Abstract
The initial impact of spinal cord injury (SCI) often results in inflammation leading to irreversible damage with consequent loss of locomotor function. Minimal recovery is achieved once permanent damage has occurred. Using a mouse model of SCI we observed a transitory increase followed by a rapid decline in gene expression and protein levels of nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of cellular anti-oxidative genes. Immediate treatment with diarylpropionitrile (DPN), a non-steroidal selective estrogen receptor ß ligand, resulted in a significant increase in Nrf2 levels, and reduction of inflammation and apoptosis compared to untreated SCI animals. Furthermore, DPN-treatment improved locomotor function within 7 days after induction of SCI. DPN acted through activation of PI3K/ Akt pathway, known to be involved in down-regulation of apoptosis and up-regulation of cell survival in injured tissues. These findings suggest that immediate activation of cellular anti-oxidative stress mechanisms should provide protection against irreversible tissue damage and its profound detrimental effect on locomotor function associated with SCI.
Keywords: Spinal cord injury, nuclear factor erythroid 2-related factor 2, diarylpropionitrile, antioxidant, apoptosis, anti-inflammation
Introduction
Pathogenesis of spinal cord injury (SCI) is characterized as being biphasic, consisting of primary injury which causes the initial spinal cord trauma and is considered irreversible, and secondary injury which refers to the molecular and biochemical events that occur following the primary injury (Rowland et al., 2008[16]). Earlier studies suggested that local inflammatory response and oxidative stress following SCI are the major contributors to further damage (David et al., 2012[4]). Thus resident microglia in the central nervous system as well as infiltrating macrophages from the periphery may further contribute to inflammation by releasing pro-inflammatory cytokines, such as interleukin-1β (IL-1β), as well as building up reactive oxygen species (ROS) (Pajoohesh-Ganji and Byrnes, 2011[14]). Inflammatory response resulting from astrogliosis around the injury site inhibits the tissue repair process (Rolls et al., 2009[15]), and the primary function of neuroprotection after SCI is to counteract the mechanisms of inflammation and oxidative stress to minimize their pathological effects. One mechanism of neural repair and prevention of cell death is via the estrogen signaling pathway through binding to estrogen receptors ERα or ERβ for the activation of downstream kinases, such as PI3K/Akt. Treatment of SCI with 17β-estradiol, which binds to both ERα and ERβ, attenuates the inflammatory response and reduces neuronal cell death (Tiwari-Woodruff et al., 2007[19]).
Diarylpropionitrile (DPN) (2,3-bis(4-hydroxyphenyl)-propionitrile), a non-steroidal selective high affinity ligand of ERβ (80-300 folds over ERα) (Carroll et al., 2012[3]), has neuroprotective effects in a number of neurological diseases (Kumar et al., 2013[11]). DPN has been shown to exert anti-inflammatory role in neuroprotection by attenuating inflammatory cytokines IL-1β and IL-6 expression in mouse brain during neuroinflammation (Brown et al., 2010[2]). DPN also increases the expression of a panel of bioenergetic enzymes and antioxidant proteins in primary rat cultured hippocampal neurons (Irwin et al., 2012[7]). Nuclear factor erythroid 2-related factor 2 (Nrf2) has been recognized for its role in detoxification during the build-up of ROS and in the activation of phase II anti-oxidative genes in a number of CNS diseases and disorders (Zhang et al., 2013[21]), as well as in traumatic brain injury (Jin et al., 2008[8], 2009[9]) and SCI (Mao et al., 2010[13]; Wang et al., 2012[20]). In this study, we examined the ameliorating effects of DPN in mice from the early stage of SCI up to 7 days by measuring the mRNA expressions and protein levels of Nrf2 and of proteins involved in inflammation, apoptosis, and the pathway related to DPN neuroprotection for SCI.
Materials and Methods
Animals and preparation of SCI
A total of 114 C57Bl/6 female 3-5 month-old mice (30-35 g) were supplied by the Department of Laboratory Animal Medicine, UCLA. These mice were housed at 20-22 °C, in relative humidity of 40-60 %, and with illumination from 6 a.m. to 6 p.m. and allowed ad libitum access to food and water. The standard environmental enrichment for mice included social housing (3 animals/ cage) in traditional wire-topped cages (800 cm2) with 1-cm layer of wood chip bedding. Animal care, handling and treatments were carried out according to the rules and regulations of UCLA Animal Research Committee and Department of Laboratory Animal Medicine. Mice were anesthetized with inhalation of isoflurane in oxygen-rich air. After dorsal midline incision and exposure of the lumbar vertebrae, laminectomy was performed at the L1/L2 level to expose the spinal cord. Moderate crush SCI was made using No.5 Dumont forceps (Fine Science Tools) ground down to a tip width of 0.4 mm and modified with a spacer so that at maximal closure a 0.4 mm space remained (Faulkner et al., 2004[5]). The behavior was tested before and every 24 h after performing SCI.
Drug administration
After induction of SCI, animals were immediately subcutaneously injected laterally to the injury site with 8 mg DPN (Tocris Bioscience, Ellisville, MO)/kg of body weight (Tiwari-Woodruff et al., 2007[19]), or with vehicle (Miglyol 812 N liquid oil; gift from Sasol North America, Houston, TX) and this treatment was repeated every 24 h until tissue collection. Normal mice were used as controls for comparison with DPN-treated and -untreated SCI animals. Tissue samples from three mice for each experiment were collected at 6, 12, 24, 48, 72 h and 7 days after induction of SCI.
Nrf2 and IL-1ß mRNA expression in the injured spinal cord of mice as detected by Real-Time PCR
The region of injured spinal cord tissue was dissected, immediately frozen in liquid nitrogen and stored at -80 °C until processed. Total tissue RNA was prepared using RNeasy Mini kit (Qiagen, CA) and cDNA synthesis was performed using RETROscript® reverse transcription kit (Ambion, TX) as previously described (Kumar et al., 2009[10]). Quantitative (q)-PCR was conducted in SsoAdvancedTM SYBR® Green Supermix (Bio-Rad, CA) containing 300 nM of each of the following primers; Cyclophilin A (PPIA) forward: 5’-CGAGCTGTTTGCAGACAAA G-3’ and reverse 5’-TCTGTGAAAGGAGG AACCCTTA-3’ primers; Nrf2 forward 5’-TGGAGAACATTGTCGAGCTG-3’ and reverse 5’-CCGCCTTTTCAGTAGATGGA-3’ primers; or IL-1ß forward 5’-GTGAAATG CCACCTTTTGACA-3’; and reverse 5’CAA AGGTTTGGAAGCAGCC-3’ primers (Kumar et al., 2009[10]). Thermocycling (CFX96, Bio-Rad, CA) of triplicate samples was performed as follows: 5 min at 95 °C; 45 cycles of 30 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C; followed by a melting curve analysis of 1 min at 95 °C, 1 min at 55 °C, 60 cycles of 5 s with temperature increase of 0.5 °C/cycle starting from 65 °C to 95 °C. The expression level of the gene of interest was normalized to that of PPIA using the formula 2-ΔΔCT. The results were analyzed using BioRad CFX manager software (Bio-Rad, CA).
Nrf2, IL-1ß, activated caspase-3, PI3K and Akt protein expression in the injured spinal cord of mice as detected by Western blot analysis
Equal amounts of protein from tissue lysate were separated on 10 % SDS-PAGE under reducing condition, and transferred onto polyvinylidene fluoride membranes. The membranes were incubated with 1:1000 rabbit anti-Nrf2 (GeneTex), 1:200 rabbit anti-IL-1ß (Santa Cruz), 1:500 rabbit anti-PI3K,
-p-PI3K, -Akt, and -p-Akt (Cell Signaling), 1:200 rabbit anti-activated caspase-3 (Chemicon), or 1:20000 mouse anti-GAPDH (Ambion), and then incubated with a HRP-conjugated secondary antibody (Zymed). The signal was detected with ECL Western blotting substrate (Bio-Rad) and captured on HyperfilmTM (Amersham Pharmacia Biotech).
Score of locomotor function of mouse hind limbs
Recovery of locomotor function was evaluated to assess the gross voluntary use of the hind limbs using open field locomotor test every 24 h after SCI induction. The behavior of animals was observed for 5 min by three individuals blinded to the experimental conditions. A simple six-point scale was used, which is found to be more appropriate for the model of upper lumbar SCI (Herrmann et al., 2008[6]), and animals received a score for voluntary movement of each hind limb. In brief, the scale involves closely monitoring limb movement, weight-bearing capability, coordinated and proper gait, movement in all joints of the hind limb, full weight support and appropriate limb position. Left and right hind limbs were scored separately and averaged.
Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Student-Newman-Keul’s post hoc test. P < 0.05 is considered statistically significant different among control, SCI, and DPN-treated groups.
Results
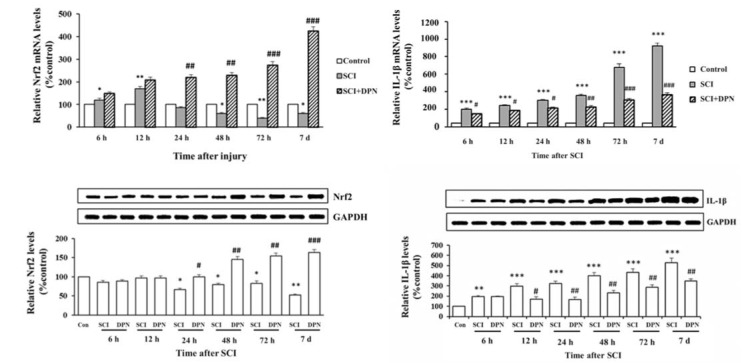
Gene expression of Nrf2, involved in detoxifying ROS, was detected in mouse spinal cord tissues within 12 h after SCI, then continuously declined after 24 h until day 7 (Figure 1A(Fig. 1)) and treatment with DPN resulted in enhancing Nrf2 gene expression, starting from 6 h up to day 7. There is a concomitant significant elevation of Nrf2 protein levels from 24 h to day 7 compared to non DPN-treated SCI group (Figure 1B(Fig. 1)). After 6 h post SCI, increased mRNA and protein levels of IL-1ß were detected in spinal cord tissues compared with normal controls. With DPN treatment, IL-1ß mRNA (Figure 1C(Fig. 1)) and protein (Figure 1D(Fig. 1)) levels significantly decline after 6 h and 12 h respectively. Similarly, levels of the apoptosis protein, activated caspase-3, show a significant increase in SCI group compared to normal control, starting from 6 h to day 7, which was attenuated by DPN treatment after 24 h (Figure 2(Fig. 2)). After 12 h following SCI, phosphorylated-PI3K (p-PI3K) and phosphorylated-Akt (p-Akt) levels are significantly higher in DPN-treated mice compared to untreated SCI group (Figure 3(Fig. 3)). As for the open field locomotor test, DPN-treated SCI mice show significant elevation of locomotor score after 48 h compared to untreated SCI group and continuously improved till the end of the test period (Figure 4(Fig. 4)).
Figure 1. Neuroprotective effects of DPN in activation of Nrf2 following SCI. DPN can significantly elevate Nrf2 mRNA (A) and protein (B) levels. DPN treatment also attenuates inflammatory cytokine, IL-1ß, mRNA (C) and protein (D) levels induced by SCI. Results are expressed as mean ± SEM of 3 independent experiments.
* p < 0.05, ** p < 0.01, *** p < 0.001 compared to normal control; # p < 0.05, ## p < 0.01, ### p <0.001 compared to SCI group.
Figure 2. DPN protects spinal cord tissue against SCI-induced apoptosis. Treatment of DPN significantly reduces activated caspase-3 level after 24 h following SCI induction. The results are expressed as mean ± SEM of 3 independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to normal control; # p < 0.05, ## p < 0.01 compared to SCI group.
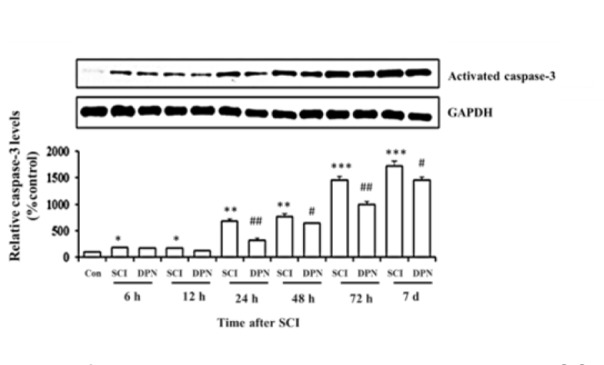
Figure 3. DPN treatment significantly activates phosphorylation of PI3K (A) and Akt (B) after 12 h following SCI. The results are expressed as mean ± SEM of 3 independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to normal control; # p < 0.05, ## p < 0.01, ### p < 0.001 compared to SCI group.
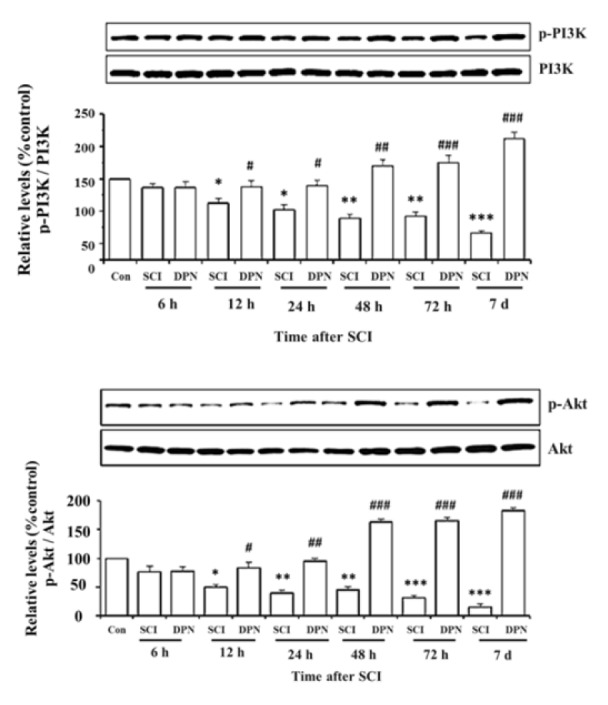
Figure 4. DPN effects on recovery of hind limb locomotor function after SCI. Locomotor recovery as measured by open field locomotor score. Animals were evaluated daily after SCI for 7 days. The results are expressed as mean ± SEM of 3 independent experiments. * p < 0.05, ** p < 0.01 compared to normal control; # p < 0.05, ## p < 0.01 compared to SCI group.
Discussion
In this study, we demonstrate that oxidative stress and inflammation (as evidenced by transient induction of anti-oxidative Nrf2 gene expression and a more sustained stimulation of inflammatory cytokine IL-1β mRNA and protein levels) occurred within 6-12 hours from the induction of SCI, which induced neuronal apoptotic death and dysfunction in open field locomotor activity. Treatment with DPN, a ligand of ERβ, immediately following SCI resulted in elevation of Nrf2 level, attenuation of IL-1β and activated caspase-3 levels, and improvement of locomotor function. Wang et al. (2012[20]) have demonstrated that Nrf2-deficient mice are more susceptible to oxidative stress and show more severe neurological dysfunctions and spinal cord edema after SCI. Moreover, administration of Nrf2-activator sulforaphane provides a neuroprotective effect in SCI rats (Benedict et al., 2012[1]). Sribnick et al. (2005[18]) demonstrated that estrogen treatment in SCI rats attenuated inflammatory response by reducing the levels of IL-1β and NF-κB. Kumar et al. (2013) showed a decrease in activated caspase-3 after DPN treatment in experimental autoimmune encephalomyelitis mice and that DPN increases oligodendrocyte progenitor cell numbers and improves myelination. The improvement in locomotor function in DPN-treated SCI mice in our study was similar to that reported with estrogen (Sribnick et al., 2010[17]).
DPN-induced neuroprotection is dependent on kinase activities, including the PI3K/Akt signaling pathway (Mannella and Brinton, 2006[12]). DPN treatment of SCI mice elevated levels of p-PI3K and p-Akt compared to untreated SCI animals. Activation of PI3K/Akt pathway mediates cell survival in a multiple sclerosis animal model (Kumar et al., 2013[11]). In addition, PI3K/Akt pathway plays a key role in the regulation of Nrf2-dependent neuroprotection by inducing Nrf2 to translocate into the nucleus and activate anti-oxidative genes (Zhang et al., 2013[21]), and inhibition of PI3K with wortmanin attenuates Nrf2 expression (Zou et al., 2013[23]). Besides its anti-oxidative effects through the activation of PI3K/Akt pathway, DPN likely mediates neuroprotection against SCI through its direct interaction with ERβ. To clarify whether ERβ plays a significant role in the neuroprotective effects of DPN, Zhao et al. (2011[22]) implemented (R,R)-THC, a specific ERβ inhibitor, to confirm that the neuroprotective effects of DPN against Aβ1-42 neurotoxicity are mediated through ERβ pathway in rat cultured hippocampal neurons. The findings from this study indicated that the oxidative stress, apoptosis and inflammatory processes accompanying SCI can, in part, be ameliorated by the immediate DPN administration, leading to partial restoration of locomotor function.
Acknowledgements
This research project was supported by The Thailand Research Fund (TRF) through the Royal Golden Jubilee Ph.D. Program (Grant no. PHD/0354/2550) and the Office of the Higher Education Commission, Ministry of Education, Thailand. We thank Prof. Michael Sofroniew and his colleagues for performing SCI procedure and Ms. Poonam Sachan for assistance in laboratory and animal studies. We are grateful to Prof. Prapon Wilairat, Mahidol University, for the valuable suggestions that improved the quality of this manuscript.
References
- 1.Benedict AL, Mountney A, Hurtado A, Bryan KE, Schnaar RL, Dinkova-Kostova AT, Talalay P. Neuroprotective effects of sulforaphane after contusive spinal cord injury. J Neurotrauma. 2012;29:2576–2586. doi: 10.1089/neu.2012.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors alpha and beta. Endocrinology. 2010;151:4916–4925. doi: 10.1210/en.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll VM, Jeyakumar M, Carlson KE, Katzenellenbogen JA. Diarylpropionitrile (DPN) enantiomers: synthesis and evaluation of estrogen receptor β-selective ligands. J Med Chem. 2012;55:528–537. doi: 10.1021/jm201436k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David S, Zarruk JG, Ghasemlou N. Inflammatory pathways in spinal cord injury. Int Rev Neurobiol. 2012;106:127–152. doi: 10.1016/B978-0-12-407178-0.00006-5. [DOI] [PubMed] [Google Scholar]
- 5.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin RW, Yao J, To J, Hamilton RT, Cadenas E, Brinton RD. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J Neuroendocrinol. 2012;24:236–248. doi: 10.1111/j.1365-2826.2011.02251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin W, Wang H, Yan W, Xu L, Wang X, Zhao X, et al. Disruption of Nrf2 enhances upregulation of nuclear factor-kappaB activity, proinflammatory cytokines, and intercellular adhesion molecule-1 in the brain after traumatic brain injury. Mediators Inflamm. 2008;2008:725174. doi: 10.1155/2008/725174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin W, Wang HD, Hu ZG, Yan W, Chen G, Yin HX. Transcription factor Nrf2 plays a pivotal role in protection against traumatic brain injury-induced acute intestinal mucosal injury in mice. J Surg Res. 2009;157:251–260. doi: 10.1016/j.jss.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Biancotti JC, Matalon R, de Vellis J. Lack of aspartoacylase activity disrupts survival and differentiation of neural progenitors and oligodendrocytes in a mouse model of Canavan disease. J Neurosci Res. 2009;87:3415–3427. doi: 10.1002/jnr.22233. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Patel R, Moore S, Crawford DK, Suwanna N, Mangiardi M, et al. Estrogen receptor β ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol Dis. 2013;56:131–144. doi: 10.1016/j.nbd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao L, Wang H, Qiao L, Wang X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-α, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010;2010:238321. doi: 10.1155/2010/238321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pajoohesh-Ganji A, Byrnes KR. Novel neuroinflammatory targets in the chronically injured spinal cord. Neurotherapeutics. 2011;8:195–205. doi: 10.1007/s13311-011-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 16.Rowland JW, Hawryluk GWJ, Kwon B. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 17.Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, et al. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosci Res. 2010;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari-Woodruff SK, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)α and ERβ ligand treatment. Proc Natl Acad Sci USA. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, de Rivero Vaccari JP, Wang H, Diaz P, German R, Marcillo AE, et al. Activation of the nuclear factor E2-related factor 2/antioxidant response element pathway is neuroprotective after spinal cord injury. J Neurotrauma. 2012;29:936–945. doi: 10.1089/neu.2011.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Yao J, Mao Z, Chen S, Wang Y, Brinton RD. 17β-Estradiol regulates insulin-degrading enzyme expression via an ERβ/PI3K pathway in hippocampus: relevance to Alzheimer's prevention. Neurobiol Aging. 2011;32:1949–1963. doi: 10.1016/j.neurobiolaging.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou W, Chen C, Zhong Y, An J, Zhang X, Yu Y, et al. PI3K/Akt pathway mediates Nrf2/ARE activation in human L02 hepatocytes exposed to low-concentration HBCDs. Environ Sci Technol. 2013;47:12434–12440. doi: 10.1021/es401791s. [DOI] [PubMed] [Google Scholar]