Abstract
Potassium tetraborate (PTB) is a product resulting from the controlled reaction of potassium hydroxide, water and boric acid (BA). It is used in many areas of industry such as disinfectant, detergent and treatment of contact lenses. PTB is one of the boron compounds which is most commonly used in many areas of industry although very limited information is available concerning its toxicity. Therefore, in this study, it is aimed to determine genetic and biochemical effects of PTB in human blood cell cultures (n=4). PTB was added into culture tubes at various concentrations (0-1280 µg/ml). Micronucleus (MN) and chromosomal aberration (CA) tests were performed for genotoxic damage influences estimation. In addition, biochemical parameters (total antioxidant capacity (TAC) and total oxidative status (TOS) were examined to determine oxidative effects. The results indicated that all tested concentrations of PTB were found to be non-genotoxic. In addition, low concentrations (1.25, 2.5 and 5 µg/ml) of PTB caused increases of TAC levels. Furthermore, all concentrations of PTB were not changed the TOS levels in cultured human blood cells. Based on these results, in this study it has been reported for the first time that PTB is not genotoxic and it increases the antioxidant capacity in human peripheral blood lymphocytes.
Keywords: boron, potassium tetraborate, genotoxicity, oxidative stress, antioxidant
Introduction
Boron compounds play important biological roles including improving arthritis, plasma lipid profiles and brain function (Colak et al., 2011[4]; Devirian and Volpe, 2003[5]). So far, boron compounds have attracted much interest with respect to their protective effect against free radical damage that may be the cause of many diseases including cancer (Barranco and Eckhert, 2006[1]; Scorei et al., 2008[18]). Furthermore, it was found that boron compounds were capable of protecting against heavy metal toxicity in cultured human blood cells (Turkez et al., 2012[25]). Besides, it was reported that boron compounds like borax (BX) and BA showed antioxidant activity in cultured human blood cells (Turkez et al., 2007[25], 2012[22]). On the other hand, the cellular and genetic toxicity of boron compounds was not investigated in mammalian cells, al-though the effects of boron compounds on reproductive system were studied in detail (Bolt et al., 2102[3]; Duydu et al., 2012[6]). Potassium tetraborate (K2B4O7, PTB) is a product resulting from the controlled reaction of potassium hydroxide, water and BA. It is used in many industrial processes including oil (Kazuhiro et al., 2004[14]) and glass (Toyoyuki et al., 2003[19]) industries. As far as we know, there is no study about genotoxic and oxidative potentials of PTB in human lymphocytes culture. With this aim, not only chromosomal aberrations (CA) frequencies but also micronuclei (MN) formations have been established as related to the doses of PTB on human blood cultures. Moreover, total antioxidant capacity (TAC) and total oxidative status (TOS) assays were examined to determine oxidative effects.
Materials and Methods
Experimental design
The heparinized blood samples were collected from four healthy males aged from 25 to 30 years old, respectively, non-smoking, non-alcoholic, not under drug therapy and with no recent history of exposure to mutagens. Human peripheral blood cultures were set up according to a slight modification of the protocol described by Evans and O’Riordan (1975[7]). The heparinized blood (0.5 ml) was cultured in 6 ml of culture medium (Chromosome Medium B, Biochrom, Berlin, Germany) with 5 mg/ml of phytohemagglutinin (Biochrom). Potassium tetraborate (K2B4O7, CAS Number 12045-78-2) was synthetized previously described by Marezio et al. (1963[15]) and dissolved in distilled water and it was added to the cultures just before incubation in concentrations of 1.25, 2.5, 5, 10, 20, 40, 80, 160, 320, 640 and 1280 µg/ml. Each individual blood culture without PTB was studied as a control group. The concentrations were selected according to the works of Turkez et al. (2007[25]). Mitomycin C (MMC; 10-7 M, Sigma-Aldrich®) was used as the positive control in CA and MN assays. Ascorbic acid (10 µM, Sigma-Aldrich®) and hydrogen peroxide (25 µM, Sigma-Aldrich®) were also used as the positive controls in TAC and TOS analysis, respectively.
Micronuclei (MN) assay
Human lymphocytes were stimulated by potassium tetraborate and cultured for about 72 h; cytochalasin B (Sigma; final concentration of 6 mg ml-1) 44 h after PTB stimulation was added. MN was scored in 1000 binucleated cells and the frequency of cells with micronuclei was determined (Fenech, 2000[8]; Turkez et al., 2010[27]).
Chromosomal aberration assay
Chromosomal aberration (CA) tests were performed not only to study the cytotoxicity of the material on cells but also to determine the aberrations induced by the particular material on chromosomes of the human lymphocytes cell line. For each treatment, 30 well-spreaded metaphases were analyzed to detect the presence of chromosomal aberrations (Turkez and Sisman, 2010[26]).
Biochemical assays
The levels of TAC and TOS were determined by commercially available kits (Rel Assay Diagnostics®, Turkey) in culture samples.
Results
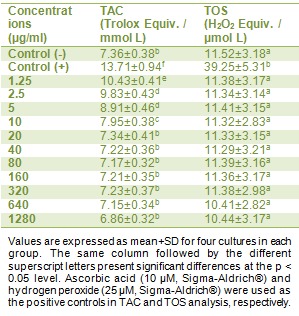
Table 1(Tab. 1) shows the biochemical data obtained from various concentrations of PTB, on human peripheral blood cell cultures. As shown from the results presented in Table 1(Tab. 1), 1.25, 2.5 and 5 µg/ml concentrations of PTB treatment caused significant increases of TAC levels. On the other hand, PTB did not change the TOS levels in cultured human lymphocytes at all concentrations.
Table 1. In vitro levels of TAC (as mmol Trolox Equiv./L) and TOS (as mmol H2O2 Equiv./L) in cultured human lymphocytes maintained in the presence of potassium tetraborate (PTB) for 24 h.
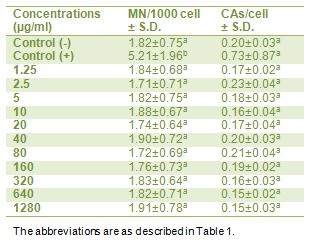
Results obtained from the analysis of MNs and CAs in human lymphocytes cultured with different PTB concentrations are shown in Table 2(Tab. 2). PTB at tested concentrations did not induce significant (p<0.05) number of MNs and CAs. However, the MMC applied culture (as positive control) showed about three fold increases of both parameters as compared to control group.
Table 2. In vitro levels of TOS (H2O2 Equiv. / µmol L-1) in cultured human lymphocytes maintained in the presence of potassium tetraborate for 24 h.
Discussion
Antioxidant capacity has important role in daily life of human and animals (Geyikoglu et al., 2005[10]; Bohloli et al., 2007[2]; Turkez and Togar, 2010[28]). In the present study, for assessing the antioxidant/oxidant effects of PTB, total antioxidant capacity (TAC) and total oxidant status (TOS) assays were performed for the first time. Our results reported that PTB applications at its low concentrations (1.25, 2.5 and 5µg/ml) caused increase of TAC levels, while TOS level were not affected. There is no data in literature about the antioxidant/oxidant effect of different PTB concentrations on cultured human lymphocytes cells and any other cell lines. The evidence of the previous studies suggested that boron compounds have the ability of strengthening the tissue antioxidant defences in human and animals (Hunt, 1994[11]; Pawa and Ali, 2006[16]). In accordance with present findings, previous studies have shown that boron compounds (such as BA, BX, colemanite, ulexite) had antioxidant property at lower concentrations than 40 mg/L (Turkez et al., 2007[25], 2012[24]). Besides, Hunt and Idso (1999[12]) stated that boron prevents oxidative damage by increasing of glutathione and its analogs or by supporting the other neutralizing agents. Likewise, Pawa and Ali (2006[16]) administrated BX (4 mg/kg), to rats as a boron source followed by administration of thioacetamide (a toxic chemical). As a result they reported that boron may have positive effects on the oxidant/antioxidant balance. In addition, Ince et al. (2010[13]) demonstrated that boron supplementation decreases lipid peroxidation and enhances antioxidant defence mechanism. Moreover, it was reported that boron prevents oxidative damage by increasing of glutathione and its analogs or by supporting the other neutralizing agents (Hunt and Idso, 1999[12]).
In this study, genotoxicity assessments were carried out using CAs and MN tests to provide more safety information on PTB. There were no significant differences in the incidence of chromosomal aberrations, even after a 72 h treatment with the all concentration of PTB. Also, the tested PTB was shown to lack mutagenic activity in cultured human lymphocytes when assessed for induction of DNA damage in the MN assay. In parallel to our finding, Turkez and Geyikoglu (2006[21]) studied the mutagenic properties of some boron compounds in cultured human lymphocytes. They reported that the used boron compounds did not change the SCE frequencies or MN formations. In addition, negative results in a large number of mutagenicity assays indicate that boron compounds especially BA and BX are not genotoxic (Turkez et al., 2007[25]). In the same way, Geyikoglu and Turkez (2008[9]) and Turkez (2008[20]) reported that BA and BX showed protective activity against vanadium (IV) tetraoxide and titanium dioxide induced DNA damage on peripheral lymphocytes. Besides, it was found that the dramatic reduction of aflatoxin B1 (AFB1)-induced SCEs and MN formations were due to the protective roles of BX (Turkez et al., 2012[22]). Furthermore, Turkez et al. (2013[23]) studied the protective role of BX on genotoxicity induced by aluminum (Al) in rat liver, using liver MN assay as an indicator of genotoxicity. They have reported that BX has beneficial influences and has the ability to antagonize Al toxicity. On the other hand, recent studies reported that BX was found to be genotoxic in human lymphocyte cells (Pongsavee, 2009[17]).
In conclusion, the present study has clearly demonstrated that PTB has anti-oxidative and non-genotoxic activities for the first time. However, further studies are needed to further elucidate the mechanisms of PTB to improve our understanding of their antioxidant effect using in vivo and in vitro different cell models.
Acknowledgements
The authors are grateful to all volunteers for the blood samples.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Barranco WT, Eckhert CD. Cellular changes in boric acid-treated DU–145 prostate cancer cells. Brit J Cancer. 2006;94:884–90. doi: 10.1038/sj.bjc.6603009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohloli M, Uzun H, Aytac E, Toklu AS, Paksoy M, Durak H, et al. Hyperbaric oxygen (HBO) therapy after partial hepatectomy: an experimental study on oxidative stress in rats. Scand J Lab Anim Sci. 2007;34:131–40. [Google Scholar]
- 3.Bolt HM, Başaran N, Duydu Y. Human environmental and occupational exposures to boric acid: reconciliation with experimental reproductive toxicity data. J Toxicol Environ Health. 2012;75:508–514. doi: 10.1080/15287394.2012.675301. [DOI] [PubMed] [Google Scholar]
- 4.Colak S, Geyikoglu F, Keles ON, Turkez H, Topal A, Unal B. The neuroprotective role of boric acid on aluminum chloride-induced neurotoxicity. Toxicol Ind Health. 2011;27:700–10. doi: 10.1177/0748233710395349. [DOI] [PubMed] [Google Scholar]
- 5.Devirian TA, Volpe SL. The physiological effects of dietary boron. Crit Rev Food Sci Nutr. 2003;43:219–31. doi: 10.1080/10408690390826491. [DOI] [PubMed] [Google Scholar]
- 6.Duydu Y, Başaran N, Bolt HM. Exposure assessment of boron in Bandırma boric acid production plant. J Trace Elem Med Biol. 2012;26:161–164. doi: 10.1016/j.jtemb.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Evans HJ, O’Riordan ML. Human peripheral blood lymphocytes for the analysis of chromosome aberrations in mutagen tests. Mutat Res. 1975;31:135–48. doi: 10.1016/0165-1161(75)90082-5. [DOI] [PubMed] [Google Scholar]
- 8.Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/s0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 9.Geyikoglu F, Turkez H. Boron compounds reduce vanadium tetraoxide genotoxicity in human lymphocytes. Environ Toxicol Pharmacol. 2008;26:342–347. doi: 10.1016/j.etap.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Geyikoglu F, Turkez H, Keles SM. The role of fruit juices in the prevention of aluminum sulphate toxicity in human blood in vitro. Fresenius Environ Bull. 2005;14:878–883. [Google Scholar]
- 11.Hunt C. The biochemical effects of physiologic amounts of dietary boron in animal nutrition models. Environ Health Perspect. 1994;102:35–43. doi: 10.1289/ehp.94102s735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunt CD, Idso J. Dietary boron as a physiological regulator of the normal inflammatory response: a review and current research progress. J Trace Elem Exp Med. 1999;12:221–33. [Google Scholar]
- 13.Ince S, Kucukkurt I, Cigerci IH, Fidan AF, Eryavuz A. The effects of dietary boric acid and borax supplementation on lipid peroxidation, antioxidant activity, and DNA damage in rats. J Trace Elem Med Biol. 2010;24:161–4. doi: 10.1016/j.jtemb.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Kazuhiro Y, Jinichi I, Takeo K. Lubricating oil composition for internal combustion engine. Patent no: EP1439217. 004-07-21.2004. [Google Scholar]
- 15.Marezio M, Plettinger H, Zarchariasen W. The crystal structure of potassium tetraborate tetrahydrate. Acta Crystallogr. 1963;16:975–980. [Google Scholar]
- 16.Pawa S, Ali S. Boron ameliorates fulminant hepatic failure by counteracting the changes associated with the oxidative stress. Chem Biol Interact. 2006;160:89–98. doi: 10.1016/j.cbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Pongsavee M. Genotoxic effects of borax on cultured lymphocytes. Southeast Asian J Trop Med Public Health. 2009;40:411–418. [PubMed] [Google Scholar]
- 18.Scorei R, Ciubar R, Ciofrangeanu CM, Mitran V, Cimpean A, Iordachescu D. Comparative effects of boric acid and calcium fructoborate on breast cancer cells. Biol Trace Elem Res. 2008;122:197–205. doi: 10.1007/s12011-007-8081-8. [DOI] [PubMed] [Google Scholar]
- 19.Toyoyuki T, Hisashi O, Hiroaki K, Jun H, Kazuhiro D, Hiroaki Y. Glass article, method for handling glass article and handling tool for glass article. European Patent, Patent No: US6503630. 2003-01-07.2003. [Google Scholar]
- 20.Turkez H. Effects of boric acid and borax on titanium dioxide genotoxicity. J Appl Toxicol. 2008;28:658–664. doi: 10.1002/jat.1318. [DOI] [PubMed] [Google Scholar]
- 21.Turkez H, Geyikoglu F. 3rd International Boron Symposium, Ankara. Ankara: 2006. Protective effects of Kernite and Probertite against titanium dioxide genotoxicity in vitro; pp. 475–479. [Google Scholar]
- 22.Turkez H, Geyikoğlu F, Dirican E, Tatar A. In vitro studies on chemoprotective effect of borax against aflatoxin B1-induced genetic damage in human lymphocytes. Cytotechnology. 2012;64:607–612. doi: 10.1007/s10616-012-9454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turkez H, Geyikoğlu F, Tatar A. Borax counteracts genotoxicity of aluminum in rat liver. Toxicol Ind Health. 2013;29:775–779. doi: 10.1177/0748233712442739. [DOI] [PubMed] [Google Scholar]
- 24.Turkez H, Geyikoglu F, Tatar A, Keles MS, Kaplan I. The effects of some boron compounds against heavy metal toxicity in human blood. Exp Toxicol Pathol. 2012;64:93–101. doi: 10.1016/j.etp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Turkez H, Geyikoğlu F, Tatar A, Keleş S, Ozkan A. Effects of some boron compounds on peripheral human blood. Z Naturforsch. 2007;C62:889–896. doi: 10.1515/znc-2007-11-1218. [DOI] [PubMed] [Google Scholar]
- 26.Turkez H, Sisman T. Toxicologic evaluation of imazalil with particular reference to genotoxic and teratogenic potentials. Toxicol Ind Health. 2010;26:641–648. doi: 10.1177/0748233710375951. [DOI] [PubMed] [Google Scholar]
- 27.Turkez H, Tatar A, Hacimuftuoglu A, Ozdemir E. Boric acid as a protector against paclitaxel genotoxicity. Acta Biochim Pol. 2010;57:95–97. [PubMed] [Google Scholar]
- 28.Turkez H, Togar B. The genotoxic and oxidative damage potential of olanzapine in vitro. Toxicol Ind Health. 2010;26:583–588. doi: 10.1177/0748233710373090. [DOI] [PubMed] [Google Scholar]


