Abstract
Purpose: Much work has been carried out to investigate the genetic and epigenetic basis of endometriosis and proposed that endometriosis has been described as an epigenetic disease. The purpose of this study was to extract the imprinting genes that are associated with endometriosis development.
Methods: The information on the imprinting genes can be accessed publicly from a web-based interface at http://www.geneimprint.com/site/genes-by-species.
Results: In the current version, the database contains 150 human imprinted genes derived from the literature. We searched gene functions and their roles in particular biological processes or events, such as development and pathogenesis of endometriosis. From the genomic imprinting database, we picked 10 genes that were highly associated with female reproduction; prominent among them were paternally expressed genes (DIRAS3, BMP8B, CYP1B1, ZFAT, IGF2, MIMT1, or MIR296) and maternally expressed genes (DVL1, FGFRL1, or CDKN1C). These imprinted genes may be associated with reproductive biology such as endometriosis, pregnancy loss, decidualization process and preeclampsia.
Discussion: This study supports the possibility that aberrant epigenetic dysregulation of specific imprinting genes may contribute to endometriosis predisposition.
Keywords: endometriosis, imprinting gene, pathogenesis
Introduction
Although endometriosis occurs in ~10 % of women of reproductive age and in ~50 % of women with infertility, the etiology is poorly understood. There is accumulating evidence supporting a concept that endometriosis is a disease associated with a genetic (Albertsen et al., 2013[2]; Xiao et al., 2010[90]) and also an epigenetic disorder (Izawa et al., 2013[36]; Nasu et al., 2011[60]; Guo, 2009[29]; Colón-Díaz et al., 2012[17]). Genetic mechanisms have been ascribed important roles in endometriosis (Albertsen et al., 2013[2]; Xiao et al., 2010[90]). Genetic and network-based pathway analysis of endometrial and endometriotic tissues revealed that the unique endometriosis susceptibility genes include genes encoding cell cycle, growth factors, signal transduction, transcription factors, hormones, cytokines, chemokines and (pro)inflammation, proteases, cell adhesion and motility, stress response and detoxification, immune response and metabolism (Khan et al., 2012[44]; Kobayashi et al., 2013[46]). Kobayashi et al. (2013[46]) recently showed the overlapping genetic signatures between endometriosis development and decidualization process, suggesting that insufficient decidualization may underline this disorder. Epigenetic alterations reported to date in endometriosis include the genomic DNA methylation of progesterone receptor (PGR)-B, E-cadherin (CDH1), homeobox A10 (HOXA10) (Cakmak and Taylor, 2011[12]), estrogen receptor-beta (ESR2), steroidogenic factor-1 (NR5A1), aromatase (CYP19A1) (Nasu et al., 2011[60]), histone deacetylase inhibition (HDACi) (Colón-Díaz et al., 2012[17]), CDKN2A/B (Kawano et al., 2011[43]), IGFBP-1 (Cakmak and Taylor, 2011[12]), leukemia inhibitory factor (LIF) (Cakmak and Taylor, 2011[12]) and DNA-methyltransferase (DNMTs) (Wu et al., 2007[89]).
There are no data, however, when and how the disruption of such epigenetic changes occurs. DNA methylation lies at the basis of genomic imprinting by epigenetic processes. The parentally imprinting-related epigenetic basis of endometriosis is poorly understood. Imprinted genes are expressed mainly from one parental allele due to an epigenetic mechanism while the other allele is inactivated. The paternally expressed / maternally imprinted genes such as insulin-like growth factor (IGF)-2 were related to promoting cell proliferation, differentiation and metabolism, and are involved in regulating placental size and birth weight (Haggarty et al., 2013[30]). Maternally expressed / paternally imprinted genes reduce the flow of resources to the fetus and are associated with fetal growth restriction, supporting the "parental conflict hypothesis".The previous studies have not yet provided convincing evidence for any susceptibility genomic imprinting genes of endometriosis.
In the present study, from the genomic imprinting database, we search for the first time the parentally imprinted genes that are reported to be involved in the reproductive process including endometriosis.
Materials and Methods
The genomic imprinting database is now freely accessible at http://www.geneimprint.com/site/what-is-imprinting.
This database search identified all the existing publications on the imprinting events. In the current version, the database contains 150 human imprinted genes, including information such as gene name, aliases, gene location and expressed allele. Particular emphasis was given on the imprinting genes associated with female reproduction, including endometriosis. Additional information was manually collected by keyword searches of the biomedical literature database PubMed (http://www.ncbi.nlm.nih.gov/pubmed).
Results
The genomic imprinting database contains 90 paternally expressed genes (Figure 1(Fig. 1)) and 60 maternally expressed genes (Figure 2(Fig. 2)). Supplementary data show biological functions of a total of 150 imprinted genes, with preferential expression from the paternal or maternal allele. Biological functions of each imprinting gene were manually searched by PubMed. By analyzing the cellular functions of these 150 imprinted genes, they play an essential role in
Figure 1. Paternally expressed/maternally imprinted genes.
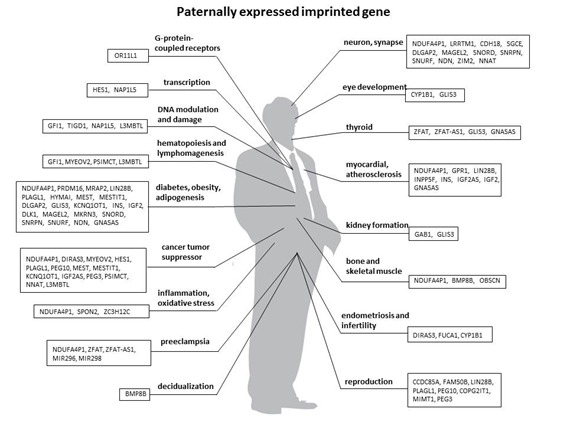
List of paternally expressed and maternally imprinted genes in humans. Imprinting gene information has been gathered from NCBI database (http://www.geneimprint.com/site/genes-by-species)
Figure 2. Maternally expressed/paternally imprinted genes.
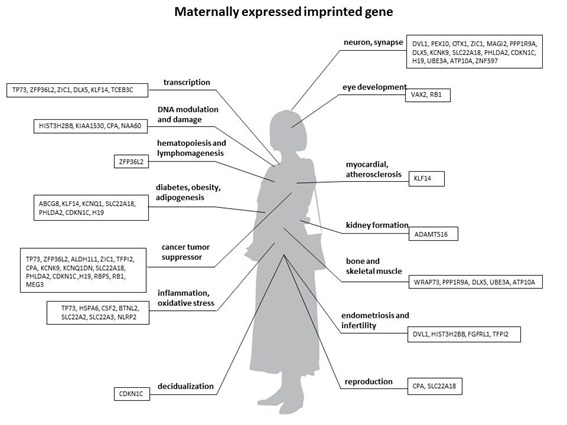
List of maternally expressed and mpaternally imprinted genes in humans. Imprinting gene information has been gathered from NCBI database (http://www.geneimprint.com/site/genes-by-species)
1. multiple metabolism pathways including
diabetes (Mackay and Temple, 2010[56]; El Hajj et al., 2013[22]; Santin and Eirizik, 2013[71]; Travers et al., 2013[82]; Hamed et al., 2012[31]),
hyperphagia (Zhang et al., 2012[95]),
adipogenesis (Hudak and Sul, 2013[35]),
obesity (Zhang et al., 2012[95]; Liu et al., 2013[52]; Do et al., 2013[20]),
atherosclerosis, cardiovascular disease, myocardial ischemia and reperfusion, hypertension (Karagiannis et al., 2013[42]; Small et al., 2011[74]; Zhu et al., 2009[98]),
Prader-Willi syndrome (Zhang et al., 2012[95]; Rodriguez-Jato et al., 2013[70]; Gallagher et al., 2002[26]; Rieusset et al., 2013[68]),
Angelman syndrome (Rodriguez-Jato et al., 2013[70]; Mabb et al., 2011[55]),
Wilms tumor (Jacobi et al., 2013[37]; Hubertus et al., 2013[34]; Xin et al., 2000[91]) and
Beckwith-Wiedemann syndrome (Zhang et al., 2012[95]; Rodriguez-Jato et al., 2013[70]; Gallagher et al., 2002[26]; Rieusset et al., 2013[68]; Mabb et al., 2011[55]; Hubertus et al., 2013[34]; Xin et al., 2000[91]
2. the emotional, social and neurological behavior and the appearance of certain neurodegenerative diseases such as schizophrenia, parkinsonism, Huntington disease, auitism, and mature synaptic function (Becanovic et al., 2010[6]; Gos, 2013[28]),
3. malignancies such as Wilms tumor, rhabdomyosarcoma, adrenocortical carcinoma, and lung, ovarian, and breast cancer (Jacobi et al., 2013[37]; Hubertus et al., 2013[34]; Xin et al., 2000[91]; Ozdemir, 2012[63]; Fernández Massó et al., 2013[24]; Zhong et al., 2012[96]; Kim et al., 2012[45]; Britschgi et al., 2013[10]; Ho et al., 2007[33]; Peltomäki and Bützow, 2011[66]),
4. female reproductive system including placental development, fetal growth, infertile, decidualization and endometrial function, menarche, puberty, pregnancy loss such as spontaneous miscarriages or fetal deaths, preeclampsia, endometriosis, sexual behaviors, and spermatogenesis (Albertsen et al., 2013[2]; Izawa et al. 2013[36]; Nasu et al., 2011[60]; Guo, 2009[29]; Colón-Díaz et al., 2012[17]; Khan et al., 2012[44]; Kobayashi et al., 2013[46]; Cakmak and Taylor, 2011[12]; Kawano et al., 2011[43]; Wu et al., 2007[89]; Peltomäki and Bützow, 2011[66]; He et al., 2004[32]; Li et al., 2011[48]; Brandelli and Passos, 1998[9]; Wetendorf and DeMayo, 2012[88]; Sonderegger et al., 2010[75]; Monteiro et al., 2014[58]; Tiberi et al., 2010[80]; Li and Wang, 2009[50]; Kang et al., 2010[41]; Choi et al., 2013[15]; Vinatier et al., 2000[85]; Nyholt et al., 2012[61]), and
5. other important spheres including
developmental (Jacobi et al., 2013[37]; Wetendorf and DeMayo, 2012[88]; Kajimura et al., 2010[39]; Matsumoto et al., 2006[57]; Yuen et al., 2011[94]; Bergman et al., 2013[7]; Lambertini et al., 2012[47]; Godfrey et al., 2011[27]),
regulatory (Hubertus et al., 2013[34]; Sturrock et al., 2013[77]),
transcriptional, G-protein signaling processes, inflammatory responses (Santin and Eirizik, 2013[71]; Arnett et al., 2007[3]; Liu et al., 2013[51]),
oxidative stress (Srinivasan and Avadhani, 2012[76]),
DNA replication and transcription (Nasu et al., 2011[60]; Haggarty et al., 2013[30]; El Hajj et al., 2013[22]; Gos, 2013[28]; Yuen et al., 2011[94]; Du et al., 2013[21]; Schwertman et al., 2013[73]; Tobi et al., 2011[81]; Calicchio et al., 2013[13]),
chromatin remodeling (Du et al., 2013[21]),
bone and skeletal diseases (Nakabayashi et al., 2004[59]),
muscle function (Karagiannis et al., 2013[42]; Nakabayashi et al., 2004[59]; Devaney et al., 2007[18]),
energy expenditure (Zhou et al., 2012[97]),
eye development and hypothyroidism (Figure 1(Fig. 1) and Figure 2(Fig. 2)).
Aberrant genomic imprinting is an important epigenetic process involved in regulating metabolic disease, the emotional and social behavior, malignancies, placental and fetal growth, reproductive disorders and other important biological processes in later life.
Among 150 differentially expressed parentally imprinted genes, we extracted ten genes that showed a reproductive biology in the set of imprinted genes in human. They include paternally expressed genes (DIRAS3 [DIRAS family, GTP-binding RAS-like 3], BMP8B [bone morphogenetic protein 8b], CYP1B1 [cytochrome P450, family 1, subfamily B, polypeptide 1], ZFAT [zinc finger and AT hook domain containing], IGF2 [insulin-like growth factor-2], MIMT1 [MER1 repeat containing imprinted transcript 1], or MIR296 [microRNA 296]) and maternally expressed genes (DVL1 [dishevelled segment polarity protein 1], FGFRL1 [fibroblast growth factor receptor-like 1], or CDKN1C [cyclin-dependent kinase inhibitor 1C (p57, Kip2)]) (Table 1(Tab. 1)). Biological functions of seven paternally expressed genes associated with female reproduction (Table 1(Tab. 1)):
Table 1. The imprinted gene candidates associated with the pathogenesis of endometriosis.
DIRAS3, DIRAS family, GTP-binding RAS-like 3
DIRAS3 is a member of the Ras superfamily, and appears to be a putative tumor suppressor gene (http://www.ncbi.nlm.nih.gov/gene/9077). Up-regulation of DIRAS3 expression is associated with infertility and endometriosis (Li et al., 2011[48]).
BMP8B, bone morphogenetic protein 8b
BMP is a part of the transforming growth factor-beta (TGFB) superfamily that induces ectopic bone growth (http://www.ncbi.nlm.nih.gov/gene/656). Progesterone-dependent Ihh, Wnt, and BMP signaling pathways within the endometrium play a role in decidualization, implantation and embryo attachment (Wetendorf and DeMayo, 2012[88]).
CYP1B1, cytochrome P450, family 1, subfamily B, polypeptide 1
The cytochrome P450 metabolizes procarcinogens and synthesizes cholesterol, steroids and other lipids (http://www.ncbi.nlm.nih.gov/gene/1545). This enzyme is involved in eye development. The gene polymorphisms of CYP1B1 in exon 2 codon 119 are also an associated risk factor for endometriosis (Li and Wang, 2009[50]).
ZFAT, zinc finger and AT hook domain containing
ZFAT-AS1, ZFAT antisense RNA 1
The encoded protein, ZFAT, is a DNA binding protein and functions as a transcriptional regulator involved in cell survival and apoptosis (http://www.ncbi.nlm.nih.gov/gene/57623). SNPs of the ZFAT gene are associated with an increased risk of autoimmune thyroid disease. ZFAT expressed in syncytiotrophoblasts is downregulated in placentas from preeclampsia (Barbaux et al., 2012[5]). The ZFAT-AS1 gene encodes a small antisense RNA that regulates the sense strand locus, ZFAT (http://www.ncbi.nlm.nih.gov/gene/594840)
IGF2, insulin-like growth factor 2 (somatomedin A)
The IGF2 gene is involved in cell proliferation, growth, migration, differentiation and survival. Epigenetic changes at this locus are associated with Wilms tumor, Beckwith-Wiedemann syndrome, rhabdomyosarcoma, Silver-Russell syndrome and cardiovascular disease (Bergman et al., 2013[7]) (http://www.ncbi.nlm.nih.gov/gene/3481). IGF2 gene was among the most regulated genes in endometriosis.
MIMT1, MER1 repeat containing imprinted transcript 1
MIMT1 is a non-protein coding gene that forms part of the imprinted PEG3 (paternally expressed gene 3) domain. Loss of paternal MIMT1 expression results in the phenotype of late term abortion and stillbirth in cattle (Flisikowski et al., 2010[25]).
MIR296, microRNA 296
Several miRNAs including MIR296 are found to be dysregulated in placenta of preeclampsia patients (Choi et al., 2013[15]). MIR296 is important for the pathogenesis of preeclampsia (Choi et al., 2013[15];. MIR296 lies within the GNASAS transcription units (Robson et al., 2012[69]). DNA methylation of GNASAS gene might be associated with small for gestational age and myocardial infarction among women (Tobi et al., 2011[81]).
Biological functions of three maternally expressed genes associated with female reproduction (Table 1)
DVL1, disheveled segment polarity protein 1
Wnt stimulation induces recruitment of DVL to the G-protein coupled frizzled (FZD) receptors (Kawano et al., 2011[43]). DVL plays a key role in relaying cellular information for several developmental pathways such as cell proliferation, migration, polarity, terminal differentiation, and the self-renewal of stem cells (Dillman et al., 2013[19]). DVL1 encodes a cytoplasmic phosphoprotein and is a substrate of NR1I2 (nuclear receptor subfamily 1, group I, member 2), which is a family of serine / threonine kinases that have been associated with differentiation of epithelial and neuronal cells (http://www.ncbi.nlm.nih.gov/gene/1855) (Elbert et al., 2006[23]). Charcot-Marie-Tooth disease has been mapped to the same region as DVL1. This disease is the hereditary neuropathy characterized by muscular atrophy and weakness in the distal parts of the legs (Ostern et al., 2013[62]). Failures in Wnt signalling are a cause of infertility and endometriosis (Sonderegger et al., 2010[75]).
FGFRL1, fibroblast growth factor receptor-like 1
FGFRL1 influences mitogenesis and differentiation (http://www.ncbi.nlm.nih.gov/gene/53834). The FGF2 754C/G polymorphism may be closely associated with a risk of developing endometriosis (Kang et al., 2010[41]). This gene stimulates cell proliferation at the ectopic endometriotic site.
CDKN1C, cyclin-dependent kinase inhibitor 1C (p57, Kip2)
The encoded protein is a strong G1 cyclin/Cdk-dependent inhibitor and a negative regulator of cell proliferation, suggesting a tumor suppressor candidate (http://www.ncbi.nlm.nih.gov/gene/1028). Mutations in this gene are implicated in sporadic cancers and Beckwith-Wiedemann syndorome. CDKN1C plays a role in endometrial stromal cell differentiation in the process of decidualization (Qian et al., 2005[67]).
These ten genes are mainly associated with the control of resource usage and reproductive biology such as not only endometriosis, but also abortion, stillbirth, infertility, decidualization, preeclampsia, metabolic syndrome, diabetes, coronary heart disease, eye development, autoimmune thyroid disease, tumor suppression, Wilms tumor, Beckwith-Wiedemann syndrome, rhabdomyosarcoma, Silver-Russell syndrome and Charcot-Marie-Tooth disease (supplementary data).
Discussion
Using the genomic imprinting database, we identified 10 imprinted genes, of which 7 were paternally expressed, and found that these genes are associated with female reproductive functions, including decidualization (BMP8B and CDKN1C), implantation (BMP8B), embryo attachment (BMP8B), abortion (MIMT1), stillbirth (MIMT1 and MIR296), preeclampsia (ZFAT), infertility (DIRAS3 and DVL1) and endometriosis (DIRAS3, CYP1B1, IGF2, DVL1 and FGFRL1). These parentally imprinted genes are necessary for female reproductive system such as normal endometrial development, decidualization, placentation and endometriosis. As described previously, the endometriosis susceptibility genes include growth factors (DIRAS3, IGF2 and FGFRL1), Wnt signal transduction (DVL1), metabolism (CYP1B1), and stress response and detoxification (CYP1B1) (Khan et al., 2012[44]; Kobayashi et al., 2013[46]). Some biological aspects of endometriosis may be explained from a dysregulation of parentally imprinted gene.
Recent advances in sequencing, profiling and pathway technologies allow genome-scale approaches to endometriosis-susceptibility gene discovery, which enables us to look for evidence in support of the genetic hypothesis (Albertsen et al., 2013[2]; Khan et al., 2012[44]; Nyholt et al., 2012[61]; Yuen et al., 2011[94]). Endometriosis is also thought to be an epigenetic disease (Izawa et al., 2013[36]; Nasu et al., 2011[60]; Guo, 2009[29]; Colón-Díaz et al., 2012[17]; Kobayashi et al., 2013[46]; Kawano et al., 2011[43]; Wu et al., 2007[89]; Calicchio et al., 2013[13]). The previous study has shed new light on the overlapping genetic and epigenetic signatures between endometriosis development and insufficient decidualization process, indicating that a number of genes are essential for the decidualization and implantation processes, but up-regulation of a small number of them, including IGF, IGFBP, PRL, HOXA10, FOXO1, C/EBPbeta, IL11 and LIF, are important for this process (Kobayashi et al., 2013[46]). Downregulation of the specific genes related to embryogenesis (the downstream targets of HOXA10) and immuno-endocrine behavior (IL11, LIF, TGF-beta, FKBP4, COX2, PGs, FOXO1 and C/EBPbeta) might appear critical to the development of endometriosis (Izawa et al., 2013[36]; Nasu et al., 2011[60]; Guo, 2009[29]; Colón-Díaz et al., 2012[17]; Khan et al. 2012[44]; Kobayashi et al., 2013[46]; Kawano et al., 2011[43]; Wu et al., 2007[89]; Peltomäki and Bützow, 2011[66]; Tiberi et al., 2010[80]; Vinatier et al., 2000; Nyholt et al., 2012; Calicchio et al., 2013;). Kobayashi et al. (2013) reported that the upregulated genes in endometriosis may evolve for the benefit of the endometrial growth, whereas the downregulated genes evolve as a protective mechanism for the endometrial decidualization. The irreversible programming or epigenetics may cause insufficient decidualization, which in turn results in infertility and endometriosis.
Previous studies identified several susceptibility genes that have highlighted the important role of endometriosis development, including IGF, IGFBP, PRL, HOXA10, FOXO1, C/EBPbeta, IL11, LIF, TGF-beta, FKBP4, COX2, and prostaglandins (Izawa et al., 2013; Nasu et al., 2011; Guo, 2009; Colón-Díaz et al., 2012[17]; Khan et al. 2012[44]; Kobayashi et al., 2013[46]; Kawano et al., 2011[43]; Wu et al., 2007[89]; Peltomäki and Bützow, 2011[66]; Tiberi et al., 2010[80]; Vinatier et al., 2000[85]; Nyholt et al., 2012[61]; Calicchio et al., 2013[13]). We tried to summarize the recent literature that supports a direct or indirect relationship between the novel candidate imprinting genes and the previously reported endometriosis susceptibility genes.
Epigenetic changes induced by various environmental stress factors including nutrition or ecosystem components play a role in interactions between exposed species and chemicals. Chemicals such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and benzo(a)pyrene modulate the mRNA levels of CYP1B1, C/EBPbeta, IL11 and PRL (Cao et al., 2011[14]; Vogel and Matsumura, 2013[86]; Ueng et al., 2005[84]). CYP1B1 is responsible for tumor progression in estrogen receptor-positive breast and endometrial cancers via estrogen metabolism. Allelic polymorphisms at codons 119 and 432 of CYP1B1 gene increases the risk of estrogen-dependent cancer (Sasaki et al., 2003[72]). CYP1B1, IGF2 and HOXA10 genes are reported to be hypermethylated in breast and gastric cancer (Kang et al., 2008[40]; Park et al., 2012[64]). CYP1B1 is transcriptionally regulated by steroidogenic factor-1 (SF-1) (Tsuchiya et al., 2006[83]) or PGE2, the main product of COX-2 (Yuan et al., 2012[93]). CYP1B1 reduces expression of CDH1 or IGFBP1 (Achary et al., 2000[1]; Collins et al., 2009[16]). Aromatase (CYP19A1) mRNA expression is stimulated by IGF2. ESR2 and Smads, downstream signaling cascades of TGF-beta, participates in the establishment of parent-of-origin-specific expression of IGF2 (Pathak et al., 2009[65]; Szabó et al., 2004[78]; Bergström et al., 2010[8]). IGF2 induced through the transactivation of C/EBPbeta is involved in PRL signaling (Tao et al., 2013[79]; Wang et al., 2011[87]). LIF inhibits the glial cell-derived neurotrophic factor (GDNF)-dependent alteration of the genomic imprinting of Igf2 in mice (Jung et al., 2010[38]). There is bidirectional regulation of insulin receptor signaling and FOXO1 (Liu et al., 2007[53]). DVL1 is a downstream molecule of Wnt signaling (Li et al., 2013[49]). DIRAS3 modulates estrogen and progesterone receptor expression and inhibits PRL-induced mammary gland development and lactation, which results in decreased fertility (Xu et al., 2000[92]). DIRAS3 also induces CDH1 expression and acts as a tumor suppressor gene (Lyu et al., 2013[54]). Taken together, ESR2 and TGF-beta downstream targets, Smads, co-localize to the IGF2 imprinting control region (Bergström et al., 2010[8]).
No convincing evidence has been provided to suggest that these imprinting genes would control the endometriosis susceptibility genes, although some indirect indications are available. At this time, no attempt was made to ascertain whether any of these 10 genes had a “driver” role or had a role of merely a by-stander in the development of endometriosis. Since there are no known driver genes in endometriosis, complex genomic alterations may be responsible for the endometriosis phenotype. The quest for driver imprinting genes or complex genomic alterations can now open new avenues to better understand the mechanisms of endometriosis development.
In conclusion, this study supports the possibility that aberrant epigenetic dysregulation of specific imprinting genes may contribute to endometriosis predisposition. Further investigations are needed to provide biological evidence for the direct association between the novel candidate imprinting genes and the previously reported endometriosis susceptibility genes.
Acknowledgements
The present review was supported by grant-in-aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan to the Department of Obstetrics and Gynecology, Nara Medical University (to H.K.).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
References
- 1.Achary MP, Jaggernauth W, Gross E, Alfieri A, Klinger HP, Vikram B. Cell lines from the same cervical carcinoma but with different radiosensitivities exhibit different cDNA microarray patterns of gene expression. Cytogenet Cell Genet. 2000;91:39–43. doi: 10.1159/000056815. [DOI] [PubMed] [Google Scholar]
- 2.Albertsen HM, Chettier R, Farrington P, Ward K. Genome-wide association study link novel loci to endometriosis. PLoS One. 2013;8:e58257. doi: 10.1371/journal.pone.0058257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnett HA, Escobar SS, Gonzalez-Suarez E, Budelsky AL, Steffen LA, Boiani N, et al. BTNL2, a butyrophilin/B7-like molecule, is a negative costimulatory molecule modulated in intestinal inflammation. J Immunol. 2007;178:1523–1533. doi: 10.4049/jimmunol.178.3.1523. [DOI] [PubMed] [Google Scholar]
- 4.Balsa E, Marco R, Perales-Clemente E, Szklarczyk R, Calvo E, Landázuri MO, et al. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 2012;16:378–386. doi: 10.1016/j.cmet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Barbaux S, Gascoin-Lachambre G, Buffat C, Monnier P, Mondon F, Tonanny MB, et al. A genome-wide approach reveals novel imprinted genes expressed in the human placenta. Epigenetics. 2012;7:1079–1090. doi: 10.4161/epi.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becanovic K, Pouladi MA, Lim RS, Kuhn A, Pavlidis P, Luthi-Carter R, et al. Transcriptional changes in Huntington disease identified using genome-wide expression profiling and cross-platform analysis. Hum Mol Genet. 2010;19:1438–1452. doi: 10.1093/hmg/ddq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman D, Halje M, Nordin M, Engström W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology. 2013;59:240–249. doi: 10.1159/000343995. [DOI] [PubMed] [Google Scholar]
- 8.Bergström R, Savary K, Morén A, Guibert S, Heldin CH, Ohlsson R, et al. Transforming growth factor beta promotes complexes between Smad proteins and the CCCTC-binding factor on the H19 imprinting control region chromatin. J Biol Chem. 2010;285:19727–19737. doi: 10.1074/jbc.M109.088385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandelli A, Passos EP. Glycosidases in the peritoneal fluid from infertile women with and without endometriosis. Clin Biochem. 1998;31:181–186. doi: 10.1016/s0009-9120(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 10.Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S, et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA. 2013;110:E1026–E1034. doi: 10.1073/pnas.1217072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger H, Zoumaro-Djayoon A, Boersma AW, Helleman J, Berns EM, Mathijssen RH, et al. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2) Br J Pharmacol. 2010;159:898–908. doi: 10.1111/j.1476-5381.2009.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17:242–253. doi: 10.1093/humupd/dmq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calicchio R, Doridot L, Miralles F, Méhats C, Vaiman D. DNA methylation, an epigenetic mode of gene expression regulation in reproductive science. Curr Pharm Des. 2013 Jul. Curr Pharm Des. 2013 Jul 19;:[Epub ahead of print]. doi: 10.2174/13816128113199990517. [DOI] [PubMed] [Google Scholar]
- 14.Cao J, Patisaul HB, Petersen SL. Aryl hydrocarbon receptor activation in lactotropes and gonadotropes interferes with estradiol-dependent and -independent preprolactin, glycoprotein alpha and luteinizing hormone beta gene expression. Mol Cell Endocrinol. 2011;333:151–159. doi: 10.1016/j.mce.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi SY, Yun J, Lee OJ, Han HS, Yeo MK, Lee MA, et al. MicroRNA expression profiles in placenta with severe preeclampsia using a PNA-based microarray. Placenta. 2013;34:799–804. doi: 10.1016/j.placenta.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Collins LL, Lew BJ, Lawrence BP. TCDD exposure disrupts mammary epithelial cell differentiation and function. Reprod Toxicol. 2009;28:11–17. doi: 10.1016/j.reprotox.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colón-Díaz M, Báez-Vega P, García M, Ruiz A, Monteiro JB, Fourquet J, et al. HDAC1 and HDAC2 are differentially expressed in endometriosis. Reprod Sci. 2012;19:483–492. doi: 10.1177/1933719111432870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devaney JM, Hoffman EP, Gordish-Dressman H, Kearns A, Zambraski E, Clarkson PM. IGF-II gene region polymorphisms related to exertional muscle damage. J Appl Physiol. 2007;102:1815–1823. doi: 10.1152/japplphysiol.01165.2006. [DOI] [PubMed] [Google Scholar]
- 19.Dillman AR, Minor PJ, Sternberg PW. Origin and evolution of dishevelled. G3 (Bethesda) 2013;3:251–262. doi: 10.1534/g3.112.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do DN, Strathe AB, Ostersen T, Jensen J, Mark T, Kadarmideen HN. Genome-wide association study reveals genetic architecture of eating behavior in pigs and its implications for humans obesity by comparative mapping. PLoS One. 2013;8:e71509. doi: 10.1371/journal.pone.0071509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du P, Tang F, Qiu Y, Dong F. GFI1 is repressed by p53 and inhibits chromatin damage induced apoptosis. PLoS One. 2013;8:e73542. doi: 10.1371/journal.pone.0073542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Hajj N, Pliushch G, Schneider E, Dittrich M, Müller T, Korenkov M, et al. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes. 2013;62:1320–1328. doi: 10.2337/db12-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elbert M, Cohen D, Müsch A. PAR1b promotes cell-cell adhesion and inhibits dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3345–3355. doi: 10.1091/mbc.E06-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández Massó JR, Oliva Argüelles B, Tejeda Y, Astrada S, Garay H, Reyes O, et al. The antitumor peptide CIGB-552 increases COMMD1 and inhibits growth of human lung cancer cells. J Amino Acids. 2013;2013:251398. doi: 10.1155/2013/251398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flisikowski K, Venhoranta H, Nowacka-Woszuk J, McKay SD, Flyckt A, Taponen J, et al. A novel mutation in the maternally imprinted PEG3 domain results in a loss of MIMT1 expression and causes abortions and stillbirths in cattle (Bos taurus) PLoS One. 2010;5:e15116. doi: 10.1371/journal.pone.0015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher RC, Pils B, Albalwi M, Francke U. Evidence for the role of PWCR1/HBII-85 C/D box small nucleolar RNAs in Prader-Willi syndrome. Am J Hum Genet. 2002;71:669–678. doi: 10.1086/342408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godfrey KM, Inskip HM, Hanson MA. The long-term effects of prenatal development on growth and metabolism. Semin Reprod Med. 2011;29:257–265. doi: 10.1055/s-0031-1275518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gos M. Epigenetic mechanisms of gene expression regulation in neurological diseases. Acta Neurobiol Exp. 2013;73:19–37. doi: 10.55782/ane-2013-1919. [DOI] [PubMed] [Google Scholar]
- 29.Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15:587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- 30.Haggarty P, Hoad G, Horgan GW, Campbell DM. DNA methyltransferase candidate polymorphisms, imprinting methylation, and birth outcome. PLoS One. 2013;8:e68896. doi: 10.1371/journal.pone.0068896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamed M, Ismael S, Paulsen M, Helms V. Cellular functions of genetically imprinted genes in human and mouse as annotated in the gene ontology. PLoS One. 2012;7:e50285. doi: 10.1371/journal.pone.0050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Wang Z, Sun Y. Reduced amount of cytochrome c oxidase subunit I messenger RNA in placentas from pregnancies complicated by preeclampsia. Acta Obstet Gynecol Scand. 2004;83:144–148. doi: 10.1111/j.0001-6349.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- 33.Ho JC, Cheung ST, Poon WS, Lee YT, Ng IO, Fan ST. Down-regulation of retinol binding protein 5 is associated with aggressive tumor features in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2007;133:929–936. doi: 10.1007/s00432-007-0230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hubertus J, Zitzmann F, Trippel F, Müller-Höcker J, Stehr M, von Schweinitz D, et al. Selective methylation of CpGs at regulatory binding sites controls NNAT expression in Wilms tumors. PLoS One. 2013;8:e67605. doi: 10.1371/journal.pone.0067605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudak CS, Sul HS. Pref-1, a gatekeeper of adipogenesis. Front Endocrinol (Lausanne) 2013;4:79. doi: 10.3389/fendo.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izawa M, Taniguchi F, Terakawa N, Harada T. Epigenetic aberration of gene expression in endometriosis. Front Biosci. 2013;5:900–910. doi: 10.2741/e669. [DOI] [PubMed] [Google Scholar]
- 37.Jacobi CL, Rudigier LJ, Scholz H, Kirschner KM. Transcriptional regulation by the Wilms tumor protein, Wt1, suggests a role of the metalloproteinase Adamts16 in murine genitourinary development. J Biol Chem. 2013;288:18811–18824. doi: 10.1074/jbc.M113.464644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung YH, Gupta MK, Oh SH, Uhm SJ, Lee HT. Glial cell line-derived neurotrophic factor alters the growth characteristics and genomic imprinting of mouse multipotent adult germline stem cells. Exp Cell Res. 2010;316:747–761. doi: 10.1016/j.yexcr.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang GH, Lee S, Cho NY, Gandamihardja T, Long TI, Weisenberger DJ, et al. DNA methylation profiles of gastric carcinoma characterized by quantitative DNA methylation analysis. Lab Invest. 2008;88:161–170. doi: 10.1038/labinvest.3700707. [DOI] [PubMed] [Google Scholar]
- 41.Kang S, Li SZ, Wang N, Zhou RM, Wang T, Wang DJ, et al. Association between genetic polymorphisms in fibroblast growth factor (FGF)1 and FGF2 and risk of endometriosis and adenomyosis in Chinese women. Hum Reprod. 2010;25:1806–1811. doi: 10.1093/humrep/deq128. [DOI] [PubMed] [Google Scholar]
- 42.Karagiannis GS, Weile J, Bader GD, Minta J. Integrative pathway dissection of molecular mechanisms of moxLDL-induced vascular smooth muscle phenotype transformation. BMC Cardiovasc Disord. 2013;13:4. doi: 10.1186/1471-2261-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawano Y, Nasu K, Li H, Tsuno A, Abe W, Takai N, Narahara H. Application of the histone deacetylase inhibitors for the treatment of endometriosis: histone modifications as pathogenesis and novel therapeutic target. Hum Reprod. 2011;26:2486–2498. doi: 10.1093/humrep/der203. [DOI] [PubMed] [Google Scholar]
- 44.Khan MA, Sengupta J, Mittal S, Ghosh D. Genome-wide expressions in autologous eutopic and ectopic endometrium of fertile women with endometriosis. Reprod Biol Endocrinol. 2012;10:84. doi: 10.1186/1477-7827-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JW, Kim ST, Turner AR, Young T, Smith S, Liu W, et al. Identification of new differentially methylated genes that have potential functional consequences in prostate cancer. PLoS One. 2012;7:e48455. doi: 10.1371/journal.pone.0048455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi H, Uekuri C, Shigetomi H. Towards an understanding of the molecular mechanism of endometriosis: unbalancing epithelial-stromal genetic conflict. Gynecol Endocrinol. 2013 Sep 03;:epub ahead of print. doi: 10.3109/09513590.2013.831832. [DOI] [PubMed] [Google Scholar]
- 47.Lambertini L, Marsit CJ, Sharma P, Maccani M, Ma Y, Hu J, et al. Imprinted gene expression in fetal growth and development. Placenta. 2012;33:480–486. doi: 10.1016/j.placenta.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Wang SJ, Sun L, Li YL. Differential expression of the anti-oncogene ARHI between patients with and without endometriosis. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:796–800. [PubMed] [Google Scholar]
- 49.Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T, et al. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/β-catenin signal network. Cancer Lett. 2013;336:379–389. doi: 10.1016/j.canlet.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 50.Li YG, Wang X. Association of the CYP1B1 gene polymorphism with susceptibility to endometriosis. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2009;26:66–69. doi: 10.3760/cma.j.issn.1003-9406.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Zhou Z, Huang S, Guo Y, Fan Y, Zhang J, et al. Zc3h12c inhibits vascular inflammation by repressing NF-κB activation and pro-inflammatory gene expression in endothelial cells. Biochem J. 2013;451:55–60. doi: 10.1042/BJ20130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu T, Elmquist JK, Williams KW. Mrap2: an accessory protein linked to obesity. Cell Metab. 2013;18:309–311. doi: 10.1016/j.cmet.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu TJ, Lai HC, Ting CT, Wang PH. Bidirectional regulation of upstream IGF-I/insulin receptor signaling and downstream FOXO1 in cardiomyocytes. J Endocrinol. 2007;192:149–158. doi: 10.1677/joe.1.07020. [DOI] [PubMed] [Google Scholar]
- 54.Lyu T, Jia N, Wang J, Yan X, Yu Y, Lu Z, et al. Expression and epigenetic regulation of angiogenesis-related factors during dormancy and recurrent growth of ovarian carcinoma. Epigenetics. 2013;8:1330–1346. doi: 10.4161/epi.26675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mabb AM, Judson MC, Zylka MJ, Philpot BD. Angelman syndrome: insights into genomic imprinting and neurodevelopmental phenotypes. Trends Neurosci. 2011;34:293–303. doi: 10.1016/j.tins.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mackay DJ, Temple IK. Transient neonatal diabetes mellitus type 1. Am J Med Genet C Semin Med Genet. 2010;154C:335–342. doi: 10.1002/ajmg.c.30272. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monteiro JB, Colón-Díaz M, García M, Gutierrez S, Colón M, Seto E, et al. Endometriosis is characterized by a distinct pattern of histone 3 and histone 4 lysine modifications. Reprod Sci. 2014;21:305–318. doi: 10.1177/1933719113497267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakabayashi K, Makino S, Minagawa S, Smith AC, Bamforth JS, Stanier P, et al. Genomic imprinting of PPP1R9A encoding neurabin I in skeletal muscle and extra-embryonic tissues. J Med Genet. 2004;41:601–608. doi: 10.1136/jmg.2003.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasu K, Kawano Y, Tsukamoto Y, Takano M, Takai N, Li H, et al. Aberrant DNA methylation status of endometriosis: epigenetics as the pathogenesis, biomarker and therapeutic target. J Obstet Gynaecol Res. 2011;37:683–695. doi: 10.1111/j.1447-0756.2011.01663.x. [DOI] [PubMed] [Google Scholar]
- 61.Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 2012;44:1355–1359. doi: 10.1038/ng.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostern R, Fagerheim T, Hjellnes H, Nygård B, Mellgren SI, Nilssen O. Diagnostic laboratory testing for Charcot Marie Tooth disease (CMT): the spectrum of gene defects in Norwegian patients with CMT and its implications for future genetic test strategies. BMC Med Genet. 2013;14:94. doi: 10.1186/1471-2350-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ozdemir F, Altinisik J, Karateke A, Coksuer H, Buyru N. Methylation of tumor suppressor genes in ovarian cancer. Exp Ther Med. 2012;4:1092–1096. doi: 10.3892/etm.2012.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SY, Kwon HJ, Choi Y, Lee HE, Kim SW, Kim JH, et al. Distinct patterns of promoter CpG island methylation of breast cancer subtypes are associated with stem cell phenotypes. Mod Pathol. 2012;25:185–196. doi: 10.1038/modpathol.2011.160. [DOI] [PubMed] [Google Scholar]
- 65.Pathak S, D'Souza R, Ankolkar M, Gaonkar R, Balasinor NH. Potential role of estrogen in regulation of the insulin-like growth factor2-H19 locus in the rat testis. Mol Cell Endocrinol. 2009;314:110–117. doi: 10.1016/j.mce.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Peltomäki P, Bützow R. Pathogenesis of endometriosis and its relationship to gynecological cancers. Epigenomics. 2011;3:689–690. doi: 10.2217/epi.11.96. [DOI] [PubMed] [Google Scholar]
- 67.Qian K, Chen H, Wei Y, Hu J, Zhu G. Differentiation of endometrial stromal cells in vitro: down-regulation of suppression of the cell cycle inhibitor p57 by HOXA10? Mol Hum Reprod. 2005;11:245–251. doi: 10.1093/molehr/gah147. [DOI] [PubMed] [Google Scholar]
- 68.Rieusset A, Schaller F, Unmehopa U, Matarazzo V, Watrin F, Linke M, et al. Stochastic loss of silencing of the imprinted Ndn/NDN allele, in a mouse model and humans with prader-willi syndrome, has functional consequences. PLoS Genet. 2013;9:e1003752. doi: 10.1371/journal.pgen.1003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robson JE, Eaton SA, Underhill P, Williams D, Peters J. MicroRNAs 296 and 298 are imprinted and part of the GNAS/Gnas cluster and miR-296 targets IKBKE and Tmed9. RNA. 2012;18:135–144. doi: 10.1261/rna.029561.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodriguez-Jato S, Shan J, Khadake J, Heggestad AD, Ma X, Johnstone KA, et al. Regulatory elements associated with paternally-expressed genes in the imprinted murine Angelman/Prader-Willi syndrome domain. PLoS One. 2013;8:e52390. doi: 10.1371/journal.pone.0052390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santin I, Eizirik DL. Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and β-cell apoptosis. Diabetes Obes Metab. 2013;15(Suppl 3):71–81. doi: 10.1111/dom.12162. [DOI] [PubMed] [Google Scholar]
- 72.Sasaki M, Tanaka Y, Kaneuchi M, Sakuragi N, Dahiya R. CYP1B1 gene polymorphisms have higher risk for endometrial cancer, and positive correlations with estrogen receptor alpha and estrogen receptor beta expressions. Cancer Res. 2003;63:3913–3918. [PubMed] [Google Scholar]
- 73.Schwertman P, Vermeulen W, Marteijn JA. UVSSA and USP7, a new couple in transcription-coupled DNA repair. Chromosoma. 2013;122:275–284. doi: 10.1007/s00412-013-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Small KS, Hedman AK, Grundberg E, Nica AC, Thorleifsson G, Kong A, et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat Genet. 2011;43:561–564. doi: 10.1038/ng.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sonderegger S, Pollheimer J, Knöfler M. Wnt signalling in implantation, decidualisation and placental differentiation - review. Placenta. 2010;31:839–847. doi: 10.1016/j.placenta.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med. 2012;53:1252–1263. doi: 10.1016/j.freeradbiomed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sturrock M, Hellander A, Matzavinos A, Chaplain MA. Spatial stochastic modelling of the Hes1 gene regulatory network: intrinsic noise can explain heterogeneity in embryonic stem cell differentiation. J R Soc Interface. 2013;10:20120988. doi: 10.1098/rsif.2012.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szabó PE, Pfeifer GP, Mann JR. Parent-of-origin-specific binding of nuclear hormone receptor complexes in the H19-Igf2 imprinting control region. Mol Cell Biol. 2004;24:4858–4868. doi: 10.1128/MCB.24.11.4858-4868.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tao S, Connor EE, Bubolz JW, Thompson IM, do Amaral BC, Hayen MJ, et al. Effect of heat stress during the dry period on gene expression in mammary tissue and peripheral blood mononuclear cells. J Dairy Sci. 2013;96:378–383. doi: 10.3168/jds.2012-5811. [DOI] [PubMed] [Google Scholar]
- 80.Tiberi F, Tropea A, Romani F, Apa R, Marana R, Lanzone A. Prokineticin 1, homeobox A10, and progesterone receptor messenger ribonucleic acid expression in primary cultures of endometrial stromal cells isolated from endometrium of healthy women and from eutopic endometrium of women with endometriosis. Fertil Steril. 2010;94:2558–2563. doi: 10.1016/j.fertnstert.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 81.Tobi EW, Heijmans BT, Kremer D, Putter H, Delemarre-van de Waal HA, Finken MJ, et al. DNA methylation of IGF2, GNASAS, INSIGF and LEP and being born small for gestational age. Epigenetics. 2011;6:171–176. doi: 10.4161/epi.6.2.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Travers ME, Mackay DJ, Dekker Nitert M, Morris AP, Lindgren CM, Berry A, et al. Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes. 2013;62:987–992. doi: 10.2337/db12-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsuchiya Y, Nakajima M, Takagi S, Katoh M, Zheng W, Jefcoate CR, et al. Binding of steroidogenic factor-1 to the regulatory region might not be critical for transcriptional regulation of the human CYP1B1 gene. J Biochem. 2006;139:527–534. doi: 10.1093/jb/mvj055. [DOI] [PubMed] [Google Scholar]
- 84.Ueng TH, Hung CC, Kuo ML, Chan PK, Hu SH, Yang PC, et al. Induction of fibroblast growth factor-9 and interleukin-1alpha gene expression by motorcycle exhaust particulate extracts and benzo(a)¬pyrene in human lung adenocarcinoma cells. Toxicol Sci. 2005;87:483–496. doi: 10.1093/toxsci/kfi251. [DOI] [PubMed] [Google Scholar]
- 85.Vinatier D, Cosson M, Dufour P. Is endometriosis an endometrial disease? Eur J Obstet Gynecol Reprod Biol. 2000;91:113–125. doi: 10.1016/s0301-2115(99)00263-8. [DOI] [PubMed] [Google Scholar]
- 86.Vogel CF, Matsumura F. Interaction of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with induced adipocyte differentiation in mouse embryonic fibroblasts (MEFs) involves tyrosine kinase c-Src. Biochem Pharmacol. 2013;66:1231–1244. doi: 10.1016/s0006-2952(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 87.Wang J, Liu X, Li T, Liu C, Zhao Y. Increased hepatic Igf2 gene expression involves C/EBPβ in TCDD-induced teratogenesis in rats. Reprod Toxicol. 2011;32:313–321. doi: 10.1016/j.reprotox.2011.06.117. [DOI] [PubMed] [Google Scholar]
- 88.Wetendorf M, DeMayo FJ. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol. 2012;357:108–118. doi: 10.1016/j.mce.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril. 2007;87:24–32. doi: 10.1016/j.fertnstert.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 90.Xiao X, Zhang ZX, Li WH, Feng K, Sun Q, Cohen HJ, et al. Low birth weight is associated with components of the metabolic syndrome. Metabolism. 2010;59:1282–1286. doi: 10.1016/j.metabol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xin Z, Soejima H, Higashimoto K, Yatsuki H, Zhu X, Satoh Y, et al. A novel imprinted gene, KCNQ1DN, within the WT2 critical region of human chromosome 11p15.5 and its reduced expression in Wilms' tumors. J Biochem. 2000;128:847–853. doi: 10.1093/oxfordjournals.jbchem.a022823. [DOI] [PubMed] [Google Scholar]
- 92.Xu F, Xia W, Luo RZ, Peng H, Zhao S, Dai J, et al. The human ARHI tumor suppressor gene inhibits lactation and growth in transgenic mice. Cancer Res. 2000;60:4913–4920. [PubMed] [Google Scholar]
- 93.Yuan L, Jiang R, Yang Y, Ding S, Deng H. 1,25-Dihydroxyvitamin D3 inhibits growth of the breast cancer cell line MCF-7 and downregulates cytochrome P4501B1 through the COX-2/PGE2 pathway. Oncol Rep. 2012;28:2131–2137. doi: 10.3892/or.2012.2031. [DOI] [PubMed] [Google Scholar]
- 94.Yuen RK, Jiang R, Peñaherrera MS, McFadden DE, Robinson WP. Genome-wide mapping of imprinted differentially methylated regions by DNA methylation profiling of human placentas from triploidies. Epigenetics Chromatin. 2011;4:10. doi: 10.1186/1756-8935-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Q, Bouma GJ, McClellan K, Tobet S. Hypothalamic expression of snoRNA Snord116 is consistent with a link to the hyperphagia and obesity symptoms of Prader-Willi syndrome. Int J Dev Neurosci. 2012;30:479–485. doi: 10.1016/j.ijdevneu.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhong J, Chen S, Xue M, Du Q, Cai J, Jin H, et al. ZIC1 modulates cell-cycle distributions and cell migration through regulation of sonic hedgehog, PI(3)K and MAPK signaling pathways in gastric cancer. BMC Cancer. 2012;12:290. doi: 10.1186/1471-2407-12-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou W, Li JD, Hu WP, Cheng MY, Zhou QY. Prokineticin 2 is involved in the thermoregulation and energy expenditure. Regul Pept. 2012;179:84–90. doi: 10.1016/j.regpep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Zhu W, Trivedi CM, Zhou D, Yuan L, Lu MM, Epstein JA. Inpp5f is a polyphosphoinositide phosphatase that regulates cardiac hypertrophic responsiveness. Circ Res. 2009;105:1240–1247. doi: 10.1161/CIRCRESAHA.109.208785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



