Abstract
The present study was carried out to evaluate the anti-inflammatory activities of polyphenols isolated from the leaves of mistletoe (Loranthus micranthus Linn.) parasitic on Hevea brasiliensis. The anti-inflammatory properties of the isolated compounds were evaluated on the basis of their ability to inhibit the production of nitric oxide (NO) and tumuor necrosis factor-α (TNF-α) in lipopolysaccharide (LPS) activated RAW 264.7 mouse macrophages. Semi-preparative HPLC separation of the ethyl acetate (EtOAc) and butanol (n-BuOH) fractions of the leaves of mistletoe (Loranthus micranthus Linn) parasitic on Hevea brasiliensis led to the isolation of four polyphenols: 3-O-(3,4,5-trimethoxybenzoyl)-(-)-epicatechin (TMECG) (1); (-)-epicatechin-3-O-(3″-O-methyl)-gallate (ECG3″Me) (2); rutin (3) and peltatoside (4). Compounds 1-4 were isolated for the first time from this plant while 1 was isolated for the first time in nature. These compounds (1-4) were readily identified by comparison of their spectroscopic data with those reported in the literature. The polyphenols proved to have anti-inflammatory activity as evidenced by the suppression of inducible nitric oxide (iNO) and cytokine (TNF-α) levels in the culture supernatant of lipopolysaccharide (LPS)-stimulated RAW 264.7 murine macrophages. However, the study showed that the quercetin diglycosides showed stronger inhibition of proinflammatory mediators than the epicatechin derivates. These data provide evidence that polyphenolic compounds isolated from the mistletoe parasitic on Hevea brasiliensis may contribute to its anti-inflammatory properties by inhibiting the expression of inducible nitric oxide and proinflammatory cytokines such as tumour necrosis factor-α.
Keywords: mistletoe, structural elucidation, polyphenols, inflammation, inducible nitric oxide, tumour necrosis factor-alpha, TNF-alpha, RAW 264.7 murine macrophages
Introduction
Mistletoes are hemiparasitic plants growing on different host trees and shrubs. They depend on their host plant for water and mineral nutrition, even though they produce their own carbohydrates through photosynthesis (Ali et al., 2005[2]). Mistletoe grows on many host trees like Kola acuminata, Baphia nitida, Persia americana, Irvingia gabonensis, Citrus simensis, Pentacletra macrophylla, Treculiar africana, and Ficus exaperata (Ali et al., 2005[2]; Osadebe et al., 2012[24]). The leaves of mistletoes are traditionally used in folkloric medicine of Nigeria for the treatment of diarrhoea, epilepsy, hypertension and rheumatism (Griggs, 1991[12]). The leaves of African mistletoe have been reported to have anti-inflammatory activity in vivo on Wistar albino rats (Patrick-Iwuanyanwu et al., 2010[25]). Inflammation is a bodily response to injury, infection or destruction characterized by heat, redness, pain, swelling and disturbed physiological functions (Black and Berman, 1999[5]). It is the body response to inactivate or destroy the invading organisms, to remove the irritants and set the stage for tissue repair. Inflammation is triggered by the release of chemical mediators from injured tissue and migrating cells (Sangita et al., 2012[29]). A number of chemical mediators have been postulated to play important roles in the inflammatory process. The most common sources of chemical mediators include neutrophils, basophil, mast cells, platelets, macrophages and lymphocytes. Activated macrophages secrete a number of different inflammatory mediators, including interleukin-1β (IL-1β), interleukin-6 (IL-6), prostaglandin E2 (PGE2), nitric oxide (NO), and tumour necrosis factor-α (TNF-α) (Boscá et al., 2005[7]; Lawrence et al., 2002[17]; Kaplanski et al., 2003[14]). However, if inflammation is not treated it leads to onset of diseases like vasomotor rhinorrhoea, rheumatoid arthritis and atherosclerosis (Henson and Murphy, 1989[13]). In this study four polyphenolic compounds isolated from Loranthus micranthus parasitic on Hevea brasiliensis were tested, in vitro, for anti-inflammatory activity. The compounds were tested for their ability to inhibit inducible nitric (iNO) oxide and tumour necrosis factor-α (TNF-α) in murine macrophage cell line (RAW264.7). Inhibition of these two important mediators of inflammation is important in our understanding of the possible mechanism through which these compounds may be involved in mediating any anti-inflammatory effect shown by mistletoe (Nathan, 1992[21]; Nworu et al., 2012[22]; Lee et al. 2012[18]).
Materials and methods
Plant material
Fresh leaves of mistletoe (Loranthus micranthus Linn.) parasitic on Hevea brasiliensis were collected from Enugu-Ezike in Enugu State, Nigeria in January. 2012. The plant material was identified and authenticated by Mr. A. O. Ozioko of the Bioresources Conservation and Development Program (BDCP), Nsukka. A voucher specimen (LM1610) was deposited at the herbarium of the Institute.
Preparation of extract
The dried leaves (500 gm) of Loranthus micranthus Linn. parasitic on Hevea brasiliensis were macerated with 3.0 L of 100 % methanol (MeOH) and extracted at room temperature for 48 h with agitation. The resulting methanol extract was concentrated in vacuum at 40 °C to obtain the dry methanol extract.
Isolation of the polyphenols
The dry methanol extract (50 gm) was dissolved in 400 mL of 10 % methanol-water and the resulting mixture (i. e., the aqueous layer) partitioned with 3.0 L n-hexane (6 x 500 mL), 3.0 L ethyl acetate (6 x 500 mL) and 1.0 L n-butanol (2 x 500 mL) using separating funnel to obtain n-hexane (HF, 3.90 gm, 7.8 %), ethyl acetate (EF, 13.8 gm, 27.6 %), n-butanol (BF, 12.6 gm, 25.2 %) and water (WF, 1.02 g, 2.04 %) fractions respectively. The ethyl acetate fraction (5 gm) was purified by vacuum liquid chromatography using silica gel (230 – 400 mesh, 3.0×30 cm, 500 gm) as the stationary phase and eluted with a gradient of n-hexane in ethyl acetate (10:0, 8:2, 6:4, 4:6, 2:8, 0:10, each 500 mL) and of dichloromethane (DCM) in methanol (9:1, 7:3, 5:5, 3:7,1:9, each 1000 mL) to afford 11 sub-fractions (EF1-EF11). Fraction EF6 (348.5 mg) was further fractionated on Sephadex LH-20 (100 % MeOH) to afford seven sub-fractions (EF6A–EF6G). Fraction EF6E (36.3 mg) was purified using semi-preparative HPLC with MeOH–H2O as the mobile phase to yield TMECG (2.9 mg). Similarly, fractions EF6F (39.0 mg) were purified in a similar way to afford ECG3"Me (15.8 mg). Also fraction EF9 (600 mg) was subjected to Sephadex LH-20 column chromatography (3×110 cm) eluted with 100 % MeOH to give nine sub-fractions (EF9A–EF9I).
Rutin (5.6 mg) was obtained from sub- fractions EF9B (77.3 mg) by semi-preparative HPLC. The n-butanol fraction (10 g) was purified by vacuum liquid chromatography over silica gel (230 – 400 mesh, 3.0×30 cm, 800 g) and eluted with a gradient of DCM in MeOH (9:1, 8:2, 7:3, 6:4, 5:5,4:6, 3:7, 2:8, 1:9,0:10, each 1000 mL) to give 10 fractions (BF1-BF10). Fraction BF3 was subjected to Sephadex LH-20 column chromatography eluting with 100 % MeOH (3×110 cm) to afford seven sub-fractions (BF3A–BF3G). Sub-fraction BF3A (65.6 mg) was purified by semi-preparative HPLC (MeOH–H2O) to give peltatoside (2.6 mg).
Cell viability assay
The cytotoxicity effects of the compounds were evaluated in RAW 264.7 cells using MTT assays (Mosmann, 1983[20]). Laboratory stock of RAW264.7 mouse macrophage cell line (ATCC, MD, USA) was cultured in R-10 medium, consisting of RPMI 1640 medium (Corning cellgro® RPMI; Mediatech Inc., Manassas, VA, USA) supplemented with 10 % heat-FBS, 50 µM 2-mercaptoethanol (Gibco, Invitrogen, USA), 100 U/ml penicillin (Gibco, Invitrogen, USA), and 100 µg/ml streptomycin (Gibco, Invitrogen, USA) in a 5 % CO2 humidified atmosphere at 37 °C. The effect of TMECG, ECG3"Me, rutin and peltatoside on the viability of RAW264.7 mouse macrophages was determined using a modification of the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cytotoxicity assay originally described by Mosmann (1983[20]). The cells were seeded in triplicate into 96-culture well plates at a density of 104 cells/well in 100 µL. After 24 h of seeding, the cells were treated with graded concentrations (5, 25, 100, 250, and 500 µM) of the compounds. R-10 medium was used as the “no-drug” control. After 48 h of incubation at 37 °C under 5 % CO2, a solution of MTT (20 µL per well of 5 mg/ml solution) was added and further incubated for 4 h to allow formazan formation in viable cells. Thereafter, the MTT-containing media were removed, and the reduced formazan dye was solubilized by adding 150 µL of dimethyl sulfoxide (DMSO) to each well. The plate was agitated for 15 min on a shaker. Then the optical density (OD) was determined at 570 nm using a multi-well plate reader (Bio-Kinetic Reader-E312e®; Bio-Tech Instruments, Winooski, VT). Each value of the triplicate determination was expressed as a percentage of the mean of the no-drug control wells.
Nitrite determination
Nitric oxide production and release by RAW264.7 cells was measured indirectly by determining nitrite accumulation in culture supernatant using the Griess reaction as described by Kim et al. (2013[15]). RAW 264.7 cells (5×105 cells/well) were seeded in 24-well plate for 24 h. The cells were pre-treated for 2 h with 0, 5, 25, and 100 µM of TMECG, ECG3"Me, rutin and peltatoside. Thereafter, lipopolysaccharide, LPS (1 µg/ mL) (serotype 0128: B 12, L 4255; Sigma, St Louis, MO, USA) was added into each well and incubated for an additional 24 h. The quantity of nitrite generated was measured using the Griess reagent system. Equal volumes of cell-free culture supernatants and freshly prepared Griess reagent were added to the 96-well plate and incubated at room temperature for 10 min. The absorbance was measured on a microplate reader at 570 nm (Bio-Kinetic Reader-E312e®; Bio-Tech Instruments, Winooski, VT). Nitrite concentrations were extrapolated from a standard NaNO2 curve included within each assay plate.
Measurement of TNF-α
The concentration of TNF-α in the conditioned culture supernatants from macrophage cultures was measured by cytokine enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (R&D Systems, Inc., Minneapolis, MN, USA) according to the method described by Ravipati et al. (2012[26]). RAW 264.7 cells (5×105 cells/well) were seeded in 24-well plate for 24 h. The cells were pre-treated for 2 h with 0, 5, 25, and 100 µM of TMECG, ECG3"Me, rutin, and peltatoside respectively before LPS (1 µg/mL) was added to the wells. After incubation, cell-free supernatant was harvested and stored at -20 °C for future analysis. The concentration of TNF-α was calculated from a standard curve of the standard mouse TNF-α included ELISA assay.
Results
Semi-preparative HPLC separation of the ethyl acetate (EtOAc) and butanol (n-BuOH) fractions of the leaves of mistletoe (Loranthus micranthus Linn.) parasitic on Hevea brasiliensis led to the isolation of four polyphenols 1-4 (Figure 1(Fig. 1)).
Figure 1. Structures of the isolated compounds 1-4.
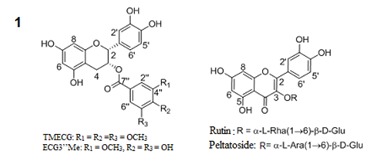
The characterizations of the isolated compounds were done by comparison with reported values.
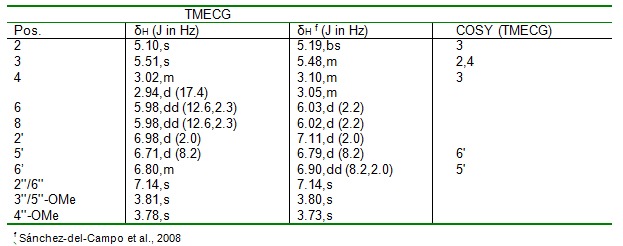
3-O-(3,4,5-trimethoxybenzoyl)-(-)-epicatechin (1) was obtained as a yellow amorphous powder; [α]D20 = -106.9 o (c 0.10, MeOH); UV (MeOH) λmax 271.1 and 211.8 nm; HR-ESIMS: m/z 485.14423 [M+H] +; 1H and 13C NMR spectroscopic data (Table 1(Tab. 1)).
Table 1. 1H-NMR (500 MHz) data of compound 1 in MeOH-d4; J in Hz.
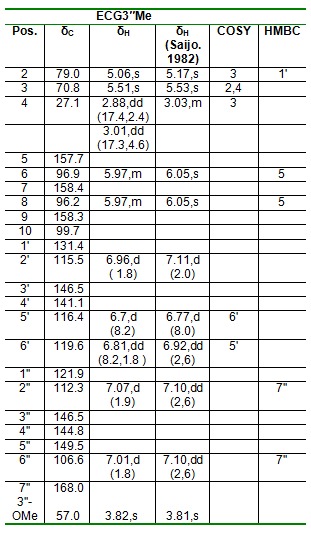
Epicatechin-3-O-(3"-O-methyl)-gallate (2) was obtained as a yellow amorphous powder; [α]D20 = 181.8 o (c 0.10, MeOH); UV (MeOH) λmax (MeOH) 216.4, 278.8 nm; ESI-MS: m/z 457 [M+H] +; 1H and 13C NMR spectroscopic data (Table 2(Tab. 2)).
Table 2. 1H (500 MHz) and 13C-NMR (125 MHz), COSY and HMBC Data of compound 2.
Rutin (3) was obtained as a yellow amorphous powder; [α]D20 = -11.8 o (c 0.10, MeOH); UV (MeOH) λmax. 203.0, 256.9 and 355.8nm; ESI-MS: m/z 611.0 [M+H] +; 1H and 13C NMR spectroscopic data (Table 3(Tab. 3)).
Table 3. Table 3: 1H-NMR (500MHz) data of compounds 3 and 4 in MeOH-d4; J in Hz.
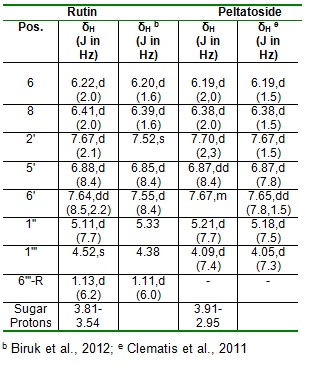
Peltatoside (4) was obtained as a yellow amorphous powder; [α]D20 = -15.0 o (c 0.10, MeOH); UV (MeOH) λmax. 203.0, 256.8 and 355.5 nm; ESI-MS: m/z 597 [M+H] +; 1H and 13C NMR spectroscopic data (Table 3(Tab. 3)).
Cell viability MTT assay
The compounds were measured at treatment concentrations in the cell culture system.
Nitrite determination
The inhibitory effects of the compounds were evaluated on the production of nitrite, a stable metabolite of NO in RAW 264.7 cells that had been challenged with LPS in the presence or absence of the test compounds.
Measurement of TNF-α
The isolated polyphenols were evaluated and compared with regard to the production of TNF-α (major pro-inflammatory cytokines) in LPS-stimulated RAW 264.7 cells.
Discussion
Compound 1 was obtained as a yellow amorphous powder (2.90 mg, tR = 21.2 min). The UV spectrum showed absorption maxima λmax (MeOH) 271.1 and 211.8 nm. The molecular formula C25H24O10 was deduced from the HR-ESIMS with peak ion at m/z 485.14423 [M+H] +, indicating fourteen degree of unsaturation. The 1H NMR (500 MHz, CD3OD) spectral revealed an ABX-type aromatic protons at δH 6.71 (1H, d, J =8.2Hz, H-5'), 6.80 ( 1H,m,H-6') and 6.98 (1H, d, J =2.0 Hz, H-2') and two-meta coupled aromatic protons signals at δH 5.98 (2H, dd, J = 12.6, 2.3 Hz) attributed to the epicatechin protons in ring B. However, the galloyl moiety has a para methoxyl group with chemical shift δH 3.78 (3H, s, 4''-OMe) and two equivalent methoxyl groups with chemical shift δH 3.81 (6H, s, 3''/5''-OMe). In the 1H-1H COSY spectrum, H-3 proton (δH 5.10, s) was found to correlate to the two germinal protons (H2-4) of C-4 and the aromatic proton (δH 6.71 d, H-5') was found to correlate with H-6' (δH 6.80, m). Compound 1 was isolated for the first time from a natural source. The NMR data of compound 1 agrees with literature values (Sánchez-del-Campo et al., 2008[28]). Thus, the structure of compound 1 was established as 3-O-(3,4,5-trimethoxybenzoyl)-(-)-epicatechin (TMECG).
Compound 2 isolated from the EtOAc fraction as a yellow amorphous powder (15.80 mg, tR =16.9 min). It showed UV absorbance at λmax (MeOH) 216.4, 278.8 nm. Positive and negative ESI-MS showed molecular ion peaks at m/z 457 [M+H] + (base peak) and m/z 455 [M-H] - (base peak) respectively, indicating a molecular mass of 456 g/mol and a molecular weight of C23H20O10. The 1H NMR (500 MHz, CD3OD) spectrum of ECG3"Me shows a 3,4-disubstitution on the ring B of the epicatechin unit with chemical shifts of δH 6.96 (d, J = 1.8 Hz, H-2'), 6.81 (dd, J = 8.2,1.8 Hz, H-6') and 6.70 (d, J = 8.2 Hz, H-5') . The 1H NMR also showed an AX-type aromatic protons at δH 7.02 (d, J= 1.8 Hz, H-2’’) and 7.07 (d, J= 1.9 Hz, H-6') on the ring C, as well as one doublet of doublet at 5.97 ppm integrating as 2H suggesting that ring A is a tetra-substituted benzene ring. In the 1H-1H COSY spectrum, H-2 (δH 5.16, s) was found to correlate with H-3 (δH 5.51, s), which in turn correlates with H2-4 (δH 2.88 and 3.01, each dd), in the ring A thus revealing a typical pattern of epicatechin nucleus. Also in the ring B, the aromatic proton H-5' (δH 6.7, d) was found to correlate with H-6' (δH 6.81, dd). The attachments of the methoxy group at position C-3'' in the galloyl moiety was confirmed by the long range correlation of -OCH3-3 to C-3'' (δC 146.5) in the HMBC spectrum. The HMBC spectrum shows the correlation of H-2’’ (δH 7.07) and H-6'' (δH 7.01) with δC 168.0 (C-7''), as well as the correlation of 3''-OMe (δH 3.82) to C-7'' (δC 57.0) suggesting that the oxymethyl group was connected to the benzene ring via C-3''. The 13C NMR (125 MHz, MeOH-d4) spectrum showed 23 carbon signals, including one methylene (δC27.1), one oxymethyl group (δC 57.0), nine methines, 11 quaternary carbons and one ester carbonyl group (δC 168.0) as aided by the DEPT-135 experiment (Table 2(Tab. 2)). The NMR spectral data compares favourably with literature values (Saijo,1982[27]), thus compound 2 is named as (-)-Epicatechin 3-O-(3-O-Methyl) gallate (ECG3"Me).
Rutin (3) was isolated as a yellow amorphous powder (5.60 mg, tR =20.2 min). The HPLC-ESIMS spectrum of 3 exhibited a peak ion at m/z 611.0 [M+H] +, indicating a molecular formula of C27H30O16, containing thirteen degrees of unsaturation. The UV spectrum showed absorbance maxima λmax. 203.0, 256.9 and 355.8 nm indicative of a quercetin diglycoside unit. In the 1H NMR (500 MHz, CD3OD) spectrum the aromatic protons exhibited an ABX coupling system at δH 7.70 (d, J=2.1Hz) for H-2', δH 7.67 (dd, J=8.5, 2.2Hz) for H-6' and δH 6.91 (d, J= 8.4 Hz) for H-5'. The other AX coupling system at δH 6.43 (d, J=2.0Hz) and δH 6.24 (d, J=2.0 Hz) was assigned to H-8 and H-6 protons respectively, characteristic of quercetin moiety. From the mass spectrum data, fragments at m/z 465 [M-146 + H] + (loss of rhamnose); 303 [M-146-162 + H] + (loss of rhamnose and glucose), together with two anomeric protons at δH 5.11 (d, J=7.7 Hz) and δH 4.52 (1H, s) for glucose and rhamnose respectively, indicated the presence of two sugar residues in the molecule. The presence of a rhamnose moiety was further confirmed by the presence of a methyl doublet at δH 1.13 (3H, d, J = 6.2 Hz) downfield in the 1H NMR spectrum. The rest of the protons in the sugar moiety resonated between 3.57 and 3.84 ppm. The 1H NMR data recorded on MeOH-d4 were in good agreement with published values (Al-Sawi and Sleem, 2010[3]; Biruk et al., 2012[4]). Hence, the compound was identified unequivocally as rutin (3,3',4', 5,7-pentahydroxyflavone-3-rhamnoglucoside).
Peltatoside (4) was obtained as a yellow amorphous powder (2.60 mg, tR =19.8 min). The HPLC-ESIMS spectrum of 4 exhibited a peak ions at m/z 597 [M+H] +, and m/z 595 [M-H] - indicating a molecular formula of C26H28O16, containing thirteen degrees of unsaturation. The UV spectrum showed absorbance maxima λmax. 203.0, 256.8 and 355.5 nm indicating a quercetin diglycoside unit. In the 1H NMR (500 MHz, MeOH-d4) three aromatic protons (δH 7.67, m); (δH 6.87, dd, J= 8.4 Hz) and (δH 7.70, d, J=2.3 Hz) assignable to H-6', H-5' and H-2' respectively. Also the 1H NMR revealed two meta-coupled protons at δH 6.19, d (J =2.0 Hz) and δH 6.38 (d, J= 2.0 Hz) for H-6 and H-8 respectively. From the mass spectrum data, fragments at m/z 465 [M-132 + H] + (loss of arabinose); 303 [M- 132-162 + H] + (loss of arabinose and glucose), together with the presence of arabinose and glucose moieties with the proton signals at δH 4.09, d, J=7.4 Hz and δH 5.21, d, J=7.7 Hz for the arabinose and glucose anomeric protons respectively, indicated the presence of two sugar residues in the molecule. The rest of the protons in the sugar moieties resonated between 2.95 and 3.91 ppm. The proton 1H NMR data recorded in MeOH-d4 were in good agreement with the published values (Clematis et al., 2011[9]). Hence, the compound was identified as peltatoside.
Cell viability assays of the isolated compounds were assessed using MTT assay. The compounds were measured at treatment concentrations in the cell culture system. The polyphenols did not cause significant loss in viability of RAW264.7 cells even at concentration up to 500 µM after 24 h of incubation. The result showed that at 24 h incubation, the viability of the RAW262.7 cells remained greater than 90 % at the highest treatment dose of the test compounds, showing that the test compounds have less toxicity on the mouse macrophage cells (Table 4(Tab. 4)).
Table 4. Effect of compounds 1-4 on viability of RAW264.7 mouse macrophage cells.

The inhibitory effects of the compounds were evaluated on the production of nitrite, a stable metabolite of NO in RAW 264.7 cells that had been challenged with LPS in the presence or absence of the test compounds. The test compounds effectively suppressed LPS-induced nitrite production in a dose-dependent manner. The NO in LPS-stimulated RAW 264.7 cells exposed to 100 µM of the test compounds inhibited iNO production by 89.8, 96.5, 98.7 and 99.2 % (% of the control), for TMECG, ECG3" rutin and peltatoside respectively (Figure 2(Fig. 2)).
Figure 2. Suppression of LPS-induced NO production in RAW264.7 cells by the polyphenols of mistletoe parasitic on Hevea brasiliensis. Cells were treated for 24 h with 0, 5, 25, or 100 μM of the polyphenols in the presence of 1.0 μg/mL LPS. The normal group was treated with media only. The results are expressed as the mean ± SEM from three independent experiments.
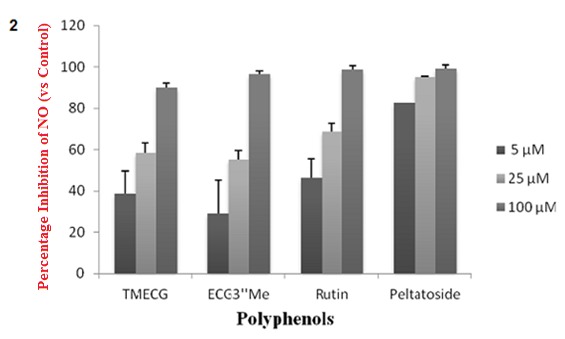
The IC50 values of the polyphenols indicate that rutin and peltatoside have a higher iNO inhibitory activity compared to the catechin gallates with values of 0.013, 8.05,14.67 and 11.18 µM for peltatoside, rutin, ECG3’’Me and TMECG, respectively. These results show that these polyphenols could be exerting an anti-inflammatory effect by ameliorating the production of inflammatory mediators, including NO.
The isolated polyphenols were evaluated and compared with regard to the production of TNF-α (major pro-inflammatory cytokines) in LPS-stimulated RAW 264.7 cells. At 24 h, the polyphenols at treatment dose of 5 to 100 µM produced significant dose-dependent decrease in TNF-α production. In particular, the inhibition in the production of TNF-α by the catechin gallate derivatives at treatment dose of 100 µM revealed comparable inhibition in the production of proinflammatory cytokine of 88.2 and 82.4 % (% of the control) for TMECG and ECG3"Me, respectively. This trend was also observed in the inhibition of the cytokine by the quercetin diglycosides at the same treatment dose with percentage of inhibition of 91.3 and 99.6 % (% of the control) for rutin and peltatoside respectively (Figure 3(Fig. 3)). Also, the quercetin diglycosides had a lower IC50 (11.84 and 7.75 µM) when compared to the IC50 of the catechin gallates (14.94 and 22.60 µM) (Table 5(Tab. 5)).
Figure 3. Figure 3: Suppression of LPS-induced cytokine production (TNF-α) in RAW264.7 cells by the polyphenols of mistletoe parasitic on Hevea brasiliensis. RAW264.7 cells were treated for 24h with 0, 5, 25, or 100μM of the polyphenols in the presence of 1.0μg/mL LPS. The normal group was treated with media only. The cell culture media were then collected. The results are expressed as the mean ± SE from three independent experiments.
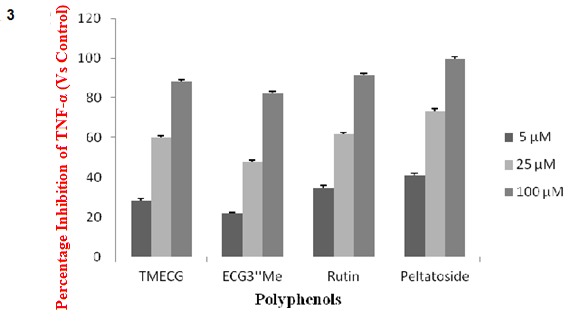
Table 5. IC50 a values of compounds 1-4.

Statistical analyses
The data were expressed as mean ± SEM of at least triplicate determinations (n = 3). To demonstrate statistical significance of data, a One-way Analysis of Variance (ANOVA) using GraphPad Prism 5 software was performed followed by Dunnett’s posthoc test. Differences between test and control treatments are considered significant at P < 0.05.
Conclusions
The four polyphenolic compounds isolated from mistletoe and investigated in this study showed potent inhibition of inducible release of important proinflammatory mediators, NO and TNF-α. Mistletoe is used in alternative and complementary medicine for a variety of indications including treatment of degenerative inflammation of the joints (Blumenthal, 1998[6]). In a recent study, the anti-oxidative property of these polyphenols has been reported (Agbo et al., 2013[1]).
Polyphenols can exert their anti-inflammatory properties through the modulation of mitogen-activated protein kinases (MAPK) signalling pathways (Kong et al., 2000[16]; Wiseman et al., 2001[32]) and NF-κB and AP-1 transcription factors (Manna et al., 2000[19]), inhibition of the production of inflammatory cytokines and chemokines, suppressing the activity of cyclooxygenase (COX) (O'Leary et al., 2004[23]) and inducible nitric oxide synthase (iNOS) (Donnelly et al., 2004[11]) and thereby decreasing the production of reactive oxygen and nitrogen species (ROS/RNS). Previous studies have shown that quercetin inhibits lipopolysaccharide (LPS)-stimulated TNF-α production and NF-κß activation in RAW 264.7 macrophage (Comalada et al., 2005[10]; Cho et al., 2003[8]; Wadsworth and Koop, 1999[31]). Recently, a study showed that oral administration of rutin reduced, in a dose-dependent manner, the polymorphonuclear neutrophils chemotaxis to FMLP in a model of rat paw oedema (Sunita et al., 2011[30]). Cytotoxicity profiles for the isolated polyphenols were assayed in parallel using the MTT protocol (Table 1(Tab. 1)). The result of the cytotoxicity assay showed that the inhibition of proinflammatory mediators caused by the compounds was not due to loss in viability of the treated murine macrophages at the concentration range used.
Acknowledgements
The authors are grateful to Fulbright for Postdoctoral Research Fellowship award to Dr C.S. Nworu and to Mr. Alfred Ozioko (Bioresources Conservation and Development Program (BDCP), Nsukka, Nigeria) for identifying the plant material.
References
- 1.Agbo MO, Lai D, Okoye FBC, Osadebe PO, Proksch P. Antioxidative polyphenols from Nigerian mistletoe Loranthus micranthus (Linn.) parasitizing on Hevea brasiliensis. Fitoterapia. 2013;86:78–83. doi: 10.1016/j.fitote.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Ali FH, Intesar TN, Khylood WA, Saad AH. Hematopoietic toxicity of Loranthus europaeus chloroform extract: in vitro study. Int J Compr Pharm. 2005;17:345–52. [Google Scholar]
- 3.Al-Sawi SA, Sleem AA. Flavonoids and hepatoprotective activity of leaves of Senna surattensis (Burm.f.) in CCl4 induced hepatotoxicity in rats. Aust J Basic Appl Sci. 2010;4:1326–1334. [Google Scholar]
- 4.Biruk S, Kaleab A, Raghavendra Y. Radical scavenging activities of the leaf extracts and a flavonoid glycoside isolated from Cineraria abyssinica. J Appl Pharm Sci. 2012;2(4):44–49. [Google Scholar]
- 5.Black HP, Berman SA. Stress and inflammation. In: Faith ER, Murgo JA, Good AR, editors. Cytokines: stress and immunity. Boca Raton, FL: CRC Press; 1999. pp. 115–132. [Google Scholar]
- 6.Blumenthal M, editor. The complete German Commission E monographs: Therapeutic guide to herbal medicines. The American Botanical Council. London: Churchill Livingstone; 1998. [Google Scholar]
- 7.Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–258. doi: 10.1016/j.tox.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaβ pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem. 2003;243:153–60. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- 9.Clematis F, Tedeschini J, Dolci M, Lanzotti V, Cangelosi B, Fascella S, et al. Phenol composition and susceptibility to Fusarium Oxysporum Dianthi in carnation. J Life Sci. 2011;5:921–925. [Google Scholar]
- 10.Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Galvez J, et al. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-kappaB pathway. Eur J Immunol. 2005;35:584–92. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RH, Ito K, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;87:774–783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 12.Griggs P. Mistletoe: myth, magic and medicine. Biochemist. 1991;13:3–4. [Google Scholar]
- 13.Henson PM, Murphy RC. Mediators of the inflammatory process. Amsterdam: Elsevier; 1989. p. 404. [Google Scholar]
- 14.Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003;24:25–29. doi: 10.1016/s1471-4906(02)00013-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim SK, Kim H, Kim SA, Park HK, Kim W. Anti-inflammatory and anti-superbacterial activity of polyphenols isolated from black raspberry. Korean J Physiol Pharmacol. 2013;17:73–79. doi: 10.4196/kjpp.2013.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong AN, Yu R, Chen C, Mandlekar S, Primiano T. Signal transduction events elicited by natural products: role of MAPK and caspase pathways in homeostatic response and induction of apoptosis. Arch Pharm Res. 2000;23:1–16. doi: 10.1007/BF02976458. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–795. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 18.Lee TH, Jung M, Bang MH, Chung DK, Kim J. Inhibitory effects of a spinasterol glycoside on lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines via down-regulating MAP kinase pathways and NF-κB activation in RAW264.7 macrophage cells. Int Immunopharmacol. 2012;13:264–270. doi: 10.1016/j.intimp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assay. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 22.Nworu CS, Akah PA, Okoye FB, Esimone CO. Inhibition of pro-inflammatory cytokines and inducible nitric oxide by extract of Emilia sonchifolia L. aerial parts. Immunopharmacol Immunotoxicol. 2012;34:925–931. doi: 10.3109/08923973.2012.696202. [DOI] [PubMed] [Google Scholar]
- 23.O'Leary KA, de Pascual-Tereasa S, Needs PW, Bao YP, O'Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res. 2004;551:245–254. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Osadebe PO, Abba CC, Agbo MO. Antimotility effects of extracts and fractions of Eastern Nigeria mistletoe (Loranthus micranthus Linn) Asian Pac J Trop Med. 2012;5:412–20. doi: 10.1016/S1995-7645(12)60098-4. [DOI] [PubMed] [Google Scholar]
- 25.Patrick-Iwuanyanwu KC, Onyeike EN, Wegwu MO. Anti-inflammatory effect of crude methanolic extract and fractions of African mistletoe Tapinanthus bangwensis (Engl. & K. Krause) on wistar albino rats. Der Pharmacia Lettre. 2010;2(6):76–83. [Google Scholar]
- 26.Ravipati AS, Zhang L, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement Altern Med. 2012;12:173. doi: 10.1186/1472-6882-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saijo R. Isolation and chemical structures of two new catechins from fresh tea leaf. Agric Biol Chem. 1982;46:1969–1970. [Google Scholar]
- 28.Sánchez-del-Campo L, Otón F, Tárraga A, Cabezas-Herrera J, Chazarra S, Rodríguez-López JN. Synthesis and biological activity of a 3,4,5-trimethoxybenzoyl ester analogue of epicatechin-3-gallate. J Med Chem. 2008;51:2018–2026. doi: 10.1021/jm701346h. [DOI] [PubMed] [Google Scholar]
- 29.Sangita C, Priyanka C, Protapaditya D, Sanjib B. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac J Trop Biomed. 2012;6:178–180. [Google Scholar]
- 30.Sunita P, Jha S, Pattanayak SP. Anti-inflammatory and in-vivo antioxidant activities of Cressa cretica Linn, a halophytic plant. Middle-East J Sci Res. 2011;8:129–140. [Google Scholar]
- 31.Wadsworth TL, Koop DR. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem Pharmacol. 1999;15:941–9. doi: 10.1016/s0006-2952(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 32.Wiseman S, Mulder T, Rietveld A. Tea flavonoids: bioavailability in vivo and effects on cell signaling pathways in vitro. Antioxid Redox Signal. 2001;3:1009–1021. doi: 10.1089/152308601317203549. [DOI] [PubMed] [Google Scholar]


