Abstract
A series of phenyl-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carbothioamides (TTa-TTg) were synthesized by the ring closure reaction of phenyl-1-(thiophen-2-yl) prop-2-en-1-ones with thiosemicarbazide in alcoholic basic medium. All the final derivatives were evaluated for their antidepressant and neurotoxicity screening. The structures of the compounds were characterized by IR, 1H NMR, 13C NMR, Mass and elemental analyses. Preclinical evaluation of the compounds were ascertained by in silico toxicity, blood-brain barrier and human oral absorption prediction. In this series, 5-(4-hydroxyphenyl)-3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazole-1 carbothioamide (TTg) reduced immobility time 61.17 and 62.05 % in both force swimming and tail suspension test respectively at 10 mg/kg dose level when compared to the standard Imipramine without influencing the baseline locomotion. Moreover it was observed that the titled scaffold possessing electron withdrawing chlorine atom in the 4th position of aromatic ring of the scaffold also showed good the antidepressant activity. In conclusion, the behavioural investigation revealed that thiophene based pyrazolines having a carbothioamide tail unit in the N1 position may be therapeutically useful as potential antidepressant medications.
Keywords: 2-Acetyl thiophene, pyrazoline, total polar surface area, Force swimming test, neurotoxicity
Introduction
In the five membered nitrogen containing heterocyclic family, pyrazoline could be encountered as the most promising scaffold for antidepressant activity (Palaska et al., 2001[11]; Prasad et al., 2005[14]; Ozedemir et al., 2007[10]; Kalpancikli et al., 2010[4]; Gok et al., 2010[3]). Recently this nucleus endowed with remarkable attention in the inhibition of MAO-A, which is considered as the effective target for the management of depressive disorders (Chimenti et al., 2004[2]; Karuppasamy et al., 2010[5]; Nayak et al., 2013[9]; Salgin-Goksen et al., 2013[15]; Mathew et al., 2013[8]; Kumar et al., 2013). In 1974 Parmar et al. identified the therapeutic benefit of pyrazoline nucleus towards MAO-inhibition and has anticonvulsant action (Parmar et al., 1974[12]). In the five membered nitrogen containing heterocyclic family, pyrazolines could be considered a valid pharmacophore for the synthesis of selective monoamine oxidase (MAO) inhibitors because they were developed by the cyclization of the early hydrazine derivatives such as isocarboxazid. The literature survey revealed that the substitutions of pyrazoline nucleus preferably at the N1, C3, and C5 positions showed remarkable effect in the central nervous system.
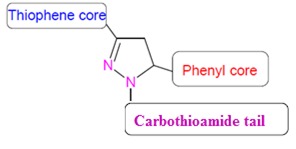
In the organic perspective view the N-substituted pyrazolines have been synthesized from the cyclization reaction with chalcones and substituted hydrazine derivatives in basic alcoholic medium. Among various nitrogen containing heterocycles that have been explored for developing pharmaceutically important molecules, pyrazolines have attracted a greater attention towards the antidepressant action. Considering that pyrazoline are promising class of antidepressants and in the light of aforementioned findings, we aimed to synthesize a thiophene based pyrazolines. In the present work, it has been planned to attach a carbothioamide tail unit in the N1 position with a thiophene and substituted phenyl system in the 3rd and 5th position of pyrazoline template and evaluate their antidepressant action (Figure 1(Fig. 1)). Preclinical evaluation involves the toxicity prediction and pharmacokinetic evaluation of the titled compounds by using computational tools. Most of the synthesized candidates fail in clinical trials because of reasons unrelated to potency against intended drug target. Pharmacokinetics and toxicity issues are blamed for more than half of all failures in clinical trials. Many computational predictions are nowadays available to overcome such scenario in the drug discovery process. In the present study, an attempt has been carried out to determine the in silico ADMETox prediction of the titled synthesized compounds to ensure their drug-likeness properties.
Figure 1. Design of thiophene based pyrazoline.
Materials and Methods
Chemistry
2-Acetyl thiophene was procured from Sigma–Aldrich USA. All other chemicals and reagents purchased from SD-fine and Nice chemicals. Melting points of all the synthesized derivatives were determined by open-capillary tube method and values were uncorrected. IR spectra were recorded on Shimadzu FT/IR spectrometer on KBr pellets recorded in cm-1 values.1H NMR and 13C NMR spectra were recorded on a Bruker 400 mhz NMR spectrometer using CDCl3 as the solvent. Mass spectra were recorded on a JEOL GCmate mass spectrometer.
Synthesis of 3- substituted phenyl-1-(thiophen-2-yl) prop-2-en-1-ones (Ta-Tg)
A mixture of 2-acetyl thiophene (0.01 mol), aromatic aldehyde (0.01 mol) and 40 % aqueous potassium hydroxide (15 ml) in ethanol (30 ml) was stirred at room temperature for about 2 hr. The resulting product was kept overnight in refrigerator. The mixture was poured into crushed ice and acidified with 5 % HCl. The solid separated was filtered, washed with water and recrystallized from ethanol. Synthesis of 5- substituted phenyl-3-(thio-phen-2-yl)-4, 5-dihydro-1H-pyrazole-1-car-bothioamide (TTa-TTg).
A mixture of 3-substituted phenyl-1-(thiophen-2-yl) prop-2-en-1-ones (Ta-Tg) (0.01), sodium hydroxide (1 g, 0.025 mol) in ethanol 50 ml and thiosemicarbazide (0.012 mol) were magnetically stirred for 4 hrs. The resulting homogenous mixture refluxed for 8-10 hrs in a water bath. The products were poured into crushed ice and solid mass which was separated out was filtered, washed with water, dried and recrystallized from methanol: benzene (6:4). In case of TTf&TTg formation conc. HCl is added slightly for the recovery of the products.
5-phenyl-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carbothioamide (TTa): Dark brown, Yield 70 %, M.p. 120 °C, TLC Rf= 0.65(CHCl3/CH3OH,8/2); FT-IR (KBr) vmax/cm-1: 3439(NH2), 1573(C=N), 1471(-CH2-pyrazoline), 1352(C=S), 698(C-S-C). 1H NMR (CDCl3, 400 MHz, δ ppm): 3.22(d, 1H, HA), 3.86(d, 1H, HB), 5.55(d, 1H, HX), 6.05(s, 2H, S=C-NH2), 7.03-7.47(m, 8H, Ar-H& Th-H). 13C-NMR (400 MHz, CDCl3, δ ppm): 42.71(CH2, Pyr-C4), 60.20(CH, Pyr-C5), 125.44-126.78(CH&C, Th-C2& Th-C5), 126.85-142.24(CH, Ar-CH& Th-CH), 144.27(C, Ar-C1), 147.49(C, Pyr-C3), 154.95(C=S, CSNH2). MS: m/z (M+1) +281. Anal. calcd. for C14H13N3S2: C: 58.51, H: 4.56, N: 14.62, S: 22.31. Found C: 58.33, H: 4.76, N: 14.52, S: 22.22.
5-(4-chlorophenyl)-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carboxamide (TTb): Cream white, Yield 61 %, M.p.180 °C, TLC Rf= 0.61(CHCl3/CH3OH, 8/2); FT-IR (KBr) vmax/cm-1: FT-IR (KBr) vmax/cm-1: 3427(NH2), 1579(C=N), 1469(-CH2-pyrazoline), 1350(C=S), (Ar-Cl), 713 (C-S-C). 1H NMR (CDCl3, 400 MHz, δ ppm): 3.26(d, 1H, HA), 3.89(d, 1H, HB), 5.67(d, 1H, HX), 6.02(s, 2H, S=C-NH2), 7.07-7.49(m, 7H, Ar-H&Th-H). 13C-NMR (400 MHz, CDCl3, δ ppm): 41.78(CH2, Pyr-C4), 60.68(CH, Pyr-C5), 124.43-125.38 (CH&C, Th-C2& Th-C5), 126.36-141.85 (CH, Ar-CH& Th-CH), 142.32(C, Ar-C1), 146.54(C, Pyr-C3), 155.27(C=S, CSNH2). MS: m/z (M+2) +321. Anal. calcd. for C14H12N3S2Cl: C: 52.25, H: 3.76, N: 11.06, S: 19.93. Found C: 52.13, H: 3.58, N: 11.26, S: 19.23.
5-(4-methoxyphenyl)-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carboxamide (TTc): Brown, Yield 67 %, M.p.150 °C, TLC Rf= 0.73(CHCl3/CH3OH, 8/2); FT-IR (KBr) vmax/cm-1: 3429(NH2), 1602(C=N), 1510(-CH2-pyrazoline), 1352(C=S), 1250 (C-O), 712(C-S-C). 1H NMR (CDCl3, 400 MHz, δ ppm): 3.31(d, 1H, HA), 3.77(s, 3H, OCH3), 3.85(d, 1H, HB), 5.47(d, 1H, HX), 6.02(s, 2H, 2H, S=C-NH2), 6.87-7.60(m, 7H, Ar-H&Th-H). 13C-NMR (400 MHz, CDCl3, δ ppm): 43.64(CH2, Pyr-C4), 55.28 (CH3, O-CH3), 59.74(CH, Pyr-C5), 114.03-114.27(CH, Ar-C3&Ar-C5), 126.79-135.07 (CH, Ar-CH& Th-CH), 147.48(C, Ar-C4), 154.95(C, Pyr-C3), 159.04(C=S, CSNH2). MS: m/z (M+1) +317. Anal. calcd. for C15H15N3S2O: C: 56.76, H: 4.76, N: 13.24, S: 20.20. Found C: 56.31, H: 4.64, N: 13.22, S: 20.13.
5-[4-(dimethylamino) phenyl]-3-(thio-phen-2-yl)-4, 5-dihydro-1H-pyrazole-1-car-boxamide (TTd): Greenish black, Yield 52 %, M.p.129 °C, TLC Rf= 0.84 (CHCl3/ CH3OH, 8/2); FT-IR (KBr) vmax /cm-1: 3470 (NH2), 1593(C=N), 1521(-CH2-pyrazoline), 1352(C=S), 725(C-S-C). 1H NMR (CDCl3, 400 MHz, δ ppm): 2.91(s, 6H, (N(CH3)2), 3.21(d, 1H, HA), 3.79(d, 1H, HB), 5.96(d, 1H, HX), 6.85(s, 2H, 2H, S=C-NH2), 7.09-7.83(m, 7H, Ar-H&Th-H). 13C-NMR (400 MHz, CDCl3, δ ppm): 40.51(CH3, N(CH3)2), 43.72(CH2, Pyr-C4), 63.27(CH, Pyr-C5), 111.83-112.63(CH, Ar-C3&Ar-C5), 116.37-132.82(CH, Ar-CH& Th-CH), 145.00(C, Ar-C4), 150.05(C, Pyr-C3), 155.00(C=S, CSNH2). MS: m/z (M+1) +330. Anal. calcd. for C16H18N4S2: C: 58.15, H: 5.49, N: 16.95, S: 19.41. Found C: 58.32, H: 5.76, N: 16.59, S: 19.42.
5-(3-nitrophenyl)-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carboxamide (TTe): Brown, Yield 70 %, M.p.144 °C, TLC Rf= 0.82(CHCl3/CH3OH, 8/2); FT-IR (KBr) vmax /cm-1: 3361(NH2), 1581(C=N), 1527(-CH2-pyrazoline), 1350(C=S), 736(C-S-C). 1H NMR (CDCl3, 400 MHz, δ ppm): 3.22(d, 1H, HA), 3.98(d, 1H, HB), 6.15(d, 1H, HX), 7.10(s, 2H, 2H, S=C-NH2), 7.19-8.14(m, 7H, Ar-H&Th-H). 13C-NMR (400 MHz, CDCl3, δ ppm): 43.47(CH2, Pyr-C4), 62.86(CH, Pyr-C5), 120.84-131.92(CH, Ar-C3&Ar-C5), 144.27(C, Ar-C3), 147.49(C, Pyr-C3), 155.95(C=S, CSNH2). MS: m/z (M+1) +332. Anal. calcd. for C14H12N4S2O2: C: 50.59, H: 3.64, N: 16.86, S: 19.29. Found C: 50.69, H: 3.76, N: 16.73, S: 19.52.
5-(2-hydroxyphenyl)-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1 carboxamide (TTf): Dark brown, Yield 42 %, M.p.140 °C, TLC Rf= 0.73(CHCl3/CH3OH, 8/2); FT-IR (KBr) vmax /cm-1: 3491(NH2), 3212(Ar-OH), 1582(C=N), 1496(-CH2-pyrazoline), 732(C-S-C). 1H NMR (CDCl3, 400 MHz, δ ppm): 3.34(d, 1H, HA), 3.97(d, 1H, HB), 5.21(s, 1H, Ar-OH), 5.65(d, 1H, HX), 7.01(s, 2H, 2H, S=C-NH2), 7.23-7.68(m, 7H, Ar-H&Th-H). 13C-NMR (400 MHz, CDCl3, δ ppm): 43.79(CH2, Pyr-C4), 61.13(CH, Pyr-C5), 118.23-119.16(CH, Ar-C1&C3) 127.43-135.68(CH, Ar-CH& Th-CH), 144.75(C, Ar-C2), 147.68(C, Pyr-C3), 157.35(C=S, CSNH2). MS: m/z (M+1) +281. Anal. calcd. for C14H13N3S2O: C: 55.42, H: 4.32, N: 13.85, S: 22.14. Found C: 55.32, H: 4.18, N: 13.66, S: 22.24.
5-(4-hydroxyphenyl)-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carboxamide (TTg): Light brown, Yield 75 %, M.p.140 °C, TLC Rf= 0.58(CHCl3/CH3OH, 8/2); FT-IR (KBr) vmax/cm-1: 3494(NH2), 3210 (Ar-OH), 1586(C=N), 1495(-CH2-pyrazoline), 730(C-S-C). 1H NMR (DMSO-d6, 400 MHz, δ ppm): 1H NMR (CDCl3, 400 MHz, δ ppm): 3.28(d, 1H, HA), 3.91(d, 1H, HB),5.27(s,1H,Ar-OH), 5.55(d, 1H, HX), 7.06(s, 2H, 2H, S=C-NH2), 7.28-7.72(m, 7H, Ar-H&Th-H). 13C-NMR (400 MHz, CDCl3, δ ppm): 43.71(CH2, Pyr-C4), 60.03(CH, Pyr-C5), 116.03-119.26(CH, Ar-C3&C5) 127.53-133.66(CH, Ar-CH& Th-CH), 144.05(C, Ar-C4), 145.69(C, Pyr-C3), 158.15(C=S, CSNH2). MS: m/z (M+1) +281. Anal. calcd. for C14H13N3S2O: C: 55.42, H: 4.32, N: 13.85, S: 22.14. Found C: 55.36, H: 4.16, N: 13.56, S: 22.28.
Preclinical evaluation
Toxicity prediction of the newly designed scaffold was retrieved from a web-based application for Organic Chemistry Portal (http://www.organic-chemistry.org/prog) (Sander et al., 2009[16]). Blood brain barrier prediction was done by utilizing (www.cbligand.org/BBB/). The percentage of absorption (%ABS) was calculated by using topological polar surface area (TPSA) of the molecule. Absorption (%ABS) was calculated by:
%ABS = 109-(0.345 × TPSA) (Zaho et al., 2002[19]).
Pharmacological screening
The experimental protocol for the pharmacological screening on mice were done with an Institutional Animal Ethics Committee, K.M. College of Pharmacy, Madurai, India (Reg no: 661/02/c/ CPCSEA). Swiss albino mice (18-25 g) and albino rats (80-120 g) of either sex were used for the study. The animals were obtained from the K.M. College of Pharmacy, Madurai. The animals were housed in colony cages at an ambient temperature of 25 ± 2 °C, 12 hr light/dark cycle and 50 ± 5 % relative humidity with free access to food and water ad libitum. Food, but not water, was deprived overnight and during the experiment. All the experiments were carried out during the light period (9.00-16.00 hrs). Each group consisted of six animals.
Antidepressant activity
Forced swim test (FST)
The development of immobility when the rats are placed in an inescapable cylinder filled with water reflects the cessation of persistent escape-directed behaviour. The cylindrical container (diameter 10 cm, height 25 cm) was filled to a 19-cm depth with water at (25 ± 1 °C). The duration of immobility during the 6 min test was scored. Each rat was judged to be immobile when it ceased struggling and remained floating motionless in the water, making only those movements necessary to keep its head above water. (Porsolt et al., 1977[13])
Tail suspension test (TST)
The total duration of immobility by the Tail suspension test was measured according to the previously described method (Steru et al., 1985[18]). Rats were isolated both acoustically and visually and suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was observed during a 6 min test for animals of all groups. Rats were considered to be immobile when they hung passively and were completely motionless. The percentage change relative to control was calculated by the following equation.
% Change of immobility = [(test/control) ×100) - 100]
Evaluation of motor coordination activity
The motor coordination and performance of each mouse was evaluated 30 min after i.p. administration of (TTa-TTg) (10 mg/kg) and standard diazepam (4 mg/kg) in a rota-rod apparatus. This equipment has a bar 2.5 cm in diameter and divided into six parts, and it is placed at a height of 50 cm, rotating at 20 rpm. Latency to fall from the rotating bar was registered.
Results and Discussion
Chemistry
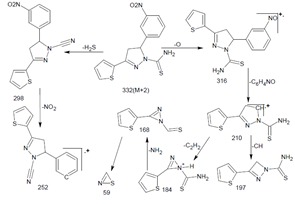
The intermediate of thiophene based heteroaryl chalcones have been synthesized by Claisen–Schmidt condensation between 2-acetyl thiophene and appropriate phenyl and substituted phenyl aldehydes in presence of alcoholic basic medium (Satyanarayana et al., 2013[17]; Mathew et al., 2013[7]). The formation of pyrazoline nucleus involves the nucleophilic addition of the amino group of thiosemicarbazide to the keto group of thiophene chalcone afford imine linkage. It was followed by the cyclisation with β-carbon unit of 1-(2-thienyl)-3-aryl-2-propen-1-one (Aboul-Enein et al., 2012[1]). The synthetic route involved in the formation of titled compounds were outlined in Figure 2(Fig. 2). The IR spectra of TTe showed the disappearance of C=O band and appearance of a new band at 1581 cm-1 recommended the formation of C=N. This functional group transformation indicates the ring closure. The band at 3361 and 1527 cm-1 corresponds to the NH2 and CH2 of pyrazoline nucleus respectively. In proton NMR, pyrazoline ring protons (HA and HB) showed at around 3.22 and 3.98 ppm as a doublet and also vicinal methine proton HX showed triplet/doublet at about 6.15 ppm due to vicinal coupling with the two magnetically non-equivalent protons of the methylene group HA and HB. NH2 protons of the carbamoyl group were seen at 7.10 ppm in the case of TTe. In 13C-NMR spectra the peaks for pyrazoline at C3, C4 and C5 are in the range of δ 145.69-154.95 ppm, δ 43.79-40.51 ppm and δ 59.74-63.27 ppm respectively. In Mass spectra of TTe, the fragment peaks which corresponds to loss of H2S, O and NO2 molecular ion are consistent with the postulated structure. The splitting of an oxygen atom from the TTe derivative showed a peak of 316 which correspond to the molecular formula of C14H12N4OS2 gave a full agreement of the formation of 5-(3-nitrophenyl)-3- (thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carbothioamide.The details of the mass fragmentation pattern of TTe is shown in Figure 3(Fig. 3).
Figure 2. Synthetic route of the titled derivatives.
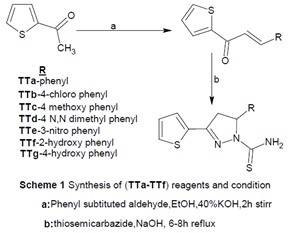
Figure 3. Mass fragmentation pattern of TTe.
Preclinical evaluation
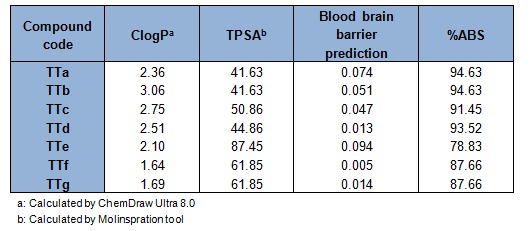
The toxicity assessment of all the final synthesized derivatives were shown in Table 1(Tab. 1). It is quite interesting to see that all the compounds were free from all type of toxicity issues mentioned in the software package except TTe which exhibited a medium risk mutagenic effect. Pharmacokinetic profile of the synthesized molecules were shown in Table 2(Tab. 2). The positive values of the candidates towards the blood brain barrier indicated their ability in acting in the CNS. The unsubstituted and the presence of lipophilic halogen or electron donating groups in the phenyl nucleus of titled derivatives showed a TPSA below 51. The low score of TPSA suggested that these molecule preferentially act as hydrophobic in nature and can easily transport through the blood brain barrier. It can be observed that all the titled thiophene analogues exhibited a good %ABS ranging from 78.83 to 94.63 %.
Table 1. Toxicity prediction of titled derivatives (TTa-TTg).
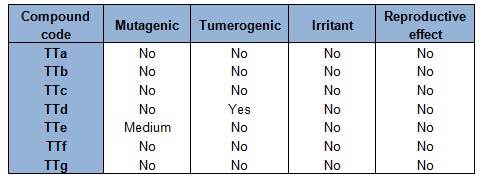
Table 2. Physicochemical properties of titled derivatives (TTa-TTg).
Antidepressant activity
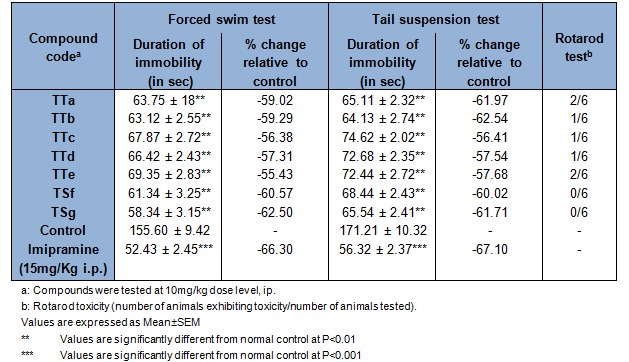
The antidepressant activities for the synthesized derivatives (TTa-TTg) were carried by Force swimming test (FST) and Tail suspension test (TST) in rat at dose of 10 mg/kg in comparison with the standard drug imipramine (15 mg/kg). Antidepressant activity was determined as mean immobility time in seconds and data has been presented as mean ± S.E.M in Table 3(Tab. 3). The results suggested that all the compounds showed good antidepressant potential in the experimental animal models. In the series of thiophene based pyrazoline carbthioamide derivatives, 5-(4-hydroxyphenyl)-3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazole-1 carbothioamide (TTg) reduced immobility time 61.17 and 62.05 % in both FST and TST respectively at 10 mg/kg dose level when compared to the standard Imipramine. The preliminary SAR of newly synthesized carboxamides suggested that the compounds possessing electron-releasing groups such as hydroxy either in the ortho or para and withdrawing chlorine in the 4th position of aromatic rings of the scaffold showed good the antidepressant activity. Neurotoxicity of the compounds were accessed by rotarod test. Interestingly, all the compounds in the series have passed neurotoxicity test.
Table 3. In vivo antidepressant activity and neurotoxicity of the (TTa-TTg) on wistar albino rats.
Conclusion
The present study has proved that the synthesized 5-substituted Phenyl-3-(Thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carbothioamides showed promising antidepressant action and can be potential candidates for the management of depression. The substitution pattern of thiophene based pyrazoline derivatives were carefully selected to confer the effect of substituent in the phenyl system. Thus electron donating groups to phenyl system such as methoxy, dimethyl amino, hydroxyl and electron withdrawing group such as chloro and nitro were chosen as substituents on the chemical structure of the target compounds. Preclinical evaluation for the synthesized derivatives evidence assurance of the drug-likeness property. Moreover, it is extensively reported that the inhibition of MAO-A level in the brain could be used to treat depression state. We hypothesised that the thiophene containing pyrazoline carbothioamides can efficiently knockout the MAO system in mice and have revealed that they exhibit a typical reduction in immobility in the forced swim test by increasing the swimming behaviour. However, to establish the detailed mechanism of these candidates, molecular interaction studies are necessary, which is expected to be carried out separately as an extension of the present study. Moreover all effective 5- substituted phenyl-3-(thiophen-2-yl)-4, 5-dihydro-1H-pyrazole-1-carbothioamides did not show acute neurotoxicity in the rotarod screen.
Acknowledgements
The present work is a part of Ph. D programme by Mr. Bijo Mathew doing in J.N.T.U, Hyderabad, India. The authors are highly thankful to Grace College of Pharmacy for the support they have provided in carrying out the current work. Authors also acknowledge the help of IIRBS, Mahatma Gandhi University, Kottayam & SAIF IIT, Chennai for carrying out the spectral analysis. Our sincere thanks to K.M. College of Pharmacy, Madurai, India for carrying out the pharmacological work.
References
- 1.Aboul-Enein MN, El-Azzouny AA, Attia MI, Maklad YA, Amin KM, Abdel-Rehim M, et al. Design and synthesis of novel stiripentol analogues as potential anticonvulsants. Eur J Med Chem. 2012;47:360–369. doi: 10.1016/j.ejmech.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Chimenti F, Bolasco A, Manna F, Secci D, Chimenti P, Befani O, et al. Synthesis and selective inhibitory activity of 1-Acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole derivatives against Monoamine oxidase. J Med Chem. 2004;47:2071–2074. doi: 10.1021/jm031042b. [DOI] [PubMed] [Google Scholar]
- 3.Gok S, Demet MM, Ozdemir A, Turan-Zitouni G. Evaluation of antidepressant-like effect of 2-pyrazoline derivatives. Med Chem Res. 2010;19:94–101. [Google Scholar]
- 4.Kaplancıklı ZA, Özdemir A, Turan-Zitouni G, Altıntop MD, Can OD. New pyrazoline derivatives and their antidepressant activity. Eur J Med Chem. 2010;45:4383–4387. doi: 10.1016/j.ejmech.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Karuppasamy M, Mahapatra S, Yabanoglu S, Ucar G, Sinha BN, Basu A, et al. Development of selective and reversible pyrazoline based MAO-A inhibitors: Synthesis, biological evaluation and docking studies. Bioorg Med Chem. 2010;18:1875–1881. doi: 10.1016/j.bmc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Jain S, Parle M, Jain N, Kumar P. Aryl-1-phenyl-1H-pyrazole derivatives as new multitarget directed ligands for the treatment of alzheimer’s disease, with acetylcholinesterase and monoamine oxidase inhibitory properties. EXCLI J. 2013;12:1030–1042. [PMC free article] [PubMed] [Google Scholar]
- 7.Mathew B, Suresh J, Anbazhagan S. Antitumor activity of 5-(2E)-1-(1H- benzimidazol-2-yl)-3-substituted phenylprop-2-en-1-ylidene pyrimidine-2, 4, 6 (1H, 3H, 5H) triones against Dalton’s Ascitic Lymphoma in mice. Med Chem Res. 2013;22:3911–3917. [Google Scholar]
- 8.Mathew B, Suresh J, Anbazhagan S, Mathew GE. Pyrazoline: A promising scaffold for the inhibition of Monoamine oxidase. Cent Nerv Syst Agents Med Chem. 2013;13:195–206. doi: 10.2174/1871524914666140129122632. [DOI] [PubMed] [Google Scholar]
- 9.Nayak BV, Ciftci-Yabanoglu S, Singh S, Jagrat M, Sinha BN, Ucar G, et al. Monoamine oxidase inhibitory activity of 3,5-biaryl-4,5-dihydro-1H-pyrazole-1-carboxylate derivatives. Eur J Med Chem. 2013;69:762–767. doi: 10.1016/j.ejmech.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir Z, Kandilci HB, Gumusel B, Calis U, Bilgin AA. Synthesis and studies on anti-depressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur J Med Chem. 2007;42:373–379. doi: 10.1016/j.ejmech.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Palaska E, Aytemir M, Uzbay IT, Erol D. Synthesis and antidepressant activities of some 3, 5-diphenyl-2-pyrazolines. Eur J Med Chem. 2001;36:539–543. doi: 10.1016/s0223-5234(01)01243-0. [DOI] [PubMed] [Google Scholar]
- 12.Parmar SS, Pandey BR, Dwivedi C, Harbison RD. Anticonvulsant activity and monoamine oxidase inhibitory properties of1, 3, 5-trisubstituted pyrazolines. J Pharm Sci. 1974;63:1152–1155. doi: 10.1002/jps.2600630730. [DOI] [PubMed] [Google Scholar]
- 13.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 14.Prasad YR, Rao AL, Prasoona L, Murali K, Kumar PR. Synthesis and antidepressant activity of some 1,3,5-triphenyl-2-pyrazolines and 3-(2-hydroxy naphthalen-1-yl)-1,5-diphenyl-2-pyrazolines. Bioorg Med Chem Lett. 2005;15:5030–5034. doi: 10.1016/j.bmcl.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Salgın-Goksen U, Yabanoglu-Ciftci S, Ercan A, Yelekci K, Ucar G, Gokhan-Kelekci N. Evaluation of selective human MAO inhibitory activities of some novel pyrazoline derivatives. J Neural Transm. 2013;120:863–873. doi: 10.1007/s00702-013-0980-6. [DOI] [PubMed] [Google Scholar]
- 16.Sander T, Freyss J, Korff MV, Reich JR, Rufener C. OSIRIS, an entirely in-house developed drug discovery informatics system. J Chem Inf Model. 2009;49:232–46. doi: 10.1021/ci800305f. [DOI] [PubMed] [Google Scholar]
- 17.Satyanarayana D, Revanasiddappa BC, Nemma KV. Synthesis and biological evaluation of some novel pyrazoline derivatives. Indian J Heterocyclic Chem. 2013;22;353-6. [Google Scholar]
- 18.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 19.Zaho YH, Abraham MH, Le J, Hersey A, Luscombe CN, Beck G, et al. Rate-limited steps of human oral absorption and QSAR studies. Pharm Res. 2002;19:1446–1457. doi: 10.1023/a:1020444330011. [DOI] [PubMed] [Google Scholar]


