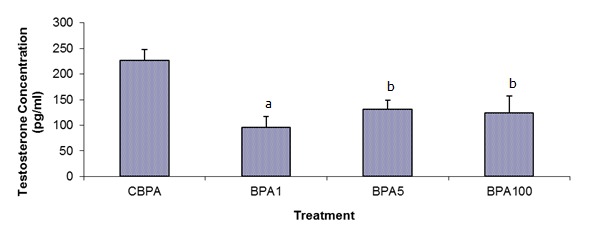
Figure 2. Effects of varying doses of BPA on plasma free 17ß-oestradiol levels (mean ± SEM). Differences were statistically significant compared with the control value at a P < 0.01 and b P < 0.05.
Morphological changes
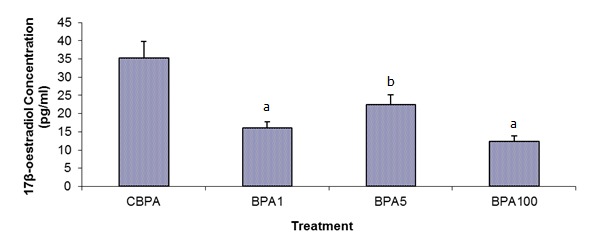
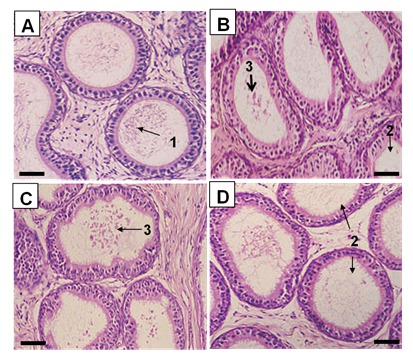
The testes of the BPA-treated animals exhibited morphological changes compared to the control testes. While normal patterns of spermatogenesis were observed in the control animals (Figure 3A(Fig. 3)), those in the BPA-treated groups clearly indicated loosening of the intercellular bridges between germ cells as well as between germ cells and Sertoli cells. This was supported by the presence of immature germ cells in the lumen of the seminiferous tubules even with the lowest dose of BPA (Figure 3B(Fig. 3)). Majority of animals in the treated groups did not have mature sperms in their seminiferous tubular lumen. At least 50 % of rats from BPA5 exhibited sloughing of germ cells into the tubular lumen (Figure 3C(Fig. 3)), and BPA100 animals exhibited the most disruption where the seminiferous tubular lumen were filled with cellular debris and immature germ cells (Figure 3D(Fig. 3)). Furthermore, the epididymal tubules of the control animals contained maturing spermatozoa (Figure 4A(Fig. 4)), while those of the BPA-treated animals showed empty lumen or lumen filled with cellular debris (Figure 4B-D(Fig. 4)).
Figure 3. Photomicrographs of testis from control group (A), BPA1 (B), BPA5 (C) and BPA100 (D). Note: 1, maturing spermatids; 2, disruption of intercellular junctions between germ cells; 3, sloughing of germ cells; and 4, lumen filled with cellular debris and immature germ cells. Scale bar (i) = 120 µm; (ii) = 60 µm.
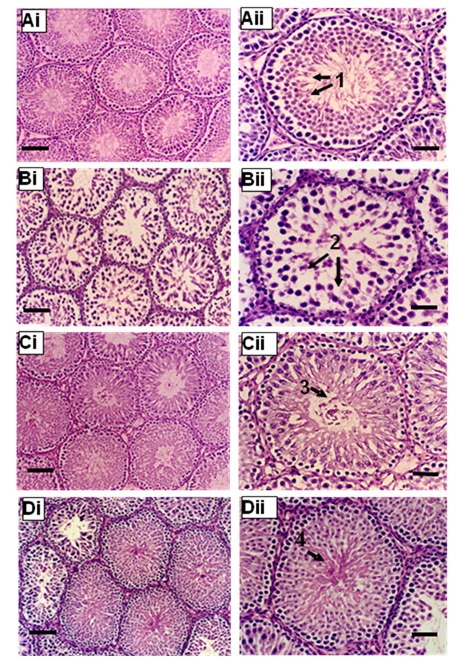
Figure 4. Photomicrographs of epididymis from control group (A), BPA1 (B), BPA5 (C) and BPA100 (D). Note: 1, lumen filled with spermatozoa; 2, lumen filled with debris and immature germ cells; 3, empty lumen. Scale bar = 60 µm.
Discussion
Male infertility is one of the major reproductive disorders that warrant public concern. This disorder has been associated with oestrogenic compounds such as BPA (Toppari et al., 1996[29]; Oliva et al., 2002[18]). BPA and its derivatives may leach into food, beverages and utensils from products containing BPA. This happens because of incomplete polymerization during manufacturing or depolymerization caused by increase of temperature induced intentionally (sterilisation purpose) or unintentionally (storage in warehouse) (Brotons et al., 1995[4]; Olea et al., 1996[17]; Biles et al., 1997[3]).
The results of the present study indicate that exposure of prepubertal male rats to BPA below, at and above NOAEL (PND 28-70) consistently showed some degree of disruption in the morphology and function of the testes. However, there were no significant changes in the absolute and relative weight of the reproductive organs suggesting that BPA did not alter the general growth of their reproductive organs. Earlier research studies did not observe any changes in the weight of testis of rats treated with BPA at 40 and 200 mg/kg BW (Yamasaki et al., 2002[33]). However, a study on adult male Holtzman rats (W13) administered with BPA 20 µg/kg BW showed a reduction in the testis weight, while another study reported BPA to cause an increase in the prostate weight, decrease in the epididymal weight and a reduction in the sperm production in rodents during pubertal development (Sakaue et al., 2001[23]).
A decrease in the weight of reproductive organs in perinatal studies with chronic exposure to BPA was associated with a reduction in the testicular testosterone level. This may be due to dependency of these glands on androgen for development and maintenance (Akingbemi et al., 2004[1]). Despite the significant decrease in the plasma testosterone level observed in the BPA-treated animals, the present study did not show any difference in the weight of the reproductive organs of the experimental animals compared to the control animals. Earlier research results showed that prenatal exposure to BPA and arochlor increased the size of the prostate and decreased the epididymal weight (Welshons et al., 1999[32]). There are reports of altered differentiation pattern of periductal stromal cells of the ventral prostate following prenatal exposure to BPA (Ramos et al., 2001[22]). However, a significant reduction was observed in the diameter of the seminiferous tubules of the animals treated with the highest dose of BPA (100 mg/kg BW). Interestingly, this observation has been reported previously by other researchers but at much higher doses of exposure to BPA (235-950 mg/kg BW) (Takahashi and Oishi, 2001[27]).
In the present study, it was noted that spermatogenesis was disrupted in all the BPA-treated SD rats. The seminiferous tubules were devoid of spermatozoa even in animals treated with the lowest dose. Furthermore, the seminiferous epithelium appeared to be disintegrating with loosening of the intercellular bridges between germ cells. Immature germ cells and degenerating germ cells were observed in the tubular lumen. The smaller seminiferous tubule diameter may be attributed to the thinning seminiferous germinal epithelium with the highest dose of BPA.
The process of spermatogenesis begins with differentiation of spermatogonia which requires testosterone action. The present results showed significant lower level of free plasma testosterone and 17β-oestradiol in the BPA-treated animals. It is postulated that the low testosterone level may have caused failure of spermatogenesis and disruption of the seminiferous epithelium. The low plasma testosterone level in BPA-treated animals was probably due to interference of proliferative activity and development of Leydig cells in rat (Nanjappa et al., 2012[16]). The same study opined that BPA acts as a mitogen in Leydig cells (Nanjappa et al., 2012[16]). The low level of 17β-oestradiol on the other hand may be responsible for apoptotic degeneration of male germ cells as described by Pentikäinen and co-researchers (Pentikäinen et al., 2000[19]). This may explain the lack of spermatozoa and the presence of cellular debris in the lumen of the epididymis of BPA-treated rats. In addition, BPA at lower doses has been reported to induce complete degeneration of epididymal epithelium with reduction in the number of spermatozoa of male Wistar rats. This observation was postulated to be due to either a decrease in serum testosterone or dihydrotestosterone (DHT) or even as a result of reduction in 5a-reductase, an enzyme required to convert testosterone to DHT (Chitra et al. 2003[6]).
Akingbemi and co-researchers (2004[1]) also reported lower levels of free plasma testosterone and 17β-oestradiol in BPA-treated animals (PND 21-35) although the duration of exposure differed from the present study (PND 28-70) (Akingbemi et al., 2004[1]). They concluded that testosterone production was reduced possibly due to a direct action of BPA on Leydig cells. This speculation was based on their finding that BPA suppressed aromatase gene expressions which lead to reduced 17β-oestradiol biosynthesis. Aromatase is an enzyme necessary for aromatization of testosterone to 17β-oestradiol. A recent study reported that BPA caused a reduction in the number of Leydig cells as well as the testosterone levels following 6 weeks of subcutaneous administration (Nakamura et al., 2010[15]). The research findings indicated that BPA exposure is not only affecting the development and function of the reproductive organs at puberty but also throughout adulthood (Akingbemi et al., 2004[1]).
Conclusion
The results of the present study provide further evidence to the adverse effects of BPA on the pubertal development of the reproductive system of male SD rats even at a level lower than NOAEL. The most significant effects of BPA were: - (1) arresting spermatogenesis; (2) disruption of seminiferous epithelium and (3) reduction in free plasma testosterone and 17β-oestradiol levels. In line with the recommendation by the World Health Organization, The Malaysian Health Ministry has imposed a ban on the use of polycarbonate infant bottles containing this xenoestrogen beginning early 2014. Campaigns for the awareness of BPA containing products such as canned food, plastic food containers and dental sealant and the possible detrimental effects may be increased.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors would like to acknowledge SUCXeS (Shimadzu-UMMC Centre for Xenobiotic Studies) Laboratory and Departments of Anatomy and Physiology, Faculty of Medicine, University Malaya for providing the facilities for this study and University of Malaya Fundamental Research grant [SF096/2007A].
References
- 1.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol a is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 2.Anjum S, Rahman S, Kaur M, Ahmad F, Rashid H, Ansari RA, et al. Melatonin ameliorates bisphenol A-induced biochemical toxicity in testicular mitochondria of mouse. Food Chem Toxicol. 2011;49:2849–2854. doi: 10.1016/j.fct.2011.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Biles JE, McNeal TP, Begley TH, Hollifield HC. Determination of bisphenol-A in reusable polycarbonate food-contact plastics and migration to food-simulating liquids. J Agric Food Chem. 1997;45:3541–3544. [Google Scholar]
- 4.Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabado AG, Aldea S, Porro C, Ojea G, Lago J, Sobrado C, et al. Migration of BADGE (bisphenol A diglycidylether) and BFDGE (bisphenol F diglycidylether) in canned seafood. Food Chem Toxicol. 2008;46:1674–1680. doi: 10.1016/j.fct.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Chitra KC, Rao KR, Mathur PP. Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: a histological and biochemical study. Asian J Androl. 2003;5:203–208. [PubMed] [Google Scholar]
- 7.Chung SY, Kwon H, Choi YH, Karmaus W, Merchant AT, Song KB, et al. Dental composite fillings and bisphenol A among children: a survey in South Korea. Int Dent J. 2012;62:65–69. doi: 10.1111/j.1875-595X.2011.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Cruz SC, Jubendradass R, Jayakanthan M, Rani SJ, Mathur PP. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: an in vivo and in silico study. Food Chemical Toxicol. 2012;50:1124–1133. doi: 10.1016/j.fct.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Domoradzki JY, Thornton CM, Pottenger LH, Hansen SC, Card TL, Markham DA, et al. Age and dose dependency of the pharmacokinetics and metabolism of bisphenol A in neonatal Sprague-Dawley rats following oral administration. Toxicol Sci. 2004;77:230–242. doi: 10.1093/toxsci/kfh054. [DOI] [PubMed] [Google Scholar]
- 10.Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 11.Inoue H, Tsuruta A, Kudo S, Ishii T, Fukushima Y, Iwano H, et al. Bisphenol a glucuronidation and excretion in liver of pregnant and nonpregnant female rats. Drug Metab Dispos. 2005;33:55–59. doi: 10.1124/dmd.104.001537. [DOI] [PubMed] [Google Scholar]
- 12.Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: A report of the US EPA-sponsored workshop. Environ Health Perspect. 1996;104:715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto J, Yokota H, Yuasa A. Developmental increases in rat hepatic microsomal UDP-glucurono-syltransferase activities toward xenoestrogens and decreases during pregnancy. Environ Health Perspect. 2002;110:193–196. doi: 10.1289/ehp.02110193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naciff JM, Hess KA, Overmann GJ, Torontali SM, Carr GJ, Tiesman JP, et al. Gene expression profile induced by 17 alpha ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat.Toxicol Sci. 2002;68:184–199. doi: 10.1093/toxsci/68.1.184. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura D, Yanagiba Y, Duan Z, Ito Y, Okamura A, Asaeda N, et al. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol Lett. 2010;194:16–25. doi: 10.1016/j.toxlet.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in ratLeydig cells. Biol Reprod. 2012;86:135. doi: 10.1095/biolreprod.111.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olea N, Pulgar R, Pérez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, et al. Estrogenicity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliva A, Giami A, Multigner L. A study in a consulting population. J Androl. 2002;23:546–550. [PubMed] [Google Scholar]
- 19.Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab. 2000;85:2057–2067. doi: 10.1210/jcem.85.5.6600. [DOI] [PubMed] [Google Scholar]
- 20.Poole A, van Herwijnen P, Weideli H, Thomas MC, Ransbotyn G, Vance C. Review of the toxicology, human exposure and safety assessment for bisphenol A diglycidylether (BADGE) Food Addit Contam. 2004;21:905–919. doi: 10.1080/02652030400007294. [DOI] [PubMed] [Google Scholar]
- 21.Ramos JG, Varayoud J, Kass L, Rodríguez H, Costabel L, Muñoz-De-Toro M, et al. Bisphenol A induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats. Endocrinology. 2003;144:3206–3215. doi: 10.1210/en.2002-0198. [DOI] [PubMed] [Google Scholar]
- 22.Ramos JG, Varayoud J, Sonnenschein C, Soto AM, Muñoz De Toro M, Luque EH. Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol Reprod. 2001;65:1271–1277. doi: 10.1095/biolreprod65.4.1271. [DOI] [PubMed] [Google Scholar]
- 23.Sakaue M, Ohsako S, Ishimura R, Kurosawa S, Kurohmaru M, Hayashi Y, et al. Bisphenol-A affects spermatogenesis in the adult rat even at a low dose. J Occup Health. 2001;43:185–190. [Google Scholar]
- 24.Sakuma S, Nakanishi M, Morinaga K, Fujitake M, Wada S, Fujimoto Y. Bisphenol A 3,4-quinone induces the conversion of xanthine dehydrogenase into oxidase in vitro. Food Chem Toxicol. 2010;48:2217–2222. doi: 10.1016/j.fct.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 25.Sakuma S, Nakanishi M, Morinaga K, Fujitake M, Wada S, Fujimoto Y. Bisphenol A 3,4-quinone induces the conversion of xanthine dehydrogenase into oxidase in vitro. Food Chem Toxicol. 2010;48:2217–2222. doi: 10.1016/j.fct.2010.05.051. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenschein C, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol. 1998;65:143–150. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi O, Oishi S. Testicular toxicity of dietary 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) in F344 rats. Arch Toxicol. 2001;75:42–51. doi: 10.1007/s002040000204. [DOI] [PubMed] [Google Scholar]
- 28.Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouseprostate and urethra. Proc Natl Acad Sci USA. 2005;102:7014–7019. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ Jr, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104:741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viberg H Lee I. A single exposure to bisphenol A alters the levels of important neuroproteins in adult male and female mice. Neurotoxicology. 2012;33:1390–1395. doi: 10.1016/j.neuro.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Welshons WV, Nagel SC, Thayer KA, Judy BM, Vom Saal FS. Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice. Toxicol Ind Health. 1999;15:12–25. doi: 10.1177/074823379901500103. [DOI] [PubMed] [Google Scholar]
- 33.Yamasaki K, Sawaki M, Noda S, Imatanaka N, Takatsuki M. Subacute oral toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the 'Enhanced OECD Test Guideline no. 407'. Arch Toxicol. 2002;76:65–74. doi: 10.1007/s00204-001-0319-1. [DOI] [PubMed] [Google Scholar]


