Abstract
The present study was aimed to investigate the possible protective effects of thymoquinone (TQ) and curcumin (Cur) on gentamicin (GM)-induced nephrotoxicity in rats. Rats were divided into four groups as follows: group 1 received normal saline and served as normal controls, group 2 received GM only, group 3 concurrently received GM and TQ and group 4 concurrently received GM and Cur. At day 21, rats were sacrificed and samples were collected for assaying serum tumor necrosis factor alpha (TNF-α), urea and creatinine levels, and renal lipid peroxidaion, glutathione (GSH) content as well as glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities. In addition, kidneys were collected for histopathological examination and immunohistochemical determination of the antiapoptotic protein, B-cell lymphoma 2 (Bcl-2). The biochemical results showed that GM-induced nephrotoxicity was associated with a significant increase in serum TNF-α, urea and creatinine as well as renal lipid peroxidation. On the other hand, renal GSH content and GPx and SOD activities were significantly declined. Concomitant administration of either TQ or Cur efficiently alleviated the altered biochemical and histopathological features. In conclusion, both TQ and Cur showed more or less similar marked renoprotective effect against GM-induced nephrotoxicity through their antioxidant, anti-inflammatory and anti-apoptotic efficacies.
Keywords: gentamicin, nephrotoxicity, thymoquinone, curcumin, inflammation, apoptosis, Bcl-2
Introduction
The use of nephrotoxic drugs has been implicated as a causative factor in up to 25 % of all cases of severe acute renal failure in critically ill patients (Pannu and Nadim, 2008[43]). Aminoglycoside antibiotics are employed clinically because of their potent bactericidal activities, less bacterial resistance, post-antibiotic effects and low cost. However, drugs belong to this class are well-known to cause nephrotoxicity, which limits their frequent clinical exploitation (Balakumar et al., 2010[9]). Gentamicin (GM), an aminoglycoside antibiotic, is effective against gram-negative bacterial infections (Martinez-Salgado et al., 2007[37]). In spite of inducing nephrotoxicity, GM is used clinically due to its wide spectrum of activities against gram-negative bacterial infections caused by Pseudomonas, Proteus, and Serratia (Miglioli et al., 1999[38]; Hendriks et al., 2004[22]).
Oxidative stress has been proposed to contribute to nephrotoxicity, and it has been suggested that reactive oxygen species (ROS) is the central key in the mechanisms that lead to tubular necrosis and decrease of glomerular filtration rate (Lopez-Novoa et al., 2011[34]). GM increases generation of ROS such as superoxide anions (Nitha and Janardhanan, 2008[40]; Kalayarasan et al., 2009[25]; Yaman and Balikci, 2010[62]), hydroxyl radicals, hydrogen peroxide, and reactive nitrogen species in the kidney (Balakumar et al., 2008[8]). It has been shown that GM exerts its adverse renal effect by generation of ROS (Kadkhodaee et al., 2005[24]) which results in sever tissue damage (Kaul et al., 1993[31]). Therefore, ROS scavengers and antioxidant molecules have the capacity to partially reduce or eliminate the deleterious effects induced by GM.
Curcumin (Cur) is a yellow colored phenolic pigment obtained from powdered rhizome of Curcuma longa Linn. Studies have shown that curcumin has multiple pharmacological actions, such as antioxidant (Feinstein et al., 2005[16]), anti-inflammatory (Barinaga, 1998[10]), and anticancer properties (Dutta et al., 2005[14]). Thymoquinone (TQ) has been found as the main bioactive constituent of the volatile oil of Nigella sativa seeds (Padhye et al., 2008[42]). Recently, clinical and experimental studies have demonstrated many therapeutic effects of TQ including immunomodulative (Salem, 2005[52]), anti-inflammatory (Al-Ghamdi, 2001[3]), anti-tumor (Rooney and Ryan, 2005[51]), gastroprotective (Kanter et al., 2005[27]), cardioprotective (Kanter, 2011[26]) and antimicrobial (Harzallah e al., 2011[21]). Thus, the intention of the present study was to demonstrate the efficacy of Cur and TQ in the modulation of oxidative stress, inflammation and cell damage associated with GM-induced nephrotoxicity in experimental animals.
Materials and Methods
Chemicals
GM was supplied from Memphis for Pharmaceutical Chemical Industries Co. (Egypt). Cur and TQ were purchased from Sigma Chemicals Co. (USA), stored at 2-4 °C and protected from sunlight. All other chemicals were of analytical grade and were obtained from standard commercial supplies.
Experimental animals
White male albino rats (Rattus norvegicus), 8 weeks old, weighing about 130-150 g were used. They were obtained from the animal house of the National Research Center, El-Giza, Egypt. They were kept under observation for about 15 days before the onset of the experiment to exclude any intercurrent infection. The chosen animals were housed in plastic well-aerated cages (4 rats/cage) at normal atmospheric temperature (25 ± 5 °C) and normal 12-hour light/dark cycle. Moreover, they had free access to water and were supplied daily with standard diet of known composition ad libitum. All animal procedures were in accordance with the recommendations of the Canadian Committee for Care and Use of Animals (Canadian Council on Animal Care, 1993[13]).
Experimental design
Nephrotoxicity was induced by the intraperitoneal (ip) administration of GM at a dose of 100 mg/kg body weight 3 days/ week for 3 weeks. Both Cur and TQ were dissolved in 1 % carboxymethylcellulose (CMC) and were orally administered 3 days/week. The experimental animals were divided into four groups, each group comprising six rats as detailed follows:
Group 1: served as normal (N) and injected with saline.
Group 2: GM only.
Group 3: GM + TQ (20 mg/kg b.wt. every other day for 21 days) (El-Wakf et al., 2011[15]).
Group 4: GM + Cur (20 mg/kg b.wt. every other day for 21 days) (Attia et al., 2010[5]).
At day 21, rats were sacrificed and samples were collected for assaying serum TNF-α, urea and creatinine levels, and renal lipid peroxidaion, reduced glutathione (GSH) as well as glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities. In addition, kidneys were collected for histopathological examination and immunohistochemical determination of Bcl-2.
Biochemical assays
Serum levels of the proinflammatory cytokine, TNF-α, was determined by specific ELISA kits (R&D Systems, USA) according to the manufacturer's instructions. The concentration of TNF-α was determined spectrophotometrically at 450 nm. Standard plot was constructed by using standard cytokine and the concentrations for unknown samples were calculated from the standard plot.
Serum creatinine concentration (Young, 1995[63]) and urea level (Kaplan, 1984[28]) were determined using reagent kits purchased from Spinreact (Spain). Lipid peroxidation, GSH, and SOD and GPx activities were also measured in kidney homogenate according to the methods of Preuss et al. (1998[48]), Beutler et al. (1963[11]), Marklund and Marklund (1974[36]) and Kar and Mishra (1976[29]), respectively.
Histopathological study
After sacrifice, decapitation and dissection, kidneys from each rat were rapidly excised and then perfused in saline solution. Pieces from the kidneys of rats of different groups were fixed in 10 % neutral buffered formalin for 24 hours. The fixed kidneys were cut to slices approximately 1-mm thick, and after tissue processing, paraffin sections (5-µm thickness) were prepared and stained by hematoxylin and eosin (H&E).
Immunohistochemical staining of BCL-2
Immunolocalization technique for Bcl-2 was prepared on 3-4 µm thickness sections according to Pedrycz and Czerny (2008[45]). In brief, mouse anti-Bcl-2 (diluted 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), were incubated with sections for 60 min. Primary antibodies were diluted in Tris-buffered saline with 1 % bovine serum albumin. Then a biotinylated secondary antibody directed against mouse immunoglobulin (Dako Cytomation, USA) was added and incubated for 15 min, followed by horseradish peroxidase conjugated with streptavidin for further 15 min incubation. At the sites of immunolocalization of the primary antibodies, a reddish to brown color appeared after adding 3-amino-9-ethyl-carbasole (Dako Cytomation, USA) for 15 min. The specimens were counterstained with hematoxylin for 1 min and mounted using the Aquatex fluid (Merk KGaA, Germany). All sections were incubated under the same conditions with the same concentration of antibodies and at the same time, so the immunostaining was comparable among the different experimental groups.
Statistical analysis
Statistical analysis was performed using SPSS v.16. Results were articulated as mean ± standard error (SE) and all statistical comparisons were made by means of one-way ANOVA test followed by Duncan’s multiple range test post hoc analysis. A P value <0.05 was considered significant.
Results
As shown in Table 1(Tab. 1), ip injection of GM resulted in marked impairment of renal functions as reflected by significant (P < 0.001) increase in the levels of serum urea and creatinine as compared with normal control rats. On the other hand, concomitant oral administration of TQ as well as Cur produced an efficient protective effect on the altered renal functions. Both TQ and Cur have more or less similar potentials in improving renal function markers deleteriously perturbed in GM-administered rats.
Table 1. Effect of TQ and Cur on serum urea and creatinine levels in GM-administered rats.
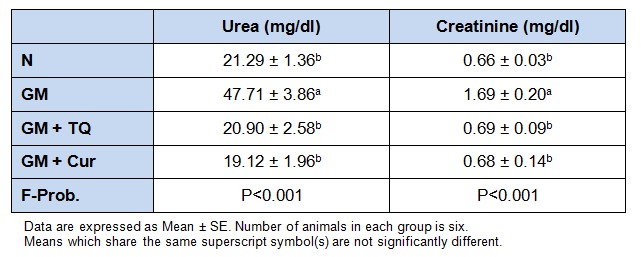
The effects of oral administration of TQ and Cur on the level of serum TNF-α of normal and GM-injected rats were depicted in Figure 1(Fig. 1). The level of TNF-α was elevated significantly (P < 0.001) in GM group as compared with normal one. Upon oral treatment with TQ and Cur, this altered level was significantly ameliorated.
Figure 1. Effect of TQ and Cur on serum TNF-α in GM-administered rats. Data are expressed as Mean ± SE. Means which share the same superscript symbol(s) are not significantly different, P < 0.001.
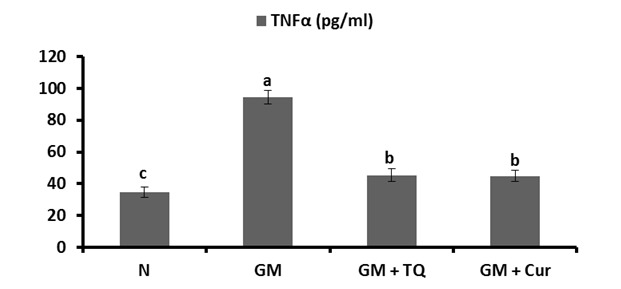
In the same regard, GM induced a remarkable elevation (P < 0.001) in renal lipid peroxidation, assayed as malondialdehyde (MDA) level. Oral administration of either TQ or Cur produced a significant decrease in the elevated MDA levels (Figure 2(Fig. 2)).
Figure 2. Effect of TQ and Cur on renal malondialdehyde (MDA) levels in GM-administered rats. Data are expressed as Mean ± SE. Means which share the same superscript symbol(s) are not significantly different, P < 0.001.
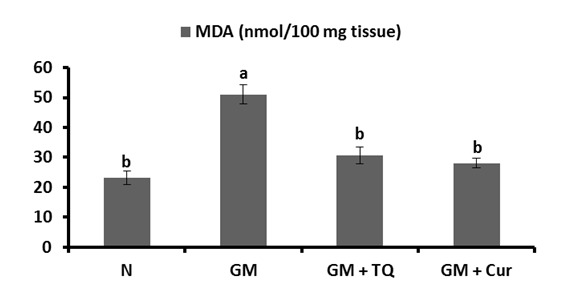
The effects of TQ and Cur supplementation on the level of renal tissue GSH content were depicted in Figure 3(Fig. 3). Following GM injection, renal tissue GSH was significantly (P < 0.001) declined in GM control rats as compared to normal ones. TQ or Cur administrations significantly ameliorated the altered GSH content.
Figure 3. Effect of TQ and Cur on renal GSH levels in GM-administered rats. Data are expressed as Mean ± SE. Means which share the same superscript symbol(s) are not significantly different, P < 0.001.
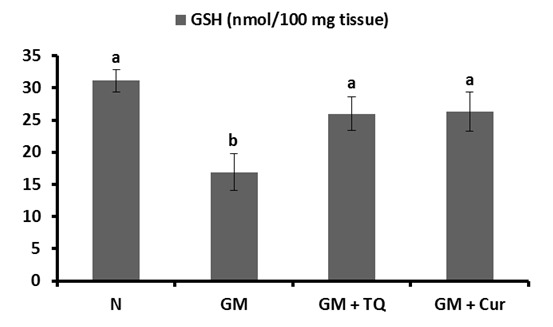
The activities of GPx and SOD in renal tissue were represented in Figures 4(Fig. 4) and 5(Fig. 5), respectively. GM control rats revealed a significant reduction in renal GPx and SOD activities in comparison with normal control rats. The co-administration of TQ and Cur successfully prevented the profound decrease in both enzyme activities; TQ seemed to be more potent in this regard.
Figure 4. Effect of TQ and Cur on renal GPx activity in GM-administered rats. Data are expressed as Mean ± SE. Means which share the same superscript symbol(s) are not significantly different, P < 0.001.
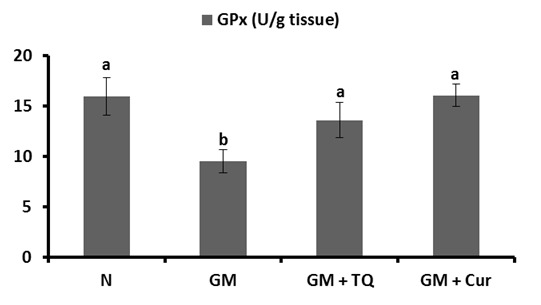
Figure 5. Effect of TQ and Cur on renal SOD activity in GM-administered rats. Data are expressed as Mean ± SE. Means which share the same superscript symbol(s) are not significantly different, P < 0.001.
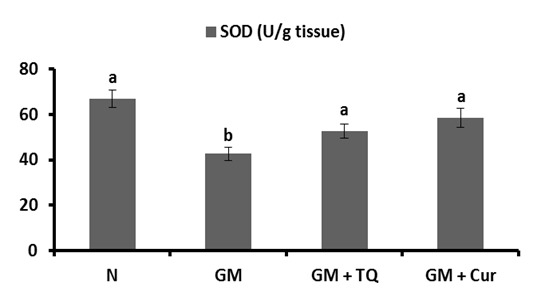
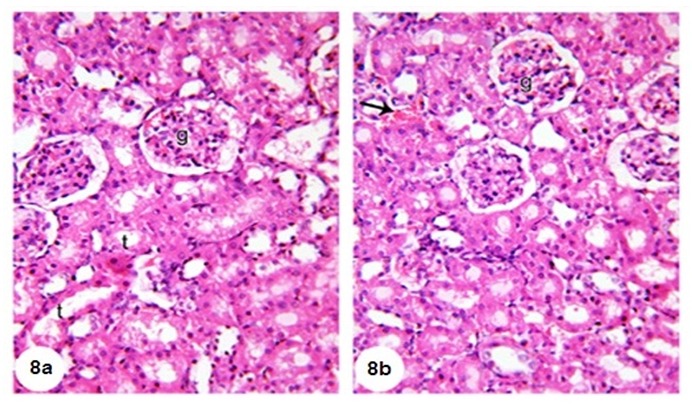
Microscopical examination of the kidney of normal rats (Figure 6a and b(Fig. 6)) revealed normal histological structure. The kidney is divided into an outer cortex and inner medulla. The injection of GM led to perturbed histological changes and several lesions including dilated hypermic vein (Figure 7a(Fig. 7)), damaged and dilated tubules, inflammatory cells infiltration (Figure 7b(Fig. 7)), desquamation of tubular epithelium with cytoplasmic vaculations, complete atrophy of some glomeruli (Figure 7c(Fig. 7)) and shrinkage of glomerular capillaries in others, oedema, proximal tubular necrosis, interstitial hemorrhage and fibroblast proliferation (Figure 7d(Fig. 7)). In addition, the kidneys of GM-treated rats showed interstitial haemorrhage accompanied proximal tubular necrosis, Lymphocytic infiltration has increased and shrinkage of glomerular capillary (Figure 7e and f(Fig. 7)). The administration of TQ and Cur prevented the GM-induced histological alterations. Only interstitial hemorrhage is observed in Cur-treated rats (Figure 8a and b(Fig. 8)).
Figure 6. Photomicrographs of kidney sections of normal rat showing glomerulus (G), proximal tubules (p) and distal tubules (d). (6a) (X100) and (6b) (X400).
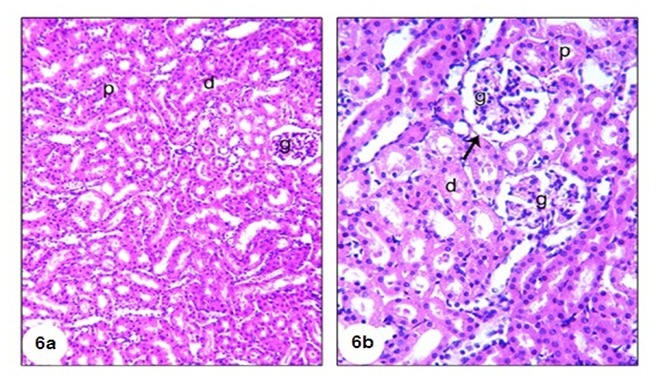
Figure 7. A photomicrograph of H&E stained kidney section of GM-administered rats (Group 2) showing dilated hypermic portal vein (hg), inflammatory cells infiltration (if) (7a), damaged and dilated tubule (t) with if (7b), complete atrophy of some glomeruli (arrow), desquamation of tubular epithelium with cytoplasmic vaculations (v) (7c), odema (o) with a number of inflammatory cells, proximal tubular necrosis (arrow) (7d), and interstitial haemorrhage (arrow) accompanied with proximal tubular necrosis and shrinkage of glomerular capillary (7e and f), (X400).
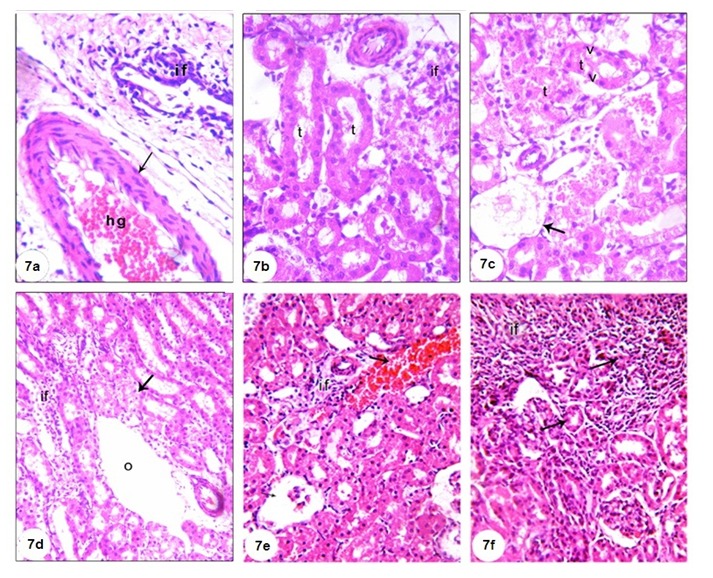
Figure 8. (8a) Kidney section of group 3 (GM + TQ) showing nearly normal renal tubules (t) and renal corpuscles (g), (8b) kidney section of group 4 (GM + Cur) showing normal renal corpuscles (g), normal renal tubules (t) with interstitial haemorrhage (arrow), (H&E, X400).
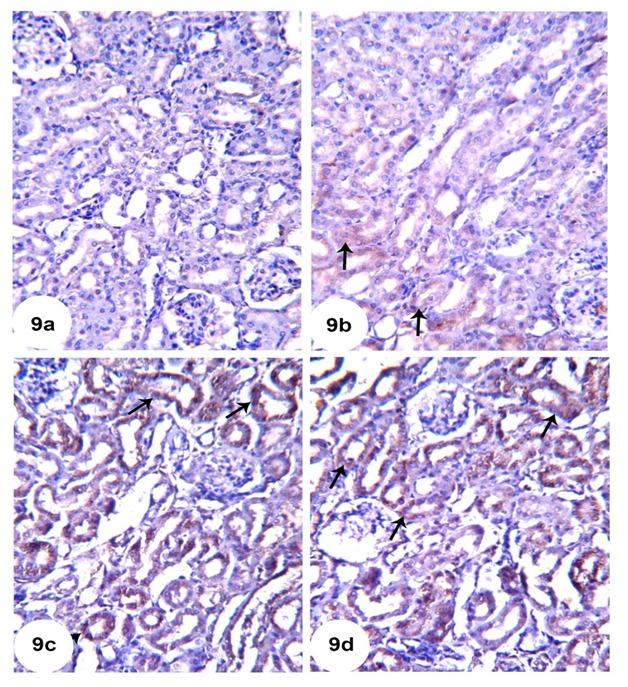
Immunohistochemical photographs for detection of the anti-apoptotic protein, Bcl-2, revealed moderate expression in renal tubular cells of the normal control rats (Figure 9a(Fig. 9)). On the other hand, Bcl-2 expression was obviously decreased in GM-injected rats (Figure 9b(Fig. 9)). The administration of either TQ or Cur potentially increased Bcl-2 expression in renal tubular epithelial cells as demonstrated in Figures 9c and 9d, respectively.
Figure 9. Representative Bcl-2 immunohistochemistry of kidney sections of N (9a), GM (9b), GM + TQ (9c) and GM + Cur (9d).
Discussion
The mechanisms of GM-induced nephrotoxicity are not completely known. However, proposed pathological mechanisms include induction of oxidative stress, apoptosis, necrosis, elevation of endothelin I and increase of monocyte/macrophages infiltration (Geleilete et al., 2002[18]; Balakumar et al., 2010[9]). GM-induced nephrotoxicity is characterized functionally by increased serum creatinine, increased blood urea nitrogen, and decreased glomerular filtration rate (Mysior and Stefanczyk, 2007[39]; Romero et al., 2009[50]), and morphologically characterized by proximal tubule epithelial desquamation, tubular necrosis, epithelial edema, and glomerular hypertrophy (Lakshmi et al., 2009[33]).
The current study revealed a significant increase in serum TNF-α of GM-administered rats as compared to normal control rats. The importance of the contribution of the immune system to drug-induced kidney and liver toxicity has been well recognized over the past years. Several nephrotoxicants and hepatotoxicants have been shown to induce an inflammatory response, which participated in the organ injury (Pabla and Dong, 2008[41]; Quiros et al., 2011[49]; Araujo et al., 2012[4]). It is believed that during kidney toxicity, the initial insult by the toxicant results in tissue damage, which leads to generation of inflammatory mediators by the injured cells as well as by immune cells. Subsequently, these inflammatory mediators induce migration and infiltration of leukocytes into the injured organs and aggravate the primary injury induced by the toxicant (Luster et al., 2001[35]; Akcay et al., 2009[2]). This evidence is supported by the histological results of the present study which revealed the presence of inflammatory cells infiltration in kidney sections of GM-administered rats. For kidneys, the pro-inflammatory cytokine TNF-α is the main orchestrator of this inflammatory response and in several cases has been shown to aggravate the toxicant-induced pathophysiological responses (Shaw et al., 2007[55]; Zou et al., 2009[64]; Fredriksson et al., 2011[17]; Piao et al., 2012[46]). Alleviation of the altered serum TNF-α following TQ and Cur administration might be attributed to the anti-inflammatory properties of the tested agents.
Results from many studies have shown that intraperitoneal injections of GM resulted in development of destructive renal injury that was associated with significant elevation in serum urea and creatinine levels (Pedraza-Chaverrí et al., 2004[44]; Silan et al., 2007[56]; Soliman et al., 2007[57]). In addition, the findings of histopathological examinations confirmed the biochemical data and showed the clear signs of nephrotoxicity in the form of marked glomerular and tubular degenerative changes and necrosis, tubulointerstitial nephritis and dilatation of the tubular lumen. These biochemical and histopathological observations of GM-induced nephrotoxicity run in consistency with those reported earlier in human patients (Baciewicz et al., 2003[6]) and experimental animals (Silan et al., 2007[56]). On the other hand, either TQ or Cur concurrently administered with GM efficiently protected the rat kidneys from the serious nephrotoxic effects of GM. These results support those of earlier studies that demonstrated the protective properties of TQ and Cur against nephrotoxicity induced by other chemotherapeutic agents (Badary, 1999[7]; Tirkey et al., 2005[59]; Sayed, 2008[53]).
Oxidative stress has been proposed to contribute to nephrotoxicity, and it has been suggested that ROS is the central key in the mechanisms that lead to tubular necrosis and decrease of glomerular filtration rate. ROS activates nuclear factor kappa B that plays a key role in the inception of inflammatory process. It may be said that the central role of GM-induced nephrotoxicity is oxidative stress and inflammation; a loop of damage amplification and a connection between mechanisms of tubular and glomerular changes (Lopez-Novoa et al., 2011[34]).
According to our results, in the GM treated group, renal tissue GSH content as well as antioxidant enzymes activity were declined significantly when compared with the control group, similar to what reported by other researchers (Polat et al., 2006[47]; Nitha and Janardhanan, 2008[40]; Karadeniz et al., 2008[30]; Kalayarasan et al., 2009[25]). The increased production of ROS in GM-induced nephrotoxicity may cause inactivation of antioxidant enzymes such as SOD and GPx (Karadeniz et al., 2008[30]). In addition, it has been reported that the GM nephrotoxicity involves renal free radical generation, reduction in antioxidant defense mechanisms, acute tubular necrosis and glomerular congestion (Geleilete et al., 2002[18]; Martinez-Salgado et al., 2007[37]; Abdel-Raheem et al., 2009[1]), resulting in diminished glomerular filtration rate and renal dysfunction. Our results showed that oral administration of TQ and Cur notably alleviated renal tissue lipid peroxidation, GSH, GPx and SOD in comparison with GM control group. These results were reported by others who treated animals with different antioxidant agents (Sener et al., 2002[54]; Nitha and Janardhanan, 2008[40]; Kalayarasan et al., 2009[25]).
Apoptosis is an essential process in the development and tissue homeostasis of most multicellular organisms. There are experimental data suggesting that the neprotoxicity may be closely associated with activation of proapoptotic proteins (Han et al., 2006[20]). Bcl-2 may play an important role in protection from apoptosis (Huang et al., 2000[23]) and has been one focus in present research. This proto-oncogene is localized on the mitochondrial membrane, endoplasmic reticulum membrane and nuclear pores where it functions to prolong the cell life span, inhibit the apoptosis during cell proliferation and protect from apoptosis of non-proliferating cells (Hale et al., 1996[19]). Transfection of Bcl-2 gene can prolong the cell life span by inhibiting the stimulating factors-induced cell apoptosis, which may be caused by radiative damages, c-myc or p53 genes, chemotherapeutic drugs, decreased cell growth factors, etc. (Korsmeyer, 1999[32]). Studies have confirmed that the over-expression of Bcl-2 inhibits the apoptosis of liver cancer cells (Tsujimoto et al., 1997[60]). Takahashi et al. (1999[58]) found that the over-expression of Bcl-2 proteins produced by the transfection of Bcl-2 gene into the vascular smooth muscle cell (VSMC) inhibited the apoptosis of VSMC induced by nitric oxide (NO). Thus, the increased expression of Bcl-2 following TQ and Cur administration reflected their anti-apoptotic and renoprotective effects. Our findings are in consistent with Bhattacharyya et al. (2007[12]) who reported that Cur prevents tumor induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction and Ullah et al. (2012[61]) who demonstrated that TQ provided protection against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons.
Conclusion
Results of the present study suggests that concurrent administration of either TQ or Cur attenuated the development of GM-induced nephrotoxicity by mechanisms related to their ability to decrease lipid peroxidation and potentiating the antioxidant defense system. In addition, their renoprotective effects were also attributed to their anti-inflammatory and antiapoptotic efficacies.
References
- 1.Abdel-Raheem IT, Abdel-Ghany AA, Mohamed GA. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009;32:61–67. doi: 10.1248/bpb.32.61. [DOI] [PubMed] [Google Scholar]
- 2.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Ghamdi MS. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76:45–48. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 4.Araujo LP, Truzzi RR, Mendes GE, Luz MA, Burdmann EA, Oliani SM. Annexin A1 protein attenuates cyclosporine-induced renal hemodynamics changes and macrophage infiltration in rats. Inflamm Res. 2012;61:189–196. doi: 10.1007/s00011-011-0400-z. [DOI] [PubMed] [Google Scholar]
- 5.Attia A, Ragheb A, Sylwestrowicz T, Shoker A. Attenuation of high cholesterol-induced oxidative stress in rabbit liver by thymoquinone. Eur J Gastroenterol Hepatol. 2010;22:826–834. doi: 10.1097/MEG.0b013e328336000d. [DOI] [PubMed] [Google Scholar]
- 6.Baciewicz AM, Sokos DR, Cowan RI. Aminoglycoside-associated nephrotoxicity in the elderly. Ann Pharmacother. 2003;37:182–186. doi: 10.1177/106002800303700203. [DOI] [PubMed] [Google Scholar]
- 7.Badary OA. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J Ethnopharmacol. 1999;67:135–142. doi: 10.1016/s0378-8741(98)00242-6. [DOI] [PubMed] [Google Scholar]
- 8.Balakumar P, Chakkarwar VA, Kumar V, Jain A, Reddy J, Singh M. Experimental models for nephropathy. J Renin Angiotensin Aldosterone Syst. 2008;9:189–195. doi: 10.1177/1470320308098343. [DOI] [PubMed] [Google Scholar]
- 9.Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62:179–186. doi: 10.1016/j.phrs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Barinaga M. Stroke-damaged neurons may commit cellular suicide. Science. 1998;281(5381):1302–1303. doi: 10.1126/science.281.5381.1302. [DOI] [PubMed] [Google Scholar]
- 11.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Mid. 1963;61:882–888. [PubMed] [Google Scholar]
- 12.Bhattacharyya S, Mandal D, Saha B, Sen GS, Das T, Sa G. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem. 2007;282:15954–15964. doi: 10.1074/jbc.M608189200. [DOI] [PubMed] [Google Scholar]
- 13.Canadian Council on Animal Care, editor. Guide to the care and use of experimental animals. Vol. 2. Ottawa, Ontario, Canada: CCAC; 1993. [Google Scholar]
- 14.Dutta S, Padhye S, Priyadarsini KI, Newton C. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg Med Chem Lett. 2005;15:2738–2744. doi: 10.1016/j.bmcl.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.El-Wakf AM, Elhabiby EM, El-kholy WM, Abd El-Ghany E. Use of tumeric and curcumin to alleviate adverse reproductive outcomes of water nitrate pollution in male rats. Nature Sci Sleep. 2011;9:229–239. [Google Scholar]
- 16.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, et al. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Fredriksson L, Herpers B, Benedetti G, Matadin Q, Puigvert JC, de Bont H, et al. Diclofenac inhibits tumor necrosis factor-alpha-induced nuclear factor-kappaB activation causing synergistic hepatocyte apoptosis. Hepatology. 2011;53:2027–2041. doi: 10.1002/hep.24314. [DOI] [PubMed] [Google Scholar]
- 18.Geleilete TJ, Melo GC, Costa RS, Volpini RA, Soares TJ, Coimbra TM. Role of myofibroblasts, macrophages, transforming growth factor-beta, endothelin, angiotensin-II, and fibronectin in the progression of tubulointerstitial nephritis induced by gentamicin. J Nephrol. 2002;15:633–42. [PubMed] [Google Scholar]
- 19.Hale AJ, Smith CA, Sutherland LC, Stoneman VE, Longthorne VL, Culhane AC, et al. Apoptosis: molecular regulation of cell death. Eur J Biochem. 1996;236:1–26. doi: 10.1111/j.1432-1033.1996.00001.x. [DOI] [PubMed] [Google Scholar]
- 20.Han SY, Chang EJ, Choi HJ, Kwak CS, Park SB, Kim HC, et al. Apoptosis by cyclosporine in mesangial cells. Transplant Proc. 2006;38:2244–2246. doi: 10.1016/j.transproceed.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Harzallah HJ, Kouidhi B, Flamini G, Bakhrouf A, Mahjoub T. Chemical composition, antimicrobial potential against cariogenic bacteria and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chem. 2011;129:1469–1474. [Google Scholar]
- 22.Hendriks JGE, van Horn JR, van der Mei HC, Busscher HJ. Backgrounds of antibiotic loaded bone cement and prosthesis-related infection. Biomaterials. 2004;25:545–56. doi: 10.1016/s0142-9612(03)00554-4. [DOI] [PubMed] [Google Scholar]
- 23.Huang LH, Zhang GQ, Ye RG, Li YJ, Chen XH, Guan WM. The role of cell proliferation and apoptosis in renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Chin J Nephrol. 2000;16:24–27. [Google Scholar]
- 24.Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol. 2005;90:571–576. doi: 10.1113/expphysiol.2004.029728. [DOI] [PubMed] [Google Scholar]
- 25.Kalayarasan S, Prabhu PN, Sriram N, Manikandan R, Arumugam M, Sudhandiran G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur J Pharmacol. 2009;606:162–171. doi: 10.1016/j.ejphar.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 26.Kanter M. Thymoquinone attenuates lung injury induced by chronic toluene exposure in rats. Toxicol Ind Health. 2011;27:387–395. doi: 10.1177/0748233710387630. [DOI] [PubMed] [Google Scholar]
- 27.Kanter M, Demir H, Karakaya C, Ozbek H. Gastroprotective activity of Nigella sativa L oil and its constituent, thymoquinone, against acute alcohol-induced gastric mucosal injury in rats. World J Gastroenterol. 2005;11:6662–6666. doi: 10.3748/wjg.v11.i42.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan A. Urea. In: Kaplan LA, Pesce AJ, editors. Clinical chemistry: theory, analysis and correlation. St. Louis, Miss.: Mosby; 1984. pp. 1257–1260. [Google Scholar]
- 29.Kar M, Mishra D. Catalase, peroxidase and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 1976;57:315–319. doi: 10.1104/pp.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karadeniz A, Yildirim A, Simsek N, Kalkan Y, Celebi F. Spirulina platensis protects against gentamicin-induced nephrotoxicity in rats. Phytother Res. 2008;22:1506–1510. doi: 10.1002/ptr.2522. [DOI] [PubMed] [Google Scholar]
- 31.Kaul N, Siveski-Iliskovic N, Hill M, Slezak J, Singal PK. Free radicals and the heart. J Pharmacol Toxicol Methods. 1993;30:55–67. doi: 10.1016/1056-8719(93)90008-3. [DOI] [PubMed] [Google Scholar]
- 32.Korsmeyer SJ. Bcl-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693–1700. [PubMed] [Google Scholar]
- 33.Lakshmi BV, Neelima N, Kasthuri N, Umarani V, Sudhakar M. Protective effect of Bauhinia purpurea on gentamicin-induced nephrotoxicity in rats. Indian J Pharm Sci. 2009;71:551–554. doi: 10.4103/0250-474X.58196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79:33–45. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- 35.Luster MI, Simeonova PP, Gallucci RM, Bruccoleri A, Blazka ME, Yucesoy B. Role of inflammation in chemical-induced hepatotoxicity. Toxicol Lett. 2001;120:317–321. doi: 10.1016/s0378-4274(01)00284-3. [DOI] [PubMed] [Google Scholar]
- 36.Marklund SL, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Salgado C, Lopez-Hernandez FJ, Lopez-Novoa JM. Glomerular nephrotoxicityof aminoglycosides. Toxicol Appl Pharmacol. 2007;223:86–98. doi: 10.1016/j.taap.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Miglioli PA, Silini R, Carzeri O, Grabocka E, Allerberger F. Antibacterial activity of gentamicin and ciprofloxacin against gram-negative bacteria: interactions with pig and calf sera. Pharmacol Res. 1999;39:321–3. doi: 10.1006/phrs.1998.0447. [DOI] [PubMed] [Google Scholar]
- 39.Mysior M, Stefanczyk L. Doppler ultrasound criteria of physiological flow in asymmetrical vertebral arteries. Med Sci Monit. 2007;13(Suppl 1):73–77. [PubMed] [Google Scholar]
- 40.Nitha B, Janardhanan KK. Aqueous-ethanolic extract of morel mushroom mycelium Morchella esculenta, protects cisplatin and gentamicin induced nephrotoxicity in mice. Food Chem Toxicol. 2008;46:3193–3199. doi: 10.1016/j.fct.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 42.Padhye S, Banerjee A, Ahmad A, Mohammad R, Sarkar FH. From here to eternity - the secret of pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- 43.Pannu N, Nadim M. An overview of drug induced acute kidney injury. Crit Care Med. 2008;36(Suppl 4):S216–S223. doi: 10.1097/CCM.0b013e318168e375. [DOI] [PubMed] [Google Scholar]
- 44.Pedraza-Chaverrí J, Barrera D, Maldonado PD, Chirino YI, Macías-Ruvalcaba NA, Medina-Campos ON, et al. S-allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin Pharmacol. 2004;4:5–18. doi: 10.1186/1472-6904-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedrycz A, Czerny K. Immunohistochemical study of proteins linked to apoptosis in rat fetal kidney cells following pregnancy Adriamycin administration in mother. Acta Histochem. 2008;110:519–523. doi: 10.1016/j.acthis.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Piao RL, Liu YY, Tian D, Ma ZH, Zhang M, Zhao C, et al. Adefovir dipivoxil modulates cytokine expression in Th1/Th2 cells in patients with chronic hepatitis B. Mol Med Rep. 2012;5:184–189. doi: 10.3892/mmr.2011.627. [DOI] [PubMed] [Google Scholar]
- 47.Polat A, Parlakpinar H, Tasdemir S, Colak C, Vardi N, Ucar M, et al. Protective role of aminoguanidine on gentamicin-induced acute renal failure in rats. Acta Histochem. 2006;108:365–371. doi: 10.1016/j.acthis.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Preuss HG, Jarrell ST, Scheckenbach R, Lieberman S, Anderson RA. Comparative effect of chromium vanadium and Gymnema sylvestre on sugar-induced blood pressure elevation in SHR. J Am Coll Nutr. 1998;17:116–123. doi: 10.1080/07315724.1998.10718736. [DOI] [PubMed] [Google Scholar]
- 49.Quiros Y, Vicente-Vicente L, Morales AI, Lopez-Novoa JM, Lopez-Hernandez FJ. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol Sci. 2011;119:245–256. doi: 10.1093/toxsci/kfq267. [DOI] [PubMed] [Google Scholar]
- 50.Romero F, Perez M, Chavez M, Parra G, Durante P. Effect of uric acid on gentamicin-induced nephrotoxicity in rats - role of matrix metalloproteinases 2 and 9. Basic Clin Pharmacol Toxicol. 2009;105:416–424. doi: 10.1111/j.1742-7843.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 51.Rooney S, Ryan MF. Modes of action of hederin and thymoquinone, active constituents of Nigella sativa, against HEp-2 cancer cells. Anticancer Res. 2005;25:4255–4259. [PubMed] [Google Scholar]
- 52.Salem ML. Immunomodulatory and therapeutic properties of Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Sayed AA. Thymoquinine protects renal tubular cells against tubular injury. Cell Biochem Funct. 2008;26:374–380. doi: 10.1002/cbf.1454. [DOI] [PubMed] [Google Scholar]
- 54.Sener G, Sehirli AO, Altunbas HZ, Ersoy Y, Paskaloglu K, Arbak S, et al. Melatonin protects against gentamicin-induced nephrotoxicity in rats. J Pineal Res. 2002;32:231–236. doi: 10.1034/j.1600-079x.2002.01858.x. [DOI] [PubMed] [Google Scholar]
- 55.Shaw PJ, Hopfensperger MJ, Ganey PE, Roth RA. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol Sci. 2007;100:259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- 56.Silan C, Uzun O, Comunoglu NU, Gokçen S, Bedirhan S, Cengiz M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull. 2007;30:79–83. doi: 10.1248/bpb.30.79. [DOI] [PubMed] [Google Scholar]
- 57.Soliman KM, Abdul-Hamid M, Othman AI. Effect of carnosine on gentamicin-induced nephrotoxicity. Med Sci Monit. 2007;13:73–83. [PubMed] [Google Scholar]
- 58.Takahashi M, Saito H, Okuyama T, Miyashita T, Kosuga M, Sumisa F, et al. Overexpression of Bcl-2 protects human hepatoma cells from Fas-antibody-mediated apoptosis. J Hepatol. 1999;31:315–322. doi: 10.1016/s0168-8278(99)80230-x. [DOI] [PubMed] [Google Scholar]
- 59.Tirkey N, Kaur G, Vij G, Chopra K. Curcumin a diferuloylmethane, attenuates cyclosporine induced renal dysfunction and oxidative stress in rat kidneys. J Biosci. 2005;22:233–246. doi: 10.1186/1471-2210-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsujimoto Y, Shimizu S, Eguchi Y, Kamiike W, Matsuda H. Bcl-2 and Bcl-XL block apoptosis as well as necrosis possible involvement of common mediators in apoptosic and necrotic signal transduction pathways. Leukemia. 1997;11:380–382. [PubMed] [Google Scholar]
- 61.Ullah I, Ullah C, Naseer MI, Lee HY, Kim MO. Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neuroscience. 2012;13:11. doi: 10.1186/1471-2202-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yaman I, Balikci E. Protective effects of nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62:183–190. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Young DS. Effects of drugs on clinical laboratory tests. 4th ed. Washington, DC: The American Association for Clinical Chemistry, AACC Press; 1995. [Google Scholar]
- 64.Zou W, Beggs KM, Sparkenbaugh EM, Jones AD, Younis HS, Roth RA, et al. Sulindac metabolism and synergy with tumor necrosis factor-alpha in a drug-inflammation interaction model of idiosyncratic liver injury. J Pharmacol Exp Ther. 2009;331:114–121. doi: 10.1124/jpet.109.156331. [DOI] [PMC free article] [PubMed] [Google Scholar]


