Abstract
This review will concentrate on the clinical pharmacology, in particular pharmacodynamic data, related to atypical antipsychotics, clozapine, risperidone, paliperidone, olanzapine, que¬tiapine, amisulpride, ziprasidone, aripiprazole, asenapine, iloperidone, lurasidone and cariprazine. A summary of their acute pharmacokinetics properties are also reported. Four new second-generation antipsychotics are available: iloperidone, asenapine, lurasidone and in the next future cariprazine. Similar to ziprasidone and aripiprazole, these new agents are advisable for the lower propensity to give weight gain and metabolic abnormalities in comparison with older second-generation antipsychotics such as olanzapine or clozapine. Actually lurasidone seems to be best in terms of minimizing unwanted alterations in body weight and metabolic variables. Therapeutic drug monitoring is not strictly necessary for all of the new antipsychotic drugs because there are no unequivocal data supporting a relationship between plasma drug levels and clinical outcomes or side effects. The exception can be represented by clozapine for which plasma levels of 350-420 ng/ml are reported to be associated with an increased probability of a good clinical response. Also for olanzapine an established therapeutic range (20-50 ng/ml) is proposed to yield an optimal response and minimize side effects.
Introduction
The primary distinction between classical and second-generation antipsychotics has been made on clinical basis. Actually conventional or “typical” antipsychotics are characterized by undesirable side effects such as extrapyramidal symptoms (EPS), hyperprolactinaemia, tardive dyskinesia and possible neuroleptic malignant syndrome. These symptoms are specific to the group as a whole and generally associated with high doses but in some cases also at clinically effective dosages. The second-generation or “atypical” antipsychotic drugs can be differentiated from traditional antipsychotics by their low or negligible levels of these unwanted side effects, by effectiveness and in general supposed increased safety. This latter has been recently questioned for the incidence of symptoms linked to metabolic syndrome.
The multiple clinical and adverse effects of different antipsychotics depend on the combination of receptors occupancy, but the dopamine pathway is still considered the primary common target for all antipsychotic drugs. More specifically, no drug has yet been identified with antipsychotic action without a significant affinity for D2 receptors.
There are 5 types of dopamine receptors in human beings: types 1 and 5 are similar in structure and drug sensitivity, and these two receptors are referred to as the "D1like" group or class of receptors. Dopamine receptor types 2, 3, and 4 are also similar in structure and are, therefore, grouped together as the "D2like" group. Dopamine receptors 2, 3 and 4, however, have significantly different sensitivities to antipsychotic drugs.
Although the D1like receptors are mentioned as a primary target for antipsychotic drugs, several findings indicate that they are not clinically relevant.
Of the 3 D2like receptors, only the D2 receptor itself is blocked by antipsychotic drugs in direct relation to their clinical antipsychotic potencies. In particular the clinical efficacy of antipsychotics is associated with a blockade of 60 % to 80 % of D2 receptors in the brain as measured by positron emission tomography (PET) or single photon emission tomography (SPET).
D2 receptor blockade in the brain is a general pharmacodynamic property of all antipsychotics, and without it a drug will not show any antipsychotic properties.
With conventional antipsychotics the level of D2 receptor blockade is directly related to the antipsychotic effect but with atypical agents the situation is more complicated (Seeman, 2002[131]; Meltzer, 2002[102]).
Three theories for atypical antipsychotic action are reported. The "fast-off-D2" theory proposes that typical antipsychotics bind more tightly than dopamine to the dopamine D2 receptor in its functional high-affinity state, with dissociation constants lower than that for dopamine. On the contrary, the atypicals bind more loosely than dopamine to the dopamine D2 receptor, with dissociation constants higher than that for dopamine. A typical example is represented by clozapine and quetiapine (Seeman, 2002[131]; Meltzer, 2002[102]).
Rapid dissociation from D2 receptors is one explanation for the improved EPS profile of atypical antipsychotics, and one that is also consistent with the theory of a lower affinity for D2 receptors for these drugs (Miyamoto et al., 2005[107]; Horacek et al., 2006[67]).
The dopamine-serotonin antagonism theory generally predicts a separation between typicals and atypicals, except that out of 20 antipsychotics there are apparent exceptions to this theory: amisulpride and remoxipride are an important exception.
Blockade of 5HT2A and D2 receptors was, in 1989, first labelled a pharmacodynamic mechanism that differentiated conventional from atypical antipsychotics. Meltzer (2002[102]) defined atypical antipsychotics as drugs showing a higher affinity for 5HT2A receptors than for D2 receptors and a lower affinity for D2 receptors than was seen with conventional antipsychotics. For the nigro-striatal dopaminergic pathway, a model was suggested in which blockade of 5HT2A receptors should lead to increased output of dopaminergic neurons into the striatum leading to displace the antipsychotic drug from its binding to D2 receptors. This could decrease the risk of EPS development (Horacek et al., 2006[67]) (Figure 1(Fig. 1), modified by Seeman, 2002[131]).
Figure 1. Atypical antipsychotics: proposed mechanisms of action (modified by Seeman, 2002).

There is the theory which predicts that new antipsychotics stimulate S-HT2A receptors by inverse agonism. Although it has long been known that the stimulation of 5HT1A receptors in animals can alleviate catalepsy caused by D2 blockade, there do not appear to be any antipsychotics that have this 5HT1A stimulating action combined with D2 blocking action. It has been proposed that the stimulation of 5HT2A receptors by an inverse action is an important contribution to atypical antipsychotic action. However, although some authors proposed that M100,907 has the desired stimulating action, this compound has shown no antipsychotic activity in humans.
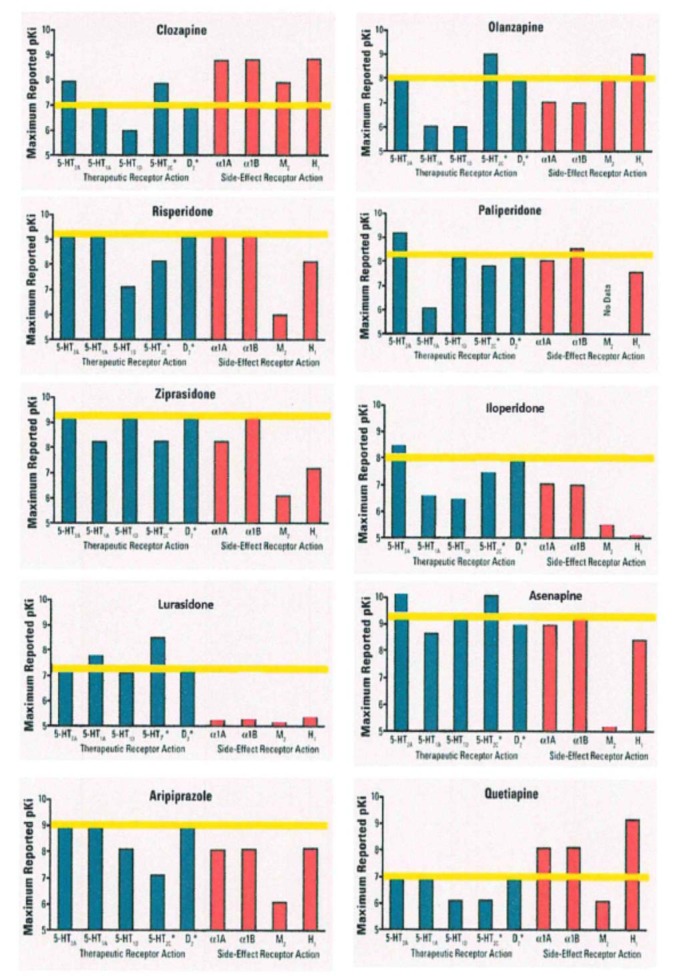
On the other hand the original classification of antipsychotics according to their chemical structure (i.e. phenothiazines, thioxanthenes, butyrophenones and diphenylpiperidines) and prevailing sedative or antipsychotic (incisiveness) potential is still relevant for the conventional antipsychotic agents. The classification of atypical anti-psychotics is linked essentially to their pharmacodynamic properties, which reflect their affinities for specific receptors. Atypical antipsychotics with a high selec-tivity for serotonin 5-HT2A receptors and dopamine D2 receptors (and also α1-adrenoceptors) are called serotonin-dopamine antagonists (SDA) (risperidone, its metabolite paliperidone, ziprasidone, iloperidone, lurasidone). Drugs showing an affinity for 5-HT2A, D2 and receptors of other systems (cholinergic, histaminergic, 5-HT1A, 5-HT1C and others) are designated as multi-acting receptor-targeted antipsychotics (MARTA) (clozapine, olanzapine, quetiapine, asenapine). Drugs that preferentially block D2 and D3 subtypes of the D2-like receptors are classified as combined D2/D3 receptor antagonists (amisulpride). A final class of atypical antipsychotics are the partial dopamine receptor agonists (aripiprazole and cariprazine) (Horacek et al., 2006[67]) (Figure 2(Fig. 2), modified by Cutler et al., 2008[35], Table 1(Tab. 1), modified by Horacek et al., 2006[67]).
Figure 2. Simplified receptor binding affinity profiles for atypical antipsychotics.
Blue, potentially therapeutics, red, potentially side effects, receptor binding affinities. The two receptors with asterisks 5HT2C and D2 should be both therapeutic and side effects inducing. The yellow line indicates the D2 affinity level for each drug (modified by Cutler et al., 2008)
Table 1. Proposed mechanisms of action of atypical antipsychotics (modified by Horacek et al., 2006).
Neuroplasticity refers to the ability of the nervous system to adapt to environmental changes and includes both synaptic plasticity consisting of remodelling of the synapses or development of new neuronal connections and neurogenesis with the development of new neurons. Atypical antipsychotics are reported to induce restructuring of neuronal networks by inducing neuroplastic changes. This effect contributes to a more substantial description of the interaction between antipsychotics and the neurodevelopmentally altered function and structure of the brain in schizophrenic patients. Moreover it can explain the longterm antipsychotic effect, suggesting a remodelling of neuronal structures (Miyamoto et al., 2005[107]).
On the other hand there are evidences to indicate dysfunctions in the connectivity of glutamatergic neurons in schizophrenia. For example, reductions in the density of prefrontal cortex pyramidal cell dendrites, the number of glutamatergic synaptosomes and the expression of messenger RNA (mRNA) for the synaptic density marker synaptophysin are found post mortem in patients who had schizophrenia (Horacek et al., 2006[67]). The theory of glutamatergic dysfunction in schizophrenia is also supported by the fact that phencyclidine and other antagonists of glutamatergic NMDA receptors may, from the phenomenological point of view, model the schizophrenic symptoms in healthy persons better than serotonergic psychotomimetic drugs (e.g. LSD). Moreover these substances can aggravate or exacerbate psychosis in patients with schizophrenia. All the antipsychotic drugs affect the glutamatergic system directly as partial agonists at the NMDA receptor-associated glycine recognition site and indirectly by the blockade of glycine and glutamate transporters at the synaptic level. By this mechanism, deficient glutamatergic signalling would be potentiated (Table 2(Tab. 2), modified by Miyamoto et al., 2005[107]).
Table 2. Potential clinical efficacy and benefits related to the mechanisms of action of antipsychotics (modified by Miyamoto et al., 2005).
This explanation is supported by the finding that the glutamate coagonist glycine and its derivatives (D-serine, D-cycloserine), as well as inhibitors of glycine reuptake, might potentiate the clinical effect of conventional antipsychotics.
Actually, bitopertin is an oral, small molecule first-in-class glycine reuptake inhibitor (GRI). Bitopertin enhances N-methyl-D-aspartate (NMDA) receptor activity, thereby targeting at least theoretically an important pathway in the treatment of psychiatric disorders, especially schizophrenia (Green et al., 2011[59]). However, clinical trials on this type of molecules do not seem to always answer to desired expectations.
The aim of this review is to update the data relating to the pharmacodynamics and pharmacokinetics of atypical antipsychotics. It will consider the following currently available second-generation antipsychotics, clozapine, risperidone, paliperidone, olan-zapine, quetiapine, aripiprazole, ziprasidone, amisulpride, asenapine, iloperidone, lura-sidone and cariprazine.
Clozapine
Clozapine (CLZ), still actually the main stone of atypical antipsychotics, belongs to the chemical class of the dibenzodiazepines. It has many unique clinical advantages over typical neuroleptic agents, including its efficacy in treatment-refractory schizophrenia, a low propensity to induce extrapyramidal symptoms (EPS), particularly tardive dyskinesia, and it does not increase serum prolactine levels. In addition to being efficacious against positive symptoms, CLZ may also be beneficial in treating negative symptoms. It has been demonstrated to prevent suicidal tendencies and some types of cognitive deficits associated with schizophrenia (Meltzer et al., 1989[104]).
CLZ has much greater antagonistic activity on cortical and limbic dopamine D4 than D2 receptors. It also antagonizes 5HT2 serotoninergic receptor subtypes (5HT2A 5HT2C) and adrenergic (α1), histamine (H1) and muscarinic receptors (M1) (Meltzer, 1992[102]).
In an extended series of patients, PET studies (Nordstrom et al., 1995[110]) confirm that CLZ is atypical with regard to degree of D2 receptor occupancy, a finding that may explain the lack of extrapyramidal side effects. The combination of relatively high D1, low D2, and very high 5-HT2 receptor occupancy rates is unique to CLZ.
Furthermore, binding studies have shown that the concentrations capable of blocking 75 % of D4 dopamine receptors match the therapeutic concentrations (Seeman and Van Tool, 1995[132]). On the other hand, another study suggested that inherited variants of D4 may explain some of the interindividual variation seen in patients’ response to CLZ (Zhao et al., 2005[150]).
From the pharmacokinetic point of view, CLZ is rapidly absorbed after oral administration. Food does not seem to affect the amount of drug absorbed. Only 27-50 % of the dose reaches the systemic circulation unchanged due to extensive first-pass metabolism.
CLZ shows a wide interindividual range with mean half-lives ranging from 9.1 to 17.4 h. The time to peak plasma concentration is between 1.1 and 3.6 h, plasma clearance between 8.7 and 53.3 L/h and distribution volume between 1.6 and 7.3 L/kg (Jann et al., 1993[68]). Steady-state plasma concentrations are reached after 7-10 days of dosing. CLZ is 95 % bound to plasma proteins, primarily alpha1-glycoprotein (Choc et al., 1990[26]).
Several studies have assessed the relationship between plasma CLZ levels and clinical responses (Perry et al., 1991[117]; Potkin et al., 1994[121]; Kronig et al., 1995[76]; Mauri et al., 2004[95]). It has been suggested that monitoring plasma CLZ concentrations might be useful in clinical management, but the effective plasma CLZ window remains debated (Freeman and Oyewumi, 1997[52]; Khan and Preskorn, 2005[75]). Most researchers find that a threshold plasma level of 350-420 ng/ml of CLZ is associated with an increased probability of a good clinical response to the drug. Moreover, much of the reviewed data indicate that increasing the oral CLZ dose in non responder patients to achieve a plasma level of at least 350-420 ng/ml can increase the number of CLZ responders (Potkin et al., 1994[121]; Fabrazzo et al., 2002[48]; Mauri et al., 2007[97]). Plasma CLZ concentrations (and the probability of reaching a given threshold) may be influenced by many factors such as age, gender and smoking (Schulte, 2003[130]).
Therapeutic drug plasma level monitoring (TDM) is useful in the case of suspected poor compliance and for patients with altered pharmacokinetics, such as those characterized by ultrarapid CYP1A2 activity. This could lead to low plasma CLZ levels and a non-response that requires the use of very high CLZ doses or the co-administration of a strong CYP1A2 inhibitor (e.g. fluvoxamine) (Eap et al., 2004[44]). Moreover, the high interindividual and low intraindividual variability of plasma CLZ levels confirm the usefulness of monitoring them.
Risperidone
Risperidone (RSP) is an approved antipsychotic agent for the treatment of schizophrenia and the acute manic phase of bipolar disorder. It is effective in both the short- and longterm treatment of schizophrenia, has a rapid onset of action, and is efficacious in treating both positive and negative symptoms (Rabinowitz and Davidson, 2001[124]). It has a lower acute incidence of side effects than haloperidol at least at therapeutical dosages of 4-6 mg/day (Peuskens, 1995[120]; Emsley, 1999[46]).
RSP is a benzisoxazole derivative, and has a strong binding affinity for serotonin (5-HT2A and 5-HT7) and dopamine D2 receptors (its affinity for D3 and D4 receptors is three times lower), with a 5HT2A/D2 affinity ratio of about 20. It also has a high affinity for adrenergic (α1 and α2) receptors, and a low affinity for histamine (H1) receptors (Ereshefsky and Lacombe, 1993[47]; Leysen et al., 1998[80]).
From a pharmacokinetic point of view RSP is rapidly absorbed after oral administration, with peak plasma concentrations being reached in about one hour; its rather good oral bioavailability is about 70-85 %. It mainly undergoes 9-hydroxylation in the liver that yields the active 9-hydroxy-risperidone metabolite (9-OH-RSP), a step that is mainly catalysed by CYP2D6 and, to a lesser extent, CYP3A4; alicyclic dehydroxylation and oxidative N-dealkylation are minor metabolic pathways (Mannens et al., 1993[90]). As the pharmacological properties of 9-OH-RSP are similar to those of RSP, both are regarded as being able to contribute to the drug’s overall antipsychotic effects in the treatment of schizophrenia, and thus represent “the active moiety”.
Genetic influences, such as CYP2D6 status, play an important role in determining the variability of its pharmacokinetic parameters. The mean half-life of RSP is three hours in extensive metabolizers (the majority of population), and 22 hours in poor metabolisers; the mean half-life of the “active moiety” (RSP and its main metabolite) is almost constant at about 22 hours in both groups. Steady-state levels are reached within five days of treatment. RSP and its main metabolite are 89 % and 77 % bound to plasma proteins (Leysen et al., 1998[80]). The mean plasma clearance of RSP is 5.4 ± 1.4 ml/min/kg, and the distribution volume is 1.1 l/kg. Most of the 9-OH-RSP is removed by means of renal excretion; it undergoes little further hepatic metabolism.
Aravagiri et al. (2003[2]) showed a large intra- and interindividual variation in the plasma concentrations of both RSP and 9-OH-RSP. The latter seems to be the major circulating active moiety and its plasma concentrations are 22-fold higher than those of RSP. The authors described a weak relationship between the daily dose and plasma concentrations of RSP, but a close relationship with the concentrations of 9-OH-RSP and those of the total active moiety. The same authors showed that the administered dose and duration of treatment did not have any effect on the metabolism of RSP to 9-OH-RSP. The concentrations of RSP seemed to vary more widely than those of its metabolite, and the relationship of both 9-OH-RSP and the active moiety to the administered dose was closer than that of RSP. The authors concluded that measuring the plasma levels of the parent compound (RSP) alone may lead to erroneous interpretations in plasma level monitoring studies (Aravagiri et al., 1998[3], 2003[2]).
Other authors have also reported that there is a linear but often weak relationship between the RSP dose and mean plasma RSP, 9-OH-RSP and active moiety levels (Ereshefsky and Lacombe, 1993[47]; Grant et al., 1994[58]; Spina et al., 2001[137]; Riedel et al., 2005[126]) but Mauri et al. (2001[95]) and Chen et al. (2004[24]) found no significant relationship.
It is available also a long-acting injectable RSP (RSP microsphere). RSP has been the first atypical antipsychotic agent to be available in a long-acting formulation: the intramuscular depot formulation called "long-acting injectable (LAI) RSP" is an aqueous suspension in a matrix of glycolic acid-lactate polymer.
Gefvert et al. (2005[54]) studied the relationship between brain D2 receptor occupancy and LAI RSP in 13 patients with schizophrenia two weeks after the third and fifth injections. Injections of 25, 50 and 75 mg respectively led to D2 receptor occupancy rates of 25-48 %, 59-83 % and 62-72 %, and plasma active moiety levels of 4.4-8.8, 15.0-31.1 and 22.5-26.3 ng/ml. The results indicate that brain D2 receptor occupancy at steady-state after injections of LAI RSP is within the range observed in patients effectively treated with 2-6 mg of oral RSP.
From a pharmacokinetic point of view the plasma concentrations of free RSP increase gradually, peak after about five weeks, and are detectable for up to seven weeks after a single intramuscular injection. Administration intervals of approximately two weeks are commonly used (Kelleher et al., 2002[73]).
The single dose pharmacokinetic profiles of LAI and oral RSP were extrapolated to steady-state in a study by Mannaert et al. (2005[89]). Plasma concentrations of the active moiety (unchanged RSP+9-OH-RSP) were measured by radioimmunoassay up to 72 h after a single oral dose of RSP 1 mg in 12 healthy volunteers, and up to 84 days after a single intramuscular injection of LAI RSP 50 mg in 26 schizophrenic patients. The most interesting results obtained at steady-state were that LAI RSP a lower than predicted peak plasma level (46 vs 62 ng/ml) and a lower than predicted degree of fluctuation between Cmax and Cmin (53 vs 145 %). The pharmacokinetic profile of LAI RSP administered every two weeks ensures the maintenance of steady-state.
It has been demonstrated a bioequivalence of the LAI and oral formulations, but the mean peak steady-state peak concentrations of the active moiety were 25-32 % lower with LAI than oral RSP, and their fluctuations were 32-42 % lower (Eerdekens et al., 2004[45]; Nesvag et al., 2006[109]).
In some patients who had previously received oral RSP, switching to the LAI formulation led to an average increase in the C/D ratio of 33 % (range 12 %-68 %) (Castberg and Spigset, 2005[23]).
The available data do not make it possible to establish the value of RSP therapeutic drug monitoring in absolute terms. The overall pharmacological effects of RSP depend on the sum of the plasma concentrations of it and its 9-OH-RSP metabolite (the “total active moiety”), and so monitoring the plasma levels of the parent compound (RSP) alone can lead to erroneous interpretations (Aravagiri et al., 2003[2]). It seems that monitoring the plasma levels of the active moiety may be useful, but further investigations are required in order to clarify the discrepancies in the results obtained so far.
It is important to understand whether there is a positive correlation between clinical responses and plasma levels of active moiety: some authors have reported a negative correlation (Riedel et al., 2005[126]) which conflicts with previously published data (Mauri et al., 2001[95]; Odou et al., 2000[111]). This discrepancy may be due to the large variability in plasma drug levels and the lack of studies using fixed dosages. No therapeutic plasma level range for RSP has yet been clearly established: the plasma threshold for parkinsonian side effects has been found to be of 74 ng/ml (Peuskens et al., 1995[120]; Marder and Meibach, 1994[91]) but it is not clear what is the minimum effective plasma concentration of the active moiety. Some data suggest that the normal range is between 15 and 60 ng/ml, but they need to be confirmed (Mauri et al., 2001[95]; Odou et al., 2000[111]; Darby et al., 1997[38]). According to the AGNP consensus guidelines, RSP TDM is recommended and the proposed plasma RSP concentrations are between 20 and 60 ng/ml. Moreover, RSP TDM should be particularly useful when medication is switched from the oral to the depot form, or vice versa (Baumann et al., 2004[8]).
Paliperidone
Paliperidone is a novel antipsychotic agent belonging to the benzisoxazole-derivatives class. Paliperidone is the major active metabolite of risperidone (OH-RSP).
Paliperidone is a centrally active dopamine D2 antagonist with predominant serotonergic 5-HT2A antagonistic activity. Paliperidone is also active as an antagonist at α1 and α2 adrenergic receptors and H1 histaminergic receptors. Paliperidone has no affinity for cholinergic muscarinic or ß1- and ß2-adrenergic receptors. The pharmacological activity of the (+)– and (-)- paliperidone enantiomers is qualitatively and quantitatively similar.
Paliperidone extended-release (ER) is approved in the United States, Europe, and globally for the treatment of schizophrenia in adults and in adolescents aged 12-17 years. It is also approved for the treatment of adults with schizoaffective disorder, both as a monotherapy and as adjunctive therapy to mood stabilizers and/or antidepressants.
It is formulated using OROS® (osmotic controlled-release system, ALZA Corporation, CA, USA) technology, which reduces peak-to-trough variations in plasma concentrations and eliminates the need for initial dose titration (Conley et al., 2006[34]). Paliperidone ER is also minimally metabolized in the liver and thus has a lower potential for clinically significant pharmacokinetic drug interactions with drugs that are metabolized by the cytochrome P450 (CYP450) enzyme system. Furthermore, no dose adjustment of paliperidone ER is needed in patients with mild-to-moderate hepatic impairment.
In a 6-week dose-finding study of paliperidone ER in male patients with schizophrenia using positron emission tomography, paliperidone ER at 6-9 mg/day resulted in an estimated dopamine D2 receptor occupancy of between 70 and 80 % in the striatum and in the temporal cortex (Citrome, 2012[32]).
Paliperidone palmitate is the long-acting injectable (LAI) formulation of the approved oral antipsychotic paliperidone ER for the treatment of schizophrenia.
Paliperidone palmitate is an ester in an aqueous-based nano-suspension. It has very low water solubility. After injection, paliperidone palmitate slowly dissolves and is hydrolyzed to paliperidone by esterases in the muscle tissue. It is intended for once-monthly intramuscular injection and it does not require any oral supplementation (Gilday and Nasrallah, 2012[57]).
Olanzapine
Olanzapine (OLZ) is a thienobenzodiazepine derivate structurally similar to clozapine that is effective in treating schizophrenia and acute manic episodes, and in preventing the recurrence of bipolar disorders (McCormack and Wiseman, 2004[100]). It is as effective and safe as haloperidol in acute reducing the psychopathological symptoms of psychosis and has been shown to have some therapeutic advantages over other classic antipsychotics in terms of symptom reduction and its adverse event profile. It has a low propensity to cause extrapyramidal effects or sustained increases in prolactin levels (Lieberman et al., 2003[81]). Nevertheless, treatment with OLZ (like CLZ) is associated with a higher risk of weight gain and, more extensively, metabolic syndrome than other typical and atypical antipsychotics (Lambert et al., 2005[77]; Duggan et al., 2005[43]).
OLZ shares higher affinity to 5-HT2A receptors than D2 receptors (high 5-HT2A/D2 ratio). In comparison to the other atypicals, olanzapine presents high affinity for serotoninergic 5-HT2A, 5-HT2C, 5-HT3, and 5-HT6 receptors, medium affinity for dopaminergic D1, D2, D3, D4, D5, and muscarinic M1–M5 receptors, 18 low affinity for adrenergic α1 and α2 receptors, and the highest affinity for histamine H1 receptors (olanzapine is the most potent histamine H1 antagonist known) (Bymaster et al., 1999[17]).
OLZ selectively reduces the activity of dopaminergic mesolimbic (A10) neurons but not dopaminergic striatal (A9) neuron fire and, in animal studies, counteracts conditioned avoidance behavior (test of antipsychotic efficacy) at a dose that is not sufficient to induce catalepsy (test of motor side effect) (Chiodo and Bunney, 1983[25]; Stockton and Rasmussen, 1996[142]; Frampton, 2010[51]). More recently, preclinical studies have shown that OLZ efficacy on psychotic and cognitive symptoms of schizophrenia may be represented by its facilitating effect on N-methyl-D-aspartic acid, which can favor brain derived neurotrophic factor (BDNF) expression (Horacek et al., 2006[67]). According to another study, treatment with OLZ markedly restored the reduction of both BDNF and tyrosine kinase (TrkB) receptors in hippocampus, associated with previous treatment with haloperidol (Parikh et al., 2004[114]). Other studies mentioned that OLZ efficacy on negative and depressive symptoms of schizophrenia might be related to inhibition of norepinephrine transporter and modulation of cytokine plasma level, as interleukin-2 declined after 8-week olanzapine treatment (Hikaru et al., 2007[66]; Schatzberg and Nemeroff, 2013[128]).
Approximately 85 % of an oral OLZ dose is absorbed but, as about 40 % is inactivated by first-pass hepatic metabolism, its oral bioavailability is about 60 %. OLZ has a mean half-life in healthy individuals of 33 hours (range 21-54 hours). Peak plasma concentrations are reached within six hours. The drug is approximately 93 % bound to plasma proteins, mainly albumin (90 %) and alpha 1-acid glycoprotein (77 %). Its distribution volume is 16.4 ± 5.1 L (SD). Mean apparent plasma clearance is 26 L/h (range 12-47 L/h). Smokers and men show greater OLZ clearance than women and non-smokers (Callaghan et al., 1999[21]). After the administration of [14C]-OLZ in a single load pharmacokinetic study, approximately 87 % of the radioactivity was excreted, with 30 % appearing in the feces and 57 % in the urine (Kassahun et al., 1997[72]). Approximately half of the radiocarbon was excreted within three days, and >70 % of the dose was recovered within seven days.
OLZ is metabolized to its 10-N- and 4'-N-glucuronides, 4'-N-desmethylolanzapine (by CYP1A2), and olanzapine-N-oxide (by flavin mono-oxygenase 3). Metabolism to 2-hydroxymethylolanzapine via CYP2D6 is a minor pathway. The 10-N-glucuronide is the most abundant metabolite (Callaghan et al., 1999[21]).
OLZ does not inhibit CYP isozymes. No clinically significant metabolic interactions have been found between OLZ and diazepam, alcohol (ethanol), imipramine, racemic mixture - R and –S warfarin, aminophylline, biperiden, lithium or fluoxetine. Fluvoxamine, an inhibitor of CYP1A2, increases the plasma concentrations of OLZ, whereas the inducers of CYP1A2 (including tobacco smoking) and UDP-glucuronyltransferase and CYP3A4 (such as carbamazepine) decrease them (Kassahun et al., 1997[72]; Callaghan et al., 1999[21]).
A number of studies have investigated the relationship between the daily OLZ dose and plasma OLZ concentrations showing that the latter increase linearly with the daily oral dose (0.12<r<0.68) (Kelly et al., 1999[74]; Gex-Fabry et al., 2003[56]; Mauri et al., 2005[96]). Moreover, some authors have demonstrated a linear relationship between the prescribed daily dose and the plasma concentration of the major N-desmethylolanzapine metabolite (Olesen and Linnet, 1999[113]).
These studies showed that mean plasma OLZ concentrations vary widely, depending on factors such as the prescribed daily dose and the duration of treatment. Given their lower OLZ clearance, women have significantly higher mean plasma OLZ levels, which become evident after the fifth week of treatment (Kelly et al., 1999[74]; Gex-Fabry et al., 2003)[56]. At commonly used daily OLZ doses (5-30 mg/day), mean plasma concentrations can range from 10 to 54 ng/ml. Considerable inter-patient variability has sometimes been observed, possibly due to unreported co-medications, non-compliance, and intrinsic interindividual variability in drug metabolism and/or clearance (Gex-Fabry et al., 2003[56]; Fellows et al., 2003[50]).
Only short-term studies of the relationships between clinical responses and plasma OLZ levels have been carried out, none of which had a treatment period of more than six weeks. The reviewed studies strongly indicate a relationship between clinical outcomes and plasma OLZ concentrations (Perry et al., 1997[118]; Lane et al., 2002[78]; Mauri et al., 2005[96]). Furthermore, given the large inter-patient variability in plasma OLZ levels at the same doses, the monitoring of blood OLZ concentrations can be considered very useful in assessing therapeutic efficacy and controlling adverse events. A therapeutic range of between 20 ng/ml and 50 ng/ml has been found (Lane et al., 2002[78]; Fellows et al., 2003[50]; Mauri et al., 2005[96]).
PET studies by Kapur et al. (1998[71]) analyzed the differences in D2 receptor occupancy on the basis of OLZ dose/plasma OLZ levels, and found that the relationship between plasma OLZ levels and D2 occupancy was described by a saturating rectangular hyperbola. OLZ is a potent 5-HT2 blocker and shows greater 5-HT2 than D2 occupancy at all doses. At a plasma concentration of 10.3 ng/ml, OLZ occupied 50 % of the available D2 receptors. Within the usual clinical range of 10-20 mg/day, D2 occupancy varies from 71 % to 80 % (Kapur et al., 1998[71]). Recently, Attarbaschi et al. (2007[6]) have explored the relationship between striatal D2 receptor occupancy and EPS in 17 bipolar patients receiving OLZ 5-45 mg/day for at least 14 days, and found a significant correlation between plasma levels and occupancy (R2= 0.55, P=0.001). The bipolar patients did not show any EPS at D2 occupancy levels of 28-80 %.
According to the AGNP-TDM consensus group, OLZ TDM is strongly recommended as an established therapeutic range (20-50 ng/ml) is proposed to yield an optimal response and minimize side effects (Baumann et al., 2004[8]).
Intramuscular OLZ is a fast-acting formulation that is also indicated for use in patients with agitation associated with schizophrenia or bipolar mania. This OLZ formulation is at least as effective as intramuscular haloperidol or lorazepam in treating patients with acute agitation associated with schizophrenia or bipolar mania, and has a faster onset of action (Wright et al., 2003[148]; Lindborg et al., 2003[82]). At doses of between 2.5 and 10.0 mg per injection, the response is dose related. The drug has a favourable safety profile (Breier et al., 2002[13]).
It is now available also OLZ long-acting formulation. OLZ long-acting injection (OLAI) is a crystalline salt composed of olanzapine and pamoic acid, that is suspended in an aqueous solution which permits a depot intramuscular formulation of olanzapine. Once injected in the gluteal muscle, the two components of the salt slowly dissociate into separate molecular compounds, olanzapine and pamoic acid. The rate of dissolution of the salt is slow, allowing for a gradual release of olanzapine into the circulation over 2 to 4 weeks.
The half-life of olanzapine pamoate is 30 days, and its steady state is reached approximately at 12 weeks. Oral supplementation of olanzapine should not be required during OLAI initiation, although a study indicates that =60 % of D2 receptor occupancy was reached only by the fifth injection cycle (Lindenmayer, 2010[83]; Di Lorenzo and Brogli, 2010[42]).
Quetiapine
Quetiapine (QTP) is a dibenzothiazepine derivate approved for the management of acute and chronic psychotic disorders, the acute phase of mania and bipolar depression. QTP has been shown to be at least as efficacious as the typical antipsychotics chlorpromazine and haloperidol in the short-term treatment of schizophrenia (Arvanitis et al., 1997[5]; Peuskens and Link, 1997[119]). Controlled clinical trials have shown that it is well tolerated, not associated with a sustained increase in plasma prolactin concentrations, and has a low propensity to cause extrapyramidal symptoms (Small et al., 1997[135]).
QTP and the active human plasma metabolite, norquetiapine (N-desalkylquetiapine) (N-QTP), interact with a broad range of neurotransmitter receptors. QTP and N-QTP exhibit affinity for brain serotonin (5HT2) and dopamine D2 receptors. QTP has a lower affinity for D1 receptors. This combination of receptor antagonism with a higher selectivity for 5HT2 relative to D2 receptors seems to contribute to the clinical antipsychotic properties and low extrapyramidal undesirable effect (EPS) liability of QTP compared to typical antipsychotics. Additionally, N-QTP has high affinity for the norepinephrine transporter (NET). QTP and N-QTP also have high affinity at histaminergic and adrenergic α1 receptors, with a lower affinity at adrenergic α2 and serotonin 5HT1A, 5HT2A and 5HT2C receptors. QTP has no appreciable affinity at muscarinic or benzodiazepine receptors (Small et al., 2002[136]).
On the other hand the dissociation time-course of QTP at dopamine D2 receptors and at serotonin (5-HT) receptors should be considered: QTP such as other atypicals, CLZ, OLZ, ziprasidone and amisulpride all bind more loosely than dopamine to the dopamine D2 receptors and have dissociation constants higher than that for dopamine. These tight and loose binding data agree with the rates of antipsychotic dissociation from the human-cloned D2 receptor. For instance, radioactive haloperidol and chlorpromazine, all dissociate very slowly over a 30-minute time span, while radioactive QTP, CLZ and amisulpride dissociate rapidly, in less than 60 seconds. These data also match clinical brain-imaging findings that show haloperidol remaining constantly bound to D2 in humans undergoing 2 positron emission tomography (PET) scans 24 hours apart. Conversely, the occupation of D2 by QTP or CLZ has mostly disappeared after 24 hours (Seeman, 2002[131]).
At doses within the recommended therapeutic range, QTP instant release (IR) has a linear pharmacokinetic profile. QTP is rapidly absorbed following the oral administration of all dose levels, with a median time to Tmax of 1-1.5 hours. Its coadministration with food only marginally increases Cmax and AUC values (DeVane and Nemeroff 2001[41]). There are no absolute bioavailability data because of the lack of an injectable formulation, but preliminary data indicate that about 70 % is absorbed. Apparent oral clearance ranges from 55 to 87 l/h, and Cmax values of 53-117 µg/L were observed after the single administration of a 25 mg dose. Multiple dose studies show Cmax values of 778-1080 ng/ml at doses of 250 mg/day. The apparent oral volume of distribution ranges from 513 to 710 L. The drug is approximately 83 % bound to plasma proteins, and is eliminated with a mean half-life of approximately six hours. The primary route of elimination is hepatic metabolism (DeVane and Nemeroff, 2001[41]).
QTP is extensively metabolized (at least 20 metabolites have been identified): only two, 7-hydroxy-quetiapine and 7-hydroxy-N-desalkyl-quetiapine (N-QTP) are pharmacologically active (Grimm et al., 1997[61]). In particular, N-QTP, a potent norepinephrine reuptake inhibitor and partial serotonin 5-HT1A receptor agonist, has been suggested as a putative mediator of QTP antidepressant activity (Altamura et al., 2012[1]).
After single subclinical doses, the mean plasma clearance of QTP is reduced by only 25 % in patients with severe renal impairment (creatinine clearance 10-30 ml/min/ 1.73 m2) or hepatic impairment (stable alcoholic cirrhosis) (Gunasekara and Spencer, 1998[62]). The oral clearance of QTP seems to be lower (30-50 %) in elderly patients (63-85 years) receiving 300-750 mg/day than in younger patients on similar regimens (Grimm et al., 2006[60]). It shoud be noted that an age of >70 years has been found to be associated with a marked decline in the hepatic content of CYP3A4. Dose titration may therefore need to be slower in the elderly, and the daily dose lower than in younger patients. QTP does not generally show any clinically relevant gender-related pharmacokinetic changes (DeVane and Nemeroff 2001[41]; Grimm et al., 2006)[60] but Mauri et al. (2004[98]) found higher levels/doses per kg in women than men.
The coadministration of drugs that are known to induce CYP3A4 (e.g. phenytoin, carbamazepine) may lead to a clinically relevant decrease in QTP levels (Grimm et al., 2006[60]).
Only a few studies have investigated the relationship between plasma QTP levels and clinical responses, all of which are short-term and none have used a treatment period of more than six weeks. Although some data argue in favour of the existence of a relationship between plasma QTP levels and clinical responses, they only provide some preliminary information about the meaning of plasma QTP levels (Gefvert et al., 1998[53]; Mauri et al., 2004[98]; Mauri et al., 2007[99]). Other authors have failed to identify an optimal therapeutic range for QTP (Fabre et al., 1995[49]; Small et al., 1997[135]).
A suggested therapeutic range of 70-170 ng/ml has been proposed by AGNP guidelines. TDM is therefore recommended as “useful” in order to check whether plasma concentrations are plausible for a given dose and optimize the clinical response in non responder patients with low concentrations (Baumann et al., 2004).
Given the few data concerning the relationship between clinical responses and plasma QTP levels, some clues can be extrapolated from PET studies of receptor blockades. A number of studies have investigated the relationship between the time-course of CNS dopamine and serotonin receptor blockade and plasma drug concentrations after the discontinuation of QTP treatment, and shown that the disappearance of QTP from plasma is much more rapid than the decrease in serotonin receptor occupancy (Gefvert et al., 1998[53]; Kapur et al., 2000[70]; Gefvert et al., 2001[55]). A better, but not perfect, correspondence was observed between D2 receptor dissociation and plasma QTP half-life. These data indicate a discrepancy between the time-course of receptor occupancy and plasma QTP concentrations (Gefvert et al., 2001[55]).
Kapur et al. (2000[70]) found a clear curvilinear relationship between 5-HT2A receptor occupancy and plasma QTP levels, but only a weak relationship between plasma QTP levels and D2 receptor occupancy. In an albeit very small patient sample (n=5), Gevfert et al. (2001[55]) found an apparently clear correlation between plasma QTP levels and D2 receptor occupancy in a study that used PANSS, CGI and SAS to assess clinical outcomes. The results showed that the data at QTP doses of <300 mg/day and >450 mg/ day were collected from individuals who differed clinically in that the former were uncontrolled and the latter were controlled on QTP treatment. These results suggest that a certain threshold plasma level and definite D2 occupancy is required for the efficacy of the treatment.
It is also available QTP extended release (XR) formulation: once-daily dosing of the XR formulation showed peak and trough plasma levels and central D2 receptor occupancy comparable to twice-daily dosing of the IR formulation (Mamo et al., 2008[88]). More recently XR exhibited a less pronounced D2 receptor occupancy peak receptor occupancy levels remaining higher for longer compared with IR formulation (Bui et al., 2013[15]).
Once-daily quetiapine XR produced a similar area under the plasma concentration-time curve (AUC), minimum plasma concentration (Cmin) and a slightly lower maximum plasma concentration (Cmax) than the equivalent dose of IR formulation given twice daily. In a crossover, head-to-head study, total daily exposure, measured by AUC at steady state, was less variable with XR versus IR (percent coefficient of variation 39.2 % versus 51.2 %, respectively). Compared with fasting, a high-fat meal increased the AUC and Cmax for XR formulation, whereas a light meal had no significant effect on these parameters (Bui et al., 2013[15]).
Amisulpride
Amisulpride (AMI) might be considered from a clinical point of view a second-generation antipsychotic which has been approved for the treatment of schizophrenia. It seems to be as effective as haloperidol in controlling the positive symptoms of schizophrenia, and more effective than haloperidol in controlling negative and depressive symptoms (Carriere et al., 2000[22]).
AMI is a substituted benzamide derivative, and highly selective dopamine D2 and D3 receptor antagonist. In ex vivo binding studies, it is twice as selective for D3 receptors as D2 receptors. It has no affinity for the D1, D4 and D5 receptor subtypes, and little affinity for adrenergic, histaminergic, serotonergic or cholinergic receptors (Perrault et al., 1997[116]). It has also demonstrated preferential affinity for presynaptic D2 and D3 receptors at low doses (<10 mg/kg), leading to enhanced dopamine transmission, whereas higher doses antagonise postsynaptic D2 and D3 receptors, thus reducing dopamine transmission.
On the other hand atypicality of AMI, such as other atypical antipsychotics, could be attributed by transiently occupying D2 receptors and then rapidly dissociating to allow normal dopamine neurotransmission. This could help also AMI to keep prolactin levels generally normal, to spare cognition and to obviate EPS (Seeman, 2002[131]).
Finally, it shows preferential affinity for limbic rather than nigro-striatal regions (Perrault et al., 1997[116]).
AMI is rapidly absorbed after oral administration, and has an absolute bioavailability of 50 %. Cmax is 42-56 µg/L, and is reached in 1-4 hours (Tmax); steady-state is reached after 2-3 days. Its distribution volume is 5.8 L/kg and plasma protein binding is about 17 %. Its plasma elimination half-life is 12 hours, with renal clearance of 17-20 L/h. Excretion occurs mainly via the kidneys, with 22-25 % of an oral dose being recovered in the urine as unchanged drug. In patients with renal impairment, the drug‘s half-life is unchanged but systemic clearance is reduced by one-third and so dose adjustments are required (Rosenzweig et al., 2002[127]).
AMI undergoes minimal metabolism in the liver, and produces only two main metabolites, both of which are inactive. It is mainly eliminated renally and, interestingly enough, its rate of renal excretion is about 2.5 times higher than that which might be expected from mere glomerular filtration. It is therefore likely that active drug secretion occurs.
AMI is unlikely to interact with other drugs, and does not affect the activity of the cytochrome P450 system (Rosenzweig et al., 2002[127]).
Age and gender have a significant effect on dose-corrected AMI plasma concentrations, which are higher in older patients and women (Bergemann et al., 2004[10]), possibly because of a gender difference in the drug’s renal clearance. Co-medication with lithium and clozapine increases dose-corrected AMI plasma concentrations (Mauri et al., 1996[92]; Bergemann et al., 2004[10]).
Little is known about the therapeutic significance of plasma AMI concentrations. Only one study has considered the range of therapeutic plasma AMI levels, and the correlation beween daily oral doses and plasma concentrations (Bergemann et al., 2004[10]). There was a positive correlation (r = 0.50, p <0.001) between the daily AMI dose and plasma concentrations. As in the case of most antipsychotics, patients commonly show great interindividual variance in plasma AMI levels. The therapeutic plasma AMI levels of about 367 ng/ml were associated with stable clinical improvement. The therapeutic range of 100-400 ng/ml has been proposed by the AGNP-TDM consensus guidelines, which is based on data from non-systematic clinical experiences (Baumann et al., 2004[8]).
Ziprasidone
Ziprasidone (ZPS) is a benzothiazolylpiperazine developed from the chemically-related antipsychotic tiospirone, and has been reported to be effective on positive, negative and depressive symptoms in the short-term treatment of schizophrenia (Caley and Cooper, 2002[20]). It has been associated with a low incidence of sedative effects, little likelihood of extrapyramidal symptoms and postural hypotension, no anticholinergic effects, and only mild transient hyperprolactinaemia (Gunasekara et al., 2002[63]). Unlike most atypical antipsychotic drugs, ZPS is not associated with weight gain, hyperlipidemia or high plasma glucose levels (Caley and Cooper, 2002[20]).
This clinical profile may be related to its unique combination of pharmacological activities at human receptors: it is a dopamine D2 and serotonin 5-HT2A receptor antagonist (with greater in vitro affinity for the 5-HT2A receptor) (Schmidt et al., 2001[129]; Caley and Cooper, 2002[20]), a 5-HT1A agonist, and a 5-HT2C and 5-HT1B/D antagonist. Like antidepressant drugs, it also inhibits the reuptake of serotonin and noradrenaline (Schmidt et al., 2001[129]; Gunasekara et al., 2002[63]).
A significant positive correlation between plasma ZPS levels and the occupancy of both D2 and 5-HT2 receptors has been reported, with the relationship described by a hyperbolic curve (Mamo et al., 2004[87]).
From a pharmacokinetic point of view the absolute bioavailability of a 20 mg oral dose of ZPS under fed conditions is 60 %. The duration and extent of ZPS absorption may be as much as doubled in the presence of food, whereas its half-life is shorter than under fasting conditions. ZPS has a mean apparent distribution volume of 1.5 L/kg. It is highly bound to plasma proteins, primarily albumin and alpha-1-acid glycoprotein. It’s pharmacokinetics seem to be linear as both AUC and Cmax linearly increase with increasing doses, and its half-life of ZPS at steady-state has been reported to be 8-10 hours (Miceli et al., 2000[105]; Wilner et al., 2000[146]). Age and gender do not have a clinically significant influence on the pharmacokinetics of ZPS (Stimmel et al., 2002[140]).
ZPS is highly metabolized in humans, with less than 5 % of the administrated dose being excreted in unchanged form. The initial metabolic pathway involves CYP3A4, which is responsible for two alternative oxidation pathways: oxidation at the sulphur atom of the benzisothiazole ring to yield ZPS sulfoxide, and oxidative cleavage to yield benzisothiazole piperazine (BITP) (Beedham et al., 2003[9]). These metabolites themselves undergo varying degrees of subsequent metabolism. CYP3A4 is also thought to be the enzyme responsible for the formation of ZPS sulfone, the only metabolite that may contribute to the clinical activity of ZPS. There are 12 different circulating metabolites of ZPS, four of which are major: BITP-sulfoxide, BITP-sulfone, ZPS-sulfoxide and S-methyldihydro ZPS (Prakash et al., 1997[122]).
Clinical studies have shown mean plasma concentrations of 35-109 ng/ml at the doses commonly used in clinical settings (80-160 mg/day), with steady-state being reached by the seventh day (Daniel et al., 1999[37]; Mauri et al., 2007[93]). There are no published studies of the relationships between plasma ZPS levels and clinical responses, and so the optimal therapeutic range is still poorly defined. According to the AGNP consensus guidelines, TDM is “probably useful” in patients receiving ZPS because of the current lack of valid clinical data. It may be useful to check whether plasma concentrations are plausible for a given dose (Baumann et al., 2004[8]).
It is available the ZPS mesylate intramuscular formulation. Its pharmacokinetics include the rapid attainment of therapeutic drug levels (Tmax <60 minutes post-dosing), a mean terminal elimination half-life of 2-5 hours, approximately 100 % bioavailability, drug exposure that increases in a dose-related manner, and little drug accumulation even after three days of repeated intramuscular administrations, with low concentrations of ZPS 12-18 hours after the last intramuscular injection (Preskorn, 2005[123]). The rapid plasma clearance of ZPS after intramuscular administration leads to little or no persistence of plasma drug levels when switching from intramuscular to oral drug administration. No clinically significant age, sex or race related effects on the pharmacokinetics of intramuscular ZPS have been reported (Miceli et al., 2005[106]).
Aripiprazole
Aripiprazole (ARI) might be considered a “third-generation” antipsychotic agent. ARI has been reported to be effective and well tolerated in short-term and longer-term studies in patients with schizophrenia or schizoaffective disorders (Swainston and Perry, 2004[143]; Stip and Tourjman, 2010[141]). In addition, a potential role for ARI in acute mania so as in depression is supported by the findings of clinical trials in patients with bipolar I or major depressive disorder (Weber et al., 2008[145]; De Fazio et al., 2010[39]; Brown et al., 2013[14]; Stewart et al., 2014[139]).
The most frequent reported adverse effects are headache, anxiety, insomnia, nausea, vomiting, and lightheadedness. The incidence of extrapyramidal symptoms is lower than with haloperidol. The increase in prolactin levels and QTc prolongation are reported to be similar in patients treated with ARI and placebo.
ARI is a quinolinone derivative. It shows a high affinity for dopamine D2 and D3 receptors, and serotonin 5-HT1A, 5-HT2A and 5-HT2B receptors. In particular ARI has partial-agonist activity at dopamine D2 and D3 receptors and serotonin 5-HT1A receptors, and antagonist activity at 5-HT2A receptors (Lawler et al., 1999[79]; Jordan et al., 2002[69]; Burris et al., 2002[16]).
Really the mechanism of action of ARI is not yet known, but evidence suggests that its efficacy in the treatment of the positive and negative symptoms of schizophrenia and its lower propensity for extrapyramidal symptoms (EPS) may be attributable to ARI partial agonist activity at dopamine D2 receptors. At serotonin 5-HT1A receptors, in vitro studies have shown that aripiprazole acts as a partial agonist whereas at serotonin 5-HT2A receptors aripiprazole is an antagonist. The main active metabolite, dehydro-aripiprazole, has affinity for dopamine D2 receptors and thus has some pharmacological activity similar to that of the parent compound.
A positron emission tomography study in patients with schizophrenia found that at 10 mg/day ARI had high mean occupancy at striatal D2 receptors (putamen, 87 %; caudate, 93 %; ventral striatum, 91 %), lower mean occupancy at 5-HT2 receptors (54 %–60 %), and lower mean occupancy at 5-HT1A receptors (16 %). A study that analyzed the relationship between occupancy at the D2 receptor and clinical response in schizophrenia suggested that at least 60 % of receptors must be blocked for psychotic symptoms to be reduced (Mamo et al., 2007[86]).
As it has partial agonist activity on dopamine D2 and serotonin 5-HT1A receptors, and also antagonizes 5-HT2A receptors, is considered a "dopamine-serotonin stabilizer" (Yokoi et al., 2002[149]; McGavin and Goa, 2002[101]).
From a pharmacokinetic point of view ARI is well absorbed, with peak plasma concentrations occurring within 3-5 hours of administration. Oral availability is 87 %. The mean elimination half-life is about 75 hours for ARI and 94 hours for its active metabolite. A linear pharmacokinetic profile has been observed at all doses between 5 and 30 mg/day, with peak plasma concentrations being reached within 3-5 hours and steady-state plasma concentrations being reached by day 14. The elimination half-life is 48-68 hours, distribution volume 404 L (4.9 L/kg), and oral clearance 3.3-4.0 L/h. Protein binding is extensive (>99 %), primarily to albumin (Winans, 2003[147]).
It has been found that therapeutic doses of lithium and divalproex have no clinically significant effects on the pharmacokinetics of ARI in patients with schizophrenia or schizoaffective disorder (Citrome et al., 2005[33]; DeLeon et al., 2004[40]).
Metabolism takes place in the liver via CYP3A4 and CYP2D6, primarily as a result of dehydrogenation, hydroxylation and N-dealkylation. At steady-state, about 40 % of the plasma ARI level is represented by the major metabolite, dehydroARI. Although it is known that the affinity of dehydroARI to D2 receptors is similar to that of ARI, its contribution to ARI’s clinical effects has not been assessed. Excretion occurs via the kidney and liver, with 25 % of the dose being recovered in the urine (<1 % unchanged) and 55 % in the feces (18 % unchanged) (Citrome et al., 2005[33]; Mallikaarjun et al., 2004[85]). According to the manufacturer, no dose adjustments are required on the grounds of hepatic or renal impairment, age, gender, race or smoking status.
The dosage adjustment of ARI is necessary when it is coadministered with CYP3A4 and CYP2D6 inhibitors (since aripiprazole concentration is increased) and with inducers of CYP3A4 (since aripiprazole concentration is decreased) (Mallikaarjun et al., 2004[85]).
There are no published data concerning plasma ARI levels, and so it is not known if a therapeutic plasma concentration range even exists: studies of the relationships betwen plasma levels and clinical responses are required.
There are two ARI formulations for intramuscular use with different dosages, dosing frequencies, and indications.
ARI injection (9.75 mg per vial) is a short-acting formulation indicated for agitation in patients with schizophrenia or mania.
ARI Depot is a long-acting ARI formulation, available in several countries in Europe and USA, with 4 week dosing intervals indicated for the treatment of schizophrenia.
ARI long-acting activity is presumably primarily due to the parent drug, aripiprazole, and to a lesser extent, to its major metabolite, dehydro-aripiprazole, which has been shown to have affinities for D2 receptors similar to the parent drug and represents about 29 % of the parent drug exposure in plasma.
ARI absorption into the systemic circulation is slow and prolonged following intramuscular injection due to low solubility of ARI particles. Following a single intramuscular dose, the plasma concentrations of ARI gradually rise to reach maximum plasma concentrations at a median Tmax of 5-7 days. The mean ARI terminal elimination half-life is 29.9 days and 46.5 days after every 4-week injection of ARI long-acting 300 mg and 400 mg, respectively, and steady state concentrations are attained by the fourth dose. Approximate dose-proportional increases in ARI and dehydro-aripiprazole concentrations and AUC parameters are observed after every four week ARI long-acting injections of 300 mg and 400 mg.
Elimination of aripiprazole is mainly through hepatic metabolism involving two P450 isozymes, CYP2D6 and CYP3A4. Aripiprazole is not a substrate of CYP1A1, CYP1A2, CYP2A6, CYP2B6,CYP2C8, CYP2C9, CYP2C19, or CYP2E1 enzymes.
Asenapine
Asenapine (ASN), available as an orally disintegrating tablet administered sublingually, differs from other oral antipsychotics in that it is absorbed through the oral mucosa.
ASN has recently been added as a treatment option for schizophrenia for adults by the US Food and Drug Administration and as monotherapy or adjunctive therapy with lithium or valproate in the treatment of manic or mixed episodes associated with bipolar I disorder. ASN is also indicated in the European Union for the treatment of moderate to severe manic episodes associated with bipolar I disorder (Szegedi et al., 2012[144]; Bobo, 2013[12]). A sedative profile of the drug is generally proved.
The human receptor signature of ASN is characterized by high affinity for serotonin (5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5, 5-HT6, 5-HT7), α-adrenergic (α1, α2A, α2B, α2C), dopamine (D1, D2, D3, D4) and histamine (H1, H2) receptors, but minimal affinity for muscarinic receptors (Peeters et al., 2011[115]).
In particular, ASN has substantially higher affinity (Ki in nM) to serotonin 5-HT2C (0.03), 5-HT2A (0.06), 5-HT7 (0.13), 5-HT2B (0.16), 5-HT6 (0.25), and dopamine D3 (0.42) receptors than to dopamine D2 receptors (1.3). Binding affinity to histamine H1 (1.0), dopamine D4 (1.1), norepinephrine α1 (1.2), and norepinephrine α2 (1.2) receptors approximates that for dopamine D2 receptors. Low affinity to muscarinic receptors would theoretically predict a low propensity for causing anticholinergic side effects. The remaining complex pharmacodynamic profile of ASN is of potential interest, particularly for the serotonin 5-HT7 receptor where there are pre-clinical findings of a possible pro-cognitive effect (Ballaz et al., 2007[7]). Antagonism at serotonin 5-HT2C receptors can also theoretically be expected to produce desirable clinical effects, including improvements in both cognition and mood (Shayegan and Stahl, 200[134]4; Citrome, 2013[27]).
In vitro studies have demonstrated that ASN is metabolized to N-desmethylasenapine, asenapine N-oxide, 11-hydroxyasenapine. The main metabolite, N-desmethylasenapine, and the phase II metabolite, asenapine N+-glucuronide, show affinities that are 10- to 100-fold lower than that of asenapine for the most relevant of these receptors; an exception is 5-HT1A, for which the affinity of N-desmethylasenapine is similar to that of ASN (Peeters et al., 2011[115]).
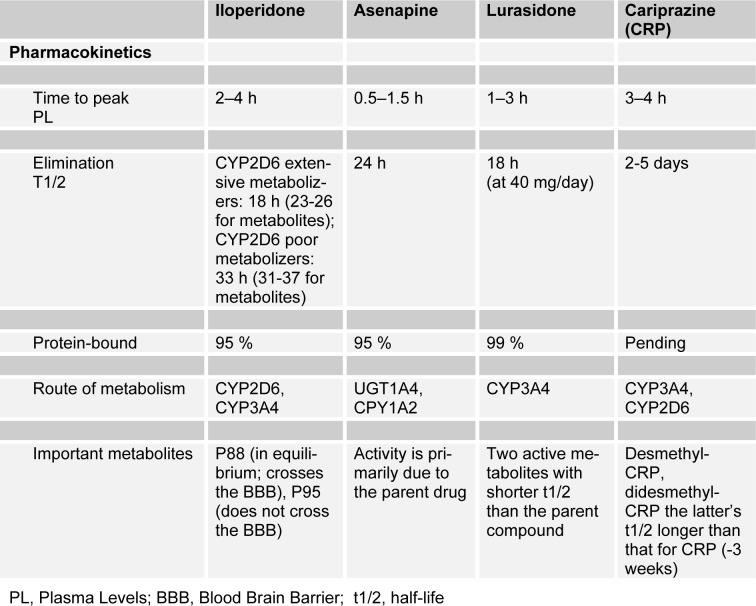
The pharmacokinetic characteristics of ASN are reported in Table 3(Tab. 3) (modified by Citrome, 2013[32]). It is rapidly absorbed. Absolute bioavailability is 35 %, mean Cmax is approximately 4 ng/mL, and Tmax is 0.5 to 1.5 h. Steady-state concentrations are reached within 3 days of twice-daily dosing. Food or water may decrease asenapine exposure. It is rapidly distributed throughout the body. Vd is approximately 20 to 25 L/kg; 95 % bound to plasma proteins. ASN is metabolized in the liver by direct glucuronidation by UGT1A4 and by oxidative metabolism by CYP-450 isoenzymes (predominantly CYP1A2). Elimination is 50 % in urine and 40 % in feces. The mean terminal half-life is approximately 24 h. Plasma Cl after IV administration is 52 L/h. Pharmacokinetics are similar in patients with varying degrees of renal impairment compared with healthy patients. The effect of dialysis on the pharmacokinetics of asenapine has not been studied. Severe hepatic impairment exposure was 7 times higher than in healthy patients; mild or moderate hepatic impairment exposure was 12 % higher than in healthy patients. In elderly patients ASN Cl is decreased, increasing exposure by 30 % to 40 % (Peeters et al., 2011[115]; Citrome, 2014[28]).
Table 3. Pharmacokinetic characteristics of new oral antipsychotics (modified by Citrome, 2013).
Lurasidone
Chemically, lurasidone (LRS) is structurally related to perospirone and ziprasidone and other benzisothiazoles including the benzisoxazole derivatives risperidone, its active metabolite paliperidone, and iloperidone. LRS has shown efficacy in the treatment of acute exacerbation of schizophrenia in a series of short-term placebo controlled studies and in a more recent longterm studies (Bobo, 2013[12]; Stahl et al., 2013[137]).
LRS has high affinity for the dopamine D2 and 5-HT2A receptors. However, it has the highest affinity of any atypical antipsychotic for the 5-HT7 receptor and its potent 5-HT7 receptor antagonism, might be beneficial for mood and cognition (Harvey et al., 2011[65]; Luoni et al., 2014[84]]
LRS also has high affinity for the 5-HT1A subtype, with which it interacts as a partial agonist such as the benzisothiazoles perospirone and ziprasidone, but not risperidone and iloperidone (Harvey et al., 2011[65]). 5-HT1A receptors, widely expressed in the central nervous system and upregulated in the frontal cortex, have been implicated in the enhancement of cognitive function in the schizophrenic patients. The stimulation of these receptors could normalize frontal cortex function and reduce side effects induced by dopamine D2 receptor blockade such as EPS, including dystonia and diskinesia, and the iperprolactinemia (Citrome, 2012[31]).
In addition to the other unique binding profiles of other monoamine receptors, the lack of affinity for the dopamine D4 receptor by LRS might also contribute, at least partly, to its cognitive enhancing effect (Murai et al., 2014[108]).
LRS has affinity for α2C-adrenergic receptors and low affinity for α1-adrenergic receptors (orthostatic hypotension) (Citrome, 2013[29]).
It has minimal affinity for 5-HT2C receptors and it has negligible affinity for histamine H1 and muscarinic receptors, which are linked to sedation and weight gain, and have negative cognitive effects.
From a pharmacokinetic point of view LRS is rapidly absorbed, reaching peak concentrations within 1.5–3 hours (Tmax) after single and multiple oral doses. Its pharmacokinetics was linear in the range of 20 to 160 mg in healthy and schizophrenic subjects, but interindividual variability was high (30 %–60 %) in terms of plasma maximum concentrations (Cmax) and area under the curve (AUC). Absorption increased when the drug was taken with food AUC and Cmax increasing two- to three-fold. LRS has high binding to human plasma albumin and alpha-1-glycoprotein (99 %).
Elimination is essentially by metabolism, primarily involving CYP3A4. The two main metabolites, the acidic derivative ID-20219 and its hydroxylated derivative ID-20220, have negligible affinity for the D2 receptors and 5-HT1A, 5-HT2A, and 5-HT7 receptors.
The mean terminal half-life at steady state in patients with schizophrenia ranges 28.8 and 37.4 hours. Thus, after repeated oral doses in schizophrenic patients, steady-state concentrations of lurasidone were achieved within 7 days (Caccia et al., 2012[19]; Citrome, 2013[29]) (Table 3(Tab. 3), modified by Citrome, 2013[29]).
Iloperidone
Iloperidone (ILP) was recently approved by the US Food and Drug Administration for the acute treatment of schizophrenia (Arif and Mitchell, 2011[4]; Rado and Janicak, 2014[125]).
ILP shows high affinity for dopamine D3 receptors, 5HT2A receptors, norepinephrine α1 receptors and an intermediate to high affinity for norepinephrine α2C receptors. It shows an intermediate affinity for dopamine D2A and D4 receptors, 5HT1A, 5HT2C, and 5HT6 receptors. Moreover ILP shows low affinity for norepinephrine α2A, α2B, ß1, ß2, receptors, histamine H1 receptors, dopamine D1 and D5 receptors. Negligible affinity is for muscarinic µ1–µ5 receptors, dopamine and norepinephrine re-uptake transporters (Rado and Janicak, 2014[125]).
Of clinical importance is the relatively high affinity for noradrenergic α1 receptors (Ki 0.36 nM), compared with the affinity for serotonin 5-HT2A and dopamine D2 receptors (Ki 5.6 and 6.3 nM, respectively). This is the explanation for iloperidone’s potential for dizziness and orthostatic hypotension and this potent effect at noradrenergic α1 receptors is the principal reason for the requirement that ILP be titrated to its therapeutic target dose range of 12–24 mg/day.
The randomized controlled trials of ILP tested several doses of ILP vs placebo, and all employed twice daily (bid) dosing even though the elimination half-life approximates 24 h, which would possibly justify once-daily dosing once titration to a tolerable dose has taken place. An open-label extension study did suggest that 12 mg given once daily at bedtime was efficacious and tolerable (Cutler et al., 2013[36]).
Other receptor-binding characteristics may be important clinically. Low affinity to muscarinic receptors would theoretically predict a low propensity for causing anticholinergic side effects, including cognitive dysfunction and gastrointestinal disturbances, at clinically relevant doses (Citrome, 2013[27]). Low affinity to histamine H1 receptors would theoretically predict a low propensity for causing sedation or weight gain (Shayegan and Stahl, 2004[134]). However, proof that these receptor binding affinities are clinically relevant in the day-to-day treatment of patients requires the conduct of clinical trials to test these hypothesized effects.
Pharmacokinetic studies have determined that iloperidone is well absorbed orally, with a bioavailability of 96 %. Peak plasma concentration (Cmax) of ILP is reached 2–4 hours after oral administration. Its elimination half-life ranges from 18 hours for extensive cytochrome P450 2D6 (CYP2D6) metabolizers to 33 hours for poor metabolizers. It is metabolized in the liver by the CYP3A4 and CYP2D6 enzyme pathways and circulates 95 % bound to serum proteins. ILP has two major metabolites: P88-8991 and P95-12113. P88-8991 shares similar receptor binding affinities with the parent compound. P95-12113 exhibits a lower affinity for the 5HT2A receptor, does not cross the blood–brain barrier, and therefore does not contribute to the clinical effects of ILP. P95-12113 binds to receptors with a significantly lower affinity than ILP. The main pharmacokinetic characteristics of iloperidone are reported in Table 3(Tab. 3) (Citrome, 2013[32]; Rado and Janicak, 2014[125]).
Cariprazine
Cariprazine is a potential antipsychotic awaiting approval from the US Food and Drug Administration. CRP is in late-stage clinical development for the treatment of schizophrenia, bipolar disorders and as an adjunctive agent for the treatment of major depressive disorder. Clinical trials showed that CRP should be titrated to target doses (3-9 mg/day) (Citrome, 2013[29]).
Cariprazine (CRP) is a dopamine D2- and D3-receptor partial agonist, with higher affinity for D3 receptors, as opposed to the D2 antagonism of most older antipsychotic agents (Seneca et al., 2011[133]; Caccia et al., 2013[18]). In particular CRP is a dopamine D3-preferring D3/D2 receptor partial agonist presently considered for the treatment of schizophrenia and bipolar disorders (Citrome, 2013[27],[29] ,[30]). Binding affinities (Ki) for dopamine D3 receptors (0.085) are an order of magnitude higher than for D2 receptors (0.49–0.69). At present, ARI and CRP are the only dopamine D2 partial agonists commercially available for the treatment of psychiatric disorders (Citrome, 2013[29]).
CRP is also a partial agonist at serotonin 5-HT1A receptors with a Ki of 3. Differing from many other second generation antipsychotics, the binding of CRP at serotonin 5- HT2A receptors is relatively weaker, with a Ki of 19. Theoretically, dopamine D3-preferring agents may exert pro-cognitive effects, as evidenced in animal studies (Gyertyán et al., 2011[64]). Serotonin 5-HT1A partial agonism, a property CRP also shares with ARI and LRS, is also thought to possibly benefit negative symptoms and cognitive deficits (Blier and Ward, 2003[11]; Ohno, 2011[112]).
CRP is rapidly absorbed, reaching peak concentrations between 3 and 4 hours after oral dosing in healthy subjects. Food marginally delayed the absorption of cariprazine, but did not affect the extent of its absorption after a single 2 mg oral dose. Its pharmacokinetics were linear in terms of area under the concentration–time curve (AUC) but maximum concentrations were more than proportional within the dose range from 3 to 5 mg in healthy subjects. Mean half-life was 2–5 days (1.5–12.5 mg/day). Cariprazine is then primarily cleared by hepatic metabolism, as are most lipophilic antipsychotics. There are two active metabolites of note: desmethyl-cariprazine and didesmethyl-cariprazine. The half-life of didesmethyl- cariprazine is substantially longer than that of cariprazine, and systemic exposure to didesmethyl-cariprazine can be several times higher than that for CRP (Caccia et al., 2013[18]). The pharmacokinetic properties of CRP are reported in Table 3(Tab. 3) (modified by Citrome, 2013[32]).
Generals conclusions
The choice of the best antipsychotic treatment for an individual patient appears complex. It should be considered the pharmacological anamnestic history of the patients including the previous history of therapeutic response and tolerability with other medications so as individual patient preferences. Patients may also have specific sensitivities to certain adverse effects of medication, such as akathisia, sedation, or weight gain. Having different options in order to optimize efficacy and tolerability for the individual patient is desirable.
Four new second-generation antipsychotics are available iloperidone, asenapine, lurasidone and in the next future cariprazine. Similar to ziprasidone and aripiprazole, these new agents have a lower propensity for weight gain and metabolic abnormalities than older second-generation antipsychotics such as olanzapine or clozapine; lurasidone is reported be best in class in terms of minimizing untoward alterations in body weight and metabolic variables. Asenapine generally have a more sedative profile.
However, iloperidone, asenapine, lura-sidone, (and cariprazine) differ among themselves in terms of on-label dosing frequency, once daily for lurasidone (and cariprazine), versus twice daily for iloperidone and asenapine, the need for initial titration to a therapeutic dose for iloperidone (and cariprazine), requirement to be taken sublingually for asenapine, requirement for administration with food for lurasidone. They also differ for lengthening of the ECG QT interval, greater for iloperidone than for asenapine while no effect observed with lurasidone. Adverse effects like akathisia have been observed with cariprazine, lurasidone and asenapine but not with iloperidone. Sedation was reported more frequenly with asenapine.
Therapeutic drug monitoring is not strictly necessary for all of the new antipsychotic drugs because there are no unequivocal data supporting a relationship between plasma drug levels and clinical outcomes or side effects, with the exception of the concentration-dependent pro-convulsant effects of CLZ. On the other hand, there are no clinical pharmacokinetic data, particularly long-term data concerning some of the other atypical antipsychotics, and this will require future research. It must be remembered that optimal plasma level ranges for CLZ, RSP and OLZ are proposed by some authors, but not all. Studies of QTP and AMI provide limited information, and there are no direct data for ZPS, ARI and the more recent second-gen¬eration antipsychotics.
In any case, it is necessary to consider the value of acute pharmacokinetic data to characterize the new drug and the heuristic value of plasma level determination together with its medico-legal importance in the case of intoxication, etc. Moreover, the importance of drug plasma level monitoring remains when it comes to identifying “pseudo-pharmacoresistance” problems such as poor compliance, high individual levels of metabolism, excessive water consumption by patients, excessive smoking, drug abuse, as well as the appearance of unpredictable side effects and possible drug interactions.
References
- 1.Altamura AC, Moliterno D, Paletta S, Buoli M, Dell’Osso B, Mauri MC, et al. Effect of quetiapine and norquetiapine on anxiety and depression in major psychoses using a pharmacokinetic approach: a prospective observational study. Clin Drug Investig. 2012;32:213–219. doi: 10.2165/11597330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Aravagiri M, Marder SR, Nuechterlein KH, Gitlin MJ. Intra- and interindividual variations in steady-state plasma concentrations of risperidone and 9-hydroxyrisperidone in schizophrenic patients treated chronically with various doses of risperidone. Ther Drug Monit. 2003;25:657–664. doi: 10.1097/00007691-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Aravagiri M, Marder SR, Wirshing D, Wirshing WC. Plasma concentrations of risperidone and its 9-hydroxy metabolite and their relationship to dose in schizophrenic patients: simultaneous determination by a high performance liquid chromatography with electrochemical detection. Pharmacopsychiatry. 1998;31:102–109. doi: 10.1055/s-2007-979308. [DOI] [PubMed] [Google Scholar]
- 4.Arif SA, Mitchell MM. Iloperidone: A new drug for the treatment of schizophrenia. Am J Health Syst Pharm. 2011;68:301–308. doi: 10.2146/ajhp100079. [DOI] [PubMed] [Google Scholar]
- 5.Arvanitis LA, Miller BG. Multiple fixed doses of "Seroquel" (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42:233–246. doi: 10.1016/s0006-3223(97)00190-x. [DOI] [PubMed] [Google Scholar]
- 6.Attarbaschi T, Sacher J, Geiss-Granadia T, Klein N, Mossaheb N, Lanzenberger R, et al. Striatal D(2) receptor occupancy in bipolar patients treated with olanzapine. Eur Neuropsychopharmacol. 2007;17:102–107. doi: 10.1016/j.euroneuro.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Ballaz SJ, Akil H, Watson SJ. The 5-HT7 receptor: role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience. 2007;149:192–202. doi: 10.1016/j.neuroscience.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 8.Baumann P, Hiemke C, Ulrich S, Eckermann G, Gaertner I, Gerlach M, et al. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry. 2004;37:243–265. doi: 10.1055/s-2004-832687. [DOI] [PubMed] [Google Scholar]
- 9.Beedham C, Miceli JJ, Obach RS. Ziprasidone metabolism, aldehyde oxidase, and clinical implications. J Clin Psychopharmacol. 2003;23:229–232. doi: 10.1097/01.jcp.0000084028.22282.f2. [DOI] [PubMed] [Google Scholar]
- 10.Bergemann N, Kopitz J, Kress KR, Frick A. Plasma amisulpride levels in schizophrenia or schizoaffective disorder. Eur Neuropsychopharmacol. 2004;14:245–250. doi: 10.1016/j.euroneuro.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- 12.Bobo WV. Asenapine, iloperidone and lurasidone: critical appraisal of the most recently approved pharmacotherapies for schizophrenia in adults. Expert Rev Clin Pharmacol. 2013:1–91. doi: 10.1586/ecp.12.70. [DOI] [PubMed] [Google Scholar]
- 13.Breier A, Meehan K, Birkett M, David S, Ferchland I, Sutton V, et al. A double-blind, placebo-controlled dose-response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Arch Gen Psychiatry. 2002;59:441–448. doi: 10.1001/archpsyc.59.5.441. [DOI] [PubMed] [Google Scholar]
- 14.Brown R, Taylor MJ, Geddes J. Aripiprazole alone or in combination for acute mania. Cochrane Database Syst Rev. 2013;17:12. doi: 10.1002/14651858.CD005000.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bui K, Earley W, Nyberg S. Pharmacokinetic profile of the extended-release formulation of quetiapine fumarate (quetiapine XR): clinical implications. Curr Med Res Opin. 2013;29:813–825. doi: 10.1185/03007995.2013.794774. [DOI] [PubMed] [Google Scholar]
- 16.Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 17.Bymaster F, Perry KW, Nelson DL, Wong DT, Rasmussen K, Moore NA, et al. Olanzapine: a basic science update. Br J Psychiatry. 1999;37:36–40. [PubMed] [Google Scholar]
- 18.Caccia S, Invernizzi RW, Nobili A, Pasina L. A new generation of antipsychotics: pharmacology and clinical utility of cariprazine in schizophrenia. Therap Clin Risk Manag. 2013;9:319–28. doi: 10.2147/TCRM.S35137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caccia S, Pasina L, Nobili A. Critical appraisal of lurasidone in the management of schizophrenia. Neuropsych Dis Treat. 2012;8:155–68. doi: 10.2147/NDT.S18059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caley CF, Cooper CK. Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacotherap. 2002;36:839–851. doi: 10.1345/aph.1A053. [DOI] [PubMed] [Google Scholar]
- 21.Callaghan JT, Bergstrom RF, Ptak LR, Beasley CM. Olanzapine: pharmacokinetic and pharmacodynamic profile. Clin Pharmacokinet. 1999;37:177–193. doi: 10.2165/00003088-199937030-00001. [DOI] [PubMed] [Google Scholar]
- 22.Carriere P, Bonhomme D, Lemperiere T. Amisulpride has a superior risk/benefit profile to haloperidol in schizophrenia: results of a multicentre, double-blind study. Amisulpride study Group. Eur Psychiatry. 2000;15:321–329. doi: 10.1016/s0924-9338(00)00401-6. [DOI] [PubMed] [Google Scholar]
- 23.Castberg I, Spigset O. Serum concentrations of risperidone and 9-hydroxyrisperidone after administration of the long-acting injectable form of risperidone: evidence from a routine therapeutic drug monitoring service. Ther Drug Monit. 2005;27:103–106. doi: 10.1097/00007691-200502000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Chen PS, Yang YK, Su SF, Liao YC, Chang JW, Yeh TL. Correlation between scores on continuous performance test and plasma concentration for schizophrenic patients on risperidone. Psychiatry Clin Neurosci. 2004;58:168–172. doi: 10.1111/j.1440-1819.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- 25.Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choc MG, Hsuan F, Honigfeld G, Robinson WT, Ereshefsky L, Crismon ML, et al. Single- vs multiple-dose pharmacokinetics of clozapine in psychiatric patients. Pharm Res. 1990;7:347–351. doi: 10.1023/a:1015859103824. [DOI] [PubMed] [Google Scholar]
- 27.Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27:879–911. doi: 10.1007/s40263-013-0105-7. [DOI] [PubMed] [Google Scholar]
- 28.Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10:893–903. doi: 10.1517/17425255.2014.908185. [DOI] [PubMed] [Google Scholar]
- 29.Citrome L. Cariprazine in schizophrenia: clinical efficacy, tolerability, and place in therapy. Adv Ther. 2013;30:114–126. doi: 10.1007/s12325-013-0006-7. [DOI] [PubMed] [Google Scholar]
- 30.Citrome L. Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability. Expert Opin Drug Metab Toxicol. 2013;9:193–206. doi: 10.1517/17425255.2013.759211. [DOI] [PubMed] [Google Scholar]
- 31.Citrome L. Lurasidone in schizophrenia: new information about dosage and place in therapy. Adv Ther. 2012;29:815–25. doi: 10.1007/s12325-012-0052-6. [DOI] [PubMed] [Google Scholar]
- 32.Citrome L. Oral paliperidone extended-release: chemistry, pharmacodynamics, pharmacokinetics and metabolism, clinical efficacy, safety and tolerability. Expert Opin Drug Metab Toxicol. 2012;8:873–888. doi: 10.1517/17425255.2012.693160. [DOI] [PubMed] [Google Scholar]
- 33.Citrome L, Josiassen R, Bark N, Salazar DE, Mallikaarjun S. Pharmacokinetics of aripiprazole and concomitant lithium and valproate. J Clin Pharmacol. 2005;45:89–93. doi: 10.1177/0091270004269870. [DOI] [PubMed] [Google Scholar]
- 34.Conley R, Gupta SK, Sathyan G. Clinical spectrum of the osmotic-controlled release oral delivery system (OROS), an advanced oral delivery form. Curr Med Res Opin. 2006;22:1879–1892. doi: 10.1185/030079906x132613. [DOI] [PubMed] [Google Scholar]
- 35.Cutler A, Ball S, Stahl SM. Dosing atypical antipsychotics. CNS Spectr. 2008;13(5 Suppl 9):1–16. [PubMed] [Google Scholar]
- 36.Cutler AJ, Kalali AH, Mattingly GW, Kunovac J, Meng X. Long-term safety and tolerability of iloperidone: results from a 25-week, openlabel extension trial. CNS Spectr. 2013;18:43–54. doi: 10.1017/S1092852912000764. [DOI] [PubMed] [Google Scholar]
- 37.Daniel DG, Zimbroff D, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20:491–505. doi: 10.1016/S0893-133X(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 38.Darby JK, Pasta DJ, Elfand L, Dabiri L, Clark L, Herbert J. Risperidone dose and blood level variability: accumulation effects and interindividual and intraindividual variability in the nonresponder patient in the clinical practice setting. J Clin Psychopharmacol. 1997;17:478–484. doi: 10.1097/00004714-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 39.De Fazio P, Girardi P, Maina G, Mauri MC, Mauri M, Monteleone P, et al. Aripiprazole in acute mania and long-term treatment of bipolar disorder. A critical review by an italian working group. Clin Drug Investig. 2010;30:827–841. doi: 10.2165/11584270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther. 2004;26:649–666. doi: 10.1016/s0149-2918(04)90066-5. [DOI] [PubMed] [Google Scholar]
- 41.DeVane CL, Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40:509–522. doi: 10.2165/00003088-200140070-00003. [DOI] [PubMed] [Google Scholar]
- 42.Di Lorenzo R, Brogli A. Profile of olanzapine long-acting injection for the maintenance treatment of adult patients with schizophrenia. Neuropsych Dis Treat. 2010;6:573–81. doi: 10.2147/NDT.S5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duggan L, Fenton M, Rathbone J, Dardennes R, El-Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database Syst Rev. 2005;18(2):CD001359. doi: 10.1002/14651858.CD001359.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eap CB, Bender S, Jaquenoud SE, Cucchia G, Jonzier-Perey M, Baumann P, et al. Nonresponse to clozapine and ultrarapid CYP1A2 activity: clinical data and analysis of CYP1A2 gene. J Clin Psychopharmacol. 2004;24:214–219. doi: 10.1097/01.jcp.0000116646.91923.2f. [DOI] [PubMed] [Google Scholar]
- 45.Eerdekens M, Van Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res. 2004;70:91–100. doi: 10.1016/j.schres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Emsley RA. Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Risperidone Working Group. Schizophr Bull. 1999;25:721–729. doi: 10.1093/oxfordjournals.schbul.a033413. [DOI] [PubMed] [Google Scholar]
- 47.Ereshefsky L, Lacombe S. Pharmacological profile of risperidone. Can J Psychiatry. 1993;38(Suppl 3):80–88. [PubMed] [Google Scholar]
- 48.Fabrazzo M, La Pia S, Monteleone P, Esposito G, Pinto A, De Simone L, et al. Is the time corse of clozapine response correlated to the time course of clozapine plasma levels? A one-year prospective study in drug-resistant patients with schizophrenia. Neuropsychopharmacology. 2002;27:1050–1055. doi: 10.1016/S0893-133X(02)00319-6. [DOI] [PubMed] [Google Scholar]
- 49.Fabre JR, Arvanitis L, Pultz J, Jones VM, Malick JB, Slotnick VB. ICI. 204,636, a novel atypical antipsychotic: early indication of safety and efficacy in patients with chronic and subchronic schizophrenia. Clin Ther. 1995;17:366–378. doi: 10.1016/0149-2918(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 50.Fellows L, Ahmad, Castle DJ, Dusci LJ, Bulsara MK, Ilett KF. Investigation of target plasma concentration–effect relationships for olanzapine in schizophrenia. Ther Drug Monit. 2003;25:682–689. doi: 10.1097/00007691-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Frampton JE. Olanzapine long-acting injection. A review of its use in the treatment of schizophrenia. Drugs. 2010;70:2289–2313. doi: 10.2165/11204930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Freeman DJ, Oyewumi LK. Will routine therapeutic drug monitoring have a place in clozapine therapy? Clin Pharmacokinet. 1997;32:93–100. doi: 10.2165/00003088-199732020-00001. [DOI] [PubMed] [Google Scholar]
- 53.Gefvert O, Bergstrom M, Langstrom B, Lundberg T, Lindstrom L, Yates R. Time course of central nervous dopamine D2 and 5HT2 receptor blockade and plasma drug concentration after discontinuation of quetiapine (Seroquel) in patients with schizophrenia. Psychopharmacology. 1998;135:119–126. doi: 10.1007/s002130050492. [DOI] [PubMed] [Google Scholar]
- 54.Gefvert O, Eriksson B, Persson P, Helldin L, Bjorner A, Mannaert E, et al. Pharmacokinetics and D2 receptor occupancy of long-acting injectable risperidone (Risperdal Consta) in patients with schizophrenia. Int J Neuropsychopharmacol. 2005;8:27–36. doi: 10.1017/S1461145704004924. [DOI] [PubMed] [Google Scholar]
- 55.Gefvert O, Lundberg T, Wieselgren I, Bergstrom M, Langstrom B, Wiesel F, et al. D2 and 5HT2A receptor occupancy of different doses of quetiapine in schizophrenia: a PET study. Eur Neuropsychopharmacol. 2001;11:105–110. doi: 10.1016/s0924-977x(00)00133-4. [DOI] [PubMed] [Google Scholar]
- 56.Gex-Fabry M, Balant-Gorgia AE, Balant LP. Therapeutic drug monitoring of olanzapine: the combined effect of age, gender, smoking, and comedication. Ther Drug Monit. 2003;25:46–53. doi: 10.1097/00007691-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Gilday E, Nasrallah HA. Clinical pharmacology of paliperidone palmitate a parenteral long-acting formulation for the treatment of schizophrenia. Rev Rec Clin Trials. 2012;7:2–9. doi: 10.2174/157488712799363307. [DOI] [PubMed] [Google Scholar]
- 58.Grant S, Fitton A. Risperidone. A review of its pharmacology and therapeutic potential in the treatment of schizophrenia. Drugs. 1994;48:253–273. doi: 10.2165/00003495-199448020-00009. [DOI] [PubMed] [Google Scholar]
- 59.Green MJ, Matheson SL, Sheperd A, Weickert CS, Carr VJ. Brain derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 60.Grimm SW, Richtand NM, Winter HR, Stams KR, Reel SB. Effects of cytochrome P450 3A modulators ketoconazole and carbamazepine on quetiapine pharmacokinetics. Br J Pharmacol. 2006;61:58–69. doi: 10.1111/j.1365-2125.2005.02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grimm SW, Stams KR, Bui K. In vitro prediction of potential metabolic drug interaction for seroquel. Schizophr Res. 1997;24:198. [Google Scholar]
- 62.Gunasekara NS, Spencer CM. Quetiapine. A review of its use in schizophrenia. CNS Drugs. 1998;9:325–340. doi: 10.2165/00023210-199809040-00007. [DOI] [PubMed] [Google Scholar]
- 63.Gunasekara NS, Spencer CM, Keating GM. Spotlight on ziprasidone in schizophrenia and schizoaffective disorder. CNS drugs. 2002;16:645–652. doi: 10.2165/00023210-200216090-00005. [DOI] [PubMed] [Google Scholar]
- 64.Gyertyán I, Kiss B, Sághy K, Laszy J, Szabó G, Szabados T, et al. Cariprazine (RGH-188), a potent D3/D2 dopamine receptor partial agonist, binds to dopamine D3 receptors in vivo and shows antipsychotic-like and procognitive effects in rodents. Neurochem Int. 2011;59:925–35. doi: 10.1016/j.neuint.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 65.Harvey PD, Ogasa M, Cucchiaro J, Loebel A, Keefe RS. Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. ziprasidone. Schizophr Res. 2011;127:188–94. doi: 10.1016/j.schres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Hikaru H, Reiji Y, Yasuhisa Y, Atsuko I, Masae M, Yoshishige I, et al. Effects of olanzapine on plasma levels of catecholamine metabolites, cytokines, and brain-derived neurotrophic factor in schizophrenic patients. Int Clin Psychopharmacol. 2007;22:21–27. doi: 10.1097/YIC.0b013e3280103593. [DOI] [PubMed] [Google Scholar]
- 67.Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- 68.Jann MW, Grimsley SR, Gray EC, Chang WH. Pharmacokinetics and pharmacodynamics of clozapine. Clin Pharmacokin. 1993;24:161–176. doi: 10.2165/00003088-199324020-00005. [DOI] [PubMed] [Google Scholar]
- 69.Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002;441:137–140. doi: 10.1016/s0014-2999(02)01532-7. [DOI] [PubMed] [Google Scholar]
- 70.Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia. Arch Gen Psychiatry. 2000;57:553–559. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- 71.Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA, et al. 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry. 1998;155:921–928. doi: 10.1176/ajp.155.7.921. [DOI] [PubMed] [Google Scholar]
- 72.Kassahun K, Mattiuz E, Nyhart E, Jr, Obermeyer B, Gillespie T, Murphy A, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos. 1997;25:81–93. [PubMed] [Google Scholar]
- 73.Kelleher JP, Centorrino F, Albert MJ, Baldessarini RJ. Advances in atypical antipsychotics for the treatment of schizophrenia: new formulations and new agents. CNS Drugs. 2002;16:249–261. doi: 10.2165/00023210-200216040-00004. [DOI] [PubMed] [Google Scholar]
- 74.Kelly DL, Conley RR, Tamminga CA. Differential olanzapine plasma concentrations by sex in a fixed-dose study. Schizophr Res. 1999;40:101–114. doi: 10.1016/s0920-9964(99)00053-5. [DOI] [PubMed] [Google Scholar]
- 75.Khan AY, Preskorn SH. Examining concentration-dependence toxicity of clozapine: role of therapeutic drug monitoring. J Psychiat Pract. 2005;11:289–301. doi: 10.1097/00131746-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Kronig MH, Munne RA, Szymansky S, Safferman AZ, Pollack S, Cooper T, et al. Plasma clozapine levels and clinical response for treatment-refractory schizophrenic patients. Am J Psychiatry. 1995;152:179–182. doi: 10.1176/ajp.152.2.179. [DOI] [PubMed] [Google Scholar]
- 77.Lambert M, Haro JM, Novick D, Edgell ET, Kennedy L, Ratcliffe M, et al. Olanzapine vs. other antipsycho-tics in actual out-patient settings: six months tolerability results from the European Schizophrenia Out-patient Health Outcomes study. Acta Psychiatr Scand. 2005;111:232–243. doi: 10.1111/j.1600-0447.2004.00451.x. [DOI] [PubMed] [Google Scholar]
- 78.Lane HY, Guo SC, Hwang TJ, Chen YS, Cheng JJ, Lee YC, et al. Effects of olanzapine plasma concentrations on depressive symptoms in schizophrenia: a pilot study. J Clin Psychopharmacol. 2002;22:530–532. doi: 10.1097/00004714-200210000-00019. [DOI] [PubMed] [Google Scholar]
- 79.Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, et al. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–627. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- 80.Leysen JE, Gommeren W, Eens A, de Chaffoy de Courcelles D, Stoof JC, Jansen PAJ. Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther. 1998;247:661–670. [PubMed] [Google Scholar]
- 81.Lieberman JA, Tollefson G, Tohen M, Green AI, Gur RE, Kahn R, et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160:1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 82.Lindborg SR, Beasley CM, Alaka K, Taylor CC. Effects of intramuscular olanzapine vs. haloperidol and placebo on QTc intervals in acutely agitated patients. Psychiatry Res. 2003;15;119:113–123. doi: 10.1016/s0165-1781(03)00107-0. [DOI] [PubMed] [Google Scholar]
- 83.Lindenmayer JP. Long-acting injectable antipsychotics: focus on olanzapine pamoate. Neuropsych Dis Treat. 2010;6:261–7. doi: 10.2147/ndt.s3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luoni A, Rocha FF, Riva MA. Anatomical specificity in the modulation of activity-regulated genes after acute or chronic lurasidone treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:94–101. doi: 10.1016/j.pnpbp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 85.Mallikaarjun S, Salazar DE, Bramer SL. Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol. 2004;44:179–187. doi: 10.1177/0091270003261901. [DOI] [PubMed] [Google Scholar]
- 86.Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: A triple tracer PET study. Am J Psychiatry. 2007;164:1411–1417. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- 87.Mamo D, Kapur S, Shammi CM, Papatheodorou G, Mann S, Therrien F, et al. A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry. 2004;161:818–825. doi: 10.1176/appi.ajp.161.5.818. [DOI] [PubMed] [Google Scholar]
- 88.Mamo DC, Uchida H, Vitcu I, Barsoum P, Gendron A, Goldstein J, et al. Quetiapine extended-release versus immediate-release formulation: a positron emission tomography study. J Clin Psychiatry. 2008;69:81–86. doi: 10.4088/jcp.v69n0111. [DOI] [PubMed] [Google Scholar]
- 89.Mannaert E, Vermeulen A, Remmerie B, Bouhours P, Levron JC. Pharmacokinetic profile of long-acting injectable risperidone at steady-state: comparison with oral administration. Encephale. 2005;31:609–615. doi: 10.1016/s0013-7006(05)82420-0. [DOI] [PubMed] [Google Scholar]
- 90.Mannens G, Huang ML, Meuldermans W, Hendrickx J, Woestenborghs R, Meibach R, et al. Absorption, metabolism, and excretion of Risperidone in humans. Drug Metab Disp. 1993;21:1134–1141. [PubMed] [Google Scholar]
- 91.Marder SR, Meibach R. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825–835. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- 92.Mauri MC, Bravin S, Bitetto A, Rudelli R, Invernizzi G. A risk-benefit assessment of sulpiride in the treatment of schizophrenia. Drug Safety. 1996;14:288–298. doi: 10.2165/00002018-199614050-00003. [DOI] [PubMed] [Google Scholar]
- 93.Mauri MC, Colasanti A, Rossattini M, Volonteri LS, Dragogna F, Valli A, et al. Ziprasidone outcome and tolerability: a practical clinical trial with plasma drug levels. Pharmacopsychiatry. 2007;40:1–4. doi: 10.1055/s-2007-973835. [DOI] [PubMed] [Google Scholar]
- 94.Mauri MC, Fiorentini A, Volonteri LS, Beraldo S, Dell’Osso B, Valli I, et al. Quetiapine in acute psychosis and personality disorders during hospitalization: assessment of a therapeutic range. Eur Neuropsychopharmacol. 2004;14(S3):283–284. [Google Scholar]
- 95.Mauri MC, Laini V, Boscati L, Rudelli R, Salvi V, Orlandi R, et al. Long term treatment of chronic schizophrenia with risperidone: a study with plasma levels. Eur Psychiatry. 2001;16:57–63. doi: 10.1016/s0924-9338(00)00536-8. [DOI] [PubMed] [Google Scholar]
- 96.Mauri MC, Steinhilber CPC, Marino R, Invernizzi E, Fiorentini A, Cerveri G, et al. Clinical outcome and olanzapine plasma levels in acute schizophrenia. Eur Psychiatry. 2005;20:55–60. doi: 10.1016/j.eurpsy.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 97.Mauri MC, Volonteri LS, Colasanti A, Fiorentini A, DeGaspari IF, Bareggi SR. Clinical pharmacokinetics of atypical antipsychotics. A critical review of the relationship between plasma concentrations and clinical response. Clin Pharmacokinet. 2007;46:359–388. doi: 10.2165/00003088-200746050-00001. [DOI] [PubMed] [Google Scholar]
- 98.Mauri MC, Volonteri LS, Fiorentini A, Invernizzi G. Bareggi SR. Clinical outcome and plasma levels of clozapine and norclozapine in drug-resistant schizophrenic patients. Schizophr Res. 2004;66:197–198. doi: 10.1016/s0920-9964(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 99.Mauri MC, Volonteri LS, Fiorentini A, Pirola R, Bareggi SR. Two weeks’ quetiapine treatment for schizophrenia, drug-induced psychosis and borderline personality disorder: a naturalistic study with drug plasma levels. Expert Opin Pharmacother. 2007;8:2207–2213. doi: 10.1517/14656566.8.14.2207. [DOI] [PubMed] [Google Scholar]
- 100.McCormack PL, Wiseman LR. Olanzapine: a review of its use in the management of bipolar disorder. Drugs. 2004;64:2709–2726. doi: 10.2165/00003495-200464230-00006. [DOI] [PubMed] [Google Scholar]
- 101.McGavin JK, Goa KL. Aripiprazole. CNS Drugs. 2002;16:779–788. doi: 10.2165/00023210-200216110-00008. [DOI] [PubMed] [Google Scholar]
- 102.Meltzer HY. Mechanism of action of atypical antipsychotic drugs. In: Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: the fifth generation of progress. Philadelphia, PA: Lippincott, Williams and Wilkins; 2002. pp. 819–831. [Google Scholar]
- 103.Meltzer HY. The mechanism of action of clozapine in relation to its clinical advantages. In: Meltzer, HY, editors. Novel antipsychotic drugs. New York: Raven Press; 1992. pp. 1–13. [Google Scholar]
- 104.Meltzer HY, Bastani B, Ramirez L, Matsubara S. Clozapine: new research on efficacy and mechanism of action. Eur Arch Psychiatry Neurol Sci. 1989;238:332–339. doi: 10.1007/BF00449814. [DOI] [PubMed] [Google Scholar]
- 105.Miceli JJ, Wilner KD, Hansen RA, Johnson AC, Apseloff G, Gerber N. Single- and multiple-dose pharmacokinetics of ziprasidone under non-fasting conditions in healthy male volunteers. Br J Clin Pharmacol. 2000;49:5–13. doi: 10.1046/j.1365-2125.2000.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miceli JJ, Wilner KD, Swan SK, Tensfeldt TG. Pharmacokinetics, safety, and tolerability of intramuscular ziprasidone in healthy volunteers. J Clin Pharmacol. 2005;45:620–630. doi: 10.1177/0091270005276485. [DOI] [PubMed] [Google Scholar]
- 107.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 108.Murai T, Nakako T, Ikeda K, Ikejiri M, Ishiyama T, Taiji M. Lack of dopamine D4 receptor affinity contributes to the procognitive effect of lurasidone. Behav Brain Res. 2014;261:26–30. doi: 10.1016/j.bbr.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 109.Nesvag R, Hendset M, Refsum H, Tanum L. Serum concentrations of risperidone and 9-OH risperidone following intramuscular injection of long-acting risperidone compared with oral risperidone medication. Acta Psychiatr Scand. 2006;114:21–26. doi: 10.1111/j.1600-0447.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 110.Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to Clozapine serum concentration: A PET study of schizophrenic patients. Am J Psychiatry. 1995;152:1444–1449. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- 111.Odou P, Levron JC, Luyckx M, Brunet C, Robert H. Risperidone drug monitoring. Clin Drug Invest. 2000;19:283–292. [Google Scholar]
- 112.Ohno Y. Therapeutic role of 5-HT1A receptors in the treatment of schizophrenia and Parkinson’s disease. CNS Neurosci Ther. 2011;17:58–65. doi: 10.1111/j.1755-5949.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olesen OV, Linnet K. Olanzapine serum concentrations in psychiatric patients given standard doses: the influence of comedication. Ther Drug Monit. 1999;21:87–90. doi: 10.1097/00007691-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 114.Parikh V, Khan MM, Mahadik SP. Olanzapine counteracts reduction of brain-derived neurotrophic factor and TrkB receptors in rat hippocampus produced by haloperidol. Neurosci Lett. 2004;356:135–139. doi: 10.1016/j.neulet.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 115.Peeters P, Bockbrader H, Spaans E, Dogterom P, Lasseter K, Marbury T, et al. Asenapine pharmacokinetics in hepatic and renal impairment. Clin Pharmacokinet. 2011;50:471–481. doi: 10.2165/11590490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 116.Perrault G, Depoortere R, Morel E, Sanger DJ, Scatton B. Psychopharmacological profile of amisulpride: an antipsychotic drug with presynaptic D2/D3 dopamine receptor antagonist activity and limbic selectivity. J Pharmacol Exp Ther. 1997;280:73–82. [PubMed] [Google Scholar]
- 117.Perry PJ, Miller DD, Arndt SV, Cadoret RJ. Clozapine and norclozapine plasma concentrations and clinical response treatment-refractory schizophrenic patients. Am J Psychiatry. 1991;148:231–235. doi: 10.1176/ajp.148.2.231. [DOI] [PubMed] [Google Scholar]
- 118.Perry PJ, Sanger T, Beasley C. Olanzapine plasma concentrations and clinical response in acutely ill schizophrenic patients. J Clin Psychopharmacol. 1997;17:472–477. doi: 10.1097/00004714-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 119.Peuskens J, Link CG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand. 1997;96:265–273. doi: 10.1111/j.1600-0447.1997.tb10162.x. [DOI] [PubMed] [Google Scholar]
- 120.Peuskens J Risperidone Study Group. Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Br J Psychiatry. 1995;166:712–726. doi: 10.1192/bjp.166.6.712. [DOI] [PubMed] [Google Scholar]
- 121.Potkin SG, Bera R, Gulasekaram B, Costa J, Hayes S, Jin Y, et al. Plasma clozapine concentrations predict clinical response in treatment-resistant schizophrenia. J Clin Psychiatry. 1994;55:133–136. [PubMed] [Google Scholar]
- 122.Prakash C, Kamel A, Gummerus J. Identification of the major human liver cytochrome P450 isoform(s) responsible for the formation of the primary metabolites of ziprasidone and prediction of possible drug interactions. Drug Metab Disp. 1997;25:863–872. doi: 10.1046/j.1365-2125.2000.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Preskorn SH. Pharmacokinetics and therapeutics of acute intramuscular ziprasidone. Clin Pharmacokinet. 2005;44:1117–1133. doi: 10.2165/00003088-200544110-00002. [DOI] [PubMed] [Google Scholar]
- 124.Rabinowitz J, Davidson M. Risperidone versus haloperidol in long-term hospitalized chronic patients in a double blind randomized trial: a post hoc analysis. Schizophr Res. 2001;50:89–93. doi: 10.1016/s0920-9964(00)00163-8. [DOI] [PubMed] [Google Scholar]
- 125.Rado JT, Janicak PG. Long-term efficacy and safety of iloperidone: an update. Neuropsychiat Dis Treat. 2014;10:409–15. doi: 10.2147/NDT.S37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riedel M, Schwarz MJ, Strassing M, Spellmann, Muller-Arends A, Weber K, et al. Risperidone plasma levels, clinical response and side-effects. Eur Arch Psychiatry Clin Neurosci. 2005;255:261–268. doi: 10.1007/s00406-004-0556-4. [DOI] [PubMed] [Google Scholar]
- 127.Rosenzweig P, Canal M, Patat A, Bergougnan L, Zieleniuk I, Bianchetti G. A review of the pharmaco-kinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers. Hum Psychopharmacol. 2002;17:1–13. doi: 10.1002/hup.320. [DOI] [PubMed] [Google Scholar]
- 128.Schatzberg AF, Nemeroff CB, editors. Essentials of clinical psychopharmacology. 3rd ed. Arlington, VA: American Psychiatric Pub; 2013. [Google Scholar]
- 129.Schmidt AW, Lebel LA, Howard HR, Jr, Zorn SH. Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol. 2001;17;425:197–201. doi: 10.1016/s0014-2999(01)01188-8. [DOI] [PubMed] [Google Scholar]
- 130.Schulte P. What is an adequate trial with clozapine? Therapeutic drug monitoring and time to response in treatment-refractory schizophrenia. Clin Pharmacokinet. 2003;42:607–618. doi: 10.2165/00003088-200342070-00001. [DOI] [PubMed] [Google Scholar]
- 131.Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- 132.Seeman P, Van Tool HHM. Deriving the therapeutic concentrations for clozapine and haloperidol: the apparent dissociation constant of a neuroleptic at the D2 or D4 receptor varies with the affinity of the competing radioligand. Eur J Pharmacol Mol Pharmacol. 1995;291:59–66. doi: 10.1016/0922-4106(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 133.Seneca N, Finnema SJ, Laszlovszky I, Kiss B, Horváth A, Pásztor G, et al. Occupancy of dopamine D2 and D3 and serotonin 5-HT1A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography. Psychopharmacology. 2011;218:579–587. doi: 10.1007/s00213-011-2343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shayegan DK, Stahl SM. Atypical antipsychotics: matching receptor profile to individual patient’s clinical profile. CNS Spectr. 2004;9(S11):6–14. doi: 10.1017/s1092852900025086. [DOI] [PubMed] [Google Scholar]
- 135.Small JG, Hirsch SRM, Arvanitis LA, Miller BG, Link CG. Quetiapine in patients with schizophrenia. A high- and low-dose double blind comparison with placebo. Arch Gen Psych. 1997;54:549–557. doi: 10.1001/archpsyc.1997.01830180067009. [DOI] [PubMed] [Google Scholar]
- 136.Small JG, Kellams JJ, Kolar MC. Relationship between quetiapine dose and efficacy. Presented at the 15th European College of Neuropsychopharmacology Congress; October 5–9, 2002; Barcelona, Spain. Barcelona, Spain: 2002. [Google Scholar]
- 137.Spina E, Avenoso A, Facciola G, Salemi M, Scordo MG, Ancione M, et al. Relationship between plasma risperidone and 9-hydroxyrisperidone concentrations and clinical response in patients with schizophrenia. Psychopharmacology (Berl) 2001;153:238–243. doi: 10.1007/s002130000576. [DOI] [PubMed] [Google Scholar]
- 138.Stahl SM, Cucchiaro J, Simonelli D, Hsu J, Pikalov A, Loebel A. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74:507–515. doi: 10.4088/JCP.12m08084. [DOI] [PubMed] [Google Scholar]
- 139.Stewart TD, Hatch A, Largay K, Sheehan JJ, Marler SV, Berman RM, et al. Effect of symptom severity on efficacy and safety of aripiprazole adjunctive to antidepressant monotherapy in major depressive disorder: A pooled analysis. J Affect Disord. 2014;162:20–5. doi: 10.1016/j.jad.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 140.Stimmel GL, Gutierrez MA, Lee V. Ziprasidone: an atypical antipsychotic drug for the treatment of schizophrenia. Clin Therap. 2002;24:21–37. doi: 10.1016/s0149-2918(02)85003-2. [DOI] [PubMed] [Google Scholar]
- 141.Stip E, Tourjman V. Aripiprazole in schizophrenia and schizoaffective disorder: A review. Clin Therap. 2010;32(SA):3–20. doi: 10.1016/j.clinthera.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 142.Stockton ME, Rasmussen K. Electrophysiological effects of olanzapine, a novel atypical antipsychotic, on A9 and A10 dopamine neurons. Neuropsychopharmacology. 1996;14:97–104. doi: 10.1016/0893-133X(94)00130-R. [DOI] [PubMed] [Google Scholar]
- 143.Swainston HT, Perry CM. Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 2004;64:1715–36. doi: 10.2165/00003495-200464150-00010. [DOI] [PubMed] [Google Scholar]
- 144.Szegedi A, Verweij P, van Duijnhoven W, Mackle M, Cazorla P, Fennema H. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73:1533–1540. doi: 10.4088/JCP.11r07596. [DOI] [PubMed] [Google Scholar]
- 145.Weber J, Lyseng-Williamson KA, Scott LJ. Aripiprazole in major depressive disorder. CNS Drugs. 2008;22:807–813. doi: 10.2165/00023210-200822100-00002. [DOI] [PubMed] [Google Scholar]
- 146.Wilner KD, Tensfeldt TG, Baris B, Smolarek, Turncliff RZ, Colburn WA. Single- and multiple-dose pharmacokinetics of ziprasidone in healthy young and elderly volunteers. Br J Clin Pharmacol. 2000;49(Suppl 1):15–20. doi: 10.1046/j.1365-2125.2000.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Winans E. Aripiprazole. Am J Health Syst Pharm. 2003;60:2437–2445. doi: 10.1093/ajhp/60.23.2437. [DOI] [PubMed] [Google Scholar]
- 148.Wright P, Lindborg SR, Birkett M, Meehan K, Jones B, Alaka K, et al. Intramuscular olanzapine and intramuscular haloperidol in acute schizophrenia: antipsychotic efficacy and extrapyramidal safety during the first 24 hours of treatment. Can J Psychiatry. 2003;48:716–721. doi: 10.1177/070674370304801102. [DOI] [PubMed] [Google Scholar]
- 149.Yokoi F, Grunder G, Biziere K, Stephane M, Dogman AS, Dannals RF, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C] raclopride. Neuropsychopharmacology. 2002;27:248–259. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 150.Zhao AL, Zhao JP, Zhang YH, Xue ZM, Chen JD, Chen XG. Dopamine D4 receptor gene exon III polymorphism and interindividual variation in response to clozapine.Int J Neurosci. 2005;115:1539–1547. doi: 10.1080/00207450590957863. [DOI] [PubMed] [Google Scholar]