Abstract
The current study examines the antimicrobial and antioxidant properties of different extracts of the microalga Dunaliella salina Teodoresco (Dunaliellaceae), their fatty acid composition and the antimicrobial activity of the oil. Antimicrobial and antioxidant activities were evaluated by obtaining extracts of D. salina in n-hexane, dichloromethane, ethanol, and methanol. To evaluate the antimicrobial activity, the extracts, and fatty acids from D. salina were assessed by the disc diffusion and microdilution techniques against pathogenic microorganisms including fish and clinical/food-borne. The MBC or MFC values of the extracts and fatty acids ranged from 0.63 to 10.00 mg/ml. The antioxidant activity was studied by phosphomolybdenum and DPPH assays and ß-carotene/linoleic acid tests. In addition, the total phenolic and flavonoid contents were evaluated and the fatty acid composition was determined using gas chromatography. Palmitic, alpha-linolenic, and oleic acids were discovered to be the major components of the fatty acids. These findings have demonstrated that the extracts and oil from D. salina could be used as natural antimicrobials and antioxidants in the food/feed and pharmaceutical industry and as a biodiesel because of its high unsaturated fatty acid content.
Keywords: fatty acid composition, antimicrobial, antioxidant, Lake Tuz
Introduction
Algae constitute a major group of living organisms that are an important source of a variety of useful products with widespread applications in the biodiesel, food, and pharmaceutical industries, as well as in biotechnology. Algae have developed a chemical defense system with the secondary metabolites that are synthesized by the means of different metabolic enzymes to survive in a competitive environment (Puglisi et al., 2004[45]; Barros et al., 2005[4]). In addition to their role in protecting the organism against stress conditions such as temperature, light, salinity, and drought, most components have some useful biological activities and are essential in human nutrition (Mayer and Hamann, 2004[36]).
The salinity of Lake Tuz in Turkey (Salt) ranges from 30 % to 45 % (Saygi and Demirkalp, 2002[48]). This high salinity generates extreme stress in several living organisms, and limits life in Lake Tuz. Dunaliella salina Teodoresco (Dunaliellaceae), a green alga species, is one of the rare organisms can naturally thrive in Lake Tuz. D. salina is one of the richest sources of ß-carotene, a secondary metabolite and a lipid-soluble orange pigment, used in food and feed as a coloring agent. D. salina normally contains 5–10 mg of ß-carotene per gram dry weight (Del Campo et al., 2007[15]). However, the ratio increases up to 14 % of dry weight in stress conditions such as high salinity, high light intensity, food scarcity, and extreme temperature (Jin and Melis, 2003[27]). ß-carotene exhibits several important biological activities, including preventing coronary heart disease and malignant tumors, increasing cell division of lymphocytes, expanding the immunological response, and control of growth (Wald et al., 1988[52]; Stryker et al., 1990[51]; Knekt et al., 1990[31]; Challem, 1997[11]; Buiatti, 1997[7]; Chidambara et al., 2005[13]; Raja et al., 2007[46]). In addition, the antioxidant properties of ß-carotene help eliminate free radicals (Burton and Ingold, 1984[8]). Thus, ß-carotene, which has applications in several different industrial sectors because of its numerous properties, is of great economic importance.
ß-carotene accumulates within the lipid globules in the spaces between thylakoids located in chloroplast (Ben-Amotz et al., 1982[5]). Thus, it has been proposed that the increase in fatty acid content under stress conditions can be partially attributed to the increase in ß-carotene (Mendoza et al., 1999[37]). Previous studies examining the fatty acid profile of D. salina identified high expression of palmitic (C16: 0), alpha-linolenic (C18: 3), and oleic acid (C18: 1) (Herrero et al., 2006[23]; Lamers et al., 2010[34]). Of these fatty acids, alpha-linolenic (C18: 3) essential fatty acid and ω3 fatty acids are the most well-known. ω3 fatty acids are of great importance in growth and development, brain development, and prevention of cardiovascular diseases. Marine organisms are an important source of these fatty acids (Meyer et al., 2003[38]).
Microalgae have been used for therapeutic applications for several years, and their extract and/or extracellular products present antimicrobial activity (Kellan and Walker, 1989[29]; Ozemir et al., 2004[41]; Herrero et al., 2006[23]). A study by Herrero et al. (2006[23]) discovered the antibacterial and antifungal activities of various D. salina extracts on several microorganisms, which are important in the food industry; in addition, it highlighted the fatty acid profile of these extracts. In our study, we examined the antimicrobial activity of various extracts and fatty acids of D. salina on a total of 19 fish as well as clinical and food-borne pathogens, and emphasized the potential use of these extracts in the food/feed and pharmaceutical industries.
Studies conducted with the macro- and microalgae have shown that most algae have antioxidant activity (Jiménez-Escrig et al., 2001[26]; Li et al., 2007[35]), which stems from the expression of compounds such as polyphenols, ascorbic acid, and carotenoids in the algae (Cornish and Garbary, 2010[14]).
The present study aimed to determine the oil content of D. salina naturally present in Lake Tuz, and to investigate the antimicrobial effect of the oil, as well as the antioxidant and antimicrobial effects of various organic D. salina extracts.
Materials and Methods
Sample collection
Samples were collected on July 14, 2012 during the period when D. salina bloom along with a net plankton of 8 µ. The collected samples were examined under the binocular microscope to ensure that they consisted of pure D. salina. These samples were desalinated by maintaining them in distilled water followed by several rounds of dialysis to generate desalinated pure D. salina. Subsequently, the samples were completely dried in the oven at 35 °C and then used to obtain lipids and extracts.
Lipid extraction
The lipids from D. salina were homogenized three times with 10 ml of chloroform:methanol (2:1, v/v) ultraturrax. Subsequently, the solvent was completely evaporated till it reached a constant weight, and the total lipid content was determined. The lipid fatty acid methyl esters (FAME) were prepared by saponification with 0.5 N methanolic NaOH and esterification with BF3-methanol complex (20 % solution in methanol), in accordance with IUPAC (1979[25]). Then, 1 µl of the prepared FAME was analyzed using the HP 6890N gas chromatograph (Agilent Technologies, USA), and using the HP-88 (100-m length, 0.25-mm id, and 0.2-mM film thickness) capillary column. The identification of fatty acids was conducted by comparing retention times of the peaks derived from Altech and Accu standards. Analyses were conducted following three experiments, and the mean values were provided with SD.
The organic solvent portion of the fatty acid was completely evaporated under nitrogen (N2) till it reached a constant weight, and then used to determine the antimicrobial activity of fatty acids. Subsequently, following dissolution in dimethylsulphoxide (DMSO), it was sterilized by using a 0.45 µm Millipore filter, and stored at +4 °C for use in future studies.
Preparation of extracts
Extracts were prepared using the inline extraction method, by modifying the method of Sánchez-Saavedra et al. (2010[47]). The dried D. salina, which was first dissolved in n-hexane (H), was vortexed and then sonicated on ice in an ultra-sonic bath (Bandelin Sonorex RK 100H), and was then incubated on a shaker for 2 h in the dark. After centrifugation, the pellet and supernatant were transferred to different tubes. Then, the solvent in the supernatant was evaporated in a drying oven at 50 °C, while the remaining solid extract was dissolved with DMSO. The pellet in the other tube was similarly processed using various solvents: dichloromethane (DCM), methanol (M), and ethanol (E). The solid extract obtained following DCM extraction was dissolved in DMSO, while the solid extracts obtained following methanol and ethanol extraction were dissolved with their respective solvents. The extracts were sterilized using a 0.45-µm Millipore filter and stored at +4 °C before used in the analysis of their antimicrobial and antioxidant activities.
Determination of antimicrobial activity
Microbial strains
For the determination of antimicrobial activity, the fish pathogenic strains, Yersinia ruckeri, Lactococcus garvieae, Vibrio anguillarum (M1 and A4 strains, obtained from different companies), and Vibrio alginolyticus were used. In addition, for the determination of antimicrobial activity on clinical and food-borne pathogenic microorganisms, Yersinia enterocolitica NCTC 11175, Staphylococcus aureus ATCC 25923, Listeria monocytogenes ATCC 7644, Micrococcus luteus NRRL B-4375, Bacillus cereus RSKK 863, Escherichia coli O157:H7, Escherichia coli ATCC 11229, Escherichia coli ATCC 35218, Salmonella enteritidis ATCC 13076, Pseudomonas aeruginosa ATCC 27853, Shigella sonnei Mu:57, Bacillus subtilis RSKK 244, Salmonella enteritidis RSKK 171, and Candida albicans ATCC 10231 strains were used. Nutrient agar (NA) and Tryptic Soy Agar (TSA) were used for the growth of bacteria, and YPD medium was used for the growth of yeast. Fish pathogenic bacteria were incubated at 25 °C and other bacterial cultures were incubated at 37 °C for 24 h. Yeast cultures were incubated at 30 °C for 48 h.
Determination of antimicrobial effect
The antimicrobial activity was determined by the disc diffusion technique (Murray et al., 1995[39]), in duplicate using filter paper discs (6 mm in diameter). The culture suspensions, adjusted by comparing with 0.5 McFarland, were spread on plates. The paper discs, impregnated with 10 µl of the extract (500 µg extract/disc) and 20 µl of fatty acids (250 µg fatty acid/disc), were placed on the inoculated plates. The plates were stored in a refrigerator (2 h) to enable prediffusion of the extracts into the agar, followed by incubation for 24 h and 48 h for bacterial and yeast strains, respectively. Amikacin (AK, 30 µg/disc), ampicillin (Amp, 10 µg/disc), gentamicin (CN, 10 µg/disc), and fluconazole (FCA, 25 µg/disc) were used as positive controls. Paper discs loaded with solvents were used as negative controls. The inhibition zones (mm) were used to determine the antimicrobial activity.
Determination of Minimal Bactericidal (MBC) or Fungicidal Concentration (MFC)
The MBC and MFC values of the extracts were determined with two-fold dilutions (Chandrasekaran and Venkatesalu, 2004[12]). MBC and MFC values were determined for the microorganisms that presented an inhibition zone by the disc diffusion method. The extracts were collected in tubes, with the highest concentration of 40.00 mg/ ml, followed by serial dilutions in tubes until the lowest concentration of 0.16 mg/ml was achieved. The final volume of 100 µl in each tube was attained by using the respective medium, before adding 2.5 µl of the microorganism (0.5 McFarland) to each tube. Tubes containing 2.5 µl of inoculum and growth medium were prepared as a positive control, and tubes containing 2.5 µl of extract and 100 µl of growth medium were prepared as a negative control. After thoroughly mixing the contents of the tube, it was incubated for 24 h at the appropriate incubation temperatures. Subsequently, 5 µl of the sample was withdrawn from each tube and spot inoculated on the petri containing the solid medium. The concentration that inhibited growth in its medium was recorded as MBC or MFC.
Antioxidant activity
Total phenolic and flavonoid content
Total phenolic content was determined by using the Folin–Ciocalteu reaction, in accordance with the method of Slinkard and Singleton (1977[49]). The method was applied by measuring absorbance at 765 nm after adding 1 ml Folin–Ciocalteu reagent and 2 ml of 7.5 % Na2CO3 to 0.2 ml of extract at 2 mg/ml concentration, stirring in 7 ml of deionized water, and incubating at room temperature for 2 h. The results provided the gallic acid equivalent, which is the standard phenolic compound (GAE mg/g extract).
Total flavonoid content was determined by the method of Arvouet-Grand et al. (1994[3]). In brief, 1 ml of a 2 % methanolic AlCl3 solution was mixed with 1 ml of 2 mg/ ml extract, and its absorbance was determined at 415 nm. Quercetin was used as the standard and the results were expressed as µg/g quercetin equivalent (QE).
Total antioxidant capacity
Total antioxidant capacity was evaluated by a method that uses phosphomolybdenum (Prieto et al., 1999[44]). In brief, the mixture of 0.3 ml extract (2 mg/ml) with 3 ml of reagent solution (6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate) was incubated at 95 °C for 90 min. The absorbance of the mixture was then measured against blank at 695 nm. Antioxidant capacity of the extracts was expressed as ascorbic acid (mg AE/g extract) equivalent.
Free radical scavenging activity (DPPH, 2,2-diphenyl-1-picrylhydrazyl)
The method was applied with a partial modification of the method of Kirby and Schmidt (1997[30]). In brief, 0.5 ml of the sample extract, with concentrations ranging from 0.2 to 1 mg/ml was mixed with 3 ml of a 6.10-5 M DPPH solution, and the mixture was incubated for 30 min at room temperature in the dark. The absorbance was subsequently measured at 517 nm by using the following equation and the % inhibition (I %) was determined;
I(%) = (A0 - A1)/A0 x 100 [1]
(A0 = absorbance of the control, A1 = sample/absorbance of standard)
BHT was used as the standard antioxidant. Results were expressed by calculating IC50 values. A low value of IC50 indicates a higher inhibitory activity.
ß-Carotene/linoleic acid bleaching assay
The method was applied with a partial modification of the method of Sokmen et al. (2004[50]). In brief, 0.5 mg ß-carotene was dissolved in 1 ml of chloroform, and then 25 µl of linoleic acid and 200 mg of Tween 40 were added. The chloroform was completely evaporated, distilled water was added, and quickly shaken and saturated with oxygen. To 2.5 ml of the reaction mixture, 350 µl of the extract (2 mg/ml) was added, and the reaction mixture was incubated at 50 °C for 2 h. The same procedure was used for the standard antioxidants BHA and BHT, which were used as a positive control, and for the blank. By measuring the absorbance of the mixture at 490 nm, inhibition was calculated according to the equation;
R = ln (a/b)/t [2]
(ln = natural log, a = absorbance at time 0, b = absorbance at time t (120 min)]
The antioxidant activity (AA) was calculated by the following equation as % inhibition:
AA = [(RControl - RSample)/RControl] x 100 [3]
Results
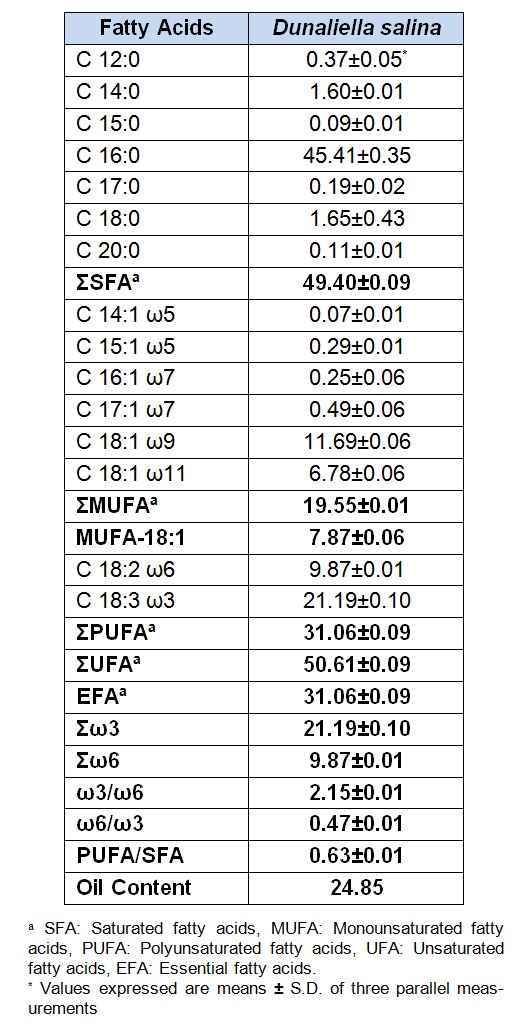
The fatty acid composition of lipids from D. salina is provided in Table 1(Tab. 1). The total oil content of D. salina was found to be 24.85 %. Fifteen prominent fatty acids were identified in D. salina, including saturated fatty acids (SFA), monounsaturated acids (MUFA), and polyunsaturated acids (PUFA). SFA and PUFA were the dominant fatty acid groups, while the levels of MUFA were relatively low. The primary fatty acids identified in the samples were palmitic (C16:0), linolenic (C18:3 ω3), and oleic (C18:1 ω9) acids.
Table 1. Fatty acid composition and oil content of Dunaliella salina.
The antimicrobial activities of n-hexane, DCM, methanol, and ethanol extracts and fatty acids of D. salina against five fish and 14 clinical and food-borne pathogenic microorganisms were determined.
Due to the increasing use of antimicrobial drugs in aquaculture, bacteria are developing resistance, and these bacteria can then pass on to humans consuming these fish (WHO, 2006[53]). Therefore, the use of natural antimicrobial agents as an alternative to synthetic antimicrobials in aquaculture is very important and public’s awareness and interest in these issues is gradually increasing. The first phase of our study was intended to reveal whether various extracts and fatty acids of D. salina have antimicrobial activity on certain fish pathogens, and its potential utilization as an alternative to synthetic antimicrobials, used to prevent various diseases or as an inhibitor. According to the results of the disc diffusion technique conducted against the pathogenic microorganisms from fish, the highest inhibitory effect was identified with the ethanol extract: 18.21 mm against L. garviae (Table 2(Tab. 2)). In general, the methanol and ethanol extracts demonstrated a better inhibition zone than n-hexane and DCM against fish pathogenic microorganisms other than V. alginolyticus. The MBC values of extracts against fish pathogens were identified as 0.63–10.00 mg/ml. In parallel with the results of the disc diffusion method, the MBC values of methanol and ethanol extracts were observed to be lower than the other extracts. The lowest MBC value of the ethanol extract was identified in V. anguillarum (strain A4) and V. alginolyticus.
Table 2. Antimicrobial activity of Dunaliella salina against fish pathogens.
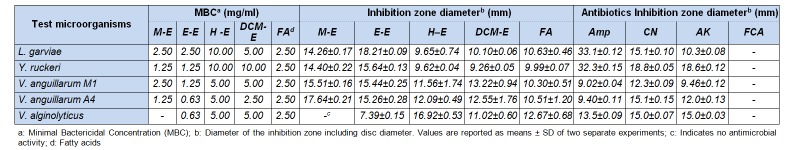
Based on the results of the disc diffusion method, the antibacterial activity of fatty acids obtained from D. salina on fish pathogenic microorganisms resulted in a 9.99-12.67 mm zone of inhibition (Table 2(Tab. 2)). However, the MBC values of the extracts were identified as 2.50 mg/ml for all bacteria. Compared with ampicillin (10 µg/disc, standard antibiotic), the fatty acids (250 µg/ disc) showed higher inhibitory activity against V. anguillarum (strain A4 and M1), as demonstrated by the disc diffusion method.
The inhibitory activity of different extracts of D. salina on clinical and food-borne pathogens was also researched in this study. According to the results of the disc diffusion method, the extract of ethanol, and then the extract of methanol showed the highest inhibitory effect for all microorganisms, with respect to other extracts (Table 3(Tab. 3)), although the methanol extract was not effective against B. subtilis RSKK 244 and S. enteritidis RSKK 171. However, Sánchez-Saavedra et al. (2010[47]) reported that the hexane extract of D. tertiolecta showed better antimicrobial activity than DCM and methanol extracts. While all three extracts in their study could inhibit S. aureus and B. subtilis, the extracts were ineffective against M. luteus, E. coli, P. aeruginosa. However, in our study, each of the three extracts showed antimicrobial activity against these three bacteria.
Table 3. Antimicrobial activity of Dunaliella salina against clinical and food-borne pathogens.
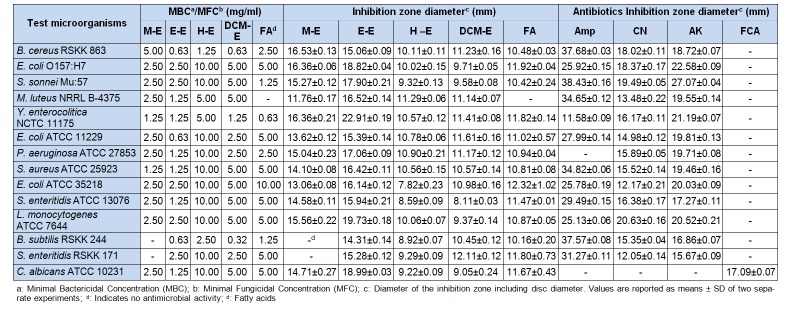
According to the results of the disc diffusion method, the ethanol extract (500 mg/ disc) showed higher inhibitory activity against C. albicans (ATCC 10231) than FCA (25 µg/disc), which is the standard antifungal antibiotic. MBC and MFC values of the extracts were determined as 0.32 to 10.00 mg/ ml. When the antimicrobial activity of the fatty acids on clinical and food-borne pathogens was studied, a maximum zone of inhibition of 11.82 mm was observed and a MBC of 0.63 mg/ml was estimated against Y. enterolitica NCTC 11175. The fatty acids demonstrated effective antimicrobial activity against all microorganisms to various degrees, except M. luteus NRRL B-4375. MBC and MFC values of the fatty acids varied between 0.63–10.00 mg/ml (Table 3(Tab. 3)).
Antioxidant activity of four different (hexane, dichlormethane, methanol, and ethanol) extracts obtained from D. salina was determined by using total antioxidant capacity, free radical scavenging activity and ß-carotene/linoleic acid bleaching assay. In addition, the total phenolic and flavonoid contents of the extracts were researched (Table 4(Tab. 4)).
Table 4. Total phenolic, flavonoid contents, antioxidant capacities, free radical scavenging activity and inhibition activity against on linoleic acid oxidation of extracts from D. salina and standard antioxidants.

The total phenolic content of D. salina was determined in hexane and DCM extracts. No phenolic content was observed in the remaining extracts (methanol and ethanol). In hexane (53.27 mg GAE/g) and DCM (56.45 mg GAE/g) extracts, a low phenolic content was found. None of the D. salina extracts expressed any flavonoids (Table 4(Tab. 4)).
The DPPH method is widely used to determine free radical scavenging activity. This activity is evaluated by determining the IC50 (the amount of extract required to scavenge 50 % of DPPH radicals) values for each extract being evaluated (Table 4(Tab. 4)). A low IC50 value indicates a high level of activity. Among the D. salina extracts, the methanol extract has the highest DPPH removal activity, with the value of IC50 = 0.45 mg/ml. The IC50 values of DCM, hexane, and ethanol extracts are 1.92, 3.07, and 3.46 mg/ml, respectively. BHT showed higher activity than other extracts, with the value of IC50 = 0.05 mg/ml.
Oxidation of linoleic acid, using the ß-carotene/linoleic acid bleaching test, produces peroxyl free radicals depending on the cleavage of the hydrogen atoms from deallic methyl (Laguerre et al., 2007[33]). The resulting free radicals cause the oxidation of ß-carotene, which is an unsaturated molecule, and its orange color becomes lighter. The ß-carotene oxidation inhibition values of different extracts of D. salina range between 25.79 %- 68.88 % (Table 4(Tab. 4)). In this test, the inhibitory rates of BHA and BHT were 89.57 % and 93.8 %, respectively. The methanol and ethanol extracts were particularly noteworthy with their high inhibition values. D. salina extracts have a high inhibitory effect because of the increased ß-carotene expression.
Discussion
Several studies have reported the total oil content of D. salina as well as the fatty acid content (Herrero et al., 2006[23]; Lamers et al., 2010[34]; Diaz-Palma et al., 2012). D. salina has been reported to have 7.9 % of oil content (Diaz-Palma et al., 2012[19]). The study by Lamers et al. (2010[34]) identified fatty acids as the major component of D. salina (C16:0, C16:4, C18:1, C18:2, and C18:3), and indicated that these fatty acids constitute approximately 95 % of the total fatty acid content. The study by Herrero et al. (2006[23]) identified palmitic, alpha-linolenic, and oleic acids as the essential fatty acid components. Our present study discovered that the fatty acid profile of the D. salina samples from Lake Tuz is primarily composed of palmitic, linolenic, and oleic acids, which complements the previous reports. The ω3/ω6 fatty acid isomer ratio is high, particularly in fishes, and it is a useful indicator for comparing the nutritional value of the fats (Piggot and Tucker, 1990[43]). Studies conducted on the fatty acid composition of fishes have identified that the ratio varies between 1.03-2.80 (Guler et al., 2007[22]; Donmez, 2009[20]; Cakmak et al., 2012[9]). In the present study, the ω3/ω6 ratio is 2.15. Accordingly, the nutritional value of the fat obtained from D. salina is higher than that of some fishes.
Herrero et al. (2006[23]) reported that ethanol and hexane extracts of D. salina showed antimicrobial activity against E. coli, S. aureus, and C. albicans, and that this antimicrobial activity might result from fatty acids such as palmitic, α-linolenic, and oleic acids, which are a major component of the extracts. Krishnika et al. (2011[32]) identified the antimicrobial activity of different extracts of eight microalgae, and reported that Dunaliella sp. showed a high degree of inhibitory effect on Shigella boydi, Salmonella paratphi A, and Pseudomonas fluorecens. However, the antimicrobial activity of DCM, hexane, ethanol, and methanol extracts on E. coli was not observed. These results contradicted those of our study; we demonstrated that the aforementioned extracts of D. salina showed inhibitory effects against different strains of E. coli. The contradictions could be explained by the different strains used in the determination of the antimicrobial activity, the different methods used in the preparation of extracts or the concentration of the extracts used. In addition, the presence of different types of Dunaliella and the difference in location might affect the antimicrobial activity.
The antimicrobial activity of fatty acids has been reported by many studies over several years (Galbraith et al., 1971[21]; Kabara and Vrable, 1977[28]; Desbois et al., 2008[16], 2009[17]; Abdelillah et al., 2013[1]). Due to this important feature, fatty acids can have potential applications in various fields such as agriculture, medicine, cosmetics, food preservation, and in particular as an alternative to the forbidden antibiotics (Desbois and Smith, 2010[18]). It is reported that the antimicrobial activity of fatty acids depends on both the chain length and the degree of unsaturation (Kabara and Vrable, 1977[28]). Fatty acids with chain length less than 8 carbon atoms are defined as short-chain and those higher than 16 carbon atoms are defined as long chain (Desbois and Smith, 2010[18]). In D. salina, only medium and long chain fatty acids were identified. Several studies have reported that medium and long chain fatty acids have antimicrobial activity (Galbraith et al., 1971[21]; Bergsson et al., 1999[6]). Also, in this study, the unsaturated fatty acid content of D. salina was found to be higher than the saturated content. It is reported that unsaturated fatty acids, because they contain double bonds, have higher antimicrobial activity in comparison with the saturated fatty acids (Ouattara et al., 1997[40]).
Microalgae need to adapt their metabolism to their environment in order to survive in different habitats, and this characteristic equips them to resist environmental stress conditions by producing various secondary metabolites. Due to these features, microalgae hold an important place in biotechnology, healthcare, food industry, and aquaculture (Andersen, 1996[2]). The stress evoked by the high salinity in Lake Tuz has sharpened the production of secondary metabolites in D. salina and, consequently, may have increased antimicrobial activity. As a result of this study, the antimicrobial potential of extracts and fatty acids obtained from D. salina against some fish and human pathogens was revealed; the isolation and characterization of the components responsible for the antimicrobial activity will be the basis for future studies.
Phenolic compounds are obtained from plant secondary metabolites, and they are major components that determine the antioxidant capacity of plants. Polyphenols have several beneficial effects, including anticarcinogenic activity (Carroll et al., 1999[10]), and reducing the risk of coronary heart diseases (Williams and Eliot, 1997[54]). There is very limited information on the phenolic content of microalgae. Li et al. (2007[35]) examined the phenolic content of hexane, ethyl acetate, and water extracts from 23 microalgae, and demonstrated that hexane extracts have 2.12-39.87 mg GAE/g, ethyl acetate extracts have 0.01-9.80 mg GAE/g, and water extracts have 1.09-10.68 mg GAE/g. Another study examining the total polyphenol content of some marine algae identified the expression of phloroglucinol equivalent (PGE) between 5.3-41.4 g PGE/kg (dry matter) (Jiménez-Escrig et al., 2001[26]). According to the phosphomolybdenum assay, the DCM extract showed the highest activity at 454.92 mg AE/g, followed by hexane (151.85 mg AE/g) and ethanol (1.08 mg AE/g) extracts. Similarly, in our previous study, Anabaenopsis sp. Woloszynska (Cyanophyceae). DCM extract demonstrated (34.15 mg AE/g) the highest total antioxidant capacity; the methanol extract (13.77 mg AE/g) had relatively low activity, while water and ethanol extracts did not show any activity (Ozusaglam et al., 2013[42]).
Hu et al. (2008[24]) compared the free radical scavenging effect of algal extracts of D. salina with some commercially carotenoids (alltrans forms of lutein, zeaxanthin, α-carotene, and ß-carotene), which is also in the composition of D. salina, and determined that the algal extract showed higher activity than each of the listed carotenoids. Accordingly, while the EC50 value of the algal extract was 8.36 mg/ml, the carotenoids demonstrated a much greater activity in the range of 22.82–24.54 mg/ml (Hu et al., 2008[24]). In our study, different extracts of D. salina showed higher activity than demonstrated in the study by Hu et al. (2008[24]).
In conclusion, our study shows that D. salina has high oil content. Analysis of the composition of this oil content concluded that more than 50 % of the total fatty acids are mono- and poly-unsaturated, and it is expected to have beneficial health effects. With a high content of ω3/ω6, D. salina may have a positive effect on the risk of coronary disease. The present study also demonstrated that this oil has antimicrobial activity. Further, the various extracts obtained from D. salina have antimicrobial and antioxidant activity. Thus, we can conclude that due to its beneficial fatty acid composition, antimicrobial and antioxidant properties, D. salina is a safe food additive, a good feed source for fish and other aquatic organisms, and it can be utilized in the field of pharmacology due to its biological activities. And also it can be used as a new sources of essential fatty acids because of its high their content.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Abdelillah A1, Houcine B, Halima D, Meriem CS, Imane Z, Eddine SD, et al. Evaluation of antifungal activity of free fatty acids methyl esters fraction isolated from Algerian Linum usitatissimum L. seeds against toxigenic Aspergillus. Asian Pac J Trop Biomed. 2013;3:443–448. doi: 10.1016/S2221-1691(13)60094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen RA. Maintaining cultures for biotechnology and industry. In: Hunter-Cervera JC, Belt A, editors. Algae. London: Academic Press; 1996. pp. 29–64. [Google Scholar]
- 3.Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardisation d’un extrait de propolis et identification des principaux constituants. J Pharm Belg. 1994;49:462–468. [PubMed] [Google Scholar]
- 4.Barros MP, Pinto E, Sigaud-Kutner TCS, Cardozo KHM, Colepicolo P. Rhythmicity and oxidative/nitrosative stress in algae. Biol Rhythm Res. 2005;36:67–82. [Google Scholar]
- 5.Ben Amotz A, Katz A, Avron M. Accumulation of β-carotene in halotolerant algae: purification and characterization of b-carotene rich globules from Dunaliella bardawil (Chlorophyceae) J Phycol. 1982;18:529–537. [Google Scholar]
- 6.Bergsson G, Steingrímsson O, Thormar H. In vitro susceptibilities of Neisseria gonorrhoeae to fatty acids and monoglycerides. Antimicrob Agents Chemother. 1999;43:2790–2792. doi: 10.1128/aac.43.11.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buiatti E. The role of chemoprevention in cancer control. Salud Publ de Mex. 1997;39:310–317. doi: 10.1590/s0036-36341997000400009. [DOI] [PubMed] [Google Scholar]
- 8.Burton GW, Ingold KU. Beta-carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 9.Cakmak YS, Zengin G, Guler GO, Aktumsek A, Ozparlak H. Fatty acid composition and ω3/ω6 ratios of the muscle lipids of six fish species in Sugla Lake, Turkey. Arch Biol Sci. 2012;64:471–477. [Google Scholar]
- 10.Carrol KK, Kurowska EM, Guthirie N. Use of citrus limonoids and flavonoids as well as tocotrienols for the treatment of cancer. Int Patent WO 9916167. 1999.
- 11.Challem J. Beta-carotene and other carotenoids: promises failures, and a new vision. J Orthomol Med. 1997;12:11–19. [Google Scholar]
- 12.Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharmacol. 2004;91:105–108. doi: 10.1016/j.jep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Chidambara KN, Vanitha A, Rajesha J, Mahadeva M, Sowmya PR, Ravishankar A. In vivo antioxidant activity of carotenoids from Dunaliella salina, a green microalga. Life Sci. 2005;76:1381–1390. doi: 10.1016/j.lfs.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Cornish ML, Garbary DJ. Antioxidants from macroalgae: potential applications in human health and nutrition. Algae. 2010;25:155–171. [Google Scholar]
- 15.Del Campo JA, García-González M, Guerrero MG. Outdoor cultivation of microalgae for carotenoid production: current state and prospectives. Appl Microbiol Biotechnol. 2007;74:1163–1164. doi: 10.1007/s00253-007-0844-9. [DOI] [PubMed] [Google Scholar]
- 16.Desbois AP, Lebl T, Yan L, Smith VJ. Isolation and structural characterisation of two antibacterial free fatty acids from the marine diatom, Phaeodactylum tricornutum. Appl Microbiol Biotechnol. 2008;81:755–764. doi: 10.1007/s00253-008-1714-9. [DOI] [PubMed] [Google Scholar]
- 17.Desbois AP, Mearns-Spragg A, Smith VJ. A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA) Mar Biotechnol. 2009;11:45–52. doi: 10.1007/s10126-008-9118-5. [DOI] [PubMed] [Google Scholar]
- 18.Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 19.Díaz-Palma P, Stegen S, Queirolo F, Arias D, Araya S. Biochemical profile of halophilous microalgae strains from high-andean extreme ecosystems (NE-Chile) using methodological validation approaches. J Biosci Bioeng. 2012;113:730–736. doi: 10.1016/j.jbiosc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Donmez M. Determination of fatty acid compositions and cholesterol levels of some freshwater fish living in Porsuk Dam, Turkey. Chem Nat Compd. 2009;45:14–17. [Google Scholar]
- 21.Galbraith H, Miller TB, Paton AM, Thompson JK. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J Appl Bacteriol. 1971;34:803–813. doi: 10.1111/j.1365-2672.1971.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 22.Guler GO, Aktumsek A, Citil OB, Arslan A, Torlak E. Seasonal variations on total fatty acid composition of fillets of zander (Sander lucioperca) in Beysehir Lake (Turkey) Food Chem. 2007;103:1241–1246. [Google Scholar]
- 23.Herrero M, Ibañez E, Cifuentes A, Reglero G, Santoyo S. Dunaliella salina microalga pressurized liquid extracts as potential antimicrobials. J Food Prot. 2006;69:2471–2477. doi: 10.4315/0362-028x-69.10.2471. [DOI] [PubMed] [Google Scholar]
- 24.Hu CC, Lin JT, Lu FJ, Chou FP, Yang DJ. Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem. 2008;109:439–446. doi: 10.1016/j.foodchem.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Paquot C, editor. IUPAC. Standard methods for the analysis of oils, fats and derivatives. 6th ed. Oxford: Pergamon Press; 1979. pp. 59–66. [Google Scholar]
- 26.Jiménez-Escrig A, Jiménez-Jiménez I, Pulido R, Saura-Calixto F. Antioxidant activity of fresh and processed edible seaweeds. J Sci Food Agric. 2001;81:530–534. [Google Scholar]
- 27.Jin E, Melis A. Microalgal biotechnology: carotenoid production by the green alga Dunaliella salina. Biotechnol Bioprocess Eng. 2003;8:331–337. [Google Scholar]
- 28.Kabara JJ, Vrable R. Antimicrobial lipids: natural and synthetic fatty acids and monoglycerides. Lipids. 1977;12:753–759. doi: 10.1007/BF02570908. [DOI] [PubMed] [Google Scholar]
- 29.Kellan SJ, Walker JM. Antibacterial activity from marine microalgae. Br J Phycol. 1989;23:41–44. [Google Scholar]
- 30.Kirby AJ, Schmidt RJ. The antioxidant activity of Chinese herbs for eczema and of placebo herbs. J Ethnopharmacol. 1997;56:103–108. doi: 10.1016/s0378-8741(97)01510-9. [DOI] [PubMed] [Google Scholar]
- 31.Knekt P, Aromaa A, Maatela J, Aaran RK, Nikkari T, Hakama M, et al. Serum vitamin A and subsequent risk of cancer incidence follow-up of the finish mobile clinic health examination survey. Am J Epidermiol. 1990;132:857–870. doi: 10.1093/oxfordjournals.aje.a115728. [DOI] [PubMed] [Google Scholar]
- 32.Krishnika A, Bhanupriya PB, Beena B. Antibacterial activity of eight marine microalgae against a few gram negative bacterial pathogens. J Pharm Res. 2011;4:3024–3026. [Google Scholar]
- 33.Laguerre M, Lecomte J, Villeneuve P. Evaluation of the ability of antioxidant to counteract lipid oxidation: existing methods, new trends and challenges. Prog Lipid Res. 2007;46:244–282. doi: 10.1016/j.plipres.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Lamers PP, van de Laak CC, Kaasenbrood PS, Lorier J, Janssen M, De Vos RC, et al. Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng. 2010;106:638–648. doi: 10.1002/bit.22725. [DOI] [PubMed] [Google Scholar]
- 35.Li HB, Cheng KW, Wong CC, Fan KW, Chen F, Jiang Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007;102:771–776. [Google Scholar]
- 36.Mayer AMS, Hamann MT. Marine pharmacology in. 2000: marine compounds with antibacterial, antico-agulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antituberculosis, and antiviral activities; affecting the cardiovascular, immune, and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol (NY) 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendoza H, Martel A, Jiménez Del Rio M, Garcia-Reina G. Oleic acid is the main fatty acid related with carotenogenesis in Dunaliella salina. J App Phycol. 1999;11:15–19. [Google Scholar]
- 38.Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, Howe PRC. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids. 2003;38:391–398. doi: 10.1007/s11745-003-1074-0. [DOI] [PubMed] [Google Scholar]
- 39.Murray PR, Baron EJ, Pfalle MA, Tenover FC, Yolke RH. Manual of clinical microbiology. 6th ed. Washington, DC: ASM Press; 1995. [Google Scholar]
- 40.Ouattara B, Simard RE, Holley RA, Piette GJP, Begin A. Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int J Food Microbiol. 1997;37:155–162. doi: 10.1016/s0168-1605(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 41.Ozemir G, Karabay NU, Dalay MC, Pazarbasi B. Antibacterial activity of volatile components and various extracts of Spirulina platensis. Phytother Res. 2004;18:754–757. doi: 10.1002/ptr.1541. [DOI] [PubMed] [Google Scholar]
- 42.Ozusaglam MA, Darilmaz DO, Erzengin M, Teksen M, Erkul SK. Antimicrobial and antioxidant activities of two endemic plants from Aksaray in Turkey. Afr J Tradit Complement Altern Med. 2013;10:449–457. doi: 10.4314/ajtcam.v10i3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piggot GM, Tucker BW. Effects of technology on nutrition. New York: Marcel Dekker; 1990. [Google Scholar]
- 44.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphor molybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 45.Puglisi MP, Tan LT, Jensen PR, Fenical W. Capisterones A and B from the tropical green alga Penicillus capitatus: unexpected anti-fungal defenses targeting the marine pathogen Lindra thallasiae. Tetrahedron. 2004;60:7035–7039. [Google Scholar]
- 46.Raja R, Hemaiswarya S, Balasubramanyam D, Rengasamy R. Protective effect of Dunaliella salina against experimentally induced fibrosarcoma on Wistar rats. Microbiol Res. 2007;162:177–184. doi: 10.1016/j.micres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Sánchez-Saavedra MD, Licea-Navarro A, Bernáldez-Sarabia J. Evaluation of the antibacterial activity of different species of phytoplankton. Rev Biol Mar Oceanogr. 2010;45:531–536. [Google Scholar]
- 48.Saygi YB, Demirkalp FY. Effects of temperature on survival and growth of artemia from Tuz Lake, Turkey. Isr J Aquac Bamidgeh. 2002;54:125–133. [Google Scholar]
- 49.Slinkard K, Singleton VL. Total phenol analysis: Automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 50.Sokmen A, Gulluce M, Akpulat HA, Daferera D, Tepe B, Polissiou M, et al. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control. 2004;15:627–634. [Google Scholar]
- 51.Stryker WS, Stampfer MJ, Stein E, Kaplan AL, Louis T, Sober A, et al. Diet, plasma levels of beta-carotene and alpha-tocopherol, and risk of malignant melanoma. Am J Epidemiol. 1990;131:597–611. doi: 10.1093/oxfordjournals.aje.a115544. [DOI] [PubMed] [Google Scholar]
- 52.Wald NJ, Thompson SG, Denseml JW, Borehaml J, Bailey A. Serum beta-carotene and subsequent risk of cancer: Results from the BUPA Study. Br J Cancer. 1988;57:428–433. doi: 10.1038/bjc.1988.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.WHO. Antimicrobial use in aquaculture and antimicrobial resistance. Consultations and workshops. Report of a Joint FAO/OIE/WHO, Seoul, Republic of Korea; Geneva: World Health Organization; Jun 13-16, 2006. p. 97. [Google Scholar]
- 54.Williams RL, Elliot MS. Antioxidants in grapes and wine: Chemistry and health effects. In: Shaihidi F, editor. Natural antioxidants: chemistry, health effects and applications. Urbana, IL: AOCS Press; 1997. pp. 150–173. [Google Scholar]


