Abstract
Vaginal delivery is a risk factor for stress urinary incontinence (SUI). Mesenchymal stem cells (MSCs) home to injured organs and can facilitate repair. The goal of this study was to determine if MSCs home to pelvic organs after simulated childbirth injury and facilitate recovery from SUI via paracrine factors. Three experiments were performed. Eighteen female rats received vaginal distension (VD) or sham VD and labeled intravenous (IV) MSCs to investigate if MSCs home to the pelvic organs. Whole-organ imaging and immunofluorescence were performed 1 week later. Thirty-four female rats received VD and IV MSCs, VD and IV saline, or sham VD and IV saline to investigate if MSCs accelerate recovery of continence. Twenty-nine female rats received VD and periurethral concentrated conditioned media (CCM), VD and periurethral control media, or sham VD and periurethral control media to investigate if factors secreted by MSCs accelerate recovery from VD. Urethral histology and function were assessed 1 week later. Significantly more MSCs were observed in the urethra, vagina, and spleen after VD compared to sham VD. Continence as measured by leak point pressure (LPP) was significantly reduced after VD in rats treated with saline or control media compared to sham VD but not in those given MSCs or CCM. External urethral sphincter (EUS) function as measured by electromyography (EMG) was not improved with MSCs or CCM. Rats treated with MSCs or CCM demonstrated an increase in elastin fibers near the EUS and urethral smooth muscle more similar to that of sham-injured animals than rats treated with saline or control media. MSCs homed to the urethra and vagina and facilitated recovery of continence most likely via secretion of paracrine factors. Both MSCs and CCM have promise as novel noninvasive therapies for SUI.
Keywords: Paracrine factors, Urethra, External urethral sphincter (EUS), Electromyography (EMG), Female, Stress urinary incontinence (SUI), Elastin
INTRODUCTION
Stress urinary incontinence (SUI) is a significant medical problem, with prevalence rates of 4–35% among women (24). Vaginal delivery has been correlated with SUI development (29), likely via injury of the muscles, organs, and extracellular matrix (ECM) responsible for continence (34). Treatments include physiotherapy, continence pessaries, injectable bulking agents, and surgery—the mainstay for cases that are nonresponsive to conservative measures (2). Although surgery can improve SUI, it does not necessarily repair the underlying pathophysiology.
Clinical trials have utilized autologous muscle-derived progenitor cells injected directly into the urethra of women with SUI and have demonstrated that adult stem cells are safe and can improve urethral function (4,5). Preclinical studies hypothesized that the stem cells act by differentiating into smooth and or striated muscle (6). Alternatively, it has been proposed that paracrine mechanisms may explain improvements with stem cell therapy (14,23).
Hematopoetic and mesenchymal stem cells (HSCs and MSCs) circulate in the bloodstream and home to sites of injury following chemokine gradients (32). Animal studies have demonstrated MSC homing and benefit in several disease models, including cardiac injury (3,20), renal failure (26), and skin wounds (30), highlighting the clinical potential of these cells.
Preclinical studies demonstrated that simulating the second phase of labor via vaginal distension (VD) in rats results in decreased leak point pressure (LPP), taken to be indicative of SUI (13,22). Previously, chemokine (C-C motif) ligand 7 [CCL7; the homing factor for MSCs, formerly known as monocyte-specific chemokine-3 (MCP-3)] was shown to be upregulated in the urethra and vagina after VD, suggesting that MSCs will home to these organs and facilitate recovery (37,38). The goal of this study was to determine if intravenously infused MSCs home to pelvic organs after VD and facilitate recovery via secreted paracrine factors.
MATERIALS AND METHODS
Age-matched virgin female Sprague–Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) weighing 225–250 g were utilized in three experiments as approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
To investigate if MSCs home to pelvic organs after VD, rats were randomized into two groups and underwent ex vivo whole organ fluorescence imaging (n = 10) or immunofluorescence (n = 8) 1 week after either VD (n = 9) or sham VD (n = 9) followed by intravenous (IV) infusion of 2 million green fluorescent protein-labeled MSCs (GFP+) in 1 ml saline via the lateral tail vein 1 h after VD. Three additional animals that did not receive cells were used as negative controls in each outcome.
To determine if IV-infused MSCs accelerate recovery of continence after VD, rats were randomized into three groups and underwent IV infusion of MSCs 1 h after VD (VD + MSC; n = 11) as noted above, IV infusion of 1 ml saline via the lateral tail vein 1 h after VD (VD + saline; n = 12), or sham VD (sham VD + saline; n = 11). Five animals in each group underwent histological assessment of the urethra 1 week after injury. The others underwent LPP and external urethral sphincter electromyography (EUS EMG) recording 1 week after injury.
In order to ascertain if factors secreted by MSCs accelerate functional recovery after VD, rats were randomized into three groups and underwent periurethral injection of 300 μl concentrated conditioned media (CCM) 1 h after VD (VD + CCM; n = 10) via a low midline incision to expose the urethra, periurethral injection of 300 μl concentrated control media (CM) 1 h after VD (VD + CM; n = 9) or sham VD (sham VD + CM; n = 10). Five animals in each group underwent histological assessment of the urethra 1 week after injury. The others underwent LPP and EUS EMG recording 1 week after injury.
Stem Cell Harvest and Culture
Bone marrow from a donor female Sprague–Dawley rat was used to create cultured MSCs as previously reported (9). In brief, marrow was aspirated from the femur and tibia by flushing the bone with saline. Cells were cultured in a normoxic incubator with 5% CO2 at 37°C. At passage 3 (P3), cells were incubated and sorted for intracellular adhesion molecule I [ICAM-1; 100 μl/ml of provided stock solution (Abcam, Cambridge, MA, USA)] to select for MSCs via flow cytometry using a BD FACAria II (BD Biosciences, San Jose, CA, USA). The MSCs were transfected with pCCLsin.ppt.hPGK.GFP.wpre [donated by Giulio Cossu’s Laboratory (San Raffaele Hospital, Milan, Italy) to Dr. Marc Penn’s laboratory], which uses a human phosphoglycerate kinase (PGK) promoter to constitutively express GFP. Transfection was performed by adding the lentivirus vector containing the plasmid and 6 μg/ml of polybene (Sigma-Aldrich, St. Louis, MO, USA) to the cells at least 10 days before sorting. Transfection was done in a manner approved by the Institutional Biosafety Committee of Cleveland Clinic. After reaching confluence, cells were resorted, and GFP-positive (GFP+) cells were collected. Cells were cultured until P14, then cryopreserved in a freezing media consisting of 90% fetal bovine serum (FBS; Life Technologies, Grand Island, NY, USA) + 10% dimethyl sulfoxide (DMSO) solution (Sigma-Aldrich). The cells were then stored overnight at −80° in a cryofreezing container and were transferred to liquid nitrogen the following day where they were stored until used. At that point, the cells were thawed by pre-warming 9 ml of media, which was added drop by drop to the freezing media/cells solution. The solution was then centrifuged at 300 × g for 2 min, supernatant was removed, the cells resuspended in media, and the cells were then plated. Media was changed every 3 days until the cells were ready for injection. Cells were injected into rats at P16, which was used to consistently ensure a sufficient number of passages for thawing, sorting, and transfection.
MSC Characterization
To confirm expression of cell surface markers, MSCs at P8 and P15 were stained with cluster of differentiation 29 (CD29 at 5 μg/ml; Cat. No. 102218, Biolegend, San Diego, CA, USA), CD90 (at 2 μg/ml; Cat. No. 561404, BD Biosciences), CD54 (100 μl/ml; Cat. No. 22389, Abcam, Cambridge, MA, USA), and CD45 (at 5 μg/ml; Cat. No. 559135, BD Biosciences) and sorted using a LSR Fortessa cell analyzer (BD Biosciences). To confirm the ability of MSCs to differentiate into mesenchymal cell types, MSCs at P16 were plated at a starting density of 50,000 cells/well and given regular MSC media, adipogenic differentiation media (A10070-01; Gibco, Gaithersburg, MD, USA), or osteogenic differentiation media (A10071-01; Gibco) every 3 days once confluent for 20–35 days. Cells in osteogenic media were fixed with 4% formaldehyde (Electron Microscopy Science, Hatfield, PA, USA) and stained with alizarin red S (Sigma-Aldrich). Cells in adipogenic media were fixed and stained with oil red O (Sigma-Aldrich) and hematoxylin (Dako, Carpinteria, CA, USA).
Preparation of Concentrated Conditioned Media (CCM)
CCM was obtained by incubating 100% confluent MSCs at P16 in serum-free Dulbecco’s modified Eagle’s medium (DMEM; Cat. No. 11885, Life Technologies) for 24 h in a normoxic incubator with 5% CO2 at 37°C. Cultured MSC supernatant was extracted and centrifuged at 2,933 × g for 45 min (50× concentrated; 300 μl) and filtered with a sterile 0.22-μm filter (Millipore, Darmstadt, Germany). CM was created by processing serum-free DMEM that had not been utilized to culture cells though all the above steps. CCM was sampled and validated by a cell count and cell proliferation assays before being used. After concentrating the CCM, it was sampled to determine if there were any cells present. The CCM was also reconstituted and added to the MSC culture, and the growth rate was compared with adding reconstituted CM. These validation tests showed no cells in the CCM and demonstrated that MSCs proliferated significantly faster in CCM than in CM.
Vaginal Distention (VD)
VD was performed as previously described (37). The vagina was dilated under isoflurane anesthesia (Piramal Healthcare, Mumbai, India) by sequentially inserting increasing sized (24–32) Otis Bougie a Boule urethral dilators (NovoSurgical, Oak Brook, IL, USA) lubricated with surgilube (Savage Laboratory, Melville, NY, USA). A modified 10-Fr Foley catheter (Bard Medical, Covington, GA, USA) was then inserted into the vagina and the balloon inflated to 3 ml for 4 h. Sham VD consisted of vaginal accommodation and catheter insertion for 4 h without balloon inflation.
Leak Point Pressure (LPP) and External Urethral Sphincter Electromyography (EUS EMG) Recording
LPP was recorded simultaneously with EUS EMG 1 week after injury, as previously described (33). Rats were anesthetized with 1.2 g/kg intraperitoneally administered urethane (Sigma-Aldrich) and the urethra exposed by bluntly opening the pubic symphysis. Bipolar parallel platinum electrodes (30-gauge needles 2 mm apart) were placed on the outside of the midurethra at the location of the EUS and connected to an amplifier (Model P511 AC Amplifier, Astro-Med, Inc., Providence, RI, USA; band pass frequencies: 3 Hz to 3 kHz) and electrophysiological recording system (DASH 8X, Astro-Med; 10 kHz sampling rate). A PE-50 polyethylene catheter (BD, Franklin Lakes, NJ, USA) was inserted into the bladder via the urethra and connected to both a pressure transducer (Astro-Med, Inc.) and syringe pump (KD Scientific, Holliston, MA, USA). Bladder pressure was referenced to air pressure at the level of the bladder. Bladder pressure and EUS EMG were recorded while the bladder was filled with saline (5 ml/h).
For LPP testing, intravesical pressure was increased when the bladder was approximately half full by gradually applying external pressure with a cotton swab until leakage at the meatus occurred. At the moment of leakage, the cotton swab was rapidly removed. If a bladder contraction was induced by LPP testing, the results were not analyzed, and the test was repeated. The test was repeated six to eight times in each animal. Values of bladder pressure just prior to LPP testing (tonic activity) and at the peak pressure (peak value) of LPP testing were determined. Quantitative assessment of EUS EMG signals was performed by determining the mean rectified amplitude and the mean firing rate of tonic activity during bladder filling and at the pressure peak of the LPP test, as previously described (10).
Ex Vivo Fluorescent Imaging
Seven days after VD or sham VD, rats were euthanized, and the bladder, urethra, vagina, rectum, lungs, and spleen were harvested. Each organ was imaged using a Xenogen IVIS 100 System (PerkinElmar, Waltham, MA, USA). Net total fluorescent flux (photons/s/cm2/steridian) in a region of interest selected to include each organ was calculated by subtracting background flux from a negative control.
Immunofluorescent Staining
To investigate engraftment of MSCs in the urethra and vagina, rats underwent intracardiac perfusion with heparinized saline (Sagent Pharmaceuticals, Schaumburg, IL, USA) with 4% formaldehyde (Electron Microscopy Science) 1 week after injury. The urethra and vagina were harvested en bloc and immersed in 20% sucrose (Sigma-Aldrich). Tissues were cryosectioned (10 μm) on a Leica CM 1950 (Leica Biosystem, Buffalo Grove, IL, USA) and stained with a smooth muscle α-actin mouse monoclonal antibody (1:50; SC1306, Santa Cruz, Inc., Santa Cruz, CA, USA) followed by goat anti-mouse Texas red conjugated secondary antibody (1:400; SC2781, Santa Cruz, Inc.). Slides were coverslipped with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Southfield, MI, USA) and imaged on a confocal microscope.
Histology
To assess anatomical recovery of the urethra, it was harvested en bloc with the anterior vagina 1 week after injury and immersion fixed, paraffin embedded, transversely sectioned (5 μm), and stained with Masson’s trichrome and elastin von Giesson stains (both from Newcomer Supply, Middleton, WI, USA). Masson’s trichrome-stained slides of EUS and urethral smooth muscle were graded independently by two blinded investigators using a semiquantitative scale, in which grade 1 represented significant disruption and grade 4 indicated normal findings. Grade 4 EUS had contiguous muscle fibers, striations, and little ECM infiltration. Grade 1 EUS demonstrated disruption of muscle fibers, no striations, and ECM infiltration. Grade 4 smooth muscle had large muscle bundles and little ECM infiltration. Grade 1 smooth muscle demonstrated very small muscle bundles and marked ECM infiltration. Grades 2 and 3 were scaled between 1 and 4. Elastin von Giesson-stained slides were analyzed for the presence or absence of elastin fibers.
Data Analysis
Mean values of each of the quantitative variables were calculated for each animal and were used to calculate a mean and standard error of the mean for each group. Results are presented as mean ± standard error of the mean of each group. Student’s t test was used to compare quantitative variables in Experiment 1 (Sigma Stat, Systat, Inc., San Jose, CA, USA). One-way ANOVA followed by a Holm–Sidak post hoc test was used to compare functional outcomes in Experiments 2 and 3. Kruskal–Wallis one-way ANOVA on ranks followed by a Dunn’s post hoc test were used to compare semiquantitative grading of histology. Data were still presented as mean ± standard error of the mean for consistency. For all statistical tests, p < 0.05 indicated a statistically significant difference between groups. Immunofluorescence and elastin von Giesson stain results were evaluated qualitatively by a blinded observer.
RESULTS
Characterization and Differentiation of Bone Marrow-Derived MSCs
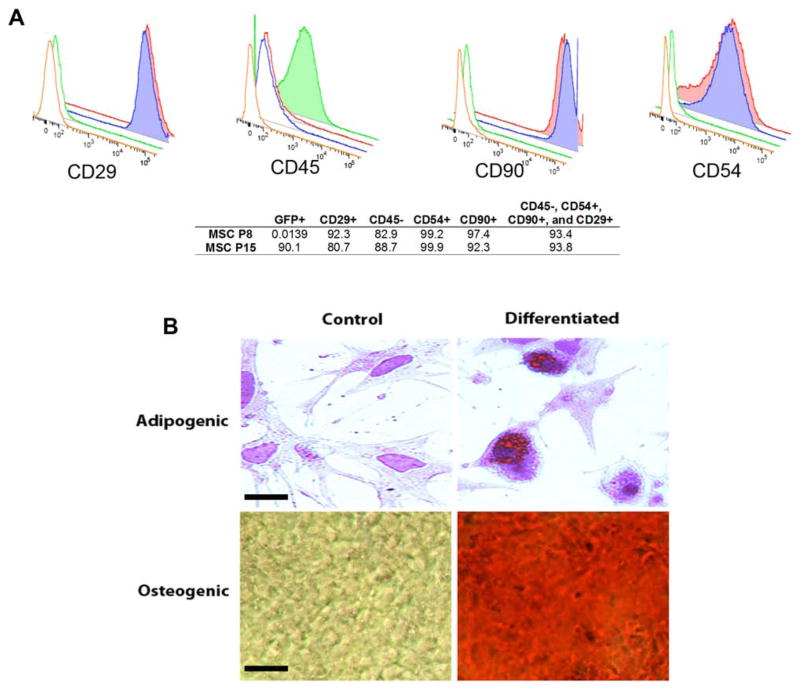
MSCs in this study expressed CD29, CD54, and CD90, but not CD45, similar to previous work with undifferentiated MSCs (15). Cells that were CD45−, CD29+, CD54+, and CD90+ accounted for 93.4% of the MSC population (Fig. 1A). Adipogenic-induced cells contained intracellular lipid droplets, stained orange by oil red O (Fig. 1B). Cells cultured under noninductive conditions did not accumulate lipid droplets. MSCs cultured in osteogenic differentiation medium changed their spindle-shaped morphology to stellate and irregular shaped (Fig. 1B). In control cultures, the cells preserved typical fibroblast morphology. These results demonstrate that the cells were predominately MSCs with multilineage differentiation potential.
Figure 1.
Characterization of mesenchymal stem cells (MSCs). (A) Example flow cytometry results showing positivity for cluster of differentiation 29 (CD29), CD90, and CD54, as well as negativity for CD45. Blue represents MSCs at P8, 0.0139% of which were green fluorescent protein positive (GFP+), since this was prior to GFP labeling. Red represents MSCs at P15, 90.1% of which were GFP+, after GFP labeling. Green and orange are negative controls; 93.8% of P15 cells were CD29+, CD90+, CD54+, and CD45−. (B) Adipogenic and osteogenic differentiation of MSCs. Adipogenic-induced cells contained intracellular lipid droplets, stained orange by oil red O (scale bar: 10 μm). Osteogenic-induced cells are red as assessed with alizarin red staining (scale bar: 100 μm).
Homing of IV-Infused MSCs
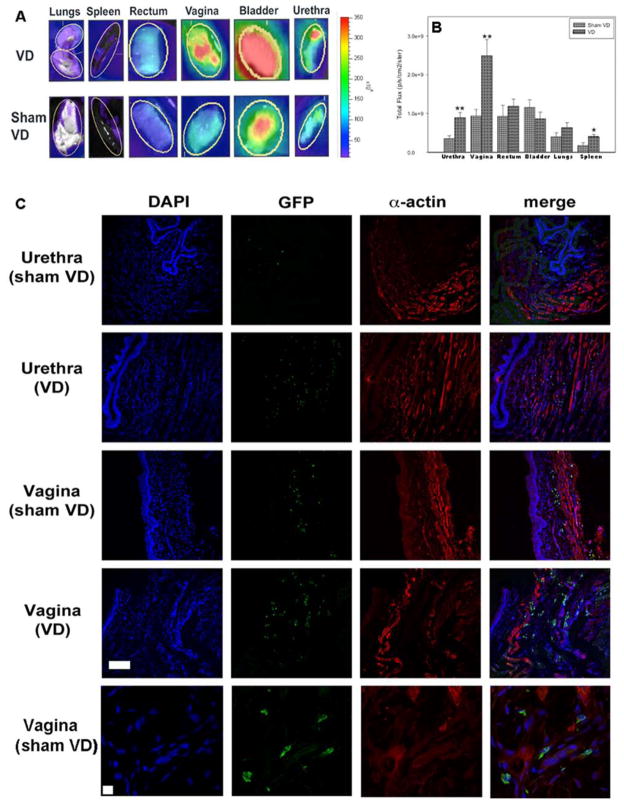
Rats that did not receive cells had only nominal total fluorescent flux. The urethra, vagina, and spleen of rats that underwent VD and received MSCs IV had significantly higher mean total fluorescent flux than rats that underwent sham VD, indicating that MSCs preferentially home to these organs after VD (Fig. 2A, B). More GFP+ cells were evident in the smooth muscle of the urethra and vagina after VD than after sham VD, suggesting that IV-infused MSCs could preferentially home to these smooth muscle layers (Fig. 2C).
Figure 2.
Homing of mesenchymal stem cells (MSCs) to pelvic organs 1 week after vaginal distension (VD) and sham VD. (A) Example ex vivo whole organ imaging results. Yellow oval indicates region of interest inside of which fluorescent flux was calculated. Pseudocolor scale indicates the quantification of level of fluorescent flux, with red being the most intense and purple being the least intense. Rats that received no cells had only nominal total fluorescent flux and are not shown. (B) Fluorescent flux of organs 1 week after VD or sham VD. Each bar indicates mean ± standard error of the mean of organs from nine rats. *A statistically significant difference compared to the same organ from the sham VD group with p < 0.05. **A statistically significant difference compared to the same organ from the sham VD group with p < 0.005. (C) Example immunofluorescence from the urethra and vagina 1 week after VD or sham VD. Blue indicates nuclei with 4′,6-diamidino-2-phenylindole (DAPI) stain; green indicates MSCs labeled with green fluorescent protein (GFP); red indicates smooth muscle with α-actin immunolabeling. Rows 1–4 are at the same magnification (scale bar: 100 μm). The last row shows an example immunofluorescence at higher magnification (scale bar: 10 μm) demonstrating dual labeling of blue and green to show association of nuclei with MSCs. Rats that did not receive cells did not show fluorescence (data not shown).
IV-Infused MSCs and Local Injection of CCM Accelerate Functional Recovery
The VD + saline group demonstrated LPP and EUS EMG firing rates and amplitudes significantly lower than those after sham VD (Fig. 3A–C), similar to previous work with untreated animals in this model (19). There was no significant difference in LPP between rats in the VD + MSC and sham VD groups, indicating that MSCs facilitated urethral recovery. In contrast, EUS EMG firing rate and amplitude were significantly lower in the VD + MSC group than in the sham VD group, suggesting that MSCs had not facilitated EUS electrophysiologic recovery by 1 week after VD (Fig. 3A–C).
Figure 3.
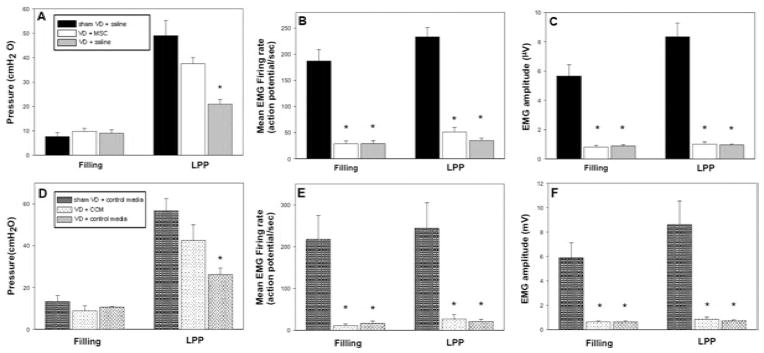
Functional testing after vaginal distension and treatment. Results of functional testing after vaginal distension (VD) or sham VD and treatment with mesenchymal stem cells (VD + MSC) or saline (A–C) or concentrated conditioned media (CCM) or control media (D–F). Bladder pressure during filling and leak point pressure (LPP) testing (A and D). External urethral sphincter electromyogram (EMG) firing rate during filling and LPP testing (B and E). External urethral sphincter EMG amplitude (C and F). Each bar indicates mean ± standard error of the mean of from five to seven rats. *A significant difference compared to the same outcome for the sham VD group with p < 0.05.
LPP in the VD + CM group was significantly decreased compared to the sham VD group. In contrast, LPP in the VD + CCM group was not significantly different from that of sham VD rats, indicating facilitation of urethral recovery by CCM. Similar to the results with MSC treatment, EUS EMG firing rate and amplitude were significantly decreased in all rats that received VD compared to rats that received sham VD, regardless of treatment, suggesting that CCM, like MSCs, did not facilitate EUS electrophysiologic recovery 1 week after VD (Fig. 3D–F).
Effects of MSCs and CCM on Urethral Anatomy
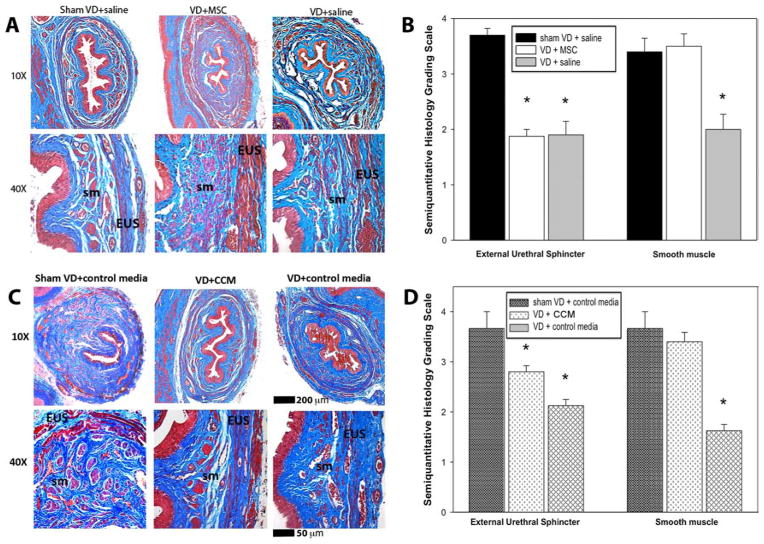
Sham VD rats had contiguous but thin EUS and urethral smooth muscle cells separated by ECM. VD caused disruption of smooth and striated urethral muscle as well as infiltration of ECM. The urethra of rats in the VD + MSC and VD + CCM groups appeared to have more complete smooth muscle layers than those in the VD + saline group (Fig. 4A, B).
Figure 4.
Urethral histology 1 week after vaginal distension and treatment. Examples of urethral histology 1 week after vaginal distension (VD) or sham VD and treatment with mesenchymal stem cells (VD + MSC) or saline (A) or concentrated conditioned media (CCM) or control media (B). Results of semiquantitative grading of urethral histology after VD or sham VD and treatment with MSCs or saline (C) or CCM or control media (D). Each bar indicates mean ± standard error of the mean from five rats. Semiquantitative data was analyzed with a Kruskal–Wallis one-way ANOVA on ranks followed by a Dunn’s post hoc test. *A significant difference compared to the same outcome for the sham VD group with p < 0.05. +A significant difference compared to the same outcome for VD + MSC group with p < 0.05. sm, smooth muscle; EUS, external urethral sphincter.
Urethral tissues from rats in the VD + MSC group were not significantly different in smooth muscle histology grade compared to sham VD rats but had a significantly higher smooth muscle histology grade than urethral tissue from the VD + saline group. Smooth muscle histology grade was not significantly different in the VD + CCM group compared to sham VD rats, while rats in the VD + CM and VD + saline groups had significantly lower histology grades than sham VD rats (Fig. 4C, D). Rats in the VD + MSC group had significantly lower EUS histology grade compared to sham VD rats, but those in the VD + CCM group did not. These results indicate that both MSCs and CCM have positive effects on urethral smooth muscle, while MSCs have little effect on the EUS, and CCM has a partial effect on the EUS at this time point (Fig. 4C, D).
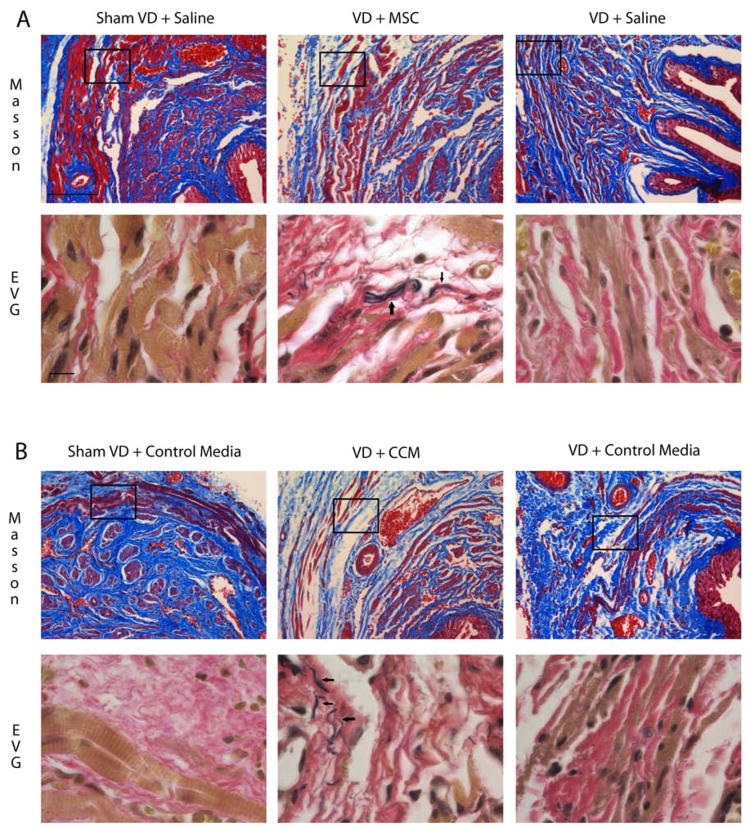
There were few to no elastin fibers in the urethra after sham VD or VD + saline. In contrast, aggregations of disorganized elastin fibers were identified near the EUS 1 week after VD + MSC (Fig. 5A). Similarly, VD + CM demonstrated little to no elastin, but aggregations of disorganized elastin fibers were identified near the EUS 1 week after VD + CCM, suggesting that MSCs facilitated production of elastin, likely by a paracrine effect on urethral smooth muscle cells and fibroblasts (Fig. 5B).
Figure 5.
Histological staining of urethral sections after vaginal distension and treatment. Example urethral cross sections stained with Masson’s trichrome (upper panels) and near sections stained with elastin von Giesson stain (EVG; lower panels) 1 week after vaginal distension (VD) or sham VD and treatment with mesenchymal stem cells (VD + MSC) or saline (A) or concentrated conditioned media (CCM) or control media (B). Inset in upper panels indicates approximate area of higher magnification EVG example placed just below it. Note that anatomical structures do not align exactly in near sections. Masson’s trichrome scale bar: 100 μm. EVG scale bar: 10 μm. Black arrows indicate disorganized elastin fibers in EVG examples.
DISCUSSION
Stem cell-based therapy has recently gained attention as a promising treatment for SUI (5,12,36). Preclinical investigations have focused on determining if stem cells differentiate and engraft in the tissue after direct injection into the urethra (12). However, successful clinical application of stem cell-based therapy may be more feasible with less invasive delivery methods. The current study demonstrates a potentially novel noninvasive MSC-based therapy for SUI after childbirth injury and suggests a mechanism for its effect.
This study confirmed our previous results that MSCs delivered IV will preferentially home to the urethra and vagina after simulated childbirth in female rats (9). The current study extends the results of our previous study and demonstrates homing to the spleen after VD, suggesting that MSCs could have a beneficial effect via immunomodulation and the systemic release of trophic factors, as has been demonstrated previously in other animal models (39).
Consistent with our urethral smooth muscle results, confocal imaging suggests that IV MSCs preferentially engraft in the smooth muscle of the urethra and vagina. In addition, our functional results demonstrated that while LPP recovered 7 days after VD with either MSC or CCM treatment, the EUS EMG did not recover at this time point. Therefore, improvement of urethral function 1 week after VD treated with either MSCs or CCM could result from facilitated recovery of smooth muscle and/or improved biomechanical properties of the urethra. The ratio of type I to type II striated muscle in rat EUS differs from that of human (27,31), which may reduce application of this conclusion to humans. Nonetheless, this study could provide rationale to justify a clinical investigation.
An alternative rat model of SUI that involves vaginal delivery and ovariectomy has been utilized to demonstrate that stem cells increase elastin fibers in the urethra and have a therapeutic effect even when the majority of the transplanted cells remained undifferentiated (23), suggesting the therapeutic actions of stem cells on the urethra are likely mediated by secreted factors from the cells. Although our animal model of VD does not involve the hormonal changes of pregnancy and delivery, our study supports the prior result since treatment with CCM after VD improved SUI recovery and altered the morphology of the elastin fibers in the urethra. Both studies therefore highlight the likely paracrine actions of MSCs as a potential mechanism of action and, although neither model is a perfect simulacrum of human delivery, together they suggest this mechanism may provide therapeutic benefit clinically.
Functional improvements from stem cell-based therapy in other fields have been shown to be due in part to paracrine actions in the host tissue rather than differentiation, repopulation, or fusion (1). CCM does not exactly replicate the paracrine actions of MSCs in vivo since local environmental factors affect cell function (25). Nonetheless, it remains the best estimate of such secreted factors. Therefore, CCM was used in this study as an approximation of the secretome of MSCs in vivo and had an effect similar to that of the MSCs.
Both MSCs and CCM likely improve continence via multiple mechanisms, including trophic effects on smooth muscle, angiogenesis, neuroregeneration, and promotion of ECM repair (18,28). Increased elasticity of the urethra could potentially account, in part, for improved LPP in the absence of improved EUS function 1 week after injury. However, the elastic fibers observed in this study were not highly organized and therefore probably contributed little in this regard. In contrast to previous work (23), we did not observe elastin fibers in urethras of sham-treated rats. This may be due to the more distal location at which we studied the urethra, since elastin in the urethra peaks proximally, near the vesicourethral junction (11). Furthermore, we assessed the animals 1 week after VD and treatment with MSCs or CCM, which is insufficient time to develop fully organized elastin fibers (17). Thus, it is possible that a bulking or inflammatory effect accounts for improved continence 1 week after MSC treatment; likewise, a similar bulking effect, although unlikely, could occur with periurethral CCM treatment that produced a similar extent of recovery. However, bulking does not explain improved smooth and striated muscle. Therefore, longer term quantitative studies should be done to fully investigate the mechanism of therapeutic benefit and anatomical changes with MSC or CCM treatment.
We did not identify which secreted factors play an important role in the improvement of urethral function; however, MSCs secrete a number of factors that could have a therapeutic effect, including angiogenic and cytoprotective factors, such as vascular endothelial growth factor (VEGF), and factors that inhibit fibroblasts and can remodel the ECM, such as TIMP tissue inhibitor of metalloproteinase 1 (TIMP-1), TIMP-2, and matrix metalloproteinase enzymes (MMPs) (28). Prior work has shown that CCM can inhibit breakdown of elastin by MMPs, which may indicate a role for TIMP-1 and/or TIMP-2 in our model (18). Since inflammatory processes may account for improved LPP 1 week after injury in the absence of improved EUS EMG, MSC-secreted factors may play a significant role. Future studies will be aimed at investigating which cytokines have a therapeutic effect with the goal of developing a clinical therapeutic strategy.
We used an acute injury model of SUI in female rats where VD simulates childbirth injury. Our results suggest a potential therapy for persistent postpartum SUI. Furthermore, if an MSC- or CCM-based therapy is free of adverse effects, it could serve as a prophylactic therapy for women at risk for later development of SUI. This work also has potential to develop into a clinical therapy for older women with SUI at a time remote from delivery, where injection of CCM or its key components may induce elastogenesis or other reparative effects.
Autologous muscle-derived progenitor cells have demonstrated therapeutic potential for SUI both in animal models (21) and in clinical trials (4,5). Adipose-derived stem cells delivered IV can also improve urethral function after simulated childbirth injury in rats (23). Stem cells from different tissue sources have been shown to display similar, although not identical, properties, such as their capacity for differentiation toward adipogenic, osteogenic, and chrondrogenic lineages (7). Therefore, the therapeutic effect of these cells and their secreted factors might be similar to each other. MSCs have been found to be immune privileged (35), suggesting their potential for use as an off-the-shelf therapy. To this end, a recent clinical trial has demonstrated safety and efficacy of non-type-matched MSCs as a therapy for ischemic heart disease (16).
Since rat MSCs were used in this study, the results of this study can only be applied to a rat model of injury treated with rat cells. Adult human stem cells need to be investigated in future studies, which can hopefully build upon this work. We used P16 cells to allow time for thawing, sorting, and transfecting in our laboratory. Lower passages of MSCs have been demonstrated to have greater tissue-protective effects (8). Therefore, our investigation could represent a worst case scenario, which nonetheless, demonstrated a significant therapeutic effect. As lower passage MSCs may have an even greater effect, future research should test this idea.
In conclusion, MSCs delivered IV facilitate recovery of the urethra after simulated childbirth injury, likely via secreted paracrine factors. In addition, local application of these paracrine factors stimulated an equivalent recovery to MSC treatment. These findings have the potential to increase the range of therapeutic applications of MSCs and hold promise as a novel, noninvasive therapy for SUI. Further investigations are needed to determine the long-term effects of MSC and CCM therapy, to identify those secreted factors responsible for the observed results and the mechanisms by which they act, as well as to determine if these findings also apply to humans.
Acknowledgments
We would like to thank Giulio Cossu’s Lab at San Raffaele Hospital (Milan, Italy) for the pCCLsin.ppt. hPGK.GFP.pre vector used in this experiment. This study was completed with financial support from the State of Ohio. The content reflects the views of the authors and does not purport to reflect the views of the State of Ohio. This award continues to support the commercialization of innovative therapies and technologies at the Center for Stem Cell and Regenerative Medicine at Case Western Reserve University, Cleveland, OH. Financial support was also obtained from the Cleveland Clinic, the Rehabilitation Research and Development Service of the Department of Veterans Affairs, and the National Science Foundation (grant #0755263). This work was also made possible by the Case Western Reserve University/Cleveland Clinic CTSA Grant from the NIH/National Center for Research Resources (UL1 RR024989).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berghmans LC, Hendriks HJ, Bo K, Hay-Smith EJ, de Bie RA, van Waalwijk van Doorn ES. Conservative treatment of stress urinary incontinence in women: A systematic review of randomized clinical trials. Br J Urol. 1998;82:181–191. doi: 10.1046/j.1464-410x.1998.00730.x. [DOI] [PubMed] [Google Scholar]
- 3.Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol. 2007;122:17–28. doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Carr LK, Magalic R, Kultgen PL, Herschorn S, Birch C, Murphy M, Chancellor MB. Autologous muscle derived cell therapy for stress urinary incontinence: A prospective, dose ranging study. J Urol. 2013;189:595–601. doi: 10.1016/j.juro.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, Erickson J, Huard J, Chancellor MB. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J. 2008;19:881–883. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 6.Corcos J, Loutochin O, Campeau L, Eliopoulos N, Bouchentouf M, Blok B, Galipeau J. Bone marrow mesenchymal stromal cell therapy for external urethral sphincter restoration in a rat model of stress urinary incontinence. Neurourol Urodynam. 2011;30:447–455. doi: 10.1002/nau.20998. [DOI] [PubMed] [Google Scholar]
- 7.Covas DT, Panepucci RA, Fontes AM, Silva WA, Orellana MD, Freitas MCC, Neder L, Santos ARD, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146(+) perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Crisostomo PR, Wang M, Wairiuko GM, Morrell ED, Terrell AM, Seshadri P, Nam UH, Meldrum DR. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 9.Cruz MA, Dissaranan C, Cotleur A, Kiedrowski M, Penn M, Damaser MS. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gynecol Int. 2012;2012:612946. doi: 10.1155/2012/612946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz Y, Jiang HH, Zaszczurynski PJ, Juarez R, Pastelin C, Damaser MS. Electromyography of pelvic floor muscles in rats. In: Mizrahi J, editor. Advances in applied electromyograms. Rijeka, Croatia: Intech Publishing; 2011. pp. 189–212. [Google Scholar]
- 11.Dass N, McMurray G, Brading AF. Elastic fibres in the vesicourethral junction and urethra of the guinea pig: Quantification with computerised image analysis. J Anat. 1999;195:447–453. doi: 10.1046/j.1469-7580.1999.19530447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dissaranan C, Cruz MA, Couri BM, Goldman HB, Damaser MS. Stem cell therapy for incontinence: Where are we now? What is the realistic potential? Curr Urol Rep. 2011;12:336–344. doi: 10.1007/s11934-011-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill BC, Moore C, Damaser MS. Postpartum stress urinary incontinence: Lessons from animal models. Exp Rev Obstet Gynecol. 2010;5:567–580. doi: 10.1586/eog.10.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnecchi M, He HM, Noiseux N, Liang OD, Zhang LM, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 15.Gnecchi M, Melo LG. Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 16.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cell (prochymal) after acute myocardial infusion. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashizume R, Yamawaki-Ogata A, Ueda Y, Wagner WR, Narita Y. Mesenchymal stem cells attenuate angiotensin II-induced aortic aneurysm growth in apolipoprotein E-deficient mice. J Vasc Surg. 2011;54:1743–1752. doi: 10.1016/j.jvs.2011.06.109. [DOI] [PubMed] [Google Scholar]
- 18.Jeon YK, Jang YH, Yoo DR, Kim SN, Lee SK, Nam MJ. Mesenchymal stem cells’ interaction with skin: Wound-healing effect on fibroblast cells and skin tissue. Wound Repair Regen. 2010;18:655–661. doi: 10.1111/j.1524-475X.2010.00636.x. [DOI] [PubMed] [Google Scholar]
- 19.Jiang HH, Pan HQ, Gustilo-Ashby MA, Gill B, Glaab J, Zaszczurynski P, Damaser MS. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodynam. 2009;28:229–235. doi: 10.1002/nau.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang WH, Ma AQ, Wang TZ, Han K, Liu Y, Zhang YM, Zhao XG, Dong AP, Du Y, Huang X, Wang J, Lei XJ, Zheng XP. Intravenous transplantation of mesenchymal stem cells improves cardiac performance after acute myocardial ischemia in female rats. Transplant Int. 2006;19:570–580. doi: 10.1111/j.1432-2277.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim SO, Na HS, Kwon D, Joo SY, Kim HS, Ahn Y. Bone-marrow-derived mesenchymal stem cell transplantation enhances closing pressure and leak point pressure in a female urinary incontinence rat model. Urol Int. 2011;86:110–116. doi: 10.1159/000317322. [DOI] [PubMed] [Google Scholar]
- 22.Lin AS, Carrier S, Morgan DM, Lue TF. The effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143–151. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 23.Lin GT, Wang GF, Banie L, Ning HX, Shindel AW, Fandel TM, Lue TF, Lin CS. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12:88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luber KM. Risk factors for stress urinary incontinence. Rev Urol. 2004;6:S3–S9. [PMC free article] [PubMed] [Google Scholar]
- 25.Metallo CM, Mohr JC, Detzel CJ, de Pablo JJ, van Wie BJ, Palecek SP. Engineering the stem cell microenvironment. Biotech Prog. 2007;23:18–23. doi: 10.1021/bp060350a. [DOI] [PubMed] [Google Scholar]
- 26.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 27.Praud CSP, Mondet F, Sebille A. The striated urethral sphincter in female rats. Anat Embryol. 2003;207:169–175. doi: 10.1007/s00429-003-0340-7. [DOI] [PubMed] [Google Scholar]
- 28.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Urinary incontinence after vaginal delivery or cesarean section. New Engl J Med. 2003;348:900–907. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by trans-differentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 31.Schroder HD, Reske-Kielsen E. Fiber types in the striated urethral and anal sphincters. Acta Neuropathol. 1983;60: 278–282. doi: 10.1007/BF00691877. [DOI] [PubMed] [Google Scholar]
- 32.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42–S45. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 33.Steward JE, Clemons JD, Zaszczurynski PJ, Butler RS, Damaser MS, Jiang HH. Quantitative evaluation of electrodes for external urethral sphincter electromyography during bladder-to-urethral guarding reflex. World J Urol. 2010;28:365–371. doi: 10.1007/s00345-009-0463-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sultan AH, Monga AK, Stanton SL. MRCOG - The pelvic floor sequelae of childbirth. Br J Hosp Med. 1996;55:575–579. [PubMed] [Google Scholar]
- 35.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 36.Wang HJ, Chuang YC, Chancellor MB. Development of cellular therapy for the treatment of stress urinary incontinence. Int Urogynecol J. 2011;22:1075–1083. doi: 10.1007/s00192-011-1432-1. [DOI] [PubMed] [Google Scholar]
- 37.Woo LL, Hijaz A, Kuang M, Penn MS, Damaser MS, Rackley RR. Over expression of stem cell homing cytokines in urogenital organs following vaginal distention. J Urol. 2007;177:1568–1572. doi: 10.1016/j.juro.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 38.Wood HM, Kuang M, Woo L, Hijaz A, Butler RS, Penn M, Rackley R, Damaser MS. Cytokine expression after vaginal distention of different durations in virgin Sprague–Dawley rats. J Urol. 2008;180:753–759. doi: 10.1016/j.juro.2008.03.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Zheng C, Ren X, Li J, Liu M, Zhang L, Liang L, Du W, Han ZC. Intravenous administration of bone marrow mesenchymal stem cells benefits experimental autoimmune myasthenia gravis mice through an immunomodulatory action. Scand J Immunol. 2010;72:242–249. doi: 10.1111/j.1365-3083.2010.02445.x. [DOI] [PubMed] [Google Scholar]