Abstract
Immunomodulatory cytotoxins are prominent virulence factors produced by Staphylococcus aureus, a leading cause of bacterial sepsis, skin infection, and pneumonia. S. aureus α-toxin is a pore-forming toxin that utilizes a widely expressed receptor, ADAM10, to injure the host epithelium, endothelium, and immune cells. As each host tissue is characterized by a unique composition of resident cells and recruited immune cells, the outcome of α-toxin-mediated injury may depend on the infected tissue environment. Utilizing myeloid lineage-specific Adam10 knockout mice, we show that α-toxin exerts tissue-specific effects on innate immunity to staphylococcal infection. Loss of ADAM10 expression exacerbates skin infection, yet affords protection against lethal pneumonia. These diverse outcomes are not related to altered immune cell recruitment, but rather correlate with a defect in toxin-induced IL-1β production. Extension of these studies through analysis of ADAM10 double-knockout mice affecting both the myeloid lineage and either the skin or lung epithelium highlight the prominence of toxin-induced injury to the epithelium in governing the outcome of infection. Together, these studies provide evidence of tissue specificity of pore-forming cytotoxin action in the modulation of host immunity, and illustrate that the outcome of infection is a collective manifestation of all effects of the toxin within the tissue microenvironment.
Key Words: ADAM10, Innate immunity, Myeloid lineage-specific knockout, Pathogenesis, Staphylococcus aureus, α-Toxin
Introduction
Staphylococcus aureus is a versatile human pathogen capable of causing disease in multiple body tissues [1]. Predominantly associated with skin and soft tissue infection, S. aureus remains a leading cause of severe invasive disease, including bacteremia and sepsis, pneumonia, and osteomyelitis. While the severity of skin infection is often limited, deep tissue infection can be life threatening. Confounding the treatment of clinical staphylococcal disease, S. aureus demonstrates a remarkable capacity for rapid development of resistance to antimicrobials [2]. The once potent class of β-lactam antimicrobials has become nearly obsolete in the treatment of modern-day S. aureus infection, given the rampant spread of methicillin-resistant S. aureus (MRSA) strains. In the US alone, MRSA was responsible for nearly 100,000 invasive infections and 20,000 deaths in 2005 [3]. Updated Centers for Disease Control estimates document >80,000 cases of drug-resistant S. aureus invasive disease and >11,000 deaths in 2011, leading to the classification of this pathogen as a serious public health threat [4].
The diverse clinical manifestations of S. aureus disease highlight the complex interaction of bacterial virulence factors with host tissues. Site-specific defenses against infection including tissue barrier function and innate immune cells represent one of the most formidable challenges to early pathogen survival and host invasion. S. aureus encodes an array of toxins that target these defenses, including pore-forming leukotoxins, hemolysins, and a family of small peptides termed the phenol-soluble modulins [5, 6]. A predominant action of these toxins is host immune cell lysis, injuring neutrophils, macrophages, and platelets in addition to T and B cells that contribute to adaptive immunity. S. aureus α-toxin (α-hemolysin, Hla) is a chromosomally encoded pore-forming toxin secreted as a water-soluble monomer. Upon binding to the host cell membrane, Hla rapidly assembles into a homoheptamer and inserts a β-barrel across the lipid bilayer to create a 1- to 2-nm cytolytic pore in toxin-sensitive cells. Hla exhibits the broadest range of cell specificity among the staphylococcal toxins, contributing to lysis and host cell signaling in immune cells, platelets, and epithelial and endothelial cells [6]. A disintegrin and metalloprotease 10 (ADAM10) is encoded by the gene Adam10, and functions as a cellular receptor for Hla [7]. The expression of ADAM10 on host alveolar epithelial cells, keratinocytes, and endothelial cells in vitro renders these cells susceptible to lytic injury [8, 9, 10]. Further, subcytolytic concentrations of Hla result in the rapid upregulation of ADAM10 catalytic activity, inducing both epithelial and endothelial barrier disruption through the pathologic cleavage of native ADAM10 substrates E-cadherin and VE-cadherin, respectively [8, 9, 10]. Conditional knockout of Adam10 in the alveolar epithelium and the epidermis confirms the role of the toxin-receptor interaction in disease, as these mice are protected against lethal lung infection and severe dermonecrotic skin injury [8, 9]. While epithelial Adam10-knockout studies demonstrated a link between barrier tissue injury and disease outcome, S. aureus survival was not significantly modified in these mice. In contrast, active and passive immunization strategies targeting Hla also confer protection against severe skin and lung infection, but are associated with a reduction in bacterial recovery [11, 12]. Together, these findings suggest that Hla may have distinct cellular actions in the complex tissue microenvironment that collectively yield disease manifestations.
The principal host immune response to S. aureus skin and lung infection is an acute inflammatory cell infiltrate [13, 14]. An array of studies indicates that Hla can target both neutrophils and monocytes. While neutrophils are relatively resistant to toxin-induced cell death [15, 16], monocytes sustain membrane injury and undergo cell death following exposure to Hla [16, 17, 18]; these findings are consistent with relatively lower levels of ADAM10 expression on human neutrophils [18]. Human monocytes and macrophages secrete IL-1β in response to stimulation with low concentrations of the toxin [18, 19, 20], and primary mouse neutrophils exhibit a dampened IL-1β response to live S. aureus in the presence of anti-toxin antisera [21]. Several studies have shed light on IL-1β secretion in response to S. aureus Hla, demonstrating that the toxin can contribute to two distinct cellular signals - first, the generation of pro-IL-1β is enhanced in macrophages by stimulation of NOD2, an intracellular pattern recognition receptor that senses staphylococcal peptidoglycan in the presence of Hla, dependent on the ability of the toxin to facilitate cytosolic exposure to peptidoglycan [22]. Second, processing of pro-IL-1β to its mature, secreted form then occurs through Hla-stimulated assembly of the NLRP3 inflammasome, resulting in caspase-1 activation and cleavage of pro-IL-1β [20, 23]. As such, exposure of innate immune cells to the toxin in the context of live or heat-killed S. aureus (HKSA) elicits more robust IL-1β production than toxin alone [21, 23] and facilitates the increased generation of IL-6, an inflammatory cytokine that can enhance neutrophil killing of staphylococci [22]. Hla-deficient S. aureus strains exhibit defects in macrophage IL-1β production [20]; similarly, Nlrp3−/− macrophages display impaired IL-1β production in response to S. aureus supernatants and these mice lack IL-1β in tissue homogenates in response to staphylococcal skin and lung infection [20, 24]. S. aureus lung infection of Nlrp3−/− mice is associated with improved outcome relative to wild-type control mice, further suggesting that inflammasome activation by Hla patterns the host response to infection [24].
Efforts to understand the Hla-innate immune cell interaction in vivo have relied on S. aureus mutants lacking Hla expression, toxin-neutralizing immunization strategies, or deletion of host signaling proteins that are responsive to multiple bacterial factors such as other pore-forming S. aureus toxins [11, 12, 20, 23, 24, 25, 26, 27, 28]. As such, these approaches confound the direct analysis of the tissue-specific effects of Hla in patterning host innate immune responses. To circumvent these limitations, this study examines S. aureus infection in mice harboring a selective knockout of Adam10 in myeloid lineage cells, leading to the observation that toxin-induced injury to innate immune cells contributes to tissue-specific patterning of host outcome of infection.
Materials and Methods
Bacterial Strains and Growth Conditions
S. aureus LAC/USA300 and the isogenic hla::erm mutant that does not express Hla [11] were grown at 37°C with shaking in tryptic soy broth or on tryptic soy agar plates without antibiotics as previously described [29]. For mouse infections, staphylococcal cultures were prepared as described previously for skin and lung infection [8, 9]. Briefly, 50 ml of S. aureus culture grown to mid-log phase (OD660 0.5) were sedimented, washed with phosphate-buffered saline (PBS), and resuspended in 1,000-1,500 μl PBS for intranasal infection [2-3 × 108 colony forming units (CFU)/30 μl inoculum] or 17-50 ml PBS for intradermal infection (1-3 × 107 CFU/50 μl inoculum).
Cell Culture, Antibodies, and Reagents
All cultured cells were grown at 37°C in a 5% CO2 incubator. To obtain primary murine bone marrow-derived macrophages, bones of the hind legs were dissected from adult mice, cleaned, sterilized by submersion in ethanol, and flushed with cold PBS. The resulting marrow suspension was passed once through a 40-μm mesh filter, centrifuged, and plated into tissue culture dishes for passaging over 7 days in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS), L-glutamine, and 20–25% conditioned L-929 media to promote differentiation and maturation of macrophages. To obtain L-929 conditioned media containing murine M-CSF, L-929 murine fibroblasts were grown past confluence in DMEM/F12 medium supplemented with FBS and L-glutamine. Media from these cultures were harvested, filter sterilized, and stored at −80°C until use.
Primary mouse neutrophils were isolated from bone marrow suspensions by positive selection with Ly-6G microbeads (Miltenyi) according to the manufacturer's instructions. For experimental verification of Adam10 knockout, primary neutrophils were further purified by FACS sorting on the basis of Gr-1 positivity.
Conjugated antibodies for flow cytometry were purchased from Biolegend and included APC-Cy7-CD45, APC-Gr-1, APC-CD11b, PE-F4/80, and corresponding fluorophore-conjugated rat IgG isotype controls. Purified anti-mouse CD16/32 (Biolegend) was used for Fc receptor blocking. For flow-cytometric evaluation of ADAM10 protein, cells were stained with anti-ADAM10 primary antibody (R&D Systems) followed by Alexa488 donkey anti-rat secondary antibody (Invitrogen). Flow cytometry was performed on BD FACSCanto, LSR II, and FACSAria II instruments. Rat primary antibodies for Ly-6G (eBioscience), MoMa-2 (AbD Serotec) or rat IgG2B isotype control (Biolegend) followed by Alexa488 donkey anti-rat secondary antibody (Invitrogen) were used for immunofluorescence staining of frozen sections.
Myeloperoxidase activity was quantified using a colorimetric assay and purified human myeloperoxidase standards (Sigma-Aldrich) as described [21]. In vitro cytotoxicity was assessed using an LDH release assay (Roche) as previously described [7]. Where indicated, total protein concentrations in samples were measured by DC protein assay (Bio-Rad). Murine IL-1β levels were measured by ELISA (R&D Systems) according to the manufacturer's instructions. Ex vivo neutrophil chemotaxis assays were performed with a modified Boyden Transwell assay [30]. In brief, the bottom chambers of a 96-well Transwell plate (Corning, 0.3-mm pore size) were prepped by adding 150 μl of media alone, HBSS containing 0.5% FBS, or with the addition of 1 × 106 CFU S. aureus USA300/LAC. Just prior to assay, primary neutrophils purified from bone marrow of control or Adam10−/− mice were pre-incubated for 30 min in RPMI containing 1% FBS at 37°C in a 5% CO2 incubator. Cells were pelleted and resuspended to ∼1.5 × 106 cells/ml in HBSS plus 0.5% FBS; 100 μl of the cell suspension were added to the top chamber of each Transwell and incubated at 37°C in a 5% CO2 incubator for 50 min after which time EDTA (0.5 M, pH 7.4) was added to the bottom chambers and incubated at 4°C for 10 min. Migrated cells were counted using a hemocytometer.
Animal Strains and Breeding
All animal work was performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Chicago. LysMcre transgenic C57Bl/6 mice [31] (Jackson Laboratories) were bred to Adam10loxP/loxP transgenic C57Bl/6 mice [32] (Jackson Laboratories) to generate LysMcre Adam10loxP/loxP mice (Adam10−/−). To verify Cre-mediated excision of the loxP-flanked Adam10 locus, bone marrow-derived macrophages and primary neutrophils were isolated as described above, and a region of the mouse Adam10 gene spanning part of the excised genomic fragment was amplified by PCR with the following primer pair: 5′ ACCTCTTAGCGATACCACAAGCC and 5′ CCATGGAAGTGTCCCTCTTCATTCGTAGG. A second pair amplifying an intact portion of the Adam10 genomic sequence (exon 11) was used as a control: 5′ GGCCAGCCTATCTGTGGAAAC and 5′ GTTGGCATCGAAGCAGCAATC.
Mice harboring a conditional knockout of Adam10 in the alveolar epithelium [8] or epidermal keratinocytes [9] have been described previously. LysMcre mice were crossed with these lines to generate double-knockout animals deficient in ADAM10 in either the pulmonary epithelium and myeloid lineage cells (LysM/SpcTet Adam10−/−), or the epidermis and myeloid lineage cells (LysM/K14 Adam10−/−).
Animal Infections and Procedures
Prior to infections and procedures, animals were anesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg) in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Chicago.
Skin Infection. The right flanks of 5- to 7-week-old male mice were trimmed with an electric razor and treated with hair removal cream prior to infection. For LysM/K14 Adam10−/− double-knockout studies, the exposed flank was treated topically for 5 days prior to infection with 1 mg/day 4-hydroxytamoxifen (Sigma) or 99.5% ethanol as a vehicle control as previously described [9]. Mice were anesthetized, infected via subcutaneous injection with 50 μl of S. aureus using a 28-gauge insulin syringe, and monitored for 14 days after infection with daily recordings of lesion sizes (abscess and dermonecrosis). Lesion area was calculated using the equation (A = [π/2] × l × w) as described [27]. In a small percentage of mice, the inoculum disrupted the tissue planes in the subcutaneous space, causing diffuse spread immediately upon injection and resulting in the formation of excessively large lesions within 1 day after infection. These animals were not included in subsequent analyses.
Lung Infection. Six to 8-week-old female mice (weight 15-19 g) were weighed, anesthetized, and infected via the intranasal route with 30 μl S. aureus as described previously [14]. Animals were monitored for morbidity and mortality at regular intervals for ≥96 h after infection. A small percentage of mice routinely succumbed within the first 2 h following infection, likely from the combined effects of aspiration and anesthesia. These animals were not included in subsequent analyses.
Harvesting and Analysis of Infected Animal Tissues. Animals were sacrificed and tissues harvested using sterile surgical tools. Skin lesions were harvested using disposable 8-mm punch biopsies as described [21]. Lesional Hla protein content was determined by sandwich ELISA. For flow-cytometric analysis of inflammatory infiltrates to the lung, the pulmonary circulation was first perfused by injection of 3 ml PBS into the right atrium prior to harvesting lung tissue. Lung and skin samples were homogenized using a rotor stator homogenizer (PRO Scientific). For bronchoalveolar lavage (BAL), the trachea was exposed surgically and a 20-gauge catheter (Exelint Medical Products) was inserted; 1 ml sterile PBS was infused into the lungs and withdrawn 10 s later. For evaluation of bacterial burden, homogenized tissues were serially diluted in sterile PBS and plated onto tryptic soy agar. Whole blood obtained from 7-week-old female mice by retroorbital bleed was anticoagulated with EDTA, and complete blood counts were performed by Comparative Clinical Pathology Services, LLC (Columbia, Mo., USA). Spleens and metatarsi from adult mice were harvested and fixed in 10% formalin for histologic evaluation of hematopoietic phenotypes. Paraffin embedding, mounting, sectioning, and hematoxylin-eosin staining were performed by the Human Tissue Resource Center at the University of Chicago or Nationwide Histology (Veradale, Wash., USA).
Immunofluorescence Staining and Microscopy
Tissue specimens were harvested into cassettes with OCT Compound (Tissue-Tek) and immediately snap-frozen in a dry ice/ethanol bath. Samples were stored at or below −20°C until sectioning. Sections (15 μm) were cut using a Microm HM550 cryostat (Thermo Scientific) and mounted onto glass slides. Slides were stored at −20°C until staining. Frozen tissue sections were blocked in PBS containing 1% BSA, 1:20 human IgG (Sigma), and 1:20 normal donkey serum (Jackson Immuno Research). After staining with primary and secondary antibodies, slides were mounted with glass coverslips using ProLong Gold Antifade Reagent with DAPI (Invitrogen). Images were obtained on an Olympus IX81 TIRF inverted microscope with a Hamamatsu Orca Flash 4.0 camera and processed using NIH ImageJ software.
In vitro Analysis of Cytokine Production
Primary mouse neutrophils and bone marrow-derived macrophages were obtained as described above. Cells were distributed to 96-well plates (105 cells/200 μl) in RPMI supplemented with 10% FBS and incubated for 6 h with 25 μg/ml of LPS-free recombinant Hla [33], 2 × 107 HKSA USA300/LAC, or both. IL-1β levels in the culture supernatants were measured by ELISA (R&D Systems).
Statistical Analysis and Calculations
Statistical analyses were performed using GraphPad Prism software. Pairwise comparisons were performed using Student's unpaired t test or the Gehan-Breslow-Wilcoxon test (for comparison of survival following Kaplan-Meier estimation). Chemotactic migration, skin lesion abscess size, and dermonecrosis area comparisons were performed using two-way ANOVA followed by Bonferroni post hoc testing. Repeated measures were indicated when appropriate. Values of p < 0.05 were considered significant. Error bars represent ± SEM.
Results
Myeloid Lineage Adam10 Knockout Exacerbates S. aureus Skin Infection
To examine the contribution of the Hla-ADAM10 complex on innate immune cells during disease pathogenesis, we generated myeloid lineage-specific Adam10 knockout mice. Mice harboring floxed alleles of Adam10 exon 3 (Adam10loxP/loxP) were crossed to mice expressing the Cre recombinase under control of the lysozyme M (LysM) promoter, primarily expressed in neutrophils and macrophages (online suppl. fig. 1a; for all online suppl. material, see www.karger.com/doi/10.1159/000360006). Excision of the floxed genomic region (online suppl. fig. 1b) and loss of ADAM10 expression was documented in primary neutrophils (online suppl. fig. 1c) and macrophages (online suppl. fig. 1d) harvested from Adam10-/- mice compared to control wild-type mice. Previously, Adam10 conditional knockouts in T and B cell lineages have been demonstrated to impair T cell thymic maturation [32], germinal center formation, and humoral immune function following antigen challenge, respectively [34]. Additionally, Adam10 knockout driven by an Mx-1 promoter-driven Cre recombinase abrogates expression in a wide array of hematopoietic lineages, and is associated with the development of a myeloproliferative disorder, characterized by splenomegaly, hypercellular bone marrow, and abnormal peripheral blood counts [35]. In contrast to these phenotypes, LysM-driven recombination in our mice did not result in hematopoietic abnormalities, as control and Adam10−/− mice exhibited identical peripheral leukocyte differentials (online suppl. fig. 2a), normal red blood cell (online suppl. fig. 2b) and platelet (online suppl. fig. 2c) indices, and splenic size (online suppl. fig. 2d). In addition, normal cellularity and architecture of the spleen and bone marrow cavity was observed in Adam10−/− mice (online suppl. fig. 2e). These results are consistent with the observation that the myeloproliferative disorder seen in Mx-1creAdam10loxP/loxP mice is largely due to non-cell-autonomous effects [35, 36].
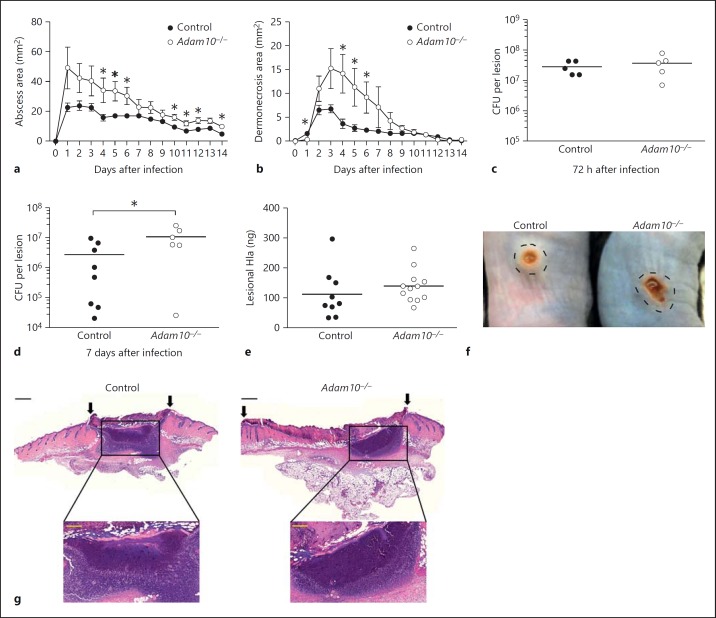
The innate immune response to S. aureus infection is required for clearance of both cutaneous and invasive infection in humans, as individuals with chronic granulomatous disease in which phagocyte oxidative burst is impaired frequently suffer staphylococcal skin and lung infection [37]. Subcutaneous infection of control and Adam10−/− mice with S. aureus USA300, an epidemic MRSA clinical isolate, revealed an increased abscess area in knockout mice (fig. 1a). Consistent with this finding, Adam10−/− mice suffered from larger dermonecrotic skin lesions that overlie the abscess site (fig. 1b), a pathophysiologic process previously demonstrated to depend on Hla-mediated injury to the epithelium [9, 27]. Confirming that this phenotype is dependent on the Hla-ADAM10 toxin-receptor interaction, infection with the isogenic Hla-deficient S. aureus strain hla::erm yielded identical non-necrotizing lesions in control and Adam10−/− mice (online suppl. fig. 3). CFU recovery from harvested skin lesions was unaltered in Adam10−/− mice relative to controls when examined 72 h after infection (fig. 1c). By 7 days after infection, however, the staphylococcal burden was found to be higher in Adam10−/− mice than in controls (fig. 1d, online suppl. fig. 4a), suggesting a moderate defect in innate host immune control of infection. An examination of Hla content in skin lesion biopsies harvested 24 h after infection revealed a nonsignificant trend toward increased Hla content in the tissue of Adam10−/− mice (fig. 1e, online suppl. fig. 4b), likely an early reflection of the subtle advantage of the pathogen in the Adam10−/− host. While gross pathologic specimens imaged 4 days after infection demonstrate the exacerbated lesions of Adam10−/− mice (fig. 1f), histopathologic analysis of infected tissues revealed abscess lesions that were similar in overall appearance and cellularity between both control and Adam10−/− mice at 4 days (fig. 1g). Dermonecrotic lesions overlying the abscess site were visibly larger in Adam10−/− mice, indicative of increased epidermal injury (fig. 1g, arrows).
Fig. 1.
Myeloid lineage knockout of Adam10 worsens clinical outcome in S. aureus skin infection. Abscess (a) and dermonecrosis (b) area in control and Adam10−/− mice following subcutaneous infection with 1 × 107 CFU S. aureus USA300/LAC. One representative experiment of 10 using 6-10 individual mice per group is shown. Lesion sizes were recorded by noninvasive measurements and the lesion area calculated according to the formula A = ([π/2] × l × w). * p < 0.05, Student's t test. c, d Mice were infected as in a and skin lesions were harvested 72 h (c) or 7 days (d) after infection with an 8-mm punch biopsy tool, then homogenized in PBS for quantification of tissue bacterial burden by serial dilution analysis. e Hla protein content in lesion homogenates 24 h after infection, measured by sandwich ELISA. f Gross pathologic image of control and Adam10−/− skin lesions 4 days after infection, where the abscess area (delineated by a dotted circle) surrounds the central dermonecrosis lesion. g HE-stained tissues of day 4 lesions in infected control and Adam10−/− mice. Black scale bar (low magnification images) = 500 μm; yellow scale bar (high magnification images) = 200 μm.
Myeloid Lineage Adam10 Knockout Mitigates S. aureus Pneumonia
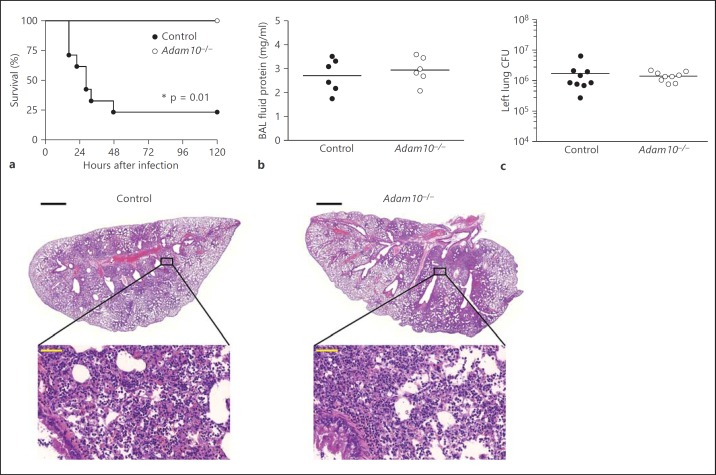
While the results described above are consistent with a model in which toxin action on myeloid innate immune cells augments host defense against S. aureus skin infection, Adam10−/− mice subjected to intranasal infection with S. aureus USA300 were highly protected against lethal staphylococcal pneumonia relative to control mice (fig. 2a). A hallmark of acute lung injury is leakage of proteinaceous fluid into the alveolar space, a consequence of both epithelial injury and inflammation-induced pulmonary vascular permeability [38]. Both control and Adam10−/− mice displayed similar levels of proteinaceous edema in response to infection, quantified in BAL fluid (fig. 2b). Similar to the skin, bacterial recovery from the lungs early in the course of infection was not affected by Adam10 deletion (fig. 2c) and the histopathologic appearance of infected lung tissue in both groups of mice demonstrated significant injury with large areas of consolidation and loss of airspace (fig. 2d). These findings are distinct from those observed following deletion of Adam10 in the alveolar epithelium, wherein the improved clinical course of disease was paralleled by a reduction in the recruitment of inflammatory cells to the lung parenchyma, and an overall improvement in pathologic findings of disease despite the lack of significant changes in S. aureus recovery from the tissues [8]. The tissue-specific impact of myeloid lineage Adam10 deletion on disease outcome, together with the histopathologic findings in both skin and lung infection that do not differ in cellularity between control and Adam10−/− mice, suggest that Hla action on innate immune cells must alter clinical disease outcome in a manner that cannot be inferred solely from pathologic observations of immune cell recruitment to the infected tissue.
Fig. 2.
Myeloid lineage knockout of Adam10 reduces lethality from S. aureus pneumonia. a Survival of control and Adam10−/− mice following intranasal infection with 2-3 × 108 CFU S. aureus USA300/LAC, scored over 5 days. One representative experiment of 6 using 7-13 mice per group is shown. * p = 0.01, Gehan-Breslow-Wilcoxon test. b BAL fluid protein concentration in mice infected as in a, harvested for analysis 24 h after infection. c S. aureus recovery from left lung tissue harvested 24 h after infection and homogenized for bacterial CFU quantification. d Lung histopathology in control and Adam10−/− mice infected as in a, harvested 24 h after infection and stained with HE. Black scale bar (low magnification images) = 1 mm; yellow scale bar (high magnification images) = 50 μm.
Modulation of Cellular Immune Responses in Myeloid Lineage Adam10 Knockout
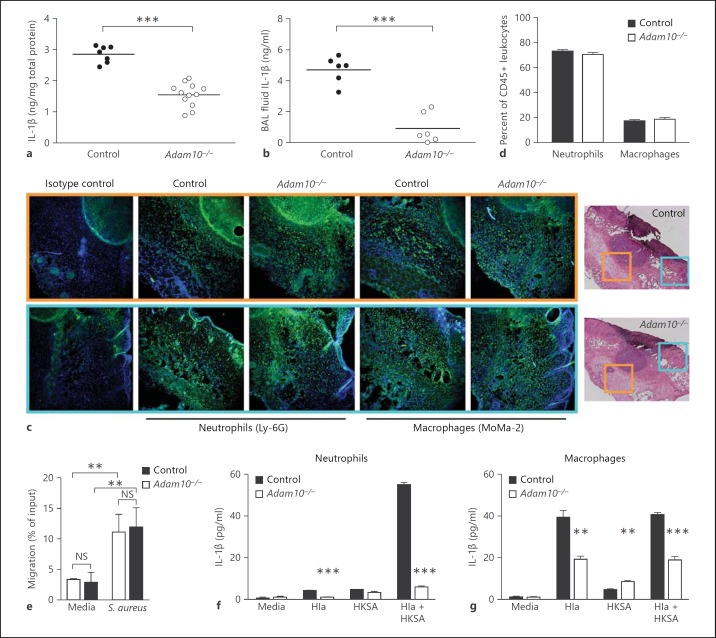
The production of inflammatory cytokines and chemokines by innate immune cells is one of the earliest measurable host responses to S. aureus. The IL-1β response to S. aureus skin infection inversely correlates with disease severity. Mice lacking IL-1β, IL-1R1 (the functional IL-1β receptor), or the MyD88 signaling adaptor critical for transduction of IL-1 responses display increased susceptibility to S. aureus skin infection, marked by profound defects in neutrophil recruitment to the site of infection [21, 39, 40]. In the context of lung infection, both keratinocyte-derived chemokine and macrophage inflammatory protein-2 were increased by 5 h in mice infected with wild-type S. aureus in contrast to Hla- staphylococci [25]. Passive immunization with neutralizing antisera targeting Hla protects against lethal S. aureus pneumonia, associated with a reduction in the generation of IL-1β [11]. Given these findings, we examined the host IL-1β response to S. aureus infection. Loss of myeloid lineage ADAM10 expression was associated with a decreased tissue IL-1β response in both the skin (fig. 3a) and the lung (fig. 3b) following S. aureus infection. As neutrophils are both recruited to the infected tissue through the action of IL-1β and are an important source of IL-1β during skin infection [21], we examined innate immune cell infiltrates in infected skin and lung tissues. While the defect in tissue IL-1β accumulation was measurable by 8 h after infection, neutrophil and macrophage recruitment to the site of skin infection was similar in control and Adam10−/− mice, as demonstrated by immunofluorescence microscopy (fig. 3c). Innate immune cell recruitment was comparable at two distinct sites examined in the infected tissue, within and immediately adjacent to the abscess lesion (orange boxed area) and in the overlying epidermis (teal boxed area). Similarly, the percentage of neutrophils and macrophages enumerated in lung tissue from control and Adam10−/− mice was identical (fig. 3d). Consistent with these findings, neutrophils from Adam10−/− mice also exhibited normal ex vivo chemotaxis in a Transwell-based assay quantifying movement towards live S. aureus (fig. 3e).
Fig. 3.
Hla-mediated production of IL-1β by myeloid lineage cells is impaired in Adam10−/− mice. Tissue IL-1β levels in control and Adam10−/− mice were assessed by ELISA in skin homogenates (a) and BAL fluid (b) from mice infected with S. aureus. To standardize against slight variations in the degree of tissue homogenization, tissue IL-1β was standardized to total protein in each sample. Results are 1 representative experiment of 4 from skin and 5 from BAL fluid. *** p < 0.001, Student's t test. c Recruitment of neutrophils and macrophages to S. aureus-infected skin tissue, examined by immunofluorescence microscopy performed on frozen skin sections. Control and Adam10−/− mice were examined 48 h after infection, demonstrating similar neutrophil recruitment by Ly-6G staining and macrophage infiltration by MoMa-2 staining. The imaged area immediately adjacent to and encompassing the abscess is highlighted with an orange box, while lesions from the overlying epidermis are highlighted in blue boxes. Images are representative of results obtained from analysis of 9 independent mice from 3 experiments. d Flow cytometric analysis of neutrophil and macrophage populations in lung homogenates prepared 8 h following intranasal infection with S. aureus. Cell populations were examined in 9 independent mice from 3 experiments as a function of all CD45+ cells, where the data are a representative experiment. e Ex vivo chemotaxis assay performed on primary neutrophils harvested from control and Adam10−/− mice where chemotaxis in response to media alone or 1 × 106 CFU S. aureus was scored by counting neutrophils that migrated across a Transwell filter toward the chemotactic stimulus. Migration was recorded as a percentage of total neutrophil input into the assay. ** p < 0.01, two-way ANOVA. NS = Not significant. f, g IL-1β production in response to 6-hour culture of primary neutrophils (f) or macrophages (g) from control or Adam10−/− mice with purified, endotoxin-free Hla (25 μg/ml), HKSA (equivalent to 2 × 107 staphylococci), or Hla + HKSA. ** p < 0.01, *** p < 0.001, Student's t test.
IL-1β secretion depends on the generation of increased pro-IL-1β transcript and production of pro-IL-1β, which can be stimulated by pathogen-associated molecules such as staphylococcal lipopeptides or peptidoglycan that induce TLR2 and NOD2-dependent signaling, respectively [20, 22]. Cleavage of pro-IL-1β by caspase-1 in the presence of α-toxin yields the mature, secreted form of the cytokine [20, 23]. To examine the role of the Hla-ADAM10 complex in stimulation of IL-1β production, we purified primary neutrophils and bone marrow-derived monocytes from control and Adam10−/− mice and quantified IL-1β secretion by ELISA in response to treatment with either the toxin alone or the toxin in combination with HKSA. Adam10−/− cells were impaired in the ability to secrete IL-1β in response to both stimuli (fig. 3f, g). The magnitude of this defect was more prominent in primary neutrophils than macrophages, consistent with the finding that control macrophages displayed a greater response to toxin alone and that IL-1β secretion in both control and Adam10−/− macrophages was not strongly increased by the addition of HKSA (fig. 3g). Importantly, these toxin-induced signaling events in neutrophils and macrophages were observed upon treatment of the cells with 25 μg/ml LPS-free Hla, a concentration that causes minimal cellular injury as measured by LDH release (online suppl. fig. 5).
Toxin-Induced Epithelial Injury Is a Primary Contributor to Outcome of Skin and Lung Infection
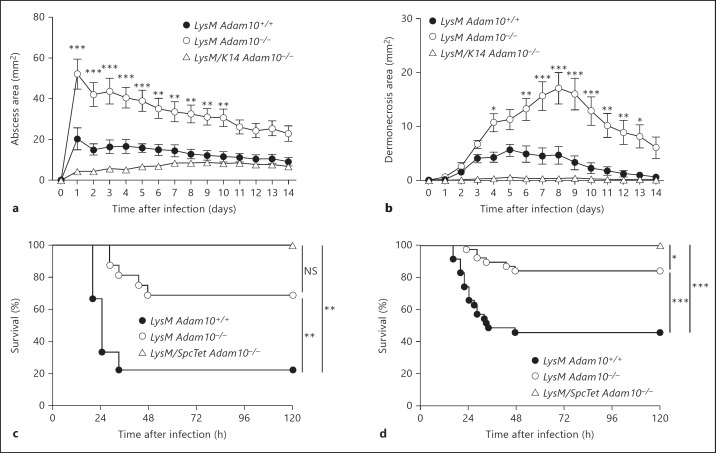
The finding that myeloid lineage knockout of Adam10 was associated with tissue-specific disease outcomes in which immune cell recruitment and bacterial burden were unchanged suggested that outcome may depend on the balance of inflammatory stimuli in the tissue microenvironment as a whole. Toxin-induced injury to epithelial cells as well as immune cells should occur in a simultaneous fashion within S. aureus-infected tissue, eliciting a host response that is the summation of these distinct events. In this context, one cell type may be the predominant contributor to the disease phenotype. To address this, we generated two lines of double lineage knockout mice, the first of which harbored deletion of Adam10 in the epidermis and the myeloid lineage and the second of which harbored a conditional deletion of Adam10 in the alveolar epithelium and the myeloid lineage. In response to S. aureus skin infection, the epidermal-myeloid lineage knockout mice (LysM/K14 Adam10−/−) demonstrated small tissue abscesses (fig. 4a) and dermonecrotic lesions (fig. 4b), reversing the increased disease susceptibility conferred by myeloid lineage knockout alone (LysM Adam10−/−). Given the minimal lesion sizes observed in both control (LysM Adam10+/+) and LysM/K14 Adam10−/−, we did not observe statistically significant differences in outcome of infection between these groups. Similarly, alveolar epithelial-myeloid lineage double-knockout mice (LysM/SpcTet Adam10−/−) showed complete protection against lethal S. aureus pneumonia (fig. 4c), displaying improvement over that observed with myeloid lineage knockout alone when animals from three independent studies were analyzed together (fig. 4d).
Fig. 4.
Epithelial and myeloid lineage expression of ADAM10 co-modulate the host response to tissue infection. a, b Mice harboring a double knockout of Adam10 in the epidermis and myeloid lineage (LysM/K14 Adam10−/−) are more resistant to S. aureus-induced abscess formation (a) and development of dermonecrotic lesions (b) following skin infection relative to mice harboring myeloid lineage-specific Adam10 deletion alone (LysM Adam10−/−). One representative experiment of 3 using 7-15 individual mice per group is shown, where statistical significance was calculated by repeated-measures two-way ANOVA with Bonferroni post hoc tests to compare each group to the wild-type control. c, d Mice harboring a double knockout of Adam10 in the alveolar epithelium and myeloid lineage (LysM/SpcTet Adam10−/−) are more resistant to lethal S. aureus pneumonia than mice harboring myeloid lineage-specific Adam10 deletion alone (LysM Adam10−/−). c One representative experiment of 3 using 8-16 mice per group is shown. NS = Not significant. d Three independent experiments in which results from 25-38 mice per group were pooled to assess for significant differences in mortality between LysM Adam10−/− and LysM/SpcTetAdam10−/− mice. Statistical significance in these experiments was calculated by pairwise comparisons using the Gehan-Breslow-Wilcoxon test. * p < 0.05, ** p < 0.01, *** p < 0.001.
Discussion
The recent definition of the cellular receptors for a number of S. aureus immunomodulatory cytotoxins, including Hla [7], Panton Valentine leukocidin [41], leukocidin AB/leukocidin GH [42], leukocidin ED [43], and the phenol-soluble modulins [44], provides keen new insight into the cell type specificity of toxin action. While each of these toxins can target distinct immune cells to facilitate disease, we currently have a limited understanding of the action of any single toxin on a specific immune cell population in disease pathogenesis. Further, it remains unclear if the effects of a single toxin on an immune cell population vary based on the host tissue. Through myeloid-lineage knockout of Adam10, these studies illustrate that the effects of Hla on neutrophils and monocytes are relevant to the pathogenesis of infection, an observation suggested by prior studies, now investigated through a host genetic strategy targeting the toxin receptor. We also provide evidence that the consequence of immune cell intoxication by Hla varies dependent on the nature of the host tissue - while pro-inflammatory signaling marked by increased IL-1β is beneficial to the host during S. aureus skin infection, this same signal is deleterious in the lung. Interestingly, these data demonstrate that tissue-specific disease outcomes observed in Adam10−/− mice were not the result of altered immune cell recruitment, but rather were linked to a neutrophil and macrophage cell-intrinsic defect in the production of IL-1β in response to Hla.
While decreased cellular production of IL-1β in Adam10−/− mice correlates with exacerbation of skin infection and improved clinical outcome in pneumonia, the precise molecular mechanisms by which this inflammatory cytokine shapes the host response during staphylococcal infection remain unclear [21]. Considerable investigation of the role of IL-1β and the pro-inflammatory state has been conducted in murine models of S. aureus skin infection and pneumonia. Mice deficient in IL-1β and IL-1R1 exhibit a profound defect in S. aureus clearance in the skin [21, 39, 40], accumulating increased numbers of bacteria at the infection site owing to a near-complete absence of recruited neutrophils. By comparison, knockouts of the upstream pathogen-sensing machinery including TLR2 and NOD2 produce dampened IL-1β responses to S. aureus skin infection, suggesting a level of redundancy of pathogen-induced signaling that leads to IL-1β secretion [22, 39]. The level of IL-1β produced in these knockout mice facilitates normal neutrophil recruitment, however is insufficient to drive normal, robust host defense. Both Tlr2−/− and Nod2−/− mice exhibit an increased S. aureus load in the tissues 7 days after infection, consistent with the phenotype we observe in myeloid lineage Adam10−/− animals. Initial neutrophil recruitment to the site of infection promotes neutrophil-dependent production of IL-1β, further enhancing the protective host response to infection [21]. Together, these data suggest that toxin-induced immunostimulation establishes a pro-inflammatory skin environment that benefits the host, limiting the consequences of S. aureus infection. In contrast to these findings, a recent study examining athymic nude mice noted decreased staphylococcal skin lesion size in these mice [45]. The authors concluded that local inflammation promotes increased severity of S. aureus skin infection, a finding that will require further mechanistic investigation in light of the more substantial alterations in immunologic function in nude mice.
Abtin et al. [46] recently reported on a murine model of subcutaneous S. aureus infection in the ear, observing that Hla interferes with normal neutrophil recruitment to sites of infection. Inoculation of an Hla-deficient S. aureus mutant led to increased neutrophilic infiltrates at the site of infection, which correlated with maintenance of a perivascular macrophage population that appears to promote neutrophil trafficking to the site. Parallel ex vivo studies of distinct macrophages harvested from the blood or peritoneal space suggested that Hla directly targets and kills macrophages, providing a potential mechanism for loss of the perivascular macrophage population in response to wild-type S. aureus infection. While these findings are distinct from our observations in which loss of myeloid lineage sensitivity to Hla exacerbates infection, it must be recognized that Hla is a key virulence factor that interacts with a wide variety of host cell types in the course of infection, including innate immune cells, epithelial cells, and endothelial cells. Infection with toxin-deficient staphylococci is therefore expected to provide a phenotypic readout of the aggregate effects of Hla in the entire tissue, perhaps the most significant of which in the context of skin infection is the dominant effect of the toxin in the induction of severe epithelial injury (fig. 4a, b) [47]. Host cell lineage-specific perturbations, such as the one we report here, therefore afford a precisely targeted and robust genetic approach to examine cell type-specific mechanisms of toxin action. Together, this intriguing series of observations will undoubtedly help to guide further investigation of the virulence properties of Hla in the context of the local tissue microenvironment.
In the lung, germline NLRP3 knockout mitigates the pathologic findings observed in S. aureus pneumonia and in response to purified Hla, consistent with a role for inflammasome signaling in immunopathogenesis [24]. In this setting, neutrophil recruitment and the generation of IL-1β are decreased. In contrast, myeloid lineage Adam10−/− knockout mice display normal immune cell recruitment to the lung, implicating the direct effect of the toxin on recruited cells in the observed alterations of disease outcome. An IL-1β response to both infectious and noninfectious lung insults is associated with pathologic injury to the lung, related to the induction of pyroptotic cell death of recruited immune cells and the potent inflammatory response that causes bystander injury to the lung parenchyma [48, 49]. Consistent with these observations, passive immunization with Hla-neutralizing polyclonal antisera dampens the host IL-1β response to S. aureus pneumonia and mitigates pathology [11], providing further evidence that toxin-generated signaling in host immune cells promotes the production of IL-1β, thereby contributing to acute lung injury.
Our studies highlight a number of areas for further investigation, namely understanding the differential contribution of the neutrophil and macrophage populations in patterning tissue-specific, toxin-mediated responses to infection. While IL-1β is well recognized as a marker of the proinflammatory state that affords protection against S. aureus skin infection and contributes to lung injury, the precise molecular mechanisms that drive disease outcome are not well defined. Specifically, further investigation on the effector functions of neutrophils and macrophages in the presence of the toxin are anticipated to provide additional insights on how the toxin impacts disease progression in a tissue-specific manner. Genetic perturbation of Adam10 affords a novel opportunity to examine innate immune cell signaling pathways in vivo and define both the effector pathways that underlie the host response and graded host responses as a function of localized toxin concentration on a diverse array of toxin-sensitive cells.
Multiple studies in addition to our findings have now illustrated a lack of direct, temporal correlation between S. aureus recovery from the infected tissue and measurements of disease severity [8, 9, 22, 45, 50], suggesting that the clinical manifestations of disease represent the aggregate balance of bacterial insults to resident and recruited cells and the host inflammatory response [51]. This response can be well directed to facilitate pathogen clearance in the given tissue, or overly aggressive, leading to exacerbated injury. Our studies on Hla illustrate that bacterial virulence factors can play an important role in the tissue microenvironment to establish this balance, both causing injury and facilitating immunostimulation. These findings illustrate the need to further investigate how multiple virulence factors interface within the tissue context and shape the host response, recognizing that a clear ‘circuitry map’ of these interactions would enable the design of novel preventative and therapeutic strategies that simultaneously dampen injurious responses while promoting beneficial responses.
Supplementary Material
Supplementary data
Acknowledgments
We thank Mr. Michael Powers for assistance with animal experimentation and Dr. Ray Roos for the gift of L-929 cells. This work was supported by NIH award AI097434-01, and the Burroughs Wellcome Foundation Investigators in the Pathogenesis of Infectious Disease Fellowship to J.B.W. The authors acknowledge membership in and support from the Region V ‘Great Lakes’ RCE (NIH award 2-U54-AI-057153). R.E.N.B. is a trainee of the National Institutes of Health Medical Scientist Training Program at the University of Chicago (grant GM007281). B.J.B. was partially supported by a National Institutes of Health Training Grant (grant GM007183).
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Antibiotic Resistance Threats in the United States Washington, US Department of Health and Human Services. 2013 [Google Scholar]
- 5.Alonzo F, 3rd, Torres VJ. Bacterial survival amidst an immune onslaught: the contribution of the Staphylococcus aureus leukotoxins. PLoS Pathog. 2013;9:e1003143. doi: 10.1371/journal.ppat.1003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berube BJ, Bubeck Wardenburg., J Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins. 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilke GA, Bubeck Wardenburg., J Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci USA. 2010;107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg., J A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoshima N, Wang Y, Wardenburg JB. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J Invest Dermatol. 2012;132:1513–1516. doi: 10.1038/jid.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg., J ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2012;206:352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady RA, Mocca CP, Prabhakara R, Plaut RD, Shirtliff ME, Merkel TJ, Burns DL. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of Staphylococcus aureus infection. PLoS One. 2013;8:e63040. doi: 10.1371/journal.pone.0063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 2000;68:6162–6167. doi: 10.1128/iai.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bubeck Wardenburg J, Patel RJ, Schneewind O. Surface proteins and exotoxins are required for the pathogenesis of Staphylococcus aureus pneumonia. Infect Immun. 2007;75:1040–1044. doi: 10.1128/IAI.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valeva A, Walev I, Pinkernell M, Walker B, Bayley H, Palmer M, Bhakdi S. Transmembrane beta-barrel of staphylococcal alpha-toxin forms in sensitive but not in resistant cells. Proc Natl Acad Sci USA. 1997;94:11607–11611. doi: 10.1073/pnas.94.21.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Malone C, Griffith S, Horswill AR, Torres VJ, Voyich JM. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One. 2012;7:e36532. doi: 10.1371/journal.pone.0036532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGee MP, Kreger A, Leake ES, Harshman S. Toxicity of staphylococcal alpha toxin for rabbit alveolar macrophages. Infect Immun. 1983;39:439–444. doi: 10.1128/iai.39.1.439-444.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nygaard TK, Pallister KB, Zurek OW, Voyich JM. The impact of alpha-toxin on host cell plasma membrane permeability and cytokine expression during human blood infection by CA-MRSA USA300. J Leukoc Biol. 2013;94:971–979. doi: 10.1189/jlb.0213080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhakdi S, Muhly M, Korom S, Hugo F. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hruz P, Zinkernagel AS, Jenikova G, Botwin GJ, Hugot JP, Karin M, Nizet V, Eckmann L. NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc Natl Acad Sci USA. 2009;106:12873–12878. doi: 10.1073/pnas.0904958106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PloS One. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis. 2012;205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartlett AH, Foster TJ, Hayashida A, Park PW. Αlpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–1535. doi: 10.1086/592758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel AH, Nowlan P, Weavers ED, Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55:3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, de Leo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank KM, Zhou T, Moreno-Vinasco L, Hollett B, Garcia JG, Bubeck Wardenburg., J Host response signature to Staphylococcus aureus alpha-hemolysin implicates pulmonary Th17 response. Infect Immun. 2012;80:3161–3169. doi: 10.1128/IAI.00191-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 30.Neutrophil Methods and Protocols Totowa, Humana. 2007 [Google Scholar]
- 31.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 32.Tian L, Wu X, Chi C, Han M, Xu T, Zhuang Y. ADAM10 is essential for proteolytic activation of Notch during thymocyte development. Int Immunol. 2008;20:1181–1187. doi: 10.1093/intimm/dxn076. [DOI] [PubMed] [Google Scholar]
- 33.Ragle BE, Bubeck Wardenburg., J Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun. 2009;77:2712–2718. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaimowitz NS, Martin RK, Cichy J, Gibb DR, Patil P, Kang DJ, Farnsworth J, Butcher EC, McCright B, Conrad DH. A disintegrin and metalloproteinase 10 regulates antibody production and maintenance of lymphoid architecture. J Immunol. 2011;187:5114–5122. doi: 10.4049/jimmunol.1102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoda M, Kimura T, Tohmonda T, Uchikawa S, Koba T, Takito J, Morioka H, Matsumoto M, Link DC, Chiba K, Okada Y, Toyama Y, Horiuchi K. Dual functions of cell-autonomous and non-cell-autonomous ADAM10 activity in granulopoiesis. Blood. 2011;118:6939–6942. doi: 10.1182/blood-2011-06-357210. [DOI] [PubMed] [Google Scholar]
- 36.Weber S, Wetzel S, Prox J, Lehmann T, Schneppenheim J, Donners M, Saftig P. Regulation of adult hematopoiesis by the a disintegrin and metalloproteinase 10 (ADAM10) Biochem Biophys Res Commun. 2013;442:234–241. doi: 10.1016/j.bbrc.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 38.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O'Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 41.Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJ, van Kessel KP, Vandenesch F, Lina G, van Strijp JA. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe. 2013;13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Dumont AL, Yoong P, Day CJ, Alonzo F, 3rd, McDonald WH, Jennings MP, Torres VJ. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci USA. 2013;110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alonzo F, 3rd, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, Myszka DG, Landau NR, Unutmaz D, Torres VJ. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493:51–55. doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee SS, Joo HS, Duong AC, Dieringer TD, Tan VY, Song Y, Fischer ER, Cheung GY, Li M, Otto M. Essential Staphylococcus aureus toxin export system. Nat Med. 2013;19:364–367. doi: 10.1038/nm.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montgomery CP, Daniels MD, Zhao F, Spellberg B, Chong AS, Daum RS. Local inflammation exacerbates the severity of Staphylococcus aureus skin infection. PLoS One. 2013;8:e69508. doi: 10.1371/journal.pone.0069508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abtin A, Jain R, Mitchell AJ, Roediger B, Brzoska AJ, Tikoo S, Cheng Q, Ng LG, Cavanagh LL, von Andrian UH, Hickey MJ, Firth N, Weninger W. Perivascular macrophages mediate neutrophil recruitment during bacterial skin infection. Nat Immunol. 2014;15:45–53. doi: 10.1038/ni.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoshima N, Wang Y, Bubeck Wardenburg., J Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J Invest Dermatol. 2012;132:1513–1516. doi: 10.1038/jid.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 2013;123:1630–1637. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 50.Martin FJ, Parker D, Harfenist BS, Soong G, Prince A. Participation of CD11c(+) leukocytes in methicillin-resistant Staphylococcus aureus clearance from the lung. Infect Immun. 2011;79:1898–1904. doi: 10.1128/IAI.01299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data