Abstract
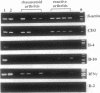
A major immunoregulatory mechanism in inflammatory infections and allergic diseases is the control of the balance of cytokines secreted by Th1/Th2 subsets of T helper (Th) cells. This might also be true in autoimmune diseases; a Th2 pattern that prevents an effective immune response in infections with intracellular bacteria may favor immunosuppression in autoimmune disease. The pattern of cytokine expression was compared in the synovial tissue from patients with a typical autoimmune disease, rheumatoid arthritis, and with a disorder with similar synovial pathology but driven by persisting exogenous antigen, reactive arthritis. We screened 12 rheumatoid and 9 reactive arthritis synovial tissues by PCR and in situ hybridization for their expression of T-cell cytokines. The cytokine pattern differs significantly between the two diseases; rheumatoid arthritis samples express a Th1-like pattern whereas in reactive arthritis interferon gamma expression is accompanied by that of interleukin 4. Studying the expression of cytokines by in situ hybridization confirmed the results found by PCR; they also show an extremely low frequency of cytokine-transcribing cells. In a double-staining experiment, it was demonstrated that interleukin 4 is made by CD4 cells. These experiments favor the possibility of therapeutic intervention in inflammatory rheumatic disease by means of inhibitory cytokines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Modlin R. L., Salgame P. Stigma variations: observations on suppressor T cells and leprosy. Annu Rev Immunol. 1992;10:453–488. doi: 10.1146/annurev.iy.10.040192.002321. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. The power of negative thinking. J Clin Invest. 1993 Apr;91(4):1265–1266. doi: 10.1172/JCI116322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. L., Kay T. W., Oxbrow L., Harrison L. C. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991 Feb;87(2):739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E., Keystone E. C., Fish E. N. Restricted cytokine expression in rheumatoid arthritis. Arthritis Rheum. 1993 Jul;36(7):901–910. doi: 10.1002/art.1780360706. [DOI] [PubMed] [Google Scholar]
- Cooper C. L., Mueller C., Sinchaisri T. A., Pirmez C., Chan J., Kaplan G., Young S. M., Weissman I. L., Bloom B. R., Rea T. H. Analysis of naturally occurring delayed-type hypersensitivity reactions in leprosy by in situ hybridization. J Exp Med. 1989 May 1;169(5):1565–1581. doi: 10.1084/jem.169.5.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournoyer D., Caskey C. T. Gene therapy of the immune system. Annu Rev Immunol. 1993;11:297–329. doi: 10.1146/annurev.iy.11.040193.001501. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990 Jun;33(6):768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987 Aug;30(8):864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. The role of T cells in rheumatoid arthritis. Ann Rheum Dis. 1993 Oct;52(10):765–765. doi: 10.1136/ard.52.10.765-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell W. M., Warren C. J., Cook N. J., Cawley M. I., Smith J. L. Detection of IL-2 at mRNA and protein levels in synovial infiltrates from inflammatory arthropathies using biotinylated oligonucleotide probes in situ. Clin Exp Immunol. 1991 Dec;86(3):393–398. doi: 10.1111/j.1365-2249.1991.tb02943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Karp C. L., el-Safi S. H., Wynn T. A., Satti M. M., Kordofani A. M., Hashim F. A., Hag-Ali M., Neva F. A., Nutman T. B., Sacks D. L. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993 Apr;91(4):1644–1648. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Khoury S. J., Hancock W. W., Weiner H. L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992 Nov 1;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley G., Sieper J. Current perspectives in reactive arthritis. Immunol Today. 1993 Aug;14(8):387–391. doi: 10.1016/0167-5699(93)90139-C. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Induction, regulation and function of T-cell subsets in leishmaniasis. Chem Immunol. 1992;54:117–135. [PubMed] [Google Scholar]
- Merilahti-Palo R., Söderström K. O., Lahesmaa-Rantala R., Granfors K., Toivanen A. Bacterial antigens in synovial biopsy specimens in yersinia triggered reactive arthritis. Ann Rheum Dis. 1991 Feb;50(2):87–90. doi: 10.1136/ard.50.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Morahan G., Allison J. Extrathymic acquisition of tolerance by T lymphocytes. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):807–813. doi: 10.1101/sqb.1989.054.01.094. [DOI] [PubMed] [Google Scholar]
- Miltenburg A. M., van Laar J. M., de Kuiper R., Daha M. R., Breedveld F. C. T cells cloned from human rheumatoid synovial membrane functionally represent the Th1 subset. Scand J Immunol. 1992 May;35(5):603–610. doi: 10.1111/j.1365-3083.1992.tb03260.x. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Melancon-Kaplan J., Young S. M., Pirmez C., Kino H., Convit J., Rea T. H., Bloom B. R. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Saito H., Kasanuki J., Tamura Y., Yoshida S. Cytokine production in patients with inflammatory bowel disease. Gut. 1992 Jul;33(7):933–937. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Parronchi P., De Carli M., Manetti R., Simonelli C., Piccinni M. P., Macchia D., Maggi E., Del Prete G., Ricci M., Romagnani S. Aberrant interleukin (IL)-4 and IL-5 production in vitro by CD4+ helper T cells from atopic subjects. Eur J Immunol. 1992 Jun;22(6):1615–1620. doi: 10.1002/eji.1830220640. [DOI] [PubMed] [Google Scholar]
- Pirmez C., Yamamura M., Uyemura K., Paes-Oliveira M., Conceiço-Silva F., Modlin R. L. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993 Apr;91(4):1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990 Dec 1;172(6):1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M. J., Jaramillo A., Zipris D., Lazarus A. H., Serreze D. V., Leiter E. H., Cyopick P., Danska J. S., Delovitch T. L. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993 Jul 1;178(1):87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98(4):279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- Salk J., Bretscher P. A., Salk P. L., Clerici M., Shearer G. M. A strategy for prophylactic vaccination against HIV. Science. 1993 May 28;260(5112):1270–1272. doi: 10.1126/science.8098553. [DOI] [PubMed] [Google Scholar]
- Schlaak J., Hermann E., Ringhoffer M., Probst P., Gallati H., Meyer zum Büschenfelde K. H., Fleischer B. Predominance of Th1-type T cells in synovial fluid of patients with Yersinia-induced reactive arthritis. Eur J Immunol. 1992 Nov;22(11):2771–2776. doi: 10.1002/eji.1830221103. [DOI] [PubMed] [Google Scholar]
- Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993 Apr 23;260(5107):496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- Scott P. Selective differentiation of CD4+ T helper cell subsets. Curr Opin Immunol. 1993 Jun;5(3):391–397. doi: 10.1016/0952-7915(93)90058-z. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E., Davis M. M., Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992 Oct 1;176(4):1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Coffman R. L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- Sher A., Gazzinelli R. T., Oswald I. P., Clerici M., Kullberg M., Pearce E. J., Berzofsky J. A., Mosmann T. R., James S. L., Morse H. C., 3rd Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992 Jun;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Sieper J., Kingsley G., Palacios-Boix A., Pitzalis C., Treharne J., Hughes R., Keat A., Panayi G. S. Synovial T lymphocyte-specific immune response to Chlamydia trachomatis in Reiter's disease. Arthritis Rheum. 1991 May;34(5):588–598. doi: 10.1002/art.1780340511. [DOI] [PubMed] [Google Scholar]
- Simon A. K., Seipelt E., Wu P., Wenzel B., Braun J., Sieper J. Analysis of cytokine profiles in synovial T cell clones from chlamydial reactive arthritis patients: predominance of the Th1 subset. Clin Exp Immunol. 1993 Oct;94(1):122–126. doi: 10.1111/j.1365-2249.1993.tb05988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel H., Herbst H., Niedobitek G., Foss H. D., Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol. 1992 Jan;140(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- Sun J., Link H., Olsson T., Xiao B. G., Andersson G., Ekre H. P., Linington C., Diener P. T and B cell responses to myelin-oligodendrocyte glycoprotein in multiple sclerosis. J Immunol. 1991 Mar 1;146(5):1490–1495. [PubMed] [Google Scholar]
- Waalen K., Sioud M., Natvig J. B., Førre O. Spontaneous in vivo gene transcription of interleukin-2, interleukin-3, interleukin-4, interleukin-6, interferon-gamma, interleukin-2 receptor (CD25) and proto-oncogene c-myc by rheumatoid synovial T lymphocytes. Scand J Immunol. 1992 Dec;36(6):865–873. doi: 10.1111/j.1365-3083.1992.tb03148.x. [DOI] [PubMed] [Google Scholar]
- Wood N. C., Dickens E., Symons J. A., Duff G. W. In situ hybridization of interleukin-1 in CD14-positive cells in rheumatoid arthritis. Clin Immunol Immunopathol. 1992 Mar;62(3):295–300. doi: 10.1016/0090-1229(92)90106-x. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yssel H., Shanafelt M. C., Soderberg C., Schneider P. V., Anzola J., Peltz G. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J Exp Med. 1991 Sep 1;174(3):593–601. doi: 10.1084/jem.174.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J. E., de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H. Do human TH1 and TH2 CD4+ clones exist? Res Immunol. 1991 Jan;142(1):59–63. doi: 10.1016/0923-2494(91)90014-a. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]