Abstract
Prior exposure to social disruption (SDR) stress exacerbates Theiler’s murine encephalomyelitis virus (TMEV) infection, a model of multiple sclerosis. Here we examined the impact of SDR on T cell responses to TMEV infection in SJL mice. SDR impaired viral clearance and exacerbated acute disease. Moreover, TMEV infection alone increased CD4 and CD8 mRNA expression in brain and spleen while SDR impaired this response. SDR decreased both CD4+ and CD8+ virus-specific T cells in CNS, but not spleen. These findings suggest that SDR-induced suppression of virus-specific T cell responses contributes to impairments in viral clearance and exacerbation of acute disease.
Keywords: Social stress, Multiple Sclerosis, Theiler’s murine encephalomyelitis virus, TMEV, adaptive immune response, T cell
1. Introduction
Multiple Sclerosis (MS) is a chronic disorder of the central nervous system (CNS) characterized by inflammation, demyelination, and neurodegeneration, which result in sensory and motor deficits (Mohr & Dick 1998; Stinissen et al., 1997). Although the etiology MS is not known, both genetic and environmental factors have been linked with disease development and severity (Noseworthy et al., 2000). Epidemiological studies have linked exposure to several common viruses (e.g., herpes, rubella, Epstein-Barr) with an increased likelihood of MS diagnosis, and a number of studies report a greater incidence of stressful life events prior to initial diagnosis and exacerbations (Ackerman er al., 2002; Brown et al., 2006a/b; Grant et al., 1989; Li et al., 2004; Warren, Greenhill, & Warren, 1982). While human studies have been instrumental in identifying these relationships, animal models of MS offer the opportunity to experimentally manipulate these variables to elucidate the mechanisms by which they may interact to determine disease susceptibility.
Theiler’s murine encephalomyelitis virus (TMEV) infection, a well-characterized model of MS (Lipton, 1975; Oleszack et al., 2004), results in a biphasic disease consisting of an acute polioencephalitic phase followed by a chronic demyelinating phase in susceptible strains of mice (Oleszack et al., 2004). Following intracranial inoculation, genetically resistant strains of mice mount an effective immune response, clear the virus from the CNS and do not develop demyelination. Susceptible strains (e.g., SJL) fail to effectively clear the virus during early infection, which results in viral persistence in the CNS and, eventually, immune-mediated demyelination (Theiler, 1937; Clatch et al., 1990; Zheng et al., 2001). Effective clearance of TMEV depends on a number of factors, including the H-2D locus (Lipton & Melvold, 1984). This suggests a role for CD8+ T cells in the clearance of TMEV. Further evidence suggests that depletion of CD8+ cell populations or altering the functionality of these cells increases disease susceptibility (Borrow et al., 1992). Others have reported a vital role of CD4+ T cells in the clearance of TMEV as mice with reduced CD4+ T cell populations show more severe inflammation, demyelination and axonal degeneration (Lin et al., 2004; Murray et al., 1998) as well as increased mortality (Welsh et al., 1987). The development of subsequent demyelinating disease is characterized by viral persistence in glia and macrophages, the recruitment of TMEV-specific T cells to the CNS and antibody formation against myelin epitopes resulting in autoimmunity, all of which are exacerbated when early viral replication is not limited effectively (Sieve et al., 2004, 2006; Johnson et al., 2004). Taken together, these data indicate that a well-orchestrated T cell response is vital to early viral clearance, and, as a result, to reducing susceptibility for chronic demyelination.
We have shown that social disruption (SDR) stress prior to TMEV infection exacerbates both the acute and chronic phases of disease (Johnson et al., 2004, 2006, Meagher et al., 2007; Vichaya et al., 2011), however, the mechanism by which this occurs remains to be fully elucidated. SDR exposure results in enhanced CNS inflammation, increased behavioral signs of encephalomyelitis, and impaired clearance of the virus from CNS during early infection (Meagher et al., 2007). More recently, we have demonstrated that SDR increases both baseline and infection-induced proinflammatory cytokine mRNA levels in the CNS (Vichaya et al., 2011). Social defeat is potent modulator of both macrophage trafficking from the periphery into the CNS and the inflammatory potential of microglia and CNS macrophages, both of which might be expected to improve viral clearance by ramping up the immune response to a given challenge (Wohleb et al., 2012). When SDR exposure occurs prior to TMEV infection, increased circulating levels of antibodies to Theiler’s virus and myelin epitopes are observed in the chronic phase of the disease (Johnson et al., 2004), levels of which have been correlated with disease severity (Johnson et al., 2006). Given the SDR-induced impairment of viral clearance previously reported in Balb/cJ mice (Johnson et al., 2004, 2006; Meagher et al., 2007), a T cell dependent process, the present studies evaluate the hypothesis that SDR impairs the adaptive immune response to CNS infection with TMEV. We demonstrate that social disruption stress increases CNS viral load, behavioral signs of sickness, and impairs both CD4+ and CD8+ T cell responses within the CNS during TMEV infection.
2. Methods
2.1. Subjects
2.1.1. Resident Mice
In order to differentiate between effects in these two populations, we needed to use a strain of mouse in which the sequences recognized by the two populations had been identified. This constraint necessitated a move to the SJL strain of mouse in which the T cell specific sequences are known, unlike the Balb/cJ mouse used previously. Four-week old male SJL mice were purchased from Harlan (Indianapolis, IN). Experimental mice were housed 3/cage for all experiments. All resident mice were given one week to acclimate to their housing environment prior to commencing experimental manipulations. Mice were maintained on a 12-h light:dark cycle with access to food and water ad libitum. All animal care protocols were in accordance with NIH Guidelines for Care and Use of Laboratory Animals and were approved by the Texas A&M University Laboratory Animal Care and Use Committee.
2.1.2. Dominant mice
Intruders were retired SJL male breeders 6-9 months of age. Intruders were screened for aggressive behaviors by placing them in the home cage of another mouse. Only intruders who consistently attacked peers within 30 s were used in these experiments.
2.2. Social Disruption and infection
Cages of three resident mice were randomly assigned to either control or SDR groups. Control mice remained undisturbed in their home cages, while SDR mice were exposed to aggressive male intruders. Intruders were introduced into the home cage of the resident mice at the onset of the dark cycle for a period of 2 hours for a total of six SDR sessions. SDR occurred for three consecutive sessions, then one night off, followed by three additional consecutive sessions (Stark et al., 2001; Johnson et al., 2004, 2006). SDR sessions were monitored to ensure that the intruder attacked the residents and that the residents demonstrated submissive behaviors. All intruders attacked within 10 minutes of SDR initiation. While physical contact between the intruders and resident mice was seen during SDR, no visible wounds or injuries were noted, in agreement with previous studies from our lab suggesting that when adolescent residents are subjected to SDR, wounding is reduced compared to SDR sessions in which the intruder and resident(s) are age-matched (Johnson et al., 2006; Vichaya et al., 2010). Two hours after the final SDR session, all subjects were anesthetized with isoflurane (MWI, Meridian, ID) and were either injected with 5.0×105 plaque forming units (PFU) of BeAn strain of TMEV or sham-infected with sterile PBS into the right mid-parietal cortex at a depth of approximately 1.5 mm (Campbell et al., 2001).
2.3. Virus and viral peptides
The BeAn 8638 strain of Theiler’s virus (kindly provided by Dr. H.L. Lipton, Department of Microbiology-Immunology, University of Illinois at Chicago, IL) was initially propagated in lung tumor (L2) cells (Welsh et al., 1987). Distinct populations of T cells (e.g. CD4+ and CD8+) recognize different immunodominant viral peptide sequences. The immunodominant CD4+ T cell peptide QEAFSHIRIPLPH corresponding to TMEV VP274-86 was used to determine CD4+ cell specific responses (Gerety et al., 1991 & 1994). Immunodominant CD8+ T cell peptide FNFTAPFI corresponding to VP3159-166 was used to determine CD8+ T cell specific responses to TMEV (Kang et al., 2002). The non-specific peptide sequence RLNRITKDSYPNS was used as a control peptide to determine non-specific immune responses (Steelman et al., 2009). All peptides were purchased from Genemed Synthesis (San Antonio, TX).
2.4. CNS Viral Clearance
Because SJL mice were required for the virus-specific T cell analyses planned, we first needed to establish that previous findings regarding SDR effects in acute TMEV in the Balb/cJ mice translated to a similar exacerbation in this new strain. Day 8 pi has previously been shown to be the peak of the adaptive immune response following TMEV infection after which time uninfected mice will show a steady decrease in T cell populations as the virus is cleared (Steelman et al., 2009). On day 8 post-infection (pi), brain and spinal cords were homogenized in Dulbecco’s modified Eagles’ medium DMEM (Gibco BRL, Grand Island, NY), centrifuged and the supernatant used in viral assays. BHK-21 cells were grown in Dulbecco’s modified Eagles’ medium DMEM supplemented with 10% fetal bovine serum (FBS) (Irvine Scientific, Irvine, CA.) for growth and 1% FBS for post inoculation maintenance of cell cultures. The titration of viruses was performed in BHK-21 cells by cytopathic effect (CPE). Cells were seeded in 96-well plates at a concentration of 105 cells per well. When the cells reached confluency, each well was washed once with serum-free IMDM and then inoculated in quadruplicate with 50 μL virus suspension per well, in tenfold dilutions (in DMEM). After viral adsorption the cells were washed in serum-free IMDM and incubated with 1% FBS-containing IMDM. The CPE was assessed daily and the TCID-50 was calculated by the Reed and Muench formula (Reed and Muench, 1938).
2.5. Behavioral Measures of Sickness
SJL mice are typically asymptomatic during the acute phase of TMEV infection, however for experiments 2 and 3, body weight and sucrose preference were monitored following infection [(day 0 to day 8 post-infection (pi)].
2.5.1. Body weight
Body weight was measured daily between 0900 hours and 1000 hours using a scale sensitive to 0.01 g.
2.5.2. Sucrose preference
Anhedonia, a loss of interest in pleasure seeking and well-characterized behavioral indicator of sickness, was assessed by evaluating preference for a 2% sucrose solution (Maier and Watkins, 1998; Pollak et al., 2000). We have previously shown a loss of sucrose preference to be a sensitive behavioral readout of sickness syndrome during acute TMEV infection (Johnson et al., 2006; Meagher et al., 2007). Twenty-four hours after arrival, all mice were given a bottle containing 2% sucrose solution for 72 hours to reduce neophobia. Following this habituation period and prior to infection, mice were provided with a two-bottle choice (one bottle contained 2% sucrose solution and the other contained tap water) for 24 hours. The percent sucrose consumption was determined by the following equation: [grams of sucrose solution consumed/(grams of sucrose solution+grams of water consumed)]*100. All cages exhibited a sucrose preference (i.e. percent sucrose consumption equal to or greater than 60%) prior to SDR and infection. Beginning 12 hours after infection, all mice were again presented with the two-bottle choice for 24 hours. The percent sucrose consumed during this period was calculated.
2.6. Quantitative RT-PCR for CD4 and CD8 mRNA
We had unpublished preliminary findings that TMEV-induced CD4 and CD8 mRNA was suppressed in the brains of Balb/cJ mice when they were exposed to social stress prior to infection. Prior to evaluating the virus-specificity of the T cell responses, we first replicated our findings from the Balb/cJ strain in a strain that is more amenable to the study of the T cell response to TMEV infection. On day 8 pi, subjects were injected with a lethal dose of Beuthanasia special 150mg/kg (Schering-Plough Animal Health) (Welsh et al., 2004). All mice were perfused through the left ventricle with ice cold RNAse free PBS. Brains were isolated and the cortex and meninges were microdissected using a mouse brain matrix (PlasticsOne, Roanoke, VA). The cortex and meninges were used for PCR analysis because previous research has shown elevated inflammatory cell infiltration in the cortex and meninges during acute TMEV infection in susceptible strains (Murray et al. 2000). Total RNA from samples was isolated and purified by column and DNase digestion using the Qiagen RNeasy mini kit according to manufacturer’s instructions (Qiagen). cDNA was generated using a High Capacity RNA-to-cDNA kit (Applied Biosystems) according to the manufacturer’s instructions. Real-time PCR was used to test for CD4 and CD8 mRNA expression and normalized to β-actin using methods described previously (Mi et al., 2006). All TaqMan gene expression assay primer and probe sets were purchased from Applied Biosystems.
2.7. Determination of Virus-Specific T cell responses
2.7.1. Preparation of Feeder Cells
Spleens were aseptically removed from aged-matched unstressed/uninfected controls and single cell suspensions prepared. Feeder cells were irradiated with 3000 rads (Co60 source, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University).
2.7.2. Tissue isolation
On day 8 pi, all subjects were injected with a lethal dose of Beuthanasia special 150 mg/kg (Schering-Plough Animal Health) (Welsh et al., 2004). Mice were perfused through the left ventricle with cold Hanks Balanced Salts solution containing heparin (10U/mL) buffered at pH 7.2. After perfusion, spleens, brains and spinal cords were aseptically removed. Single cell suspensions were prepared as described previously (Welsh et al., 2004; Steelman et al., 2009). Spleen cells were used to evaluate the peripheral response to TMEV infection due to the abundant lymphocyte population, and responses within the spleen have been shown to correlate with those in the cervical lymph nodes that drain the brain (Johnson et al., 1999). CNS infiltrating lymphocytes (CNS-ILs) were prepared from CNS tissue using nylon mesh and incubating in complete RPMI (RPMI-1640 containing 250 μg/mL collagenase Type IV, 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin) for 45 minutes at 37°C and 5% CO2 (Kang et al., 2002). Following incubation, CNS-ILs were isolated by 35/70% percoll gradient centrifugation and resuspended in complete RPMI-1640.
2.7.3. ELISPOT assay
The effects of social disruption on CNS and spleen T cell effector function to TMEV were assessed, in part, by the ability to CNS-ILs to generate IFN-γ in response to either the immunodominant CD4+ or CD8+ T cell specific peptide (VP274-86 or VP3159-166, respectively) as previously described (Steelman et al., 2009). Briefly, 96-well filtration plates (MAIPS4510;Millipore, Bedford MA) were coated per well overnight at 4°C with 1.0 μg per well of anti-mouse IFN-γ capture antibody (AN-18; eBioscience). After blocking with complete RPMI-1640 for 2 hours, 2.0 × 104 CNS-ILs were mixed with 1.0×106 irradiated feeder cells, in 150 μL of complete RPMI-1640 containing a final concentration of 2.0 μM peptide (CD4+, CD8+, or non-specific). Following an incubation at 37°C and 5.0% CO2 for 24 h, the plates were washed with PBS containing 0.05% Tween-20 (PBST) and rinsed once with water purified by reverse osmosis (RO H2O). 100 μL assay diluent (PBS with 10% FBS) containing 0.1 μg of the biotin labeled anti-INF-γ detection antibody (R4-6A2; eBioscience) was added to each well, and the plates were incubated at room temperature for 2 h. Subsequently, the plates were washed 6 times in PBST and 100 μL of avidin-HRP (horseradish peroxidase) (eBioscience) diluted 1/1000 in assay diluent was added and the plates were incubated for 30 mins at room temperature. Following additional washes, spots were developed using 100 μL of 3-amino-9-ethyl-carbazole (AEC) substrate solution (1.0mL AEC, 1.0mL dimethylformamide, 14 mL 0.1M citrate-phosphate buffer pH 5.0, and 10.0 μL H2O2). Plates were rinsed 3 times with 200 μL of RO H2O and read with an ELISPOT plate reader (AID EliSpot Reader System, Straberg, Germany). The effects of SDR on splenic T cell effector function were determined using the methods described above. However, for these assays, 1.0×106 isolated spleen cells were used in the absence of feeder cells. All samples were run in triplicate. The generation of spots to the non-specific peptide was used as a measure of background and was subtracted from CD4+ and CD8+ T cell virus-specific peptide responses (Steelman et al., 2009). All data are presented (and analyzed) as the mean number of spot forming cells (SFCs) for a given sample.
2.8. Statistical Analyses
Data are presented as mean ± SEM. Viral titers were analyzed by Student’s t-test. For Experiments 2 and 3, behavioral data were analyzed using analysis of variance (ANOVA) followed up by post hoc analyses with Fisher’s LSD where appropriate. RT-PCR data were analyzed using multivariate analysis of variance (MANOVA) while differences in ELISPOT assays were analyzed using ANOVA.
3. Results
3.1. Experiment 1: SDR impairs viral clearance from the CNS
To determine whether SDR prior to infection impairs viral clearance in SJL mice, subjects were exposed to either SDR or they remained undisturbed in their home cage prior to intracranial inoculation with TMEV. On day 8 pi, brain and spinal cord samples were collected from all subjects for the determination of viral titer. A Student’s t-test revealed significantly higher CNS viral titer in subjects that underwent SDR prior to infection (t = 4.589, p < 0.01) (Figure 1).
Figure 1.
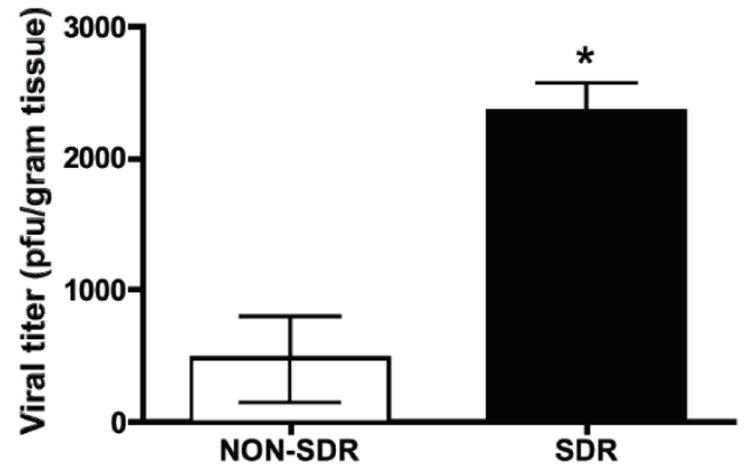
Mice exposed to social stress prior to TMEV infection have elevated CNS viral load (titer) on day 8 post-infection compared to non-stressed controls (asterisk denotes significance).
3.2. Experiment 2: SDR increases behavioral signs of acute TMEV infection
We hypothesized that, in agreement with prior findings in Balb/cJ mice, SDR would exacerbate behavioral signs of illness during acute TMEV infection in SJL mice. In order to evaluate this hypothesis, body weight and sucrose preference (consumption of 2% sucrose solution compared to total fluid consumption for a 24 hour period) were measured. Typically, SJL mice show few signs of acute TMEV infection, although restraint stress prior to infection has previously been shown to result in small but significant decreased body weight shortly after infection (Steelman et al., 2009; Young et al., 2008). Sucrose preference has previously been shown to be a sensitive measure of SDR-induced exacerbations in early TMEV infection in Balb/cJ mice but had not been used to characterize SJLs. Behavioral data for Experiments 2a and 2b were combined to improve statistical power. In the present study, prior exposure to social disruption exacerbated weight loss following TMEV infection. An ANOVA showed significant main effects of stress (F (1, 44) = 7.025, p < 0.05) and infection (F (1, 44) = 22.310, p < 0.001) as well as a significant stress x infection interaction (F (1, 44) = 7.838, p < 0.01) on the change in body weight at D1pi (Figure 2A). Post hoc analyses with Fisher’s LSD indicate that subjects exposed to stress prior to infection lost more weight than all other groups. SDR exposure prior to infection resulted in reduced sucrose preference, an indicator of anhedonia, at D1pi (Figure 2B). An ANOVA confirmed a significant main effect of infection (F (1, 12) = 5.822, p < 0.05). Post hoc analysis with Fisher’s LSD indicates that the effect of infection may be partially explained by significant differences between the SDR/Infected subjects and the uninfected subjects (both stressed and non-stressed).
Figure 2.
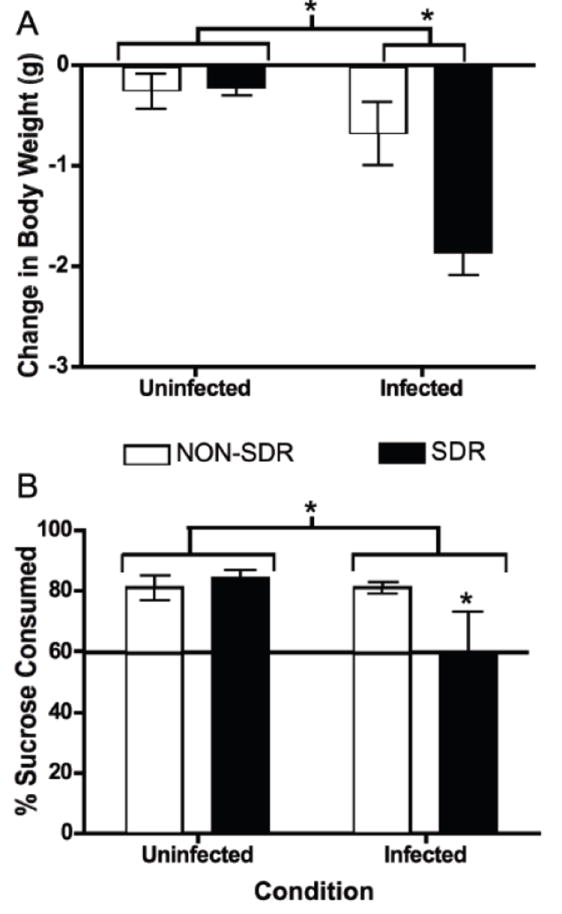
SDR prior to TMEV infection exacerbated body weight loss and anhedonia. Panel A. Infection resulted in a significant reduction in body weight at day 1 post-infection (denoted by asterisk). SDR exposure prior to TMEV infection exacerbated body weight loss on day 1 post-infection (significant difference between NON-SDR/Infected and SDR/Infected conditions denoted by asterisk). The SDR/Infected condition exhibited greater reduction in body weight (-1.8 g, 10.1%) than NON-SDR/Infected (-0.676, g, 3.1%), NON-SDR/Uninfected (-.258 g, 1.6%) and SDR/Uninfected (-0.225 g, 1.4%) conditions. Panel B. Sucrose preference was reduced as a result of infection (asterisk denotes significant difference). SDR/Infected mice consumed significantly less sucrose compared to all other conditions (denoted by asterisk). The horizontal line indicates the minimum sucrose consumption indicative of sucrose preference.
3.3. Experiment 2a: SDR decreases CNS CD4 and CD8 mRNA expression following TMEV infection
TMEV infection increased cortical/meningeal CD4 and CD8 mRNA levels at day 8 pi and SDR prior to infection with TMEV attenuated this increase (see Figure 3). An ANOVA for CD4 mRNA expression showed a significant main effect of infection, F (1, 21) = 4.726, p < 0.05. No other significant main effects or interactions reached significance. An ANOVA for CD8 mRNA expression showed no significant main effects or interactions, however there was a trend for infection to increase CD8 mRNA expression (p = 0.106). A MANOVA revealed a significant stress x infection interaction (F (2, 21) = 3.644, p < 0.05) on the cortical/meningeal levels of CD4 and CD8 mRNA detection. This significant interaction implies that SDR reduced the number of T cells in the meninges/parenchyma. However, CD4 is expressed on other CNS resident cell populations (e.g. microglia) and its expression can change following activation of these cells (Perry & Gordon, 1987). Taken together with a the recent report that restraint stress impairs viral clearance by attenuating the T cell response to TMEV infection, the present data are suggestive of an SDR-induced reduction in the number of CD4+ T cells infiltrating the CNS in response to TMEV infection. However, future studies using convergent measures of T cell populations are needed before we can conclude that reductions in CD4+T cells mediate these changes in viral clearance.
Figure 3.
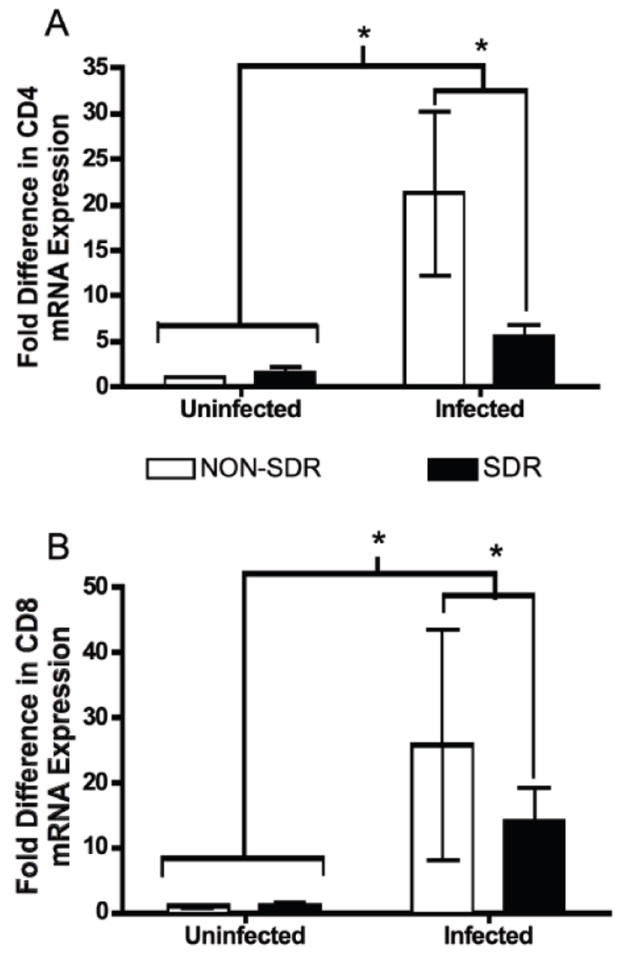
SDR exposure significantly reduced TMEV-induced CD4 and CD8 mRNA expression in samples of cortex and meninges (cortical/meningeal combined samples) at day 8 post-infection. Panel A. CD4 mRNA expression was significantly elevated as a result of TMEV infection (denoted by asterisk). This effect was attenuated by exposure to SDR prior to infection (asterisk denotes significant difference between SDR/Infected and NON-SDR/Infected conditions). Panel B. CD8 mRNA expression was significantly elevated as a result of TMEV infection (denoted by asterisk). SDR exposure attenuated the TMEV-induced elevation in CD8 mRNA (asterisk denotes significant difference between SDR/Infected and NON-SDR/Infected conditions).
3.4. Experiment 2b: SDR impairs T cell responses in the CNS but not spleen at day 8 pi
T cell responses, particularly the CD8+ T cell response, have been shown to play a vital role in the clearance of TMEV from the CNS. IFN-γ plays a vital role in the clearance of TMEV as reflected by the finding that IFN-γ-/- mice exhibit increased viral persistence and increased susceptibility to chronic phase demyelination (Rodriguez et al., 2003). Within the CNS, fewer cells responded to the CD4+ immunodominant viral peptide (VP274-86) than to the CD8+ immunodominant epitope (VP3159-166), in agreement with previous data suggesting that the population of capsid specific Th1 cells in TMEV infected SJL mice is negligible at the end of the first week post-infection (Mohindru et al., 2006; Steelman et al., 2009). We hypothesized that SDR prior to infection would impair the virus-specific T cell response resulting in fewer IFN-γ producing T cells in the CNS. In order to evaluate the effects of SDR on virus-specific IFN-γ producing T cells in the CNS and periphery, we stimulated CNS and spleen cells with immunodominant CD4+ and CD8+ TMEV epitopes in an ELISPOT assay. In this assay, following the application of detection antibodies, the number of spot forming units (SFC) is counted for each standardized sample. The number of SFCs indicates the number of IFN-γ producing cells present. TMEV infection increased the number of IFN-γ producing cells responding to both CD4+ and CD8+ immunodominant peptides while SDR profoundly decreased T cell responses in the CNS. Infection increased virus-specific CD4+ cells in the CNS, but this increase was attenuated in subjects previously exposed to social disruption. ANOVA verified significant main effects of stress (F (1, 20) = 18.668, p < 0.001) and infection (F (1, 20) = 17.552, p < 0.001) as well as a significant stress x infection interaction (F (1, 20) = 18.417, p < 0.001) for virus-specific CD4+ T cells in the CNS (Figure 4A). Post hoc analyses indicated that only the non-stressed/infected subjects had s elevated numbers of virus-specific CD4+ T cells in the CNS and verified a significant difference between the NON-SDR/Infected condition and the SDR/Infected condition.
Figure 4.
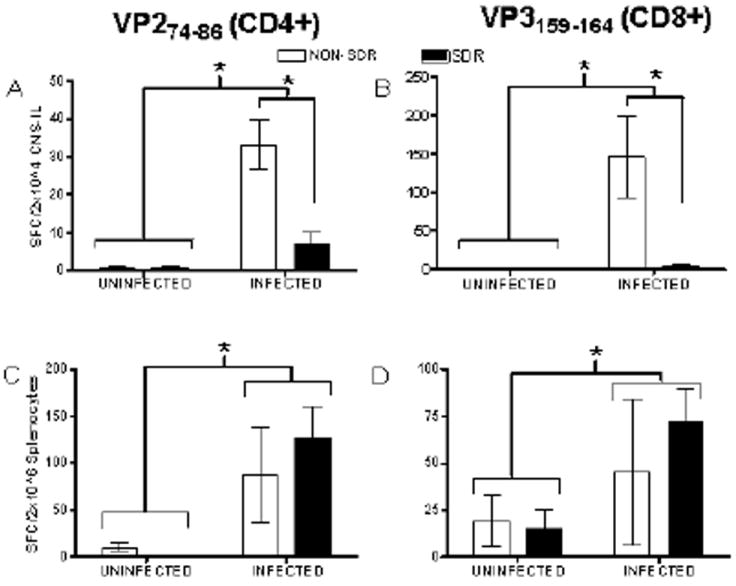
Data are presented as mean number of spot forming cells (SFCs) detected with IFN-γ detection antibodies (mean ± SEM) after SDR (or NON) and infection. Infection significantly increased the number of virus-specific CD4+ T cells (see Panel A) and virus-specific CD8+ T cells (see Panel B) detected in isolated CNS infiltrating lymphocytes (CNS-ILs; denoted by asterisk) while SDR exposure prior to infection prevented this increase (asterisk denotes significant difference between NON-SDR/Infected and SDR/Infected conditions). Infection significantly increased the number of virus-specific CD4+ T cells (see Panel C) and virus-specific CD8+ T cells (see Panel D) at day 8 post-infection (denoted by asterisk). SDR exposure did not significantly attenuate the elevation in either CD4+ or CD8+ T cells resulting from infection.
Additionally, infection increased virus-specific CD8+ cells in the CNS, an effect that was also significantly attenuated by prior exposure to social disruption. ANOVA verified significant main effects of stress (F (1, 20) = 10.197, p < 0.01) and infection (F (1, 20) = 12.338, p < 0.01) as well as a significant stress x infection interaction (F (1, 20) = 10.344, p < 0.01) (Figure 4B). Again, post hoc analyses indicate that the non-stressed/infected mice had significantly higher numbers of virus-specific CD8+ T cells compared to all other conditions and confirmed a significant difference between the NON-SDR/Infected condition and the SDR/Infected condition.
Similar to what was observed in the CNS, infection was found to increase the number of virus-specific CD4+ T cells in the spleen (F (1, 19) = 8.833, p < 0.01). However, this effect was not significantly altered by SDR exposure prior to infection (all other F’s < 0.862) (Figure 4C). Also, a main effect of infection on virus-specific CD8+ T cell populations in the spleen approached significance (F (1, 18) = 3.905, p = 0.064), but no other significant main effects or interactions were present (all other F’s < 0.574) (Figure 4D).
4. Discussion
The present findings suggest that prior exposure to social disruption stress exacerbates acute Theiler’s virus infection by suppressing T cells responses in the CNS. In order to evaluate the effects of social disruption on the virus-specific adaptive immune response, we found it necessary to use a mouse strain for which the viral peptide sequences recognized by CD4+ (Gerety et al., 1991, 1994) and CD8+ (Kang et al., 2002) T cell populations have been established. This necessitated a move to the SJL strain and, further required a demonstration that SDR exacerbates acute phase TMEV and impairs viral clearance from the CNS in SJL mice. This validation of the model in the new strain is valuable on its own because it shows the SDR-induced exacerbation of TMEV can be generalized across genetically distinct populations of mice. Specifically, we found that SJL mice show a pattern of increased viral load and behavioral exacerbation when exposed to SDR prior to TMEV infection, an effect similar to the pattern previously seen in Balb/cJ mice (Johnson et al., 2004; Meagher et al., 2007).
Once the basic finding of impaired viral clearance and behavioral exacerbation was established, we could then use the SJL strain to test our hypotheses related to T cell suppression following SDR. Chronic SDR impaired the TMEV-induced increase in CD4 and CD8 mRNA expression in CNS. CNS-ILs isolated from stressed mice (SDR) and re-stimulated in culture with either the immunodominant CD4+ (VP274-86) or CD8+ (VP3159-166) T cell epitopes had significantly reduced numbers of virus-specific IFN-γ producing CD4+ and CD8+ T cells compared to unstressed controls (NON-SDR). Lymphocyte populations isolated from the spleen did not show an SDR-induced reduction in virus-specific CD4+ or CD8+ T cells. Taken together with previous findings linking severity of behavioral signs of disease and viral load during acute infection to viral persistence and demyelination during late disease (Borrow et al., 1992, 1993; Johnson et al., 2006; Sieve et al., 2004, 2006; Young et al., 2010), the present results suggest that social stress-induced suppression of central virus-specific T cell responses may contribute to exacerbation of chronic phase disease severity.
4.1. TMEV and adaptive immunity
Following intracranial infection with TMEV, susceptible mice fail to generate a well-orchestrated immune response leading to viral persistence and initiation of a subsequent chronic demyelinating disease (Theiler, 1937; Clatch et al., 1990; Zheng et al., 2001). Both CD4+ and CD8+ T cells have been shown to play a vital role in the early clearance of TMEV from the CNS. For example, susceptible strains of mice exhibit a significant reduction in virus-specific IFN-γ producing CD4+ T cells in the CNS during acute TMEV infection (Mohindru et al., 2006). Along these same lines, Lin et al. (2004) report that clearance of the DA strain of TMEV is CD4+ T cell dependent and the inhibition of this response may be a contributing factor to disease susceptibility. In addition, selective deletion of CD4+ T cells during the time of infection has been shown to elevate mortality during the acute phase in mice (Welsh et al., 1987). Evidence has also established a role for CD8+ T cells in early viral clearance. Borrow et al. (1992) reported a higher acute phase viral load as well as accelerated onset and increased severity for the chronic phase following selective depletion of CD8+ T cells prior to infection. Further supporting a role for CD8+ T lymphocytes, both β-2 microglobulin deficient (Begolka et al., 2001; Fiette et al., 1993; Miller et al., 1995; Pullen et al., 1993) and perforin-deficient (Palma et al., 2001; Murray et al., 1998; Rossi et al., 1998) mice are rendered susceptible to chronic demyelination, presumably due to a disruption of antigen presentation and altered CD8+ T cell effector function leading to impaired viral clearance.
4.2. Social stress and the adaptive immune response
Research with human subjects suggests that psychological stress can increase T lymphocyte apoptosis (Sakami et al., 2002) and decrease virus-specific T cell proliferation in the periphery (Glaser et al., 1993). In addition, both the glucocorticoid and catecholamine responses to stress have been shown to promote a shift from Th1-mediated cellular immunity towards Th2-mediated humoral immunity and thereby suppress virus-specific T cell responses (Elenkov & Chrousos, 1999; Panina-Bordignon et al., 1997). In the present studies, social stress suppressed type 1 CD4+ and CD8+ T cell responses to viral infection of the CNS. Interestingly, this effect was not seen in the periphery (e.g. spleen) where the number of virus-specific T cells was elevated by infection but was not impaired by prior social stress exposure. Recently, Steelman et al. (2009) reported overall immunosuppression of TMEV-induced Th1 and Th2 responses as well as CD4+ and CD8+ T cell effector functions in the periphery and CD8+ T cells responses in the CNS following chronic restraint stress. While our pattern of results differs somewhat from that reported by Steelman et al. (2009) following chronic restraint, this may be due to stressor-specific differences in the physiological response to the stressor.
Stressors can be differentiated by method of induction (e.g. restraint, social hierarchy disruption, etc) as well as by the pattern of resulting activation of the HPA axis, SNS and immune systems (Bowers et al., 2007; Cohen & Hamrick, 2003; Sheridan et al., 2004). Like other stressors, the impact of SDR on immune function depends on the type of challenge (e.g. viral, bacterial, endotoxic), the route of exposure/location of challenge (e.g. systemic, skin, lung, CNS), and the timing of the stressor exposure in relation to the challenge. For example, we have shown that changing only the timing of SDR in relation to infection with TMEV determines whether SDR exposure has adverse or beneficial effects on the immune response to infection (Johnson et al., 2004, 2006). Even if only studies in which SDR exposure occurs prior to infection with a virus are considered, the direction of the behavioral, physiological and immunological effects varies across disease models. A recent report by Sommershof and colleagues (2011) indicates SDR-induced suppression of T cell function during peripheral infection with lymphocytic choriomeningitis virus (LCMV) but with no effect on viral clearance. However, SDR has also been shown to increase myeloid cells in peripheral tissues (bone marrow, blood, and spleen) while decreasing lymphocytes at the same sites (Engler et al., 2004). A reduction in lymphocytes could impair viral clearance particularly if the reduction were in CD8+ cells making IFN-γ. We detected suppression of CD4+ and CD8+ T cell populations with profound implications for viral clearance from the CNS. These divergent effects may reflect the independent requirements for effective clearance of different viruses. On the other hand, these seemingly incongruous findings may reflect differential effects of SDR in the periphery (LCMV) and in the CNS (TMEV) or differential dependence on T cell populations in clearing a virus from different immune compartments.
In the present study, we report suppression of infection-induced T cell activity in the CNS following SDR. Interestingly, previous research has shown that rats exposed to social defeat have reduced accumulation of T cells in peripheral lymphoid tissues for at least a week following cessation of the stressor (Engler & Stefanski, 2003; Stefanski et al., 2003). This initial suppression of T cell numbers was followed by a subsequent increase in the proliferative response to a challenge. Along these same lines, Mays et al. (2010) also recently reported that SDR increased numbers of CD8+ memory T cells 6-12 weeks following influenza vaccination. SDR has also been shown to enhance the local population of immune cells present in the LPS infected brain (Quan et al., 2001), infiltrating the naïve lung (Mays et al., 2010), and present in the skin following inflammatory challenge (Young et al., 2009). Social stress has repeatedly been shown to be a powerful modulator of the innate and adaptive immune responses, though the mechanism may differ between models. With respect to the current findings during acute TMEV infection, the immediate suppression of T cell activity likely allows unchecked viral replication, but as T cell function recovers CD4+ and CD8+ T cell proliferation could rebound. Given that mortality is not significantly increased when SDR precedes acute TMEV infection, it is likely that T cell populations do recover at some later time, which would be in agreement with findings by Steelman et al. (2009) that the effects of restraint dissipate after the stressor is discontinued. In the context of our previous reports that SDR increases susceptibility to the chronic demyelinating phase of TMEV infection, our present findings would seem to suggest that an early and transient disruption of T cell function is enough to augment subsequent disease susceptibility. We discuss potential mechanisms by which this early impairment of adaptive immunity may contribute to subsequent disease susceptibility.
First, suppression of virus-specific adaptive immunity likely increases the risk for viral persistence which, by itself, would contribute to increased chronic phase disease severity. Both direct viral lysis of oligodendrocytes (Roos & Wollman, 1988) and immune responses directed against virus-expressing cells in the CNS (Clatch et al., 1987; Welsh et al., 1987) has been reported in chronic TMEV. SDR suppresses plasma IFN-γ and reduces CD8+ T cell proliferation in the spleen following peripheral viral infection (Sommershof et al., 2011). These effects on IFN-γ production have important implications for the present data as IFN-γ plays a critical role in viral clearance during acute TMEV infection. INF-γ-/- mice exhibit increased mortality following TMEV infection (Murray et al., 2002; Rodriguez et al., 2003), likely due to impaired antiviral activity and higher CNS viral load (Welsh et al., 1995).
Second, SDR-induced sensitization of the proinflammatory cytokine IL-6 may alter the subsequent differentiation of naïve T cells resulting in ineffective anti-viral responding. Social stress has previously been shown to stimulate and/or sensitize the innate proinflammatory cytokine response (Meagher et al., 2007; Quan et al., 2001; Vichaya et al., 2011). In the TMEV model specifically, we have found that SDR alone increases circulating and CNS levels of the pro-inflammatory cytokine IL-6 and blocking IL-6 activity during SDR exposure attenuates the exacerbation of the acute TMEV disease (Meagher et al., 2007; Vichaya et al., 2010). Moreover, IL-6 has also been shown to facilitate a shift in CD4+ T cell populations from Th1 to Th2 or Th17 (Zou & Restifo, 2010). Both Th2 and Th17 responses have been linked with autoimmune disease susceptibility in animal models (Bettelli et al., 2007; Carding et al., 1993; Chen et al., 2006; Komiyama et al., 2006; Sutton et al., 2006; Ramsey et al., 1993; Rangachari et al., 2006) as well as impaired viral clearance (Hou et al., 2009). The current findings are consistent with the hypothesis that sensitization of the early proinflammatory cytokine response within the CNS translates into an impaired adaptive immune response (Vichaya et al., 2011).
A third possibility is that stress-induced thymic atrophy, which increases apoptosis of glucocorticoid sensitive CD4+ and CD8+ thymic cells, could impair the process of negative selection that thymocytes undergo during maturation which in turn may increase the number of autoreactive T cells (Tarcic et al., 1998; Suvas et al., 2003). In this case, the SDR-induced impairment of acute viral clearance would be incidental, merely reflecting the reduction in T cells but not representing the mechanism by which this suppression acts to increase chronic disease susceptibility. Social defeat has been shown to cause lymphopenia and thymic atrophy (Stefanski & Engler, 2003), two processes that may work synergistically to increase autoimmunity. In the NOD model of autoimmune diabetes, mice are lymphopenic and the remaining CD4+ and CD8+ T cells exhibit a “memory-like” phenotype that is more likely to be autoreactive (Le Saout et al., 2008). In the case of TMEV, an increased population of autoreactive T cells could contribute to subsequent demyelination through a virus-independent process. This is the first demonstration that SDR can suppress the adaptive response to viral infection of the CNS and further investigation into the mechanisms by which suppression during the acute TMEV infection results in exacerbation during the chronic phase are warranted.
Implications for MS
Epidemiological studies have suggested roles for viral infection and stress in the etiology of MS, though no one virus or specific stressor type has been identified. It appears that the viruses with the strongest relationship with MS are those that persist in the CNS even after the initial response to infection has resolved (Allen & Brankin, 1993). These findings are in agreement with data from our laboratory showing a relationship between impaired viral clearance/increased viral load and susceptibility to chronic autoimmune-mediated demyelination. This suggests that those individuals diagnosed with a viral infection who are also experiencing abnormal levels of stress may be at increased risk for later life demyelination as a result of altered innate and adaptive immune responses to the viral pathogen. Alternatively, stress may act in a more indirect fashion. For instance, it is well-known that infection increases the risk of exacerbation in MS patients (Buljevac et al., 2002). Stress occurring during the natural history of MS may increase susceptibility to infections (e.g. Rhinoviral infections) that can then trigger relapse (Cohen, 1991).
Acknowledgments
This research was supported by NIH/NINDS R01-NS060822 awarded to MWM and CJW. The authors would like to thank Dr. Colin Young, Department of Veterinary Integrative Biosciences Texas A&M University, for his technical expertise and assistance. In addition, we would like to thank Kristen Montgomery and Adriana Mariscal for their work on behavioral data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman KD, Heyman R, Rabin BS, Anderson BP, Houck PR, Frank E, Baum A. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2003;64:916–920. doi: 10.1097/01.psy.0000038941.33335.40. [DOI] [PubMed] [Google Scholar]
- Allen I, Brankin B. Pathogenesis of multiple sclerosis-the immune diathesis model and the role of viruses. J Neuropathol Exp Neurol. 1993;52:95–105. doi: 10.1097/00005072-199303000-00001. [DOI] [PubMed] [Google Scholar]
- Begolka WS, Haynes LM, Olson JK, Padilla J, Neville KL, Dal Canto M, Palma J, Kim BS, Miller SD. CD8-deficient SJL mice display enhanced susceptibility to Theiler’s virus infection and increased demyelinating pathology. J Neurovirol. 2001;7(5):409–420. doi: 10.1080/135502801753170264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. Th17 cells in the circle of immunity and autoimmunity. Nature Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-derived nitric oxide regulates T cell activation via reversile disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Borrow P, Tonks P, Welsh CJ, Nash AA. The role of CD8+T cells in the acute and chronic phases of Theiler’s murine encephalomyelitis virus-induced disease in mice. J Gen Virol. 1992;73(7):1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- Borrow P, Welsh CJR, Dean D, Tonks P, Blakemore WF, Nash AA. Investigation of the role of autoimmune responses to myelin in the pathogenesis of TMEV-induced demyelinating disease. Immunol. 1998;93:478–484. doi: 10.1046/j.1365-2567.1998.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Welsh CJR, Nash AA. Study of mechanisms by which CD4+ T cells contribute to protection in Theiler’s murine encephalomyelitis. Immunology. 1993;80(3):502–506. [PMC free article] [PubMed] [Google Scholar]
- Bowen JL, Olson JK. Innate immune CD11b+Gr-1+ cells, suppressor cells, affect the immune response during Theiler’s virus-induced demyelinating disease. J Immunol. 2009;183(11):6971–6980. doi: 10.4049/jimmunol.0902193. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2007;22(1):105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RF, Tennant CC, Sharrock M, Hodgkinson S, Dunn SM, Pollard JD. Relationship between stress and relapse in multiple sclerosis: part 1. Important features. Mult Scler. 12:453–464. doi: 10.1191/1352458506ms1295oa. [DOI] [PubMed] [Google Scholar]
- Brown RF, Tennant CC, Sharrock S, Hodgkindon S, Dunn SM, Pollard JD. Relationship between stress and relapse in multiple sclerosis: part II. Direct and indirect relationships. Mult Scler. 2006;12:465–475. doi: 10.1191/1352458506ms1296oa. [DOI] [PubMed] [Google Scholar]
- Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, Welsh CJR. The effects of restraint on the neuropathogenesis of Theiler’s virus infection: I. Acute disease. Brain Behav Immun. 2001;15:235–254. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- Carding SR, Allan W, McMickle A, Doherty PC. Activation of cytokine genes in T cells during primary and secondary murine influenza pneumonia. J Exp Med. 1993;177:475–482. doi: 10.1084/jem.177.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, Mckenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatch RJ, Miller SD, Metzner R, Dal Canto MC, Lipton HL. Monocytes/macrophages isolated from the mouse central nervous system contain infectious Theiler’s murine encephalomyelitis virus (TMEV) Virology. 1990;176:244–254. doi: 10.1016/0042-6822(90)90249-q. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick H. Stable individual differences in physiological response to stressors: implications for stress-elicited changes in immune related health. Brain Behav Immun. 2003;17:407–414. doi: 10.1016/s0889-1591(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Curry JM, Hanke ML, Piper MG, Bailey MT, Bringardner BD, Sheridan JF, Marsh CB. Social disruption induces lung inflammation. Brain Behav Immun. 2010;24(3):394–402. doi: 10.1016/j.bbi.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al Quran SZ, Swan R, Chung C-S, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, LaFace D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204(6):1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto MC, Calenoff MA, Miller SD, Vanderlugt CL. Lymphocytes from mice chronically infected with Theiler’s murine encephalomyelitis virus produce demyelination of organotypic cultures after stimulation with the major encephalitis epitope of myelin proteolipid protein. Epitope spreading in TMEV has functional activity. J Neuroimmunol. 2000;104:79–84. doi: 10.1016/s0165-5728(99)00230-1. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, Pro/Anti inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripehral blood, and spleen. J Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Engler H, Stefanski V. Social stress and T cell maturation in male rats: transient and persistent alterations in thymic function. Psychoneuroendocrinology. 2003;28(8):951–969. doi: 10.1016/s0306-4530(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Muller U, Huang S, Aguet M, Brahic M, Bureau JF. Theiler’s virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J Exp Med. 1995;181:2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick LR, Barreiro Arcos ML, Rapanelli M, Zappia MP, Brocco M, Mongini C, Genaro AM, Cremaschi GA. Chronic restraint stress impairs T-cell immunity and promotes tumor progression in mice. Stress. 2009;12(2):134–143. doi: 10.1080/10253890802137437. [DOI] [PubMed] [Google Scholar]
- Fujinami RW, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19(1):80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Pearson GR, Bonnear RH, Esterling BA, Atkinson C, Kiecolt-Glaser JK. Stress and the memory T-cell response to the Epstein-Barr virus in healthy medical students. Health Psychol. 1993;12:435–442. doi: 10.1037//0278-6133.12.6.435. [DOI] [PubMed] [Google Scholar]
- Gornikiewicz A, Sautner T, Brostjan C, Schmierer B, Fugger R, Roth E, Muhlbacher F, Bergmann M. Catecholamines up-regulate lipopolysaccharide-induced IL-6 production in human microvascular endothelial cells. FASEB. 2000;14:1093–1100. doi: 10.1096/fasebj.14.9.1093. [DOI] [PubMed] [Google Scholar]
- Grant I, Brown GW, Harris T, McDonald WI, Patterson T, Trimble MR. Severely threatening events and marked life difficulties preceding onset or exacerbation of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989;52:8–13. doi: 10.1136/jnnp.52.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J Exp Med. 2009;206(2):313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AJ, Njenga MK, Hansen MJ, Kuhns ST, Chen L, Rodriguez M, Pease LR. Prevalent Class I-restricted T-cell response to the Theiler’s virus epitope Db: VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J Virol. 1999;73(5):3702–2708. doi: 10.1128/jvi.73.5.3702-3708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RR, Prentice TW, Bridegam P, Young CR, Steelman AJ, Welsh TH, Welsh CJR, Meagher MW. Social stress alters the severity and onset of the chronic phase of Theiler’s virus infection. J Neuroimmunol. 2006;175:39–51. doi: 10.1016/j.jneuroim.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Storts R, Welsh TH, Welsh CJR, Meagher MW. Social stress alters the severity of acute Theiler’s virus infection. J Neuroimmunol. 2004;148:74–85. doi: 10.1016/j.jneuroim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Kaminsky SG, Milisauskas V, Chen PB, Nakamura I. Defective differentiation of natural killer cells in SJL mice. Role of the thymus. J Immunol. 1987;138:1020–1025. [PubMed] [Google Scholar]
- Kang BS, Palma JP, Lyman MA, Dal Canto M, Kim BS. Antibody response is required for protection from Theiler’s virus-induced encephalitis in C57BL/6 mice in the absence of CD8+ T cells. Virology. 2005;340:84–94. doi: 10.1016/j.virol.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR. The role of regulatory T cells in chronic and acute viral infections. Clin Inf Dis. 2008;46(7):1046–1052. doi: 10.1086/529379. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Le Saout C, Mennecht S, Taylor N, Hernandez J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc Natl Acad Sci. 2008;105(49):19414–19419. doi: 10.1073/pnas.0807743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Johansen C, Bronnum-Hansen H, Stenager E, Koch-Henriksen N, Olsen J. The risk of multiple sclerosis in bereaved parents: A nationwide cohort study in Denmark. Neurology. 2004;62:726–729. doi: 10.1212/01.wnl.0000113766.21896.b1. [DOI] [PubMed] [Google Scholar]
- Lin X, Ma X, Rodriguez M, Roos RP. CD4+ T cells are important for clearance of DA strain of TMEV from the central nervous system of SJL/J mice. Int Immunol. 2004;16:1237–1240. doi: 10.1093/intimm/dxh125. [DOI] [PubMed] [Google Scholar]
- Lipton HL. Theiler’s virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton HL, Melvold R. Genetic analysis of susceptibility to Theiler’s virus-induced demyelinating disease in mice. J Immunol. 1984;132:1821–1825. [PubMed] [Google Scholar]
- Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320(5880):1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays JW, Bailey MT, Hunzeker JT, Powell ND, Papenfuss T, Karlsson EA, Padgett DA, Sheridan JF. Influenza virus-specific immunological memory is enhanced by repeated social defeat. J Immunol. 2010;184(4):2014–2025. doi: 10.4049/jimmunol.0900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Johnson RR, Young EE, Vichaya EG, Lunt S, Hardin EA, Connor MA, Welsh CJR. Interleukin-6 as a mechanism for the adverse effects of social stress on acute Theiler’s virus infection. Brain Behav Immun. 2007;21(8):1083–1095. doi: 10.1016/j.bbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Prentice TW, Young CR, Johnson RR, Sieve AN, Meagher MW, Welsh CJ. Restraint stress decreases virus-induced pro-inflammatory cytokine mRNA expression during acute Theiler’s virus infection. J Neuroimmunol. 2006;178:49–61. doi: 10.1016/j.jneuroim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Rivera-Quinones C, Njenga MK, Leibowitz J, Rodriguez M. Spontaneous CNS remyelination in beta 2 microglobulin-deficient mice following virus-induced demyelination. J Neurosci. 1995;15(12):8345–8352. doi: 10.1523/JNEUROSCI.15-12-08345.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SD, VanDerlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler’s virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- Mohindru M, Kang B, Kim BS. Initial capsid-specific CD4+ T cell responses protect against Theiler’s murine encephalomyelitis virus-induced demyelinating disease. Eur J Immunol. 2006;36:1–10. doi: 10.1002/eji.200535785. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Dick LP. Multiple Sclerosis. In: Camic PM, Knight S, editors. Clinical Handbook of Health Psychology: a Practical Guide to Effective Interventions. Hogrefe and Huber; Seattle: 1998. pp. 313–348. [Google Scholar]
- Murray PD, McGavern DB, Pease LR, Rodriguez M. Cellular sources and targets of IFN-γ-mediated protection against viral demyelination and neurological deficits. Eur J Immunol. 2002;32:606–615. doi: 10.1002/1521-4141(200203)32:3<606::aid-immu606>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PD, McGavern DB, Lin X, Njenga MK, Leibowitz J, Pease LR, Rodriguez M. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J Neurosci. 1998a;18(18):7306–7314. doi: 10.1523/JNEUROSCI.18-18-07306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PD, Pavelko KD, Leibowitz J, Lin X, Rodriguez M. CD4(+) and CD8(+) T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J Virol. 1998b;72:7320–7329. doi: 10.1128/jvi.72.9.7320-7329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseworthy JH, Licchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. Theiler’s virus infection: a model for multiple sclerosis. Clin Microbiol Rev. 2004;17:174–207. doi: 10.1128/CMR.17.1.174-207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma JP, Lee HG, Mohindru M, Kang BS, Dal Canto M, Miller SD, Kim BS. Enhanced susceptibility to Theiler’s virus-induced demyelinating disease in perforin-deficient mice. J Neuroimmunol. 2001;116(2):125–135. doi: 10.1016/s0165-5728(01)00293-4. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, Mazzeo D, Lucia PD, D’Ambrosio D, Lang R, Fabbri L, Self C, Sinigaglia F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 1997;100(6):1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak Y, Ovadia H, Goshen I, Gurevich R, Monsa K, Avitsur R, Yirmiya R. Behavioral aspects of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;104:31–36. doi: 10.1016/s0165-5728(99)00257-x. [DOI] [PubMed] [Google Scholar]
- Pullen LC, Miller SD, Dal Canto MC, Kim BS. Class I-deficient resistant mice intracerebrally inoculated with Theiler’s virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur J Immunol. 1993;23(9):2287–2293. doi: 10.1002/eji.1830230935. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Califiui M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Ramsay AJ, Ruby J, RAmshaw IA. A case for cytokines as effector molecules in the resolution of viral infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203(8):2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hygiene. 1938;27:493–497. [Google Scholar]
- Rodriguez M, Zoecklein LJ, Howe CL, Pavelko KD, Gamez J, Nakane S, Papke LM. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler’s murine encephalomyelitis virus infection. J Virol. 2003;77:12252–12265. doi: 10.1128/JVI.77.22.12252-12265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi CP, McAllister A, Tanguy M, Kagi D. Theiler’s virus infection of perforin-deficient mice. J Virol. 1998;72(5):4515–4519. doi: 10.1128/jvi.72.5.4515-4519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakami S, Nakata A, Yamamura T, Kawamura N. Psychological stress increases human T cell apoptosis in vitro. Neuroimmunomodulation. 2002;10(4):224–231. doi: 10.1159/000068326. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Padgett DA, Avitsur R, Marucha PT. Experimental models of stress and wound healing. World J Surg. 2004;28:327–330. doi: 10.1007/s00268-003-7404-y. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJR, Meagher MW. Chronic restraint stress during early Theiler’s virus infection exacerbates the subsequent demyelinating disease in SJL mice. J Neuroimmunol. 2004;155:103–118. doi: 10.1016/j.jneuroim.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJR, Meagher MW. Sex dependent effects of chronic restraint stress during early Theiler’s virus infection on the subsequent demyelinating disease in CBA mice. J Neuroimmunol. 2006;177:46–62. doi: 10.1016/j.jneuroim.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Sommershof A, Basler M, Reither C, Engler H, Groettrup M. Attenuation of the cytotoxic T lymphocyte response to lymphocute choriomeningitis virus in mice subjected to chronic social stress. Brain Behav Immun. 2011;25(2):340–348. doi: 10.1016/j.bbi.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Smith JB, Herschman HR. Identification of inflammatory mediators by screening for glucocorticoid-attentuated response genes. Methods Enzymol. 1997;287:250–265. doi: 10.1016/s0076-6879(97)87019-x. [DOI] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1799–1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Stefanski V, Peschel A, Reber S. Social stress affects migration of blood T cells into lymphoid organs. J Neuroimmunol. 2003;138(1-2):17–24. doi: 10.1016/s0165-5728(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Steelman AJ, Dean DD, Young CR, Smith R, III, Prentice TW, Meagher MW, Welsh CJR. Restraint stress modulates virus specific adaptive immunity during acute Theiler’s virus infection. Brain Behav Immun. 2009;23:830–843. doi: 10.1016/j.bbi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond) 2001;101:185–192. [PubMed] [Google Scholar]
- Stinissen P, Rau J, Zhang J. Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies. Crit Rev Immunol. 1997;17:33–75. doi: 10.1615/critrevimmunol.v17.i1.20. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh N, Mills KHG, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse B. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198(6):889. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki A, Huang QH, Somogyvari-Vigh A, Arimura A. Immobilization stress may increase plasma interleukin-6 via central and peripheral catecholamines. Neuroimmunomodulation. 1994;1:335–342. doi: 10.1159/000097185. [DOI] [PubMed] [Google Scholar]
- Tarcic N, Ovadia H, Weiss DW, Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol. 1998;82(1):40–46. doi: 10.1016/S0165-5728(97)00186-0. [DOI] [PubMed] [Google Scholar]
- Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler M. Spontaneous Encephalomyelitis of Mice, a New Virus Disease. J Exp Med. 1937;65:705–719. doi: 10.1084/jem.65.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J-N, Mathieu J, Mailfert Y, Multon E, Drouet C, Jouan A, Drouet E. Chronic restraint stress induces severe disruption of the T-cell specific response to tetanus toxin vaccine. Immunology. 2001;102(1):87–93. doi: 10.1046/j.1365-2567.2001.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Kuang L-Q, Fujinami RS. Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J Neuropathol Exp Neurol. 1996;55:673–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Young EE, Esperon MF, Cook JL, Welsh CJR, Meagher MW. Social disruption induced priming of CNS inflammatory response to Theiler’s virus is dependent upon stress-induced IL-6 release. J Neuroimmunol. 2011;239(1-2):44–52. doi: 10.1016/j.jneuroim.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S, Greenhill S, Warren KG. Emotional stress and the development of multiple sclerosis: case-control evidence of a relationship. J Chronic Dis. 1982;35:821–831. doi: 10.1016/0021-9681(82)90047-9. [DOI] [PubMed] [Google Scholar]
- Welsh CJ, Bustamante L, Nayak M, Welsh TH, Dean DD, Meagher MW. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection II: NK cell function and cytokine levels in acute disease. Brain Behav Immun. 2004;18:166–174. doi: 10.1016/S0889-1591(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Welsh CJR, Tonks P, Nash AA, Blakemore WF. The effect of L3T4 cell depletion on the pathogenesis of Theiler’s murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68:1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Psychoneuroendocrinology. 2012;37(9):1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EE, Sieve AN, Vichaya EG, Carcoba LM, Young CR, Ambrus A, Storts R, Welsh CJ, Meagher MW. Chronic restraint stress during early Theiler’s virus infection exacerbates the subsequent demyelinating disease in SJL mice: II. CNS disease severity. J Neuroimmunol. 2010;220:79–89. doi: 10.1016/j.jneuroim.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EE, Prentice TW, Satterlee D, McCullough H, Sieve AN, Johnson RR, Welsh TH, Welsh CJR, Meagher MW. Glucocorticoid exposure alters the pathogenesis of Theiler’s murine encephalomyelitis virus during acute infection. Physiol Behav. 2008;95:63–71. doi: 10.1016/j.physbeh.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Calenoff MA, Dal Canto MC. Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler’s murine encephalomyelitis virus infection leading to demyelination. J Neuroimmunol. 2001;118:256–267. doi: 10.1016/s0165-5728(01)00338-1. [DOI] [PubMed] [Google Scholar]
- Zou W, Restifo NP. Th17 cells in tomour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]


