Abstract
Drought is the main abiotic constraint that affects crop species behaviour regarding bio-chemical responses. The current study was conducted to examine the effect of water deficit on growth, phenolic and carotenoid contents as well as the antioxidant and antimicrobial activities of two Carthamus tinctorius varieties (Jawhara and 104) flowers. Hence, plants were treated with different levels of water deficit: control (100 %), moderate water deficit (50 %) and severe water deficit (25 %). Obtained results showed that plant growth was significantly (p < 0.05) reduced under 50 %. Drought increased flower phenolic acids contents especially gallic acid where they increased significantly (p < 0.05) by 2.73 fold (104) and by 2.87 fold (Jawhara) with respect to controls under 50 %. However, the amounts of this major compound were reduced at 25 % by 9.66 % (104) and 3.83 % (Jawhara). Similar to phenolic compounds, total carotenoid content was at its highest level especially for Jawhara with an increase by 35.19 % at 50 %. On the other hands, C. tinctorius flowers extracts exhibited high antiradical activity as compared to BHT. A gradual increase by 35.29 % (Jawhara) and 33.33 % (104) especially under 25 % was observed. Moreover, under 50 %, the antimicrobial activity increased significantly (p < 0.05) by 30 % and 10.05 % against Aspergillus carbonarus and Pseudomonas aerogenosa, respectively. Taken together, our findings suggest that C. tinctorius could be a raw material for production of natural dyes under moderate salinity conditions.
Keywords: Carthamus tinctorius flower, drought, phenolic composition, carotenoids, antioxidant activity, antimicrobial activity
Introduction
A number of both biotic and abiotic factors have been shown to influence the content of secondary metabolites in plant tissues. Water deficit is considered to be a major abiotic factor affecting many aspects of plant physiology and biochemistry causing a significant reduction in agricultural production (Charles et al., 1994[8]). Drought stress significantly limits plant growth, crop productivity and changes its behaviour regarding the biosynthesis of bioactive compounds such as phenolic compounds (Al-cázar et al., 2006[3]).
Indeed, water deficit has been suggested to cause both increases and decreases in the concentration of various phenolic components (Horner, 1990[18]). In addition, the phenylpropanoid pathway, which is responsible for the synthesis of a diverse array of phenolic metabolites, is often induced by stress and plays specific roles in plant protection (Janzik et al., 2005[22]). In this way, drought stress may increase the formation of reactive oxygen species (ROS) which can damage plants by oxidizing photosynthetic pigments, membrane lipids, proteins, and nucleic acids (Reddy et al., 2004[39]).
To counteract these damages, plants have developed their own machinery through production of enzymatic and non enzymatic free radical scavengers (Hojati et al., 2011[17]). Secondary metabolites play a major role in the adaptation of plants to the changing environment and in overcoming stress constraints. This flows from the large complexity of chemical types and interactions underlying various functions such as structure stabilizing which is determined by polymerisation and condensation of phenols and quinines (Edreva et al., 2008[12]). Hence, all field crops respond differently at different phenological stages to changing water status of the soil under deficit irrigation, which means that plants are more sensitive to water deficit at some stages than others and the most sensitive one is flowering (Kirda, 2002[26]). When subjected to this constraint, plants manifest a wide range of behaviours varying from great sensitivity to high tolerance (Guerfel et al., 2008[15]).
An example of an aromatic plant is safflower (Carthamus tinctorius L.), a member of Asteraceae family, is a multi-purpose crop for oil, medicinal and industrial uses. It has been grown for centuries for its colourful petals to use as a food colouring and flavouring agent, for vegetable oils and also for preparing textile dye in the Far East, Central and Northern Asia and European Caucasian (Esendal et al., 2008[13]). Flowers are used to stimulate blood circulation and phlegm reduction, to promote the healing fractures and in treatment of cardiovascular, cerebrovascular and gynecological diseases (Kafka, 1965[24]). Safflower often suffers from water deficit, where the severity of the resulted damage varies depending on the intensity and the duration of water stress. This crop is considered as drought-resistant and a tap-rooted annual crop which can tolerate environmental stresses including salinity and water stress (Hojati et al., 2011[17]). However, studies regarding the impact of abiotic constraints on bioactive compounds of C. tinctorius are very scare and limited to the impact of water deficit on growth and oil yield (Nabipour et al., 2007[33]). In addition, to the best of our knowledge, there are no published investigations focusing on water deficit impact on phenolic and carotenoid composition, and biological potentials of safflower. Therefore, the present work was carried out in aiming to evaluate the effects of different irrigation levels on changes in phenolic and carotenoid contents, the antioxidant and antimicrobial behaviours in C. tinctorius flowers in pot culture at full flowering stage.
Materials and Methods
Chemicals
All solvents used in the experiments from Merck (Darmstadt, Germany). Sodium hydroxide (NaOH), chlorhydric acid (HCl), disodium hydrogen phosphate (Na2HPO4), sodium monobasic phosphate (NaH2PO4H2O), sodium carbonate (Na2CO3), sodium nitrite (NaNO2), butylated hydroxytoluene (BHT), ß-carotene, linoleic acid, ethylenediaminetetraacetic acid (EDTA), 3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine (ferrozine), iron(II) tetrahydrate, iron(II), iron(III), 1,1-diphenyl-2-picrylhydrazyl (DPPH), polyvinyl polypyrolidone Folin-Ciocalteu reagent, potassium ferricyanide (K3Fe(CN)6) and aluminium chloride (AlCl3) were purchased from Sigma-Aldrich (Steinheim, Germany). Authentic standards of phenolic and carotenoid compounds were purchased from Sigma and Fluka. Stock solutions of these compounds were prepared in HPLC-grade methanol. These solutions were wrapped in aluminium foil and stored at 4 °C. All other chemicals used were of analytical grade.
Plant material and water deficit treatment
Seeds of safffower (C. tinctorius L.) were obtained from plants grown in the National Institute of Agricultural Research in Tunisia. Safflower plants were cultivated in clay pots (20 cm diameter) and kept in a greenhouse under controlled conditions: 14 h day length (photosynthetic photon flux density PPFD) at 26-30 °C, 10 h at night at 8 °-12 °C, relative humidity of 60 %. During 30 days of pretreatment, plants were irrigated with tap water, and then divided into three lots subjected to different water levels: 100 % [control (C)], 50 % [moderate water deficit (MWD)] and 25 % [severe water deficit (SWD)]. Water treatment was started when the second leaf emerged. Water deficit was realised by withholding irrigation during different times according to the desired stress. Watering was suspended for six days for mildly stressed plants (MWD, flower water potential = -4.5 ± 4 KPa) and 10 days for severely stressed ones (SWD, flower water potential = -7 KPa). Control plants (C, well hydrated) were irrigated twice a week and maintained a flower water potential (-3.9 KPa). Flower water potentials were measured by a pressure chamber (Scholander et al., 1965[43]). After seven weeks of treatment, a sample of six plants at flowering stage was used. During the whole growth period, weeds and insects were effectively controlled.
Growth and water potential measurements
For each water treatment, measurements of plant height, fresh and dry weights were monitored. Plants were harvested at the soil surface, immediately weighed (fresh weight) and then oven-dried at 75 °C for 48 h and reweighed (dry weight). Their dry matter contents were computed using the following equation:
DM (%) = (DMW/FMW) × 100
where DM: dry matter,
FMW: fresh matter weight and
DMW: dry matter weight.
Moreover, for each treatment yield components such as flower heads number per plant, seeds number per plant and petals yield were determined at the end of the experiment. Measurements of water potential were realized on the aerial parts of different plants (C, MWD and SWD) at the water potential was measured by Scholander pressure chamber (Scholander et al., 1965[43]).
Determination of total phenolic, flavonoid and proanthocyanidins
Colorimetric quantification of total phenolics was determined, as described by Dewanto et al. (2002[11]). Briefly, 125 µL of suitable diluted sample extract were dissolved in 500 µL of distilled water and 125 µL of Folin-Ciocalteu reagent. The mixture was shaken, before adding 1250 µL of Na2CO3 (7 g/100 mL), adjusting with distilled water to a final volume of 3 mL and mixed thoroughly. After incubation for 90 min at 23 °C in darkness, the absorbance versus a prepared blank was read at 760 nm. Total phenolic contents of flower were expressed as mg gallic acid equivalents per gram (mg GAE/g) through the calibration curve with gallic acid. The calibration curve range was 0-400 µg/mL (R2 = 0.99). All samples were performed in triplicate.
Total flavonoid contents were measured according to Dewanto et al. (2002[11]). An aliquot of diluted sample was added to 75 µL of NaNO2 solution (5 %) and mixed for 6 min, before adding 0.15 mL of AlCl3 (10 %). After 5 min, 0.5 mL of 1 M NaOH solution was added. The final volume was adjusted to 2.5 mL, thoroughly mixed, and the absorbance of the mixture was determined at 510 nm. Total flavonoid contents of flower extracts were expressed as mg catechin equivalents (CE) per gram of dry weight respectively (mg GAE/g DW) through the calibration curve ranging from 0-500 µg/mL (R2= 0.85).
In the presence of concentrated HCl, condensed tannins were transformed by the reaction with vanillin to anthocyanidols (Sun and Ho, 2005[47]). 50 µL of the extract appropriately diluted was mixed with 3 mL of vanillin (4 %) and 1.5 mL of HCl. After 15 min, the absorbance was measured at 500 nm. Condensed tannin contents of flowers were expressed as mg catechin equivalents (CE) per gram of dry weight through the calibration curve with catechin. The calibration curve range was 0-500 µg/mL. (R2=0.85).
Carotenoids content
Extraction
The plant material was ground in an Ultraturax homogenizer and extracted with a mixture of ethyl acetate:methanol:petroleum ether (1:1:1/v/v) (Breithaupt and Schwack, 2000[6]). Extraction was carried out under continuous agitation, in diminished light, in the presence of buthylated hydroxytoluene as antioxidant and of sodium bicarbonate, added for the prevention of epoxidic rearrangements. The extraction was repeated until the material became colorless. The combined extracts were filtered and washed in a separation funnel with 5 % of NaCl solution. The upper phase, consisting in ethyl acetate and petroleum ether was then evaporated to dryness in a rotary evaporator at 35 ºC. The residue was dissolved in diethyl-ether for saponification and spectrophotometric quantification of carotenoids.
Saponification
Saponification is necessary to release carotenoids from their ester forms and to remove the saponifiable lipids. The diethyl-ether carotenoid extract was treated with an equal volume of 30 % w/v KOH in methanol. Saponification was carried out in the dark under permanent stirring for 8 h. The extract was washed with 5 % w/v of NaCl solution until the pH of the aqueous phase was neutral. The diethyl-ether fraction containing the carotenoids was separated, evaporated to dryness and kept at -20 ºC until further chromatographic analysis.
Spectrophotometric determination of total carotenoids
The carotenoid contents were calculated by using the following formula (Kirk and Allen, 1965[27]).
Carotenoid contents (mg 100 -1g) =
A 480 x volume of extract x 10 x 100
2500 x weight of plant material
Analysis of carotenoids by HPLC method
The petal extracts (3 mL) were filtered through a 0.45 µm membrane filter. The carotenoid extracts were analyzed using a Hewlett-Packard 1100 HPLC with diode array detector (DAD). The carotenoid analyses were carried out by using reversed phase C18 column Nucleosil ODS (250 x 4.6 mm), 5 µm. The mobile phase consisted of mixtures of acetonitrile: water (9:1, v/v) with 0.25 % triethylamine (A) and ethyl acetate with 0.25 % triethylamine (B). The gradient started with 90 % A at 0 min to 50 % A at 10 min. The percentage A decreased from 50 % at 10 min to 10 % of solvent A at 20 min. The flow rate was 1 ml/min and the chromatogram was monitored at 450 nm.
Carotenoids in the flower extracts were identified by their retention times in HPLC and by their UV/Visible absorption spectra compared to reference standards. Carotenoid standards were lutein, zeaxanthin, lycopene, ß-carotene, neoxanthin and violaxanthin. The purity of these standards was estimated by HPLC and was: 95 %-ß-carotene, 98.5 %-lutein, 97.7 %-lutein and neoxanthin and violaxanthin. These standards were purchased from Sigma Aldrich.
Analysis of individual phenolic compounds by analytical RP-HPLC-MS
The HPLC-DAD-MS analyses were performed using an Agilent 1100 Series LC/MSD system with a diode array detector (DAD) coupled to a mass spectrometer (MS) (quadrupole analyser) equipped with an electrospray ionization interface (ESI, Agilent). The separation was carried out on a 250 x 4.6 mm, 4 µm hypersil ODS C18 reversed phase column. The mobile phase consisted of acetonitrile (solvent A) and water with 0.2 % sulfuric acid (solvent B). The flow rate was kept at 0.5 mL/min. The gradient program was as follows:
15 % A/85 % B 0-12 min,
40 % A/60 % B 12-14 min,
60 % A/40 % B 14-18 min,
80 % A/20 % B 18-20 min,
90 % A/10 % B 20-24 min,
100 % A 24-28 min.
The injection volume was 20 µL and peaks were monitored at 280 nm. Mass spectrometry parameters were as follows: capillary voltage, 400 v; fragmentor, 160 v; drying gas temperature, 350° C, gas flow (N2), 10 L/min; nebulizer pressure, 50 psig. The instrument was operated in positive ion mode scanning from m/z 100 to 800 at a scan rate of 1.43 s/cycle. Peaks were identified by congruent retention times compared with those of authentic standards. Phenolic compound contents were expressed in micrograms per gram of dry plant material weight.
Antioxidant activity
DPPH assay
The electron donation ability of the flower extracts was measured by bleaching of the purple-colored solution of 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) according to the method of Hanato et al. (1988[16]). 0.5 mL of 0.2 mM DPPH methanolic solution was added to flower extracts of C. tinctorius (2 mL, 10-1000 µg/mL). After an incubation period of 30 min at room temperature, the absorbance was read against a blank at 517 nm. The inhibition percentage of free radical DPPH (IP%) was calculated as follows:
IP % = [(Ablank – Asample)/Ablank] x 100
where Ablank is the absorbance of the control reaction and extract concentration providing 50 % inhibition (IC50) was calculated from the regression equation prepared from the concentration of the extracts and the inhibition percentage. BHT was used as a positive control (R2 = 0.93).
Reducing power
The method of Oyaizu (1986[36]) was used to assess the reducing power of C. tinctorius flower extracts. These extracts (1 mL) were mixed with 2.5 mL of a 0.2 M sodium phosphate buffer (pH = 6.6) and 2.5 mL of 1 % potassium ferricyanide (K3Fe (CN)6) and incubated in a water bath at 50 °C for 20 min. Then, 2.5 mL of 10 % trichloroacetic acid was added to the mixture that was centrifuged at 650 g for 10 min. The supernatant (2.5 mL) was then mixed with 2.5 mL of distilled water and 0.5 mL of 0.1 % iron (III) chloride solution. The intensity of the blue green color was measured at 700 nm. The extract concentration at which the absorbance was 0.5 for the reducing power (EC50) was obtained from the linear regression equation prepared from the concentrations of the extracts and the absorbance values. High absorbance indicates high reducing power. Ascorbic acid was used as a positive control (R2 = 0.97).
Metal-chelating power
According to Zhao (2006[53]), 0.1 mL of flower extracts was added to 0.05 mL of 2 mM FeCl2. The reaction was initiated by the addition of 0.1 mL of 5 mM ferrozine and 2.75 mL of distilled water. The mixture was shaken vigorously and left at room temperature for 10 min. The absorbance of the solution was then measured at 562 nm. The scavenging activity was calculated as follows:
IP % = [(Ablank – Asample)/Ablank] x 100
where Ablank is the absorbance of the control reaction and Asample is the absorbance in the presence of flower extract. IC50 was calculated from the plot of inhibition percentage against extract concentration. EDTA was used as a positive control (R2= 0.88).
ß-Carotene-linoleic acid assay
Antioxidant capacity of C. tinctorius extracts was evaluated by ß-carotene linoleic acid assay. The ß-carotene bleaching method is based on the loss of the yellow color of ß-carotene due to its reaction with radicals formed by linoleic acid oxidation in an emulsion. The rate of ß-carotene bleaching can be slowed down in the presence of antioxidants (Kulisic et al., 2004[28]). First, ß-carotene (0.2 mg) was dissolved in 1.0 mL of chloroform. Afterward, 200 µL of linoleic acid and 0.2 mL of Tween 80 were added and the mixture was left at room temperature for 15 min. After evaporation of chloroform, 50 mL of oxygenated distilled water was added and the mixture was shaken to form an emulsion (ß-carotene-linoleic acid emulsion). Aliquots of 3.0 mL of this emulsion were transferred into test tubes containing 0.2 mL of different extract concentrations. The tubes were shaken and incubated at 50 °C in a water bath. As soon as the emulsion was added to each tube, the zero time absorbance (A0) was measured at 470 nm using a spectrophotometer. A second absorbance (A1) was measured after 120 min. A blank, without ß-carotene, was prepared for background subtraction. Lipid peroxidation (LPO) inhibition was calculated using the following equation (Soares et al., 2009[45]):
LPO inhibition (%) = (A1/A0) x 100
The extract concentration providing 50 % antioxidant activity (IC50) was calculated from the graph of antioxidant activity percentage against extract concentration. BHT was used as standard (R2 = 0.93).
Screening of antibacterial and antifungal activities
Antibacterial activity was analyzed by the disc diffusion method (Rios and Recio, 2005[40]) against seven human pathogenic bacteria including Gram-positive Methicillin-resistant Staphylococcus ATCC 25923, Bacillus cereus ATCC 14759, Listeria monocytogenes ATCC 19195, Staphylococcus aureus ATCC 25923 and Gram-negative bacteria Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853. All bacteria were grown on Mueller Hinton plate at 30 °C for 18-24 h previous inoculation onto the nutrient agar. A loop of bacteria from the agar slant stock was cultivated in nutrient broth overnight and spread with a sterile cotton swap onto Petri dishes containing 10 mL of API suspension medium and adjusted to the 0.5 McFarland turbidity standards with a Densimat (BioMérieux). Sterile filter paper disks (6 mm in diameter) impregnated with 20 µL of plant extract (5 mg/mL) was pla-ced on the cultured plates. After 1-2 h at 4 °C, the treated Petri dishes were incubated at 25 °C or 37 °C for 18-24 h. The solvent pure methanol without extracts served as negative controls and Gentamycin was used as the positive one. The antimicrobial activity was evaluated by measuring the diameter of the growth inhibition zone around the discs. Each experiment was carried out in triplicate and the mean diameter of the inhibition zone was recorded.
The same agar-disc diffusion method was used for screening the antifungal activity of C. tinctorius flower extracts. Five yeast strains (Candida albicans, Aspergillus carbonarus and Sclerotonia sclerotoria) were first grown on Sabouraud chloramphenicol agar plate at 30 °C for 18-24 h. Several colonies of similar morphology of the clinical yeast were transferred into Api suspension medium and adjusted to 2 McFarland turbidity standard with a Densimat (BioMérieux). The inocula of the respective yeast was streaked on to Sabouraud chloramphenicol agar plates at 30 °C using a sterile swab and then dried. A sterilized 6 mm paper disc was loaded with 20 µL of flower extracts. The treated Petri dishes were placed at 4 °C for 1-2 h and then incubated at 37 °C for 18-24 h. The inhibition of fungal growth was also evaluated by measuring the diameter of the transparent inhibition zone around each disc. The average of three measurements was taken. The susceptibility of the standard was determined using a disc paper containing 300 µg of Nystatine.
Statistical analysis
All analyses were performed in triplicate, and the results are expressed as mean values ± standard deviations (SD). The data were subjected to statistical analysis using statistical program package STATISTICA (Statsoft, 1998[46]). The one-way analysis of variance (ANOVA) followed by the Duncan multiple range test was employed and the differences between individual means and each solvent used were deemed to be significant at p < 0.05.
Results and Discussion
Effect of water stress on plant growth
Water deficit severely affected all growth parameters. In fact, plants subjected to 25 % presented thinner stems with fewer, dry and smaller leaves than the control ones. As shown in Table 1(Tab. 1), there was a general decline in plant height with increasing water deficit independently of safflower variety. Under 50 %, the plant height was slightly reduced by 9.40 % and 9.25 %, respectively in 104 and Jawhara varieties. While, under 25 % this effect was more pronounced by about 47.05 % (104) and 46.02 % (Jawhara) respectively in respect to 100 %. Also, plant dry biomass was significantly (p < 0.05) affected by water treatments and was inhibited by 54.37 % and 56.96 % (104) and by 24.21 % and 29.50 % (Jawhara) under 50 % and 25 %, respectively. In addition, with the water constraint sharpness, the flower head number by plant was significantly (p < 0.05) reduced. This reduction was more pronounced in 104 (88.46 %) than Jawhara (56.25 %) under 25 % with respect to 100 %. Furthermore, this constraint induced by 50 % and 25 % led to a substantial decline in seeds number by flower head in the two varieties and Jawhara seems to be less affected by the level of drought as compared to 104. Under 50 % and 25 % respectively, seeds number was reduced by 22.22 % and 37.77 % for 104 and by 16.27 % and 37.2 % for Jawhara. In addition, petals yield decreased with water stress increase. The reduction was 29.11 % and 44.30 % for 104 and 14.08 % and 22.53 % for Jawhara under 50 % and 25 %, respectively (Table 1(Tab. 1)).
Table 1. Drought effect on C. tinctorius plant growth.
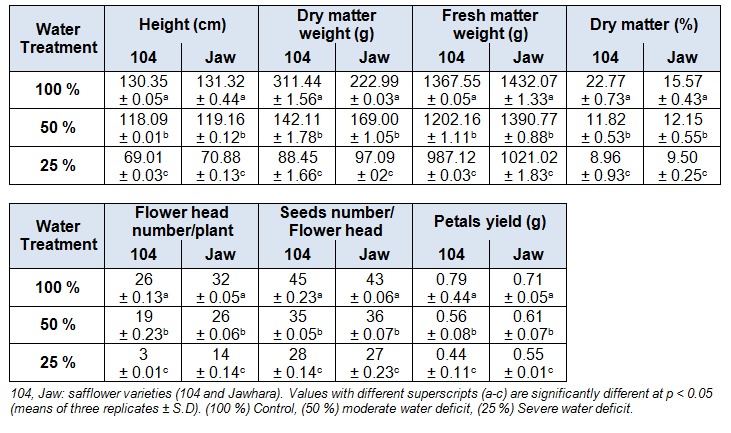
Similar results were obtained by Movahhedy-Dehnavy et al. (2009[32]) who showed a decrease in seeds yield, total biomass, number of capitula per plant and plant height in each growing season by withholding irrigation especially when water deficit stress was imposed at the flowering stage. Drought effect also increased root length of the two varieties under 50 % and 25 % with comparison to 100 % (data not shown). This increase was more pronounced in Jawhara which has deep and thick roots. In fact, root characteristics, especially root length are important for a plant to have comparatively well-established above-grounded parts by exploiting the available water (Hojati et al., 2011[17]). In contrast, Sikuku et al. (2010[44]) hadn’t the same results with the rice root where plants well-watered recorded higher root lengths than stressed ones. The authors explained that this root inhibition may be attributed to reduced extensibility of the root tip tissue and the hardening of the expanding cell walls. The general reduction in plant height and its biomass production (Table 1(Tab. 1)) in safflower agrees with previous findings on other aromatic crops conducted at single field or in pots (Laribi et al., 2009[30]). Indeed, growth involves development which is a process consisting of cell division, cell enlargement and differentiation and these processes are very sensitive to water deficit. In one hand, the inhibition of cell expansion is usually followed closely by a reduction in cell wall synthesis (Sikuku et al., 2010[44]). This may affect plant height of safflower. In another hand and in order to diminish consumption and increase absorption of water, plants often decrease their biomass production and contribute more biomass to roots in dry conditions (Albouchi et al., 2003[2]). In terms of plant growth, safflower is a tap-rooted annual crop which can tolerate environmental stresses including water stress (Movahhedy-Dehnavy et al., 2009[32]). Jawhara was the most tolerant variety among the two safflower varieties tested.
Effect of water stress on phenolic composition
Water stress doesn’t affect only the morphology, but it also severely affects the metabolism of the plant. Phenolic compounds are known to be secondary metabolic substances with anti-oxidant and anticancer properties, as well as conferring resistance to environmental disasters (Chung et al., 2006[10]). In this study, quantitative differences in different classes of polyphenols, flavonoids and proanthocyanidins of C. tinctorius flowers under water stress have been observed. Figure 1(Fig. 1) shows a noticeable change in phenolic contents of 104 and Jawhara in response to drought. In fact, total polyphenol content imposed a significant increase (p < 0.05) of about 52.39 % (104) and 51.69 % (Jawhara) under 50 % followed by a sharply decrease of these contents estimated by 19.79 % (104) and 13.45 % (Jawhara) under 25 % with respect to 100 %.
Figure 1. Effect of water stress on polyphenol, flavonoid, proanthocyanidin contents of Carthamus tinctorius varieties (a; Jawhara, b; 104). (100 %) Control, (50 %) moderate water deficit, (25 %) Severe water deficit.
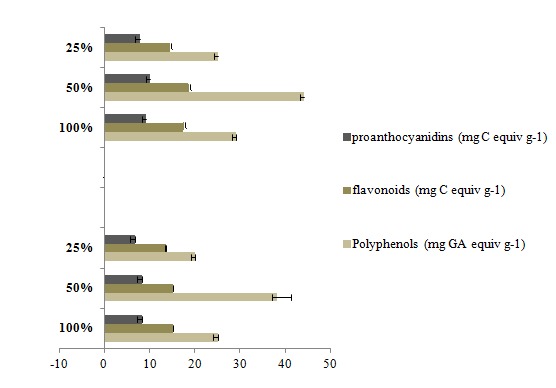
Similarly, flavonoid-content declined by about 10.31 % (104) and 15.68 % (Jawhara) under 25 % with respect to 100 %. Concerning proanthocyanidin content, this latter increased by 9.20 % in Jawhara under 50 % (Figure 1(Fig. 1)).
Our results agreed with those of Ojeda et al. (2002[35]), where biosynthesis of flavonols in Vitis vinifera berry was greater only under 50 % regime. It is thought that the decrease in phenolic compounds might result from a decline of key enzymes activity related to their biosynthesis (Chung et al., 2006[10]).
Conversely, in the case of Arabidopsis thaliana leaves, Jung (2004[23]) reported that flavonoid content increased in response to drought treatment. This suggests that abiotic constraint may increase phenolic compounds biosynthesis, as a response to the oxidative stress. This latter is generated by the formation of (ROS) in these hostile environments (Navarro et al., 2006[34]). Based on these results, it seems that drought tolerance of C. tinctorius is related to the capacity of the plant to modulate its phenolic contents in order to face to oxidative stress caused by water limiting conditions. In addition, this drought tolerant crop might have accumulated smaller amounts of free radicals and hence it produced lesser amount of antioxidant compounds. The less production of phenolics, flavonoids as well as total antioxidants by safflower may be due to an appreciably higher repair mechanism of free radical damage, which is a prominent feature of drought tolerant genotype (Kumar et al., 2011[29]). Alternatively, other antioxidants may compensate the need of low molecular weight antioxidants in drought tolerant genotypes. These results show that moderate water stress can induce shikimate pathway in tolerant cultivar (Sánchez-Rodríguez et al., 2011[41]).
Effect of water stress on phenolic compounds
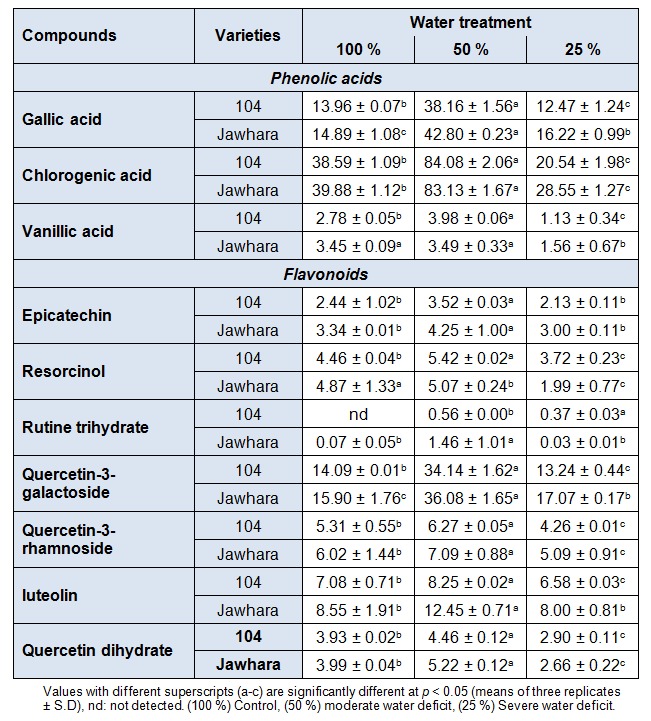
For a clear understanding of phenolic compounds responses under water deficit, it is necessary to follow changes of these individual compounds in C. tinctorius flowers with extending constraint severity. Table 2(Tab. 2) shows that changes of ten main phenolic compounds were different under drought. In fact, under 50 %, the level of the most abundant phenolic acid, gallic acid, increased by 2.73 fold (104) and by 2.87 fold (Jawhara) with respect to 100 %. The same trend was also found in flavonoids such as quercetin-3-galactoside (2.26 fold) and luteolin (1.45 fold) contents. This improvement suggested the drought tolerance of safflower. These results coincided with those reported by other authors. Indeed, the content of phenolic compounds increased under water stress conditions in the cucumber (Cucumis sativus), although no effect was found in the radish (Raphanus sativus) (Tevini et al., 1983[49]). The increase of phenolic acids content may be linked to the lignification of cell walls and, in part to the synthesis of certain amino acids maintaining osmotic adjustment in cell (Ayaz et al., 1999[4]). Therefore, this feature seems to be a biochemical response to stress conditions to remove the different toxic molecules such as ROS. On the other hand, severe water deficit (25 %) showed a slightly decrease in these phenolic compounds especially gallic acid by 9.66 % (104) and by 3.83 % (Jawhara) in comparison to 100 %. Drought was also found to enhance the biosynthesis of a new flavonoid; rutin trihydrate (0.56 ± 0.00 and 1.46 ± 1.01 mg/g DW for 104 and Jawhara respectively with comparison to 100 %). Hence this decrease may suggest drought sensitivity of safflower since the accumulation of these metabolites has been associated with increased stress tolerance (Wahid and Ghazanfar, 2006[52]).
Table 2. Contents of individual phenolic compounds (mg/g DW) of C. tinctorius flowers under water deficit.
Effect of water stress on carotenoids
Photosynthetic pigments are important to plants mainly for harvesting light and production of reducing power. Carotenoids are a large class of isoprenoid molecules that are synthesized de novo by all photosynthetic and many non-photosynthetic organisms (Jaleel et al., 2009[21]). The retention times of carotenoids from the analysed samples have been compared with those of the reference substances separated at the same conditions (Figure 2(Fig. 2)).
Figure 2. HPLC profile of carotenoids in the flower extract of C. tinctorius (1 = neoxanthin, 2 = violaxanthin, 3 = lutein, 4 = zeaxanthin, 5 = lycopene, 6 = ß-carotene) at full flowering stage.
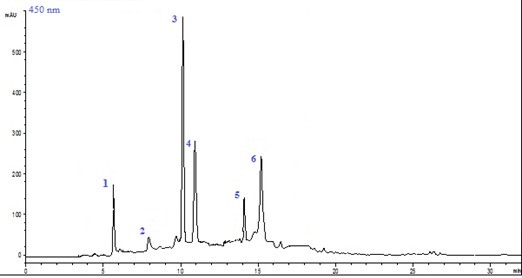
Table 3(Tab. 3) summarizes the variation of carotenoid composition of C. tinctorius flowers, grouped by families under water deficit. Jawhara was the richest variety for different carotenoid classes and lutein was the main component of xanthophylls with amounts varying from 144.85 µg/100 g DW (104) to 158.98 µg/100 g DW (Jawhara). This major component was also found to be the main compound of carotenoids of many other Asteraceae species such as Tagetes erecta (83 % and 88 % (w/w) of total carotenoids in fresh and dried extracts), Melampodium divaricatum (76 % (w/w) and Cosmos bipinnatus (77 % (w/w) (Tinoi et al., 2006[50]). While, violaxanthin had the lowest value among all carotenoid compounds which varied from 24.11 (104) to 30.22 µg/100 g DW (Jawhara) (Table 3(Tab. 3)). This difference in carotenoid amounts is related to flower colour.
Table 3. Contents of individual carotenoids (µg/100 g DW) of C. tinctorius flowers under water deficita.
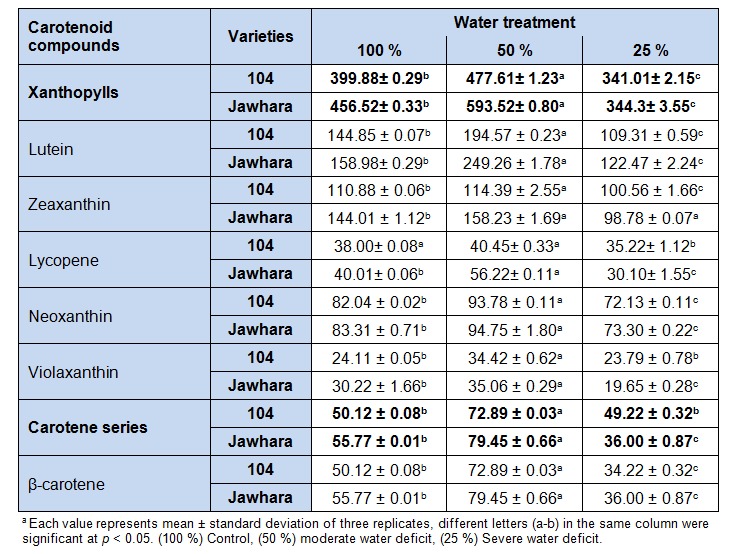
Under water deficit, the maximum increase of the total carotenoid content was observed as 18.95 % (104) and 35.19 % (Jawhara) at 50 %, while this amount was drastically reduced by 29.85 % (104) and by 45.79 % (Jawhara) at 25 % at full flowering stage. This effect was greater for ß-carotene component where its amount reduced by 35.44 % at 25 % for Jawhara. Plants retained a higher functional state of the photosynthetic machinery, justifying a reduced need for photoprotective mechanisms (non-photochemical quenching and a maintenance of the balance between energy capture and dissipative pigments (Ramalho et al., 2013[38]).
Similarly, severe water deficit (25 %) (-1.9 MPa) almost completely inhibited photosynthesis of Pisum sativum, decreased chlorophylls, ß-carotene, neoxanthin, and lutein contents and caused further conversion of violaxanthin to zeaxanthin, suggesting a damage of the photosynthetic apparatus (Iturbe-Ormaetxe et al., 1998[20]). Many other studies have been conducted aiming to establish the sensitivity of crops to water deficit. Indeed, carotenoids of rice cultivars were drastically degraded when subjected to 25 % (Cha-UM et al., 2010[9]). However, these photosynthetic pigments remained relatively constant in soybean (Tzenova et al., 2010[51]).
Carotenoids show multivarious roles in drought tolerance including light harvesting and protection from oxidative damage caused by drought (Jaleel et al., 2009[21]). Thus, increased contents specifically of carotenoids are important for safflower tolerance against water stress.
Under water deficit, the maximum increase of the total carotenoid content was observed as 18.95 % (104) and 35.19 % (Jawhara) at 50 %, while this amount was drastically reduced by 29.85 % (104) and by 45.79 % (Jawhara) at 25 % at full flowering stage. This effect was greater for ß-carotene component where its amount reduced by 35.44 % at 25 % for Jawhara. Plants retained a higher functional state of the photosynthetic machinery, justifying a reduced need for photoprotective mechanisms (non-photochemical quenching ) and a maintenance of the balance between energy capture and dissipative pigments (Ramalho et al., 2013[38]).
Similarly, severe water deficit (25 %) (-1.9 MPa) almost completely inhibited photosynthesis of Pisum sativum, decreased chlorophylls, ß-carotene, neoxanthin, and lutein contents and caused further conversion of violaxanthin to zeaxanthin, suggesting a damage of the photosynthetic apparatus (Iturbe-Ormaetxe et al., 1998[20]). Many other studies have been conducted, aiming to establish the sensitivity of crops to water deficit. Indeed, carotenoids of rice cultivars were drastically degraded when subjected to 25 % (Cha-UM et al., 2010[9]). However, these photosynthetic pigments remained relatively constant in soybean (Tzenova et al., 2010[51]).
Carotenoids show multivarious roles in drought tolerance including light harvesting and protection from oxidative damage caused by drought (Jaleel et al., 2009[21]). Thus, increased contents specifically of carotenoids are important for safflower tolerance against water stress.
Effect of water stress on antioxidant activity
In several herbaceous plant species, antioxidative systems were induced in response to drought stress, suggesting that water deficit required an increase of protective systems for stress compensation (Jung, 2004[23]). The effect of drought on the antioxidant activities is shown in Table 4(Tab. 4). The free-radical-scavenging activity of flower extracts was assessed using DPPH and ABTS tests. We noticed that all extracts exhibiting IC50 values from 1.45 ± 0.21 to 3.4 ± 1.21 µg/mL, were more effective to scavenge the DPPH radical than the synthetic antioxidant BHT (IC50 = 16 µg/mL). Therefore C. tinctorius showed appreciable repair to free radical damages and hence a prominent drought tolerance. These results confirmed that C. tinctorius is well adapted to water stress.
Table 4. Table 4: Antioxidant properties against ABTS.+, DPPH. radicals as well as chelating, lipid peroxidation inhibition (IC50 in µg/mL) and reducing power (EC50 mg/mL) of C. tinctorius flower extracts under water deficit.
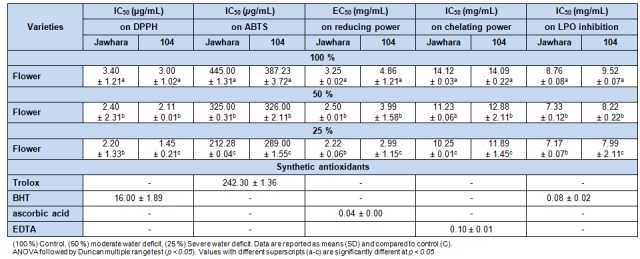
Radical scavenging activity against DPPH and ABTS radicals showed a gradual increase by 35.29 % and 33.33 % (DPPH) and by 52.35 % and 25.36 % (ABTS) especially under 25 %, respectively for Jawhara and 104. A similar trend was also found in reducing power assay where levels increased by 23.07 % and 31.69 % (Jawhara) and by 17.955 % and 38.69 % (104) under 50 % and 25 %, respectively.
Concerning chelating ability assay, although safflower extract showed low ability to chelate irons, its capacity increased by 20.46 % and 27.40 % (Jawhara) and by 8.58 % and 15.61 % (104) under 50 % and 25 %, respectively in comparison to 100 %. Finally, ß-carotene-linoleate bleaching assay showed that 50 % increased the prevention of ß-carotene bleaching ability by 1.22 and 1.19 fold, respectively for Jawhara and 104. Several studies have pointed out that drought-tolerant species increased their antioxidant enzyme activities in response to drought treatment, whereas drought-sensitive species failed to do so (Masoumi et al., 2011[31]). In addition, safflower can be considered as a candidate crop in dryland agro-ecosystems due to its potential of growth under water stress and its economical value in terms of both oil and seeds (Kar et al., 2007[25]). Generally, the increase of antioxidant activities under drought may contribute to the stability of the plant to survive in the adverse arid environment.
Effect of water stress on antimicrobial activity
The effect of water stress on the antimicrobial activity of C. tinctorius flower extracts was investigated. The methanolic extracts of two safflower varieties using agar-well diffusion method were tested against five Gram-positive bacteria (Escherichia coli, Staphylococcus aureus, Bacillus cereus, Staphylococcus metecilin resistant, Listeria monocytogenes), one Gram-negative bacteria (Pseudomonas aeruginosa) and three yeast strains (Candida albicans, Aspergillus carbonarus and Sclerotonia sclerotoria). The analysis of the data revealed that among the tested extracts, Jawhara extracts exhibited the highest rates of antibacterial activity than 104.
As shown in Table 5(Tab. 5), results displayed that safflower extracts have a moderate antibacterial activity with an inhibition zone varying between 8 to 12 mm against bacterial strains. While a slight effect was observed against yeast strains with an inhibition zone between 6 to 7 mm. It was interesting to note that the yeasts showed less sensitivity to the investigated extracts than the other Gram-positive and negative bacteria. Indeed, Akroum et al. (2009[1]) showed that methanolic extracts of leaves and flowers of Algerian safflower showed high antimicrobial activity against Staphylococcus aureus, Bacillus cereus, Candida albicans (MIC = 0.090 mg/mL) and Escherichia coli (MIC = 0.100 mg/mL). Similarly, Taskova et al. (2002[48]) have found that H2O/MeOH fraction of Carthamus lanatus aerial parts exhibited noticeable antimicrobial activity against Staphylococcus aureus (inhibition zone = 22.33 mm), Escherichia coli (inhibition zone = 12 mm) and Candida albicans (inhibition zone = 13.33 mm) but lower than streptomycin (inhibition zone = 28 mm) used as reference. However, C. tinctorius extracts showed higher antibacterial activity than other species in the literature such as water extract of the stem bark of Jatropha curcas against Pseudomonas aeruginosa (inhibition zone = 4 mm), Escherichia coli (inhibition zone = 7 mm) and Bacillus cereus (inhibition zone = 5 mm) (Igbinosa et al., 2009[19]). As for drought effect, it significantly (p < 0.05) reduced the antimicrobial activity of safflower extracts either with Jawhara and 104 varieties. In fact, there was noticeable increase in the inhibition zone due to the water treatment by 30 % and 10 % especially for Aspergillus carbonarus and Pseudomonas aerogenosa under 50 %.
Table 5. Antibacterial and antifungal activities of C. tinctorius flower extractsA against human .
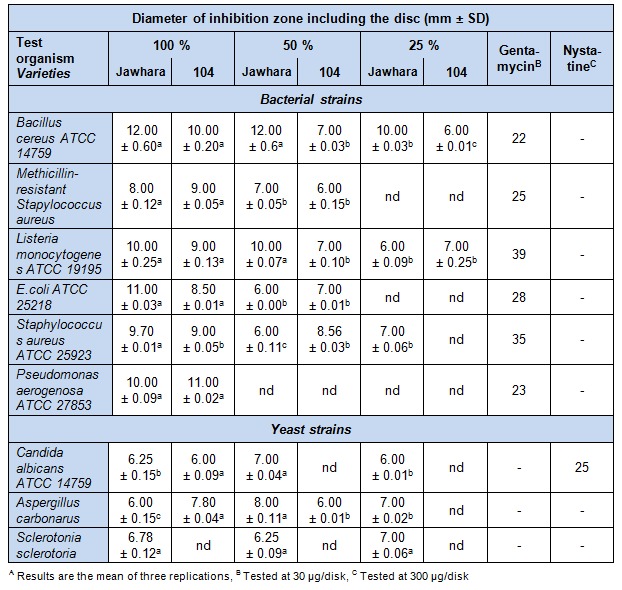
pathogenic bacteria and fungi strains under water deficit.
However, a decrease of the antimicrobial activity of other flower extracts was showed especially at 25 % (Table 5(Tab. 5)). Thus, drought reduced the bioactive substances in the flowers which in turn affected the ability of the extracts to inhibit pathogenic activity, thus affecting the efficacy of the plants as medicinal plants. In addition, our phytochemical screening revealed the presence of tannins, phenolic acids and flavonoids in the methanolic extract which could be responsible for this noteworthy activity (Cano et al., 2008[7]). In fact, the antibacterial activity of flavonoids is probably due to their ability to react with extracellular and soluble proteins and to complex with bacterial cell walls (Schinor et al., 2007[42]). This may justify the decrease of the antibacterial activity under water stress due to the decrease in phenol composition. Moreover, under stress, a decline in the acquisition of a resource produces a change in the C/N ratio that corresponds with a change in the balance of chemical compounds (Bouaziz et al., 2009[5]). In contrast, the antibacterial activity of crude extract of Chrysanthemum indicum was increased by 2 fold under osmotic stress (5 % NaCl w/v) against B. cereus and Monocytogenes. This can be explained by an inhibition in growth rate and a reduction in regulated proteins in adaptation to osmotic stress (Pitinidhipat and Yasurin, 2012[37]). Generally, this investigation showed that antibacterial activity was determined by a complex mix of factors including hydrogen bonding parameters, water solubility and molecular size (Griffin, 2000[14]).
Conclusion
In summary, our results highlight the capacity of safflower cultivar to withstand drought conditions. The application of water deficit was found to significantly (p < 0.05) influence the secondary metabolites production especially under 50 %. Moreover, it stimulates antioxidant activities of flower extracts in response to increasing water deficit level. Hence, greater synthesis of phenolic compounds and the majority presence of flavonoids would be a key in the protection against damage caused by water stress. This fact could be considered as one of C. tinctorius adaptation aspect in drought conditions. Therefore, the development of more drought-resistant crops is necessary to alleviate future threats to food availability in the world.
Acknowledgements
The authors are grateful to Pr. Abderrazak Smaoui for the botanic identification and to Pr. Chedly Abdelly (Borj-Cedria Technopol) for antioxidant activity assays.
References
- 1.Akroum S, Satta D, Lalaoui K. Antimicrobial, antioxidant, cytotoxic activities and phytochemical screening of some Algerian plants. Eur J Sci Res. 2009;31:289–295. [Google Scholar]
- 2.Albouchi A, Bejaoui Z, El Aouni ZMH. Influence d’un stress hydrique modereou severe sur la croissance de jeunes plants de Casuarina glauca. Secheresse. 2003;14:137–142. [Google Scholar]
- 3.Alcázar R, Marco F, Cuevas JC, Patrón M, Ferrando A, Carrasco P, et al. Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett. 2006;28:1867–1876. doi: 10.1007/s10529-006-9179-3. [DOI] [PubMed] [Google Scholar]
- 4.Ayaz FA, Kadioglu AR, Turgut R. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. J Plant Sci. 1999;80:373–378. [Google Scholar]
- 5.Bouaziz M, Dhouib A, Loukil S, Boukhris M, Sayadi S. Polyphenols content, antioxidant and antimicrobial activities of extracts of some wild plants collected from the south of Tunisia. Afr J Biotechnol. 2009;8:7017–7027. [Google Scholar]
- 6.Breithaupt DE, Schwack W. Determination of free and bound carotenoids in paprika (Capsicum annuum L.) by LC/MS. Eur Food Res Technol. 2000;211:52–55. [Google Scholar]
- 7.Cano A, Medinaan A, Bermejo A. Bioactive compounds in different Citrus varieties. Discrimination among cultivars. J Food Comp Anal. 2008;21:377–381. [Google Scholar]
- 8.Charles O, Joly R, Simon JE. Effect of osmotic stress on the essential oil content and composition of peppermint. Phytochem. 1994;29:2837–2840. [Google Scholar]
- 9.Cha-UM S, Yooyongwech S, Supaibulwatana K. Water deficit stress in the reproductive stage of four indica rice (Oryza Sativa L.) genotypes. Pak J Bot. 2010;42:3387–3398. [Google Scholar]
- 10.Chung MI, Kim JJ, Lim JD, Yu CY, Kim SH, Hahna SJ. Comparison of resveratol, SOD activity, phenolic compounds and free amino acids in Rhemannia glutinosa under temperature and water stress. Env Exp Bot. 2006;56:44–53. [Google Scholar]
- 11.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Sci Food Agric. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 12.Edreva A, Velikova V, Tsonev T, Dagnon S, Gürel A, Aktaş L, et al. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen Appl Plant Physiol. 2008;34:67–78. [Google Scholar]
- 13.Esendal E, Istanbulluoglu A, Arslan B, Pasa C. Safflower: unexploited potential AND world adaptability. The 7th International Safflower Conference, 2008, 3-6 Nov. New South Wales, Australia: Australian National Wine and Grape Industry Training Centre Wagga Wagga; 2008. Effect of water stress on growth components of winter safflower (Carthamus tinctorius L.) pp. 1–6. [Google Scholar]
- 14.Griffin S. Aspects of antimicrobial activity of terpenoids and the relationship to their molecular structure. Ph. D. Thesis. Sydney, AUS: University of Western Sydney; 2000. p. Aspects of antimicrobial activity of terpenoids and the relationship to their molecular structure. [Google Scholar]
- 15.Guerfel M, Baccouri O, Boujnah D, Zarrouk M. Changes in lipid composition, water relations and gas exchange in leaves of two young ‘Chemlali’ and ‘Chetoui’ olive trees in response to water stress. Plant Soil. 2008;311:121–129. [Google Scholar]
- 16.Hanato T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effect. Chem Pharmacol. 1988;36:1090–1097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- 17.Hojati M, Modarres-Sanavy SAM, Karimi M, Ghanati F. Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Physiol Plant. 2011;33:105–112. [Google Scholar]
- 18.Horner JD. Nonlinear effects of water deficits on foliar tannin concentration. Biochem System Ecol. 1990;18:211–213. [Google Scholar]
- 19.Igbinosa OO, Igbinosa EO, Aiyegoro OA. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn) Afr J Pharm Pharmacol. 2009;3:58–62. [Google Scholar]
- 20.Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M. Oxidative damage in Pea plants exposed to water or paraquat. Plant Physiol. 1998;116:173–181. [Google Scholar]
- 21.Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-juburi JH, Somasundaram R, et al. Drought stress in plants: A review on morphological characteristics and pigments composition. Inter J Agric Biol. 2009;11:100–105. [Google Scholar]
- 22.Janzik S, Preiskowski H, Kneifel H. Ozone has dramatic effects on the regulation of the prechorismate pathway in tobacco (Nicotiana tabacum L. cv. Bel W3) Planta. 2005;223:20–27. doi: 10.1007/s00425-005-0060-8. [DOI] [PubMed] [Google Scholar]
- 23.Jung S. Variation in antioxidant metabolism of young and mature leaves of Arabidopsis thaliana subjected to drought. Plant Sci. 2004;166:459–466. [Google Scholar]
- 24.Kafka S. Safflower production in California. Agric Nat Res. 1965:4–5. [Google Scholar]
- 25.Kar G, Kumar A, Martha M. Water use efficiency and crop coefficients of dry season oilseed crops. Agric Water Manag. 2007;87:73–82. [Google Scholar]
- 26.Kirda C. Deficit irrigation scheduling based on plant growth stages showing water stress tolerance.Deficit irrigation practices. Rome: FAO; 2002. pp. 3–10. (FAO Corporate Document Repository 22). [Google Scholar]
- 27.Kirk JOT, Allen RL. Dependence of chloroplast pigment on actidione. Arch Biochem Biophys Res Commun. 1965;21:523–530. doi: 10.1016/0006-291x(65)90516-4. [DOI] [PubMed] [Google Scholar]
- 28.Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633–640. [Google Scholar]
- 29.Kumar A, John MM, Gul MZ, Bimolata W, Ghazi IA. International Conference on Food Engineering and Biotechnology. IPCBEE. Singapore: IACSIT Press; 2011. Differential responses of non-enzymatic antioxidative system under water deficit condition in Rice (Oryza sativa L.) pp. 176–179. [Google Scholar]
- 30.Laribi B, Bettaieb I, Kouki K, Sahli A, Mougou A, Marzouk B. Water deficit effects on caraway (Carum carvi L.) growth, essential oil and fatty acid composition. Ind Crop Prod. 2009;30:372–379. [Google Scholar]
- 31.Masoumi H, Darvish F, Daneshian J, Normohammadi G, Habibi D. Effects of water deficit stress on seed yield and antioxidants content in soybean (Glycine max L.) cultivars. Afr J Agric Res. 2011;6:1209–1218. [Google Scholar]
- 32.Movahhedy-Dehnavy M, Modares-Sanavy SAM, Mokhtassi-Bidgoli AM. Foliar application of zinc and manganese improves seed yield and quality of safflower (Carthamus tinctorius L.) grown under water deficit stress. Ind Crops Prod. 2009;30:82–92. [Google Scholar]
- 33.Nabipour M, Meskarbashee M, Yousefpour H. The effect of water deficit on yield components of safflower (Carthamus tinctorius L.) Pak J Biol Sci. 2007;10:421–426. doi: 10.3923/pjbs.2007.421.426. [DOI] [PubMed] [Google Scholar]
- 34.Navarro JM, Flores P, Garrido C, Martinez V. Changes in the contents of antioxidant compounds in pepper fruits at ripening stages, as affected by salinity. Food Chem. 2006;96:66–73. [Google Scholar]
- 35.Ojeda H, Andary C, Kraeva E, Carbonneau A, Deloire A. Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera cv. Shiraz. Am J Enol Viticul. 2002;53:261–267. [Google Scholar]
- 36.Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction. Jap J Nut. 1986;44:307–315. [Google Scholar]
- 37.Pitinidhipat N, Yasurin P. Antibacterial activity of Chrysanthemum indicum, Centella asiatica and Andrographis paniculata against Bacillus cereus and Listeria monocytogenes under osmotic stress. Assumption Univ J Technol. 2012;15:239–245. [Google Scholar]
- 38.Ramalho JC, Zlatev ZS, Leitão AE, Pais IP, Fortunato AS, Lidon FC. Moderate water stress causes different stomatal and non-stomatal changes in the photosynthetic functioning of Phaseolus vulgaris L. genotypes. Plant Biol. 2013 doi: 10.1111/plb.12018.. Available from: http://dx.doi.org/10.1111/plb.12018. [DOI] [PubMed] [Google Scholar]
- 39.Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161:1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Rios JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Sánchez-Rodríguez E, Moreno AD, Ferreres F, Rubio M, Wilhelmi M, Ruiz JM. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry. 2011;72:723–729. doi: 10.1016/j.phytochem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Schinor EC, Salvador MJ, Ito IY, Dias DA. Evaluation of the antimicrobial activity of crude extracts and isolated constituents from Chresta scapigera. Braz J Microbiol. 2007;38:145–149. [Google Scholar]
- 43.Scholander PF, J Hammel H, Hemmingsen EA, Bradstreet ED. Sap pressure in vascular plants. Sci. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- 44.Sikuku PA, Netondo GW, Onyango J C, Musyimi DM. Effects of water deficit on physiology and morphology of three varieties of Nerica rainfed rice (Oryza sativa L.) J Agric Biol Sci. 2010;5:23–28. [Google Scholar]
- 45.Soares AA, Marques de Souza CG, Daniel FM, Ferrari GP, Gomes da Costa SM, Peralta RM. Antioxidant activity and total phenolic content of Agaricus brasiliensis (Agaricus blazei Murril) in two stages of maturity. Food Chem. 2009;112:775–781. [Google Scholar]
- 46.Statsoft, Inc., editor STATISTICA for Windows (Computer program electronic 703 manual). Tulsa. Tulsa: Statsoft, Inc.; 1998. [Google Scholar]
- 47.Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90:743–749. [Google Scholar]
- 48.Taskova R, Mitova M, Najdenski H, Tzvetkova I, Duddeck H. Antimicrobial activity and cytotoxicity of Carthamus lanatus. Fitoterapia. 2002;73:540–543. doi: 10.1016/s0367-326x(02)00184-3. [DOI] [PubMed] [Google Scholar]
- 49.Tevini M, Iwanzik W, Teramura AH. Effects of UVB radiation on plants during mild water stress II. Effects on growth, protein and flavonoid content. Z Pflanzenphysiol. 1983;110:459–467. [Google Scholar]
- 50.Tinoi J, Rakariyatham N, Deming RL. Determination of major carotenoid constituents in petal extracts of eight selected flowering plants in the North of Thailand. Chiang Mai J Sci. 2006;33:327–334. [Google Scholar]
- 51.Tzenova V, Kirkova Y, Stoimenov G. BALWOIS, 2010-Ohrid, Republic of Macedonia-25, 29 May, 2010. BALWOIS. Water deficit influence during different growth stages on the soybean yield. [Google Scholar]
- 52.Wahid A, Ghazanfar A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J Plant Physiol. 2006;163:723–730. doi: 10.1016/j.jplph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Zhao H, Dong J, Lu J, Chen J, Li Y, Shan L, et al. Effect of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in Barely (Hordeum vulgare L.) J Agric Food Chem. 2006;54:7277–7286. doi: 10.1021/jf061087w. [DOI] [PubMed] [Google Scholar]


