Abstract
Accurate diagnosis of malignant pleura mesothelioma (MPM) is challenging. Differential diagnosis of MPM versus lung adenocarcinoma (AD) is particularly difficult, yet clinically important since the two neoplasias call for different treatment approaches. Circulating miRNA-profiling to identify miRNAs that can be used to distinguish MPM from AD has not been reported.
We conducted a wide screening study of miRNA profiles in serum pools of MPM patients (N = 11), AD patients (N = 36), and healthy subjects (N = 45) to identify non-invasive biomarkers for differential diagnosis of MPM and AD, using deep sequencing.
Sequencing detected up to 300 known miRNAs and up to 25 novel miRNAs species in the serum samples. Among known miRNAs, 7 were upregulated in MPM and 12 were upregulated in AD compared to healthy controls. Of these, eight were distinctive for AD and three were unique for MPM. Direct comparison of the miRNA profiles for MPM and AD revealed differences in miRNA levels that could be useful for differential diagnosis. No differentially expressed novel miRNAs were found. Further bioinformatics analysis indicated that three upregulated miRNAs in MPM are associated with the p38 pathway.
There are unique alterations in serum miRNAs in MPM and AD compared to healthy controls, as well as differences between MPM and AD profiles. Differing miRNA levels between MPM and AD may be useful for differential diagnosis. A potential association to p38 pathway of three upregulated miRNAs in MPM was revealed.
Keywords: circulating miRNAs, malignant pleural mesothelioma, lung adenocarcinoma, differential diagnosis, non-invasive biomarkers
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive tumor arising from pleural mesothelial cells that is difficult to diagnose and unresponsive to conventional therapy (Bagheri et al., 2011[2]). The gold standard diagnosis of MPM requires tissue sample analysis of tissue markers and relies on a panel of immunohistochemical stains since there is no single tissue marker with 100 % diagnostic accuracy (Husain et al., 2009[11]). This diagnostic process is not only difficult and time-consuming, but also requires a tissue sample that must be obtained through an invasive procedure (Ray and Kindler, 2009[22]). Due to the difficulty in obtaining an accurate pathological diagnosis, final diagnoses include a combined analysis of clinical data, x-ray results, and pathology findings (Husain et al., 2009[11]). In addition, the most frequent subtype of MPM (epithelioid) can be difficult to distinguish from peripheral lung adenocarcinoma (AD). Distinguishing MPM from AD is clinically important since the two tumor types call for different treatment approaches.
Recently, microRNAs (miRNAs) have been studied as potential diagnostic markers for MPM. MiRNAs are small non-coding RNAs that target mRNAs to regulate post-transcriptional gene expression. They regulate many key cellular processes, including cell cycle progression, differentiation, proliferation, apoptosis, and hematopoiesis (Carthew and Sontheimer, 2009[4]). Notably, alterations in miRNA expression levels have been detected in solid tumors, distinguishing healthy tissue from tumor tissue (Avila-Moreno et al., 2011[1]). Nevertheless, to examine a miRNA profile for diagnostic purposes, tumor tissue has to be obtained through an invasive procedure. An alternative is to study miRNAs in blood circulation. Circulating, as opposed to local tissue, miRNAs have the potential to be used as non-invasive biomarkers. Moreover, evidence indicates that miRNAs are secreted in a selective way as a component of cell-cell communication mechanisms and it has been hypothesized that cancer cells secrete miRNAs to affect their cellular environment (Pigati et al., 2010[21]; Wang et al., 2010[24]).
In the present study, we conducted a broad screening study of miRNA profiles in pooled serum samples from MPM patients (11), AD patients, and healthy control subjects using deep sequencing. Our aim was to identify non-invasive biomarkers principally for the differential diagnosis of MPM and AD. This study included a gene and pathway enrichment analysis by bioinformatics to determine the potential relevance of the differentially expressed miRNAs to carcinogenesis mechanisms. Serum was chosen over plasma to avoid a bias related to miRNAs in platelets (McDonald et al., 2011[19]); and deep sequencing was used because it allows identification of all detectable known and novel miRNAs in blood circulation.
Material and Methods
Participants and samples
Patients
Patients with histological diagnosis of malignant pleural mesothelioma (MPM) subtype epithelioid, and No-Small Cell Lung Cancer (NSCLC) subtype adenocarcinoma (AD) with primary lung-origin, were recruited from the Pneumology-Oncology Service at the Instituto Nacional de Enfermedades Respiratorias (INER), Mexico City. The patients included in this study did not receive any adjuvant chemotherapy, surgery or radiation therapy previous to the sample collection. All patients were > 35 years old. The clinical data, smoking status and ancestry were obtained from clinical records and by personal inquiring guided by a standardized questionary. Exclusion criteria included a diagnosis of asthma, COPD, associated cancer, no primary lung-origin adenocarcinoma, HIV infection, or upper/lower respiratory tract infection in the preceding 4 weeks.
MPM and AD diagnosis was confirmed by immunohistochemistry method by the Pathology Department at INER. MPM immunohistochemistry diagnosis uses al least four of the next markers: calretinin, TTF-1 (thyroid transcription factor-1), citokeratin 5/6, CEA (carcinoembryonic antigen), and BerEP4. Only samples with matching X-rays and clinical diagnosis of MPM were included in this study.
Healthy subjects
Healthy subjects were recruited from an anti-smoking and blood donation campaign in the Instituto Nacional de Enfermedades Respiratorias (INER), Mexico City. All the subjects included in this study were born in Mexico as well as both parents and grandparents. None of the participants had airway obstruction (by determination of FEV1 /FVC). All participants were > 35 years old. Exclusion criteria included a diagnosis of asthma, COPD, bronchiectasis, lung cancer, HIV infection, or upper/lower respiratory tract infection in the preceding 4 weeks.
Written informed consent was obtained from all participants according to Scientific and Ethical Committee at the Instituto Nacional de Enfermedades Respiratorias (INER).
Samples
For the Solexa sequencing assay, equal volumes of sera from 23 healthy male smokers (CTR-1), 22 healthy female smokers (CTR-2), 11 smokers and non-smokers MPM patients (MPM), 15 non-smokers AD patients (AD-1) and 21 smokers AD patients (AD-2), were pooled separately to complete a total volume of 4 ml per group sample for RNA extraction.
Sample processing and RNA extraction
Serum was obtained from peripheral blood samples (5-ml BD Vacutainer SST II Plus plastic serum Tube, REF 368159) by centrifugation at 4000 rpm at 4 °C for 20 min. The serum was collected and stored at -80 °C until analysis. Isolation of total RNA from serum pools was performed by a combination of Trizol LS (Invitrogen) and miRNeasy kit (Qiagen) according to the manufacturer’s instructions. RNA profiles and quantification were assessed on the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and the Small RNA chip.
Deep sequencing by Solexa (Illumina)
Small RNA library preparation and sequencing was performed at the Unidad de Secuenciación e Identificación de Polimorfismos at the Instituto Nacional de Medicina Genómica (INMEGEN), Mexico City. Briefly, total RNA (30 ng) was ligated to sequencing adaptors on both ends, and reverse-transcribed (TruSeq Small RNA Sample Prep Kit Set A, Illumina, Cat. RS-200-0012). The resulting cDNA library was PCR-amplified for 15 cycles, concentrated by ethanol precipitation and gel-purified on 6 % acrylamide gel. Fragments of 147 a 157 nt (small RNA+adaptors) were isolated from the acrylamide gel and the quality of the purified DNA was checked using the chip DNA High Sensitivity (Agilent Technologies, Cat. 5067-4626) and the Agilent 2100 Bioanalyzer. Purified DNA was used for cluster bridge generation (Bridge PCR con el kit TruSeq SR Cluster v5-CS-GS) by loading on to the Illumina cluster station (Illumina, Cat. GD-203-2001). The DNA clusters generated were sequenced using the Illumina’s Solexa Sequencer (Illumina, Cat. FC-104-5020). Sequencing reads were generated and base calls were rendered using Illumina pipeline Version 1.3.2.
Computational analysis procedure and pipeline
Computational analysis of sequencing reads (.fastq files) was performed by the Winter Genomics Company (Winter Web Internet and Network Technology for Enterprise Resources S.A. de C.V., Mexico City). Each sample went through two filtering processes.
The first process:
Filter by quality; reads with a median quality below 20 were removed.
Filter by Ns; reads with one or more N’s were removed.
Filter by polynucleotides; reads harboring more than 60 % of the same base were removed.
Trimming of the small RNA 3’ adapter utilized to prepare the sequencing library.
Filter reads with a 15 base pair length or less.
The second process:
minReadCount = numeric value: the minimum read count a read must have to be written to the output file. The numeric value chosen was 1 to increase the sensibility of the miRanalyzer output.
Quality values: to control the quality of the reads, the mean quality score must be above a given threshold. The threshold value was 0.
maxLen = value: Reads with a length bigger than 26 bp were filtered.
The identification of known and putative new miRNAs was performed by MiRanalyzer (Hackenberg et al. 2009[10], 2011[9]), which detects known miRNAs and predicts new putative miRNAs following the next criteria:
Filtering of the reads that aligned to RepBase (Jurka, 2000[12]).
Identification of all reads that came from other small non-coding RNAs by aligning the filtered reads against Rfam (Gardner et al., 2011[8]).
Detection of known miRNAs by mapping the remaining reads against miRBase (Kozomara and Griffiths-Jones, 2011[14]).
Prediction of new putative miRNAs based on a learning machine approach based on a ?random forest method.
Differential expression
The statistical significance of differences in miRNAs level expression was calculated using the miRanalyzer tool ’Differential expression analysis’ which performs a variance analysis using the Bioconductor package DESeq (Hackenberg et al., 2011[9]). For every comparison requested, differential expression was tested for unique known miRNAs, unique unobserved miRNAs and novel miRNAs. In order to determine differential expression of a miRNA species in the analysis a cutoff value of expression fold-change of log2 > 2 between the samples involved at each analysis was used and a p-value of < 0.01.
Gene set enrichment analysis
Gene and pathway enrichment analyses were performed using the miRSystem, an Integrated System for Characterizing Enriched Functions and Pathways of MicroRNA Targets (Lu et al., 2012) (http://miRSystem.cgm.ntu.edu.tw/). Using this system, queried miRNAs are first converted to the latest miRBase annotation, then several statistical methods are applied to calculate O/E ratios of gene targets, hypergeometric P-values, and empirical P-values due to permutation in order to identify significantly enriched signaling pathways. MiRSystem includes seven algorithms predicting miRNA targets: DIANA-microT, miRanda, mirBridge, PicTar, PITA, rna22, and TargetScan, and two experimentally validated databases, TarBase and miRecords of miRNA target genes were included. Five pathway databases, including Gene Ontology, KEGG, BioCarta, Pathway Interaction Database, and Reactome, were used to annotate the biological functions and canonical pathways of target genes.
Results
Characteristics of the study population
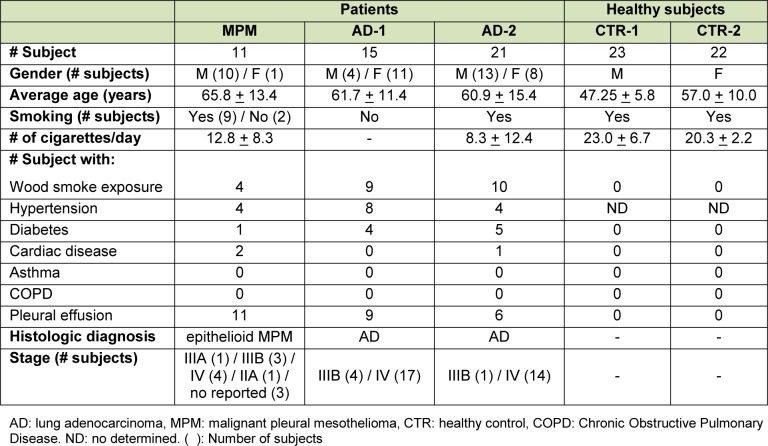
Table 1(Tab. 1) shows the distributions of demographic and clinical features in the study groups. All MPM patients presented with pleural effusion. The AD group was subdivided based on whether individuals were non-smokers or smokers into adenocarcinoma groups 1 (AD-1) and 2 (AD-2), respectively. None of the cancer patients had associated infectious respiratory diseases, associated neoplasias or received adjuvant chemotherapy, surgery or radiation therapy prior to sample collection. The control subjects were all smokers and were subdivided into male (CTR-1) and female (CTR-2) groups. None of the controls had hypertension, diabetes, or respiratory diseases, such as asthma and chronic obstructive pulmonary disease.
Table 1. Demographic and clinical features of mesothelioma patients, lung cancer patients and healthy subjects.
Identification of miRNAs in serum
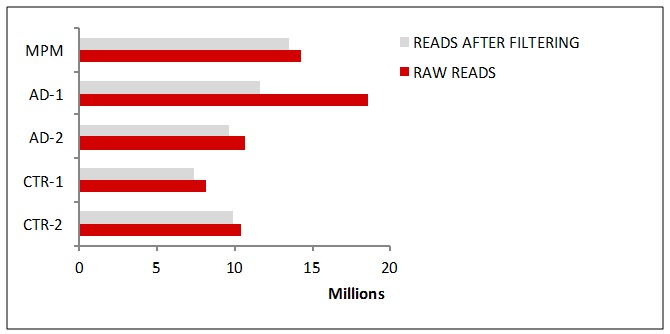
A preliminary wide screen of miRNA profiles in serum pools from 11 MPM subjects, 36 AD (AD-1 and AD-2) subjects, and 45 healthy controls (CTR-1 and CTR-2) by deep sequencing generated ~6 million raw reads for each serum pool sample (Figure 1(Fig. 1)) and detected ~300 unique known miRNAs and up to 25 novel miRNAs in all of the samples.
Figure 1. Numbers of miRNAs reads found in serum sample pools by deep sequencing.
Differential expression of serum miRNAs in lung cancer patients
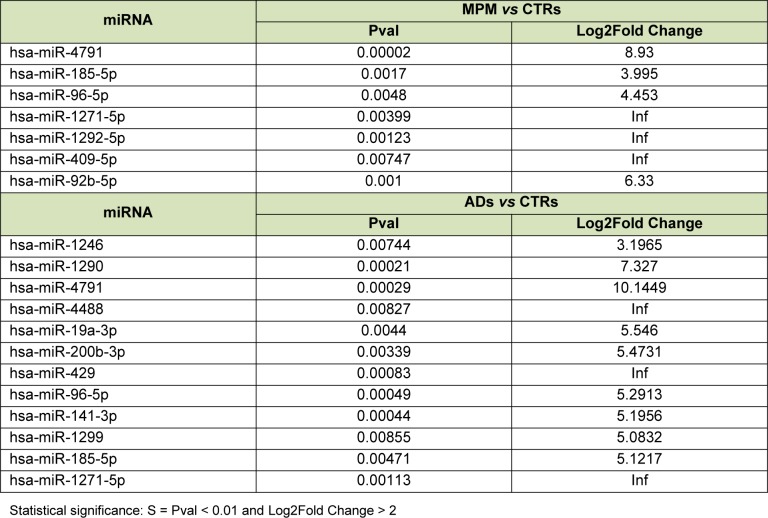
Among the known miRNAs detected, 7 were found to be upregulated in MPM serum samples and 12 were upregulated in AD serum samples, compared to controls (Table 2(Tab. 2)). There were no differentially expressed novel miRNAs in the AD and MPM groups compared to normal controls. Examination of the distribution of the deregulated miRNAs revealed that four of them were shared by both neoplasias (hsa-miR-4791, hsa-miR-185-5p, hsa-miR-96-5p, and hsa-miR-1271-5p), suggesting a common link between lung and pleural neoplasias (Figure 2(Fig. 2)). Three miRNAs were unique to the MPM patients (hsa-miR-1292-5p, has-miR-409-5p, and has-miR-92b-5p), and eight miRNAs were unique to the AD patients (hsa-miR-1246, hsa-miR-1290, hsa-miR-4488, hsa-miR-19a-3p, hsa-miR-200b-3p, hsa-miR-429, hsa-miR-141-3p, and hsa-miR-1299).
Table 2. Differentially expressed miRNAs in pooled serum samples from MPM patients and from AD patients compared to healthy controls.
Figure 2. Distributions of the deregulated miRNAs in MPM and AD samples.
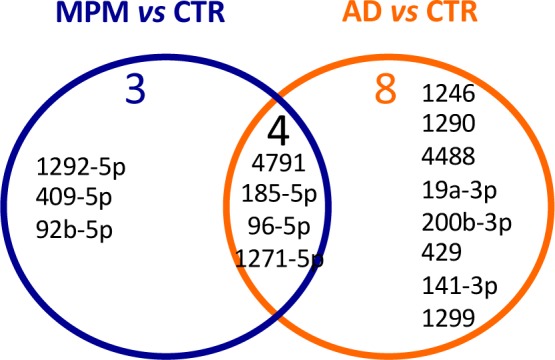
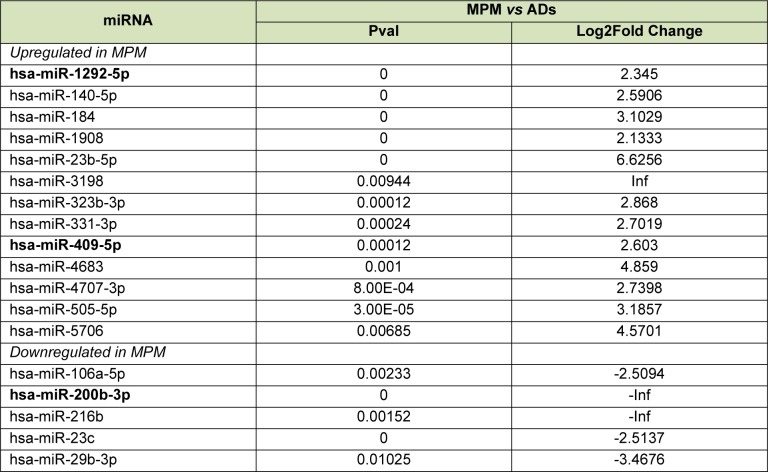
A direct comparison of miRNA levels between the two neoplasias (by miRanalyzer tool Differential expression analysis) showed 13 upregulated miRNAs (hsa-miR-1292-5p, hsa-140-5p, hsa-miR-184, hsa-miR-1908, hsa-miR-23b-5p, has-miR-3198, hsa-miR-323b-3p, hsa-miR-331-3p, hsa-miR-409-5p, hsa-miR-4683, hsa-miR-4707-3p, hsa-miR-505-5p, hsa-miR-5706), and 5 down-regulated miRNAs (hsa-miR-106a-5p, hsa-miR-200b-3p, hsa-miR-216b, hsa-miR-23c, hsa-miR-29b-3p) in MPM compared to AD (Table 3(Tab. 3)). Notably, hsa-miR-1292-5p and hsa-miR-409-5p were upregulated in MPM versus AD as well in MPM compared to healthy controls (Figure 2(Fig. 2)).
Table 3. Differentially expressed miRNAs in serum MPM patients compared to AD patients.
Biological relevance of differentially expressed miRNAs
A gene and pathway enrichment analysis conducted with MiRSystem identified 1,115 genes as predicted targets of the seven miRNAs upregulated in MPM compared to normal controls. As shown in Table 4(Tab. 4), an analysis of the biological pathways in which these
Table 4. Top three enriched pathways of miRNAs differentially expressed in MPM patients sera compared to normal controls.
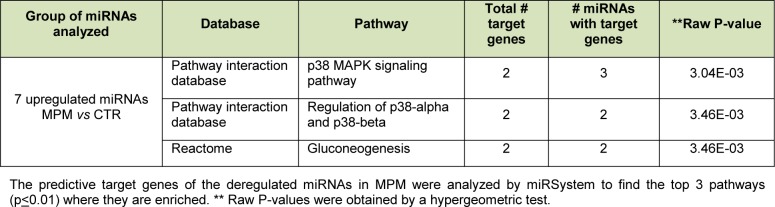
target genes participate revealed that the p38-signaling pathway, regulation of p38-alpha and P30-beta pathways, and gluconeogenesis were the top three (most significantly) enriched pathways (p < 0.01).
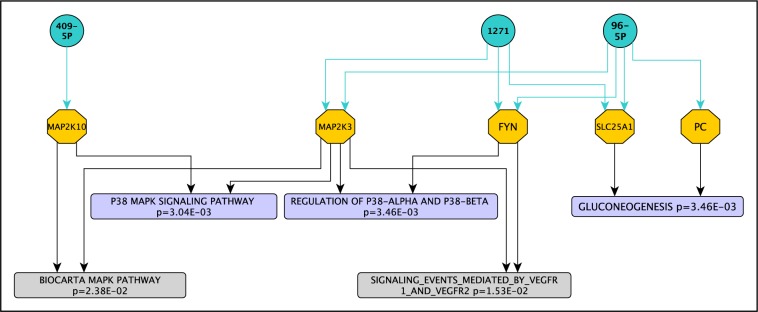
Further analysis of the p38 pathway showed that three of the seven upregulated miRNAs in MPM (hsa-miR-96-5p, hsa-miR-1271, and hsa-miR-409-5p) targeted genes in p38-related pathways, namely MAP2K3, FYN, and MAP2K10 (Figure 3(Fig. 3)). In addition, we found two more enriched pathways (p < 0.05) in the top 15 positions that were related to the three top pathways mentioned above: the signaling events mediated by VEGFR1 and VEGFR2 (raw p = 1.53E-02) and the Biocarta MAPK pathway (raw p = 2.38E-02) (Figure 3, grey font(Fig. 3)); and target genes were common to the previous three top enriched pathways (MAP2K3/Fyn and MAP2K3/MAP2K10, respectively).
Figure 3. Network of miRNA-target genes associated to p38-related pathways in MPM.
Discussion
In the present study, a wide screen of serum miRNAs identified distinctive miRNA profiles between MPM and AD patients. In particular, hsa-miR-1292-5p and hsa-miR-409-5p were upregulated in MPM versus AD as well in MPM compared to healthy controls, implicating these two miRNAs strongly as potential biomarkers for differentiating MPM from AD. In addition, the biological pathways in which the identified differentiated miRNAs’ target genes participate (hsa-miR-1271, hsa-miR-96-5p and hsa-miR-409) include most predominantly the p38 signaling pathway, regulation of p38-alpha and p30-beta pathways, and gluconeogenesis.
The p38 signaling pathway activates mitogen-activated protein (MAP) kinases associated with regulation of apoptosis, the cell cycle, and inflammation. Activation of p38 has been reported to be reduced in tumors, and this reduction may contribute to increased proliferation and increased likelihood of tumorigenic conversion (Brancho et al., 2003[3]; Zarubin and Han, 2005[25]). Gluconeogenesis seems not to be directly related to tumor development, but it could be associated with survival in low-nutrient environments.
There are two particularly interesting genes targeted by the miRNAs that were found to be upregulated in MPM. The first, MAP2K3, is an upstream activator of p38. Indeed there is evidence suggesting that MAP2K3 is the major determinant of p38 MAPK activation and it may need to be active for tumor growth suppression (Galan-Moya et al., 2011[7]; Zhu et al., 2011[26]; MacNeil et al., 2014[18]). Although studies have reported that the biological consequences of p38 activation depend on cell type and the stimulus applied (Brancho et al., 2003[3]; Zarubin and Han, 2005[25]), activation of p38 by upregulated MAP2K3 may be necessary for suppression of tumor growth (i.e. breast cancer, glioblastoma, non-small cell lung cancer [NSCLC]) (Galan-Moya et al., 2011[7]; Zhu et al., 2011[26]; MacNeil et al., 2014[18]). Hence, tumorigenesis could become more likely when p38 activity has been reduced by upregulated and secreted miRNAs from neighboring tumor cells, indicating a potential biological relevance of hsa-miR-1271, hsa-miR-96-5p and hsa-miR-409 in tumorigenesis and as potential circulating biomarkers of MPM.
Use of circulating, rather than local tissue, miRNAs as biomarkers has noteworthy advantages. Firstly, drawing blood is relatively non-invasive, especially relative to lung and pleural tissue biopsies. In addition, serum can be used rather plasma to avoid distortion of findings related to miRNAs in platelets (McDonald et al., 2011[19]). A wide screen of circulating miRNAs was performed in this study because secreted miRNA profiles have been reported to differ from tissue miRNA profiles (Ohshima et al., 2010[20]; Pigati et al., 2010[21]); indeed new miRNAs have been discovered in circulation (Chen et al., 2008[5]).
To our knowledge, this is the first report of differential miRNA profiles in sera from MPM and AD patients. This is also the first report of a wide screen for miRNAs in serum from patients with MPM. Kirschner et al. (2012[13]) reported increased circulating miR-625-3p as a potential biomarker for MPM in plasma and serum; however, they performed their first screening in plasma samples, and then examined levels of only hsa-miR-625-3p in serum and plasma samples. Their results did not match our data, perhaps because plasma samples contain cellular miRNAs from platelets that could mask cell-freel miRNAs in circulation.
On the other hand, our AD serum miRNA data differ from the data reported by Roth et al. (2012[23]), who performed serum-based miRNA profiling of 1158 known miRNAs using microfluid biochips. They reported finding 30 deregulated miRNAs in NSCLC, but did not mention if a differential analysis had been performed for the different subtypes of NSCLC analyzed. Chen et al. (2012[5]) identified ten serum miRNAs as biomarkers for NSCLC patients. Those miRNAs differed from the miRNAs identified in the present study, perhaps because they analyzed only deregulated miRNAs identified in a previous study that did not include a differential analysis between NSCLC subtypes. Not analyzing NSCLC subtypes separately might obscure differences in levels of particular miRNAs. Studies of tissue miRNAs have showed miRNA profile differences between AD and squamous cell carcinoma that are were not found when they were analyzed as a broad group of NSCLC samples (Lebanony et al., 2009[16]; Landi et al., 2010[15]).
In conclusion, our findings indicate that serum miRNA profiles may be a suitable non-invasive tool for distinguishing between MPM and AD, either through comparison to healthy controls or by comparison between the two neoplasias. These data point to at least three miRNAs as potential diagnostic biomarkers for MPM - namely hsa-miR-1271, has-miR-96-5p, and hsa-miR-409 - due to their potential association with carcinogenesis mechanisms, and - hsa-miR-1292-5p and hsa-miR-409-5p - due to their upregulation in MPM though both comparisons. Our results need to be verified by a quantitative method, such real-time polymerase chain reaction, and in experiments using individual serum samples.
Conflict of interest
None declared.
Acknowledgements
This work was supported by a grant from the Consejo Nacional de Ciencia y Tecnología (CONACyt), Project SALUD-2010-C01-141984, México. We thank to the Unidad de Secuenciación e Identificación de Polimorfismos at INMEGEN, México, and to Ricardo Grande and Verónica Jiménez Jacinto from the Unidad Universitaria de Secuenciación Masiva de DNA at UNAM for their technical support during sequencing.
References
- 1.Avila-Moreno F, Urrea F, Ortiz-Quintero B. [MicroRNAs in diagnosis and prognosis in lung cancer [Article in Spanish]]. Rev Invest Clin. 2011;63:516–535. (Ger). [PubMed] [Google Scholar]
- 2.Bagheri R, Haghi SZ, Rahim MB, Attaran D, Toosi MS. Malignant pleural mesothelioma: clinicopathologic and survival characteristic in a consecutive series of 40 patients. Ann Thorac Cardiovasc Surg. 2011;17:130–136. doi: 10.5761/atcs.oa.09.01427. [DOI] [PubMed] [Google Scholar]
- 3.Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, et al. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Hu Z, Wang W, Ba Y, Ma L, Zhang C, et al. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int J Cancer. 2014;130:1620–1628. doi: 10.1002/ijc.26177. [DOI] [PubMed] [Google Scholar]
- 7.Galan-Moya EM, de la Cruz-Morcillo MA, Llanos Valero M, Callejas-Valera JL, Melgar-Rojas P, Hernadez Losa J, et al. Balance between MKK6 and MKK3 mediates p38 MAPK associated resistance to cisplatin in NSCLC. PLoS One. 2011;6:e28406. doi: 10.1371/journal.pone.0028406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner PP, Daub J, Tate J, Moore BL, Osuch IH, Griffiths-Jones S, et al. Rfam: Wikipedia, clans and the "decimal" release.". Nucleic Acids Res. 2011;39(Database issue):D141–D145. doi: 10.1093/nar/gkq1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackenberg M, Rodríguez-Ezpeleta N, Aransay AM. miRanalyzer: an update on the detection and analysis of microRNAs in high-throughput sequencing experiments. Nucleic Acids Res. 2011;39(Web Server issue):W132–W138. doi: 10.1093/nar/gkr247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackenberg M, Sturm M, Langenberger D, Falcón-Pérez JM, Aransay AM. miRanalyzer: a microRNA detection and analysis tool for next-generation sequencing experiments. Nucleic Acids Res. 2009;37(Web Server issue):W68–W76. doi: 10.1093/nar/gkp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 12.Jurka, J Repbase update: a database and an electronic journal of repetitive elements. Trends Genet. 2000;16:418–420. doi: 10.1016/s0168-9525(00)02093-x. [DOI] [PubMed] [Google Scholar]
- 13.Kirschner MB, Cheng YY, Badrian B, Kao SC, Creaney J, Edelman JJ, et al. Increased circulating miR-625-3p: a potential biomarker for patients with malignant pleural mesothelioma. J Thorac Oncol. 2012;7:1184–1191. doi: 10.1097/JTO.0b013e3182572e83. [DOI] [PubMed] [Google Scholar]
- 14.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landi MT, Zhao Y, Rotunno M, Koshiol J, Liu H, Bergen AW, et al. MicroRNA expression differentiates histology and predicts survival of lung cancer. Clin Cancer Res. 2010;16:430–441. doi: 10.1158/1078-0432.CCR-09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebanony D, Benjamin H, Gilad S, Ezagouri M, Dov A, Ashkenazi K, et al. Diagnostic assay based on hsa-miR-205 expression distinguishes squamous from nonsquamous non-small-cell lung carcinoma. J Clin Oncol. 2009;27:2030–2037. doi: 10.1200/JCO.2008.19.4134. [DOI] [PubMed] [Google Scholar]
- 17.Lu TP, Lee CY, Tsai MH, Chiu YC, Hsiao CK, Lai LC, et al. miRSystem: an integrated system for characterizing enriched functions and pathways of microRNA targets. PLoS One. 2012;7:e42390. doi: 10.1371/journal.pone.0042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacNeil AJ, Jiao SC, McEachern LA, Yang YJ, Dennis A, Yu H, et al. MAPK kinase 3 is a tumor suppressor with reduced copy number in breast cancer. Cancer Res. 2014;74:162–172. doi: 10.1158/0008-5472.CAN-13-1310. [DOI] [PubMed] [Google Scholar]
- 19.McDonald JS, Milosevic D, Reddi HV, Grebe SK, Algeciras-Schimnich A. Analysis of circulating microRNA: preanalytical and analytical challenges. Clin Chem. 2011;57:833–840. doi: 10.1373/clinchem.2010.157198. [DOI] [PubMed] [Google Scholar]
- 20.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. 2010;5:e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, et al. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5:e13515. doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray M, Kindler HL. Malignant pleural mesothelioma: an update on biomarkers and treatment. Chest. 2009;136:888–896. doi: 10.1378/chest.08-2665. [DOI] [PubMed] [Google Scholar]
- 23.Roth C, Stückrath I, Pantel K, Izbicki JR, Tachezy M, Schwarzenbach H. Low levels of cell-free circulating miR-361-3p and miR-625* as blood-based markers for discriminating malignant from benign lung tumors. PLoS One. 2012;7:e38248. doi: 10.1371/journal.pone.0038248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 26.Zhu T, Zhao Y, Zhang J, Li L, Zou L, Yao Y, et al. ss-Elemene inhibits proliferation of human glioblastoma cells and causes cell-cycle G0/G1 arrest via mutually compensatory activation of MKK3 and MKK6. Int J Oncol. 2011;38:419–426. doi: 10.3892/ijo.2010.855. [DOI] [PubMed] [Google Scholar]