Abstract
Increased oxidative stress and hormonal imbalance have been hypothesized to underlie infertility in obese animals. However, recent evidence suggests that Ghrelin and Stem Cell Factor (SCF) play an important role in fertility, in lean individuals. Therefore, this study aimed at investigating whether changes in the levels of Ghrelin and SCF in rat testes underlie semen abnormal parameters observed in obese rats, and secondly, whether endurance exercise or Orlistat can protect against changes in Ghrelin, SCF, and/or semen parameters in diet induced obese rats. Obesity was modelled in male Wistar rats using High Fat Diet (HFD) 12-week protocol. Eight week-old rats (n=40) were divided into four groups, namely, Group I: fed with a standard diet (12 % of calories as fat); Group II: fed HFD (40 % of calories as fat); Group III: fed the HFD with a concomitant dose of Orlistat (200 mg/kg); and Group IV: fed the HFD and underwent 30 min daily swimming exercise. The model was validated by measuring the levels of testosterone, FSH, LH, estradiol, leptin, triglycerides, total, HDL, and LDL cholesterol, and final change in body weight. Levels were consistent with published obesity models (see Results). As predicted, the HFD group had a 76.8 % decrease in sperm count, 44.72 % decrease in sperm motility, as well as 47.09 % increase in abnormal sperm morphology. Unlike the control group, in the HFD group (i.e. obese rats) Ghrelin mRNA and protein were elevated, while SCF mRNA and protein were diminished in the testes. Furthermore, in the HFD group, SOD and GPx activities were significantly reduced, 48.5±5.8 % (P=0.0012) and 45.6±4.6 % (P=0.0019), respectively, while TBARS levels were significantly increased (112.7±8.9 %, P=0.0001). Finally, endurance exercise training and Orlistat administration individually and differentially protected semen parameters in obese rats. The mechanism includes, but is not limited to, normalizing the levels of Ghrelin, SCF, SOD, GPx and TBARS. In rat testes, diet induced obesity down regulates SCF expression, upregulates Ghrelin expression, and deteriorate oxidative stress levels, which are collectively detrimental to semen parameters. Exercise, and to a lesser extent Orlistat administration, protected effectively against this detrimental effect.
Keywords: stem cell factor, Ghrelin, infertility, diet induced obesity, exercise, Orlistat
Introduction
It is estimated that more than 1 billion adults around the world are overweight and at least one third of this population has a BMI that exceeds 30 kg/m2, and as such are classified as obese (Ogden et al., 2006; Pasquali, 2006[55]; Ferris and Crowther, 2006[22]). While genetic predisposition, age, and environmental factors may contribute to a person’s tendency to gain weight, it is accepted that the two primary causes of obesity are increased intake of energy-rich foods and reduced physical activity (Ogden et al., 2006[53]). While obesity has been associated with a host of cardiovascular diseases, metabolic syndrome, and a wide variety of endocrine abnormalities, recent data suggested a potential link between obesity and male infertility (Pasquali, 2006[55]; Ferris and Crowther, 2011[22]). This association has merited investigation over the past decade because of the concurrent trends of rising obesity, increasing male factor infertility, and declining semen quality (Hammoud et al., 2008[33]).
There are now several but little population-based studies showing that overweight and obese men have up to 50 % higher rate of sub-fertility when compared to men with normal weight (WHO, 2005[79]; Sunderam et al., 2009[64]). These studies have shown that overweight and obese men present hormonal changes such as lower plasma levels of sex hormone-binding globulin, total and free testosterone, luteinizing hormone (LH) and follicle-stimulating hormone (Haffner et al., 1993[31]; Magnusdottir et al., 2005[50]). Nevertheless, lower sperm count (Jensen et al., 2004[37]; Sallmen et al., 2006[61]), reduced semen quality (Magnusdottir et al., 2005[50]), decreased normal-motile sperms and increased DNA fragmentation index (Magnusdottir et al., 2005[50]) were described in men who showed increased body mass index (BMI) and diet-induced obesity (Bakos et al., 2011[7]; Fernandez et al., 2011[20]). Decreased ejaculate volumes and lower fertilization rate were also reported in rats (Bakos et al., 2011[7]; Ghanayem et al., 2010[27]), as well as increased number of couples seeking reproductive technologies (Wang et al., 2008[77]; Sunderam et al., 2009[64]). Intervention wise, Sallmen et al. (2006[61]) reported that programs including diet, exercise or medication to prevent obesity may improve men’s reproductive health and save medical costs for infertility treatment.
Ghrelin is an acylated polypeptide hormone that is secreted predominantly by P/D1 cells lining the fundus of human stomach and epsilon cells of the pancreas (Inui et al., 2004[36]). It plays a major role in the control of growth hormone, energy balance, food intake as well as body weight (Broglio et al., 2002[14]). However, recent evidence indicates that Ghrelin carries out a wide array of peripheral effects including the pancreas, lymphocytes, kidney, lung, heart, pituitary, brain, ovaries, placenta and testis (Broglio et al., 2002[14]; Gualillo et al., 2003[29]; Van der Lely et al., 2004[71]). In the testis, Ghrelin immunoreactivity has been localized mainly to Leydig cells, which release testosterone in response to LH release from the anterior pituitary. Ghrelin immunoreactivity is also found to a lesser extent in Sertoli cell precursor located in the seminiferous tubules. Those are known to express receptors for Follicular Stimulating Hormone (Gaytan et al., 2004[26]), suggesting a role in the regulation of spermatogenesis. Indeed studies have shown that Ghrelin delays pubertal onset in male rats inhibits stimulated testicular testosterone secretion and control of proliferation of Leydig cells (Tena-Sempere et al., 2002[67]; Barreiro et al., 2004[9]). In the pituitary, Ghrelin suppressed LH pulse frequency in rats, sheep, monkeys (Fernandez-Fernandez et al., 2004[21]; Harrison et al., 2008[34]; Vulliemoz et al., 2008[76]) and humans (Lanfranco et al., 2008[43]). Exogenous Ghrelin has been shown to inhibit LH secretion in rats, both in vivo and in vitro (Furuta et al., 2001[25]; Fernandez-Fernandez et al., 2004[21]) and suppresses FSH secretion in males and females (Vulliemoz et al., 2008[76]; Lanfranco et al., 2008[43]). Further, intratesticular injection of Ghrelin (15 µg for 2 days) in adult rats, inhibited SCF mRNA expression (Barreiro et al., 2004[9]). Ghrelin is a key signal for germ cell production, a putative regulator of Leydig cell development and survival factor for the different cell types in the seminiferous epithelium (e.g. spermatogonia) (Rossi et al., 2000[59]; Lanfranco et al., 2008[43]). Such inhibitory action of Ghrelin on SCF has also been detected in vitro using cultures of staged seminiferous tubules suggesting that immature Leydig cells induce differentiation in an SCF-dependent mechanism (Barreiro et al., 2004[9]).
Incidentally, little attention was directed to the role of testicular expression of SCF and/or Ghrelin in obese subjects or animals with sexual dysfunction. The hypothesis being tested here is that obesity induces Ghrelin expression, which in turn downregulates SCF and results in semen abnormalities; an effect that is reversible non-pharmacologically using endurance exercise or pharmacologically using lipase inhibitor Orlistat. If this hypothesis is correct, then this will reveal new and effective therapeutic strategies for obesity-induced reproductive impairment, including weight reduction (Strain et al., 1988[63]; Bastounis et al., 1998[10]; Kaukua et al., 2003[38]; Van Dorsten and Lindley, 2008[72]; Hammoud et al., 2009[32]; Villareal et al., 2011[75]), and/or SCF gene expression-modifiers (i.e. pharmacological and non-pharmacological).
Orlistat (Xenical), is a pharmacological agent that promotes weight loss in obese subjects via inhibiting gastric and pancreatic lipase. At three daily doses of 120 mg, it reduces fat absorption by 30 % and has been proven useful n facilitating both weight loss and weight maintenance (Tiikkainen et al., 2004[68]). In clinical use, lipase inhibitors may be effective in reducing dietary fat intake by reducing both the consumption and absorption of fat (Ellrichmann et al., 2008[18]). It has been reported that Orlistat is highly efficient when given in conjugation with a high fat diet. Indeed, higher doses of Orlistat provided to rats led to 54 % reduction in fat absorption, whereas, in humans, the expected reduction was 30 % (Filippatos et al., 2008[24]).
An alternative intervention to weight control and reduction is endurance exercise. There is a considerable body of literature supporting the use of endurance exercise as a protocol for weight loss and health improvement. Body weight reduction in humans or animals promoted by high-intensity exercise training originated from the observation that following high-intensity glycogen-depleting exercise, lipid usage during recovery periods was greatly elevated (Yoshioka et al., 2001[82]). When exercise results in glycogen depletion, muscle glycogen repletion is of high metabolic priority, resulting in the preferential use of intramuscular triacylglycerol and circulating lipids by the recovering skeletal muscle (Kiens and Richter, 1998[39]).
In this study, the effects of HFD-induced obesity on the levels of testicular Ghrelin, SCF, TBARS, activities of SOD and GPx, and on the standard semen parameters is being tested. The second part of the study focused on assessing the effect of Orlistat alone, weight loss pharmacotherapy, and exercise alone, as a non-pharmacotherapy, on the above named markers when co-administered with HFD protocol.
Materials and Methods
Materials
Orlistat was obtained from Sigma Pharmaceutical Industries (KSA) as capsules; each containing 120 mg Orlistat. ELISA kits for detecting rat serum leptin and Ghrelin were purchased from Abcam Biochemicals (USA; Cat. No. ab100773 and ab120231, respectively). ELISA kits for detecting rat serum total testosterone, estradiol and FSH were purchased from Cayman Chemical Company (USA; Cat. No. 582701, 582251, 500710, respectively). ELISA kit for detecting rat serum LH was obtained from Kamiya Biomedical Company (USA; Cat. No. KT-21064). Assay kit for determination of Malondialdehyde (MDA) in tissues was obtained supplied from NWLSS (USA; Cat. No. NWK-MDA01). Determination of tissue levels of Superoxide Dismutase (SOD) and glutathione peroxidase (GPx) activities was performed using assay kits from Caymen Chemicals (USA; Cat. No. 706002 and 703102, respectively). Serum lipids including total triglycerides (TG), total cholesterol (TC), High Density Lipoproteins (HDL) and Low Density Lipoprotein cholesterol (LDL) were determined using colorimetric Kits from Human Company (Germany).
Animals
Eight-week old male Wistar rats (n=40) weighing 280-300 g were obtained from rats breeding colony at the Animal House of the College of Medicine at King Khalid University, Abha, Saudi Arabia. Rats were housed in a 4 rat-cages. Rats in all treatment groups were preconditioned for one week prior to implementing treatment protocol. During this time, rats received standard chow diet and water ad-libitum and were kept at room temperature of 22 ± 2 °.
C, relative humidity of 55 ± 10 % and a light/dark cycle of 12 hours. All experimental procedures were conducted in strict compliance with the Animal Welfare Act, Public Health Services Policy, National Institute of Health Guide for the Care and Use of Laboratory Animals as well as the protocol approved by King Khalid University Animal Care Committee.
Experimental design
Rats were permitted to adapt for one week prior to implementing the protocol that follows. Four rat groups, 10 each, were formed: Group 1 (Control; non-obese): included rats which were fed a standard diet (12 % of calories as fat; Table 1(Tab. 1)) for 12 consecutive weeks; Group 2 (HFD): included rats which were fed HFD (40 % of calories as fat; Table 1(Tab. 1)) for 12 consecutive weeks according to the protocol of Tuzcu et al. (2011[70]); Group 3 (HFD + Orlistat): included rats which were fed the HFD and received a concomitant therapeutic dose of Orlistat (200 mg/kg) for 12 weeks according to the method of Nishioka et al. (2003[52]); and Group 4 (HFD + exercise): included rats that were fed the HFD and underwent a simultaneous daily swimming exercise (30 min/day) for 12 consecutive weeks. Swimming exercise was done in a glass tank (dimensions: 100 cm (L)*40 cm (W)*60 cm (H)) and water depth in the tank was set at 30 cm. The swimming training was always performed at water temperature of 32 °C between 10:00 to 12:00 h a.m. This method of endurance exercise training was selected because it showed advantages over other training exercise protocols including treadmill protocol; namely swimming is a natural ability in rats as well as the strength of this exercise to generate physiologically significant exertion in rats needed for this experimental design (Lee et al., 2009[45]; Da Luz et al., 2011[16]). Rats were adapted to the water environment before the beginning of the experiment by placing them in shallow water at 32 °C between 10:00 to 12:00 a.m. The adaptation period was carried out during the week prior to swimming training onset. The purpose of the water adaption was to reduce stress without promoting physical training adaption.
Table 1. Ingredients and nutrient composition of rat high-fat diet.
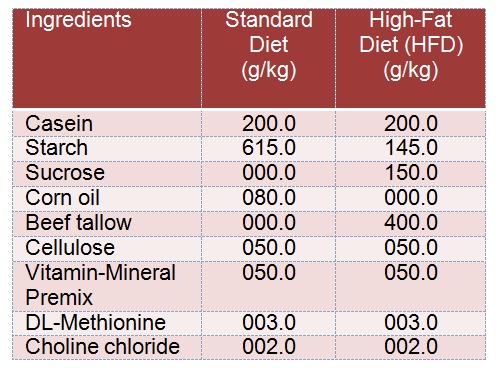
Methods
(A) Body weight gain and biochemical analysis
Body weight for all rats in every group was recorded before study initiation (Day 0) and at the end of week 12 of the protocol. Then, rats were anesthetized with diethyl ether and 3 ml blood samples were collected, using 3 ml syringe directly from the heart using ventricular puncture method, into plain 5 ml untreated glass tubes where they were allowed to clot for 15 min at RT. Samples were centrifuged at 4000 rpm for 10 min to separate the serum, which was used to determine the levels of TG, TC, HDL, LDL, testosterone, estradiol, FSH, LH, leptin as well as Ghrelin, as per manufacturer’s instructions.
(B) Adiposity index
After blood collection, animals were sacrificed by decapitation, and adipose tissue was isolated and weighed from the epididymal, visceral and retroperitoneal pad. Adiposity index was determined by the sum of epididymal, visceral and retroperitoneal fat weights divided by body weight × 100 %, then expressed as adiposity percentage (i.e. Adiposity Index; Amato et al., 2014[4]). Further, the right testis and epididymis were removed and their weights recorded (i.e. absolute weight). Epididymis relative weight to body weight was calculated, and the epididymis was used for fresh sperm count. The right testis from each rat in all 4 groups was then frozen at -80 °C for the determination of daily sperm production. At the same time, the left testis was frozen in liquid nitrogen and stored at -80 °C which was used later for the determination of Ghrelin and Ghrelin mRNA, Ghrelin and SCF protein levels, MDA levels, and the activities of SOD and GPx.
(C) Semen analysis: sperm count and motility
Right cauda epididymis from each rat in all four groups was weighed, diluted in 1:20 physiological saline solution (0.9 % NaCl) in a Petri dish and minced with a scalpel blade in the mid-to-distal region of the epididymis. The suspension was kept at 37 °C for 5 min to allow the sperms to disperse in the medium. Sperm suspension was gently mixed 20 times and placed in a hemocytometer where the total number of sperms were counted under a Nikon microscope (Nikon Eclips E600) at 400X final magnification. Sperms were counted in 5 small squares of the main large central square, where each square consists of 16 smaller squares. Therefore, a correction factor of 50 was applied to calculate the total number of sperm per ml then converted to 0.1 g tissue weight. Two samples were counted per epididymis, and one epididymis was collected from each of the 10 rats in each experimental group. Further sample analysis included counting motile and immotile sperm in a total of 400 sperm-sample and the results were expressed as a percent.
(D) Semen analysis: sperm morphology
A drop of Eosin stain was added to previously prepared sperm suspension that was kept for 5 min at 37 °C. Then, a drop of sperm suspension was placed on a clean slide and was gently spread to make a thin film. The film was air dried and then observed under a microscope for changes in sperm morphology according to the method of Feustan et al. (1989). The following sperm abnormalities were counted in two separate fields, in each of the sperm samples described above: absence of head; absence of the tail; tail bending; tail curving; tail looping; tail coiling; mid-piece curving; and mid-piece bending.
(E) Semen analysis: estimation of daily sperm production
Daily sperm production was estimated using the protocol described by Fernandes et al. (2007[19]) where resistant sperms were counted following homogenization of the testis sample. Each of the frozen right decapsulated testes was homogenized in 5 ml 0.9 % (w/v) NaCl and Triton X-100 (0.05 %, v/v) using a Waring blender. The preparation was diluted 10 folds and 4 samples were transferred to a Neubauer chamber and late spermatids were counted. The variation between duplicate testicular sperm counts was less than 10 %. Daily sperm production (DSP) values were obtained using a transit time factor of 6.1 days; the duration for which spermatids are typically present in the seminiferous epithelium.
(F) Preparation of tissue homogenates for oxidative stress experiment
Frozen parts of testes from all groups were washed with phosphate buffered saline (PBS), pH 7.4, containing 0.16 mg/ml of heparin to remove any erythrocytes and clots. Then, they were homogenized with an ultrasonic homogenizer in cold phosphate buffer, pH 7.0 with ethylenediaminetetra-acetic acid (EDTA), for thiobarbituric acid reactive substances (TBARS) measurement, and with cold 20 mM N-(2-hydroxyethyl) piperazine-N'-2-ethanesulfonic acid (HEPES) buffer, pH 7.2, containing 1 mM ethylene glycol-bis (2-aminoethoxy)-tetraacetic acid (EGTA), 210 mM mannitol, and 70 mM sucrose, for the measurement of SOD activity. Also, other parts of the kidneys and livers were homogenized in cold buffer that consists of 50 mM tris-HCl, pH 7.5, 5 mM ED-TA, 1 nM DTT to measure GPx activity. All supernatants were kept in separate tubes and stored at -20 °C.
(G) Measurement of thiobarbituric acid reactive substances (TBARS) levels
Lipid peroxidation levels in testes’ homogenates were measured by the thiobarbituric acid (TBA) reaction. This method was used to measure spectrophotometrically the colour produced by the reaction of TBA with MDA at 532 nm. To this end, TBARS levels were measured using MDA assay according to manufacture’s instruction. Briefly, tissue supernatant (50 µl) was added to test tubes containing 2 µl of butylated hydroxytoluene (BHT) in methanol. Next, 50 µl of acid reagent (1 M phosphoric acid) was added followed by 50 µl of TBA solution. Tubes were mixed vigorously and incubated for 60 min at 60 °C. The mixture was centrifuged at 10,000 × g for 3 min, and the supernatant was placed into wells on a microplate in 75 µl aliquots, and absorbance was measured with a plate reader at 532 nm. TBARS (MDA) levels were expressed as nmol/mg protein.
(H) Measurement of superoxide dismutase (SOD) activity
SOD activity in testes’ homogenates was measured using a commercial assay kit according to the manufacturer’s instructions. The SOD assay consisted of a combination of the following reagents: 0.3 mM xanthine oxidase, 0.6 mM diethylenetriamine-penta acetic acid (DETAPAC), 150 µM nitroblue tetrazolium (NBT), 400 mM sodium carbonate (Na2CO3), and bovine serum albumin (1.0g/l). The principle of the method is based on the inhibition of NBT reduction by superoxide radicals produced by the xanthine/xanthine oxidase system. For the assay, standard SOD solution and 10 µl of tissue homogenate supernatant were added to wells containing 200 µl of NBT solution, which was previously diluted by adding 19.95 ml of 50 mM Tris-HCl, pH 8.0, containing 0.1 mM DETAPAC solution and 0.1 mM hypoxanthine. Finally, 20 µl of xanthine oxidase was added to the wells at an interval of 20s . After incubation at 25 °C for 20 min, the reaction was terminated by the addition of 1.0 ml of 0.8 mM cupric chloride. The formazan was measured spectrophotometrically by reading the absorbance at 560 nm with the help of a plate reader. One unit (U) of SOD is defined as the amount of protein that inhibits the rate of NBT reduction by 50 %. The calculated SOD activity was expressed as U/mg protein.
(I) Measurement of glutathione peroxidase (GPx) activity
Glutathione peroxidase activity in testes homogenates was measured using the GPx Assay Kit as per manufacture’s instructions. The principal of the reaction is that GPx catalyzes the reduction of hydroperoxides, including hydrogen peroxide, by reduced glutathione, and functions to protect the cell from oxidative damage. With the exception of phospholipid hydroperoxide GPX, a monomer, all of the GPx enzymes are tetramers of four identical subunits. Each subunit contains a selenocysteine in the active site, which participates directly in the two-electron reduction of the peroxide substrate. The enzyme uses glutathione as the ultimate electron donor to regenerate the reduced form of the selenocysteine. The Cayman Chemical Glutathione Peroxidase Assay Kit measures GPx activity indirectly by a coupled reaction with glutathione reductase (GR). Oxidized glutathione (GSSG) is produced upon reduction of hydroperoxide by GPx and is recycled to its reduced state by GR and NADPH. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm. Under conditions in which the GPx activity is rate limiting, the rate of reduction in the A340 is directly proportional to the GPx activity in the sample. GPx activity was presented as nmol/g protein. One unit is defined as the amount of enzyme that causes the oxidation of 0.1 nmol of NADPH to NADP+/min at 25 °C.
(J) RT-PCR of SCF (soluble and mitochondrial) and Ghrelin mRNA in testicular tissue
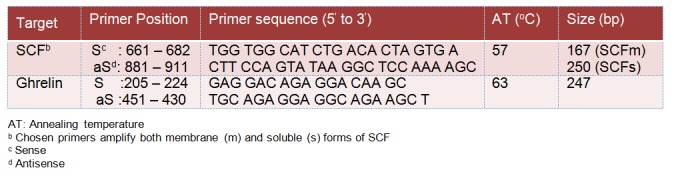
Testicular expression of SCF and Ghrelin mRNA was assessed using RT-PCR. The procedure was optimized for semiquantitative detection using the primer pairs and conditions described in Table 2(Tab. 2). Published sequence of PCR primers used for the detection of SCF and Ghrelin (Goddard et al., 2001; Barreiro et al., 2002) was used. Total RNA was extracted from testicle tissue (30 mg) using the RNeasy Mini Kit (Qiagen Pty. Ltd., Victoria, Australia) according to manufacturer’s instructions. Single strand cDNA synthesis was performed as follows: 30 µl of reverse transcription mixture containing 1.0 µg of DNase I pretreated total RNA, 0.75 µg of oligo d(T) primer, 6.0 µl of 5x RT buffer, 10 mM dithiothreitol, 0.5 mM deoxynucleotides, 50 U of RNase inhibitor, and 240 U of Reverse Transcriptase (Invitrogen). The RT reaction was carried out at 40 °C for 70 min followed by heat inactivation at 95 °C for 3 min. The tested genes, SCF and Ghrelin and that of the internal control (ß-actin) were amplified by PCR using 2 µl RT products from each sample in a 20 µl reaction containing Taq polymerase (0.01 U/ml), dNTPs (100 mM), MgCl2 (1.5 mM), and buffer (50 mM Tris-HCl). PCR reactions consisted of a denaturing cycle at 97 °C for 5 min, followed by a variable number of cycles of amplification, defined by denaturation at 96 °C for 30 sec, annealing for 30 sec, and extension at 72 °C for 1 min. A final extension cycle of 72 °C for 15 min was included. Annealing temperature was adjusted for each target: 57 °C for SCF and 63 °C for Ghrelin. 10 µl of PCR product was electrophoresed using 2 % agarose gel containing 100 ng/ml ethidium bromide, then photographed with a Polaroid camera under ultraviolet illumination.
Table 2. Primers and conditions used in PCR reactions.
(K) Expressions of SCF and Ghrelin by Western blot analysis
Frozen testes tissues from all groups of rats were homogenized in lysis buffer containing 50 mM TRIS, 150 mM NaCl, 1 % NP-40, 0.5 % sodium deoxycholate, 1 % sodium dodecyl sulfate, 1.0 mM PMSF, sodium orthovanadate, sodium fluoride, ethylenediaminetetraacetic acid, and leupeptin at 4 °C for 30 min. Homogenates were centrifuged at 12 000× g for 5 min at 4 °C, and the supernatants were collected and used as total protein. Approximately 100 µg of tissue protein extract was loaded onto each well, separated electrophoretically through a 13.5 % SDS-polyacrylamide gel and transferred onto Sequi-Blot ™ PVDF membrane (BioRad, USA) by electroblotting. Skim fast milk powder (5 % w/v) in TBS/Tween-20 buffer (137 mmol NaCl, 20 mmol Tris-HCl, pH7.4, 0.1 % Tween-20) was used to block filters for at least 1 hr at RT. 1:500 dilution of specific primary goat polyclonal antibody against Ghrelin (c-18) was used, and 1:200 dilution for primary polyclonal antibody against SCF (H-189) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and 1:1000 dilution for primary rabbit polyclonal antibodies against ß-actin (Sigma Aldrich, Germany) were used. The secondary antibody that was used was anti-goat or anti-rabbit IgG horseradish peroxidase-conjugated antibody (1:2000, Santa Cruz, USA). The separate incubation of primary antibodies was followed by 3 washes with TBS-Tween-20 buffer for 10 min. Incubation of the secondary antibody was followed by 4 washes for 10 min. Chemiluminescent based detection was performed using BM chemiluminescence blotting substrate (Boehringer, Mannheim, Germany). Thereafter, the developed membrane was exposed to X-ray film (Kodak, Wiesbaden, Germany). Comparisons between different treatment groups were made by determining the ratio of Ghrelin or SCF to ß-actin from same tissue sample using densitometry.
(L) Statistical analysis
Statistical analysis was performed by one-way ANOVA. The presence of significant difference between treatment groups was accomplished using MANOVA. Post hoc comparison (Tukey’s t-test) was also used. Data were expressed as means ± standard deviation (SD) and statistical significance was set at P ≤ 0.05.
Results
Changes in final body weight, testis weight, fat deposits and adiposity index
Results presented here show that a reproducible and reliable rat model for High Fat Diet (HFD) induced obesity was successfully established in our lab. Supporting data includes significant body size increase (Figure 1(Fig. 1)), weight increase (Figure 1(Fig. 1)), sperm analysis Figure 1(Fig. 1)), adipose tissue deposition (Figure 2(Fig. 2)) and lipid profile (Figure 3(Fig. 3)). At the end of week 12, HFD obese group rats showed a significant increase (P =0.0148) in their final body weight; a gain of 65.2±3.1 % (Figure 1A, A(Fig. 1)). There was also a significant increase in fat deposits including epididymal (80.1±6.7 %, P=0.0001), visceral (35.6± 4.5 %, P=0.0082) and retroperitoneal (59.8± 3.89 %, P=0.0045), as well as the adiposity index which was increased by 45.4±3.9 % (P=0.0053) (Figure 2A(Fig. 2), Figure 2B(Fig. 2)). Additionally, the absolute weight of the testes and the epididymis was decreased by 72.3±5.3 % (P=0.0023) and 22.5±2.1 % (P=0.0232), respectively. The relative weight of the testes and the epididymis was also decreased by 99.6±6.7 % (P=0.0001) and 33.3±1.7 % (P=0.0121), respectively (Figure 1A, B, C(Fig. 1)).
Figure 1. Figure 1A: Upper panel shows pictures to demonstrate varification of our model group. (I): represents control rat at the end of week 12 while (II) shows obese rat administered HFD for the same period. Lower pannel (A-C) represents changes in body weight and testis and epididymis absolute and relative weight (in comparsion to final total body weight) in the control and the experimental groups of rats. Values are expressed as Mean ± SD for 10 rats in each group. Values were considered significantly different at P < 0.05. *: Significantly different when compared to control group I. ß: significantly different when compared to HFD obese group. *: significantly different when compared to HFD + Orlistat group.
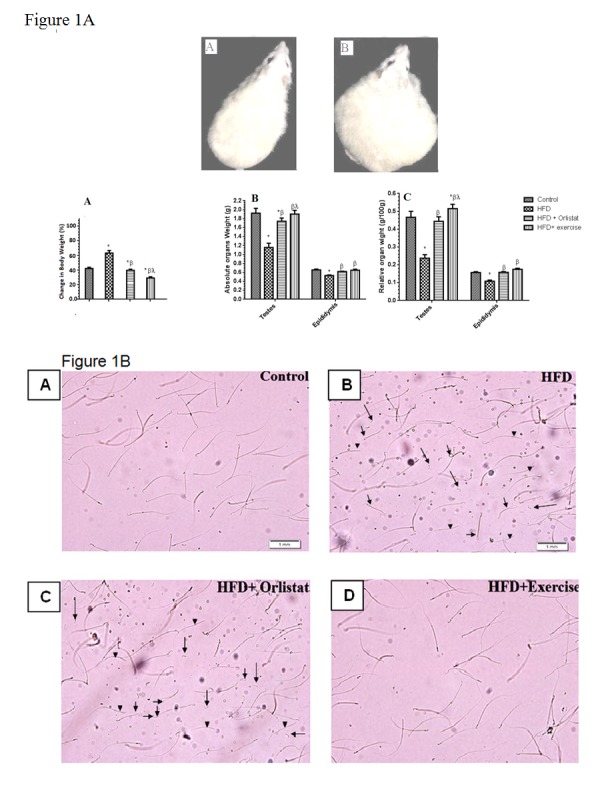
Figure 1B: Photomicrographs of sperm obtained from dissected epididymis of rats in the Control, HFD, HFD + Orlistat and HFD + exercise groups, showing increased total abnormalities including coiled tail (hort arrow), headless (arrow head), and tailless sperms (long arrow) in HFD induced obese and obese rats treated with Orlistat (B & C, respectively) and normal abnormalities in the control (A) and exercised obese group (D).
Figure 2. Weights of different fat deposits and percents of adiposity (adiposity index) in the control and the experimental groups of rats. Values are expressed as Mean ± SD for 10 rats in each group. Values were considered significantly different at P < 0.05, *: Significantly different when compared to control group I. ß: significantly different when compared to HFD obese group. *: significantly different when compared to HFD + Orlistat group.
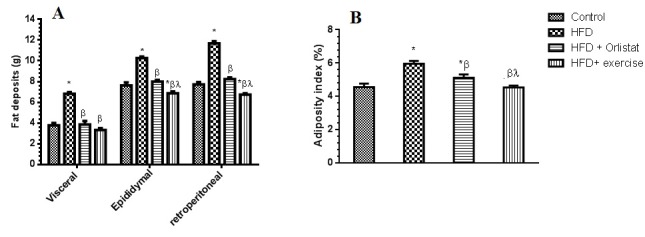
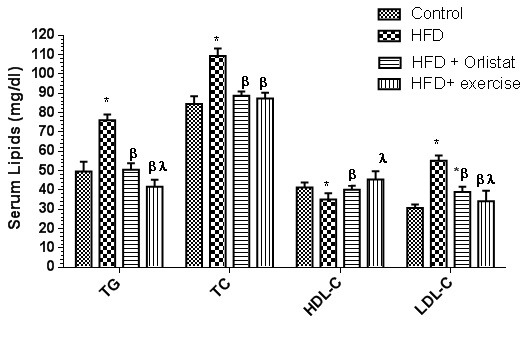
Figure 3. Serum levels of total triglycerides (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) in the control and the experimental groups of rats. Values are expressed as Mean ± SD for 10 rats in each group. Values were considered significantly different at P < 0.05. *: Significantly different when compared to control group I. ß: significantly different when compared to HFD obese group. *: significantly different when compared to HFD + Orlistat group.
Interestingly, the administration of Orlistat alone or implementation of endurance exercise training alone did significantly reduce weight gain, adiposity index and fat deposits in the HFD fed rats. Also, there was a significant increase in the testes and the epididymis absolute and relative weight (Figure 1A(Fig. 1) and Figure 2(Fig. 2)). Group 4 rats, which were exposed to endurance exercise alongside HFD had significantly decreased epididymal and retroperitoneal fat deposits (10.1±1.0 % (P= 0.0417); and 11.3±1.1 % (P=0.0431), respectively (Figure 2A(Fig. 2)). Additionally, relative testes weight was significantly increased (15.3±1.4 %) (P=0.0312) in (Figure 1A and 1C(Fig. 1)) as shown by ANOVA.
Serum lipid profile and sex hormones
Figure 3(Fig. 3) revealed the changes detected in serum lipids including TG, TC, LDL and HDL in all of the 4 experimental groups. HFD obese rats showed a significant increase (P < 0.05) in the serum levels of TG, TC and LDL with a significant reduction in HDL levels (P<0.0001). The levels of all of these serum lipids were significantly decreased in groups 3 and 4; HFD + Orlistat group or in HFD + exercise group when compared to HFD obese rats. Orlistat and exercise though reduced LDL levels compared to HFD group, their levels did return to control non obese group levels.
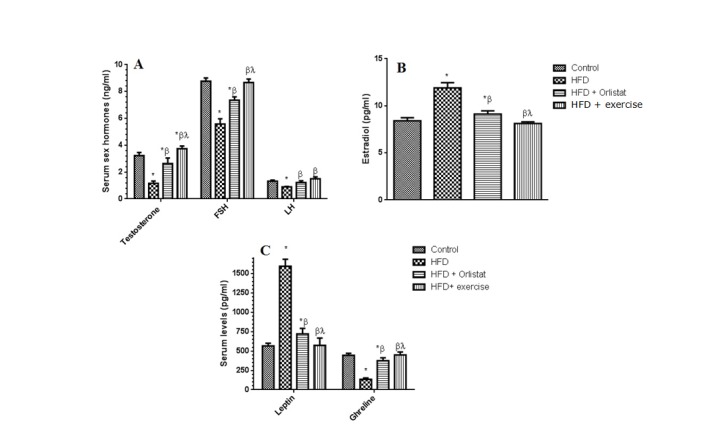
Figure 4(Fig. 4) shows that there was a significant reduction in the serum levels of circulatory testosterone (71.8±6.7 %, P=0.0026), FSH (41.8±4.1 %, P=0.0067), LH (33.3± 3.6 %, P=0.0097) (Figure 4A and 4C(Fig. 4)), Ghrelin (66.7±5.98 %, P=0.0037) accompanied by a significant increase in the levels of leptin (185.7±9.8 %, P ≤ 0.0001) (Figure 4C(Fig. 4)) and estradiol (51.8±5.8 %, P=0.0034) (Figure 4B(Fig. 4)) when compared to control non obese rats. Exercise and Orlistat normalized the detrimental effect of HFD on semen characteristics; keeping in mind the more pronounced effect of exercise when compared to Orlistat on testosterone (25.0±2.1 %, P=0.0133) and FSH.
Figure 4. Serum levels of circulatory testosterone, Follicle-stimulating hormone (FSH), Luteinizing hormone (LH), estradiol, leptin and Ghrelin in the control and the experimental groups of rats. Values are expressed as Mean ± SD for 10 rats in each group. Values were considered significantly different at P < 0.05. *: Significantly different when compared to control group I. ß: significantly different when compared to HFD obese group. *: significantly different when compared to HFD + Orlistat group.
Sperm count, motility, DSP and morphology
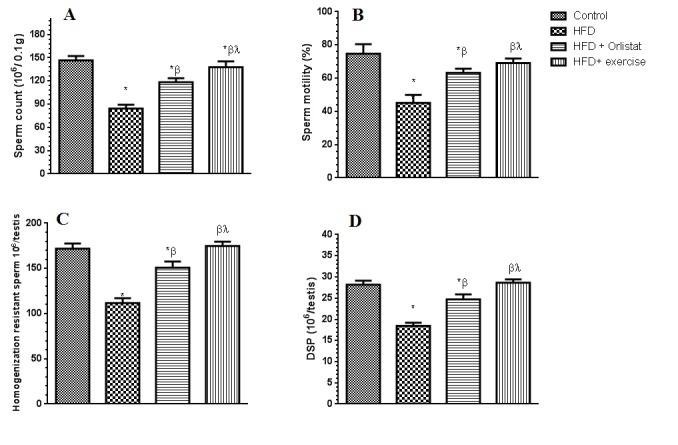
The results of epididymal sperm morphology, count, daily sperm production and general appearance are shown in Table 3(Tab. 3), Figure 1B(Fig. 1) and Figure 5(Fig. 5), respectively. There was a significant decrease in the number (76.8±7.3 %, P < 0.0001) and motility (44.7± 4.8 %, P=0.0081) of epididymal sperms (per 0.1 g of epididymis), as well as in testicular homogenization resistant sperms (46.4± 3.9 %, P=0.0039) Daily sperm production was also reduced (DSP/testis) (57.8±3.5 %, P=0.0012) in HFD obese rats as compared to control rats (Figure 5A-D(Fig. 5)). Results obtained from the morphological assessment of sperm (Figure 1B(Fig. 1)) indicated that the percentage of abnormal sperms was significantly higher (39.94%) in the HFD group as compared to control non- obese group (14.79 %). The majority of abnormalities seen were increase in tailess (4.4 folds), headless (6.7 folds) and coiled tail sperm (5.22 folds). However, Group 3 (Orlistat + HFD) rats showed significant improvement (P < 0.0001) in sperm count, motility and DSP as compared to rats in Group 2 (HFD obese) despite the fact that their absolute numbers remained significantly lower than those of the control non obese rats (17.2±1.8 %, 13.3±1.1 %, 9.3±1.2 %, respectively). The total abnormalities in these groups of rats were slightly but significantly decreased as compared to HFD group. The ANOVA analysis showed that Orlistat administration to HFD rats decreased only the percents of headless and coiled tail sperm as compared to HFD group but in spite of this decrease, headless sperm and coiled tail sperm remained significantly higher (4.6 and 2.18 folds, respectively) as compared to control non obese group. Interestingly, Group 4 (HFD + exercise) rats showed maximum recovery across sperm count, motility and all abnormalities examined and all these parameters were not significantly different when compared to their corresponding levels measured in the control non obese group of rats. However, sperm count remained slightly and significantly lower than its count seen in the control non obese rats.
Table 3. Characterization of sperm morphology in the epididymis of rats in control and experimental groups.
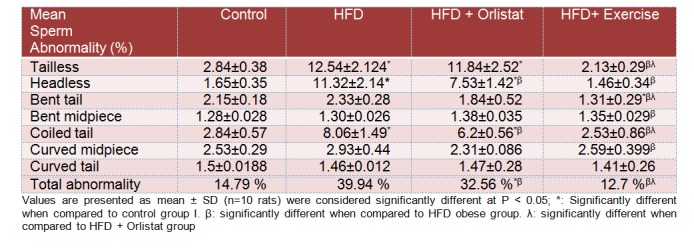
Figure 5. Epididymal sperm count and motility and testicular homogenization resistant sperm and daily testis sperm production (DSP) levels in the control and the experimental groups of rats. Values are expressed as Mean ± SD for 10 rats in each group. Values were considered significantly different at P < 0.05. *: Significantly different when compared to control group I. ß: significantly different when compared to HFD obese group. *: significantly different when compared to HFD + Orlistat group.
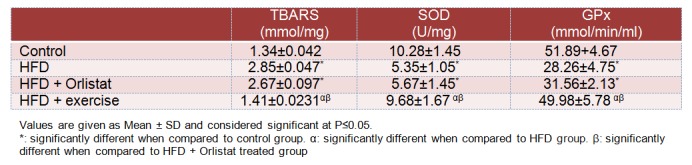
Oxidative stress markers
Table 4(Tab. 4) shows the levels of lipid peroxidation markers (TBARS) and activities of SOD and GPx; markers of protein oxidative damage in the testicular homogenates of normal and experimental animals. HFD administration to rats for 12 weeks resulted in a significant increase in TBARS (112.7± 8.9 %, P ≤ 0.0001) as well as significant decreases in the activities of SOD (48.5±5.8 %, P=0.0012) and GPx (45.6±4.6 %, P=0.0019) in testis homogenates when compared to control group rats. However, no significant change was detected in the levels of TBARS, SOD or GPx activities in Group 3 (HFD + Orlistat) rats. On the other hand, endurance exercise training for 12 weeks restored the levels of TBARS and the activities of both SOD and GPx to the control levels thus normalizing the effect of the HFD.
Table 4. Levels of TBARS and activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in the testis homogenates of all groups of rats.
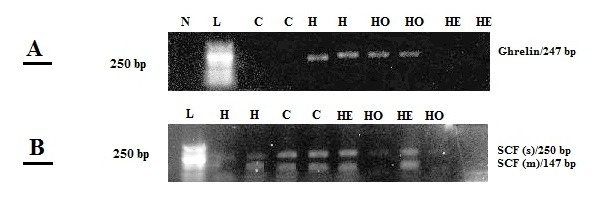
RT-PCR and Western blotting
The levels of SCF and Ghrelin mRNAs expression were assessed in all experimental groups (Figure 6(Fig. 6)). On the basis of the cDNA sequence of SCF and selected primers, two fragment products were expected to be seen on the gel: 167 bp, representing the transmembrane form of SCE (SCFm), and 250 bp, representing the soluble form of SCF (SCFs). In control non rats, both SCFs and SCFm transcripts were detected in the testes, and RT-PCR resulted in two DNA fragments similar in size to those expected (Figure 6B(Fig. 6)). As expected, SCFm had a higher level of expression. Also, Ghrelin mRNA was completely absent in the testes of control non rats as the resulted gel showed no visible band corresponding to Ghrelin in this group of rats (Figure 6A(Fig. 6)). On the other hand, HFD rats exhibited an opposite profile where Ghrelin mRNA exhibited a high level of expression (as shown by the clear band of 247 bp size), while both SCFs and SCFm mRNA were barely detectable in HFD group rats compared to control non group rats (Figure 6(Fig. 6)). Interestingly, though, Ghrelin mRNA was undetectable and both SCFs and SCFm mRNA became prominent in rats that underwent exercise (i.e. Group 4). Additionally, Orlistat had a similar effect to that of exercise on Ghrelin and SCF expression, however, to a much lesser extent since Ghrelin mRNA levels were still visible in the Orlistat receiving group. In regards to SCF mRNA in the Orlistat receiving group, SCFs was barely detectable while SCFm band was pronounced (Figure 6B(Fig. 6)).
Figure 6. RT-PCR detection of Ghrelin (A) (247 bp,) and soluble (SCFs, 250 bp) and transmembrane (SCFm, 167 bp) forms of SCF (B) mRNAs in whole testis. The RT-PCR product, obtained from all groups was separated by 2 % agarose gel electrophoresis. N: negative control. L: 50 bp ladder. C: Control non obese rats. H: high fat diet (HFD) obese group. HO: HFD + Orlistat group. HE: HFD + exercise group.
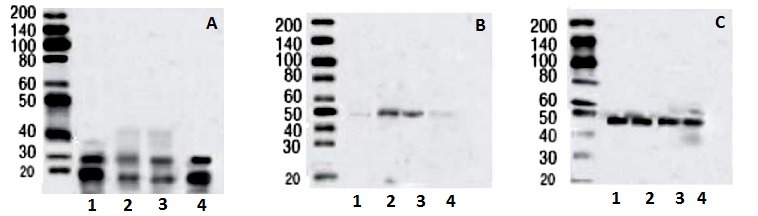
Western blotting analysis of rat testes homogenate (Figure 7(Fig. 7)) revealed two bands with immunoreactivity for SCF, at 28-kDa and 19-kDa in all experimental groups, although, with variable densities. Anti-Ghrelin antibody detected a single band of 50-kDa, while anti-β-actin antibody detected a single band of 48-kDa in all experimental groups. SCF immunoreactivity was quiet prominent in the control group (A1) rat testes homogenate, decreased precipitously in HFD (A2) obese rats, was partially protected with Orlistat treatment (A3) and was essentially fully protected in rats underwent HFD and exercise (A4) protocol. Conversely, Ghrelin protein was barely detectable in the control group (B1) but had several folds increase in HFD group (B2) rats. Interestingly, Orlistat (B3) hardly inhibited Ghrelin in a manner comparable to its partial protection of SCF (A3) protein levels. Exercise profoundly protected SCF protein levels when compared to rats in groups 1 and 2. Exercise inhibited Ghrelin (B4) fully and completely protected SCF protein levels (A4). Figure 7C(Fig. 7) is a control for equal protein loading in all lanes.
Figure 7. Western analysis of stem cell factor (SCF, A), Ghrelin (B) and ß actin (C) proteins in the testis homogenates of all groups. SCF protein detection can be seen at 2 bands of 28-KDa and 19-KDa whereas Ghrelin and ß-actin were detected at 48-KDa and 50-KDa band, respectively. 1: Control non obese rats. 2: high fat diet (HFD) obese group. 3: HFD + Orlistat group. 4: HFD+ exercise group.
Discussion
In this study, the validity and reliability of a rat model for High Fat Diet induced-obesity were first established in our lab. The evidence includes the significant waist obesity, the massive weight increase, appreciably elevated adipose tissue deposition, significantly reduced sperm count, sperm motility, and daily sperm production, hyperlipidemia, the reduced levels of testosterone, FSH, LH, and the elevated leptin. The model was utilized to test the hypothesis that obesity induces Ghrelin expression, which in turn downregulates SCF and results in semen abnormalities; an effect that can be protected against non-pharmacologically via using endurance exercise, or pharmacologically, using lipase inhibitor Orlistat. Our data clearly demonstrated that diet induced-obesity increased Ghrelin mRNA and protein, and reduced or abolished SCF mRNA and protein in the testes. It further showed a significant reduction in sperm count, sperm motility, sperm morphology and DSP as well as increased levels of TBARS and reduced activities of SOD and GPx. Complete or partial protection against these effects was enabled by exercise and Orlistat, respectively.
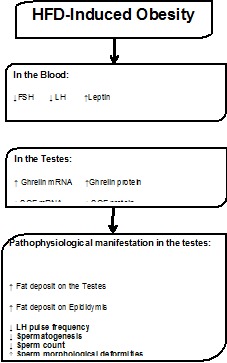
In the testes of obese rats, there exists a direct and inverse relationship between Ghrelin and SCF both at the level of mRNA and protein. This finding is significant since it sheds some light on the mechanism relating obesity to infertility. Though the current study is observational in nature, it brings to light a new molecule in the phenomenon of obesity-associated-infertility and semen abnormalities, namely, SCF in the testes. Our data argues that obesity in males negatively impacts fertility by downregulating Hypothalamic-Pituitary Gonadal (HPG) axis which results in increased Ghrelin gene expression that inhibits SCF gene expression. The inhibition of SCF gene expression induces abnormalities in already formed sperms in addition to inhibiting the formation of new sperms (Figure 8(Fig. 8)).
Figure 8. Tentative mechanistic model linking fertility to obesity.
Experimentally in our study, an obesity model induced by high-fat diet consumption was chosen since it effectively and reproducibly produced obesity in dogs, pigs, hamsters, squirrels, mice (West and York, 1998[78]), and rats (Tuzcu et al., 2011[70]). The high-fat diet used in the present study was effective in producing obesity over a period of 12 weeks, as demonstrated by increased serum levels of leptin, TG, TC, LDL, adiposity index, body weight accompanied with a significant decrease in HDL and Ghrelin levels. These findings are in agreement with various reports (Tuzcu et al., 2011[70]; West and York, 1998[78]; Tan et al., 2005[66]; Archer and Mercer, 2007[5]). However, few studies in the literature examined changes in individual organ weight in obese individuals. Here we report a direct effect of the HFD consumption on the individual weight of testis and epididymis relative to total body weight. Though variations on this issue have been published (Edmonds et al., 1982[17]; Ghanayem et al, 2010[27]), those variations can be attributed to animal model genetics as well as protocol differences. The significant decrease in the absolute and relative weights of testis and epididymis in obese rats suggests that sperm morphology and production (i.e. spermatogenesis) could also have been impaired. Therefore, a detailed sperm analysis was conducted to detect, if present, abnormalities that could shed some light on obesity-associated male infertility. Interestingly, when compared to control rats, obese rats exhibited lower daily sperm production (DSP), sperm concentration, sperm motility in addition to abnormal sperm morphology. Our findings are in agreement with Jensen et al. (2004[37]) who reported higher prevalence of oligozoospermia in overweight and obese men than in normal weight men, with a significant association between sperm count and BMI. Also, Koloszar et al. (2002[41]) reported decreased sperm count in obese normozoospermic men compared with non obese fertile subjects. Kort et al. (2006[42]) reported a negative correlation between BMI and total number of normal spermatozoa, and Sallmen et al. (2006[61]) showed that overweight and obese men had reduced sperm motility and increased sperm DNA fragmentation. Also, Sprague-Dawley rats fed a high fat diet from 21 to 90 days are presented with a reduced sperm concentration (Vigueras-Villaseñor et al., 2011[74]).
Oxidative stress may be a key mechanism linking overweight or obesity with male infertility and inducing increased sperm DNA damage. The testicular microenvironment has been reported to be exposed to oxidative stress and a positive correlation between BMI and seminal oxidative stress has been observed in both animals (Bakos et al., 2011[7]) and human (Tunc et al., 2011[69]). Although reactive oxygen species (ROS) are essential for normal reproductive functions, namely, sperm capacitation and acrosome reaction, when present at physiological concentrations. However, they become toxic and damaging at higher concentration (Allamaneni et al., 2004[3], Aitken et al., 2004[2]). Spermatids and mature spermatozoa are quite sensitive to ROS because their membranes are rich in polyunsaturated fats (Allamaneni et al., 2004[3]) which undergo lipid peroxidation and generate damaging reactive carbonyl compounds such as MDA (Allamaneni et al., 2004[3], Aitken et al., 2004[2]). Hence, sperms may be impaired, and/or die depending on how much ROS levels have increased under oxidative stress conditions (Agarwal et al., 1994[1]). Our data is in agreement with Bakos et al. (2011[7]) where significant increase in the levels of testicular lipid peroxides was observed, since we detected an increase in TBARS levels as well as a significant reduction in SOD and GPx activities in the testes of the rats fed with HFD. The results confirmed that obesity did enhance testicular oxidative stress. The present experimental design does not elucidate the obesity-infertility signal transduction pathway, nevertheless, our results argue that it includes key downstream effectors in the testes, namely, Ghrelin, SCF, SOD, GPx, and TBARS which cause semen abnormalities and could ultimately precipitate male infertility.
A disturbance in sex hormones, namely GnRH, FSH, LH, testosterone and estrogen of the HPG axis, is primarily suspected to trigger the above observed abnormalities in sex organs weight, sperm morphology and spermatogenesis (Mah and Wittert, 2010[51]). This hypothesis is based on the hormonal abnormalities observed in individuals with increased BMI where decreased levels of testosterone and increased levels of estrogen were observed in obese rats (Chavarro et al., 2010[15]). This imbalance resulted in decreased testosterone to estrogen ratio, which was shown to be caused in obese individuals by increased activity of the cytochrome p450 aromatase enzyme; the enzyme that converts androgens to estrogens (Phillips and Tanphaichitr, 2010[56]). The levels of testosterone, FSH and LH, reported here were all decreased in the HFD fed rats. Collectively, data suggests that the diet-induced obesity in rats could trigger an upstream signalling pathway starting with downregulation of testosterone, FSH and LH leading to downstream and localized upregulation of Ghrelin in the testes, that in turn downregulates SCF, gene expression and reduces SOD and GPx enzymatic activities thus casing sperm deformities and sperm death.
Ghrelin was reported to be expressed in Leydig cells and to a lesser extent in Sertoli cells of normal rat testis (Gaytan et al., 2004[26]). This observation suggested an important role in the regulation of reproduction and fertility. Functionally, Ghrelin has been shown to have multiple roles in the testes including inhibition of proliferative activity of immature Leydig cells in vivo, delay of pubertal onset in male and inhibition of testosterone secretion in stimulated rat testis (Tena-Sempere et al., 2002[67]; Barreiro et al., 2004[9]).
Incidentally, Stem Cell Factor was also found expressed by Leydig cells and spermatogonia (Loveland and Schlatt, 1997[49]), where it binds to the c-kit receptor found immunolocalized to acrosomal granules of spermatocytes and the acrosomes of spermatozoa (Sandlow et al., 1999[62]). Deletion or mutations of the c-kit gene or its ligand, SCF, have been reported to result in infertility in mice due to loss of germ cells (Besmer et al., 1993[12]). In congruence with these observations, here we report a decreased expression of SCF (both soluble and transmembranous) in the testis of obese rats.
Ghrelin has been shown to control the secretion of SCF (Barreiro et al., 2004[9]) as evident by inhibition of SCF gene expression consequent to intratesticular injection of Ghrelin (15 µg for two days) in adult rats in vivo (Barreiro et al., 2004[9]) and in vitro using cultures of staged seminiferous tubules. This suggests that Ghrelin is acting upstream of SCF in the testes of obese rats. A higher up step in the regulation of SCF is FSH, which has been shown to directly stimulate SCF synthesis (Yan et al., 1999[80]). Therefore, it is plausible to postulate that the observed decline in FSH in obese rats precipitated a decline in SCF, as well as the observed sperm deformities and sperm count.
It is noteworthy that FSH and SCF can protect germ cells from apoptosis by inhibiting the expression of key apoptotic genes such as Bok (Yan et al., 1999[80], 2000[81]), a pro-apoptotic member of the Bcl-2 gene family. In this study, we did not assess Bok expression, but Bok mRNA was detected in spermatogonia, pachytene spermatocytes and Sertoli cells in rat testis in a previous study (Suominen et al., 2001[65]). Put together, we are proposing the following hypothesis: obesity associated infertility and sexual dysfunction are controlled centrally via FSH which in turn regulates the interplay between testicular SCF and Ghrelin expression, which in turn keeps key apoptotic genes suppressed. Therefore, it is likely that the HFD induced-obesity impaired the semen parameters and decreased semen count and motility by decreasing circulatory FSH, which inhibited SCF signalling thus increasing Ghrelin expression in rats’ testes, a mechanism that could be stimulating Bok gene expression and triggering sperm apoptosis. The interplay between the trio, downregulation of SCF, reduced activities of SOD and GPx, and upregulation of Bok gene expression, leading to sperm deformation, sperm death, and ultimately infertility and sexual dysfunction, should be the focus of future research.
Rather than pursuing the molecular regulation of Ghrelin, SCF, and apoptosis in the testes, we chose to fast forward to testing if exercise and lipase inhibition individually could reverse the effect of obesity on semen abnormalities.
Orlistat (Xenical), an inhibitor of gastric and pancreatic lipase lowering fat absorption by 30 %, has been proven to be useful in facilitating both weight loss and weight maintenance (Gürsoy et al., 2007[30]). The data presented here showed that co-administration of Orlistat with the HFD, had multiple beneficial effects including partial protection against obesity associated-loss of absolute and relative weights of testes and epididymis, a significant reduction in visceral, retroperitoneal, and epididymal fat deposit, near complete normalization of lipid profile, resisting the decline in FSH, LH, testosterone and estradiol, as well as reduction of leptin and Ghrelin in the HFD fed rats. Also, it partially ameliorated oxidative stress in rats’ testes by decreasing lipid peroxidation levels and restoring SOD and GPx activities. However, it did not protect against obesity associated sperm deformities nor did it reverse Ghrelin and SCF gene expression.
In regards to exercise, its benefits have been attributed, at least in part, to counteracting the biological adaptation that drive weight gain and regain (Levin and Dunn-Meynell, 2004[47]). In the periphery, exercise promotes fat oxidation and prevents relapse-induced hypercellularity in abdominal fat pads (Levin and Dunn-Meynell, 2004[47]). As shown earlier, the effects of exercise on obese rats were unparalleled. Not only, it produced all of the effects of Orlistat described above, but changes were even more pronounced. Apart from Orlistat, exercise significantly normalized sperm deformities seen in obese rats. Furthermore, closer comparison of the effects of exercise and Orlistat revealed that while Orlistat has a partial protective action, exercise led to full protection against changes in the levels of sex hormones, lipid profile, semen parameters via abolishing testicular Ghrelin levels, increasing testicular SCF levels and correcting sperm deformities in the testis.
Concerning antioxidant effects of exercise, a significant body of literature proved that acute exercise causes oxidative and mitochondrial stress and tissue injury (Leeuwenburgh and Ji, 1995[46]; Liu et al., 2000[48]; Huang et al., 2008[35]). However, a substantial body of evidence suggests that regular exercise plays an important preventive and therapeutic role in oxidative stress-associated diseases, including ischemic heart disease, heart failure, type II diabetes, and Alzheimer’s disease (Kiraly and Kiraly, 2005[40]; Lazarevic et al., 2006[44]; Ascensão et al., 2006[6]; Belardinelli et al., 2007[11]). In animals and humans, studies have shown that exercise clearly permits significant adaptive responses such as greatly increased endurance capacity. The increased capacity is permitted by the dramatic mitochondrial biogenesis, the reduction in oxidant’s production and increased antioxidant defences (Packer and Cadenas, 2007[54]; Sachdev and Davies, 2008[60]). In 2011, Botezelli et al.[13] demonstrated that eight weeks of swim training decreased lipid peroxidation, a fact partially attributed to an improved antioxidant system with greater SOD enzyme activity. Accordingly, it has been demonstrated that endurance training up-regulates antioxidant enzymes and glutathione content improves mitochondrial respiratory function and reduces lipid peroxidation by products in various tissues (Vendittim and Di Meo, 1996[73]; Powers et al., 1998[57]; Ramires and Ji, 2001[58]). These findings are in agreement with our results where SOD and GPx activities were elevated in rat testes in Group 4 (HFD + exercise) rats. Together, besides its ameliorating effect on the Ghrelin and SCF gene expression in obese rats’ testes, endurance swimming exercise also provides protection to semen parameters by boosting antioxidant potential in the testes.
In conclusion, the significant effects of exercise and Orlistat observed here present a strategic intervention to protect against obesity induced semen abnormalities. While Orlistat seems to act through decreasing body weight, decreasing circulatory lipids and enhancing hormonal imbalance, endurance swimming exercise on the other hand, seems to act through other mechanisms including mitigation of oxidative stress, boosting hormonal imbalance and reversing the levels of Ghrelin and SCF in the testes of obese rats.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to thank the Deanship of Scientific Research at King Khalid University, Abha, and Kingdom of Saudi Arabia for funding this project.
References
- 1.Agarwal A, Hamamah S, Shekarriz M. Reactive oxygen species and fertilizing capacity of spermatozoa. Contracept Fertil Sex. 1994;22:327–30. [Google Scholar]
- 2.Aitken RJ, Ryan AL, Baker MA, McLaughlin EA. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radical Biol Med. 2004;36:994–1010. doi: 10.1016/j.freeradbiomed.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Allamaneni SSR, Naughton CK, Sharma RK, Thomas AJ, Agarwal A. Increased seminal reactive oxygen species levels in patients with Varicoceles correlate with varicocele grade but not with testis size. Fertil Steril. 2004;82:1684–6. doi: 10.1016/j.fertnstert.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 4.Amato MC, Pizzolanti G, Torregrossa V, Misiano G, Milano S. Visceral Adiposity Index (VAI) is predictive of an altered adipokine profile in patients with type 2 diabetes. PLoS One. 2014;9(3):e91969. doi: 10.1371/journal.pone.0091969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer ZA, Mercer JG. Brain responses to obesogenic diets and diet induced obesity. Proc Nutr Soc. 2007;66:124–130. doi: 10.1017/S0029665107005356. [DOI] [PubMed] [Google Scholar]
- 6.Ascensão A, Magalhães J, Soares J, Ferreira R, Neuparth M, Marques F, et al. Endurance exercise training attenuates morphological signs of cardiac muscle damage induced by doxorubicin in male mice. Basic Appl Myol. 2006;16:27–35. [Google Scholar]
- 7.Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011;34:402–410. doi: 10.1111/j.1365-2605.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 8.Barreiro ML, Gaytan F, Caminos JE, Pinilla L, Casanueva FF, Aguilar E, et al. Cellular location and hormonal regulation of Ghrelin expression in rat testis. Biol Reprod. 2002;67:1768–1776. doi: 10.1095/biolreprod.102.006965. [DOI] [PubMed] [Google Scholar]
- 9.Barreiro ML, Gaytan F, Castellano JM, Suominen JS, Roa J, Gaytan M, et al. Ghrelin inhibits the proliferative activity of immature Leydig cells in vivo and regulates stem cell factor messenger RNA expression in rat testis. Endocrinology. 2004;145:4825–4834. doi: 10.1210/en.2004-0732. [DOI] [PubMed] [Google Scholar]
- 10.Bastounis EA, Karayiannakis AJ, Syrigos K, Zbar A, Makri GG, Alexiou D. Sex hormone changes in morbidly obese patients after vertical banded gastroplasty. Eur Surg Res. 1998;30:43–47. doi: 10.1159/000008556. [DOI] [PubMed] [Google Scholar]
- 11.Belardinelli P, Ciancetta L, Staudt M, Pizzella V, Londei, Birbaumer N, et al. Cerebro-muscular and cerebro-cerebral coherence in patients with pre- and perinatally acquired unilateral brain lesions. Neuro-image. 2007;37:1301–14. doi: 10.1016/j.neuroimage.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 12.Besmer P, Manova K, Duttlinger R, Huang EJ, Packer, Gyssler C, et al. The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. In: Ingham P, Brown A, Martinez Arias A, editors. Signals, polarity and adhesion in development. Cambridge: The Company of Biologists Ltd; pp. 125–137. ((Development; Supplement)). [Google Scholar]
- 13.Botezelli JD, Cambri LT, Ghezzi AC, Dalia RA, Scariot PP, Ribeiro C, et al. Different exercise protocols improve metabolic syndrome markers, tissue triglycerides content and antioxidant status in rats. Diabetol Metabolic Syndrome. 2011;3:35. doi: 10.1186/1758-5996-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broglio F, Arvat E, Benso A, Papotti M, Muccioli G, Deghenghi, et al. Ghrelin: endocrine and non-endocrine actions. J Pediatr Endocrinol Metab. 2002;15:1219–27. [PubMed] [Google Scholar]
- 15.Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril. 2010;93:2222–2231. doi: 10.1016/j.fertnstert.2009.01.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Da Luz G, Frederico MJ, Da Silva S, Vitto MF, Cesconetto PA, de Pinho RA, et al. Endurance exercise training ameliorates insulin resistance and reticulum stress in adipose and hepatic tissue in obese rats. Eur J Appl Physiol. 2011;111:2015–23. doi: 10.1007/s00421-010-1802-2. [DOI] [PubMed] [Google Scholar]
- 17.Edmonds ES, Dallie SK, Withyachumnarnkul B. Reproductive system of the obese male zucker rat. reproductive capacity, artificial insemination and plasma testosterone levels. Biol Reprod. 1982;27:891–897. doi: 10.1095/biolreprod27.4.891. [DOI] [PubMed] [Google Scholar]
- 18.Ellrichmann M, Kapelle M, Ritter PR, Holst JJ, Herzig KH, Schmidt WE, et al. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7-36)amide-1, cholecystokinin, and peptide YY concentrations. J Clin Endocrinol Metab. 2008;93:3995–3998. doi: 10.1210/jc.2008-0924. [DOI] [PubMed] [Google Scholar]
- 19.Fernandes GS, Arena AC, Fernandez CDB, Mercadante A, Barbisan LF, Kempinas W. Reproductive effects in male rats exposed to diuron. Reprod Toxicol. 2007;23:106–112. doi: 10.1016/j.reprotox.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez CDB, Bellentani FF, Fernandes GSA, Perobelli JE, Favareto APA, Nascimento F, et al. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32. doi: 10.1186/1477-7827-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett. 2004;362:103–107. doi: 10.1016/j.neulet.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Ferris WF, Crowther NJ. Once fat was fat and that was that: our changing perspectives on adipose tissue. Cardiovasc J Afr. 2011;22:147–154. doi: 10.5830/CVJA-2010-083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feustan MH, Bodnai KR, Kerstetter SL. Reproductive toxicity of 2-methoxy ethanol applied dermally to occluded and non-occluded sides in male rats. Toxicol Appl Pharmacol. 1989;100:145–165. doi: 10.1016/0041-008x(89)90098-7. [DOI] [PubMed] [Google Scholar]
- 24.Filippatos T, Derdemezis C, Gazi I, Nakou E, Mikhailidis D, Elisaf M. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Safety. 2008;1:53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of Ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun. 2001;288:780–785. doi: 10.1006/bbrc.2001.5854. [DOI] [PubMed] [Google Scholar]
- 26.Gaytan F, Barreiro ML, Caminos JE, Chopin LK, Herington AC, Morales C Pinilla L, et al. Expression of Ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J Clin Endocrinol Metab. 2004;89:400–409. doi: 10.1210/jc.2003-031375. [DOI] [PubMed] [Google Scholar]
- 27.Ghanayem BI, Bai R, Kissling GE, Travlos G, Hoffler U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod. 2010;82:94–104. doi: 10.1095/biolreprod.109.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goddard I, Bauer S, Gougeon A, Lopez F, Giannetti N, Susini C, et al. Somatostatin inhibits stem cell factor messenger RNA expression by sertoli cells and stem cell factor-induced DNA synthesis in isolated seminiferous tubules. Biol Reprod. 2001;65:1732–42. doi: 10.1095/biolreprod65.6.1732. [DOI] [PubMed] [Google Scholar]
- 29.Gualillo O, Lago F, Gomez-Reino J, Casanueva FF, Dieguez C. Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003;552:105–9. doi: 10.1016/s0014-5793(03)00965-7. [DOI] [PubMed] [Google Scholar]
- 30.Gürsoy A, Erdoğan MF, Cin MÖ, Cesur M, Başkal N. Open-label study for the comparison of metabolic effects of Orlistat and sibutramine in women participating in an Obesity management program. Turk Jem. 2007;11:54–58. [Google Scholar]
- 31.Haffner SM, Valdez RA, Stern MP, Katz MS. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord. 1993;17:643–9. [PubMed] [Google Scholar]
- 32.Hammoud A, Gibson M, Hunt SC, Adams TD, Carrell DT, Kolotkin RL, et al. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J Clin Endocrinol Metab. 2009;94:1329–1332. doi: 10.1210/jc.2008-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Harrison JL, Miller DW, Findlay PA, Adam CL. Photoperiod influences the central effects of Ghrelin on food intake, GH and LH secretion in sheep. Neuroendocrinology. 2008;87:182–92. doi: 10.1159/000112480. [DOI] [PubMed] [Google Scholar]
- 35.Huang CC, Tsai SC, Lin WT. Potential ergogenic effects of L-arginine against oxidative and inflammatory stress induced by acute exercise in aging rats. Exp Gerontol. 2008;43:571–7. doi: 10.1016/j.exger.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–56. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- 37.Jensen TK, Andersson AM, Jørgensen N, Andersen AG, Carlsen E, Petersen JH, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–70. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 38.Kaukua J, Pekkarinen T, San T, Mustajoki P. Sex hormones and sexual function in obese men losing weight. Obes Res. 2003;11:689–694. doi: 10.1038/oby.2003.98. [DOI] [PubMed] [Google Scholar]
- 39.Kiens B, Richter EA. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am J Physiol. 1998;275:E332–7. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- 40.Kiraly MA, Kiraly SJ. The effect of exercise on hippocampal integrity: review of recent research. Int J Psychiatry Med. 2005;35:75–89. doi: 10.2190/HX7L-4B40-PQNY-2A4P. [DOI] [PubMed] [Google Scholar]
- 41.Koloszar S, Daru J, Kereszturi A, Zavaczki Z, Szollosi J, Pal A. Effect of female body weight on efficiency of donor AI. Arch Androl. 2002;48:323–7. doi: 10.1080/01485010290099156. [DOI] [PubMed] [Google Scholar]
- 42.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–452. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 43.Lanfranco F, Bonelli L, Baldi M, Me E, Broglio F, Ghigo E. Acylated Ghrelin inhibits spontaneous luteinizing hormone pulsatility and responsiveness to naloxone but not that to gonadotropin-releasing hormone in young men: evidence for a central inhibitory action of Ghrelin on the gonadal axis. J Clin Endocrinol Metab. 2008;93:3633–9. doi: 10.1210/jc.2008-0049. [DOI] [PubMed] [Google Scholar]
- 44.Lazarevic G, Antic S, Cvetkovic T, Vlahovic P, Tasic I, Stefanovic V. A physical activity programme and its effects on insulin resistance and oxidative defense in obese male patients with type 2 diabetes mellitus. Diabetes Metab. 2006;32:583–90. doi: 10.1016/S1262-3636(07)70312-9. [DOI] [PubMed] [Google Scholar]
- 45.Lee FT, Kuo TY, Liou SY, Chien CT. Chronic Rhodiola rosea extract supplementation enforces exhaustive swimming tolerance. Am J Chin Med. 2009;37:557–72. doi: 10.1142/S0192415X09007053. [DOI] [PubMed] [Google Scholar]
- 46.Leeuwenburgh C, Ji LL. Glutathione depletion in rested and exercised mice: biochemical consequence and adaptation. Arch Biochem Biophys. 1995;316:941–9. doi: 10.1006/abbi.1995.1125. [DOI] [PubMed] [Google Scholar]
- 47.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol. 2004;286:R771–R778. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Yeo HC, Overvik-Douki E, Hagen T, Doniger SJ. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous antioxidants. J Appl Physiol. 2000;89:21–8. doi: 10.1152/jappl.2000.89.1.21. [DOI] [PubMed] [Google Scholar]
- 49.Loveland KL, Schlatt S. Stem cell factor and c-kit in the mammalian testis: lessons originating from Mother Nature’s gene knockouts. J Endocrinol. 1997;153:337–44. doi: 10.1677/joe.0.1530337. [DOI] [PubMed] [Google Scholar]
- 50.Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, Heimisdottir M, Olafsdottir K. Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Human Reprod. 2005;20:208–15. doi: 10.1093/humrep/deh569. [DOI] [PubMed] [Google Scholar]
- 51.Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316:180–186. doi: 10.1016/j.mce.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Nishioka T, Hafkamp A, Havinga R, Verkade H. Orlistat treatment increases fecal bilirubin excretion and decreases plasma bilirubin concentrations in hyperbilirubinemic Gunn rats. J Pediatr. 2003:143:327–334. doi: 10.1067/s0022-3476(03)00298-1. [DOI] [PubMed] [Google Scholar]
- 53.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States,. 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 54.Packer L, Cadenas E. Oxidants and antioxidants revisited. New concepts of oxidative stress. Free Radic Res. 2007;41:951–2. doi: 10.1080/10715760701490975. [DOI] [PubMed] [Google Scholar]
- 55.Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85:1319–1340. doi: 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 56.Phillips K, Tanphaichitr PN. Mechanisms of obesity-induced male infertility. Expert Rev Endocrinol Metabol. 2010;5:229–251. doi: 10.1586/eem.09.65. [DOI] [PubMed] [Google Scholar]
- 57.Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol. 1998;5:R1468–77. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- 58.Ramires PR, Ji LL. Glutathione supplementation and training increases myocardial resistance to ischemia-reperfusion in vivo. Am J Physiol. 2001;2:H679–88. doi: 10.1152/ajpheart.2001.281.2.H679. [DOI] [PubMed] [Google Scholar]
- 59.Rossi P, Sette C, Dolci S, Geremia R. Role of c-kit in mammalian spermatogenesis. J Endocrinol Invest. 2000;23:609–15. doi: 10.1007/BF03343784. [DOI] [PubMed] [Google Scholar]
- 60.Sachdev S, Davies KJ. Production, detection, and adaptive responses to free radicals in exercise. Free Radic Biol Med. 2008;44:215–23. doi: 10.1016/j.freeradbiomed.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 61.Sallmen M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–3. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- 62.Sandlow JI, Feng HL, Zheng LJ, Sandra A. Migration and ultrastructural localization of the c-kit receptor protein in spermatogenic cells and spermatozoa of the mouse. J Urol. 1999;161:1676–80. [PubMed] [Google Scholar]
- 63.Strain GW, Zumoff B, Miller LK, Rosner W, Levit C, Kalin M, et al. Effect of massive weight loss on hypothalamic-pituitary gonadal function in obese men. J Clin Endocrinol Metab. 1988;66:1019–1023. doi: 10.1210/jcem-66-5-1019. [DOI] [PubMed] [Google Scholar]
- 64.Sunderam S, Chang J, Flowers L, Kulkarni A, Sentelle G, Jeng G, et al. Centers for Disease Control and Prevention (CDC). Assisted reproductive technology surveillance - United States,. 2006. MMWR Surveill Summ. 2009;58:1–25. [PubMed] [Google Scholar]
- 65.Suominen JS, Yan W, Toppari1 J, Kaipia A. The expression and regulation of Bcl-2-related ovarian killer (Bok) mRNA in the developing and adult rat testis. Eur J Endocrinol. 2001;145:771–8. doi: 10.1530/eje.0.1450771. [DOI] [PubMed] [Google Scholar]
- 66.Tan HM, Gundlach AL, Morris JM. Exaggerated feeding response to central galanin-like peptide administration in diet-induced obese rats. Neuropeptides. 2005;39:333–336. doi: 10.1016/j.npep.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 67.Tena-Sempere M, Barreiro ML, Gonzalez LC, Gaytan F, Zhang FP, Caminos JE, et al. Novel expression and functional role of Ghrelin in rat testis. Endocrinology. 2002;143:717–25. doi: 10.1210/endo.143.2.8646. [DOI] [PubMed] [Google Scholar]
- 68.Tiikkainen M, Bergholm R, Rissanen A, Aro A, Salminen I, Tamminen M, et al. Effects of equal weight loss with Orlistat and placebo on body fat and serum fatty acid composition and insulin resistance in obese women. Am J Clin Nutr. 2004;79:22–30. doi: 10.1093/ajcn/79.1.22. [DOI] [PubMed] [Google Scholar]
- 69.Tunc O, Bakos HW, Tremellen K. Impact of body mass index on seminal oxidative stress. Andrologia. 2011;43:121–128. doi: 10.1111/j.1439-0272.2009.01032.x. [DOI] [PubMed] [Google Scholar]
- 70.Tuzcu M, Sahin N, Orhan C, Agca CA, Akdemir F, Tuzcu Z, et al. Impact of chromium histidinate on high fat diet induced obesity in rats. Nutr Metab (Lond) 2011;8:28. doi: 10.1186/1743-7075-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of Ghrelin. Endocr Rev. 2004;25:426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 72.Van Dorsten B, Lindley EM. Cognitive and behavioral approaches in the 839 treatment of obesity. Endocrinol Metab Clin North Am. 2008;37:905–922. doi: 10.1016/j.ecl.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Vendittim P, Di Meo S. Antioxidants, tissue damage, and endurance in trained and untrained young male rats. Arch Biochem Biophys. 1996;1:63–8. doi: 10.1006/abbi.1996.0283. [DOI] [PubMed] [Google Scholar]
- 74.Vigueras-Villaseñor RM, Rojas-Castañeda JC, Chávez-Saldaña M, Gutiérrez- Pérez O, García-Cruz ME, Cuevas-Alpuche O, et al. Alterations in the spermatic function generated by obesity in rats. Acta Histochem. 2011;113:214–220. doi: 10.1016/j.acthis.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vulliemoz NR, Xiao E, Xia-Zhang L, Rivier J, Ferin M. Astressin B, a nonselective corticotropin-releasing hormone receptor antagonist, prevents the inhibitory effect of Ghrelin on luteinizing hormone pulse frequency in the ovariectomized rhesus monkey. Endocrinology. 2008;149:869–74. doi: 10.1210/en.2007-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang YA, Dean JM, Badgery-Parker T, Sullivan EA. Assisted reproductive technology in Australia and New Zealand 2006. Sydney: AIHW National Perinatal Statistics Unit and Fertility Society of Australia; 2008. (Assisted reproduction technology series, no. 12. AIHW cat. no. PER 43). [Google Scholar]
- 78.West DB, York B. Dietary fat, genetic predisposition, and obesity; lessons from animal models. Am J Clin Nutr. 1998;67(3 Suppl):505S–512S. doi: 10.1093/ajcn/67.3.505S. [DOI] [PubMed] [Google Scholar]
- 79.WHO, World Health Organization. The SuRF report 2: The Surveillance of chronic disease risk factors. Geneva: WHO; 2005. [Google Scholar]
- 80.Yan W, Linderborg J, Suominen J, Toppari J. Stage-specific regulation of stem cell factor gene expression in the rat seminiferous epithelium. Endocrinology. 1999;140:1499–504. doi: 10.1210/endo.140.3.6590. [DOI] [PubMed] [Google Scholar]
- 81.Yan W, Suominen J, Toppari J. Stem cell factor protects germ cells from apoptosis in vitro. J Cell Sci. 2000;113:161–8. doi: 10.1242/jcs.113.1.161. [DOI] [PubMed] [Google Scholar]
- 82.Yoshioka M, Doucet E, St-Pierre S, Alméras N, Richard D, Labrie A, et al. Impact of high-intensity exercise on energy expenditure, lipid oxidation and body fatness. Int J Obes Relat Metab Disord. 2001;25:332–9. doi: 10.1038/sj.ijo.0801554. [DOI] [PubMed] [Google Scholar]


