Abstract
Since, we previously demonstrated that sequentially extracted methanolic fraction showed marked antioxidant and antidiabetic property in vitro, the present study was design to evaluate the beneficial effects of Phyllanthus virgatus methanolic extract and its partially purified fraction on hyperglycemia and hyperlipidemia in streptozotocin (STZ) induced diabetic rats. The plant extract was subjected to repeated thin layer chromatographic fractionation followed by GC-MS analysis of active fraction. TLC data illustrated the presence of six prominent bands and the prelimnary screening of these bands against α-amylase inhibitory activity showed that the band with Rf value 0.514 has marked inhibitory property (IC50, 48 µg/ml). The diabetic rats were treated for four weeks with methanolic extract of P. virgatus (50 and 10 mg/rat/day), partially isolated active fraction (0.5 and 0.1 mg/rat/day) and glibenclamide (0.1 mg/rat/day). The level of fasting blood glucose (FBG), hemoglobin, glycated hemoglobin (HbA1c) and insulin were significantly alleviated in plant extract and partially purified fraction treated group after 28 days of administration. Moreover, total cholesterol (TC), triglycerides (TG), low density lipoprotein-cholesterol (LDL-C), very low density lipoprotein-cholesterol (VLDL-C) and high density lipoprotein cholesterol (HDL-C) were also markedly ameliorated in the entire treatment group, with a maximum restoration observed in group treated with partially purified fraction (0.5 mg/rat/day). The results demonstrate a strong antidiabetic and hypolipidemic impact of plant extract and its partially purified fraction coupled with their potent antioxidative property, which can provide additional benefits in the inhibition of oxidative stress and hence in the prevention and treatment of diabetes as well as diabetes linked hyperlipidemia.
Keywords: glucose, insulin, hyperlipidemia, Phyllanthus virgatus
Introduction
Diabetes mellitus is reaching as epidemic proportion across the globe, a metabolic disorder characterize by hyperglycemia resulting from defect in insulin secretion, insulin action or both (ADA, 2009[3]). Currently, there are 40 million people with diabetes in India estimated to rise to almost 70 million by 2025 (IDF, 2006[29]). Hyperglycemia is the most important factor in the onset and progress of diabetic complications mainly by producing oxidative stress (Giugliano et al., 1996[20]). Altered cellular metabolism caused by hyperglycemia has been suggested to play an important role in increasing the risk of cardiovascular, renal, ophthalmic and neurological complications of diabetes mellitus (Brownlee and Cerami, 1981[10]). The pancreatic ß cells posse the ability to respond to the minor change in the plasma glucose level and maintain the homeostasis of glucose level (Hellestrom, 1977[25]). The continuous destruction of ß-cell leads to the disturbance in glucose homeostasis. Liver and pancreas are the two main organs which plays important role in maintain the level by regulating alternative pathways between glucose uptake and gluconeogenesis (Ferre et al., 1996[16]).
The excessive non enzymatic glycosylation of proteins including low density lipoprotein (LDL), associated with substantially increased superoxide, hydrogen peroxide, and hydroxyl radical production, stimulate formation of advanced glycosylation end products and foam cells, which cause extensive cellular and tissue damage including vascular injury (Lyons, 1991[41]). Since, most animal studies of diabetes are induced by administration of streptozotocin (STZ) or alloxan, toxic agents that target ß-cells, they are thus essentially considered as models of type 1 diabetes at higher doses or type 2 diabetes at lower doses. Nevertheless, such animals show increased lipid peroxidation, hyperlipidemia and other diabetic complications seen in diabetes (Kaji et al., 1985[32]). Lipids play a major role in maintaining the integrity of the biomembrane and maintain its structure and function. Due to insulin deficiency, alteration in lipid and lipoprotein associate diabetes with the higher chance of development of pathogenesis atherosclerosis. The dyslipidemic profile of diabetics includes increased levels of triglyceride (TG), total cholesterol (TC), very low density lipoprotein (VLDL-C) and low density lipoprotein (LDL-C), increased small dense (sd-LDL), glycation of LDL and decreased plasma high density lipoprotein (HDL) concentration (Grundy et al., 1999[21]). Study indicates that altered plasma lipoprotein profile in the excess atherosclerosis associated with diabetes may be most critical, because at any TC level, diabetics have 3-to 5-fold higher coronary artery disease (CAD) mortality rates than do non diabetic subjects (Steiner, 2001[61]).
It has been found that in the developing countries where the resources are meager, the best source for the management of diabetes mellitus is green medicine (Bnouham et al., 2006[7]). It has been attributed that there antihyperglycemic potential is due to their restoring power of ß-cell, smoothing glucose fluctuation, increase insulin production and competing with the enzymes to manipulate carbohydrate metabolism (Elder, 2004[15]). Natural products, such as plants extract, either as pure compounds or as standardized extracts, provide unlimited opportunities for new drug discoveries because of the unmatched availability of chemical diversity. We previously published that Phyllanthus virgatus Forst (Eurphobacea) commonly known as Bhuiamla, has great potential as an antioxidant and antihyperglycemic agent in vitro (Hashim et al., 2013[23]). Phyllanthus has been used in Ayurvedic medicine for over 2,000 years and has a wide number of traditional uses. This family includes several plant species among all the species; P. amarus, P. urinaria, P. maderaspatensis and P. fraternus are the most popular ones due to their antioxidant properties as well as their extensive use in the treatment of disease related to kidney, liver, urinary bladder, intestinal infection, cancer, and diabetes (Kirtikar and Basu, 1933[35]; Girach et al., 2007[19]). P. virgatus is also known traditionally for its remedial properties and extensively known for antioxidant property and used in the treatment of intestinal, liver, kidney and bladder problem (Hashim et al., 2013[23]; Shabeer et al., 2009[58]; Kirtikar and Basu, 1933[35]; Calixto et al., 1998[12]; Kumaran and Joel Karunakaran, 2007[40]). P. virgatus is also rich in tannins, flavonal sulfonates, norlignan compounds (Huang et al., 1998[28]), lignans (Huang et al., 1996[27]), hypophyllanthin, isointetralin, nirathin, nirtetralin, phyltetraline, virgatusui, lactone and acids like indole-3-carboxylic acid (Calixto et al., 1998[12]), 2,4,5- trimethoxy propenylbenzene, 11-octadecenoic acid, 9,12-octadecadienoic acid, hexadecanoic acid, benzenedicarboxylic acid, tridecyl ester and 6-octadecynoic acid (Hashim et al., 2013[23]). Based on our previously published data, which delineated the in vitro antioxidant, genoprotective and -amylase inhibitory property of methanolic extract of P. virgatus, the current study was intended to investigate hypoglycemic and hypolipidemic property of P. virgatus methanolic extract and its partially isolated fraction in STZ induced diabetic-hyperlipidmic rats.
Material and Method
Solvent and chemicals
Streptozotocin (STZ) was procured from Himedia Laboratories, Mumbai, India. Porcine pancreatic α-amylase was procured from SRL Pvt. Ltd., Mumbai, India. Merckotest glucose oxidase peroxidase (GOD/POD) kit, insulin kit, TC, TG kits were obtained from Merck, India. Acarbose was procured from Bayer Pharmaceuticals and glibenclamide was obtained from Sun Pharmaceuticals. All other chemicals and solvents were of analytical grade.
Plant material and extraction procedure
P. virgatus Forst whole plant was collected from the local area around Integral University, Lucknow, India, in the months of July-August. The plant was botanically identified and authenticated by Dr. Mohd. Tariq, of National Botanical Research Institute (NBRI), Lucknow, India and a voucher specimen (98195) of the plant was submitted at NBRI. In order to obtain methanolic fraction, sequential extraction of P. virgatus whole plant was done according to the method of (Hashim et al., 2013[23]).
Isolation of bioactive fraction from P. virgatus methanolic extract
The plant extract was subjected to thin layer chromatography (TLC) in order to separate the bioactive compounds according to the procedure of Harborne (1988[22]). Using a microcapillary tube, a small drop of methanolic extract of the plant extract was placed on the TLC plate, 3 cm above the bottom. The chromatogram was developed with chloroform: methanol (9:1) and sprayed with 10 % H2SO4 and heated at 120 °C for the detection of organic components. The Rf values of the spots were calculated by the formula[img:Bild 8]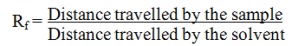 .
.
In view to isolate the bioactive fraction, repetitive preparatory TLC was prepared as described above. The bands were eluted separately with methanol and filtered free of silica gel, dried (Gayatri et al., 2011[18]) and tested for α-amylase inhibitory property.
α-amylase inhibition assay
To determine the in vitro α-amylase inhibition by various TLC fraction of P. virgatus, the procedure of Bernfeld (1955[6]) was adopted with slight modification (Hashim et al., 2013[23]). Acarbose was used as standard inhibitor.
Inhibition rates were calculated as percentage controls using the formula: % Inhibition=100 - % reaction (where % reaction = mean product in sample/mean product in control x 100).
Further, IC50 value represented the concentration of the extract that caused 50 % inhibition of α-amylase and was calculated by interpolation of linear regression analysis.
Gas chromatography and mass spectroscopy (GC-MS) analysis
In order to know the bioactive metabolites responsible for antioxidant, antidiabetic and antihyperlipidemic activity, the partially isolated fraction of P. virgatus methanolic extract was subjected to GC-MS analysis. The sample was injected into a RTX-5 col-umn (60 m x 0.25 mm i.d., film thickness 0.25 µm) of GC-MS (model GC-MS-QP-2010 plus, Shimadzu Make). Helium was used as carrier gas at a constant column flow 1.2 ml/min at 173 kpa inlet pressure. Temperature programming was maintained from 100 °C to 200 °C with constant rise of 5 °C/min and then held isothermal at 200 °C for 6 min; further the temperature was increased by 10 °C/min up to 290 °C and again held isothermal at 290 °C for 10 min. The injector and ion source temperatures were 270 °C and 250 °C, respectively. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 40 to 950 Dalton. The final confirmation of constituents was made by computer matching of the mass spectra of peaks with the National Institute of Standards and Technology (NIST) libraries mass spectral database.
Dose preparation
Sequentially extracted P. virgatus methanolic extract and its partially purified fraction with different concentrations were dissolved in 5 % dimethyl sulfoxide and homogenized with saline. The doses of extract were selected on the basis of previously published reports (Shabeer et al., 2009[58]). The reference drug glibenclamide was suspended in distilled water for oral administration. The animals in control group received buffer only.
Animals
Male wistar rats weighing between 180-220 g were procured from the Indian Institute of Toxicology Research Center, Lucknow. This study has been dully approved by Institutional animal ethics committee (IAEC) vide registration number: IU/Biotech/project/ CPCSEA/13/14. The animals were fed on normal standard pellet diet with water ad libitum, and acclimatized to the laboratory conditions for a week. Prior to experimental treatment, animals were fasted an overnight, but were allowed free access to water.
Induction of Diabetes mellitus
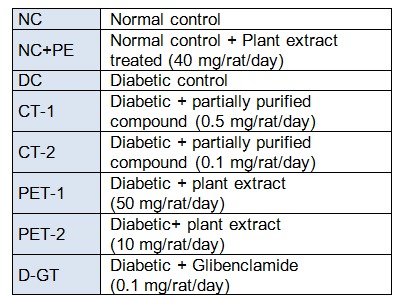
In order to induce experimental diabetes, thirty overnight fasted rats were injected with STZ, freshly dissolved in 10 mM citrate buffer, pH 4.5, 60 mg/kg body weight) intraperitonially (Zhang et al., 2004[69]). Rats in normal control group were injected with buffer only. The fasting blood glucose (FBG) level was determined after 48 h of STZ injection. The rats depicting FBG = 230 mg/dl were considered to be diabetic for investigating the hypoglycemic and hypolipidemic impacts and were divided randomly and equally (5 rats in each group) in groups as illustrated in Table 1(Tab. 1). Plant extract, its isolated fraction and glibenclamide suspension was administered through gastric intubation in two divided doses (morning and evening) of 0.5 ml each/rat/day for 28 days.
Table 1. Protocol for the treatment of STZ induced diabetic rats.
Analytical procedures
Collection of blood and packed erythrocytes
At the end of the treatment, overnight fasted rats in each group were anaesthetized and blood was drawn by cardiac puncture. The blood from each rat in a given group was collected using heparin as anticoagulant. Blood was mixed gently by inversion 2-3 times and immediately cooled to 4 °C in a refrigerator. The samples were centrifuged at 2,500 rpm for 30 min. Plasma was aliquoted and either stored at 4 °C or frozen at -20 °C for future use.
Determination of blood glucose
Fasting plasma glucose levels were determined by using GOD-POD kit by Merck, India. The method uses a modified Trinder color reaction (Trinder, 1969[66]; Tietz, 1976[65]). The color thus formed was measured at 505 nm in Eppendorf spectrophotometer, which is directly proportional to the concentration of glucose in the sample. The plasma glucose level was calculated by using a standard glusose.
Determination of glycosylated hemoglobin
The quantitative determination of glycosylated hemoglobin (HbA1c) in erythrocytes hemolysate was done by the colorimetric method of Nayak and Pattabiraman (1981[44]). Fructose was used as standard.
Determination of plasma insulin
Plasma insulin level was measured using solid phase-conjugated sandwich rat ELISA kit Merck Millipore, USA (Pitchai et al., 2009[49]).
Determination of plasma triglycerides
Triglycerides in plasma samples were determined by using glycerol-3-phosphate oxidase-peroxides (GPO-POD) kit from Merck, India. The method uses a modified Trinder color reaction to produce a fast, linear, end point reaction (Trinder, 1969[66]). The intensity of the color produced is directly proportional to the concentration of triglycerides in the sample when measured at 505 nm and results were expressed as mg/dl.
Plasma very-low-density lipoprotein (VLDL-C) was determined by dividing plasma triglyceride values (mg/dl) by a factor of 5 as described by Friedewald et al. (1972[17]).
Fractionation of plasma LDL and HDL
Plasma LDL was isolated by precipitation method as described by Wieland and Seidel (1983[67]). The precipitation buffer consisted of 64 mM trisodium citrate adjusted to pH 5.05 with 5 N HCl, containing 50,000 IU/L heparin. Before precipitation of LDL, plasma samples and precipitation reagent were allowed to equilibrate to room temperature. One ml of plasma sample was added to 7.0 ml of heparin-citrate buffer. After mixing with a vortex mixer, the suspension was allowed to stand for 10 min at 22 °C. The insoluble LDLs were then sedimented by centrifugation at 1,500 rpm for 10 min at 22 °C. The pellet was resuspended in 1.0 ml of 0.1 M sodium phosphate buffer, pH 7.4, containing 0.9 % NaCl. Isolation of HDL was done by the method of Patsch et al. (1989[48]). According to this method 100 µl of reagent containing 1.0 g of dextran sulfate dissolved in 100.0 ml of 0.5 mM MgCl2 solution was added to 1.0 ml of plasma, and vortexed-mixed for 3 sec. After an incubation period of 10 min at 22 °C, the mixture was centrifuged at 1500 rpm for 15 min at 22 °C and the supernatant containing HDL was removed. The clear supernatant was used for the analysis of HDL-Cs.
Determination of plasma cholesterol
Total cholesterol in plasma, LDL and HDL was determined by using cholesterol oxidase phenol aminophenazone (CHOD-PAP) kit, from Merck India. The intensity of the color produced is directly proportional to the concentration of cholesterol in the sample when measured at 505 nm and results were expressed as mg/dl.
Statistical analysis
The data was put across the mean ± SD. For statistical analysis of the data, group means were compared by one way analysis of variance (ANOVA) followed by Dunnett´s ´t´test which are used to identify the difference between groups. Statistical significance was expressed as *P < 0.05, **P < 0.01 and ***P < 0.001.
Results
Identification, isolation and α-amylase inhibitory property of bioactive fraction
Our previously published data illustrated that among the sequentially extracted fractions of P. virgatus, methanolic extract exhibited significant and most potent antioxidant, genoprotective and antidiabetic property in vitro (Hashim et al., 2013[23]). Therefore, in the present investigation we aim to isolate and identify the bioactive compound present in this methanolic extract and also evaluate the in vitro and in vivo antidiabetic property. In this context, partial purification and isolation was performed by repetitive preparatory TLC. The results presented in Figure 1(Fig. 1) depicted the presence of six prominent bands (1-6) with Rf value of 0.16, 0.28, 0.42, 0.541, 0.71 and 0.76. The bands were scrapped out, eluted and initial screening of various bands for in vitro α-amylase inhibitory property showed that the 1st, 4th and 6th band with Rf value of 0.76, 0.514 and 0.16 depicted significant inhibition 29.2 %, 62.11 % and 17.29 % respectively at 100 µg/ml respectively, while no significant inhibition was observed in other bands. Moreover, the 4th band (Rf 0.514) illustrated a significant IC50 value of 47.5 ± 0.56 µg/ml, which was better than the standard drug acarbose (76.88 ± 0.277 µg/ml) (Table 2(Tab. 2)). The above results confirmed that this band has most potent α- amylase inhibitory property and was subjected to GC-MS analysis for the identification of bioactive compound. Preliminary GC-MS analysis, based on retention tine and molecular mass was performed to determine the nature of phytoconstituents present in partially purified band. The GC-MS spectral results and comparison of results with library search successfully enable the identification of four compounds as illustrated in Figure 2(Fig. 2). The results revealed that hexadecanoic acid (51.11 %) and octadecanoic acid (26.64 %) were found to be the two major components in the partially isolated band, whereas 9-Octadecenoic acid (14.39 %) and benzoic acid (7.877 %) were found in less amounts (Table 3(Tab. 3)). The methanolic extract and bioactive fraction was further subjected to in vivo study.
Figure 1. TLC profile of P. virgatus methanolic extract.
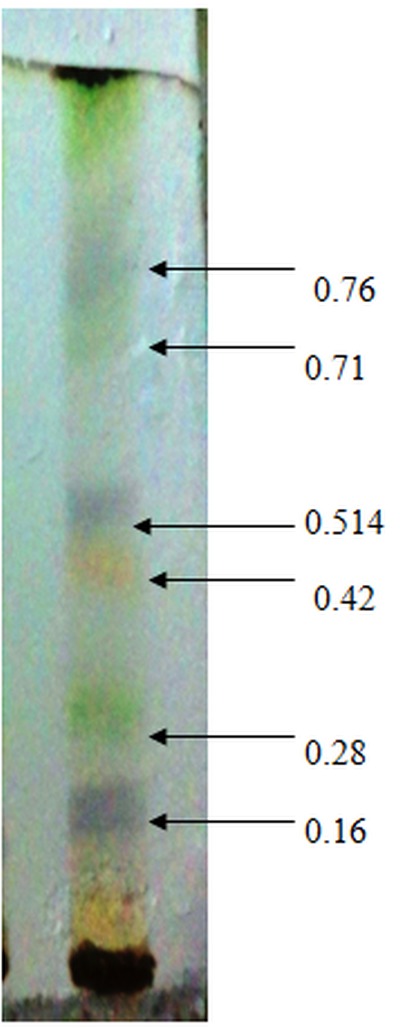
Table 2. Percentage of α-amylase inhibitory activity of various fractions of P. virgatus isolated via TLC.
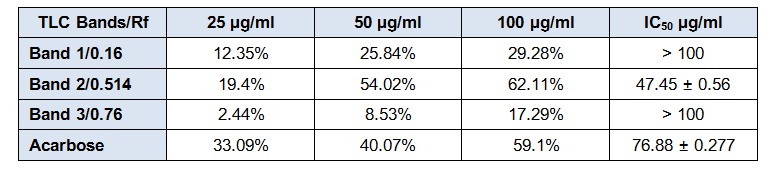
Figure 2. GC-MS analysis of isolated TLC band of P. virgatus methanolic extract.
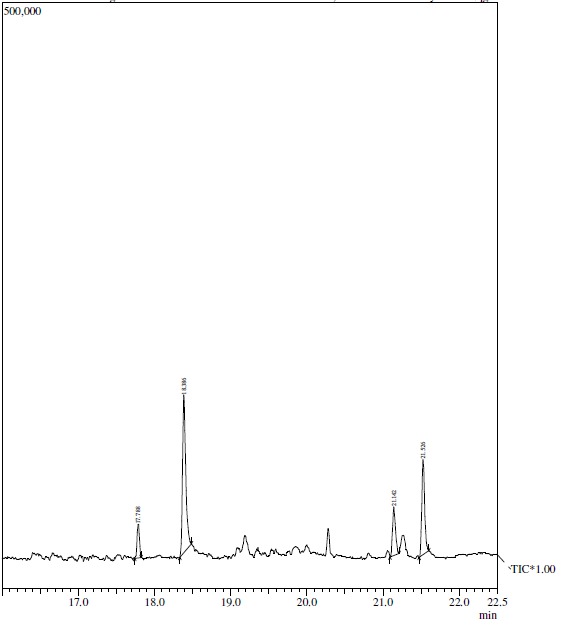
Table 3. Bioactive compounds identified via GC/MS analysis from the TLC fraction of P. virgatus methanolic extract.
Impact of P. virgatus ethanolic extract, its partially isolated fraction and glibenclamide on body weight, plasma glucose, insulin, blood hemoglobin and glycated hemoglobin in diabetic rats treated for 28 days
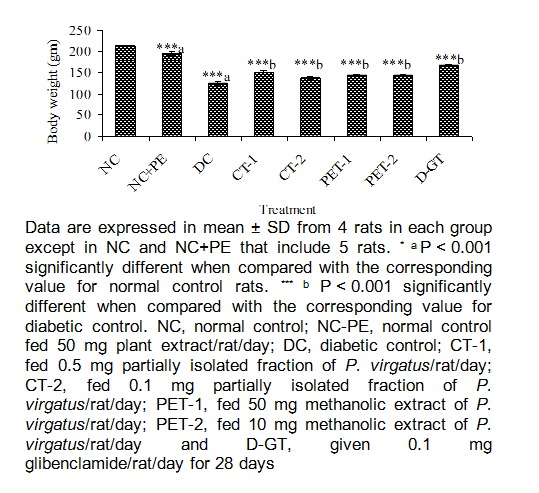
Results presented in Figure 3(Fig. 3) indicate the average body weight of diabetic rats after 28 days, of treatment with plant extract, its partially purified fraction and glibenclamide. After 28 days the body weight of diabetic rats was progressively decreased (41.5 %), as compared to normal control group. On the other hand, oral administration of different doses of P. virgatus methanolic extract and its partially purified fraction showed significant gain in body weight of diabetic rats when compared to DC rats, with a maximum increase of 34 % in glibenclamide treated rats followed by CT-1 (20 %) group.
Figure 3. Impact of P. virgatus methanolic extract, its partially purified fraction and glibenclamide on the body weight of diabetic rats after 28 days of treatment.
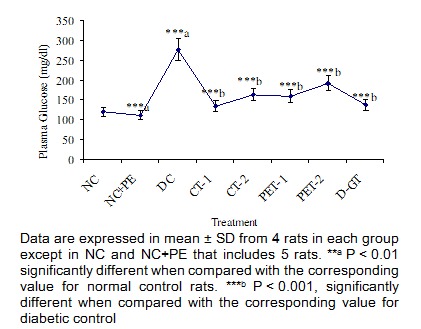
At end of experiment (28 days) plasma glucose level in DC group was further increased from initial value of 119 mg/dl to 277 mg/dl (Figure 4(Fig. 4)). In the entire treated group, plant extracts and its partially purified compound mediated a significant decrease of 52 %, 41 %, 42 % and 30 % respectively, in plasma glucose level of rats in CT-1, CT-2, PET-1 and PET-2 groups. However, a highly significant decrease in glucose level was observed in diabetic rats treated with partially purified compound which was almost comparable to the value obtained in glibenclamide treated diabetic rats.
Figure 4. Effect of P. virgatus methanolic extract, its partially purified fraction and glibenclamide on plasma glucose level in diabetic rats after 28 days of treatment.
As expected, after 28 days, Hb level in DC rats was significantly reduced (38 %) in comparison to rats in NC group. However, a highly significant increase in Hb concentration was observed in diabetic rats treated with partially purified compound, CT-1 (54 %) and CT-2 (51 %), whereas the methanolic extract treated rats also observed a marked increase in Hb concentration, when compared to DC rats (Table 4(Tab. 4)). Both partially purified fraction (CT-1) and glibenclamide (D-GT) administration to diabetic rats mediated an increase in Hb level closed to normal control value.
Table 4. Impact of P. virgatus methanolic extract, its partially purified fraction and glibenclamide on blood hemoglobin, its glycosylated form and plasma insulin in diabetic rats after 28 days of treatment.
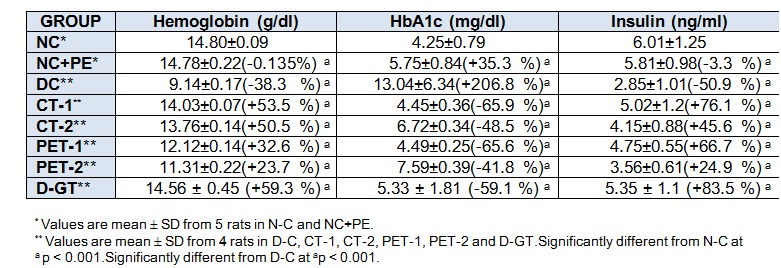
The level of HbA1c which is an index for mean blood glucose level for the previous 2-3 months, was significantly increased by 207 % in DC rats when compared to a value of 4.25 mg/dl of NC rats. After 28 days of treatment the mean HbA1c level was significantly decreased by 66 %, 48 %, 66 % and 42 % in CT-1, CT-2, PET-1 and PET-2 groups, respectively, when compared to values of DC rats. Our results also illustrated that plasma insulin level in DC rats was significantly reduced to 51 % in comparison to NC rats. On repeated admistration of P. virgatus methanolic extract and its partially isolated fraction a marked increase in insulin level of CT-1 (76 %), CT-2 (46 %), PET-1 (67 %) and PET-2 (83 %) group was observed when compared to DC group (Table 4)(Tab. 4). These results demonstrated that among all the treated groups, partially purified fraction CT-1 and CT-2 at a dose of 0.5 mg/rat/day and 0.1 mg/rat/day act as a potent hypoglycemic agent in diabetic rats.
Effect of P. virgatus methanolic extract, its partially purified fraction and glibenclamide on plasma lipid and lipoprotein levels in diabetic-hyperlipidemic rats treated for 28 days
The induction of experimental diabetes in rats by STZ had a reflective impact on lipid parameter when compared to normal rats. As shown in Figure 5(Fig. 5), TG, TC and non-HDL-C level were significantly increased from 88, 170 and 93 mg/dl in NC rats to 154, 235 and 196 mg/dl, respectively, in DC rats. After 28 days of treatment, level of TG, TC and non-HDL-C were significantly decreased in all the treated groups with a maximum decrease of 25 %, 38 % and 44 % observed in CT-1 group, when compared to the corresponding DC values. The results demonstrated that feeding of plant extract PET-1 (50 mg/rat/ day), PET-2 (10 mg/rat/day) and glibenclamide was associated with a significant reduction in lipid parameters, however the reduction was less as compared to CT-1 group, which was more effective in reducting above lipid parameter, particularly TG levels. As seen in Figure 6(Fig. 6), all the plasma lipoprotein lipids including atherogenic LDL-C were significantly increased in DC group, when compared to NC values. Our results showed that LDL-C, HDL-C and VLDL-C level were significantly increased from 76, 24 and 18 mg/dl in NC to 165 (117 %), 40 (68 %) and 31 (76 %) mg/dl, respectively in DC rats.
Figure 5. Effect of P. virgatus methanolic extract, its partially purified fraction and glibenclamide on plasma TG, TC and non-HDL-C level in diabetic rats after 28 days of treatment.
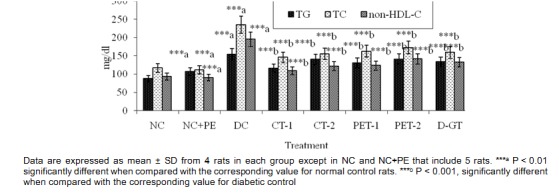
Figure 6. Effect of P. virgatus methanolic extract, its partially purified fraction and glibenclamide on plasma lipoproteins levels in diabetic-hyperlipidemic rats after 28 days of treatment.
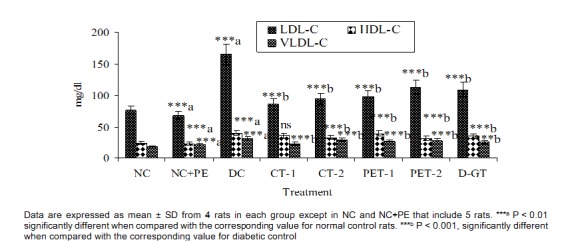
Treatment with P. virgatus methanolic extract, its partially isolated fraction and glibenclamide for 28 days showed a significant reduction of 48 %, 43 %, 41 %, 32 % and 34 % in LDL-C level of CT-1,CT-2, PET-1, PET-2 and D-GT group, respectively when compared to corresponding values in DC groups. In comparison to DC group, the decline in HDL-C level was 8 %, 17 %, 1 % and 21 %, respectively, in CT-1, CT-2, PET-1 and PET-2 group. The VLDL-C level of DC rats was also significantly increased from 18 mg/dl in NC to 31 mg/dl (76 %). All the five treated groups also exhibited a significant reduction in VLDL-C level with a maximum reduction of 24 % observed in CT-1 groups. These results exemplify that decline in plasma lipid and lipoprotein level after 28 days of treatment with P. virgatus methanolic extracts and its partially purified fraction was due to significant decrease in LDL-C level.
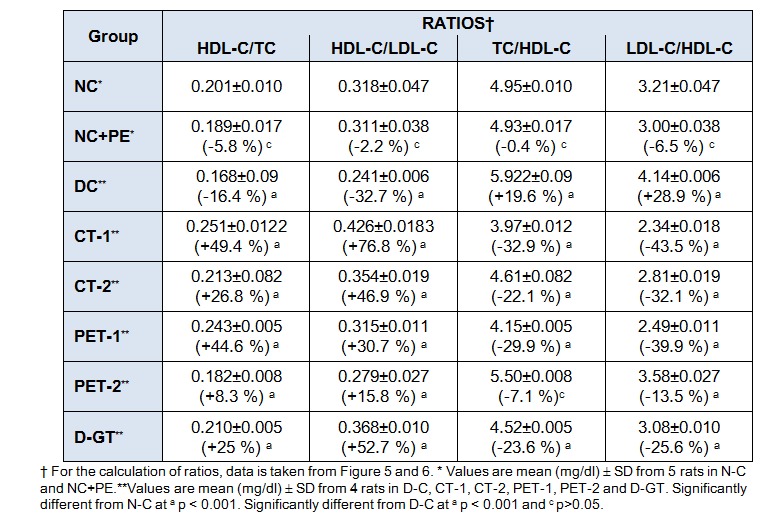
Since TC, LDL-C and HDL-C level were significantly increased in DC group and differentially decreased following treatment of diabetic-hyperlipidemic rats with plant extract and its partially purified fraction. A consistent pattern was observed in all the ratios, TC/HDL-C, HDL-C/LDL-C ratio was significantly increased by 20 % and 29 % in DC rats when compared to the control rats (Table 5(Tab. 5)). After treatment with plant extract and its partially purified fraction these ratios were significantly decreased in all the treated groups, with maximum reduction in CT-1 groups. In addition, HDL/TC and HDL-C/LDL-C ratios were significantly decreased in diabetic rats, which were significantly ameliorated after treatment with plant extract and its partially purified fraction. Here it is noteworthy to mention that these ratios which are predictor for cardiovascular disease risk were significantly and markedly restore after treatment of plant extract and partially purified fraction with a marked restoration in CT-1 group at a dose of 0.5 mg/rat/day, when compared to other treated groups.
Table 5. Impact of P. virgatus methanolic extract, its partially purified fraction and glibenclamide on the ratio of HDL-C/TC, HDL-C/LDL-C, TC/HDL-C and LDL-C/HDL-C in diabetic-hyperlipidemic rats after 28 days of treatment.
Discussion
The rationale behind this work is based on our previously published data which illustrated that among the sequentially extracted various fraction of P. virgatus, methanolic extract showed the most potent antioxidant, genoprotective and α-amylase inhibitory activity. Further, the extract showed enhanced glucose uptake in pre-adipose 3T3-L1 cell line and it was also non cytotoxic. The prelimnary GC-MS analysis of this extract revealed several bioactive compounds and their inhibitory mechanism was validated via in silico analysis (Hashim et al., 2013[23]). In the present study this methanolic extract was subjected to repetitive preparatory TLC in order to identify and isolate the partially purified fraction as well as to examine their role in STZ induced diabetes and diabetes linked hyperlipidemia in rats. Previous study also demonstrated the use of TLC mediated isolation of partially purified fraction for in vivo study (Gayathri et al., 2011[18]). Our TLC data illustrated the presence of six prominent bands and the preliminary screening of these bands against α-amylase inhibitory activity showed that the 4th band has marked inhibitory property (IC50 48 µg/ml), which depicted that this fraction might be responsible for the antidiabetic activity of sequentially extracted methanolic fraction. Our initial screening for the identification of bioactive compound in this fraction, via GC-MS analysis, showed the presence of benzoic acid, hexadecanoic acid, 9-octadecanoic acid and octadecanoic acid compounds, which are in agreement with the previously published reports indicating the presence of these compounds with antioxidant and antidiabetic properties (Kumar et al., 2010[39]; Terpstra, 2004[64]). Among these compound hexadecanoic acid has already been known to play important role in biological process (Aleryani et al., 2005[2]).
STZ is known to induce diabetes in animal models along with hyperlipidemia/atherosclerosis, diabetic nephropathy, retinopathy, and neuropathy. STZ mediated toxicity is might be due to their proposed site of action at nuclear DNA. The decomposition of STZ leads to formation of highly reactive carbonium ions, which cause DNA bases alkylation and also damage pancreatic β-cell membrane and break the DNA strand which guide to the activation of poly (ADP-ribose) synthetase and NAD depletion, which ultimately leads to cell death (Portha et al., 1989[50]). It has been previously reported that STZ selectively destroy the pancreatic cells that secrete insulin and results in substantial hyperglycemia as well as also result in weight loss, ketosis and a high rate of mortality (Arulmozhi et al., 2004[4]; Szkudelski, 2001[62]). Since, it has been shown that hyperglycemia is directly associated with decreased body weight of diabetic animals (Zafar and Naqvi, 2010[68]), our data is in agreement with this study and also demonstrated a decline in body weight in diabetic rats, that could be due to muscle destruction or degeneration of structural proteins (Salahuddin and Jalalpure, 2010[56]). Repeated oral administration of methanolic extract and its partially isolated fraction showed gain in body weight. Among the treated group, CT-1 group showed maximum gain in body weight followed by CT-2 > PET-1 > PET-2 groups which may be due to the reversal of hepatic gluconeogenesis accompanied with the suppression of lipolysis of adipose tissue (Robertson, 2007[54]; Postic et al., 2001[51]).
The present data revealed that fasting plasma glucose level was significantly increased and insulin level was decreased in STZ induced diabetic rats in comparison to rats in NC group. Among all the treatment groups, the partially isolated fraction (CT-1 group) of plant extract at a dose of 0.5 mg/ rats/day for 28 days showed a significant decrease in the plasma glucose level and increase in insulin level. The plant extract and partially purified fraction being a potent free radical scavenger and inhibitor of lipid peroxidation (Hashim et al., 2013[23]), might prevents STZ-induced oxidative stress and protects or regenerate insulin producing β-cells resulting in increased insulin secretion and decreased blood glucose levels. At present, the finding of this study is in agreement with previously published data which illustrated the antioxidant and antihyperglycemic effect of Phyllanthus simplex crude methanol extract (Shabeer et al., 2009[58]).
The extent of hemoglobin glycation is currently used as a cumulative index of glycemia over the previous few weeks in the clinical management of diabetes (Saudek and Rastogi, 2004[57]). In diabetes, there is a formation of advanced glycation end products when glucose interact with various proteins such as hemoglobin, albumin, collagen, LDL, or crystalline proteins to form labile Schiff bases, which further undergoes modification to form Amadori products (Klein, 1995[36]; Singh et al., 2001[60]; Ahmad et al., 2013[1]). Evidence showed that glycation itself may induce the formation of oxygen-derived free radicals in diabetic condition, and the level of HbA1c is considered as one of the markers of degree of oxidative stress in diabetes mellitus (Bravi et al., 2006[9]). Therefore, the measurement of HbA1c is supposed to be a very sensitive index for glycemic control (Norris, 2002[45]). Our results demonstrated a substantial decrease in hemoglobin and a significant increase in HbA1c levels in diabetic rats, which is in agreement with earlier finding (Nathan et al., 2007[43]). In the present investigation, rats in DC group showed higher levels of HbA1c, compared with those of control group. After 28 days of treatment with P. virgatus methanolic extract and its partially isolated fraction, both Hb and HbA1c levels were significantly ameliorated close to normal control values with a marked restoration in CT-1group (54 & 66 %), which could be due to an improvement in hyperglycemia. Present findings bear a resemblance with previously published data that showed the treatment of Phyllantus sellowianus (Hnatyszyn et al., 1997[26]) and Phyllanthus emblica ethanolic (Krishnaveni et al., 2010[38]) extracts to STZ induce diabetic rats significantly ameliorated blood glucose and HbA1c level.
Since, diabetes mellitus is related with increased propensity to accelerated atherogenesis, and is directly correlated with abnormalities in lipid profile, which are one of the major contributing causes of micro and macrovascular complications in diabetic patients (Ono et al., 1998[47]; Giugliano et al., 1996[20]). Oxidative stress also plays an important role in diabetes that results in increased glycation and increased oxidation of lipoprotein (Bowie et al., 1993[8]), particularly LDL, which could be one of the mechanisms for an early development of atherosclerosis in diabetes. Thus, it is worthwhile to evaluate the role of this plant and their bioactive fraction in the prevention of hyperlipidemia, which is directly related with STZ-induced diabetes in rats. As expected, our data showed a marked increase in TG, TC, and non-HLD-C levels in diabetic-hyperlipidemic rats when compared to normal control rats.
The observed increase in cholesterol level of diabetes was may be due to increase in 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase activity and is in concordant with several reports that showed increase HMG-CoA reductase activity in diabetic rats (Prince and Kannan, 2006[52]; Rydgren and Sandler, 2009[55]). This increase in cholesterol level has been shown to be associated with the deficiency in insulin which can be due to enhanced mobilization of lipid from adipose tissue to plasma (Karthikesan et al., 2010[33]).
After 28 days of treatment with plant extract and partially purified fraction at different doses, a significant reduction was observed in TG, TC and non-HDL-C level. Similarly, VLDL-C, LDL-C and HDL-C levels were significantly increased in diabetic rats, which were significantly reduced after treatment with plant extract, partially purified compound or glibenclamide. Our data showing an increase in plasma HDL-C level in DC group is consistent with other reports, where a significant increase in HDL-C level in diabetic rats has been reported (Ebara et al., 1994[14]; Kobayshi et al., 2000[37]). Further, glibenclamide treated group also depicted reduction in plasma lipid and lipoprotein level and is consistent with earlier findings (Ratzmann et al.,1983[53]; Tamai et al., 1981[63]), but the reduction was much less as compared to CT-1 & CT-2 group. Moreover glibenclamide is associated with severe side effects like mental status, seizure, coma and death (Burge et al., 1998[11]; Asplund et al., 1983[5]; Sills et al.,1997[59]).
It has been established that LDL-C/HDL-C and HDL-C/TC ratios are good predictors for the presence and severity of CAD (Drexel et al., 1992[13]). In addition to LDL-C/HDL-C and HDL-C/TC ratios, we have also presented the ratios of HDL-C/LDL-C and TC/HDL-C, which were markedly increased in D-C rats, respectively, in comparison to the corresponding ratios in N-C rats, as well as these data are in resemblance with previous reports (Heidarian and Soofiniya, 2011[24]). Treatment with plant extract and partially purified fraction resulted in a significant improvement in these ratios, indicating normalization of above lipid and lipoprotein lipid parameters. The hypolipidemic activity of plant extract and partially purified fraction might be attributed to the decrease in HMG-CoA reductase activity which could be due to an increase in insulin activity along with an associated amelioration of both glucose and lipid levels. There are several in vitro and in vivo reports which indicated that reduction in the enzymatic activity of HMG-CoA reductase by plant extract is directly associated with decrease in plasma lipid and lipoprotein levels (Iqbal et al., 2014[31], [30]; Khan et al., 2011[34]). Here, it is interesting to mention that same fraction (CT-1) which illustrated most potent hypoglycemic activity, also showed remarkable hypolipidemic property in vivo. These finding are in concordant with previously published data which illustrated the hypolipidemic activity of various species of phyllanthus in STZ induced diabetic rats (Okoli et al., 2010[46]; Mbagwu et al., 2011[42]). Thus, in addition to glycaemic control, extract of this plant may further reduce mortality from complications of the disease by ameliorating diabetes-induced hyperlipidemia. This profound activity of CT-1 and CT-2 group might be attributed to the synergistic effect of bioactive compounds viz. hexadecanoic acid and octadecanoic acid compound, present in this fraction.
Conclusion
From the data presented above, it has been extracted that methanolic extract of this plant and their partially purified fraction exert potent hypoglycemic and hypolipidemic activity. Moreover, the methanolic extract at higher dose (50 mg/rat/day) gave good response, while the partially purified fraction at a dose comparable to standard (0.5 mg/ rat/day and 0.1 mg/rat/day) illustrated improved hypoglycemic and hypolipidemic activity than the glibenclamide treated diabetic rats. Further, purification of this fraction and specific structure elucidation is necessary for development of hypoglycemic and hypolipidemic drug. The combined results has undoubtedly provided scientific confirmation and evidence for the use of P. virgatus methanolic extract and its partially purified fraction and demonstrate that strong hypoglycemic, hypolipidemic and antidiabetic impacts of this plant coupled with their potent antioxidative property, can provide additional benefits in the inhibition of oxidative stress and hence in the prevention and treatment of diabetes linked hyperlipidemia.
Conflict of interest
None declared.
Acknowledgement
The authors would like to thank University Grant commission (UGC), New Delhi, for providing predoctoral fellowship to Ms. Arshya Hashim in the form of Maulana Azad National Senior Research Fellowship. The authors would also like to thank Professor S. W. Akhtar, Vice Chancellor, for providing state-of-art research laboratory for the smooth succession of this work.
References
- 1.Ahmad S, Akther F, Moinuddin, Shahab U, Khan MS. Studies on glycation of human low density lipoprotein: A functional insight into physic chemical analysis. Int J Biol Macromol. 2013;62:167–171. doi: 10.1016/j.ijbiomac.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Aleryani SL, Cluette-Brown JE, Khan ZA, Hasaba H, Lopez de Heredia L, Laposata M. Fatty acid methyl esters are detectable in the plasma and their presence correlates with liver dysfunction. Clin Chim Acta. 2005;359:141–149. doi: 10.1016/j.cccn.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association (ADA) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:62–67. [Google Scholar]
- 4.Arulmozhi DK, Veeranjaneyulu A, Bodhankar S. Neonatal streptozotocin induced rat model of type 2 diabetes mellitus: a glance. Indian J Pharmacol. 2004;4:217–221. [Google Scholar]
- 5.Asplund K, Wiholm BE, Lithner F. Glibenclamide-associated hypoglycaemia: a report on 57 cases. Diabetologia. 1983;24:412–417. doi: 10.1007/BF00257338. [DOI] [PubMed] [Google Scholar]
- 6.Bernfeld P. Amylases a and ß. In: Colowick SP, Kaplan NO, editors. Methods in enzymology. New York: Academic Press; 1955. p. 149. [Google Scholar]
- 7.Bnouham M, Ziyyat A, Mekhfi H, Tahri A, Legssyer A. Medicinal plants with potential antidiabetic activity – A review of ten years of herbal medicine research (1990-2000) Int J Diabetes Metabol. 2006;4:1–25. [Google Scholar]
- 8.Bowie A, Owens D, Collins P, Johnson A, Tomkin GH. Glycosylated low density lipoprotein is more sensitive to oxidation: implications for the diabetic patient? Atherosclerosis. 1993;102:63–7. doi: 10.1016/0021-9150(93)90084-8. [DOI] [PubMed] [Google Scholar]
- 9.Bravi MR, Armiento A, Laurenti O, Cassano-Faldetta M, De Luca O, Morettia A, et al. Insulin decreases intracellular oxidative stress in patient with type 2 diabetes mellitus. Metabolism. 2006;55:591–596. doi: 10.1016/j.metabol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Brownlee M, Cerami A. The biochemistry of the complications of diabetes mellitus. Annu Rev Biochem. 1981;50:385–432. doi: 10.1146/annurev.bi.50.070181.002125. [DOI] [PubMed] [Google Scholar]
- 11.Burge MR, Schmitz-Fiorentino K, Fischette C. A prospective trial of risk factors for sulfonylurea-induced hypoglycemia in type 2 diabetes mellitus. JAMA. 1998;279:137–143. doi: 10.1001/jama.279.2.137. [DOI] [PubMed] [Google Scholar]
- 12.Calixto JB, Santos ARS, Filbo V, Yunes RA. Review of the plants of the genus Phyllanthus. Med Res Rev. 1998;18:225–258. doi: 10.1002/(sici)1098-1128(199807)18:4<225::aid-med2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Drexel H, Amann FW, Rentsch K, Neuenschwander C, Luethy A, Khan SI, et al. Relation of the level of high-density lipoprotein subfractions to the presence and extent of coronary artery disease. Am J Cardiol. 1992;70:436–440. doi: 10.1016/0002-9149(92)91186-8. [DOI] [PubMed] [Google Scholar]
- 14.Ebara T, Hirano T, Mamo JCL, Sakamaki R, Furukawa S, Nagano S, et al. Hyperlipidemia in streptozotocin-diabetic hamster as model from human insulin deficient diabetes: Comparasion to streptozotocin-diabetic rats. Metabolism. 1994;43:299–305. doi: 10.1016/0026-0495(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 15.Elder C. Ayurveda for diabetes mellitus: a review of the biomedical literature. Altern Ther Health Med. 2004;10:44–50. [PubMed] [Google Scholar]
- 16.Ferre T, Pujol A, Riu E, Bosch F, Valera A. Correction of diabetic alterations by glucokinase. Proc Natl Acad Sci USA. 1996;93:7225–30. doi: 10.1073/pnas.93.14.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedewal WT, Levy RI, Fredrickson DS. Estimation of concentration of low density lipoprotein cholesterol in plasma, without the use of preparative centrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Gayathri V, Asha VV, Anil John J, Subramoniam A. Protection of immunocompromised mice from fungal infection with a thymus growth-stimulatory component from Selaginella involvens, a fern. Immunopharmacol Immunotox. 2011;33:351–9. doi: 10.3109/08923973.2010.518617. [DOI] [PubMed] [Google Scholar]
- 19.Girach RD, Aminuddin AM, Brahman M. Euphobiaceae in native health practices glucose levels over time. Diabetologia. 2007;50:2239–2244. [Google Scholar]
- 20.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 21.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 22.Harborne JB. Phytochemical methods - a guide to modern techniques of plant analysis. 2nd ed. London: Chapman and Hall; 1988. p. 182ff. [Google Scholar]
- 23.Hashim A, Khan MS, Khan MS, Baig MH, Ahmad S. Antioxidant and alpha-amylase inhibitory property of Phyllanthus virgatus.: an in vitro and molecular interaction study. Biomed Res Int. 2013;2013:729393. doi: 10.1155/2013/729393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heidarian E, SoofiniyaY Hypolipidemic and hypoglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. J Med Plants Res. 2011;5:2717–2723. [Google Scholar]
- 25.Hellestrom C. Growth pattern of pancreatic islets in animals. In: Volk BV, Wellman KE, editors. The diabetic pancreas. 1st ed. New York: Plenum Medical Book Co; 1977. pp. 61–97. [Google Scholar]
- 26.Hnatyszyn O, Miño J, Gorzalczany S, Ferraro G, Coussio J, Acevedo C. Antidiabetic activity of Phyllanthus sellowianus in streptozotocin-induced diabetic rats. Phytomedicine. 1997;4:251–253. doi: 10.1016/S0944-7113(97)80076-1. [DOI] [PubMed] [Google Scholar]
- 27.Huang YL, Chen CC, Hsu FL, Chen CF. A new lignan from Phyllanthus virgatus. J Nat Prod. 1996;59:520–1. doi: 10.1021/np970336v. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y-L, Chen C-C, Hsu F-L, Chen C-F. Tannins, flavonol sulphates and a norlignan from Phyllanthus virgatus. J Nat Prod. 1998;61(1):194–197. doi: 10.1021/np970336v. [DOI] [PubMed] [Google Scholar]
- 29.International Diabetes Federation (IDF) Diabetes atlas. 3rd ed. Brussels: International Diabetes Federation (IDF); 2006. [Google Scholar]
- 30.Iqbal D, Khan MS, Khan A, Khan MS, Ahmad S, Srivastava AK, et al. In vitro screening for ß-hydroxy-ß-methylglutaryl-CoA reductase inhibitory and antioxidant activity of sequentially extracted fractions of Ficus palmata Forsk. Biomed Res Int. 2014;2014:762620. doi: 10.1155/2014/762620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iqbal D, Khan MS, Khan MS, Ahmad S, Srivastava AK. An in vitro and molecular informatics study to evaluate the antioxidative and ß-hydroxy-ß-methylglutaryl-CoA reductase inhibitory property of Ficus virens Ait. Phytother Res. 2014;28:899–908. doi: 10.1002/ptr.5077. [DOI] [PubMed] [Google Scholar]
- 32.Kaji H, Jurasaki M, Ito K. Increased lipidperoxide value and glutathione peroxidase activity in blood plasma of type 2 (non insulin dependent) diabetic women. Klin Wochenschr. 1985;63:765–768. doi: 10.1007/BF01733829. [DOI] [PubMed] [Google Scholar]
- 33.Karthikesan K, Pari L, Menon VP. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem Biol Interact. 2010;188:643–50. doi: 10.1016/j.cbi.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 34.Khan MS, Akhtar S, Al-Sagair OA, Arif JM. Protective effect of dietary tocotrienols against infection and inflammation-induced hyperlipidemia: an in vivo and in silico study. Phytother Res. 2011;25:1586–1595. doi: 10.1002/ptr.3448. [DOI] [PubMed] [Google Scholar]
- 35.Kirtikar KP, Basu BD. Indian medicinal plants. Vol. 3. Allahabad: Lalit Mohan Basu; 1933. pp. 2217–2227. [Google Scholar]
- 36.Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18:258–268. doi: 10.2337/diacare.18.2.258. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000;49:22–31. doi: 10.1016/s0026-0495(00)90588-2. [DOI] [PubMed] [Google Scholar]
- 38.Krishnaveni M, Mirunalini S, Karthishwaran S, Dhamodharan G. Antidiabetic and antihyperlipidemic properties of Phyllanthus emblica Linn (Euphorbiaceae) on streptozotocin induced diabetic rats. PJN. 2010;9:43–51. [Google Scholar]
- 39.Kumar PP, Kumaravel S, Lalitha C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biochem Res. 2010;4:191–195. [Google Scholar]
- 40.Kumaran A, Joel Karunakaran R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT - Food Sci Technol. 2007;40:344–52. [Google Scholar]
- 41.Lyons TJ. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes? Diabetic Med. 1991;8:411–9. doi: 10.1111/j.1464-5491.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 42.Mbagwu HOC, Jackson C, Jackson I, Ekpe G, Essien UEG. Evaluation of the hypoglycemic effect of aqueous extract of Phyllanthus amarus in alloxan-induced diabetic albino rats. Int J Pharm Biomed Res. 2011;2:158–160. [Google Scholar]
- 43.Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia. 2007;50:2239–2244. doi: 10.1007/s00125-007-0803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nayak SS, Pattabiraman TN. A new colorimetric method for the estimation of glycosylated haemoglobin. Int J Clin Chem. 1981;109:267–274. doi: 10.1016/0009-8981(81)90312-0. [DOI] [PubMed] [Google Scholar]
- 45.Norris SL. Self-management education for adults with type 2 diabetes. Diabetes Care. 2002;25:1159–71. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 46.Okoli CO, Ibiam AF, Ezike AC, Akah PA, Okoye TC. Evaluation of antidiabetic potentials of Phyllanthus niruri in alloxan diabetic. Afr J Biotechnol. 2010;9:248–259. [Google Scholar]
- 47.Ono Y, Aoki S, Ohnishi K, Yasuda T, Kawano K, Tsukada Y. Increased serum level of advanced glycation end-products and diabetic complications. Diabetes Res Clin Pract. 1998;41:131–137. doi: 10.1016/s0168-8227(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 48.Patsch W, Brown SA, Morrisett JD, Gotto AM, Jr, Patsch JR. A dual-precipitation method evaluated for measurement of cholesterol in high-density lipoprotein subfractions HDL2 and HDL3 in human plasma. Clin Chem. 1989;35:265–270. [PubMed] [Google Scholar]
- 49.Pitchai D, Saravana BA, Modilal R. Antihyperglycemic effects of Phyllanthus extracts in alloxan-induced diabetic rats. Int J Ph Sci. 2009;1:261–264. [Google Scholar]
- 50.Portha B, Blondel O, Serradas P, Mc Evoy R, Giroix MH, Kergoat M. The rat models of non-insulin dependent diabetes induced by neonatal streptozotocin. Diabet Metab. 1989;15:61–75. [PubMed] [Google Scholar]
- 51.Postic C, Dentin R, Girard J. Role of the liver in the control of carbohydrate and lipid homeostasis. Atherosclerosis. 2001;158:1–12. doi: 10.1016/s1262-3636(07)70133-7. [DOI] [PubMed] [Google Scholar]
- 52.Prince PSM, Kannan NK. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. J Pharm Pharmacol. 2006;58:1373–83. doi: 10.1211/jpp.58.10.0011. [DOI] [PubMed] [Google Scholar]
- 53.Ratzmann KP, Witt S, Schulz B. The effect of long-term glibenclamide treatment on glucose tolerance, insulin secretion and serum lipids in subjects with impaired glucose tolerance. Diabet Metab. 1983;9:87–93. [PubMed] [Google Scholar]
- 54.Robertson RP. Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes. 2007;56:2420–2424. doi: 10.2337/db07-0742. [DOI] [PubMed] [Google Scholar]
- 55.Rydgren T, Sandler S. The protective effect of simvastatin against low dose streptozotocin induced type 1 diabetes in mice is independent of inhibition of HMG-CoA reductase. Biochem Biophys Res Commun. 2009;379:1076–9. doi: 10.1016/j.bbrc.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Salahuddin M, Jalalpure SS. Antidiabetic activity of aqueous fruit extract of Cucumis trigonus Roxb. in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2010;127:565–67. doi: 10.1016/j.jep.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Saudek CD, Rastogi R. Assessment of glycemia in diabetes mellitus - self-monitoring of blood glucose. J Assoc Physicians India. 2004;52:809–815. [PubMed] [Google Scholar]
- 58.Shabeer J, Srivastava RS, Singh SK. Antidiabetic and antioxidant effect of various fractions of Phyllanthus simplex in alloxan diabetic rats. J Ethnopharmacol. 2009;12:34–8. doi: 10.1016/j.jep.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Sills MN, Ogu CC, Maxa J. Prolonged hypoglycemic crisis associated with glyburide. Pharmacotherapy. 1997;17:1338–1340. [PubMed] [Google Scholar]
- 60.Singh R, Barden A, Mori T, Bellin L. Advanced glycation endproducts: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 61.Steiner G. Treating lipid abnormalities in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88:37–40. doi: 10.1016/s0002-9149(01)02151-8. [DOI] [PubMed] [Google Scholar]
- 62.Szkudelski T. The mechanism of alloxan and streptozotocin action in b cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 63.Tamai T, Nakai T, Yamada S, Kobayashi T, Hayashi T, Kutsumi Y, et al. The effects of glibenclamide and insulin on plasma high-density lipoprotein in diabetes. Artery. 1981;9:477–493. [PubMed] [Google Scholar]
- 64.Terpstra AH. Effect of conjugated linoleic acid on body composition and plasma lipids in humans: an overview of the literature. Am J Clin Nutr. 2004;79:352–61. doi: 10.1093/ajcn/79.3.352. [DOI] [PubMed] [Google Scholar]
- 65.Tietz NW, editor. Clinical guide to laboratory tests. 3rd ed. Philadelphia, PA: Saunders; 1976. [Google Scholar]
- 66.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen receptor. Ann Clin Biochem. 1969;6:24–27. [Google Scholar]
- 67.Wieland H, Seidel D. A simple specific method for precipitation of low density lipoproteins. J Lipid Res. 1983;24:904–909. [PubMed] [Google Scholar]
- 68.Zafar M, Naqvi SNH. Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: a comparative study. Int J Morphol. 2010;28:135–142. [Google Scholar]
- 69.Zhang SX, Sima J, Shao C, Fant J, Chen Y, Rohrer B, et al. Plasminogen kringle 5 reduces vascular leakage in the retina in rat models of oxygen-induced retinopathy and diabetes. Diabetologia. 2004;47:124–131. doi: 10.1007/s00125-003-1276-4. [DOI] [PubMed] [Google Scholar]


