Abstract
Tight junctions (TJs), which are the most apically located of the intercellular junctional complexes, have a barrier function and a fence function. Recent studies show that they also participate in signal transduction mechanisms. TJs are modulated by intracellular signaling pathways including protein kinase C, mitogen-activated protein kinase, and NF-ϰB, to affect the epithelial barrier function in response to diverse stimuli. TJs are also regulated by various cytokines, growth factors, and hormones via signaling pathways. To investigate the regulation of TJ molecules via signaling pathways in human epithelial cells under normal and pathological conditions, we established a novel model of human telomerase reverse transcriptase-transfected human epithelial cells. In this review, we describe the recent progress in our understanding of the role of TJs for signal transduction under normal conditions in upper airway epithelium, pancreatic duct epithelial cells, hepatocytes, and endometrial epithelial cells, and in pathological conditions including cancer and infection.
Keywords: tight junctions, signal transduction, claudins, occludin, human epithelial cells
Introduction
Tight junctions (TJs), which are the most apically located of the intercellular junctional complexes, inhibit solute and water flow through the paracellular space (termed the barrier function) (Gumbiner, 1993[32]; Schneeberger and Lynch, 1992[98]). In addition, they separate the apical and basolateral cell surface domains to establish cell polarity (termed the fence function) (Figure 1(Fig. 1)) (van Meer and Simons, 1986[117]; Cereijido et al., 1998[17]). Recent evidence shows that TJs participate in signal transduction mechanisms that regulate epithelial cell proliferation, gene expression, differentiation, and morphogenesis (Balda and Matter, 2009[6]).
Figure 1. (A) Schematic diagram of a tight junction. Tight junctions are the most apically located of the intercellular junctional complexes in vertebrate epithelial cell sheets. On transmission electron microscopy, tight junctions appear as a series of very close membrane appositions (so-called kissing points) between adjacent cells. (B) Functions of tight junctions. Fence function: Separating the apical from the basolateral cell surface domains to establish cell polarity. Barrier function: Inhibiting solute and water flow through the paracellular space. Signal transduction: Regulating epithelial cell proliferation, gene expression, differentiation, and morphogenesis.
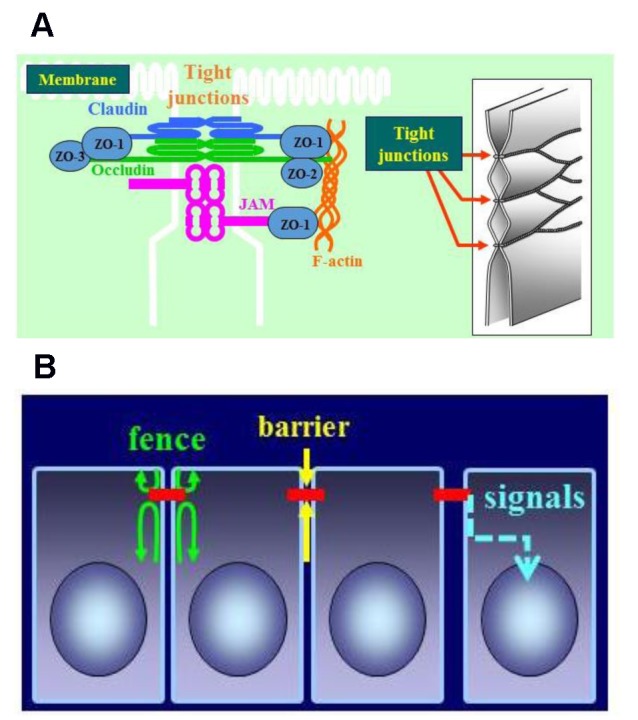
TJs are formed not only by the integral membrane proteins claudins, occludin, and junction adhesion molecules (JAMs) but also by several peripheral membrane proteins. These peripheral membrane proteins include the scaffold PDZ expression proteins zonula occludins (ZO)-1, -2, -3, multi-PDZ domain protein-1 (MUPP1), and membrane-associated guanylate kinase with inverted orientation (MAGI)-1, -2, -3. Furthermore, they comprise cell polarity molecules, such as ASIP/PAR-3, PAR-6, PALS-1, and PALS-1-associated TJ (PATJ) and the non-PDZ-expressing proteins, cingulin, symplekin, ZONAB, GEF-H1, aPKC, PP2A, Rab3b, Rab13, PTEN, and 7 H6 (Schneeberger and Lynch, 2004[99]; Tsukita et al., 2001[114]; Sawada et al., 2003[97]). The proteins ZO-1, -2, and -3 are members of the membrane-associated guanylate kinase (MAGUK) family of proteins displaying a characteristic multidomain structure comprising SH3, guanylate kinase-like (GUK), and multiple PDZ (PSD95–Dlg–ZO1) domains (Anderson, 1996[3]). ZO-1 and -2 are also closely associated with the polymerization of claudins (Umeda et al., 2006[116]). Tricellulin was first identified at tricellular contacts with three epithelial cells and has been shown to have a barrier function (Ikenouchi et al., 2005[41]). More recently, lipolysis-stimulated lipoprotein receptor (LSR) was identified as a tricellular TJ-associated membrane protein that recruits tricellulin to tricellular TJs (Masuda et al., 2011[69]).
The claudin family, which consists of at least 27 members, is solely responsible for forming TJ strands and has four transmembrane domains and two extracellular loops (Figure 1(Fig. 1)) (Tsukita et al., 2001[114]; Mineta et al., 2011[74]). The first extracellular loop is the coreceptor of hepatitis C virus (Meredith et al., 2012[71]), which influences the paracellular charge selectivity (Krug et al., 2012[57]). The second extracellular loop is the receptor of Clostridium perfringens enterotoxin (CPE) (Fujita et al., 2000[30]). In addition, because claudin-4 is also a high-affinity receptor of CPE (Katahira et al., 1997[47]), full-length CPE with a direct cytotoxic effect and the C-terminal receptor binding domain of CPE (C-CPE) without a cytotoxic effect are employed for selective treatment and drug delivery against claudin-4-expressing cells (Michl et al., 2001[73]; Saeki et al., 2009[91]).
Occludin, the first discovered integral membrane protein of TJs, is ubiquitously expressed at the most apically located basolateral membranes and is the most reliable immunohistochemical marker for TJs (Tsukita et al., 2001[114]; Furuse et al., 1993[31]). Overexpression of occludin increases the barrier function, indicated by an increase in transepithelial electric resistance (TER) in mammalian epithelial cells (Balda et al., 1996[7]; McCarthy et al., 1996[70]). However, TJ strands can be formed without occludin in some cell types, including occludin-deficient embryonic stem cells (Hirase et al., 1997[36]; Saitou et al., 1998[94]). Moreover, an occludin-deficient mouse model does not display a perturbation of epithelial barrier function. However, a complex pathophysiological phenotype is observed, with growth retardation, chronic inflammation and hyperplasia of the gastric epithelium, calcification in the brain, testicular atrophy, loss of cytoplasmic granules in striated duct cells of the salivary gland, and thinning of the compact bone (Saitou et al., 2000[95]). Compared with claudins, occludin has a relatively long cytoplasmic C terminus containing several phosphorylation sites and a coiled-coil domain that probably interacts with compounds, including PKC-z, c-Yes, connexin26, and the regulatory subunit of phosphatidylinositol 3-kinase (PI3K) (Cummins, 2012[22]), as well as occludin itself, and ZO-1 and -3 (Schneeberger and Lynch, 2004[99]; Tsukita et al., 2001[114]). Thus, research has suggested several roles of occludin in signal transduction, and its involvement in apoptosis has been reported (Murata et al., 2005[78]; Osanai et al., 2006)[86]. Furthermore, occludin is required for hepatitis C virus (HCV) infection, similar to claudins (Zeisel et al., 2011[128]).
JAMs (JAM-A, -B, -C, and -4) are immunoglobulin superfamily proteins expressed at cell junctions in epithelial and endothelial cells, as well as on the surfaces of leukocytes, platelets, and erythrocytes (Martin-Padura et al., 1998[66]). They are important for a variety of cellular processes including TJ assembly, leukocyte transmigration, platelet activation, angiogenesis, and adenovirus binding. Current evidence indicates that JAM-A dimerization is necessary for functional regulation of the cellular barrier (Monteiro and Parkos, 2012[77]).
TJs for signal transduction in normal epithelial cells
TJs and signals in the upper respiratory tract
The epithelium in the upper respiratory tract consists of pseudostratified ciliated columnar epithelial cells, including M cells (membranous or microfold cells), which are specialized for antigen uptake and form a continuous barrier against a wide variety of exogenous antigens (Holgate, 2008[37]; Fujimura, 2000[29]; Kim et al., 2011[48]; Nawijn et al., 2011[79]; Takano et al., 2008[110]), and dendritic cells (DCs), which take up transported antigens via M cells and present antigens for CD4+ T cells while maintaining the integrity of the airway epithelial barrier (Steinman et al., 1997[107]; Yamanaka et al., 2003[124]; Hammad and Lambrecht, 2011[34]). The epithelium plays a crucial role as an interface of adaptive responses and innate responses via TJs, to prevent invasion of inhaled environmental agents such as allergens and pathogens. Dynamic changes in TJs have been identified in human nasal mucosa affected by allergic rhinitis or viral infection.
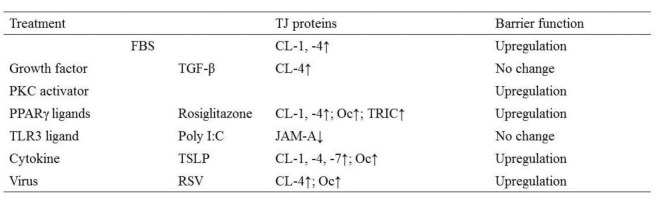
The proliferation and storage of epithelial cells in primary cultures are known to be limited. We introduced the catalytic component of telomerase, the human telomerase reverse transcriptase (hTERT) gene, into primary cultured human nasal epithelial cells (HNECs) (Kurose et al., 2007[59]). The ectopic expression of hTERT in the epithelial cells resulted in greater growth potential and a longer lifespan of the cells. The cells had a small cobblestone appearance in phase-contrast images and cilia-like structures, a differentiation marker of nasal epithelial cells, were observed on the surface of hTERT-transfected cells. The properties of the hTERT-transfected cells were similar to those of the cells in primary cultures (Kurose et al., 2007[59]). In HNECs cultured in vitro using primary cultures and our established culture systems, TJ molecules and the barrier function are upregulated by various stimuli (Table 1(Tab. 1)).
Table 1. Changes in TJ proteins and barrier function in human nasal epithelial cells (HNECs) in vitro (CL, claudins; Oc, occludin; TRIC, tricellulin).
Protein kinase C (PKC) is a family of serine–threonine kinases known to regulate the epithelial barrier function (Tsukamoto et al., 1999[113]; Andreeva et al., 2001[4]; Seth et al., 2007[100]). PKC has been shown to induce both assembly and disassembly of TJs, depending on the cell type and the conditions of activation (Stuart and Nigam, 1995[108]; Andreeva et al., 2001[4]). The activation of PKC causes an increase in permeability in renal epithelial LLC-PK1 cells and Madin–Darby canine kidney (MDCK) cells (Ellis et al., 1992[25]; Clarke et al., 2000[19]). Conversely, it causes a decrease in permeability in the human colon carcinoma cell line HT29 (Sjö et al., 2003[103]). Bryostatin enhances TJ barrier function in T84 cells through a PKC signaling pathway (Yoo et al., 2003[127]). PKC appears to regulate the subcellular localization, phosphorylation states, and transcription of several TJ-associated proteins (Banan et al., 2005[8]), although the isozyme specificity has not been clearly elucidated. The isoforms can be divided into three groups (Zeng, 2012[131]): (1) the classic or conventional isozymes (a, ßI, ßII, and α) are both Ca2+- and diacylglycerol (DAG)-dependent, (2) the novel (nPKC) isozymes (δ, ε, θ, η, and µ) are Ca2+-independent but DAG-dependent, and (3) the atypical isozymes (λ, τ, and ζ) are neither Ca2+- nor DAG-dependent. Activation of PKC by 12-O-tetradecanoylophorbol-13-acetate (TPA) is known to cause increases in transcription of occludin, ZO-1, and claudin-1 in T84 cells and melanoma cells (Weiler et al., 2005[119]; Leotlela et al., 2007[63]).
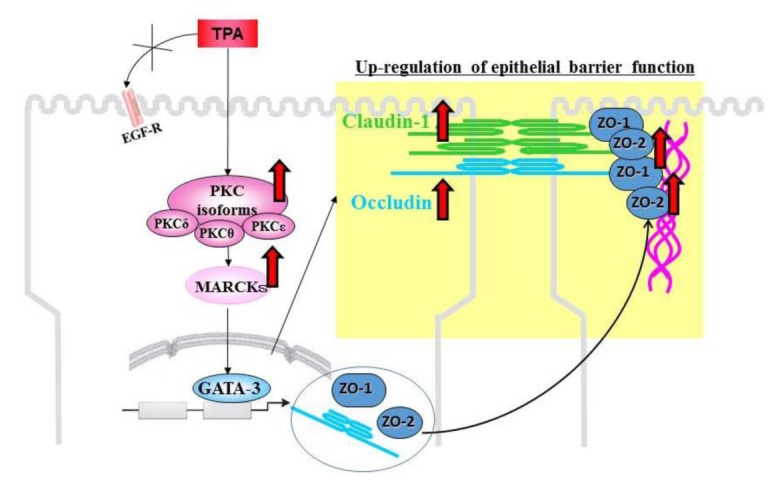
To investigate the mechanisms of regulation of TJs through a PKC signaling pathway, HNECs were treated with TPA as a PKC activator (Koizumi et al., 2008[50]). We found that short treatment with TPA greatly enhanced epithelial barrier function together with an increase in the expression of occludin, ZO-1 and -2, and claudin-1 at the transcriptional level. The upregulation of barrier function and TJ proteins was prevented by a pan-PKC inhibitor and the inhibitors of PKCδ and PKCθ but not PKCε (Koizumi et al., 2008[50]). When we focused on the transcriptional factor GATA family to investigate the transcriptional mechanisms, upregulation of ZO-1 and -2 by treatment with TPA was regulated by GATA-3 via a PKC signaling pathway (Figure 2(Fig. 2)) (Koizumi et al., 2008[50]).
Figure 2. TPA, a PKC activator, increases tight junction barrier function together with upregulation of the tight junction proteins, occludin, zona occludens (ZO)-1 and -2, and claudin-1, at the transcriptional level in human nasal epithelial cells. It also affects the subcellular localization of the tight junction proteins and the numbers of tight junction strands. PKC signaling enhances the barrier function via transcriptional upregulation of tight junction proteins.
Peroxisome proliferator-activated receptor (PPAR)γ is a nuclear hormone receptor superfamily member and a ligand-activated transcription factor that requires heterodimerization with the retinoid X receptor (RXR) to bind specific PPAR response elements (PPRE) located in promoter regions of target genes (Bensinger and Tontonoz, 2008[10]). Recently, PPARγ agonists have been shown to enhance barrier functions together with upregulation of TJ molecules in gastrointestinal epithelial cells, urothelial cells, and endothelial cells (Varley et al., 2006[118]; Dubuquoy et al., 2003[24]; Ponferrada et al., 2007[87]; Ramakers et al., 2007[89]; Huang et al., 2009[38]). To investigate the effects of PPARγ agonists on the TJ barrier of HNECs, which highly express both PPARγ and TJ proteins, HNECs were treated with the PPARγ agonists rosiglitazone and troglitazone. Treatment with the PPARγ agonists enhanced the barrier function of hTERT-transfected HNECs together with the upregulation of TJ molecules claudin-1 and -4, occludin, and tricellulin at the transcriptional level. In addition, a significant increase in TJ strands was observed after treatment with rosiglitazone. Treatment with PPARγ agonists induced the activity of phospho-PKC in hTERT-transfected HNECs. The upregulation of the TJ molecules in hTERT-transfected HNECs by rosiglitazone was inhibited not only by PPARγ antagonists but also by a pan-PKC inhibitor. These findings suggest that PPARγ agonists upregulate the barrier function of the TJs of HNECs via a PKC signaling pathway (Ogasawara et al., 2010[84]).
TJs and signals in pancreatic duct epithelial cells
To investigate the structure and regulation of TJs in normal pancreatic epithelial cells, we established a novel model of hTERT-transfected human pancreatic ductal epithelial (HPDE) cells with an extended life span (Yamaguchi et al., 2010[121]). In this culture system, hTERT–HPDE cells in serum-free conditioned medium have increased growth potential and a long lifespan. Treatment with FBS induces an increase in protein and mRNA of carbonic anhydrase (CA) II that is dependent on the FBS concentration, whereas proteins of cytokeratin (CK) 7 and 19 are stably expressed independent of the FBS concentration. Claudin-1, -4, and -7; occludin; JAM-A; and ZO-1 and -2 are induced by 10% FBS together with an increase in the barrier function, and the upregulation is inhibited by a pan-PKC inhibitor (Table 2(Tab. 2)) (Yamaguchi et al., 2010[121]).
Table 2. Changes in TJ proteins and barrier function in normal human pancreatic duct epithelial (HPDE) cells via PKC and JNK pathways (CL, claudins; Oc, occludin; TRIC, tricellulin).
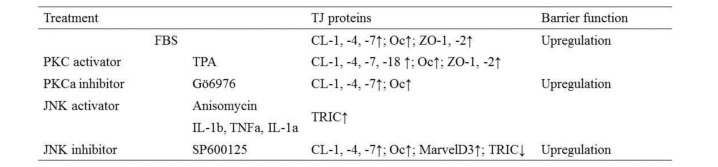
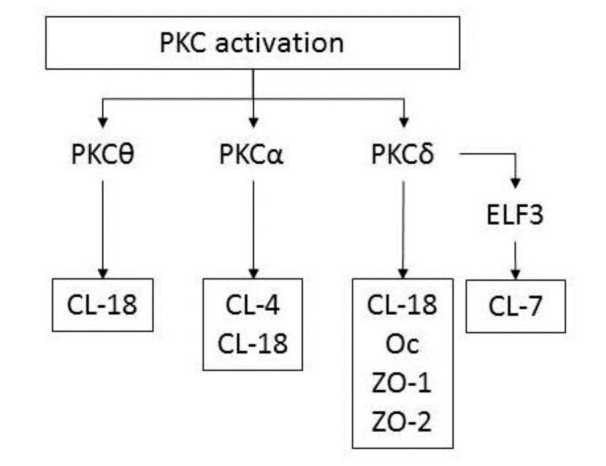
The TJ molecules and the barrier function induced by FBS in hTERT–HPDE cells are partly regulated via a PKC pathway. Treatment with TPA-enhanced expression of most TJ proteins (Yamaguchi et al., 2010[121]). In particular, claudin-4 and -18 were markedly induced by TPA at the transcriptional level, together with an increase in TJ strands (Yamaguchi et al., 2010[121]). The upregulation of TJ proteins by TPA was completely inhibited by a pan-PKC inhibitor (GF109203X). A PKCθ inhibitor (myristoylated PKCθ pseudosubstrate peptide inhibitor) prevented upregulation of claudin-18 by TPA, a PKCα inhibitor prevented upregulation of claudin-4 and -18 by TPA, and a PKCδ inhibitor (rottlerin) prevented upregulation of claudin-7 and -18, occludin and ZO-1 and -2 by TPA (Figure 3(Fig. 3)) (Yamaguchi et al., 2010[121]). According to GeneChip analysis, upregulation of transcription factor E74-like factor (ELF) 3 occurs in both fetal bovine serum- and TPA-treated cells. ELF3 belongs to the ELF subfamily of the ETS transcription factors, but it is distinguished from most ETS family members by its expression pattern, which is specific in epithelial tissues of the lung, liver, kidney, pancreas, prostate, small intestine, and colon mucosa (Tymms et al., 1997[115]). ELF3 controls intestinal epithelial differentiation (Jedlicka et al., 2008[45]) and the expression of claudin-7 in epithelial structures in synovial sarcoma is regulated by ELF3 (Kohno et al., 2006[49]). In hTERT–HPDE cells, ELF3 mRNA is increased by TPA, and a pan-PKC inhibitor prevents upregulation of ELF3 mRNA by TPA (Yamaguchi et al., 2010[121]). When knockdown of ELF3 was induced by small interfering RNAs (siRNAs) in hTERT–HPDE cells, upregulation of claudin-7 by TPA was inhibited. In summary, claudin-7 in normal HPDE cells may be regulated via a PKCδ–ELF3 pathway (Figure 3(Fig. 3)).
Figure 3. Diagram showing the regulation of tight junction molecules in hTERT–HPDE cells via PKC isoforms. CL, claudin; Oc, occludin.
TJs and signals in hepatocytes
The TJs of hepatocytes play a crucial role in keeping bile in bile canaliculi, away from the blood circulation. This role is referred to as the blood–biliary barrier (Kojima et al., 2003[55]). In primary rat hepatocytes, changes in claudin-1 and -2 and occludin induced by growth factors [epidermal (EGF), hepatocyte (HGF), and transforming (TGF-ß)] and cytokines interleukin (IL)-1ß and oncostatin M (OSM) are regulated via distinct signal transduction pathways (Table 3(Tab. 3)) (Kojima et al., 2004[54], 2008[52]; Yamamoto et al., 2004[122]; Imamura et al., 2007[43]). Furthermore, in immortalized mouse hepatocytes, upregulation of claudin-2 via phosphatidylinositol 3 (PI3) kinase and PKC by treatment with OSM, and downregulation of claudin-2 via mitogen-activated protein (MAP) kinase and p38 MAP kinase by transfection with Raf-1 are observed (Table 3(Tab. 3)) (Lan et al., 2004[60]; Imamura et al., 2007[43]). These observations indicate that in hepatocytes, the TJ proteins claudin-1 and -2, and occludin are regulated by various cytokines and growth factors via distinct signal transduction pathways. In addition, it is thought that the p38 MAP kinase pathway plays a crucial role in the regulation of claudin-1 and -2 in hepatocytes. When primary cultured rat hepatocytes were treated with the p38 MAP kinase activator anisomycin, downregulation of claudin-1 was observed (Kojima et al., 2003[55]). Furthermore, a p38 MAP-kinase inhibitor prevented downregulation of claudin-1 by EGF in primary cultured rat hepatocytes (Table 3(Tab. 3)) (Yamamoto et al., 2005[123]). In the regenerating rat liver, treatment with a p38 MAP kinase inhibitor enhanced the upregulation of claudin-1 by partial hepatectomy (Table 3(Tab. 3)) (Kojima et al., 2003[55]; Yamamoto et al., 2005[123]). Although the reason for this discrepancy between the in vitro and in vivo is yet unclear, the p38 MAP kinase pathway may be important for the formation of TJs during the proliferation of hepatocytes and regeneration of the liver.
Table 3. Signal transduction to TJ proteins in hepatocytes [EGF, epidermal growth factor; HGF, hepatocyte growth factor; OSM, oncostatin M; (1), primary cultures of rat hepatocytes; (2), Raf-1 immortalized mouse hepatocytes; PH, partial hepatectomy in rat livers.].

In primary cultures of occludin-deficient mouse hepatocytes, claudin-2 expression and apoptosis are induced by downregulation of the activation of MAP kinase and Akt. In hepatic cell lines derived from occludin-deficient mice, claudin-2 expression and serum-free induced apoptosis are also increased by downregulation of the activation of MAP kinase and Akt. Furthermore, in hepatic cell lines transiently transfected with mouse and rat occludin genes, induction of claudin-2 expression and apoptosis are inhibited, with increases in the activation of MAP kinase and Akt. These findings show that occludin plays a crucial role in claudin-2-dependent TJ function and apoptosis involving the MAP kinase–Akt and PI3-kinase–Akt signaling pathways in hepatocytes (Murata et al., 2005[78]).
The development of polarity in hepatocytes, which results in bile canalicular formation, is regulated by various kinases in response to extracellular signals. The serine–threonine kinase PAR1b/EMK1/MARK2 regulates bile canalicular formation in hepatoma cell lines, although the inhibition of bile canalicular formation by knockdown of PAR1b is weak (Cohen et al., 2004[20], 2007[21]). Furthermore, Rho kinase, myosin-II, and p44/42 MAP kinase, the first identified factors, are involved in hepatocyte-derived extracellular matrix (ECM)-mediated multicellular patterning and bile canalicular luminal morphogenesis in HepG2 cells (Herrema et al., 2006[35]). PI3 kinase and p38 MAP kinase control taurocholate (ursodeoxycholate)-induced trafficking of ATP-dependent transport to the canalicular surface in the rat liver, isolated hepatocytes, and hepatic cell lines (Misra et al., 1998[76]; Sai et al., 1999[93]; Kubitz et al., 2004[58]). The serine–threonine kinase LKB1/PAR4 is mutated in most cases of Peutz–Jeghers syndrome, in which benign hamartomas and a high frequency of malignant tumors develop (Cohen et al., 2004[20]). The phosphorylation of LKB1 acts as a master kinase that activates PAR1 polarity kinase and AMP kinase (Baas et al., 2004[5]; Xie et al., 2006[120]). AMP kinase is known to not only act as a sensor of cellular energy status but to also regulate TJ assembly and epithelial polarity (Zhang et al., 2006[130]; Mirouse et al., 2007[75]; Zheng and Cantley, 2007[131]). By knockdown of claudin-2, upregulation of pLKB1, pp44/42 MAP kinase, pAkt, and pp38 MAP kinase was unexpectedly observed, together with inhibition of bile canalicular formation (Son et al., 2009[105]). These findings indicate that signaling from TJ proteins may be important as a subcellular system of bile canalicular formation.
TJs and signals in endometrial epithelial cells
Normal human endometrial epithelial (HEE) cells show a typical polarized phenotype during the proliferative phase of the menstrual cycle, which is controlled by 17ß-estradiol (E2), and during the secretory phase, which is predominantly governed by progesterone (P4), when the epithelial cells become receptive for implantation (Lessey, 1998[64]). A redistribution of desmosomes and adherens junctions in HEE cells in vivo is observed during the menstrual cycle (Buck et al., 2012[12]). The action of E2 and P4 is primarily mediated by their nuclear receptors, abbreviated as estrogen receptor (ER) and progesterone receptor (PR). The steroids E2 and P4 interact with their respective receptors and activate them to act as transcription factors (Bulun et al., 2012[13]). However, the mechanism of sex hormone regulation of TJs in normal HEE cells remains unclear. To investigate the regulation of TJs in HEE cells, we isolated and cultured epithelial and stromal cells from human endometrium (Someya et al., 2013[104]). In primary cultured HEE cells but not stromal cells, claudin-1, -3, -4, and -7, and occludin were detected together with ERa and PR. Embryo implantation requires that the apical plasma membrane of uterine epithelial cells acquires adhesiveness and that epithelial cell polarity is maintained. This process, referred to as the fence function, is thought to play a crucial role in embryo implantation (Thie et al., 1996[111]). In primary cultured HEE cells, all TJ proteins, including claudin-3 and -4, were upregulated by P4 and the upregulation was inhibited by E2. The barrier function but not the fence function in HEE cells was downregulated by P4, which also inhibited the proliferation of HEE cells. Regardless of the upregulation of TJ proteins and the inhibition of cell proliferation by P4, a downregulation of the barrier function was observed. In addition, we found that the formation of stress fibers, demonstrated by F-actin staining, was induced by P4. P4 increases stress fiber formation via PAR1 and the alteration of the actin organization results in a decrease in the barrier function (Camussi et al., 1991[14]; Diaz et al., 2012[23]). The decrease in the barrier function caused by P4 signaling in normal HEE cells may contribute to cytoskeletal remodeling. The finding that the fence function of normal HEE cells is not affected by sex hormones may be associated with the maintenance of embryo implantation.
TJs for signal transduction in human disease
Infectious disease
An indispensable role of TJs in pathogen infection has been widely demonstrated because disruption of TJs leads to a distinct increase in paracellular permeability and polarity defects, which facilitate viral or bacterial entry and spread. In addition, some proteins comprising TJs work as receptors for viruses, and extracellular stimuli, pathogenic bacteria, and viruses target and affect the function of TJs, leading to disease (Sawada, 2013[96]).
Claudin-3 and -4 are receptors for CPE, which is a common cause of food poisoning (Katahira et al., 1997[47]; Fujita et al., 2000[30]). When CPE binds claudin-4 expressed in MDCK cells, the complexes internalize like other ligand receptor complexes, and the function of TJs becomes disordered (Sonoda et al., 1999[106]). It is interesting that this phenomenon is observed only when the basolateral surface of the cell is exposed to CPE (Maeda et al., 2012[65]). The Helicobacter pylori toxin CagA causes an increase in paracellular permeability of intestinal cells by inactivating PAR1/MARK kinase (Saadat et al., 2007[90]). As a result, the barrier function of gastric foveolar epithelium deteriorates and microvilli disappear (Suzuki et al., 2002[109]).
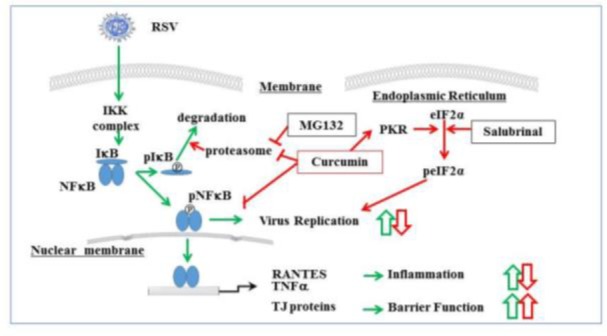
The airway epithelium, which has a well-developed barrier regulated by TJs, is the first line of defense during respiratory virus infection. Moreover, TJs include targets or receptors of viruses such as claudin-1 and occludin as coreceptors of HCV, JAM as a reovirus receptor, and the coxsackie and adenovirus receptor (CAR) (Guttman and Finlay, 2009[33]). In HNECs, rhinovirus infection decreases expression of TJ molecules ZO-1, occludin, and claudin-1 and reduces barrier function (Yeo et al., 2010[126]). Respiratory syncytial virus (RSV) is the major cause of bronchitis, asthma, and severe lower respiratory tract disease in infants and young children (Bitko et al., 1998[11]). To investigate the detailed mechanisms of replication and budding of RSV in HNECs, and the epithelial cell responses including TJs, we established an RSV-infected model using hTERT-transfected HNECs (Masaki et al., 2011[67]). The replication and budding of RSV and the epithelial cell responses in HNECs were regulated via a PKCδ/HIF–1α/NF–ϰB pathway (Masaki et al., 2011[67]) (Figure 4(Fig. 4)). In addition, it is thought that the PKCδ/HIF–1α/NF–ϰB pathway, via TGF-ß in an autocrine manner, may also be important in epithelial cell responses at a late stage after RSV infection (Masaki et al., 2011[67]) (Figure 4(Fig. 4)). The control of this pathway in HNECs may be useful not only for prevention of replication and budding of RSV but also in therapy for RSV-induced respiratory pathogenesis. In fact, we found that humulone, which is the main constituent of hop bitter acids, prevented the expression of RSV G protein, the formation of virus filaments, and the release of IL-8 and regulated on activation, normal T-cell expressed and secreted (RANTES) in a dose-dependent manner in RSV-infected HNECs. The effects of humulone in RSV-infected HNECs may be regulated via distinct signaling pathways including NF-ϰB (Fuchimoto et al., 2013[28]). Furthermore, we showed that in HNECs, curcumin [1, 7-bis (4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5dione], which is a major phenolic compound from the rhizome of the plant Curcuma longa, prevented the replication and budding of RSV, release of TNF-α and RANTES, and expression of RIG-I and MDA5 via its pharmacokinetic effects as an inhibitor of NF-ϰB, eIF-2a dephosphorylation, proteasome, and cytochrome C oxidase (COX)-2 (Obata et al., 2013[83]) (Figure 5(Fig. 5)).
Figure 4. Diagram showing signal transduction events in human nasal epithelial cells (HNECs) infected with RSV. The replication and budding of RSV and the epithelial cell responses in HNECs infected with RSV are regulated via a PKCδ–HIF-1α–NF-ϰB pathway. The PKCδ–HIF-1α–NF-ϰB pathway, via TGF-ß in an autocrine manner, may also be important in epithelial cell responses at a late stage after RSV infection.
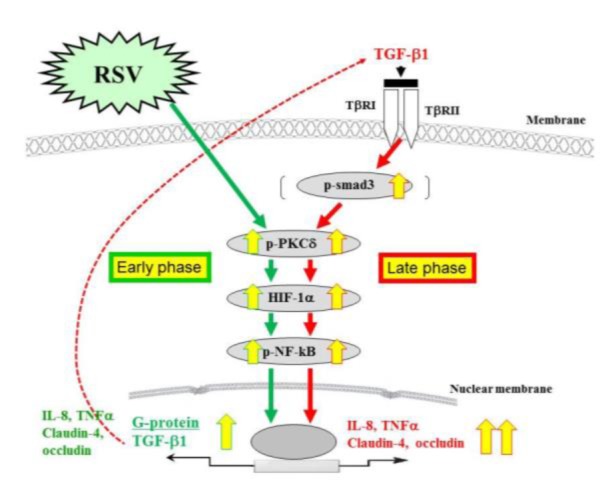
Figure 5. Overview of signal transduction events in human nasal epithelial cells (HNECs). Cells infected with RSV are indicated with green arrows, and cells treated with curcumin, salubrinal, and MG132 are indicated with red arrows. Curcumin prevented the replication and budding of RSV and the epithelial responses in HNECs and strongly increased the epithelial barrier of HNECs via its pharmacokinetic effects.
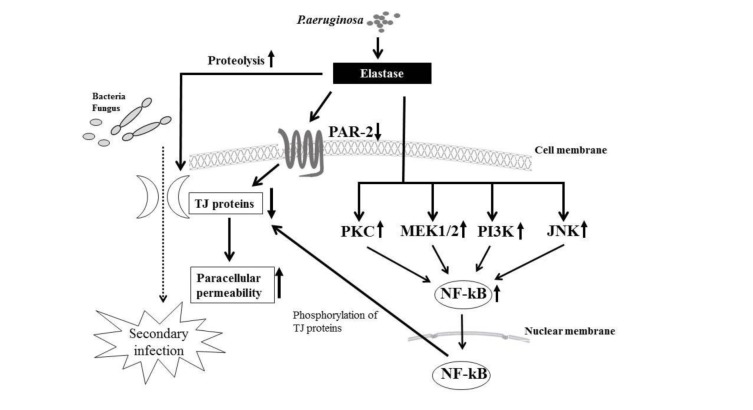
Pseudomonas aeruginosa causes chronic respiratory disease, and the elastase enzyme that it produces increases the permeability of airway epithelial cells through disruption of TJs. In addition, P. aeruginosa is implicated in prolonged chronic rhinosinusitis. We investigated the mechanisms involved in the disruption of TJs by P. aeruginosa elastase (PE) in HNECs (Nomura et al., 2014[82]). We found that PE treatment transiently disrupted the epithelial barrier and downregulated the transmembrane proteins claudin-1 and -4, occludin, and tricellulin, but not the scaffold PDZ-expression proteins ZO-1 and -2 and adherens junction proteins E-cadherin and ß-catenin. The transient downregulation of TJ proteins was controlled via distinct signal transduction pathways such as the PKC, MAPK, PI3K, p38 MAPK, c-Jun N-terminal kinase (JNK), COX-1 and -2, and NF-ϰB pathways (Figure 6(Fig. 6)). In addition, PE transiently decreased PAR2 expression, which also regulated the expression of the TJ proteins, and treatment with a PAR2 agonist prevented the downregulation of the TJ proteins after PE treatment in HNECs.
Figure 6. Overview of signaling pathways and treatment with PE. The transient downregulation of the transmembrane TJ proteins by treatment with PE is controlled via distinct signal transduction pathways including PKC, MEK1/2, PI3K, p38 MAPK, JNK, COX-1, -2, and NF-ϰB. PE also transiently downregulates mRNA of the TJ molecules in human nasal epithelial cells. PE, Pseudomonas aeruginosa elastase.
Malignant disease
TJs are central for the regulation of paracellular permeability and the maintenance of epithelial cell polarity, and their expression is altered in various cancers compared with that in normal tissues (Sawada, 2013[96]; Kondoh et al., 2011[56]). It is thought that cancer cells irreversibly and progressively lose TJs, with dedifferentiation as a result of genetic and epigenetic changes (Sawada, 2013[96]). In several human cancers, some TJ protein claudins are abnormally regulated, and therefore are promising molecular targets for diagnosis and therapy (Michl et al., 2003[72]; Karanjawala et al., 2008[46]).
Claudin-18 has two alternatively spliced variants, claudin-18a1 and -18a2, which are highly expressed in the lung and stomach, respectively (Yano et al., 2008[125]). Furthermore, a PKC–MAPK–AP-1-dependent pathway regulates claudin-18a2 expression in TPA-stimulated gastric cancer cells (Yano et al., 2008[125]). Claudin-18a2 is activated in a wide range of human malignant tumors, including gastric, esophageal, pancreatic, lung, and ovarian cancers, and can be specifically targeted by monoclonal antibodies (Sahin et al., 2008[92]). Claudin-18 is highly expressed in pancreatic intraepithelial neoplasia, including precursor PanIN lesions and pancreatic duct carcinoma, and it serves as a diagnostic marker (Karanjawala et al., 2008[46]).
PKC is known to be dysregulated in cancers of the prostate, breast, colon, pancreas, liver, and kidney (Ali et al., 2009[2]). Levels of PKCα, PKCß1, and PKCδ are higher in pancreatic cancer, whereas levels of PKCe are higher in normal tissue (El-Rayes et al., 2008[26]). Claudin-18 mRNA, indicated as claudin-18a2, was markedly induced by TPA, and in well- and moderately-differentiated human pancreatic cancer cell lines HPAFII and HPAC; the protein was also strongly increased (Ito et al., 2011[44]). The upregulation of claudin-18 by TPA in human pancreatic cancer cell lines was prevented by inhibitors of PKCδ, PKCε, and PKCα, whereas the upregulation of claudin-18 by TPA in hTERT–HPDE cells was prevented by inhibitors of PKCδ, PKCθ, and PKCα (Figure 3(Fig. 3)).
Activation of JNK is essential for disassembly of adherens junctions and TJs in human keratinocytes and colonic epithelial cells (Naydenov et al., 2009[80]; Lee et al., 2009[62]). Recently, inhibition of JNK activity was shown to enhance epithelial barrier function through differential modulation of claudin expression in murine mammary epithelial cells (Carrozzino et al., 2009[16]). To investigate whether tricellulin is regulated via a JNK pathway, human pancreatic cancer (HPAC) cells, highly expressed at tricellular contacts, were exposed to various stimuli such as the JNK activators anisomycin and the proinflammatory cytokines IL-1ß, tumor necrosis factor (TNF)-α, and IL-1ß (Kojima et al., 2010[51]). Tricellulin expression and the barrier function were upregulated together with the activity of phospho-Rac1/cdc42 and phospho-JNK by treatment with the JNK activator anisomycin, and suppressed not only by inhibitors of JNK and PKC but also by siRNAs of tricellulin (Kojima et al., 2010[51]). Tricellulin expression was induced by treatment with the proinflammatory cytokines IL-1ß, TNF-α, and IL-1ß, whereas the changes were inhibited by a JNK inhibitor (Kojima et al., 2010[51]). Furthermore, in normal HPDE cells, the responses of tricellulin expression to the various stimuli were similar to those in HPAC cells (Kojima et al., 2010[51]). Tricellulin expression in tricellular TJs is strongly regulated together with the barrier function via the JNK transduction pathway. These findings suggest that JNK may be involved in the regulation of tricellular TJs, including tricellulin expression and the barrier function, during normal remodeling of epithelial cells, and may prevent disruption of the epithelial barrier in inflammation and other disorders in pancreatic duct epithelial cells.
The transcription factor Snail has a key role in epithelial–mesenchymal transition (EMT) during development and in tumor progression via negative regulation of adherens junction and TJ molecules such as E-cadherin, claudins, and occludin (Nieto, 2002[81]; Batlle, 2000[9]; Cano et al., 2000[15]; Ikenouchi et al., 2003[42]). EMT is characterized by a loss of cell–cell contact and apicobasal polarity, which are hallmarks of dysfunction of the TJ fence (Balda et al., 1996[7]; Lee et al., 2006[61]). The repression of tricellulin is also related to Snail-induced EMT in human gastric carcinoma (Masuda et al., 2010[68]). On the other hand, MarvelD3 was transcriptionally downregulated in poorly differentiated pancreatic cancer cells (Kojima et al., 2011[53]). It was also downregulated during Snail-induced EMT of pancreatic cancer cells, in which Snail was highly expressed and the fence function downregulated, whereas it was maintained in well-differentiated HPAC cells and normal pancreatic duct epithelial cells (Kojima et al., 2011[53]). Depletion of marvelD3 by siRNAs in HPAC cells resulted in downregulation of barrier functions, indicated by a decrease in transepithelial electric resistance and an increase of permeability to fluorescent dextran tracers, whereas it did not affect the fence function of TJs (Table 2(Tab. 2)) (Kojima et al., 2011[53]). These results suggested that marvelD3 is transcriptionally downregulated in Snail-induced EMT during the progression of pancreatic cancer.
The exogenous ligand EGF is one of the important growth factors that promote biological responses, including cell proliferation, differentiation, and migration in normal cells, whereas EGF is linked to malignant transformation in epithelial cancer cells (Price et al., 1996[88]; Tobita et al., 2003[112]; Zuo et al., 2011[131]). EGF induces EMT in ovarian surface epithelium via distinct signaling transduction pathways (Ahmed et al., 2006[1]). Furthermore, EGF signaling modulates expression of claudins with changes in fence and barrier functions in various cell types (Kojima et al., 2004[54]; Singh and Harris, 2004[101]; Chen et al., 2005[18]; Flores-Benítez et al., 2007[27]; Singh et al., 2007[102]; Ikari et al., 2009[39], 2011[40]). We demonstrated that EGF downregulated claudin-3 in mucinous cystadenocarcinoma (MCAS) cells and claudin-4 in serous cystadenocarcinoma (HUOA) cells by inducing degradation of the proteins with changes in the structures and functions of TJs (Ogawa et al., 2012[85]). In addition, in HUOA cells but not MCAS cells, EGF downregulated the cytotoxic effect of CPE via claudin-4, and the mechanisms of the regulation of claudins by EGF differed between the subtypes of epithelial ovarian cancer cells in vitro (Ogawa et al., 2012[85]). In summary, although EGF modulated claudins and TJ functions via MEK–ERK and PI3K–Akt signaling pathways in epithelial ovarian cancer cells, there were different mechanisms for the regulation of claudins by EGF between the subtypes in vitro.
Concluding remarks
Cellular TJs play a crucial role in maintaining homeostasis and in preventing disease. Until now, research on TJs has been targeted at the cellular level. However, there have been few studies on TJs at the molecular level. TJ proteins are elaborately regulated by various stimuli, such as cytokines, chemokines, hormones, and growth factors, via distinct signal transduction pathways. The signaling pathways, mainly the PKC–MAPK–NF-ϰB pathway, affect the expression of TJ proteins and the barrier function in various organs or cells. The regulation of TJ molecules, including claudins, via signal transductions in normal cells is a potentially important therapeutic target in human disease. However, details of changes to signaling pathways required to regulate TJs remain unclear. Because signaling pathways are complex and many are cross-linked, it is difficult to determine the details of complete signaling pathways. Further extensive research is needed to clarify the role of TJs in signal transduction.
References
- 1.Ahmed N, Maines-Bandiera S, Quinn MA, Unger WG, Dedhar S, Auersperg N. Molecular pathways regulating EGF induced epithelio-mesenchymal transition in human ovarian surface epithelium. Am J Physiol Cell Physiol. 2006;290:C1532–42. doi: 10.1152/ajpcell.00478.2005. [DOI] [PubMed] [Google Scholar]
- 2.Ali AS, Ali S, El-Rayes BF, Philip PA, Sarkar FH. Exploitation of protein kinase C: a useful target for cancer therapy. Cancer Treat Rev. 2009;35:1–8. doi: 10.1016/j.ctrv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM. Cell signalling: MAGUK magic. Curr Biol. 1996;6:382–4. doi: 10.1016/s0960-9822(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 4.Andreeva AY, Krause E, Müller EC, Blasig IE, Utepbergenov DI. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem. 2001;276:38480–38486. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- 5.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 6.Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–7. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 7.Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banan A, Zhang LJ, Shaikh M, Fields JZ, Choudhary S, Forsyth CB, et al. theta Isoform of protein kinase C alters barrier function in intestinal epithelium through modulation of distinct claudin isotypes: a novel mechanism for regulation of permeability. J Pharmacol Exp Ther. 2005;313:962–982. doi: 10.1124/jpet.104.083428. [DOI] [PubMed] [Google Scholar]
- 9.Batlle, E The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Nature Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 10.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipidactivated nuclear receptors. Nature. 2008;454:470–7. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 11.Bitko V, Barik S. Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IkkBßß, and sequestration of protein phosphatase 2A by the viral phosphoprotein. J Virol. 1998;72:5610–8. doi: 10.1128/jvi.72.7.5610-5618.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck VU, Windoffer R, Leube RE, Classen-Linke I. Redistribution of adhering junctions in human endometrial epithelial cells during the implantation window of the menstrual cycle. Histochem Cell Biol. 2012;137:777–90. doi: 10.1007/s00418-012-0929-0. [DOI] [PubMed] [Google Scholar]
- 13.Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, et al. Role of estrogen receptor-β in endometriosis. Semin Reprod Med. 2012;30:39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camussi G, Turello E, Bussolino F, Baglioni C. Tumor necrosis factor alters cytoskeletal organization and barrier function of endothelial cells. Int Arch Allergy Appl Immunol. 1991;96:84–91. doi: 10.1159/000235539. [DOI] [PubMed] [Google Scholar]
- 15.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 16.Carrozzino F, Pugnale P, Féraille E, Montesano R. Inhibition of basal p38 or JNK activity enhances epithelial barrier function through differential modulation of claudin expression. Am J Physiol Cell Physiol. 2009;297:C775–87. doi: 10.1152/ajpcell.00084.2009. [DOI] [PubMed] [Google Scholar]
- 17.Cereijido M, Valdés J, Shoshani L, Contreras RG. Role of tight junctions in establishing and maintaining cell polarity. Ann Rev Physiol. 1998;60:161–77. doi: 10.1146/annurev.physiol.60.1.161. [DOI] [PubMed] [Google Scholar]
- 18.Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, et al. Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol. 2005;98:322–8. doi: 10.1152/japplphysiol.00681.2004. [DOI] [PubMed] [Google Scholar]
- 19.Clarke H, Soler AP, Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci. 2000;113:3187–3196. doi: 10.1242/jcs.113.18.3187. [DOI] [PubMed] [Google Scholar]
- 20.Cohen D, Brennwald PJ, Rodriguez-Boulan E, Müsch A. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J Cell Biol. 2004;164:717–727. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen D, Tian Y, Müsch A. Par1b promotes hepatic-type lumen polarity in Madin Darby canine kidney cells via myosin II- and E-cadherin-dependent signaling. Mol Biol Cell. 2007;18:2203–2215. doi: 10.1091/mbc.E07-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cummins BM. Occludin: One protein, many forms. Mol Cell Biol. 2012;32:242–250. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz J, Aranda E, Henriquez S, Quezada M, Espinoza E, Bravo ML, et al. Progesterone promotes focal adhesion formation and migration in breast cancer cells through induction of protease-activated receptor- 1. J Endocrinol. 2012;214:165–75. doi: 10.1530/JOE-11-0310. [DOI] [PubMed] [Google Scholar]
- 24.Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265–76. doi: 10.1016/s0016-5085(03)00271-3. [DOI] [PubMed] [Google Scholar]
- 25.Ellis B, Schneeberger EE, Rabito CA. Cellular variability in the development of tight junctions after activation of protein kinase C. Am J Physiol. 1992;263:F293–F300. doi: 10.1152/ajprenal.1992.263.2.F293. [DOI] [PubMed] [Google Scholar]
- 26.El-Rayes BF, Ali S, Philip PA, Sarkar FH. Protein kinase C: a target for therapy in pancreatic cancer. Pancreas. 2008;36:346–52. doi: 10.1097/MPA.0b013e31815ceaf7. [DOI] [PubMed] [Google Scholar]
- 27.Flores-Benítez D, Ruiz-Cabrera A, Flores-Maldonado C, Shoshani L, Cereijido M, Contreras RG. Control of tight junctional sealing: role of epidermal growth factor. Am J Physiol Renal Physiol. 2007;292:F828–36. doi: 10.1152/ajprenal.00369.2006. [DOI] [PubMed] [Google Scholar]
- 28.Fuchimoto J, Kojima T, Okabayashi T, Masaki T, Ogasawara N, Obata K, et al. Humulone suppresses replication of respiratory syncytial virus and release of IL-8 and RANTES in normal human nasal epithelial cells. Med Mol Morphol. 2013;46:203–209. doi: 10.1007/s00795-013-0024-1. [DOI] [PubMed] [Google Scholar]
- 29.Fujimura Y. Evidence of M cells as portals of entry for antigens in the nasopharyngeal lymphoid tissue of humans. Virchows Arch. 2000;436:560–566. doi: 10.1007/s004289900177. [DOI] [PubMed] [Google Scholar]
- 30.Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000;476:258–61. doi: 10.1016/s0014-5793(00)01744-0. [DOI] [PubMed] [Google Scholar]
- 31.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumbiner BM. Breaking through the tight junction barrier. J Cell Biol. 1993;123:1631–3. doi: 10.1083/jcb.123.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009;1788:832–41. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Hammad H, Lambrecht BN. Dendritic cells and airway epithelial cells at the interface between innate and adaptive immune responses. Allergy. 2011;66:579–587. doi: 10.1111/j.1398-9995.2010.02528.x. [DOI] [PubMed] [Google Scholar]
- 35.Herrema H, Czajkowska D, Théard D, van der Wouden JM, Kalicharan D, Zolghadr B, et al. Rho kinase, myosin-II, and p42/44 MAPK control extracellular matrix-mediated apical bile canalicular lumen morphogenesis in HepG2 cells. Mol Biol Cell. 2006;17:3291–3303. doi: 10.1091/mbc.E06-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 37.Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int. 2008;57:1–10. doi: 10.2332/allergolint.R-07-154. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Eum SY, András IE, Hennig B, Toborek M. PPARalpha and PPARgamma attenuate HIV-induced dysregulation of tight junction proteins by modulations of matrix metalloproteinase and proteasome activities. FASEB J. 2009;23:1596–606. doi: 10.1096/fj.08-121624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikari A, Atomi K, Takiguchi A, Yamazaki Y, Miwa M, Sugatani J. Epidermal growth factor increases claudin-4 expression mediated by Sp1 elevation in MDCK cells. Biochem Biophys Res Commun. 2009;384:306–10. doi: 10.1016/j.bbrc.2009.04.120. [DOI] [PubMed] [Google Scholar]
- 40.Ikari A, Takiguchi A, Atomi K, Sugatani J. Epidermal growth factor increases clathrin-dependent endocytosis and degradation of claudin-2 protein in MDCK cells. J Cell Physiol. 2011;226:2448–56. doi: 10.1002/jcp.22590. [DOI] [PubMed] [Google Scholar]
- 41.Ikenouchi J, Furuse M, Furuse K, Sasaki JH, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–45. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–67. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- 43.Imamura M, Kojima T, Lan M, Son S, Murata M, Osanai M, et al. Oncostatin M induces upregulation of claudin-2 in rodent hepatocytes coinciding with changes in morphology and function of tight junctions. Exp Cell Res. 2007;313:1951–1962. doi: 10.1016/j.yexcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Ito T, Kojima T, Yamaguchi H, Kyuno D, Kimura Y, Imamura M, et al. Transcriptional regulation of claudin-18 via specific protein kinase C signaling pathways and modification of DNA-methylation in human pancreatic cancer cells. J Cell Biochem. 2011;112:1761–72. doi: 10.1002/jcb.23095. [DOI] [PubMed] [Google Scholar]
- 45.Jedlicka P, Gutierrez-Hartmann A. Ets transcription factors in intestinal morphogenesis, homeostasis and disease. Histol Histopathol. 2008;23:1417–1424. doi: 10.14670/hh-23.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karanjawala ZE, Illei PB, Ashfaq R, Infante JR, Murphy K, Pandey A, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol. 2008;32:188–96. doi: 10.1097/PAS.0b013e31815701f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chemi. 1997;272:26652–8. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- 48.Kim DY, Sato A, Fukuyama S, Sagara H, Nagatake T, Kong IG, et al. The airway antigen sampling system: respiratory M cells as an alternative gateway for inhaled antigens. J Immunol. 2011;186:4253–4262. doi: 10.4049/jimmunol.0903794. [DOI] [PubMed] [Google Scholar]
- 49.Kohno Y, Okamoto T, Ishibe T, Nagayama S, Shima Y, Nishijo K, et al. Expression of claudin7 is tightly associated with epithelial structures in synovial sarcomas and regulated by an Ets family transcription factor, ELF3. J Biol Chem. 2006;281:38941–38950. doi: 10.1074/jbc.M608389200. [DOI] [PubMed] [Google Scholar]
- 50.Koizumi J, Kojima T, Ogasawara N, Kamekura R, Kurose M, Go M, et al. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol Pharmacol. 2008;74:432–442. doi: 10.1124/mol.107.043711. [DOI] [PubMed] [Google Scholar]
- 51.Kojima T, Fuchimoto J, Yamaguchi H, Ito T, Takasawa A, Ninomiya T, et al. c-Jun N-terminal kinase is largely involved in the regulation of tricellular tight junctions via tricellulin in human pancreatic duct epithelial cells. J Cell Physiol. 2010;225:720–33. doi: 10.1002/jcp.22273. [DOI] [PubMed] [Google Scholar]
- 52.Kojima T, Takano K, Yamamoto T, Murata M, Son S, Imamura M, et al. Transforming growth factor-beta induces epithelial to mesenchymal transition by down-regulation of claudin-1 expression and the fence function in adult rat hepatocytes. Liver Int. 2008;28:534–545. doi: 10.1111/j.1478-3231.2007.01631.x. [DOI] [PubMed] [Google Scholar]
- 53.Kojima T, Takasawa A, Kyuno D, Ito T, Yamaguchi H, Hirata K, et al. Downregulation of tight junction associated MARVEL protein marvelD3 during epithelial mesenchymal transition in human pancreatic cancer cells. Exp Cell Res. 2011;317:2288–98. doi: 10.1016/j.yexcr.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Kojima T, Yamamoto T, Lan M, Murata M, Takano K, Go M, et al. Inhibition of MAP kinase activity moderates changes in expression and function of Cx32 but not claudin-1 during DNA synthesis in primary cultures of rat hepatocytes. Med Electron Microsc. 2004;37:101–113. doi: 10.1007/s00795-003-0239-7. [DOI] [PubMed] [Google Scholar]
- 55.Kojima T, Yamamoto T, Murata M, Chiba H, Kokai Y, Sawada N. Regulation of the blood-biliary barrier: interaction between gap and tight junctions in hepatocytes. Med Electron Microsc. 2003;36:157–164. doi: 10.1007/s00795-003-0220-5. [DOI] [PubMed] [Google Scholar]
- 56.Kondoh A, Takano K, Kojima T, Ohkuni T, Kamekura R, Ogasawara N, et al. Altered expression of claudin-1, claudin-7, and tricellulin regardless of human papilloma virus infection in human tonsillar squamous cell carcinoma. Acta Otolaryngol. 2011;131:861–868. doi: 10.3109/00016489.2011.562537. [DOI] [PubMed] [Google Scholar]
- 57.Krug SM, Günzel D, Conrad MP, Lee IF, Amasheh S, Fromm M, et al. Charge-selective claudin channels. Ann N Y Acad Sci. 2012;1257:20–28. doi: 10.1111/j.1749-6632.2012.06555.x. [DOI] [PubMed] [Google Scholar]
- 58.Kubitz R, Sütfels G, Kühlkamp T, Kölling R, Häussinger D. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology. 2004;126:541–553. doi: 10.1053/j.gastro.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Kurose M, Kojima T, Koizumi J, Kamekura R, Ninomiya T, Murata M, et al. Induction of claudins in passaged hTERT-transfected human nasal epithelial cells with an extended life span. Cell Tissue Res. 2007;330:63–74. doi: 10.1007/s00441-007-0453-z. [DOI] [PubMed] [Google Scholar]
- 60.Lan M, Kojima T, Osanai M, Chiba H, Sawada N. Oncogenic Raf-1 regulates epithelial to mesenchymal transition via distinct signal transduction pathways in an immortalized mouse hepatic cell line. Carcinogenesis. 2004;25:2385–2395. doi: 10.1093/carcin/bgh248. [DOI] [PubMed] [Google Scholar]
- 61.Lee DB, Huang E, Ward HJ. Tight junction biology and kidney dysfunction. Am J Physiol Renal Physiol. 2006;290:F20–34. doi: 10.1152/ajprenal.00052.2005. [DOI] [PubMed] [Google Scholar]
- 62.Lee MH, Koria P, Qu J, Andreadis ST. JNK phosphorylates beta-catenin and regulates adherens junctions. FASEB J. 2009;23:3874–83. doi: 10.1096/fj.08-117804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leotlela PD, Wade MS, Duray PH, Rhode MJ, Brown HF, Rosenthal DT, et al. Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene. 2007;26:3846–3856. doi: 10.1038/sj.onc.1210155. [DOI] [PubMed] [Google Scholar]
- 64.Lessey BA. Endometrial integrins and the establishment of uterine receptivity. Hum Reprod. 1998;13:247–61. doi: 10.1093/humrep/13.suppl_3.247. [DOI] [PubMed] [Google Scholar]
- 65.Maeda T, Murata M, Chiba H, Takasawa A, Tanaka S, Kojima T, et al. Claudin-4-targeted therapy using Clostridium perfringens enterotoxin for prostate cancer. Prostate. 2012;72:351–360. doi: 10.1002/pros.21436. [DOI] [PubMed] [Google Scholar]
- 66.Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masaki T, Kojima T, Okabayashi T, Ogasawara N, Ohkuni T, Obata K, et al. A nuclear factor-kB signaling pathway via protein kinase C d regulates replication of respiratory syncytial virus in polarized normal human nasal epithelial cells. Mol Biol Cell. 2011;22:2144–56. doi: 10.1091/mbc.E10-11-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masuda R, Semba S, Mizuuchi E, Yanagihara K, Yokozaki H. Negative regulation of the tight junction protein tricellulin by snail-induced epithelial mesenchymal transition in gastric carcinoma cells. Pathobiology. 2010;77:106–13. doi: 10.1159/000278293. [DOI] [PubMed] [Google Scholar]
- 69.Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, et al. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci. 2011;124:548–555. doi: 10.1242/jcs.072058. [DOI] [PubMed] [Google Scholar]
- 70.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, et al. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 71.Meredith LW, Wilson GK, Fletcher NF, McKeating JA. Hepatitis C virus entry: beyond receptors. Rev Med Virol. 2012;22:182–93. doi: 10.1002/rmv.723. [DOI] [PubMed] [Google Scholar]
- 72.Michl P, Barth C, Buchholz M, Lerch MM, Rolke M, Holzmann KH, et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63:6265–71. [PubMed] [Google Scholar]
- 73.Michl P, Buchholz M, Rolke M, Kunsch S, Löhr M, McClane B, et al. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology. 2001;121:678–684. doi: 10.1053/gast.2001.27124. [DOI] [PubMed] [Google Scholar]
- 74.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011;585:606–612. doi: 10.1016/j.febslet.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 75.Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–392. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Misra S, Ujházy P, Gatmaitan Z, Varticovski L, Arias IM. The role of phosphoinositide 3-kinase in taurocholate-induced trafficking of ATP-dependent canalicular transporters in rat liver. J Biol Chem. 1998;273:26638–26644. doi: 10.1074/jbc.273.41.26638. [DOI] [PubMed] [Google Scholar]
- 77.Monteiro AC, Parkos CA. Intracellular mediators of JAM-A-dependent epithelial barrier function. Ann N Y Acad Sci. 2012;1257:115–124. doi: 10.1111/j.1749-6632.2012.06521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murata M, Kojima T, Yamamoto T, Go M, Takano K, Osanai M, et al. Down-regulation of survival signaling through MAPK and Akt in occludin-deficient mouse hepatocytes in vitro. Exp Cell Res. 2005;310:140–51. doi: 10.1016/j.yexcr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 79.Nawijn MC, Hackett TL, Postma DS, van Oosterhout AJ, Heijink IH. E-cadherin: gatekeeper of airway mucosa and allergic sensitization. Trends Immunol. 2011;32:248–255. doi: 10.1016/j.it.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Naydenov NG, Hopkins AM, Ivanov AI. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle. 2009;8:2110–21. doi: 10.4161/cc.8.13.8928. [DOI] [PubMed] [Google Scholar]
- 81.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 82.Nomura K, Obata K, Keira T, Miyata R, Hirakawa S, Takano K, et al. Pseudomonas aeruginosa elastase causes transient disruption of tight junctions and downregulation of PAR-2 in human nasal epithelial cells. Respir Res. 2014;18:15–21. doi: 10.1186/1465-9921-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Obata K, Kojima T, Masaki T, Okabayashi T, Yokota S, Hirakawa S, et al. Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PLoS One. 2013;8:e70225. doi: 10.1371/journal.pone.0070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogasawara N, Kojima T, Go M, Ohkuni T, Koizumi J, Kamekura R, Masaki T, Murata M, Tanaka S, Fuchimoto J, Himi T, Sawada N. PPARgamma agonists upregulate the barrier function of tight junctions via a PKC pathway in human nasal epithelial cells. Pharmacol Res. 2010;61:489–498. doi: 10.1016/j.phrs.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Ogawa M, Kojima T, Someya M, Nomura K, Takasawa A, Murata M, et al. Epidermal growth factor modulates claudins and tight junctional functions. Histochem Cell Biol. 2012;138:323–338. doi: 10.1007/s00418-012-0956-x. [DOI] [PubMed] [Google Scholar]
- 86.Osanai M, Murata M, Nishikiori N, Chiba H, Kojima T, Sawada N. Epigenetic silencing of occludin promotes tumorigenic and metastatic properties of cancer cells via modulations of unique sets of apoptosis-associated genes. Cancer Res. 2006;66:9125–33. doi: 10.1158/0008-5472.CAN-06-1864. [DOI] [PubMed] [Google Scholar]
- 87.Ponferrada A, Caso JR, Alou L, Colon A, Sevillano D, Moro MA, et al. The role of PPAR gamma on restoration of colonic homeostasis after experimental stress-induced inflammation and dysfunction. Gastroenterology. 2007;132:1791–803. doi: 10.1053/j.gastro.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 88.Price JT, Wilson HM, Haites NE. Epidermal growth factor (EGF) increases the in vitro invasion, motility and adhesion interactions of the primary renal carcinoma cell line, A704. Eur J Cancer. 1996;32A:1977–82. doi: 10.1016/0959-8049(96)00207-9. [DOI] [PubMed] [Google Scholar]
- 89.Ramakers JD, Verstege MI, Thuijls G, Velde AAT, Mensink RP, Plat J. The PPARgamma agonist rosiglitazone impairs colonic inflammation in mice with experimental colitis. J Clin Immunol. 2007;27:275–83. doi: 10.1007/s10875-007-9074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 91.Saeki R, Kondoh M, Kakutani H, Tsunoda S, Mochizuki Y, Hamakubo T, et al. A novel tumor-targeted therapy using a claudin-4-targeting molecule. Mol Pharmacol. 2009;76:918–926. doi: 10.1124/mol.109.058412. [DOI] [PubMed] [Google Scholar]
- 92.Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, et al. Claudin-18 splice variant 2 is a pancancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624–34. doi: 10.1158/1078-0432.CCR-08-1547. [DOI] [PubMed] [Google Scholar]
- 93.Sai Y, Nies AT, Arias IM. Bile acid secretion and direct targeting of mdr1-green fluorescent protein from Golgi to the canalicular membrane in polarized WIF-B cells. J Cell Sci. 1999;112:4535–4545. doi: 10.1242/jcs.112.24.4535. [DOI] [PubMed] [Google Scholar]
- 94.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, et al. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sawada N. Tight junction-related human diseases. Pathol Int. 2013;63:1–12. doi: 10.1111/pin.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, et al. Tight junctions and human diseases. Med Electron Microsc. 2003;36:147–56. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- 98.Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol Lung Cell Mol Physiol. 1992;262:L647–61. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- 99.Schneeberger EE, Lynch RD. The tight junction: A multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 100.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem. 2007;282:11487–11498. doi: 10.1074/jbc.M610597200. [DOI] [PubMed] [Google Scholar]
- 101.Singh AB, Harris RC. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem. 2004;279:3543–52. doi: 10.1074/jbc.M308682200. [DOI] [PubMed] [Google Scholar]
- 102.Singh AB, Sugimoto K, Dhawan P, Harris RC. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol. 2007;293:C1660–8. doi: 10.1152/ajpcell.00274.2007. [DOI] [PubMed] [Google Scholar]
- 103.Sjö A, Magnusson KE, Peterson KH. Distinct effects of protein kinase C on the barrier function at different developmental stages. Biosci Rep. 2003;23:87–102. doi: 10.1023/a:1025524323842. [DOI] [PubMed] [Google Scholar]
- 104.Someya M, Kojima T, Ogawa M, Ninomiya T, Nomura K, Takasawa A, et al. Regulation of tight junctions by sex hormones in normal human endometrial epithelial cells and uterus cancer cell line Sawano. Cell Tissue Res. 2013;354:481–494. doi: 10.1007/s00441-013-1676-9. [DOI] [PubMed] [Google Scholar]
- 105.Son S, Kojima T, Decaens C, Yamaguchi H, Ito T, Imamura M, et al. Knockdown of tight junction protein claudin-2 prevents bile canalicular formation in WIF-B9 cells. Histochem Cell Biol. 2009;131:411–424. doi: 10.1007/s00418-008-0546-0. [DOI] [PubMed] [Google Scholar]
- 106.Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, et al. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195–204. doi: 10.1083/jcb.147.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 108.Stuart RO, Nigam SK. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci U S A. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suzuki K, Kokai Y, Sawada N, Takakuwa R, Kuwahara K, Isogai E, et al. SS1 Helicobacter pylori disrupts the paracellular barrier of the gastric mucosa and leads to neutrophilic gastritis in mice. Virchows Arch. 2002;440:318–324. doi: 10.1007/s004280100430. [DOI] [PubMed] [Google Scholar]
- 110.Takano K, Kojima T, Ogasawara N, Go M, Kikuchi S, Ninomiya T, et al. Expression of tight junction proteins in epithelium including Ck20-positive M-like cells of human adenoids in vivo and in vitro. J Mol Histol. 2008;39:265–273. doi: 10.1007/s10735-008-9162-5. [DOI] [PubMed] [Google Scholar]
- 111.Thie M, Fuchs P, Butz S, Sieckmann F, Hoschützky H, et al. Adhesiveness of the apical surface of uterine epithelial cells: the role of junctional complex integrity. Eur J Cell Biol. 1996;70:221–32. [PubMed] [Google Scholar]
- 112.Tobita K, Kijima H, Dowaki S, Kashiwagi H, Ohtani Y, Oida Y, et al. Epidermal growth factor receptor expression in human pancreatic cancer: significance for liver metastasis. Int J Mol Med. 2003;11:305–9. [PubMed] [Google Scholar]
- 113.Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- 114.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–93. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 115.Tymms MJ, Ng AY, Thomas RS, Schutte BC, Zhou J, Eyre HJ, et al. A novel epithelial-expressed ETS gene, ELF3: human and murine cDNA sequences, murine genomic organization, human mapping to 1q32.2 and expression in tissues and cancer. Oncogene. 1997;15:2449–2462. doi: 10.1038/sj.onc.1201427. [DOI] [PubMed] [Google Scholar]
- 116.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight junction strand formation. Cell. 2006;126:741–54. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 117.van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5:1455–64. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Varley CL, Garthwaite MAE, Cross W, Hinley J, Trejdosiewicz LK, Southgate J. PPAR gamma-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol. 2006;208:407–17. doi: 10.1002/jcp.20676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weiler F, Marbe T, Scheppach W, Schauber J. Influence of protein kinase C on transcription of the tight junction elements ZO-1 and occludin. J Cell Physiol. 2005;204:83–86. doi: 10.1002/jcp.20268. [DOI] [PubMed] [Google Scholar]
- 120.Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, Riek U, et al. Activation of protein kinase C ˙by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem. 2006;281:6366–6375. doi: 10.1074/jbc.M511178200. [DOI] [PubMed] [Google Scholar]
- 121.Yamaguchi H, Kojima T, Ito T, Kimura Y, Imamura M, Son S, et al. Transcriptional control of tight junction proteins via a protein kinase C signal pathway in human telomerase reverse transcriptase-transfected human pancreatic duct epithelial cells. Am J Pathol. 2010;177:698–712. doi: 10.2353/ajpath.2010.091226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamamoto T, Kojima T, Murata M, Takano K, Go M, Chiba H, et al. IL-1beta regulates expression of Cx32, occludin, and claudin-2 of rat hepatocytes via distinct signal transduction pathways. Exp Cell Res. 2004;299:427–441. doi: 10.1016/j.yexcr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 123.Yamamoto T, Kojima T, Murata M, Takano K, Go M, Hatakeyama N, et al. p38 MAP-kinase regulates function of gap and tight junctions during regeneration of rat hepatocytes. J Hepatol. 2005;42:707–718. doi: 10.1016/j.jhep.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 124.Yamanaka T, Helgeland L, Farstad IN, Fukushima H, Midtvedt T, Brandtzaeg P. Microbial colonization drives lymphocyte accumulation and differentiation in the follicle-associated epithelium of Peyer's patches. J Immunol. 2003;170:816–822. doi: 10.4049/jimmunol.170.2.816. [DOI] [PubMed] [Google Scholar]
- 125.Yano K, Imaeda T, Niimi T. Transcriptional activation of the human claudin-18 gene promoter through two AP-1 motifs in PMA-stimulated MKN45 gastric cancer cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G336–43. doi: 10.1152/ajpgi.00328.2007. [DOI] [PubMed] [Google Scholar]
- 126.Yeo NK, Jang YJ. Rhinovirus infection-induced alteration of tight junction and adherens junction components in human nasal epithelial cells. Laryngoscope. 2010;120:346–52. doi: 10.1002/lary.20764. [DOI] [PubMed] [Google Scholar]
- 127.Yoo JW, Kim YS, Lee SH, Lee MK, Roh HJ, Jhun BH, et al. Serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Pharm Res. 2003;20:1690–1696. doi: 10.1023/a:1026112107100. [DOI] [PubMed] [Google Scholar]
- 128.Zeisel MB, Fofana I, Fafi-Kremer S, Baumert TF. Hepatitis C virus entry into hepatocytes: Molecular mechanisms and targets for antiviral therapies. J Hepatol. 2011;54:566–76. doi: 10.1016/j.jhep.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 129.Zeng L, Webster SV, Newton PM. The biology of protein kinase C. Adv Exp Med Biol. 2012;740:639–661. doi: 10.1007/978-94-007-2888-2_28. [DOI] [PubMed] [Google Scholar]
- 130.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A. 2006;103:17272–17277. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104:819–822. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zuo JH, Zhu W, Li MY, Li XH, Yi H, Zeng GQ, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J Cell Biochem. 2011;112:2508–17. doi: 10.1002/jcb.23175. [DOI] [PubMed] [Google Scholar]