Abstract
In this study, we illustrate computer aided drug design of new benzothiazole and pyrimido[2,1-b]benzothiazole derivatives as epidermal growth factor receptor tyrosine kinase (EGFR-TK) inhibitors. Compounds 1-5 were screened at NCI, USA, for antitumor activity against non-small cell lung cancer (NCI-H522), colon cancer (HCT-116, HCT-15 and HT29) and breast cancer (MDA-MB-468 and MDA-MB-231/ATCC) cell lines in which EGFR is overexpressed in varying levels. Results indicated that these compounds are more potent antitumor agents compared to erlotinib against HT29 and MDA-MB-231/ATCC cell lines. Compound 3 showed GI50 value of 22.3 nM against NCI-H522 cell line, while erlotinib exhibited GI50 value of 1 µM against the same cell line. In addition, these compounds were studied for their EGFR tyrosine kinase inhibitory activity. Virtual screening utilizing molecular modeling and QSAR techniques enabled the understanding of the pharmacophoric requirements for antitumor activity. Docking the designed compounds into the ATP binding site of EGFR-TK domain was done to predict the analogous binding mode of these compounds to the EGFR-TK inhibitors.
Keywords: benzothiazoles, pyrimidobenzothiazoles, antitumor activity, EGFR tyrosine kinase inhibitory activity, molecular modeling
Introduction
Protein kinases were proved to be a viable target for anticancer drug development (Wu et al., 2012[45]; Levitzki, 2012[20]; Cheng et al., 2011[6]). They are the second most important drug targets after G protein coupled receptors (GPCR’s) (Cohen, 2002[8]). Kinases are involved in many pathophysiological problems especially cancers where their overexpression can lead to different types of malignancies (Roymans and Slegers, 2001[31]; Malumbres and Barbacid, 2007[22]). In addition, EGFR-TK is one of the most important kinases that plays a fundamental role in signal transduction pathways (Peng-Cheng et al., 2010[27]). EGFR and its ligands, epidermal growth factor (EGF) and transforming growth factor-α (TGF-α) have been implicated in numerous tumors of epithelial origin (Ullrich and Schlessinger, 1990[40]). Therefore, the design of inhibitors that target EGFR-TK is an attractive approach for the development of new therapeutic agents (Bridges, 2001[5]; Grünwald and Hidalgo, 2003[16]). Gefitinib (ZD-1839, Iressa) (Tamura and Fukuoka, 2005[39]) and erlotinib (OSI-774, Tarceva) (Reck et al., 2010[30]; Smith, 2005[37]) were approved as EGFR-TK inhibitors for the treatment of non-small cell lung cancer.
Benzothiazole derivatives constitute an important class of therapeutic agents in medicinal chemistry. Literature survey revealed that this nucleus is associated with diverse pharmacological effects including, antitumor (Shi et al., 2012[36]; Elzahabi, 2011[11]; Saeed et al., 2010[32]; Hu et al., 2010[17]; Al-Soud et al., 2008[1]; Mortimer et al., 2006[25]; Brantley et al., 2006[4]) and antioxidant (Cressier et al., 2009[9]) activities. Moreover, pyrimido[2,1-b]benzothiazoles have also been extensively investigated for their pharmacological uses (Sahu et al., 2012[33]; Prasad et al., 2012[28]; Shendarkar et al., 2011[35]; El-Sherbeny, 2000[10]), some of these compounds showed antitumor activity (El-Sherbeny, 2000[10]). Taking all the above findings into consideration and in searching for new compounds of potent antitumor activity, we report herein the in vitro antitumor evaluation of new benzothiazole and pyrimido-[2,1-b]benzothiazole derivatives bearing pyrazole and oxazole moieties (Figure 1(Fig. 1)) against non-small cell lung cancer (NCI-H522), colon cancer (HCT-116, HCT-15 and HT29) and breast cancer (MDA-MB-468 and MDA-MB-231/ATCC) cell lines in which EGFR is overexpressed in varying levels. Also, their EGFR tyrosine kinase inhibitory activity was studied, along with their docking into the ATP binding site of EGFR-TK domain.
Figure 1. Structure of compounds 1-5.

Rational and design
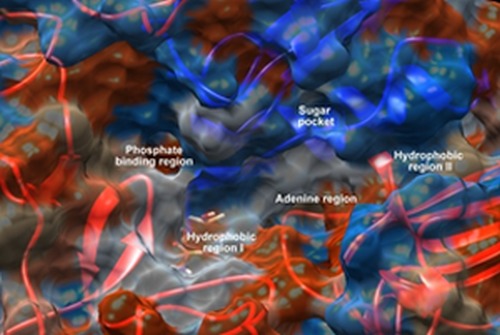
Quinazolines have emerged as a versatile template for inhibition of a diverse range of receptor tyrosine kinases. EGFR is the most widely studied receptor of tyrosine kinases and gefitinib was the first inhibitor of this receptor to be approved for the treatment of non-small cell lung cancer refractory to prior chemotherapeutic intervention (Ballard et al., 2006[2]; Ranson, 2004[29]). Subsequent research aimed at further exploration of the SAR of this novel template, led to the discovery of highly selective compounds that target EGFR. Benzothiazoles act via competing with ATP for binding at the catalytic domain of EGFR-TK (Noolvi et al., 2012[26]). The characteristic features of ATP binding site are; adenine region which contains two key hydrogen bonds formed by the interaction of N1 and N6 of the adenine ring, many potent inhibitors use one of these hydrogen bonds, sugar pocket which is a hydrophilic region, hydrophobic regions and channels, although not used by ATP but play an important role in inhibitor selectivity and binding affinity. In addition, phosphate binding region which is largely solvent exposed, can be used for improving inhibitor selectivity (Fabbro et al., 2002[12]) (Figure 2(Fig. 2)).
Figure 2. The molecular surface representation of the ATP-binding site which consists of adenine region, hydrophobic regions I and II, sugar pocket and phosphate binding region.
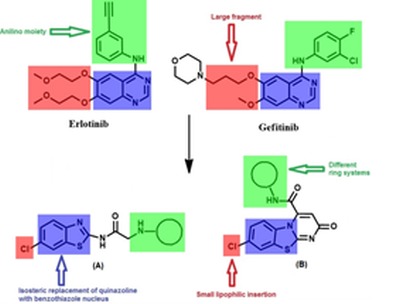
In this study, the authors present a new subfamily of compounds containing benzothiazole core as EGFR-TK inhibitors. Our strategy is directed toward designing a variety of ligands which are structurally similar to the basic skeleton, 4-anilinoquinazoline of tinibs (erlotinib and gefitinib) with diverse chemical properties (Figure 3(Fig. 3)). Accordingly, we replaced quinazoline ring with benzothiazole since both rings are isosteric with adenine portion of ATP and can mimic the ATP competitive binding regions of EGFR-TK.
Figure 3. Reported antitumor quinazolines and proposed compounds; (A) benzothiazole derivatives; (B) pyrimido[2,1-b]benzothiazole derivatives.
Materials and Methods
Source of compounds
The benzothiazole and pyrimido[2,1-b]benzothiazole derivatives 1-5 investigated in this study were previously synthesized and characterized (Gabr et al., 2014[13]). Their chemical names are:
2-(5-Amino-4-cyano-1H-pyrazol-1-yl)-N-(6-chlorobenzothiazol-2-yl)acetamide (1)
N-(6-Chlorobenzothiazol-2-yl)-2-(3-methyl-5-phenyl-1H-pyrazol-1-yl)acetamide (2)
8-Chloro-4-(3,5-dioxopyrazolidine-1-carbonyl)-2H-pyrimido[2,1-b]benzothiazol-2-one (3)
4-(5-Amino-3-oxo-2,3-dihydro-1H-pyrazole-1-carbonyl)-8-chloro-2H-pyrimido[2,1-b]benzothiazol-2-one (4)
8-Chloro-4-(5-thioxo-4,5-dihydro-[1,3,4]oxadiazol-2-yl)-2H-pyrimido[2,1-b]benzothiazol-2-one (5)
In vitro antitumor evaluation
Compounds 1-5 were evaluated for their antitumor activity in accordance with the current protocol of the National Cancer Institute (NCI), USA. They displayed lethal activity against non-small cell lung cancer (NCI-H522), colon cancer (HCT-116, HCT-15 and HT29) and breast cancer (MDA-MB-468 and MDA-MB-231/ATCC) cell lines in which EGFR is overexpressed in varying levels. They passed the preliminary in vitro one-dose anticancer assay and were further evaluated at five dose level screening. The human tumor cell lines of the cancer screening panel were grown in RPMI 1640 medium containing 5 % fetal bovine serum and 2 mM L-glutamine. For a typical screening experiment, cells were inoculated into multiwell microtiter plates at plating densities ranging from 5,000 to 40,000 cells/well depending on the doubling time of individual cell lines. After cell inoculation, the microtiter plates were incubated at 37° C, 5 % CO2, 95 % air and 100 % relative humidity for 24 hrs and then two plates of each cell line were fixed in situ with trichloroacetic acid (TCA) to represent a measurement of the cell population for each cell line at the time of compound addition. The tested compounds were solubilized in DMSO at 400-fold the desired final maximum test concentration and stored frozen prior to use. At the time of compound addition, an aliquot of frozen concentrate was thawed and diluted to twice the desired final maximum test concentration with complete medium containing 50 mg/mL gentamicin. Additional 4-, 10-fold or 1/2 log serial dilutions were made to provide a total of five compound concentrations plus control. Aliquots of 100 mL of these dilutions were added to the appropriate microtiter wells already containing 100 mL of medium, resulting in the required final compound concentrations. Following compound addition, the plates were incubated for additional 48 hrs at 37° C, 5 % CO2, 95 % air, and 100 % relative humidity. For adherent cells, the assay was terminated by the addition of cold TCA. Cells were fixed in situ by gentle addition of 50 mL of cold 50 % (w/v) TCA (final concentration, 10 % TCA) and incubated for 60 min at 4° C. The supernatant was discarded, and the plates were washed five times with tap water and air dried. Sulforhodamine B (SRB) solution (100 mL) at 0.4 % (w/v) in 1 % acetic acid was added to each well, and plates were incubated for 10 min at room temperature. After staining, unbound dye was removed by washing five times with 1 % acetic acid and the plates were air dried. Bound stain was subsequently solubilized with 10 mM trizma base and the absorbance was read on an automated plate reader at a wavelength of 515 nm (Grever et al., 1992[14]; Boyd and Paull, 1995[3]; Monks et al., 1991[24]). GI50 values were calculated for each cell line (Table 1(Tab. 1)).
Table 1. GI50 values (µM) of compounds 1-5 and erlotinib over the selected cancer cell lines.

EGFR tyrosine kinase assay
Compounds 1-5 were dissolved in DMSO and tested at a single concentration of 100 µM. They were then added to reaction plates containing the EGFR tyrosine kinase in assay buffer [20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.5, 10 mM MgCl2, 1 mM ethylene glycol tetraacetic acid (EGTA), 0.02 % Brij35, 0.02 mg/mL bovine serum albumin (BSA), 0.1 mM Na3VO4, 2 mM dithiothreitol (DTT), 1 % DMSO]. Reactions were initiated by addition of a mixture of ATP (Sigma, St. Louis, MO) and 33P ATP (Perkin Elmer, Waltham MA) to a final concentration of 10 µM. Reactions were carried out at room temperature for 120 min, followed by spotting of the reactions onto P81 ion exchange filter paper (Whatman Inc., Piscataway, NJ). Unbound phosphate was removed by extensive washing of filters in 0.75 % phosphoric acid (Ma et al., 2008[21]). Kinase activity data was reported as the percent remaining enzyme activity after subtraction of enzyme inhibitory activity of DMSO control reactions as background. Results are presented as percentage enzyme inhibition and compared to staurosporine as a reference EGFR-TK inhibitor (Table 2(Tab. 2)).
Table 2. Percentage EGFR-TK inhibitory activity of compounds 1-5.

Molecular modeling and computational studies
Protein structure preparation
The crystal structure of EGFR kinase domain (PDB ID: 1M17) in complex with an irreversible inhibitor was obtained from the protein data bank (PDB; http://www.rcsb.-org/pdb/home/home.do).
Refinement of crude PDB structure of receptor was performed. Polar hydrogens were added, Kollman charges were assigned and atomic solvation parameters were added, the internal degrees of freedom and torsions were set for all the designed small molecules. The optimized receptor was then saved as mol file and used for docking simulation.
Ligand structure prepartion
The 2D structures of the compounds were built and then converted into the 3D with the help of vLife MDS 3.0 software. The 3D structures were then energetically minimized up to the rms gradient of 0.01 using CHARMM22 force field. All conformers were then energetically minimized up to the rms gradient of 0.01 and then saved in separate folder.
Docking protocol
Docking simulation was done by Swiss-Dock software (Grosdidier et al., 2011[15]). All the conformers were virtually docked at the defined cavity of the receptor. The number of placements was fixed at 30 placements and the rotation angle was fixed at 30°. By rotation angle, the ligand gets rotated for different poses. By placements, the method will check all the 30 possible placements into the active site pocket and results out few best placements out of 30. For each ligand, all the conformers with their best placements and their dock score will be saved in output folder. The method also highlights the best placement of the best conformer of each ligand which is having the best (minimum) dock score value. After docking simulation, the best docked conformer of each ligand and receptor was merged and its complexes were then energetically optimized by defining the radius of 10 Å measured from the docked ligand.
Results and Discussion
In vitro antitumor screening
Compounds 1-5 were evaluated for their antitumor activity in accordance with the current protocol of the National Cancer Institute (NCI), USA (Grever et al., 1992[14]; Boyd and Paull, 1995[3]; Monks et al., 1991[24]). The data was reported as mean-graph of the percentage growth of the treated cells, and presented as percentage growth inhibition (GI%). They were initially screened at a single dose (10 µM) in the full NCI 60 cell lines panel assay. Results demonstrated that they satisfied the predetermined threshold inhibition criteria so they were carried over by the NCI for the five-dose screening. Non-small cell lung cancer (NCI-H522), colon cancer (HCT-116, HCT-15 and HT29) and breast cancer (MDA-MB-468 and MDA-MB-231/ATCC) cell lines were incubated with five concentrations (0.01–100 µM) of each compound and were used to create log concentration - % growth inhibition curves. The GI50 values of compounds 1-5 and erlotinib against the selected cancer cell lines are listed in Table 1(Tab. 1). Results indicated that these compounds are more potent antitumor agents compared to erlotinib against colon cancer (HT29) and breast cancer (MDA-MB-231/ATCC) cell lines. Compound 3 exhibited GI50 value of 22.3 nM against non-small cell lung cancer (NCI-H522) cell line while compound 5 showed GI50 value of 69.1 nM against breast cancer (MDA-MB-468) cell line. Moreover, compound 4 displayed potent antitumor activity with GI50 values in submicromolar range against all tested cancer cell lines.
EGFR tyrosine kinase assay
In vitro profiling of compounds 1-5 was performed at Reaction Biology Corporation to assess their inhibitory activity of EGFR-TK. Kinase activity was assessed using Hot Spot technology (Ma et al., 2008[21]), a miniaturized radioisotope-based filter binding assay. Kinase activity data was reported as the percentage remaining enzyme activity after subtraction of enzyme inhibitory activity of DMSO control reactions as background. Results are presented as percentage enzyme inhibition and compared to staurosporine as a reference EGFR-TK inhibitor (Table 2(Tab. 2)). Compound 1 showed the highest inhibitory activity against EGFR-TK with percentage enzyme inhibition value of 70.58. The attempt to improve hydrophobic interaction via incorporation of an extra phenyl moiety resulted in decreased inhibitory activity (compound 2). Furthermore, combination of [1,3,4]oxadiazole moiety with the pyrimido-[2,1-b]benzothiazole nucleus increased the activity over the other analogs with pyrazole and pyrazolidine moieties (compare 5 versus 3 and 4).
Molecular modeling and computational studies
Overactivation of receptor tyrosine kinase (RTK) signaling pathways is strongly associated with carcinogenesis. Thus, it is becoming increasingly clear that impaired deactivation of RTKs may be an oncogenic driver of cancer (Kristi et al., 2004[19]). On this basis, Computer-Aided Drug Design (CADD) tools were used to identify the interaction between the newly synthesized compounds and the active site of EGFR-TK in comparison to erlotinib as a reference EGFR-TK inhibitor. A representation of our workflow is shown in Figure 4(Fig. 4).
Figure 4. Virtual screening (VS) workflow.

Kinase inhibitors should contain the following features to gain selectivity and potency (Zhang et al., 2009[47]):
A portion that closely mimics ATP molecule and one to three hydrogen bonds with the amino acids located in the hinge region of the target kinases, as in erlotinib (Reck et al., 2010[30]), lapatinib (Wainberg et al., 2010[41]) and gefitinib (Yun et al., 2007[46]).
An additional hydrophobic binding site which is directly adjacent to the ATP binding site (allosteric site), as in imatinib (Cherry and Williams, 2004[7]) and sorafenib (Whittaker et al., 2010[43]). However, other mechanism could be achieved through binding outside the ATP binding site at an allosteric site (Weisberg et al., 2007[42]) and by forming irreversible covalent bond to the kinase active site (Sridhar et al., 2003[38]; Sharma et al., 2009[34]). In the present work, erlotinib binding mode to EGFR-TK is studied and the design of the newly synthesized compounds is based on the essential chemical features required for erlotinib binding affinity to EGFR-TK.
Similarity-based virtual screening
Similarity methods may be the simplest and most widely used tools for ligand-based virtual screening of chemical databases, where functionally similar molecules are sought by searching molecular databases for structurally similar molecules. These methods can be categorized as 2D and 3D similarity methods. However, the most common approaches are based on the 2D fingerprints, with the similarity between a reference structure and a database structure.
The synthesized compounds in SDF format were submitted to ReverseScreen3D server (Kinnings and Jackson, 2011[18]), the server uses reverse virtual screening (VS) method called ReverseScreen3D, it is a 2D fingerprint-based method to select a ligand template from each unique binding site of each protein with a target database. The target database contains only the structurally determined bioactive conformations of known ligands. The 2D comparison is followed by a 3D structural comparison to the selected query ligand using a geometric matching method in order to priotrize each target binding site in the database. The output in the form of a list of the 2D and 3D scores for protein tyrosine kinase (cluster no. 14836) is listed in Table 3(Tab. 3). Compound 4 showed the highest 3D score value of 0.573 which comes in accordance with the biological data.
Table 3. Results of molecular docking analysis of compounds 1-5 in the active site of EGFR-TK.

3D Pharmacophore elucidation
A 3-dimensional pharmacophore is defined as a critical geometric arrangement of molecular features forming a necessary but not sufficient condition for biological activity (Mason et al., 2001[23]). 3D Pharmacophore designing methods take into account both the 3-dimensional structures and binding modes of receptors and inhibitors in order to identify regions that are favorable for specific receptor-inhibitor interaction. The description of the receptor-inhibitor interaction pattern is determined by a correlation between the characteristic properties of the inhibitors and their biochemically determined enzymatic activity.
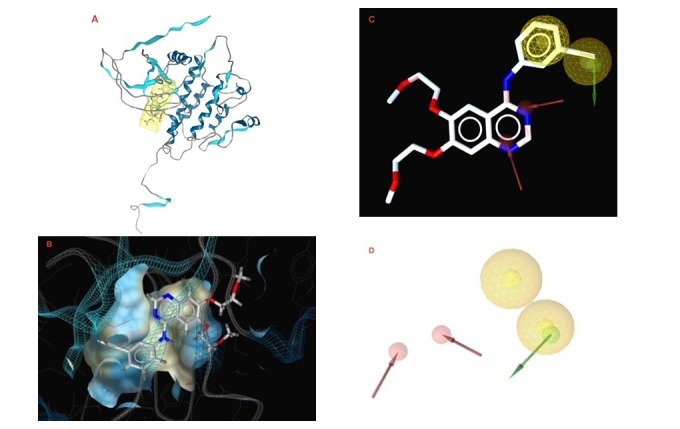
LigandScout, a program that allows the automatic construction and visualization of 3D pharmacophore from structural data of protein-ligand complex, was used in this study to create a pharmacophore for the mode of action of erlotinib, which prevents activation of EGFR kinase (Wolber and Langer, 2005[44]). The model (Figure 5(Fig. 5)) was created automatically by overlaying pharmacophoric features gathered from the crystal structure of EGFR kinase domain (PDB ID: 1M17) in complex with erlotinib (PDB; http://www.-rcsb.org/pdb/home/home.do).
Figure 5. (A) The crystal structure of EGFR kinase domain (PDB ID: 1M17) in complex with erlotinib was obtained from the protein data bank (PDB; http://www.rcsb.org/pdb/home/home.do). (B) LigandScout 3D proposed docking pose for erlotinib in the ATP binding site of EGFR kinase domain. (C) The 3D pharmacophore of erlotinib (in ball and stick representation); The pharmacophore color coding is red for hydrogen acceptor, yellow for hydrophobic regions and green for hydrogen donors. (D) The final 3D pharmacophore model for the EGFR kinase domain.
The investigated pharmacophoric features included; hydrogen bond donors and acceptors as directed vectors, positive and negative ionizable regions as well as lipophilic areas that are represented by spheres. According to the pharmacophore generated by LigandScout, the minimal structural requirements for antitumor activity consist of a hydrophobic region attached to heterocyclic ring which fits into ATP binding site, two hydrogen bond acceptors and one hydrogen bond donor. The 3D alignment of the pharmacophoric features of each of the synthesized compounds and the 3D pharmacophore of erlotinib binding pose showed that these compounds possess similar pharmacophoric features required for activity. A pharmacophore score listed in Table 3(Tab. 3) was
calculated for the alignment of the synthesized compounds into the 3D pharmacophore of erlotinib binding pose generated by LigandScout, this score reflects the similarity of the compounds to the reference pharmacophore. Compounds 1 and 5 showed the best pharmacophore score values of 116.79 and 124.75, respectively. Furthermore, compounds 3 and 4 form impressive alignment with the erlotinib binding pose model with pharmacophore score values of 97.94 and 96.20, respectively. The 3D alignment of compounds 1 and 5 with the pharmacophore model are shown in Figures 6A(Fig. 6) and 7A(Fig. 7), respectively. The detailed 2D mapping of the pharmacophore model with the structural features of compounds 1 and 5 are depicted in Figures 6B(Fig. 6) and 7B(Fig. 7), respectively.
Figure 6. The 3D and 2D alignment of compound 1 with EGFR kinase pharmacophore model. (A) The 3D alignment of compound 1 with EGFR kinase pharmacophore model. (B) The 2D representation of the structural features in compound 1 that can be aligned with the pharmacophore hypothesis. HBA, hydrogen bond acceptor; HBD, hydrogen bond donor; H, hydrophobic center.

Figure 7. The 3D and 2D alignments of compound 5 with EGFR kinase pharmacophore model. (A) The 3D alignment of compound 5 with EGFR kinase pharmacophore model. (B) The 2D representation of the structural features in compound 5 that can be aligned with the pharmacophore hypothesis. HBA, hydrogen bond acceptor; HBD, hydrogen bond donor; H, hydrophobic center.

Docking
The crystal structure of EGFR kinase domain (PDB ID: 1M17) in complex with an irreversible inhibitor was obtained from the protein data bank (PDB; http://www.rcsb.-org/pdb/home/home.do). Docking simulation was done by SwissDock software (Grosdidier et al., 2011[15]). All the conformers were virtually docked at the defined cavity of the receptor. Regarding the results of docking, the dock scores of the best conformer for each ligand are listed (Table 3(Tab. 3)). The ligand forming the most stable drug receptor complex is the one having the least dock score value. The compounds are evaluated using two scoring functions; simple fitness and full fitness. Simple fitness is a fast and efficient method to evaluate the individual binding modes which neglects the solvent effect and is used to drive the search. Simultaneously, clusters of binding modes are evaluated by the more selective yet slower full fitness, which accounts for the solvation free energy. Compound 1, the most active member in the EGFR tyrosine kinase assay showed the best full fitness score value of -1967.63 Kcal/mol. 3D Interaction of compound 1 with the binding site of EGFR-TK is shown in Figure 8(Fig. 8). The docking results support the postulation that this compound may act on the same target enzyme where EGFR-TK inhibitors act.
Figure 8. 3D Interaction of compound 1 with the binding site of EGFR-TK. The atoms are colored as following: red for oxygen atoms, blue for nitrogen atoms, yellow for sulfur atoms, white for hydrogen atoms, cyan for carbon atoms and green for chlorine atoms.

Analysis of the binding mode
The ATP-binding pocket of EGFR-TK is considerably hydrophobic; thus, hydrophobic interaction plays an important role in affinity toward EGFR-TK. Moreover, it was proposed that at least two hydrogen bonds are required for small molecules to act as EGFR-TK inhibitors (Zuccotto et al., 2010[48]).
LeadIT software was used to analyze the location and orientation of the evaluated compounds and the interactions into the binding site of EGFR-TK. 2D Interactions of compounds 1 and 3 with the binding site of EGFR-TK are shown in Figures 9(Fig. 9) and 10(Fig. 10), respectively. The docking results (Table 4(Tab. 4)) revealed that the main interaction force of the candidate compounds with the EGFR-TK active site is hydrophobic. The important residues in the hydrophobic regions that interact with the hit compounds are (Val-702, Leu-820 and Ala-719). All these residues are located near the gatekeeper residue Thr766 (Thr790 in alternative numbering in EGFR), which are the most common kinase-inhibitor interactions. The docking results showed that almost all the synthesized compounds are involved in at least two hydrogen bonding interaction with EGFR-TK binding site which is required for activity. Analysis of binding mode revealed that Phe-832 and Glu-738 are involved in hydrogen bonding interaction with compound 1 as it forms three hydrogen bonds with EGFR-TK active site, which is the highest possible number of hydrogen bonding interaction of the synthesized compounds with EGFR-TK and this indicates the correlation between the hydrogen bonding interaction and the antitumor activity. Summary of hydrophobic interaction, number of hydrogen bonds and hydrogen bonding interacting residues of compounds 1-5 is presented in Table 4(Tab. 4).
Figure 9. 2D Interaction of compound 1 with the binding site of EGFR-TK. Dashed lines represent hydrogen bonds. Hydrophobic interactions are shown by green solid lines.

Figure 10. 2D Interaction of compound 3 with the binding site of EGFR-TK. Dashed lines represent hydrogen bonds. Hydrophobic interactions are shown by green solid lines.

Table 4. Summary of hydrophobic interaction, number of hydrogen bonds and hydrogen bonding interacting residues of compounds 1-5.

Conclusion
In conclusion, compounds 1-5 exhibited more potent antitumor activity than erlotinib against colon cancer (HT29) and breast cancer (MDA-MB-231/ATCC) cell lines. In addition, compound 4 displayed the highest potency against all tested cancer cell lines with GI50 values in submicromolar range. According to the pharmacophore generated by LigandScout, the minimal structural requirements for antitumor activity consist of a hydrophobic region attached to heterocyclic ring which fits into ATP binding site, two hydrogen bond acceptors and one hydrogen bond donor. The results of the EGFR tyrosine kinase assay and the virtual screening support the postulation that the active compounds may act on the same target enzyme where EGFR-TK inhibitors act. These preliminary encouraging results of biological screening of the newly synthesized compounds could offer an excellent framework toward the discovery of new potent antitumor agents.
Acknowledgements
The authors would like to express their sincere thanks to the staff members of the Department of Health and Human Services, National Cancer Institute (NCI), Bethesda, Maryland, USA for performing the antitumor testing of the newly synthesized compounds. Thanks to Reaction Biology Corporation, USA for performing EGFR tyrosine kinase assay.
References
- 1.Al-Soud YA, Al-Sa’doni HH, Saeed B, Jaber IH, Beni-Khalid MO, Al-Masoudi NA, et al. Synthesis and in vitro antiproliferative activity of new benzothiazole derivatives. ARKIVOC. 2008;XV:225–238. [Google Scholar]
- 2.Ballard P, Bradbury RH, Harris CS, Hennequin LF, Hickinson M, Kettle JG, et al. Inhibitors of epidermal growth factor receptor tyrosine kinase: optimization of potency and in vivo pharmacokinetics. Bioorg Med Chem Lett. 2006;16:4908–4912. doi: 10.1016/j.bmcl.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Rev Res. 1995;34:91–109. [Google Scholar]
- 4.Brantley E, Antony S, Kohlhagen G, Meng LH, Agama K, Stinson SF, et al. Anti-tumor drug candidate 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole induces single-strand breaks and DNA-protein cross-links in sensitive MCF-7 breast cancer cells. Cancer Chemother Pharmacol. 2006;58:62–72. doi: 10.1007/s00280-005-0127-z. [DOI] [PubMed] [Google Scholar]
- 5.Bridges A. Chemical inhibitors of protein kinases. J Chem Rev. 2001;101:2541–2572. doi: 10.1021/cr000250y. [DOI] [PubMed] [Google Scholar]
- 6.Cheng Y, Cui W, Chen Q, Tung CH, Ji M, Zhang F. The molecular mechanism studies of chirality effect of PHA-739358 on Aurora kinase A by molecular dynamics simulation and free energy calculations. J Comput Aided Mol Des. 2011;25:171–180. doi: 10.1007/s10822-010-9408-7. [DOI] [PubMed] [Google Scholar]
- 7.Cherry M, Williams DH. Recent kinase inhibitor X-ray structures: Mechanisms of inhibition and selectivity insights. Curr Med Chem. 2004;11:663–673. doi: 10.2174/0929867043455792. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P. Protein kinases: the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 9.Cressier D, Prouillac C, Hernandez P, Amourette C, Diserbo M, Lion C, et al. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg Med Chem. 2009;17:5275–5284. doi: 10.1016/j.bmc.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 10.El-Sherbeny MA. Synthesis of certain pyrimido. [2,1-bbenzothiazole and benzothiazolo]. Arzneim Forsch. 2000;50:848–853. doi: 10.1055/s-0031-1300300. (Ger). [DOI] [PubMed] [Google Scholar]
- 11.Elzahabi HAS. Synthesis, characterization of some benzazoles bearing pyridine moiety: Search for novel anticancer agents. Eur J Med Chem. 2011;46:4025–4036. doi: 10.1016/j.ejmech.2011.05.075. [DOI] [PubMed] [Google Scholar]
- 12.Fabbro D, Ruetz S, Buchdunger E, Cowan-Jacob SW, Fendrich G, Liebetanz J, et al. Protein kinases as targets for anticancer agents: from inhibitors to useful drugs. Pharmacol Ther. 2002;93:79–98. doi: 10.1016/s0163-7258(02)00179-1. [DOI] [PubMed] [Google Scholar]
- 13.Gabr MT, El-Gohary NS, El-Bendary ER, El-Kerdawy MM. Synthesis and in vitro antitumor activity of new series of benzothiazole and pyrimido. [2,1-bbenzothiazole derivatives]. Manuscript submitted for publication, 2014. doi: 10.1016/j.ejmech.2014.07.097. (Ger). [DOI] [PubMed] [Google Scholar]
- 14.Grever MR, Schepartz SA, Chabner BA. The National Cancer Institute: cancer drug discovery and development program. Semin Oncol. 1992;19:622–638. [PubMed] [Google Scholar]
- 15.Grosdidier A, Zoete V, Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. J Comput Chem Nucleic Acids Res. 2011;9:270–277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grünwald V, Hidalgo M. Developing inhibitors of the epidermal growth factor receptor for cancer treatment. J Natl Cancer Inst. 2003;95:851–867. doi: 10.1093/jnci/95.12.851. [DOI] [PubMed] [Google Scholar]
- 17.Hu WP, Chen YK, Liao CC, Yu HS, Tsai YM, Huang SM, et al. Synthesis and biological evaluation of 2-(4-aminophenyl)benzothiazole derivatives as photosensitizing agents. Bioorg Med Chem. 2010;46:6197–6207. doi: 10.1016/j.bmc.2010.04.082. [DOI] [PubMed] [Google Scholar]
- 18.Kinnings SL, Jackson RM. ReverseScreen3D: A structure-based ligand matching method to identify protein targets. J Chem Inf Model. 2011;51:624–634. doi: 10.1021/ci1003174. [DOI] [PubMed] [Google Scholar]
- 19.Kristi GB, Thomas S, Herald S. Defective down regulation of receptor tyrosine kinases in cancer. Eur Mol Biol Org. 2004;23:2707–2712. [Google Scholar]
- 20.Levitzki A. Tyrosine kinase inhibitors: Views of selectivity, sensitivity, and clinical performance. Annu Rev Pharmacol Toxicol. 2012;53:161–185. doi: 10.1146/annurev-pharmtox-011112-140341. [DOI] [PubMed] [Google Scholar]
- 21.Ma H, Deacon S, Horiuchi K. The challenge of selecting protein kinase assays for lead discovery optimization. Expert Opin Drug Discov. 2008;3:607–621. doi: 10.1517/17460441.3.6.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Mason JS, Good AC, Martin EJ. 3-D pharmacophores in drug discovery. Curr Pharm Design. 2001;7:567–597. doi: 10.2174/1381612013397843. [DOI] [PubMed] [Google Scholar]
- 24.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 25.Mortimer CG, Wells G, Crochard JP, Stone EL, Bradshaw TD, Stevens MF, et al. Antitumor benzothiazoles. 26.(1) 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J Med Chem. 2006;49:179–185. doi: 10.1021/jm050942k. [DOI] [PubMed] [Google Scholar]
- 26.Noolvi MN, Patel HM, Kaur M. Benzothiazoles: search for anticancer agents. Eur J Med Chem. 2012;54:447–462. doi: 10.1016/j.ejmech.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Peng-Cheng LV, Zhou CF, Chen J, Liu PG, Wang KR, Mao WJ, et al. Design, synthesis and biological evaluation of thiazolidinone derivatives as potential EGFR and HER-2 inhibitors. Bioorg Med Chem Lett. 2010;18:314–319. doi: 10.1016/j.bmc.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 28.Prasad PR, Bhuvaneswari K, Kumar KP, Rajani K, Kuberkar SV. Synthesis and biological activity evaluation of some fused pyrimido-benzothiazole derivatives. J Chem Pharm Res. 2012;4:1606–1611. [Google Scholar]
- 29.Ranson M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br J Cancer. 2004;90:2250–2255. doi: 10.1038/sj.bjc.6601873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reck M, Zandwijk NV, Gridelli C, Baliko Z, Rischin D, Allan S, et al. Erlotinib in advanced non-small cell lung cancer: efficacy and safety findings of the global phase IV Tarceva Lung Cancer Survival Treatment study. J Thorac Oncol. 2010;5:1616–1622. doi: 10.1097/JTO.0b013e3181f1c7b0. [DOI] [PubMed] [Google Scholar]
- 31.Roymans D, Slegers H. Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem. 2001;268:487–498. doi: 10.1046/j.1432-1327.2001.01936.x. [DOI] [PubMed] [Google Scholar]
- 32.Saeed S, Rashid N, Jones PG, Ali M, Hussain R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur J Med Chem. 2010;45:1323–1331. doi: 10.1016/j.ejmech.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Sahu PK, Sahu PK, Gupta SK, Thavaselvam D, Agarwal DD. Synthesis and evaluation of antimicrobial activity of 4H-pyrimido. [2,1-bbenzothiazole, pyrazole and benzylidene derivatives of curcumin]. Eur J Med Chem. 2012;54:366–378. doi: 10.1016/j.ejmech.2012.05.020. (Ger). [DOI] [PubMed] [Google Scholar]
- 34.Sharma FA, Sharma R, Tyagi T. Receptor tyrosine kinase inhibitors as potent weapons in war against cancers. Curr Pharm Design. 2009;15:758–776. doi: 10.2174/138161209787582219. [DOI] [PubMed] [Google Scholar]
- 35.Shendarkar GR, Labhsetwar LB, Butle SR, Karki SS, Sharma RH, Kuberkar SV. Synthesis and antimicrobial evaluation of some fused iminopyrimidobenzothiazole derivatives. Int J Res Pharm Biomed Sci. 2011;2:1350–1356. [Google Scholar]
- 36.Shi XH, Wang Z, Xia Y, Ye T-H, Deng M, Xu Y-Z, et al. Synthesis and biological evaluation of novel benzothiazole-2-thiol derivatives as potential anticancer agents. Molecules. 2012;17:3933–3944. doi: 10.3390/molecules17043933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-small cell lung cancer. Clin Ther. 2005;27:1513–1534. doi: 10.1016/j.clinthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Sridhar S, Seymour L, Shepherd FA. Inhibitors of epidermal growth factor receptors: a review of clinical research with a focus on non small-cell lung cancer. Lancet Oncology. 2003;4:397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 39.Tamura K, Fukuoka M. Gefitinib in non-small cell lung cancer. Expert Opin Pharmacother. 2005;6:985–993. doi: 10.1517/14656566.6.6.985. [DOI] [PubMed] [Google Scholar]
- 40.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. J Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 41.Wainberg ZA, Anghel A, Desai AJ, Ayala R, Luo T, Safran B, et al. Lapatinib, a dual EGFR and HER2 kinase inhibitor, selectively inhibits HER2-amplified human gastric cancer cells and is synergistic with trastuzumab in vitro and in vivo. Clin Cancer Res. 2010;16:1509–1519. doi: 10.1158/1078-0432.CCR-09-1112. [DOI] [PubMed] [Google Scholar]
- 42.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib resistant chronic myeloid leukemia. Nat Rev Cancer. 2007;7:345–356. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 43.Whittaker S, Kirk R, Hayward R, Zambon A, Viros A, Cantarino N, et al. Gatakeeper mutations medicate resistance to BRAF targeted therapies. Sci Transl Med. 2010;2:35–41. doi: 10.1126/scitranslmed.3000758. [DOI] [PubMed] [Google Scholar]
- 44.Wolber G, Langer T. LigandScout: 3D Pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J Chem Inf Comp Sci. 2005;45:160–169. doi: 10.1021/ci049885e. [DOI] [PubMed] [Google Scholar]
- 45.Wu KW, Chen PC, Wang J, Sun YC. Computation of relative binding free energy for an inhibitor and its analogs binding with Erk kinase using thermodynamic integration MD simulation. J Comput Aided Mol Des. 2012;26:1159–1169. doi: 10.1007/s10822-012-9606-6. [DOI] [PubMed] [Google Scholar]
- 46.Yun C, Boggon TJ, Li Y, Woo MS, Greulich MS, Meyerson M, et al. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuccotto F, Ardini E, Casale E, Angiolini M. Through the “Gatekeeper Door”: Exploiting the active kinase conformation. J Med Chem. 2010;53:2681–94. doi: 10.1021/jm901443h. [DOI] [PubMed] [Google Scholar]


