Abstract
In the study, the possible effect of sodium valproate (NaVP) on urethane-induced lung tumors in mice has been evaluated. BALB/c mice (n = 120; 4–6 weeks old, both sexes) were used in the following groups: 1) urethane-treated, 2) urethane–NaVP-treated, 3) only NaVP-treated, 4) control. In the same groups, castrated male mice (n = 48) were investigated. Urethane was given by intraperitoneal injections 10 mg/mouse, twice a week, the total dose 50 mg/mouse. In NaVP-treated mice, the 0.4 % NaVP aqueous solution was offered to mice ad libitum. The duration of the experiment was 6 months. The number of tumors per mouse in urethane–NaVP-treated males was significantly higher than in those treated with urethane only (13.82 ± 1.12 vs 6.77 ± 0.43, p < 0.0001). No significant difference in the number of tumors per mouse was revealed while comparing the female urethane- and urethane–NaVP-treated groups (6.50 ± 0.79 vs 8.15 ± 0.55, p = 0.105). No difference in the number of tumors per mouse was found in urethane–NaVP-treated castrated males as compared with urethane-treated castrated males. However, in the urethane–NaVP-treated castrated males the number of tumors per mouse was significantly lower than in analogous non-castrated males (7.8 ± 1.67 vs 13.82 ± 1.12, p < 0.01). NaVP combined with urethane potentiates urethane tumorigenicity in BALB/c non-castrated but not in female and castrated male mice. These data indicate an important role of testosterone in the urethane-NaVP induced lung tumorigenesis.
Keywords: sodium valproate, urethane, mice, lung tumorigenesis, gender differences
Introduction
Urethane-induced lung tumors in mice models have been accepted for human lung adenocarcinoma investigations (Malkinson, 2001[30]). BALB/c mice are considered as a medium susceptible for the development of lung tumor by urethane and can be modified by the potential modulatory agents (Malkinson and Beer, 1983[31]; Uleckiene et al., 2005[49]). There are no studies on the effect of sodium valproate (NaVP) on urethane-induced lung tumors in mice. The aim of our study was to investigate the effect of NaVP on lung tumorigenesis induced by urethane, a chemical lung carcinogen, in BALB/c mice of both genders.
NaVP is a medicine preparation which currently is used for the treatment of a wide variety of neurological disorders (Evers, 2008[14]; Bowden, 2009[8]). NaVP increases the turnover of gamma-aminobutyric acid (GABA) and thereby potentiates GABAergic functions (Loscher, 2002[29]). GABA may affect cancer growth by activating GABA receptors. The gene expression investigation of GABA receptors in tissues of non-small cell lung cancers showed the gene expression of GABA receptor phenotypes to be correlated with cancerogenesis and clinical prognosis (Zhang et al., 2013[51]). Similarly, GABA stimulates human hepatocellular carcinoma growth through the overexpressed GABA A receptor (Li et al., 2012[28]).
The GABA A receptor is an ionotropic receptor. Its subunits form a functional chloride channel (Fritschy and Mohler, 1995[16], Bureu et al., 1997[10]). The GABA A receptor is rapidly activated by NaVP in the brain cells (Armijo et al., 2000[3]). The GABA A receptor subunits are expressed in Wistar rat kidney proximal convoluted and straight tubules and in other tissues (Sarang et al., 2001[43]; Li et al., 2012[28]; Zhang et al., 2013[51]). NaVP has shown to enhance the urinary excretion of sodium and chloride ions in Wistar rats of both genders, but the 24-hour chloriduretic response was found to be gender-related (Grikiniene et al., 2005[23]). Chloride is recognized to have an important role in tumor genesis: the intracellular chloride concentration would be one of the critical messengers in cell growth/proliferation and differentiation processes (Shiozaki et al., 2006[46]; Hiraoka et al., 2010[25]; Ohsawa et al., 2010[39]).
Furthermore, NaVP defines a novel class of HDAC inhibitors that induce differentiation of transformed cells and show antitumor properties in clinical and preclinical studies (Göttlicher et al., 2001[20]; Kawagoe, 2002[27]; Santoro et al., 2013[42]). In chronic rat and mouse studies using NaVP, an increased incidence of subcutaneous fibrosarcoma occurred in male rats at the higher dosage level, and a dose-related trend for an increased incidence of benign pulmonary adenomas was observed in male mice (McEvoy, 1991[33]). Many aspects of the contribution of the NaVP pharmacological mechanisms and their significance in gender-related tumorigenesis have not been investigated.
Our data have shown that NaVP together with urethane increases urethane tumorigenicity in male but not in female mice. In contrast, in the groups of castrated males, no synergistic NaVP and urethane effect on lung tumor numbers was found. The possible mechanisms of the NaVP gender-related effect are discussed in the article.
Materials and Methods
In the study, we used the BALB/c mouse lung adenoma assay which is one of the assays often used for investigating the possible cancer modulative agents. The examined BALB/c mice are characterized by a high sensitivity to the pulmonotropic carcinogen urethane. BALB/c mice (n = 120; 4–6 weeks old, both sexes) and castrated BALB/c male mice of similar age (n = 48) were used in the study. The animals were purchased from the Animal Facility of the Lithuanian University of Health Sciences, Veterinary Academy (Kaunas, Lithuania). The experiments were performed in compliance with the relevant laws and institutional guidelines. Permissions of the State Food and Veterinary Service of Lithuania to use experimental animals for research were obtained (No. 0177 /30/04/2008/2 & 25/07/2013). Male castration was performed by removing testes at the age of 5–6 weeks. The animals were housed in standard colony cages with free access to food and acclimatized for one week before the study; they were housed in the conditions of constant temperature (21 ± 1 °C), humidity, and the light/dark cycle (12 h/ 12 h). A commercial pellet diet and fresh drinking water were provided ad libitum.
NaVP was from Sigma-Aldrich Chemie GmbH, Germany, and urethane (ethyl carbamate) was from Fluka.
Intact male and female mice were randomly divided into eight groups – four groups for each gender (n = 15 in a group):
1) treated with urethane,
2) treated with urethane plus NaVP,
3) treated with NaVP only,
4) intact control.
In the same four groups (n = 15 per group), castrated male mice were investigated. In the urethane-treated groups, 10 mg urethane/mouse in 0.2 ml sterile physiological solution was given twice a week by intraperitoneal injections (5 doses, total dose 50 mg/mouse). The second and third groups of both genders were treated per os with NaVP 0.4 % aqueous solution (in the second group starting two weeks prior to urethane administration) for six months. The NaVP solution was offered to the animals ad libitum (in aluminum foil-wrapped bottles to avoid light-induced decomposition). The control and urethane groups were given water which was their only source of drinking. After six months, animals that survived were killed in a CO2 camera. Lungs were fixed in a 10 % buffered formalin solution. Randomly selected tumors were taken for histological evaluation. Histological examination was performed in each case when observable lung pathology (suspected not identified or malignant tumor) was present. The percentage of animals with lung tumors, the mean number of tumors per tumor-bearing mouse, tumor growth and histological type were considered in both genders only in mice that survived up to the end of the experiment. The results were statistically processed using Student`s t-test. Data were expressed a mean ± SEM. The difference at the value of p < 0.05 was considered significant.
Results
Mice survival in the study groups
Non-castrated mice. Mice of both genders in control and NaVP-treated groups survived until the end of the experiment. In the male urethane-treated group, from 15 mice 13 survived: one mouse died in the 4th and the other in the 5th month of the experiment. In the urethane–NaVP male group, 11 mice (three mice died in the 1st–3rd months and one in 5th month) survived. In the female urethane-treated group, five mice did not survive: three mice died in the 1st–3rd months, one in the 4th and one in the 5th month of the experiment. In the urethane–NaVP-treated group, two mice perished (one in the 1st and the other in the 5th month). This implies that young animals are sensitive to urethane and urethane–NaVP toxicity. Autopsies of mice that perished during the 5th month revealed that in urethane- and urethane–NaVP-treated groups benign lung tumors had developed in all cases, but data on these animals were not taken for statistical assessment.
Castrated male mice. Among castrated mice, all mice survived in the following groups: in the castrated control as well as in NaVP-treated and in urethane-treated groups. In the male urethane–NaVP-treated group, nine mice survived up to six months of the experiment (two mice died in the 2nd–3rd and four in the 3rd–4th months). The prevalence of perished mice in the urethane–NaVP-treated group is indicative of the toxicity of this combination for castrated mice.
Gender-related differences in the number of lung tumors per mouse in non-castrated mice groups
No mice with lung tumors were found in control animals of both genders and in NaVP-treated female groups. Four small (< 1 mm) tumors were found in the lungs of two mice in the NaVP-treated male group (one in one mouse and three in the other).
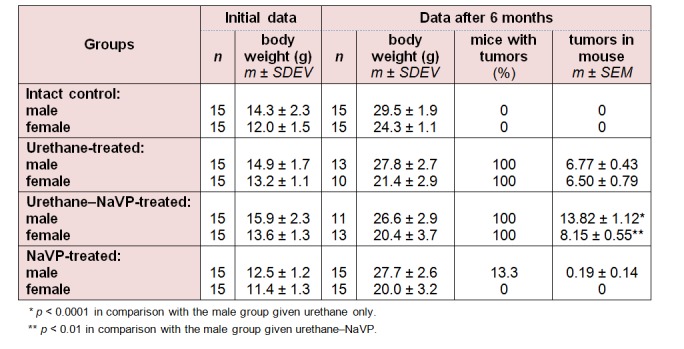
All urethane-treated mice of both genders developed tumors. No significant difference in the number of tumors (lung tumors per mouse) was detected when comparing urethane-treated male and female mice groups (Table 1(Tab. 1)).
Table 1. Incidence of lung tumors in BALB/c mice of the study groups.
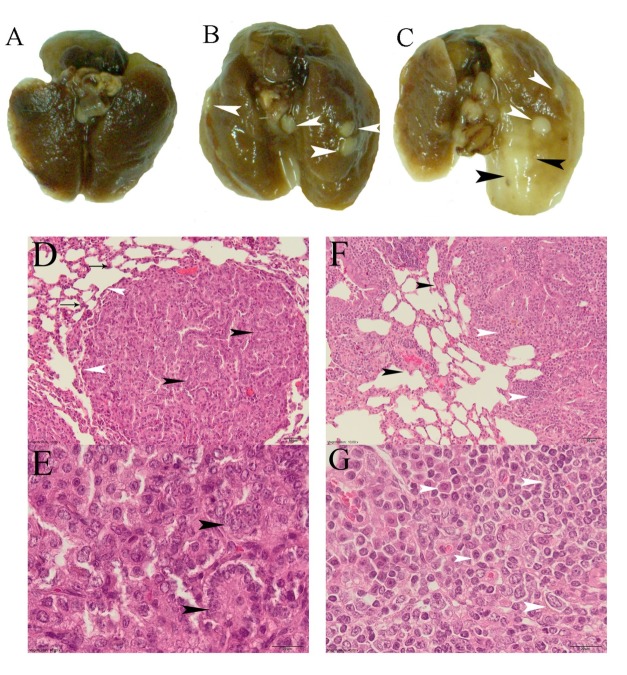
Urethane-caused adenomas may be found in any lobe of a lung and are often situated just beneath the pleura, and are recognized by their nodular, pearly, grey-white appearance contrasting with the more pink color of the normal lung parenchyma (Figure 1(Fig. 1)).
Figure 1. Lung specimens. A – control; B – urethane-induced lung multiple benign adenomas; C – lungs with advanced tumor (black arrowheads) – adenomas after urethane–NaVP treatment (white arrowheads); D – histological slide of lungs with benign adenoma. White arrowheads show well established borders of the tumor, black arrowheads – tumor cells with rounded regular nuclei, and arrows show alveolar/bronchial hyperplasia (bar 50 µm); E – adenoma, higher magnification. Tumor cells have regular oval nuclei, in some places cells have papillary arrangement (bar 20 µm); F – pulmonary adenocarcinoma. Black arrowheads show irregular borders of the tumor and invasive growth, white arrowheads – pleomorphic tumor cells (bar 50 µm); G – malignant tumor, higher magnification. Cells have pleomorphic nuclei, an increased number of nucleoli, some cells have very dark nuclei (bar 20 µm).
When comparing the number of tumors per mouse in male mice treated with urethane and those treated with urethane plus NaVP, lung tumors were found statistically significantly more often in males treated with urethane in combination with NaVP (6.77 ± 0.43 vs 13.82 ± 1.12, p < 0.0001; Table 1(Tab. 1)). The number of tumors per male mouse treated with urethane (n = 13) according to tumor diameter was per subgroup as follows: 3.85 ± 0.32 in the subgroup with the tumor diameter = 1.0 mm, 2.31 ± 0.45 in 1.0 to 2.0 mm and 0.23 ± 0.17 in the > 2.0 mm subgroup. In male urethane–NaVP-treated mice (n = 11), the number of tumors per mouse was as follows: with the diameter = 1.0 mm 8.09 ± 0.94, with the diameter 1.0 to 2.0 mm 5.45 ± 0.72, and with > 2.0 mm 0.27 ± 0.14. NaVP in mice treated with urethane caused an increase in the number of tumors per mouse in subgroups with the tumor diameter = 1.0 mm (p < 0.001) and 1.0 to 2.0 mm (p < 0.001). This fact indicates that NaVP causes the formation of new tumors and increases the progression of tumors in male BALB/c mice (Figure 2(Fig. 2)).
Figure 2. Incidence of benign lung tumors in BALB/c mice of both genders in urethane- and in ure-thane–NaVP-treated groups according to data on subgroups by tumor size (diameter = 1.0 mm, 1.0 to 2.0 mm and = 2.0 mm).
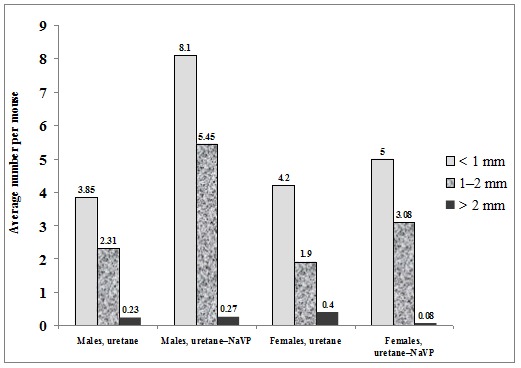
No statistically significant difference was found when comparing the number of tumors per mouse in females treated with urethane and those treated with the urethane and NaVP combination (6.50 ± 0.79 vs 8.15 ± 0.55, p = 0.105; Table 1(Tab. 1)). The number of tumors per female mouse treated with urethane (n = 10) according to tumor diameter was as follows: 4.20 ± 0.59 with tumor diameter = 1 mm, 1.90 ± 0.43 with the diameter 1.0 to 2.0 mm, and 0.4 ± 0.31 with the diameter > 2.0 mm. In the female urethane–NaVP-treated group (n = 13), the number of tumors per mouse was as follows: 5.0 ± 0.46 with the diameter = 1 mm, 3.08 ± 0.51 with the diameter 1.0 to 2.0 mm, and 0.08 ± 0.08 with the diameter > 2.0 mm (Figure 2(Fig. 2)).
No significant difference in the number of tumors per mouse was revealed while comparing the female urethane- and urethane–NaVP-treated groups, both in general and in separate subgroups.
A comparison of tumor numbers in urethane–NaVP-treated males and analogous data of the female group has shown a significant difference in the following subgroups: the number of tumors was higher in males with the tumor diameter = 1 mm (8.09 ± 0.94 vs 5.0 ± 0.46, p < 0.01), in the subgroup with the diameter 1.0 to 2.0 mm (5.45 ± 0.72 vs 3.08 ± 0.51, p < 0.01), and in the subgroup with the diameter > 2.0 mm (0.27 ± 0.14 vs 0.08 ± 0.08, p < 0.01). These data show that the NaVP effect on lung tumorigenesis is gender-related.
The number of tumors per mouse in castrated male groups
Three spontaneous tumors were found in the lungs of two control mice (two tumors in one and one more in another mouse). Three mice with lung tumors were found in the NaVP-treated castrated male group. All urethane-treated and urethane–NaVP-tretated mice developed tumors. No statistically significant difference was found when comparing the number of tumors per mouse in castrated male mice treated with urethane and in those treated with the urethane and NaVP combination. The number of tumors per castrated animals treated with urethane in subgroups according to tumor diameter showed no statistically significant difference as compared with data on the urethane–NaVP-treated group (Table 2(Tab. 2)).
Table 2. Incidence of lung tumors in castrated BALB/c male mice of the study groups.
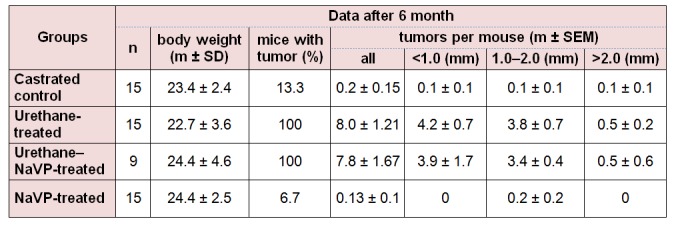
No significant difference in the number of tumors per mouse was detected when comparing intact male control and castrated male control groups as well as non-castrated and castrated urethane-treated male groups (Tables 1(Tab. 1) and 2(Tab. 2); p > 0.05). When comparing the number of tumors per mouse in castrated male mice urethane–NaVP-treated and non-castrated urethane–NaVP-treated groups, lung tumors were found statistically significantly more often in non-castrated males (7.8 ± 1.67 vs 13.82 ± 1.12, p < 0.01; Tables 1(Tab. 1) and 2(Tab. 2)).
Cases of malignant tumors
A randomly selected histological examination performed in each case of observable lung pathology when not identified or malignant tumors were suspected revealed several cases of lung tumor adenocarcinoma in non-castrated urethane- and urethane–NaVP-treated mice groups of both genders. Cases of adenocarcinoma (Figure 1(Fig. 1)) were identified by a histological examination of lungs and found to occur together with benign tumors.
In castrated male mice, a few malignant tumors were found in NaVP-treated, urethane-treated, and in urethane–NaVP-treated mice. Adenocarcinoma was revealed in cases when the tumor diameter was more than 1.5 mm (results of a detailed histological examination of adenocarcinoma cases will be published separately upon analysing all tumors in all mice groups).
Discussion
Urethane is a chemical carcinogen present in tobacco leaves, tobacco smoke (Schmetiz et al., 1978[44]), formed by the reaction of urea with alcohol (Wang et al., 2007[50]). Urethane specifically initiates the development of lung tumors from airway epithelial cells in mice. The tumorigenic effect of urethane directly depends on its dose (Malkinson, 2001[30]). BALB/c mice are considered susceptible to the development of lung tumor by urethane, and lung tumors can be modified by the influence of different modulatory agents (Malkinson and Beer, 1983[31]; Uleckiene et al., 2005[49]).
In our study model, all urethane-treated mice of both genders developed lung tumors. No gender-related difference in the number of mice with lung tumors as well as in tumors per mouse was detected between urethane-treated male and female mice. The study data show that the number of pulmonary tumors per mouse in the urethane-treated males and in males treated with urethane plus NaVP, lung tumors were observed statistically significantly more often in the non-castrated male group treated with urethane in combination with NaVP. No significant difference was found between analogous female mice groups. No lung tumors were found in untreated intact control groups of both genders. Of all male mice in the NaVP-treated group, 13.3 % developed benign lung tumors. No mice with lung tumors were found in the NaVP-treated female group.
The study data show that NaVP in the urethane-treated BALB/c mice could act synergistically with urethane as an oncogenic factor in male but not in female mice. Sex differences in mouse models of lung cancers were reported (Festing et al., 1994[15]). The importance for lung oncogenesis in both spontaneous and carcinogen-induced pulmonary cancers and spontaneous pulmonary adenomas in mice is determined by multiple genetic loci that differ between female and male mice (Berndt et al., 2011[5]). The study data show that NaVP in the urethane-treated castrated BALB/c male mice did not act synergistically with urethane as an oncogenic factor in male, and this fact indicates that testosterone plays an important role in the tumorogenic process in this model. There are no studies on NaVP influence on urethane-related lung tumorigenesis in mice.
Chronic NaVP toxicity studies showed that orally administered NaVP to Sprague Dawley rats and ICR (HA/ICR) mice at doses of 80 and 170 mg/kg/day for two years developed an increased incidence of subcutaneous fibrosarcoma in male rats at a higher NaVP dosage level, and a dose-related trend for an increased incidence of benign pulmonary adenomas was observed in male mice (McEvoy, 1991[33]).
NaVP increases the GABA turnover and potentiates GABAergic functions, reducing the activity of the GABA degrading enzymes (Godin et al., 1969[19]; Loscher, 2002[29]). NaVP may enhance neuronal responsiveness to endogenous GABA. The effect of NaVP on cellular mechanisms may be sex-specific with the different response on basal GABA levels in males and females (Dodge et al., 2000[12]). The specificity of NaVP for GABA suggests that this interaction may be an important mechanism through which NaVP exerts its pharmacological effects. The GABA A receptor is rapidly activated by NaVP in brain cells (Armijo et al., 2000[3]). The GABAergic neuronal activity was about two-fold greater in male than female rats (Grattan and Selmanoff, 1997[22]). A comparison between diestrous female rats and chronically castrated animals indicated that endogenous GABA release in ovariecomized rats was only 60–70 % of that in diestrous animals, while the basal GABA levels are also significantly reduced in estrogen-treated ovariectomized rats (Ondo et al., 1982[40]). In male rats, castration decreases the activity of GABAergic neurons, suggesting that GABAergic neurons are tonically stimulated by testosterone. Testosterone replacement prevents the castration-induced decrease in GABA (Grattan and Selmanoff, 1993[21]).
GABA A receptors are also found outside synapses and on non-neural cells, cancerous cells included. GABA A receptor Ɵ subunit (GABRQ) forms a functional ionotropic chloride channel (Fritschy et al., 1995[16]; Bureu et al., 1997[10]). Such extrasynaptic receptors have a high affinity for GABA and open the chloride channels at low ambient GABA concentrations (1 mM). This leads to changes in the membrane potential (Belelli et al., 2009[4]). Opening the GABA A receptor chloride channels leads to a chloride efflux and depolarisation of the membrane (Zhao et al., 2011[52]). The plasma cell membrane potential influences cell proliferation (Morokuma et al., 2008[36]; Sundelacruz et al., 2009[47]). Neurotransmitters that change the membrane potential contribute to the control of cell proliferation (Martins and Pearson, 2008[32]). GABA has been shown to regulate the proliferation of several cell types, including stem cells (Andang et al., 2008[2]), cortical progenitor cells (Nguyen et al., 2003[38]), immune cells (Bjurstom et al., 2008[6]; Mendu et al., 2011[35]), mouse chondrogenic ATDC5 cells (Tamayama et al., 2005[48]), modulate the proliferation, migration and differentiation of neuronal cells (Neelands et al., 1999[37]; Haydar et al., 2000[24]; Meier et al., 2003[34]).
The gene expression investigation of GABA receptors in tissues of non-small cell lung cancers and non-cancerous tissues showed that the gene expression of GABA receptor phenotypes was correlated with tumorigenesis and clinical prognosis (Zhang et al., 2013[51]). The GABRQ is overexpressed in hepatocellular carcinoma cells, but not in a normal cell line, and GABA in the liver promotes the proliferation of cancer cells through GABRQ (Li et al., 2012[28]). GABA could inhibit colon cancer migration associated with the norepinephrine-induced pathway (Joseph et al., 2002[26]). The effects of GABA and GABA receptors show that GABA-associated pathways could act positively or negatively in regulating cancer cell behavior. The injection of a combination of oestrogen and progesterone produced a greater reduction in GABA A receptor binding in mouse forebrain membranes, indicating that gonadal steroids contribute to the modulation of GABA A receptor binding in the cells of male and female mice (Akinci and Johnston, 1997[1]).
There could be other factors that may contribute to NaVP gender-related induction of cell proliferation and tumorigenesis responses, for example, the link of GABA mechanisms with the ion cotransporter activity expression regulating the intracellular chloride concentration. The depolarizing effects of GABA are promoted by the relative accumulation of chloride inside the cells, leading to chloride efflux once GABA A receptor channels open. Intracellular chloride regulation and the control of GABA A receptor signaling are effected through K-Cl co-transporter (Delpire and Mount, 2002[11]). To counteract their effects, the K-Cl cotransporter exports these ions thus decreasing intracellular chloride (Galanopoulou, 2008[18]). In male rat neurons, the K-Cl cotransporter is less expressed than in female (Galanopoulou, 2008[17]). The possible mechanisms of K-Cl cotransporter activity was demonstrated in the modulation of tumor development and progression (Shen et al., 2004[45]).
NaVP has been shown to enhance the urinary excretion of chloride in Wistar rats of both genders, but the 24-hour chloriduretic response in male was significantly higher than in female rats (Grikiniene et al., 2005[23]). GABA A receptor subunits are expressed in Wistar rat kidney proximal convoluted and straight tubules and in other tissues (Sarang et al., 2001[43]; Li et al., 2012[28]; Zhang et al., 2013[51]). Chloride is recognized to have an important role in tumor genesis: the intracellular chloride concentration would be one of the critical messengers in cell growth/proliferation and differentiation processes (Shiozaki et al., 2006[46]; Hiraoka et al., 2010[25]; Ohsawa et al., 2010[39]).
NaVP is recognized as a novel class of histone deacetylases (HDAC) inhibitors that induce the differentiation of transformed cells and show antitumor properties in clinical and preclinical studies, both in vitro and in vivo, by modulating multiple pathways including cell cycle arrest, cell differentiation, apoptosis (Göttlicher et al., 2001[20]; Kawagoe et al., 2002[27]). Researchers have summarized preclinical and clinical data on positive NaVP effects in different types of tumors (Blaheta and Cinatl, 2002[7]; Duenas-Gonzalez et al., 2008[13]). Recent data indicate that HDAC play a dual role in tumorigenesis: oncosuppressive in the early stages, and oncogenic in established tumor cells in mice models (Santoro et al., 2013[42]). Our study results show the opposite – negative – effect of NaVP on the progression of urethane-induced lung tumors in the mice model. We have found no other gender-related mice studies on NaVP effects on tumorigenesis in the literature. Furthermore, there are data to show that NaVP increases the level of histone H3 acetylation in the brain with a gender-related effect: multiple NaVP selec-tivity improved the early olfactory learning experience in male but not in female 8-day-old mice pups (Burenkova et al., 2013[9]).
It is possible to hypothesize that NaVP, by changing GABA A receptor activity, the intracellular chloride level, and possibly modulating HDAC, plays an important gender-related modulative role in urethane-induced lung tumorigenesis in mice.
In our study, there were cases of malignant lung tumor in urethane- and urethane–NaVP-treated mice groups. The effect of urethane is known to be related with benign adenoma or adenocarcinoma lung tumorigenesis (Malkinson, 2001[30]; Regala et al., 2011[41]). The data of our study show that NaVP may influence adenocarcinoma frequency in urethane-caused lung tumor genesis. This effect was observed in tumors with the diameter more than 1.5 mm (a detailed analysis of adenocarcinoma cases with the description of histological examination results will be published separately).
Data of the present study show that NaVP, used together with urethane, synergistically enhances urethane tumorigenicity in lungs of only non-castrated male BALB/c mice, but not in castrated ones. To check our findings on NaVP induction and promotion of gender-related lung tumorigenesis in the urethane mice model, the further studies on the importance of the NaVP gender-related action on pharmacological and pathophysiological mechanisms are necessary.
Acknowledgements
The work was supported by a grant of the Science Council of Lithuania (No. MIP-12224).
Conflict of interest
The authors have no conflict of interests.
References
- 1.Akinci MK, Johnston GA. Sex differences in the effects of gonadectomy and acute swim stress on GABAA receptor binding in mouse forebrain membranes. Neurochem Int. 1997;31:1–10. doi: 10.1016/s0197-0186(96)00143-x. [DOI] [PubMed] [Google Scholar]
- 2.Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, et al. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451:460–464. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- 3.Armijo JA, de las Cuevas I, Adin J. Ion channels and epilepsy. Rev Neurol. 2000;30(Suppl. 1):S25–S41. [PubMed] [Google Scholar]
- 4.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berndt A, Cario CL, Silva KA, Kennedy VE, Harrison DE, Paigen B, et al. Identification of fat4 and tsc22d1 as novel candidate genes for spontaneous pulmonary adenomas. Cancer Res. 2011;71:5779–5791. doi: 10.1158/0008-5472.CAN-11-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjurstom H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, et al. GABA, a natural immuno-modulator of T lymphocytes. J Neuroimmunol. 2008;205:44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Blaheta RA, Cinatl J., Jr Anti-tumour mechanisms of valproate: a novel role for an old drug. Med Res Rev. 2002;22:492–511. doi: 10.1002/med.10017. [DOI] [PubMed] [Google Scholar]
- 8.Bowden CL. Anticonvulsants in bipolar disorders: current research and practice and future directions. Bipolar Disord. 2009;11:20–33. doi: 10.1111/j.1399-5618.2009.00708.x. [DOI] [PubMed] [Google Scholar]
- 9.Burenkova OV, Aleksandrova EA, Zaraĭskaia IIu. Gender-dependent effects of histone deacetylase inhibitor sodium valproate on early olfactory learning in 129 mice. Ross Fiziol Zh Im I M Sechenova. 2013;99:212–220. [PubMed] [Google Scholar]
- 10.Bureu M, Laschet J, Minier F, Chauvel P. Intervention of GABAergic neurotransmission in partial epilepsies. Rev Neurol. 1997;153(Suppl 1):S46–S54. [PubMed] [Google Scholar]
- 11.Delpire E, Mount DB. Human and murine phenol¬types associated with defects incation-chloride co-transport. Annu Rev Physiol. 2002;64:803–843. doi: 10.1146/annurev.physiol.64.081501.155847. [DOI] [PubMed] [Google Scholar]
- 12.Dodge JC, Illig AM, Snyder PJ, Badura LL. GABA levels within the medial preoptic area: effects of chronic administration of sodium valproic acid. Psychoneuroendocrinology. 2000;25:519–534. doi: 10.1016/s0306-4530(00)00007-x. [DOI] [PubMed] [Google Scholar]
- 13.Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008;34:206–222. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Evers S. Treatment of migraine with prophylactic drugs. Expert Opin Pharmacother. 2008;9:2565–2573. doi: 10.1517/14656566.9.15.2565. [DOI] [PubMed] [Google Scholar]
- 15.Festing MFW, Yang A, Malkinson AM. At least four genes and sex are associated with susceptibility to urethan-induced pulmonary adenomas in mice. Genet Res. 1994;64:99–106. doi: 10.1017/s0016672300032705. [DOI] [PubMed] [Google Scholar]
- 16.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 17.Galanopoulou AS. Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci. 2008;27:1557–1567. doi: 10.1523/JNEUROSCI.5180-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galanopoulou AS. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 2008;80:99–113. doi: 10.1016/j.eplepsyres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godin Y, Heiner L, Mark J, Mandel P. Effects of di-n-propylacetate, an anticonvulsant compound, on GABA metabolism. J Neurochem. 1969;16:869–873. doi: 10.1111/j.1471-4159.1969.tb08975.x. [DOI] [PubMed] [Google Scholar]
- 20.Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differrentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grattan DR, Selmanoff M. Regional variation in gamma aminobutyric acid turnover: Effect of castration of gamma aminobutyric acid turnover in microdissected brain regions of the male rat. J Neurochem. 1993;60:2254–2264. doi: 10.1111/j.1471-4159.1993.tb03512.x. [DOI] [PubMed] [Google Scholar]
- 22.Grattan DR, Selmanoff M. Sex differences in the activity of γ-aminobutyric acidergic neurons in the rat hypothalamus. Brain Res. 1997;775:244–249. doi: 10.1016/s0006-8993(97)01069-x. [DOI] [PubMed] [Google Scholar]
- 23.Grikiniene J, Stakisaitis D, Tschaika M. Influence of sodium valproate on sodium and chloride urinary excretion in rats, gender differences. Pharmacology. 2005;75:111–115. doi: 10.1159/000087505. [DOI] [PubMed] [Google Scholar]
- 24.Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J Neurosci. 2000;20:5764–5774. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiraoka K, Miyazaki H, Niisato N, Iwasaki Y, Kawauchi A, Miki T, et al. Chloride ion modulates cell proliferation of human androgen-independent prostatic cancer cell. Cell Physiol Biochem. 2010;25:379–388. doi: 10.1159/000303042. [DOI] [PubMed] [Google Scholar]
- 26.Joseph J, Niggemann B, Zaenker KS, Entschladen F. The neurotransmitter gamma-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 2002;62:6467–6469. [PubMed] [Google Scholar]
- 27.Kawagoe R, Kawagoe H, Sano K. Valproic acid induces apoptosis in human leukemia cells by stimulating both caspase-dependent and -independent apoptotic signaling pathways. Leuk Res. 2002;26:495–502. doi: 10.1016/s0145-2126(01)00151-5. [DOI] [PubMed] [Google Scholar]
- 28.Li YH, Liu Y, Li YD, Liu YH, Li F, Ju Q, et al. GABA stimulates human hepatocellular carcinoma growth through overexpressed GABAA receptor theta subunit. World J Gastroenterol. 2012;18:2704–2711. doi: 10.3748/wjg.v18.i21.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16:669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 30.Malkinson AM. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer. 2001;32:265–279. doi: 10.1016/s0169-5002(00)00232-4. [DOI] [PubMed] [Google Scholar]
- 31.Malkinson AM, Beer DS. Major effect on susceptibility to urethan-induced pulmonary adenoma by a single gene in BALB/c mice. J Natl Cancer Inst. 1983;70:931–936. [PubMed] [Google Scholar]
- 32.Martins RA, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 33.McEvoy IPCS, editor. International Programme on Chemical Safety. Valproic acid. 1991. Relevant animal data; p. chap 7.2.2. Available from: www.inchem.org/documents/pims/pharm/pim551.htm#SectionTitle:7.2 Toxicity. [Google Scholar]
- 34.Meier J, Akyeli J, Kirischuk S, Grantyn R. GABA(A) receptor activity and PKC control inhibitory synapto-genesis in CNS tissue slices. Mol Cell Neurosci. 2003;23:600–613. doi: 10.1016/s1044-7431(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 35.Mendu SK, Akesson L, Jin Z, Edlund A, Cilio C, Lernmark A, et al. Increased GABA(A) channel subunits expression in CD8(+) but not in CD4(+) T cells in BB rats developing diabetes compared to their congenic littermates. Mol Immunol. 2011;48:399–407. doi: 10.1016/j.molimm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neelands TR, Zhang J, Macdonald RL. GABA(A) receptors expressed in undifferentiated human teratocarcinoma NT2 cells differ from those expressed by differentiated NT2-N cells. J Neurosci. 1999;19:7057–7065. doi: 10.1523/JNEUROSCI.19-16-07057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen L, Malgrange B, Breuskin I, Bettendorff L, Moonen G, Belachew S, et al. Autocrine/paracrine activation of the GABA(A) receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J Neurosci. 2003;23:3278–3294. doi: 10.1523/JNEUROSCI.23-08-03278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohsawa R, Miyazaki H, Niisato N, Shiozaki A, Iwasaki Y, Otsuji E, et al. Intracellular chloride regulates cell proliferation through the activation of stress-activated protein kinases in MKN28 human gastric cancer cells. J Cell Physiol. 2010;223:764–770. doi: 10.1002/jcp.22088. [DOI] [PubMed] [Google Scholar]
- 40.Ondo J, Mansky T, Wuttke W. In vivo GABA release from the medial preoptic area of diestrous and ovariectomized rats. Exp Brain Res. 1982;46:69–72. doi: 10.1007/BF00238099. [DOI] [PubMed] [Google Scholar]
- 41.Regala RP, Justilien V, Walsh MP, Weems C, Khoor A, Murray NR, et al. Matrix metalloproteinase-10 promotes Kras-mediated bronchio-alveolar stem cell expansion and lung cancer formation. PLoS One. 2011;10:e26439. doi: 10.1371/journal.pone.0026439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santoro F, Botrugno OA, Dal Zuffo R, Pallavicini I, Matthews GM, Cluse L, et al. A dual role for Hdac1: oncosuppressor in tumorigenesis, oncogene in tumor maintenance. Blood. 2013;121:3459–3468. doi: 10.1182/blood-2012-10-461988. [DOI] [PubMed] [Google Scholar]
- 43.Sarang SS, Plotkin MD, Gullans SR, Cummings BS, Grant DF, Schnellmann RG. Identification of the gamma-aminobutyric acid receptor beta (2) and beta (3) subunits in rat, rabbit, and human kidneys. J Am Soc Nephrol. 2001;12:1107–1113. doi: 10.1681/ASN.V1261107. [DOI] [PubMed] [Google Scholar]
- 44.Schmetiz I, Chiong KG, Hoffmann DJ. Formation and of ethyl carbamate in tobacco and tobacco smoke. Anal Toxicol. 1978;2:265–268. [Google Scholar]
- 45.Shen MR, Lin AC, Hsu YM, Chang TJ, Tang MJ, Alper SL, et al. Insulin-like growth factor 1 stimulates KCl cotransport, which is necessary for invasion and proliferation of cervical cancer and ovarian cancer cells. J Biol Chem. 2004;279:40017–40025. doi: 10.1074/jbc.M406706200. [DOI] [PubMed] [Google Scholar]
- 46.Shiozaki A, Miyazaki H, Niisato N, Nakahari T, Iwasaki Y, Itoi H, et al. Furosemide, a blocker of Na+/K+/2Cl- cotransporter, diminishes proliferation of poorly differentiated human gastric cancer cells by affecting G0/G1 state. J Physiol Sci. 2006;56:401–406. doi: 10.2170/physiolsci.RP010806. [DOI] [PubMed] [Google Scholar]
- 47.Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev. 2009;5:231–246. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamayama T, Maemura K, Kanbara K, Hayasaki H, Yabumoto Y, Yuasa M, et al. Expression of GABA(A) and GABA(B) receptors in rat growth plate chondrocytes: activation of the GABA receptors promotes proliferation of mouse chondrogenic ATDC5 cells. Mol Cell Biochem. 2005;273:117–126. doi: 10.1007/s11010-005-8159-6. [DOI] [PubMed] [Google Scholar]
- 49.Uleckiene S, Didziapetriene J, Griciute L, Sukeliene D. Evaluation of original selenium-containing com-pounds for potentially chemopreventive properties in experimental lung carcinogenesis. Trace Elem Electrol. 2005;22:33–36. [Google Scholar]
- 50.Wang D, Yang B, Zhai X, Zhou L. Synthesis of diethyl carbonate by catalytic alcoholysis of urea. Fuel Processing Technol. 2007;88:807–812. [Google Scholar]
- 51.Zhang X, Zhang R, Zheng Y, Shen J, Xiao D, Li J, et al. Expression of gamma-aminobutyric acid receptors on neoplastic growth and prediction of prognosis in non-small cell lung cancer. J Transl Med. 2013;11:102. doi: 10.1186/1479-5876-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao YL, Xiang Q, Shi QY, Li SY, Tan L, Wang JT, et al. GABAergic excitotoxicity injury of the immature hippocampal pyramidal neurons’ exposure to isoflurane. Anesth Analg. 2011;113:1152–1160. doi: 10.1213/ANE.0b013e318230b3fd. [DOI] [PubMed] [Google Scholar]