Abstract
The essential oil obtained from leaves of Melissa officinalis L. (Family of Lamiaceae) growing in Algeria, was investigated for its chemical composition and in vitro antimicrobial activity. The chemical composition was determined by hydrodistillation and analyzed by GC/MS and GC-FID. Sixty-three compounds were identified in the essential oil, representing 94.10 % of the total oil and the yields were 0.34 %. The major component was geranial (44.20 %). Other predominant components were neral (30.20 %) and citronellal (6.30 %). The in vitro antimicrobial activity was determined by paper disk agar diffusion testing and minimum inhibitory concentration (MIC) using 7 bacteria (3 Gram-positive and 4 Gram-negative), 2 yeasts and 3 fungi. The results showed that the essential oil presented high antimicrobial activity against all microorganisms targeted mainly against five human pathogenic bacteria, one yeast Candida albicans and two phytopathogenic fungi tested. The minimum inhibitory concentrations (MIC) ranged from 1.00 to 5.00 µL/mL.
Keywords: Melissa officinalis, leaves, essential oil, chemical composition, antimicrobial activity
Introduction
Melissa officinalis L. (Lamiaceae) is a perennial edible herb native to the Mediterranean region. The plant is cultivated in various parts of the world and grows especially in western Asia, south-western Serbia and North Africa. In Algeria, this plant is known locally by the names touroudjan, tindjan or bararendjabouya. It is considered as an important medicinal plant largely used in traditional medicine, for the treatment of headaches, indigestion, colic, nervousness, cardiac failure and depression (Beloued, 2009[8]). Actually, essential oils and their components are gaining increasing interest because of their relatively safe status and their potential use in many functional purposes. The main advantage in the use of such natural agents is that they do not present the phenomenon of drug-resistance, commonly encountered with the long-term use of antibiotics. Their preparations have also found applications as naturally occurring antimicrobial agents in the field of pharmacology, phytopathology and food preservation. M. officinalis essential oil is recommended for its antimicrobial activity (Romeo et al., 2008[25]; Hussain et al., 2011[19]; Vitullo et al., 2011[34]; Tullio et al., 2007[33]) and aqueous extracts exhibit antiviral (Adorjan and Buchbauer, 2010[3]; Jassim and Naji, 2003[20]) and antioxidative (Spiridon et al., 2011[30]) properties and anti-inflammatory, anti-nociceptive (Birdane et al., 2007[9]) and anti-diabetic effects (Chung et al., 2010[12]). The leaves are used as a juice or as a herbal tea for their aromatic, digestive, and antispasmodic properties in nervous disturbance of sleep and for gastrointestinal disorders (Beloued, 2009[8]). It was also reported that M. officinalis contains substances inhibiting protein biosynthesis in cancer cells (Adjorjan and Buchbauer, 2010[3]; Carvalho de Sousa et al., 2004[11]). These biological activities have been attributed to the essential oil (Adinee et al., 2008[2]; Da Silva et al., 2005[14]; Sharafzadeh et al., 2007[29]) flavonoids and phenolic acids (Constantine, 2007[13]; Ziakova et al., 2003[35]) such as rosmarinic acid (Toth et al., 2003[32]) and caffeic acids (Tagashira and Ohtake, 1998[31]), phenylpropanoid heteroside (Mulkens and Kapetanidis, 1988[22]), Triterpene (Mencherini et al., 2007[21]).
Considering the antimicrobial activity of M. officinalis oil, Romeo et al. (2008[25]) and Hussain et al. (2011[19]) reported its antibacterial effect against Staphylococcus aureus, Bacillus cereus, Bacillus subtilis, Bacillus pumilis, Pseudomonas aeruginosa, Salmonella poona, Escherichia coli and Listeria innocua. However, lemon balm oil activity against fungi Fusarium oxysporum albedinis, F. oxyxporum lini, Mucor ramannianus and the yeasts Candida albicans and Saccharomyces cerevisiae has not been reported previously. The chemical composition of Algerian M. officinalis oil has not been investigated. Therefore, the aim of this paper is to analyze the chemical composition of hydrodistilled essential oil of M. officinalis from Algeria by GC/FID and GC/MS methods, and to investigate its antimicrobial activity against selected human pathogenic and phytopathogenic microbial strains via paper disk agar diffusion method and determination of minimum inhibitory concentrations. A particular interest will be accorded to comparison with oils produced by the same species grown in different regions of the world (Dukic et al., 2004[17]; Holla et al., 1997[18]; Shalaby El-Gengaihi and Khattab, 1995[28]; Carnat et al., 1998[10]; Adzet et al., 1992[4]; Damien Dorman et al., 2000[15]; Sadraei et al., 2003[26]; Basta et al., 2005[6]; Da Silva et al., 2005[14]; Pino et al., 1999[24]). This paper is the first report on the chemical composition and antimicrobial activity of the oil of Melissa officinalis growing in Algeria.
Materials and Methods
Plant materials
The sample of M. officinalis was collected in March 2012 at Algiers (Algeria). The plant was identified in the botanical department of National Institute Agronomic of Algiers (NIA), Algeria.
Chemicals
Myrcene, Linalool, Camphor, Citronellal, β-Caryophyllene, Caryophyllene oxide, citral (isomer of neral and geranial), and the mixture of aliphatic hydrocarbons (C5-C28) were purchased from Sigma-Aldrich (Germany). All compounds were of analytical standard grade.
Extraction, isolation and identification of the essential oil
Dried leaves of M. officinalis were performed by hydrodistillation for 3 hours using a Clevenger type apparatus. The oil was collected, dried over anhydrous sodium sulphate and stored in the dark at +4 °C until analyzed.
Physico-chemical indices
The physico-chemical indices of the oil were determined following the ISO regulations. ISO 280:1976 for the refractive index, ISO 279:1981 for the specific gravity, ISO 592:1981 for the optical rotation, ISO 709:1980 for the ester value and ISO 1242:1973 for the acid value.
Gas chromatography analysis
GC-FID analysis of the volatile components was carried out using a Hewlett Packard HP5890 series II instrument coupled to an ionisation flame detector (FID). Compounds were separated on a HP-5 capillary column (5 % phenylmethylpolysiloxane, 30 m x 0.25 mm i.d., 0.25 µm film thickness) and a HP-WAX (polyethylene glycol, 30 m x 0.15 mm i.d., 0.25 µm film thickness), using the following temperature programme: 5 min at 60 °C, then rising at 3 °C/min to 250 °C, held for 5 min; injector and transfer line temperatures, 250 °C; Azot was used as the carrier gas at a flow rate of 1 mL/min; injection volume, 0.1 µL; split ratio, 1:50. A mixture of aliphatic hydrocarbons (C5-C28) (Sigma) was directly injected into the GC injector under the above temperature programme in order to calculate the retention index (as Kovàts index) of each compound. The percentage composition of the individual components were obtained from electronic integration measurements using flam ionisation detection (FID; 260 °C). n-alkenes were used as reference points in the calculation of retention indices (RI).
GC/MS analysis
The GC/MS analysis was performed with a Hewlett Packard HP5890 series II gas chromatograph coupled to a HP MSD5971, equipped with an electronic impact source at 200 °C, fitted with a fused silica-capillary column with an apolar stationary phase HP-5MS (5 % phenylmethylpolysiloxane, 30 m x 0.25 mm i.d., 0.25 µm film thickness). The temperature programme conditions were the same with GC analysis. Helium was used as the carrier gas at a flow rate of 1 mL/min; acquisition mass range, 30-600 m/z. All mass spectra were acquired in electron-impact (EI) mode with ionisation voltage of 70 eV.
Identification of the compounds
The compounds were identified by comparing the retention time, retention index and mass spectrum of the chromatographic peaks with that of the standards. The identification of other volatile components was based on computer matching with the Wiley, NIST and ADAMS libraries (Adams, 2007). Co-injections with authentic samples. Chemicals were obtained from Sigma-Aldrich chemical, Germany.
Antimicrobial activity
Antimicrobial activity of the essential oil was screened by the paper disk diffusion method and by the determination of the minimal inhibitory concentrations (MIC).
Microbial strains
The essential oil and the standard compounds were individually tested against different microorganisms including 3 Gram-positive bacteria (Bacillus subtilis ATCC 6633, Staphylococcus aureus CIP 7625, Listeria monocytogenes CIP82110) and 4 Gram-negative bacteria (Pseudomonas aeruginosa CIP A22, Escherichia coli ATCC 10536, Klebsiella pneumoniae CIP 8291, Salmonella enterica CIP 81.3), 3 filamentous fungi (Fusarium oxysporum albedinis CURZA, Fusarium oxysporum lini CINRA, Mucor ramannianus NRRL 6606) and 2 yeasts (Candida albicans IPA200, Saccharomyces cerevisiae ATCC 4226). All microorganisms were graciously supplied from stock cultures of the Microbiology Laboratory of the Department of Biology, Ecole Normale Superieure, Algiers, Algeria. The bacterial strains were cultured on Mueller-Hinton agar for 48 h at 37 °C, while fungi and yeasts were propagated on Sabouraud agar at 37 °C for 48 h to 3 days before use. All microorganisms were regenerated twice before use in the manipulations.
Paper disk diffusion assay
Paper disk-diffusion method (Bauer et al., 1966[7]) was employed for the determination of antimicrobial activity of the essential oil. Microbial suspensions were prepared in sterile 0.9 % saline and adjusted as inoculum to a final concentration of 1.0 x 108 CFU/ mL. A volume of 20 mL of Mueller-Hinton agar and Sabouraud, respectively, for bacterial and fungal strains was inoculated with 20 µL of microbial suspension and then poured into a Petri dish. The plates were left at room temperature for 30 min to allow the culture media to solidify. Each paper disk of 6 mm diameter was impregnated with 35 µg of essential oil solution (in methanol) and then applied manually on the surface of the agar plates inoculated with microorganisms. The major components of the essential oil, citral, citronellal and caryophyllene oxide were also tested. Ampicillin and Nalidixic acid (30 µg/disk) were used as positive reference standards to determine the sensitivity of Gram-positive and Gram-negative bacteria species, respectively. Nystatin (30 µg/disk) was used as positive reference standard to determine the sensitivity of fungi and yeasts species. The plates were kept at 4 °C for 2 h to allow diffusion, and then incubated for 24 h at 37 °C for bacteria, and 48 h at 30 °C for yeasts and fungi. The antimicrobial activity was determined by measuring with a ruler, the diameters of inhibition zones, including disk diameter (6 mm). All tests were carried out in triplicate.
Antimicrobial minimal inhibitory concentrations
The Minimal inhibitory concentrations (MIC) of the essential oil were determined by a conventional agar dilution method (Oki et al., 1990[23]). The microorganisms tests included the same strains of bacteria and fungi used in the screening of antimicrobial activity by the paper disk method. A stock solution of the essential oil was prepared in methanol. The agar media (Mueller Hinton for bacteria and Sabouraud for fungi) were supplemented with different concentrations of essential oil (0.1, 0.2, 0.3, 0.4, 0.5, 1, 2, 3, 4, 5, 8, 10, 20, and 50 µL/mL) and then poured onto Petri dishes. Aliquots of 1 µL of strains suspensions containing 108 CFU/mL of the indicator strain were inoculated. A negative control was included in the test by inoculating in the same conditions, the target organisms onto the media without essential oil. The plates were incubated during 24–48 h at 37 °C for bacteria and 48–72 h at 28 °C for fungi. The MICs were determined as the lowest concentration of the essential oil that inhibit the growth of the tested microorganism, and detected by lack of visual growth, matching with the negative control.
Results
Chemical composition analysis
The oil of the leaves of M. officinalis isolated by hydrodistillation was of yellow-pale color with a citron smell, in total yield of 0.34 % w/w on dry weight basis. Some physicochemical characteristics of the oil were also determined:
specific gravity[d]20 = 0.9091;
refractive index ηD20 = 1.3493;
optical rotation αD20 = +24.0;
acid value IA = 1.2;
ester value IE = 23.59.
Qualitative and quantitative analytical results were obtained using both GC and GC-MS techniques. Table 1(Tab. 1) shows the compounds identified in the oil of M. officinalis in order of elution on HP5 capillary column, the percentage content of the individual components, retention indices and chemical class distribution are summarized.
Table 1. Table1: Composition of essential oil of Melissa officinalis from Algeria.
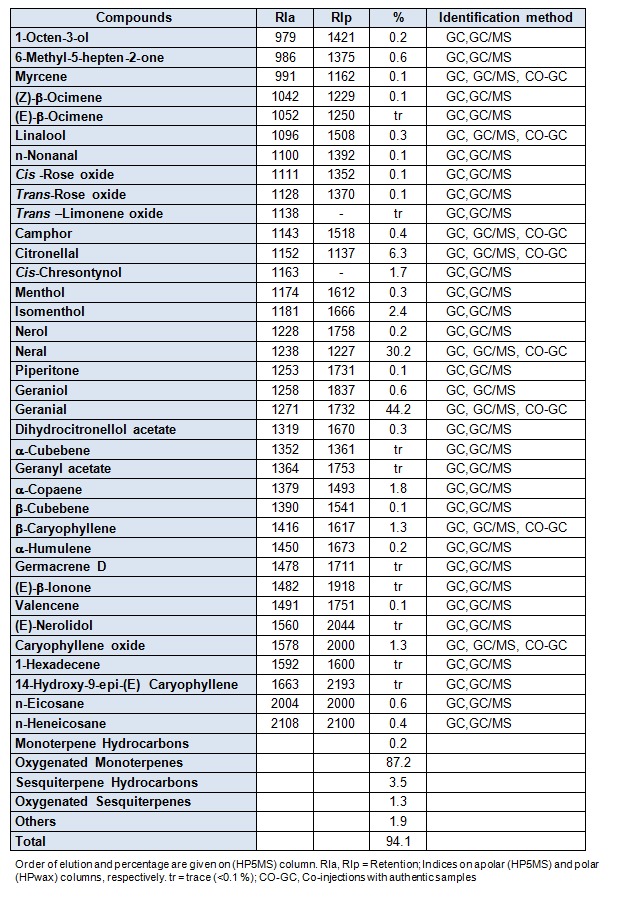
Thirty six compounds were identified, accounting for 94.10 % of the total oil. This oil was characterized by very high percentage of monoterpenes (84.4 %), especially oxygenated monoterpenes (87.2 %) in which, neral (30.2 %), geranial (44.2 %) and citronellal (6.3 %) were the major components. In contrast, the sesquiterpene fraction was lower (4.8 %); the hydrocarbons (3.5 %) represented by α-copaene (1.8 %) and β-caryophyllene (1.3 %) were detected in higher concentration than the oxygenated sesquiterpenes, such as caryophyllene oxide (1.3 %).
The above results show that our oil was characterized by the presence of three dominating components in monoterpenoid family type aldehyds, and an important fraction includes neral (30.2 %), geranial (44.2 %) and citronellal (6.3 %).
Results of the antimicrobial tests
The results of the antimicrobial activities of Algerian M. officinalis essential oil performed by paper disk method and determination of MICs are reported in Tables 2(Tab. 2) and 3(Tab. 3) respectively. The target microorganisms are considered as among human pathogenic strains (Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Salmonella enterica) and common foodborne pathogen (Listeria monocytogenes). The essential oil exhibited a strong activity against all the strains tested with very low MICs. The inhibition zones ranged between (17 and 18 mm) for the Gram-positive bacteria, and (14-21 mm) for the pathogenic Gram-negative bacteria. Generally, the essential oils are more active against Gram-positive bacteria than against Gram-negative ones. In our case, Melissa officinalis oil is more active against the Gram-negative ones.
Table 2. Results of the antimicrobial activity tests (diameter of the inhibition zones in mm) of the .
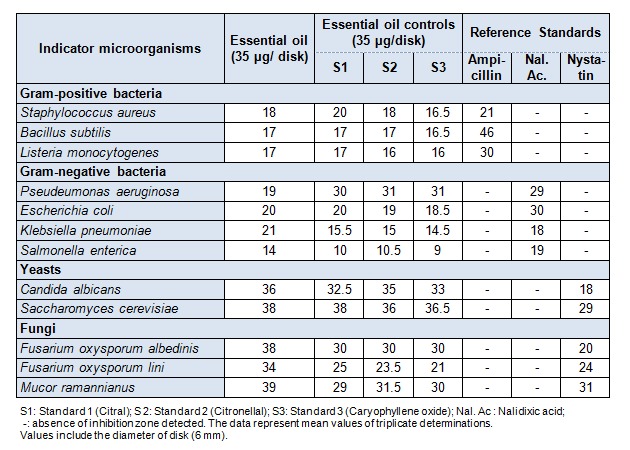
essential oil of Melissa officinalis by the paper disk method
Table 3. Antimicrobial minimal inhibitory concentrations of Melissa officinalis essential oil.
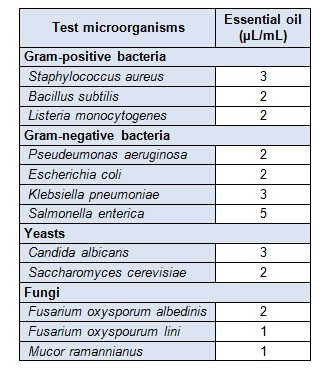
The antiyeast activity was potent against the human pathogenic Candida albicans (36 mm). The two phytopathogenic fungi tested, Fusarium oxysporum albedinis and Fusarium oxyspourum lini are the agents of vascular wilt (fusariosis) of date palm and flax, respectively. These fungi cause important deteriorations to different parts of the affected plants, including fruits. The results of antifungal activity showed that the essential oil (34-38 mm) was more potent than nystatin (20-24 mm), the reference standard used (see Table 2(Tab. 2)). These important activities may be related to the major compounds (citral, citronellal and caryophyllene oxide), which provided inhibition zones similar to that of the oil (see Table 2(Tab. 2)). The resulted whole activity involves probably some type of synergism between many active compounds. Melissa officinalis essential oil contains appreciable amounts of oxygenated compounds (monoterpenes and sesquiterpenes) (Table 1(Tab. 1)). This result is in agreement with the great diameters of inhibition obtained for all the target microorganisms. The values of minimal inhibitory concentrations (MICs) are very low. They ranged from 1 to 5 µL/mL, indicating and confirming the potent antimicrobial activity of the oil (see Table 3(Tab. 3)). The highest MIC was obtained with Salmonella enterica, the most resistant strain.
Discussion
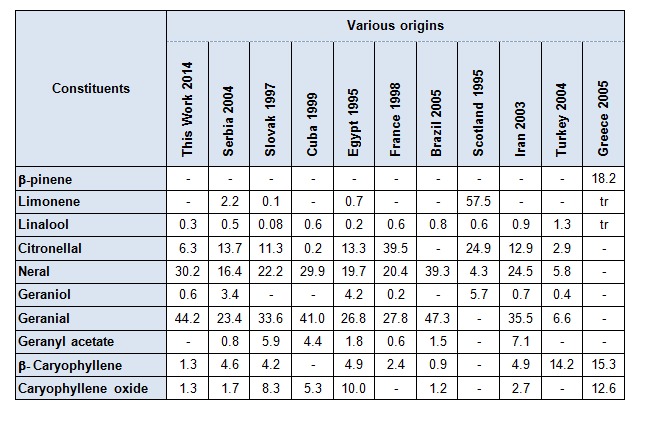
The composition of the oil from M. officinalis harvested in Algeria was dominated by neral, geranial and citronellal. This composition was qualitatively the same that the oils from Serbia (Dukic et al., 2004[17]), Slovak (Holla et al., 1997[18]), Egypt (Shalaby El-Gengaihi and Khattab, 1995[28]), France (Carnat et al., 1998[10]) and Iran (Sadraei et al., 2003[26]); as seen in Table 4(Tab. 4). However, limonene was the major component in the samples from Scotland (Damien Dorman et al., 2000[15]) (57.5 %), neral was found with only (4.3 %) and geranial was completely absent. Basta et al. (2005[6]) reported that caryophyllene oxide (12.6 %) and ß-pinene (18.2 %) were also the most abundant constituents in the oil of M. officinalis from Greece but neral and geranial were not detected in the oil. Oils from Cuba (Pino et al., 1999[24]) and Brazil (Da Silva et al., 2005[14]) were dominated by neral (29.9 % and 39.3 %) and geranial (41.0 % and 47.3 %) respectively. A low content (0.2 %) of citronellal was found in leaves of Cuba (Pino et al., 1999[24]) and it is not detected in oil from Brazil (Da Silva et al., 2005[14]). A typical composition from Turkey (Allahverdiyev et al., 2004[5]) is characterized by the occurrence of β-carophyllene (14.2 %), which is drastically different from Algerian oil. Minor compounds were punctually reported. Geraniol in Scotland (5.73 %) (Damien Dorman et al., 2000[15]), in Egypt (4.2 %) (Shalaby El-Gengaihi and Khattab, 1995[28]), and in Serbia (3.4 %) (Dukic et al., 2004[17]), against (0.6 %) of the oil from Algeria. Geranyl acetate was present at 5.9 % in Slovak (Holla et al., 1997[18]) and 7.1 % in Iran (Sadraei et al., 2003[26]). Otherwise, several sesquiterpenes have been reported at appreciable content like β-carophyllene 4.9 % in Egypt (Shalaby El-Gengaihi and Khattab, 1995[28]), 4.6 % in Serbia (Dukic et al., 2004[17]), 4.2 % in Slovak (Holla et al., 1997[18]), 4.9 % in Iran (Sadraei et al., 2003[26]), 2.4 % in France (Carnat et al., 1998[10]) against 1.3 % in our oil. An oxygenated sesquiterpene, caryophyllene oxide was identified at 10.0 % in Egypt (Shalaby El-Gengaihi and Khattab, 1995[28]), 8.35 % in Slovak (Holla et al., 1997[18]), 5.3 % in Cuba (Pino et al., 1999[24]), 2.7 % in Iran (Sadraei et al., 2003[26]), 1.7 % in Serbia (Dukic et al., 2004[17]) and 1.3 % in Algerian oil. Similar results were obtained with essential oil of M. officinalis (Sari and Ceylan, 2002[27]; Dawson et al., 1988[16]; Shalaby El-Gengaihi and Khattab, 1995[28]; Holla et al., 2000[18]; Adzet et al., 1992[4]). The most dominant constituent obtained was citral (geranial and neral).
Table 4. Main constituents of chemical composition of Melissa officinalis of various origins.
The essential oil antibacterial activity of M. officinalis was reported in some papers (Dukic et al., 2004[17]; Mencherini et al., 2007[21]; Romeo et al., 2008[25]; Tullio et al., 2007[33]). However, this is the first study of antifungal activity of M. officinalis oil against the two Fusaria species.
Conclusion
The essential oil composition of M. officinalis from Algeria was characterized by its high content of monoterpenoids with citral being major (87.2 %). The present study gives a better insight on the volatiles contained in leaves of M. officinalis which grows in Algeria and shows similitude and differences with composition oils from different countries in the world. The essential oil of M. officinalis revealed strong antimicrobial activity with large inhibition zones and small MIC values against all microorganisms tested. The essential oil represents a complex mixture of different chemical components. This antimicrobial activity of total essential oil cannot be reduced to major components. Other components can contribute to this activity. The present results also demonstrated that M. officinalis essential oil can be used in pharmaceuticals and natural therapies of infectious diseases in humans and plants, as well as in food preservation.
Acknowledgements
The authors thank Professor N. Sabaou for his valuable support in his laboratory for antimicrobial activity assays and Professor B. Meklati for his help in the GC and GC-MS analysis.
Conflict of interest
The authors report no conflict of interest. The authors are responsible for the content and writing of the paper.
References
- 1.Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL: Allured Publishing; 2007. [Google Scholar]
- 2.Adinee J, Piri K, Karami O. Essential oil component in flower of Lemon Balm (Melissa officinalis) Am J Biochem Biotechnol. 2008;4:277–278. [Google Scholar]
- 3.Adorjan B, Buchbauer G. Biological properties of essential oils: an apdated review. Flav Fragr J. 2010;25:407–426. [Google Scholar]
- 4.Adzet T, Ponz R, Wolf E, Schulte E. Content and composition of M. officinalis oil in relation to leaf position and harvest time. Planta Med. 1992;58:562–564. doi: 10.1055/s-2006-961551. [DOI] [PubMed] [Google Scholar]
- 5.Allahverdiyev A, Duran N, Ozguven M, Koltas S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine. 2004;11:657–661. doi: 10.1016/j.phymed.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Basta A, Tzakou O, Couladi M. Composition of the leaves essential oil of Melissa officinalis from Greece. Flav Fragr J. 2005;20:642–644. [Google Scholar]
- 7.Bauer AW, Kirby WMM, Sherris JC, Turk M. Antibiotic susceptibility testing by a standardized disc method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 8.Beloued A. Plantes médicinales d’Algérie. Alger: Office des Publications Universitaires,; 2009. p. (p 134). [Google Scholar]
- 9.Birdane YO, Buyukokuroglu ME, Birdane FM, Cemek M, Yavuz H. Anti-inflammatory and antinociceptive effects of Melissa Officinalis L. in rodents. Rev Méd Vét. 2007;158:75–81. [Google Scholar]
- 10.Carnat AP, Carnat A, Fraisse D, Lamaison JL. The aromatic and polyphenolic composition of lemon balm (Melissa officinalis. L. Subsp. officinalis) tea. Pharma Acta Helv. 1998;72:301–305. [Google Scholar]
- 11.Carvalho de Sousa A, Sales Alviano D, Fitzgerald Blank A, Barreto Alves P, Sales Alviano C, Rocha Gattass C. Melissa officinalis L. essential oil: anti-tumoral and antioxidant activities. J Pharm Pharmacol. 2004;56:677–681. doi: 10.1211/0022357023321. [DOI] [PubMed] [Google Scholar]
- 12.Chung MJ, Cho SY, Bhuiyan MJH, Kim KH, Lee SJ. Anti-diabetic effects of lemon balm (Melissa officcinalis) essential oil on glucose and lipid regulating enzymes in type 2 diabetic mice. Brit J Nutr. 2010;104:180–188. doi: 10.1017/S0007114510001765. [DOI] [PubMed] [Google Scholar]
- 13.Constantine DS. Extraction, separation and detection methods for phenolic acids and flavonoids. J Sep Sci. 2007;30:3268–3295. doi: 10.1002/jssc.200700261. [DOI] [PubMed] [Google Scholar]
- 14.Da Silva SS, Salgueiro Lage ACL, Da Silva San Gil RA, De Almeide Azevedo D, Esquibel MAJ. Essential oil composition of Melissa officinalis L. In vitro produced under the influence of growth regulators. Braz Chem Soc. 2005;16:1387–1390. [Google Scholar]
- 15.Damien Dorman HJ, Surai P, Deans SG. In vitro antioxidant activity of plant essential oils and phytoconstituents. J Essent Oil Res. 2000;12:241–248. [Google Scholar]
- 16.Dawson BSW, Franich RA, Meder R. Essential oil of Melissa officinalis L. subsp. altissima (Sibthr. et Smith) Arcang. Flav Fragr J. 1988;3:167–170. [Google Scholar]
- 17.Dukic NM, Bozin B, Sokovic M, Simin N. Antimicrobial and antioxidant activities of (Lamiaceae) essential oil. J Agric Food Chem. 2004;52:2485–2489. doi: 10.1021/jf030698a. [DOI] [PubMed] [Google Scholar]
- 18.Holla M, Svajdlenka E, Tekel J, Vaverkova S, Havranek E. Composition of the essential oil from Melissa officinalis L. cultived in Slovak Republic. J Essent Oil Res. 1997;9:481–484. [Google Scholar]
- 19.Hussain, AI, Anwar F, Nigam PS, Saker SD, Moore JE, Rao JR, et al. Antimicrobial activity of some Lamiaceae essential oils using resazurin as an indicator of cell growth. LWT. 2011;44:1199–1206. [Google Scholar]
- 20.Jassim SAA, Naji MA. Novel antiviral agent: a medicinal plant perspective. J Appl Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 21.Mencherini T, Picerno P, Scesa C, Aquino R. Triterpene, antioxidant and antimicrobial compounds from Melissa officinalis. J Nat Prod. 2007;70:1889–1894. doi: 10.1021/np070351s. [DOI] [PubMed] [Google Scholar]
- 22.Mulkens A, Kapetanidis I. Eugenylglucoside, a new natural phenylpropanoid heteroside from Melissa officinalis. J Nat Prod. 1988;51:496–498. doi: 10.1021/np50057a006. [DOI] [PubMed] [Google Scholar]
- 23.Oki T, Tenmyo O, Tomatsu K, Kamai H, Pradimicins AB. New antifungal antibiotics. II. In vitro and in vivo biological activities. J Antib. 1990;30:334–336. doi: 10.7164/antibiotics.43.763. [DOI] [PubMed] [Google Scholar]
- 24.Pino JA, Rosado A, Fuentes V. Composition of the essential oil of Melissa officinalis L. from Cuba. J Essent Oil Res. 1999;11:363–364. [Google Scholar]
- 25.Romeo V, Serena De Luca Piscopo A, Poiana M. Antimicrobial effect of some essential oils. J Essent Oil Res. 2008;20:373–379. [Google Scholar]
- 26.Sadraei H, Ghannadi A, Malekshahi K. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contractions. Fitoterapia. 2003;74:445–452. doi: 10.1016/s0367-326x(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 27.Sari AO, Ceylan A. Yield characteristics and essential oil composition of Lemon balm (Melissa officinalis L.) grown in the aegean region of Turkey. Turk J Agric For. 2002;26:217–224. [Google Scholar]
- 28.Shalaby El-Gengaihi AS, Khattab M J. Oil of Melissa officinalis L. as affected by storage and herb drying. J Essent Oil Res. 1995;7:667–669. [Google Scholar]
- 29.Sharafzadeh S, Khosh-Khui M, Javidnia K. Aromatic profile of leaf and stem of Lemon Balm (Melissa Officinalis) grown under greenhouse conditions. Advin Env Biol. 2007;5:547–550. [Google Scholar]
- 30.Spiridon L, Colceru S, Anghel N, Teaca CA, Bodirlau R, Armatu, A Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat Prod Res. 2011;25:1657–1661. doi: 10.1080/14786419.2010.521502. [DOI] [PubMed] [Google Scholar]
- 31.Tagashira M, Ohtake Y. A new antioxidative 1, 3-benzodioxole from Melissa officinalis. Planta Med. 1998;64:555–558. [Google Scholar]
- 32.Toth I, Mrlianova J, Tekelova D, Korenova M. Rosmarinic acid - an important phenolic active composition of lemon balm (Melissa officinalis L.) Act Facult Pharm. 2003:139–146. [Google Scholar]
- 33.Tullio V, Nostro A, Mandras N, Dugo P, Banche G, Cannatelli MA, et al. Antifungal activity of essential oils against filamentous fungi determined by broth microdilution. J Appl Microbiol. 2007;102:1544–1550. doi: 10.1111/j.1365-2672.2006.03191.x. [DOI] [PubMed] [Google Scholar]
- 34.Vitullo M, Ripabelli I, Fanelli I, Tamburro M, Delfine S, Sammarco L. Microbiological and toxicological quality of dried herbs. Lett Appl Microbiol. 2011;52:573–580. doi: 10.1111/j.1472-765X.2011.03040.x. [DOI] [PubMed] [Google Scholar]
- 35.Ziakova A, Brandsteterova E. Validation of HPLC determination of phenolic acids present in some Lamiaceae family plants. J Liquid Chromatogr. 2003;26:443–453. [Google Scholar]


