Abstract
The antifungal potential of essential oil and ethanolic leaf extracts of Lonicera japonica Thunb. was evaluated for controlling the growth of dermatophytes. The oil (1,000 ppm) and extracts (1,500 ppm) of L. japonica revealed 55.1–70.3 % and 40.1–65.5 % antidermatophytic effect against Microsporum canis KCTC 6348, 6349, 6591, Trichophyton rubrum KCTC 6345, 6352, 6375, Trichophyton mentagrophytes KCTC 6077 and 6085, respectively, along with their respective minimum inhibitory concentrations ranging from 62.5-500 and 125-1,000 µg/ml. Also, the oil had strong detrimental effect on spore germination of all the tested dermatophytes as well as concentration and time-dependent kinetic inhibition of M. canis KCTC 6348. The results demonstrated that L. japonica oil and extracts could be potential sources of natural fungicides to protect human and animals from fungal infections.
Keywords: Lonicera japonica Thunb., essential oil, ethanolic extracts, antidermatophytic activity, skin infectious fungal pathogens
Introduction
Skin infections caused by dermatophytes, a group of filamentous fungi invading and deriving nutrients from the keratinized tissues (e.g., skin, nails and hair), are the most common problem in tropical and subtropical countries (Fenner et al., 2005[10]; Portillo et al., 2001[21]). The three most important genera of dermatophytes are Trichophyton, Microsporum and Epidermophyton (Emmons et al., 1977[9]). Dermatophytoses are worldwide in distribution with highly prevalent in tropical and subtropical countries due to the hot and humid climate which favors their growth (Emmons et al., 1977[9]). The drugs used against dermatophytoses exhibit several side effects and have limited efficacy. Therefore, there is a distinct need for the discovery of new safer and more effective antifungal agents. The use of medicinal herbs in the treatment of skin diseases including mycotic infections is an age-old practice in many parts of the world (Irobi et al., 1993[11]), because herbal remedies used in traditional folk medicine may help to overcome the growing problem of resistance to antifungal drugs and their relative toxicity.
The plant Lonicera japonica Thunb. (Caprifoliaceae), is a species of honeysuckle native to eastern Asia including Japan, Korea, northern and eastern China and Taiwan, which is a major invasive species in North America. L. japonica is one of the medicinal plants traditionally used (Peng et al., 2000[20]). Pharmacological studies and clinical practices have demonstrated that L. japonica played many biological functions including hepatoprotective, cytoprotective, antimicrobial, antioxidative, antiviral and anti-inflammatory, detoxicating, dispelling noxious heat activities and it significantly increased blood neutrophil activity and promoted the neutrophil phagocytosis activities (Chang et al., 1995[5]). The constituents of this plant have been previously investigated and shown to contain iridoid glucosides (Kakuda et al., 2000[12]) and polyphenolic compounds (Peng et al., 2000[20]). The main polyphenolic components in L. japonica are hyperoside, chlorogenic acid, luteolin and caffeic acid (Kakuda et al., 2000[12]; Peng et al., 2000[20]). Some related investigations showed that hyperoside, chlorogenic acid and other flavones could be used to scavenge free radicals and have anti-inflammatory activity (Leung et al., 2005[14]). The major parts of this plant include flowers, leaves and stems, which have some medicinal properties such as flower buds have anticancer and anti-inflammatory properties (Zhang et al., 2008[30]), leaf has antioxidant and tyrosinase inhibition properties (Byun et al., 2004[2]) and stem has tyrosinase inhibition, xanthine oxidase inhibition, and nitrite scavenging activities (Byun et al., 2004[2]). However, researches on antifungal property of L. japonica are still scarce, only a few scientific articles have been reported to have some biological activities (Byun et al., 2004[2]; Shan et al., 2007[25]). Hence, efforts have been made to investigate the antidermatophytic properties of essential oil and leaf extracts of L. japonica.
Previously, we reported the chemical composition and antibacterial properties of the essential oil and various organic extracts of L. japonica against some food-borne/spoilage bacteria (Rahman and Kang, 2009[22]). In this study, we tested the antidermatophytic efficacy of the oil and ethanolic leaf extracts of L. japonica against some skin infectious fungal pathogens.
Materials and Methods
Fungal pathogens (dermatophytes)
The fungal pathogens used in this study were Microsporum canis KCTC 6348, M. canis KCTC 6349, M. canis KCTC 6591, Trichophyton rubrum KCTC 6345, T. rubrum KCTC 6352, T. rubrum KCTC 6375, Trichophyton mentagrophytes KCTC 6077 and T. mentagrophytes KCTC 6085, which were obtained from the Korean agricultural culture collection, Suwon, Republic of Korea. All the strains were maintained on Sabouraud’s agar (SBA, Difco, MI, USA) at 4 °C.
Preparation of spore suspensions and test samples
The spore suspensions of dermatophytes were obtained from their respective 10 days old cultures, mixed with sterile distilled water to obtain a homogenous spore suspension of 1 x 105 spore/ml. Essential oil and leaf extracts were dissolved in dimethyl sulfoxide (DMSO) separately to prepare the stock solutions with their respective known weights, which were further diluted to prepare test samples.
Determination of antidermatophytic activity of essential oil and ethanolic extracts
The essential oil and the extracts were bio-assayed by the poisoned food technique (Nene and Thapliyal, 1979[18]). Essential oil was mixed with dimethyl sulfoxide (DMSO) so as to ease its incorporation into the agar medium in the proportion 1 volume of oil to 9 volumes of DMSO. The essential oil was tested at 1000 ppm (that is µl/L). Organic extracts (diluted in DMSO) were tested at 1500 ppm. The oil and extracts were autoclaved and cooled (at 50 °C) SBA medium through 0.45 µm sterile Millipore filters. The medium amended with oil or extracts was then poured into sterilized Petri dishes. A mycelial disc of 5 mm in diameter of the test pathogens taken from 10 day old culture and was placed at the center of the medium with the help of a sterilized cork borer. Some plates prepared as controls without the oil or extracts but only DMSO. The plates were then sealed with parafilm and incubated at 28 ± 2 °C for 5–7 days, time period by which the growth of control would have reached the edges of the plates. Growth inhibition of each of the fungal strains was calculated as the percentage of inhibition of radial growth relative to the control along with the antidermatophytic effect on fungal mycelium. The plates were used in triplicates for each treatment.
The growth inhibition of treatment compared to control was calculated by percentage, using the following formula:
Inhibition ( %) = [(C-T)/C] ×100, where C and T are the radial growth (mm) of dermatophytes in the control and treated plates, respectively.
Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration (MIC) of essential oil and ethanolic extracts against fungal pathogens was determined by agar dilution method as described before (Mitscher et al., 1972[16]). Briefly, 10-milliliter aliquots of Sabouraud’s broth (SDB) were prepared in 25 ml Erlenmeyer flask. The oil and plant extracts were dissolved with DMSO, sterile filtered (0.45 µm) and then added to the different flasks in order to obtain concentrations of 62.5 to 2000 µg/ml of culture medium. The final concentrations of DMSO in the assay did not exceed 2 %. In addition, one flask with uninoculated oil or extract-free medium was included as a sterile control. Using a micropipette, an inoculum of 5 µl (105 spore/ml) of the spore suspension was inserted into each flask of medium containing a known concentration of samples, as well as samples-free medium. All cultures were incubated in a shaking incubator at 150 rpm for 3–9 days at 28 ± 2 °C or until good growth was apparent in the oil or extract-free control. The growth in all flasks was visually compared with that of the control in order to determine % inhibition. The growth was scored in the following manner: 4+, growth comparable to that of the sample free control; 3+, growth approximately 75 % that of the control; 2+, growth approximately 50 % that of the control; 1+, growth 25 % or less that of the control; and 0, no visible growth (data not shown). The minimum concentration at which no visible growth was observed was defined as the MIC, which was expressed in µg/ml.
Spore germination and growth kinetics assay
The essential oil which exhibited slightly higher antidermatophytic activity, as compared to organic extracts, was chosen for spore germination of the dermatophytes. Also, four dermatophytes were used on the basis of their sensitivity (the lowest MIC values) to the oil. Six concentrations of the essential oil (31.25, 62.5, 125, 250, 500 and 1000 µg/ml) and one control (0.5 % DMSO) were separately tested for spore germination of different dermatophytes (Rana et al., 1997[23]). The samples were inoculated with spore suspension of each fungal pathogen containing 105 spore/ml. From this, aliquots of 10 µl spore suspension from each were placed on separate glass slides in triplicate. Slides containing the spores were incubated in a moisture chamber at 25 °C for 24 h. Each slide was then fixed in lactophenol-cotton blue and observed under the microscope for spore germination. The spores that generated germ tubes were enumerated and percentage of spore germination was calculated. The control (0.5 % DMSO) was tested separately for spore germination of different dermatophytes. All experiments were conducted in triplicate.
M. canis KCTC 6348 which appeared to be more susceptible to the essential oil in the spore germination study was chosen as test dermatophyte for kinetic study and evaluation of antidermatophytic activity of essential oil. A 10 µl spore suspension of this fungal species was inoculated to different concentrations of the oil (31.25, 62.5 and 125 µg/ml) in a test tube and a homogenous suspension (about 2 ×105 spore/ml) was made by inverting the test tubes 3–4 times. After the specific intervals i.e., 30, 60, 90, 120 and 150 min, the reaction mixtures were filtered through Whatman No. 1 filter paper and the retained spores were washed two or three times with sterile distilled water. The filter was then removed and spores were washed off into 10 ml of sterile distilled water. From this 100 µl of spore suspension was taken onto the glass slide and incubated at 24 ± 2 °C for 24 h. About 200 spores were counted and percentage spore germination was calculated. Control sets were prepared in 0.5 % DMSO. All experiments were conducted in triplicate.
Statistical analysis
The essential oil and various extracts were assayed for antidermatophytic activity. Each experiment was run in triplicate, and mean values were calculated. A t-test was computed for the statistical significance of the results.
Results
Antidermatophytic activity of essential oil and ethanolic extracts
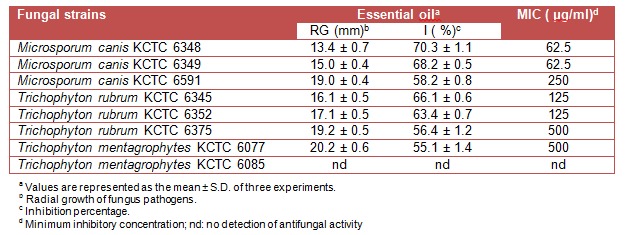
The essential oil of L. japonica exhibited a moderate to high antidermatophytic activity against all the tested dermatophytes except T. mentagrophytes KCTC 6085. As shown in Table 1(Tab. 1), 1000 ppm concentration of the oil showed potent inhibitory effect on the growth of seven dermatophytes, M. canis KCTC 6348 (70.3 %), M. canis KCTC 6349 (68.2 %), T. rubrum KCTC 6345 (66.1 %), T. rubrum KCTC 6352 (63.4 %), M. canis KCTC 6591 (58.2 %) T. rubrum KCTC 6375 (56.4 %) and T. mentagrophytes KCTC 6077 (55.1 %).
Table 1. Antidermatophytic activity of essential oil (1000 ppm) of Lonicera japonica Thunb.
The solvent extraction process using the air-dried leaves (50 g) of L. japonica yielded ethanol extract (7.2 g), which was then suspended in water and extracted successively with hexane, chloroform and ethyl acetate to give hexane fraction (2.12 g), chloroform fraction (1.23 g), ethyl acetate fraction (1.41 g) and residual ethanol subfraction (1.11 g) respectively. Ethanol extract and its hexane, chloroform, ethyl acetate fractions (1500 ppm) also showed growth inhibition against some of the dermatophytes but not for all (Table 2(Tab. 2)).
Table 2. Antidermatophytic activity of ethanolic extracts (1500 ppm) of Lonicera japonica Thunb.
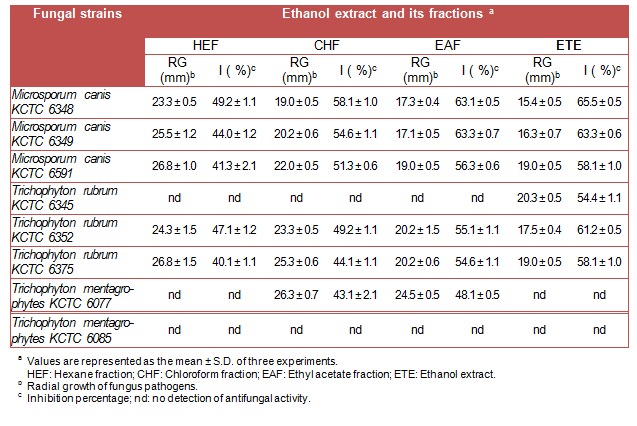
Ethanol extract showed a high potential antidermatophytic activity against M. canis KCTC 6348, M. canis KCTC 6349, M. canis KCTC 6591, T. rubrum KCTC 6345, T. rubrum KCTC 6352 and T. rubrum KCTC 6375 with inhibition of 54.4-65.5 %. Ethyl acetate fraction showed inhibition (48.1-63.3 %) against all tested pathogens except T. rubrum KCTC 6345 and T. mentagrophytes KCTC 6085. Chloroform and hexane fractions also showed moderate to weak inhibition against most of the dermatophytes with growth inhibition ranged from 40.1-58.1 %.
Minimum inhibitory concentration (MIC)
According to the results given in Table 1(Tab. 1), MIC of essential oil was found more effective against M. canis KCTC 6348 and 6349, and T. rubrum KCTC 6345 and 6352 (62.5-125 µg/ml) as compared to those of M. canis KCTC 6591, T. rubrum KCTC 6375 and T. mentagrophytes KCTC 6077 (250-500 µg/ml). On the other hand, ethanol extract and its ethyl acetate fraction were more susceptible than the hexane and chloroform fractions against the tested dermatophytes (Table 3(Tab. 3)).
Table 3. Minimum inhibitory concentration of ethanolic extracts of Lonicera japonica Thunb. against dermatophytic fungi.
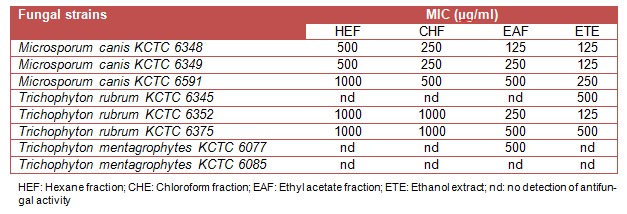
The ethanol extract displayed potent antidermatophytic activity against M. canis (KCTC 6348, 6349 and 6591) and T. rubrum KCTC (6345, 6352 and 6375) with MIC values of 125–500 µg/ml. Ethyl acetate fraction was also found to be effective (MIC: 125–500 µg/ml) against all pathogens except T. rubrum KCTC 6345 and T. mentagrophytes KCTC 6085. On the other hand, the MIC values of hexane and chloroform fractions against M. canis (KCTC 6348, 6349 and 6591) and T. rubrum KCTC (6352 and 6375) were found within the range of 250–1000 µg/ml, whereas no inhibition was observed against T. rubrum KCTC 6345 and T. mentagrophytes (KCTC 6077 and 6085).
Spore germination and growth kinetics assay
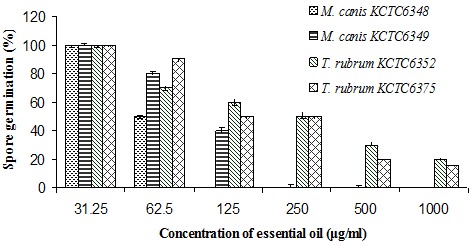
The results obtained for essential oil from the spore germination assay of each of the test fungi are shown in Figure 1(Fig. 1). DMSO (0.5 %, v/v) as a control did not inhibit the spore germination of any of the skin infectious fungal pathogens tested. There was a significant inhibition of fungal spore germination by different concentrations of essential oil. A 100 % inhibition of fungal spore germination was observed against M. canis KCTC 6348 and M. canis KCTC 6349 at 125 and 250 µg/ml concentrations of essential oil, respectively. Essential oil also exhibited a potent inhibitory effect on the spore germination of T. rubrum KCTC 6352 and T. rubrum KCTC 6375 in the range of 50–80 % at concentrations ranging from 250 to 500 µg/ml.
Figure 1. Effect of different concentrations (µg/ml) of the essential oil of Lonicera japonica Thunb. on spore germination of tested dermatophytes.
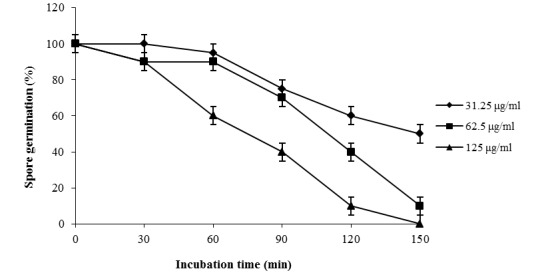
The antidermatophytic kinetics of the essential oil against M. canis KCTC 6348 is shown in Figure 2(Fig. 2). Exposure of M. canis KCTC 6348 spores to different concentrations of the essential oil for a period of 30–150 min caused varying degree of inhibition of spore germination. An increase in fungicidal activity was observed with the increase in exposure time and concentration. The essential oil at 31.25 µg/ml concentration showed antidermatophytic activity but not rapid killing and about 40 % inhibition was observed at exposure time of 120 min. However, there was a marked increase in the killing rate at 62.5 and 125 µg/ml after 30 min of exposure along with 85 % and 100 % inhibition of spore germination were observed on 150 min exposure, respectively. At low concentrations, significant rate of inhibition was the characteristic feature of the essential oil.
Figure 2. Figure 2: Kinetics of inhibition of Microsporum canis KCTC 6348 spores by the essential oil of Lonicera japonica Thunb.
Discussion
The increasing recognition of fungal infections caused by human infectious fungal pathogens means there is a constant striving to develop new antifungal agents. Research focused on plant-derived fungicides and their possible applications in pharmaceuticals, cosmetics and agriculture is being intensified as there is enormous potential to inspire and influence modern chemical methods. Essential oils and extracts from plant species have long been used for treatment of various diseases, including skin conditions, and there is at least some evidence that natural products may tend to have less deleterious side effects than corresponding synthetic drugs (Tavares et al. 2008[26]). Many essential oils and their constituents are found to exhibit antifungal properties, but the high cost of production of essential oils and the low concentration of active principles often prevent their direct use in the control of fungal diseases of animals and plants. In spite of this limitation, chemical investigation of antifungal compounds present in essential oils is considered important because of the possibility of synthesizing these compounds or their analogues which may be used in the control of fungal diseases.
The hydrodistillation of the flowers of L. japonica gave dark yellowish oil with the major components of the oil having oxygenated mono- and sesquiterpenes, and mono- and sesquiterpene hydrocarbons (Rahman & Kang, 2009[22]). In recent years, several researchers have reported that mono- and sesquiterpene hydrocarbons and their oxygenated derivatives are the major components of essential oils of plant origin, which have enormous potential to inhibit microbial pathogens (Cakir et al., 2004[4]). In general, the active antimicrobial compounds of essential oils are phenolic and alcoholic compounds such as eugenol, carvacrol, trans-nerolidol, phenyl ethyl alcohol and linalool; it would seem reasonable that their antifungal mode of action might be related to that of other compounds. Most of the studies on the working mechanism of phenolic compounds have focused on their effects on cellular membranes. In the recent years phenolic compounds have gained increasing interest because they exhibit beneficial health effects due to their potential pharmaceutical properties (Cai et al., 2004[3]).
In the present study, the essential oil of L. japonica showed remarkable antidermatophytic effect against all the tested human infectious fungal pathogens except T. mentagrophytes KCTC 6085. The antidermatophytic activity L. japonica essential oil could be contributed to the presence of some major components (e. g., trans-nerolidol, caryophyllene oxide, linalool, p-cymene, hexadecanoic acid, eugenol, geraniol, trans-linalooloxide, globulol, pentadecanoic acid, veridiflorol, benzyl alcohol and phenyl ethyl alcohol) and/or other minor components (e. g., citronellyl acetate, geranylacetone, hexahydrofarnesyl acetone, 1,8-cineole, α-cadinol and tetradecanoic acid) present in the oil (El-Sakhawy et al., 1998[8]; Melliou et al., 2007[15]; Vardar-Ünlü et al., 2008[27]).
Further, the antidermatophytic activity of ethanolic extracts could be attributed to the presence of some bioactive phenolic compounds (chlorogenic acid, luteolin, and protocatechuic acid) in L. japonica leaves and these findings are in agreement with the previous report (Chang and Hsu, 1992[6]). The antidermatophytic activity of EtOAc fraction could also be attributed to the presence of phytochemicals such as biflavonoids (3'-O-methyl loniflavone and loniflavone), luteolin and chrysin in L. japonica leaves, as evident by Kumar et al. (2005)[13]. Thus, it can be observed that phenolic compounds were abundant in leaves, while oxygenated sesquiterpenes, alcohols and phenolics were main constituents in flowers. Phenolic compounds such as chlorogenic acid, luteolin, and protocatechuic acid, 3'-O-methyl loniflavone and loniflavone were found in alcoholic extract and ethyl acetate fraction of L. japonica leaves (Chang and Hsu, 1992[6]; Kumar et al., 2005[13]) and recent studies have suggested that they may possess multiple therapeutic functions for various human diseases including liver cancer (Yip et al., 2006[29]).
This research work also described the complex effect of volatile oil on fungal spore germination and exhibited a wide range of antidermatophytic activity. During the kinetic study of M. canis KCTC 6348, it was appeared that exposure time of the essential oil had a little effect on the fungicidal activity at lower concentration but at the concentration of 62.5 and 125 µg/ml, the fungicidal action was very rapid and showed 85-100 % spore germination inhibition of M. canis KCTC 6348. This activity could be attributed to the presence of phenolic compound and oxygenated mono- and sesquiterpene as well as sesquiterpene hydrocarbons, and these finding are in agreement with the previous report (Bajpai et al., 2009[1]). Volatile compounds, such as trans-nerolidol, caryophyllene oxide, linalool, p-cymene, eugenol, veridiflorol and globulol have been claimed to contain the antifungal properties (Mockute and Judzentiene, 2003[17]; Omidbeygi et al., 2007[19]; Deba et al., 2008[7]). Those claims are further supported by our findings; indicating high contents of trans-nerolidol, caryophyllene oxide, linalool, p-cymene, eugenol, veridiflorol and globulol in L. japonica essential oil. Also, the antifungal activity of individual components of essential oils, such as caryophyllene oxide, trans-nerolidol and eugenol has been reported previously (Cakir et al., 2004[4]; Voda et al., 2003[28]). On the other hand, the components present in lower amounts, such as spathulenol, citronellyl acetate, α-cadinol and 1,8-cineole also contributed to antidermatophytic activity of the oil (Mockute and Judzentiene, 2003[17]; Salamci et al., 2007[24]). It is also possible that the minor components might be involved in some type of synergism with the other active compounds. Our research group also found similar inhibitory effect of Nandina domestica Thunb. essential oil against T. rubrum KCTC 6375 (Bajpai et al., 2009).
In conclusion, the floral essential oil and ethanolic leaf extracts of L. japonica possessed strong antidermatophytic activities. Therefore, it would also be interesting to study the effects of essential oil and extracts of L. japonica against other important fungi for developing new antifungal agents to control serious fungal infections in animal and human beings. Thus, L. japonica mediated oil and organic extracts could become an alternative to synthetic fungicides for using in pharmaceutical and cosmetic industries and also to screen and develop such novel types of selective and natural fungicides in the treatment of superficial fungal infections. A further study will evaluate the bioactive compounds present in ethanolic extracts of L. japonica.
Notes
Corresponding authors are Atiqur Rahman and Sun Chul Kang.
References
- 1.Bajpai VK, Yoon JI, Kang SC. Antifungal potential of essential oil and various organic extracts of Nandina domestica Thunb. against skin infectious fungal pathogens. Appl Microbiol Biotechnol. 2009;83:1127–33. doi: 10.1007/s00253-009-2017-5. [DOI] [PubMed] [Google Scholar]
- 2.Byun MW, Jo C, Jeon TW, Hong CH. Effects of gamma irradiation on color characteristics and biological activities of extracts of Lonicera japonica (Japanese honeysuckle) with methanol and acetone. Lebensm-Wiss Technol. 2004;37:29–33. [Google Scholar]
- 3.Cai YZ, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cakir A, Kordali S, Zengin H, Izumi S, Hirata T. Composition and antifungal activity of essential oils isolated from Hypericum hyssopifolium and Hypericum heterophyllum. Flav Fragr J. 2004;19:62–8. [Google Scholar]
- 5.Chang CW, Lin MT, Lee SS, Karin CS, Liu C, Hsu FL, et al. Differential inhibition of reverse transcriptase and cellular DNA polymerase-ce activities by lignans isolated from Chinese herbs, Phyllanthus myrtifolius Moon, and tannins from Lonicera japonica Thunb and Castanopsis hystrix. Antivir Res. 1995;27:367–374. doi: 10.1016/0166-3542(95)00020-m. [DOI] [PubMed] [Google Scholar]
- 6.Chang WC, Hsu FL. Inhibition of platelet activation and endothelial cell injury by polyphenolic compounds isolated from Lonicera japonica Thunb. Prostaglandins Leukot Essent Fatty Acids. 1992;45:307–12. doi: 10.1016/0952-3278(92)90088-z. [DOI] [PubMed] [Google Scholar]
- 7.Deba F, Xuan TD, Yasuda M, Tawata S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Cont. 2008;19:346–352. [Google Scholar]
- 8.El-Sakhawy FS, El-Tantawy ME, Ross SA, El-Sohly MA. Composition and antimicrobial activity of the essential oil of Murraya exotica L. Flav Fragr J. 1998;13:59–62. [Google Scholar]
- 9.Emmons CW, Binford CH, Utz JP, Kwon-Chung KJ. Medical mycology, 3rd ed. Philadelphia, PA: Lea and Febiger; 1977. pp. 117–167. [Google Scholar]
- 10.Fenner M, Sortinob SM, Ratesa R, Agnola S, Zacchino B. Antifungal activity of some Brazilian Hypericum species. Phytomedicine. 2005;12:236–40. doi: 10.1016/j.phymed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Irobi ON, Darambola SO. Antifungal activities of crude extract of Mitracarpus villosus (Rubiaceae) J Ethnopharmacol. 1993;40:137–140. doi: 10.1016/0378-8741(93)90059-e. [DOI] [PubMed] [Google Scholar]
- 12.Kakuda R, Imai M, Yaoita Y, Machida K, Kikuchi M. Secoiridoid glycosides from the flower buds of Lonicera japonica. Phytochemistry. 2000;55:879–81. doi: 10.1016/s0031-9422(00)00279-x. [DOI] [PubMed] [Google Scholar]
- 13.Kumar N, Singh B, Bhandari P, Gupta AP, Uniyal SK, Kaul VK. Biflavonoids from Lonicera japonica. Phytochemistry. 2005;66:2740–4. doi: 10.1016/j.phytochem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Leung HWC, Wu CH, Lin CH, Lee HZ. Luteolin induced DNA damage leading to human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol. 2005;508:77–83. doi: 10.1016/j.ejphar.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Melliou E, Stratis E, Chinou I. Volatile constituents of propolis from various regions of Greece – Antimicrobial activity. Food Chem. 2007;103:375–80. [Google Scholar]
- 16.Mitscher LA, Leu RP, Bathala MS, Wu WN, Beal JL, White R. Antimicrobial agents from higher plants. I. Introduction, rationale and methodology. Lloydia. 1972;35:157–166. [PubMed] [Google Scholar]
- 17.Mockute D, Judzentiene A. Variability of the essential oils composition of Achillea millefolium ssp. millefolium growing wild in Lithuania. Biochem Syst Ecol. 2003;31:1033–1045. [Google Scholar]
- 18.Nene YL, Thapliyal PN. Fungicides in plant disease control. New Delhi, India: Oxford and IBH Publishing Co; 1979. p. 413. [Google Scholar]
- 19.Omidbeygi M, Barzegar M, Hamidi Z, Hassanali NH. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Cont. 2007;18:1518–1523. [Google Scholar]
- 20.Peng LY, Mei SX, Jiang B, Zhou H, Sun HD. Constituents from Lonicera japonica. Fitoterapia. 2000;71:713–715. doi: 10.1016/s0367-326x(00)00212-4. [DOI] [PubMed] [Google Scholar]
- 21.Portillo A, Vila R, Freixa B, Adzet T, Canigueral S. Antifungal activity Paraguayan plant used in traditional medicine. J Ethnopharmacol. 2001;76:93–98. doi: 10.1016/s0378-8741(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 22.Rahman A, Kang SC. In vitro control of food-borne and food spoilage bacteria by essential oil and ethanol extracts of Lonicera japonica Thunb. Food Chem. 2009;116:670–675. [Google Scholar]
- 23.Rana BK, Singh UP, Taneja V. Antifungal activity and kinetics of inhibition by essential oil isolated from leaves of Aegle marmelos. J Ethnopharmacol. 1997;57:29–34. doi: 10.1016/s0378-8741(97)00044-5. [DOI] [PubMed] [Google Scholar]
- 24.Salamci E, Kordali S, Kotan R, Cakir A, Kaya Y. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem Syst Ecol. 2007;35:569–581. [Google Scholar]
- 25.Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol. 2007;117:112–9. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Tavares AC, Goncalves MJ, Cavaleira C, Cruz MT, Lopes MC, Canhoto J, et al. Essential oil of Daucus carota subsp. halophilus: composition, antifungal activity and cytotoxicity. J Ethnopharmacol. 2008;19:129–34. doi: 10.1016/j.jep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Vardar-Ünlü G, Silici S, Ünlü M. Composition and in vitro antimicrobial activity of Populus buds and poplar-type propolis. W J Microbiol Biotechnol. 2008;24:1011–7. [Google Scholar]
- 28.Voda K, Boh B, Vrtacnik M, Pohleven F. Effect of the antifungal activity of oxygenated aromatic essential oil compounds on the white-rot Trametes versicolor and the brown-rot Coniophora puteana. Int Biodeteriorat Biodegenerat. 2003;51:51–9. [Google Scholar]
- 29.Yip ECH, Chan ASL, Pang H, Tam YK, Wong YH. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol Toxicol. 2006;22:293–302. doi: 10.1007/s10565-006-0082-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Yang R, Liu CZ. Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep Purif Technol. 2008;62:480–3. [Google Scholar]


