Abstract
Supplementation of enteral nutritional formulas and parenteral nutrition lipid emulsions with omega-3 fatty acids is a recent area of research in patients with critical illness. It is hypothesized that omega-3 fatty acids may help reduce inflammation in critically ill patients, particularly those with sepsis and acute lung injury. The objective of this article is to review the data on supplementing omega-3 fatty acids during critical illness; enteral and parenteral supplementation are reviewed separately. The results of the research available to date are contradictory for both enteral and parenteral omega-3 fatty acid administration. Supplementation with omega-3 fatty acids may influence the acute inflammatory response in critically ill patients, but more research is needed before definitive recommendations about the routine use of omega-3 fatty acids in caring for critically ill patients can be made.
Keywords: Omega-3 fatty acids, fish oil, acute lung injury, sepsis, critical illness, mechanical ventilation
INTRODUCTION
The systemic inflammatory response syndrome (SIRS) and sepsis are the leading causes of death in critically ill patients in Western countries. 1 (ALI), and its more severe form the acute respiratory distress syndrome (ARDS), are inflammatory syndromes of hypoxemic respiratory failure and diffuse pulmonary infiltrates that are commonly caused by sepsis.2–3 ALI is defined by the presence of acute hypoxemia (defined as a ratio of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) of < 300 [< 200 for ARDS]) and new bilateral infiltrates on a frontal chest radiograph that are not attributable to left atrial hypertension.4 ALI is characterized by alveolar epithelial and pulmonary capillary endothelial disruption, and its pathogenesis is believed to result from uncontrolled activation of the inflammatory cascade after pulmonary and systemic insults including sepsis, pneumonia, trauma, aspiration, inhalation injury, pancreatitis, and massive blood transfusion.5–6 There are approximately 200,000 cases of ALI per year in the United States and nearly 40% of ALI patients die.3, 7 Not only is sepsis the most common risk factor for ALI, accounting for nearly 80% of cases, but patients with sepsis as their ALI risk factor generally have worse outcomes than patients with other risk factors.3, 7
One feature of uncontrolled activation of the inflammatory response involves excessive production of proinflammatory cytokines and lipid-derived inflammatory mediators termed eicosanoids.8 As such, an important and relatively new area of critical care research regards the biological and clinical effects of lipids, provided both enterally and parenterally, during critical illness. Due to their anti-inflammatory properties, omega-3 fatty acids (FA) have been a particular focus of this research.
Omega-3 FAs are essential for normal growth and development and are thought to play a fundamental role in the prevention and treatment of coronary artery disease, diabetes, hypertension, arthritis, cancer, and other inflammatory and autoimmune disorders.9 In the last decade, new research has explored whether omega-3 FAs can decrease the production of inflammatory cytokines and eicosanoids and thus may offer benefit to patients with critical illness. This review will examine the clinical research that has been conducted in which omega-3 FAs have been administered enterally or parenterally, and its scope will generally be limited to critically ill patients with sepsis or ALI who often experience a more amplified inflammatory response than post-surgical or trauma patients.
BIOLOGIC EFFECTS OF OMEGA-3 FATTY ACIDS
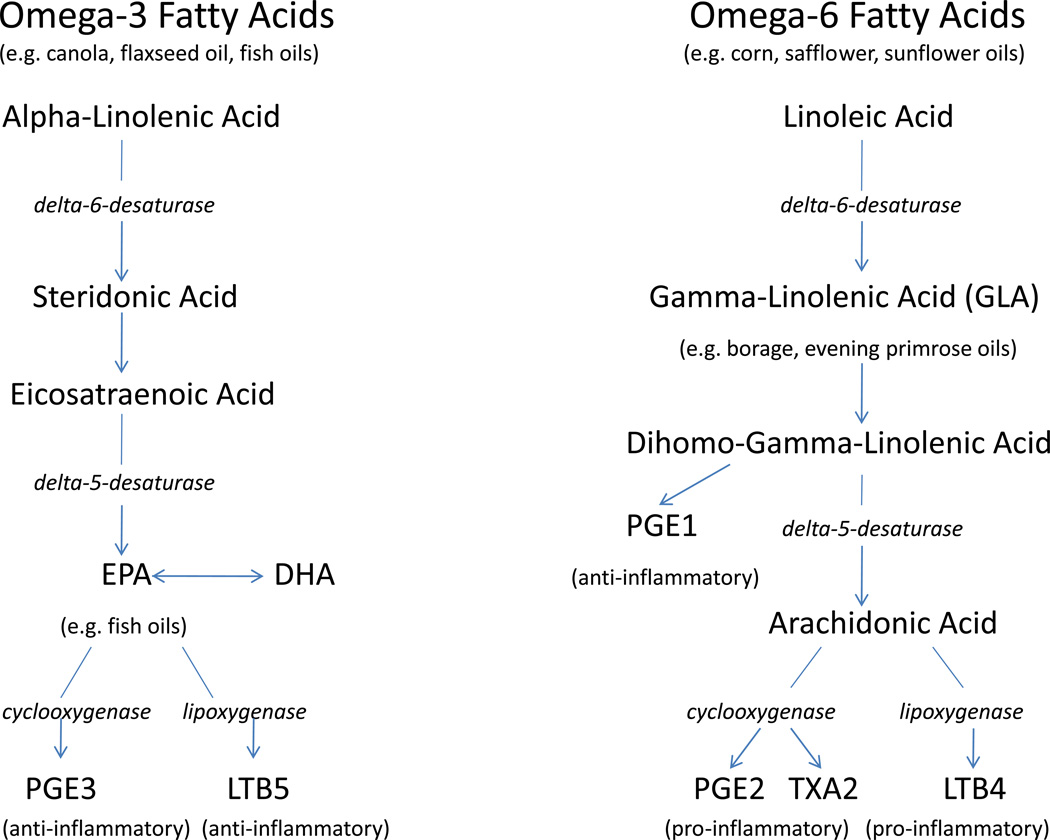
Humans are unable to synthesize omega-3 FAs de novo; they are essential. Omega-3 FAs are long-chain polyunsaturated fatty acids (PUFA) of 18–22 carbons in length with the first double-bond positioned at the third carbon atom from the methyl end of the fatty acid. Alpha-linolenic acid (ALA;18:3n-3) is the parent 18-carbon FA. The human body can synthesize longer omega-3 FAs such as eicosapentaenoic acid (EPA;20:5n-3) and docosahexanoic acid (DHA;22:6n-3) from ALA as seen in Figure 1.10 Omega-3 FAs are found in vegetable oils such as flaxseed, canola, linseed and soy oils, but fish oil is by far the richest source of EPA and DHA. Omega-6 fatty acids are also essential PUFAs with the first double-bond located at the sixth carbon from the methyl end. Linoleic acid (LA;18:2, n-6) is the shortest chain omega-6 FA and it is converted to gamma-linolenic acid (GLA;18:3, n-6) and arachidonic acid (AA;20:4, n-6). These omega-6 FAs are found in primarily in corn, safflower and sunflower oils. The chemical structure of omega-3 and omega-6 fatty acids dictates production of eicosanoid metabolic products, specifically prostaglandins, thromboxanes, leukotrienes, lipoxins and hydroxyl fatty acids which are directly involved in inflammation.11
Figure 1.
Omega-3 and omega-6 fatty acids pathways in humans.
The inflammatory response to infection or injury is extremely complex and, although normal following such an insult, can occur on a massive and uncontrolled scale, leading to additional tissue damage and potential further worsening of illness. Such uncontrolled activation of the inflammatory response has been implicated in the pathogenesis of both sepsis and ALI and is characterized by high levels of cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6.5, 12–16 Other inflammatory mediators that also characterize inflammation are derived from phospholipids composing the membranes of immune cells including macrophages, monocytes, and neutrophils. Omega-3 fatty acids are believed to have four main anti-inflammatory mechanisms of action that may be beneficial during critical illness: 1) metabolism into bioactive eicosanoid inflammatory mediators, 2) alteration of membrane lipid rafts, 3) inhibition of nuclear receptor activation (specifically NF-κB) to modulate production of inflammatory mediators, and 4) metabolism into resolvins and protectins.17
The particular FA components of the cell membrane provide substrate for production of lipid inflammatory mediators such as eicosanoids. PUFAs that are incorporated into human cellular membranes include EPA, DHA, omega-6 FA arachidonic acid (AA), and omega-9 FA oleic acid.18 Leukocyte membrane phospholipids are normally composed of 30% polyunsaturated fatty acids (PUFAs).18 With consumption of a typical Western diet, the majority of PUFAs are omega-6 FAs (primarily AA), while a small percentage are omega-3 FAs. 19 When the inflammatory cascade is activated by a stimulus, a macrophage can mobilize 25% to 40% of its membrane lipid content to produce free AA.18 Free AA is then further metabolized by cyclooxygenase (COX) and 5-lipoxygenase (LOX) into proinflammatory eicosanoids, including the 2-series prostaglandins and thromboxanes and the 4-series leukotrienes18 (Figure 1). The role of the eicosanoids in inflammation is well known, especially prostaglandin E2 (PGE2), thromboxane A2 (TXA2) and leukotriene B4 (LTB4).20 PGE2 induces fever, vascular permeability, and vasodilation during sepsis.18, 20–21 TXA2 promotes vascular permeability, platelet aggregation, leukocyte adhesion, and bronchoconstriction.21–22 TXA2 may also play a role in early sepsis and organ injury due to its pro-thrombotic effects leading to tissue ischemia.18 LTB4 activates leukocytes resulting in generation of reactive oxygen species, release of proteases such as elastase, neutrophil chemotaxis, and synthesis of lipid mediators.18
Omega-3 FAs can affect eicosanoid metabolism through a variety of mechanisms. Increased dietary consumption of EPA and DHA will result in increased incorporation into inflammatory cell membranes.23 Because omega-3 FAs replace AA in the phospholipid membrane, AA concentration is reduced, thus resulting in decreased production of the highly inflammatory AA-derived eicosanoids due to substrate restriction. In addition to replacing AA in immune cell membranes, EPA also 1) inhibits the metabolism of free AA into the inflammatory eicosanoids and 2) is metabolized itself to different eicosanoids (PGE3 and LTB5) that are considered less pro-inflammatory than those derived from AA.24–25 These mechanisms have been proven in animal studies where fish oil consumption has been shown to decrease the production of AA-derived eicosanoids by immune cells by 40 to 70%.26–27
In addition to influencing eicosanoid generation, omega-3 FAs in cell membranes can affect the fluidity of the membrane, which in turn influences the activity of membrane-bound enzymes, receptors, and transporters and lipid-based second messenger systems.28–30 Lipid rafts are detergent-resistant lipid domains within cell membranes that, upon cell activation, can compartmentalize key signaltransduction molecules, thus ensuring that signal transduction occurs properly.31 Due to its structure, it has recently been suggested that DHA may substantially influence basic properties of cell membranes, including fluidity, compressibility, and permeability,32 and thus be able to alter lipid raft behavior and function.33 This, in turn, may significantly affect cellular signal transduction and inflammatory processes. Another mechanism by which omega-3 FAs may ameliorate inflammation is via inhibition of nuclear factor kappa B (NF-κB). NF-κB is a key transcription factor involved in the up-regulation of inflammatory cytokine and adhesion molecule production.34 It is activated by phosphorylation of an inhibitory subunit (I-κB) triggered by extracellular inflammatory stimuli.35 Several recent studies have suggested that EPA and DHA may directly affect inflammatory gene expression (and thus cytokine production) by inhibiting NF-κB activation, although the exact mechanism of inhibition remains unclear.36–39
Finally, an additional anti-inflammatory mechanism of action involving EPA and DHA has recently been described. Over the past several years, it has been discovered that resolution of inflammation is an active process, rather than mere absence of inflammatory signals.40 Novel molecules called resolvins and protectins, lipid mediators derived from EPA and DHA with potent anti-inflammatory and neuroprotective properties,41–42 have been found to play an important role in the repair and resolution of inflammation.43 Resolvin (Rv) E1 and Protectin D1 are mediators effective in resolving animal models of airway inflammation and colitis.44–46 RvE1 has been found to also inhibit NF-κB – it decreased production of NF-κB by binding to an orphan receptor called ChemR23.47
Although supplementation with omega-3 FAs is generally thought to reduce the unfavorable inflammatory effects of omega-6 FAs through the mechanisms described above48, one omega-6 FA, gamma-linolenic acid (GLA), may also provide benefit in critical illness. GLA is found in evening primrose oil, black current seeds and borage oil and is rapidly converted to dihomo-GLA which is incorporated into immune cell phospholipids.49 Dihomo-GLA would then be expected to be preferentially converted to AA; however, it actually reduces the availability of AA and synthesis of AA-derived eicosanoids through mechanisms that are not yet clear.49 Dihomo-GLA is further metabolized to prostaglandin E1 (PGE1), a strong vasodilator of pulmonary and systemic circulation (Figure 1).50 GLA has been shown in animal models of critical illness to have an additive effect with EPA and DHA to decrease inflammation and organ failure.51
With this potential additive effect in mind, much of the research investigating the effects of omega-3 FAs in critically ill patients with sepsis and ALI has been conducted using a commercially available enteral feeding formula that contains EPA, DHA, GLA, and several antioxidant vitamins.52–54 This commercial feeding formula has been evaluated in several industry-sponsored animal studies. It has been shown to decrease AA concentration in inflammatory cell membranes;51, 55–59 reduce alveolar concentrations of LTB4, PGE2, and TXB2;51, 56 decrease pulmonary capillary permeability;56 and reduce alveolar neutrophil accumulation51 in endotoxemic rats.
ENTERAL OMEGA-3 FATTY ACIDS IN ACUTE LUNG INJURY AND SEPSIS
Three randomized clinical trials (RCTs) investigating a commercial feeding formula containing EPA, DHA, GLA, and antioxidants in critically ill patients have been completed and published (Table 1). Two of these RCTs studied patients with ALI 52–53 and the third studied patients with sepsis 54 (although nearly all of the patients in the third RCT had single-organ lung failure and thus had ALI). Two other similar but smaller studies have also been conducted but they have only been published in abstract form and will not be reviewed here.60–61 All three of these trials randomized patients to receive either the enteral formula containing EPA, DHA, GLA, and antioxidants (Oxepa®; Abbott Nutrition, Abbott Laboratories, Columbus, Ohio) or a control formula equal in fat, protein, and carbohydrate content but not containing fish oil. Both Oxepa® and the control formulas were considered high fat (55% of calories), low carbohydrate (28% of calories) enteral formulas as compared to standard polymeric enteral products which contain an average of 30% fat and 50% carbohydrate. Two different control formulas were used in the three RCTs, and they differed in lipid content. The lipid content of the control formula in Gadek et al. and Singer et al. was 97% corn oil, which is high in linoleic acid, an omega-6 FA.52–53 The lipid in the control formula in Pontes-Arrudes et al was 55.8% canola oil, 14% corn oil, 20%MCT, 7% high oleic safflower oil and 3.2% soy lecithin.54
Table 1.
Summary of Published Randomized Controlled Trials Comparing a Commercial Enteral Feeding Formula Containing EPA, DHA, GLA, and Antioxidants to Another Commercial High-Fat Enteral Feeding Formula Without Fish Oils
| Gadek et al. | Singer et al. | Pontes-Arruda et al. | |
|---|---|---|---|
| Population | ARDS n=146 |
ALI n=100 |
Severe Sepsis n=165 |
| Design | P, R, C, DB | P, R, C | P, R, C, DB |
| Mean Fatty Acid Intake | |||
| EPA (g/day) | 6.9 | 5.4 | 4.9 |
| DHA (g/day) | 2.9 | 2.5 | 2.2 |
| GLA (g/day) | 5.8 | 5.1 | 4.6 |
| Significant Findingsa | |||
| Improved oxygenation | Yes | Yesb | Yes |
| Reduced ICU length of stay | Yes | No | Yes |
| Reduced ventilator time | Yes | Yesc | Yes |
| Reduced 28-day mortality | No | Yes | Yes |
| Reduced new organ failure | Yes | Not assessed | Yes |
P, prospective; R, randomized; C, controlled; DB, double blind; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; GLA, gamma-linolenic acid; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; FA, fatty acid.
Statistically significant (p<0.05);
Days 4, 7 only;
Day 7 only
In the first study, subjects in the investigational group received approximately of 7g EPA, 3g DHA, and 6g GLA per day.52 Serial bronchoalveolar lavage (BAL) was done at study entry, day 4, and day 7. Of the 146 patients enrolled, 48 were excluded from main analyses of biological outcomes, although intention-to-treat analyses were done on clinical outcomes. The treatment group had improved oxygenation at days 4 and 7 (p<0.0499); reduced BAL fluid neutrophil (representing decreased lung inflammation) count at day 4 (p=0.008); and decreased duration of mechanical ventilation (p=0.027), ICU length of stay (p=0.016), and new organ failures (p=0.018). Mortality was 25% in the control group and 16% in the treatment group (p=0.165).
In the second trial, patients in the EPA/DHA/GLA/antioxidant group had a shorter, but statistically insignificant, duration of mechanical ventilation.53 Oxygenation was improved at days 4 and 7 (p<0.05) but not at day 14. Hospital length of stay was not different between the two groups. Mortality at 3 months after study enrollment was unexpectedly high at 75% in both groups..
The third trial in patients with sepsis, most of whom had ALI, found significant increases in ICU-free days (p<0.001), ventilator-free days (p<0.001), oxygenation status (p<0.0001), and 28-day survival (52% vs. 33%, p=0.04) in the treatment group.54 Patients receiving the formula enriched with EPA, DHA and GLA also had reduced development of new organ dysfunction (p<0,001). All patients tolerated near maximal enteral feeding which can be difficult to achieve in this population.
In a meta-analysis of these three studies, the data from a total of 296 patients who were considered evaluable was assessed.50 The use of the formula enriched with EPA, DHA, GLA and antioxidants was associated with a 60% reduction in the risk of 28-day all cause mortality (OR=0.40; 95% confidence interval [CI] = 0.24–0.68; p=0.001). Significant reductions were also found in the risk of developing new organ failures (OR = 0.17; 95% CI=0.08–0.34; p<0.0001), length of stay in the ICU (standardized mean difference [SMD] =0.51; 95% CI=0.27–0.74; p<0.0001), and time on mechanical ventilation (SMD=0.56; 95% CI=0.32–0.79; p<0.0001) in patients who received the formula containing omega-3 FAs. These studies have also been reviewed as part of the Canadian Clinical Practice Guidelines for enteral nutrition supplemented with fish oils, available at www.criticalcarenutrition.com.62
Two additional publications from the original RCT by Gadek et al52 have investigated mechanisms of action on EPA, DHA, GLA and antioxidants in patients with ALI. The first reported a significantly reduced level of interleukin-8 (a potent inflammatory cytokine and neutrophil chemoattractant) in BAL fluid of patients in the treatment group (p=0.05), as well as a trend toward reduced levels of IL-6, LTB4 and TNF-α.63 The second ancillary study investigated the antioxidant status of the enrolled patients with ALI and compared them to a group of healthy controls.64 At enrollment, the patients with ALI had reduced levels of antioxidants and were found to be in a state of oxidative stress (as measured by total radical antioxidant potential and lipid peroxide levels) compared to healthy controls. After receiving the treatment enteral formula, the levels of oxidative stress were not reduced at days 4 or 7, but plasma levels of β-carotene and α-tocopherol were restored to normal while lipid peroxide levels did not increase, thus suggesting the antioxidants may have protected against further lipid peroxidation. Because the above trials investigated a single enteral formula (Oxepa®) containing multiple pharmaconutrients in addition to EPA and DHA, the benefit attributed to the omega-3 FAs in particular is unknown.
Other enteral nutritional formulas enriched with omega-3 FAs that have been studied in critically ill patients have been supplemented with additional nutrients thought to affect immune function, such as the amino acids arginine or glutamine, or antioxidant vitamins (most notably selenium).65–66 It is difficult to interpret the results of studies using formulas that contain arginine because some prior studies and a meta-analysis have suggested that formulas containing arginine may benefit elective surgical patients but may be harmful to critically ill patients with sepsis.67 Use of these formulas in critically ill patients showed a trend toward higher mortality where there was no such effect in elective surgical patients.67 Therefore, a negative effect or lack of benefit with use of these arginine-containing formulas in critically ill patients may be related to the arginine and not the omega-3 FAs and these studies are therefore not included in this review.
When pharmaconutrients are added to enteral feedings and combined with macronutrients, it is difficult to generalize study results. Two recently completed, but not yet published, RCTs of omega-3 FAs in patients with ALI were designed to dissociate the pharmaconutrients from enteral feedings; the pharmaconutrients were administered as medications through enteral feeding tubes while patients received standard enteral nutrition regimens.68–69 The preliminary results of these two recent RCTs were presented orally at the American Thoracic Society (ATS) International Conference held May 15–20, 2009 in San Diego, California, and both of these trials appear to challenge the positive results found in the previous RCTs using the enteral formula containing EPA, DHA, GLA, and antioxidants. The OMEGA study was a large phase III RCT conducted by the National Institutes of Health, National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Network (ARDSnet) to investigate an enteral supplement (delivered twice daily) containing EPA, DHA, GLA, and antioxidants in patients with ALI.68 This RCT was stopped in March 2009 after accrual of 272 of the planned 1000 patients due to a lack of efficacy. The enteral supplement did not improve the outcomes of death at 60 days, ventilator-free days at day 28 or ICU-free days at day 28. We have also conducted a phase II randomized, double-blind, placebo-controlled trial of enteral fish oil (EPA and DHA) in 90 critically ill patients with ALI that is awaiting publication.69 In this RCT, fish oil or a saline placebo was given enterally as a medication, separate from enteral or parenteral nutrition. The primary endpoint was BAL fluid IL-8, and secondary endpoints included clinical outcomes as well as biomarkers of inflammation and injury, lung compliance, and oxygenation. None of the biologic or clinical endpoints were significantly different between the treatment and control groups. Based on the preliminary results available from these two RCTs, it is currently unclear if enteral omega-3 FAs are beneficial in patients with sepsis and ALI. Some limitations of the prior studies using the enteral formula enriched with EPA, DHA, GLA and antioxidants include the small number of published studies to date, and the use of a control formula in two of the studies52–53 that was high in linoleic acid, an omega-6FA. Further research will be needed before any definitive recommendations can be made about enteral omega-3 FA supplementation in critically ill patients.
INCLUSION OF OMEGA-3 FATTY ACIDS IN PARENTERAL NUTRITION
Lipid emulsions are generally administered in parenteral nutrition (PN) regimens in order to provide a source of calories and essential fatty acids, but the type of fatty acids included in the emulsions may have an impact on the inflammatory response in critical illness. The lipid traditionally used in parenteral nutrition regimens is soybean oil, in which approximately 54% of the fatty acids are the omega-6 FA linoleic acid.70 Concern has been expressed that a lipid emulsion high in linoleic acid might be pro-inflammatory, pro-coagulatory, immunosuppressive and potentially harmful. However, clinical trials using these omega-6 FA rich emulsions have provided conflicting evidence.71 A meta-analysis of two studies72–73 in which total PN was administered in critically ill patients suggested that PN with standard lipids may result in a higher infectious complication rate (p=0.02) than PN infused without lipids, although there was no difference in mortality rate.74 The suggestion that soybean oil-based lipid emulsions may not be ideal for critically ill patients has led to new formulations which replace some of the soybean oil with other oils such as medium chain triglycerides (MCT), olive oil, or fish oil.71
Recent studies have been conducted in critically ill patients using parenteral lipid formulations that contain EPA and DHA from fish oil. In a study by Mayer et al.,75 21 patients with sepsis and intolerant to enteral nutrition (i.e. requiring PN) were randomized in an open-label trial to receive an omega-3 FA rich lipid emulsion (Omegaven®; Fresenius Kabi, Bad Homburg, Germany) or a standard omega-6 rich lipid emulsion (Lipoven®; Fresenius Kabi) for 5 days. Table 2 shows the fatty acid composition of these two lipid emulsions. The administration of the omega-3 rich emulsion induced an increase in omega-3 free FAs in plasma and reversed the omega-3/omega-6 ratio, favoring EPA and DHA over AA, and reaching maximum effect in 3 days. Patients receiving the fish oil emulsion had rapid incorporation of EPA and DHA into leukocyte and monocyte cell membranes, increasing the concentration of each approximately threefold. Ex vivo, these cells produced approximately 30% less TNF-α, interleukin 1-β, IL-6, and IL-8 when stimulated by endotoxin. However, there was no difference in serum cytokine levels between the patient groups receiving PN supplemented with omega-3 FAs and the standard lipid emulsion. In another smaller study by the same authors, 10 patients with septic shock and requiring parenteral nutrition were randomly assigned to receive either fish oil fortified Omegaven® or the standard omega-6 rich Lipoven® (Table 2) for a total of 10 days.76 C-reactive protein concentration and leukocyte counts decreased in those receiving the omega-3 emulsion and increased in the group receiving the omega-6 emulsion, although the differences were not statistically significant (p=0.08 and p=0.09, respectively). Patients infused with omega-6 lipids exhibited a trend towards longer ventilation time (p=0.07). An increase in LTB5 was seen only in the group receiving omega-3 lipids, approaching an LTB4/LTB5 ratio of almost 20% by the end of the study infusion period. Trends were also seen with increases in plasma omega-3 free FAs, the TXA3/TXA2 ratio, and platelet-activating factor synthesis in the group receiving the omega-3 rich lipid emulsion. Although these studies do not have the power to definitively state that infusion of omega-3 FA in patients with septic shock will modulate inflammatory mediator production and reduce inflammation, the results tend to support this hypothesis.
Table 2.
Fatty Acid Composition of Omega-3 and Omega-6 Rich Intravenous Lipid Emulsions
| Fatty Acid Name |
Shorthand Nomenclature |
Omega-3 lipid emulsion (Omegaven®) |
Omega-6 lipid emulsion (Lipoven®) |
|---|---|---|---|
| Myristic Acid | C14:0 | 4.9 | - |
| Palmitic Acid | C16:0 | 10.7 | 12.4 |
| Palmitoleic Acid | C16:1n-7 | 8.2 | - |
| Stearic Acid | C18:0 | 2.4 | 5.0 |
| Oleic Acid | C18:1n-9 | 12.3 | 24.1 |
| Linoleic Acid (LA) | C18:2n-6 | 3.7 | 52.2 |
| Alpha-Linolenic Acid (ALA) | C18:3n-3 | 1.3 | 8.2 |
| Arachidonic Acid (AA) | C20:4n-6 | 2.6 | - |
| Eicosapentaenoic Acid (EPA) | C20:5n-3 | 18.8 | - |
| Docosapentaenoic Acid | C22:5n-3 | 2.8 | - |
| Docosahexaneoic Acid (DHA) | C22:6n-3 | 16.5 | - |
| Others | 16.1 | - |
Administration of an omega-3 FA rich infusion (Omegaven®) during PN was reported in a prospective, open label trial to improve diagnosis-related clinical outcomes in critically ill patients after major abdominal surgery (n=255) or who were experiencing peritonitis and abdominal sepsis (n=276), non-abdominal sepsis (n=16), multi-trauma (n=59), severe head injury (n=18) or other diagnoses (n=37). 77 All patients received Omegaven® which contains fish oil in doses between 0.005–0.426 gm/kg/day because the dosage was at the discretion of the attending physician. A significant reduction in the length of both ICU stay and hospital stay (both p<0.001) was found with doses of >0.05 gm fish oil/kg/day. Mortality was also significantly reduced (p<0.05) in patients receiving >0.1 gm fish oil/kg/day. A difference in mortality effect was observed for the different diagnosis groups with significant benefit seen in patients with severe head injury and multiple trauma (both p<0.0001), followed by patients with abdominal sepsis (p=0.0027). Need for antibiotic treatment was higher in those patients receiving fish oil doses <0.15 gm/kg/day. Although the study was not controlled or blinded, these results suggest clinical benefit from the inclusion of omega-3 FAs in PN regimens in these populations. The question of clinical benefit was further explored in a recent randomized, controlled trial using the PN lipid emulsions (20% Omegaven® + 80% Lipoven® or Lipoven® alone) in 40 patients with severe acute pancreatitis for 5 days.78 In this study, there were no significant differences between groups in white blood cell count, rates of organ dysfunction, the number of infections, or length of ICU stay. However, it is of interest that plasma IL-6 level was reduced in the omega-3 FA group and slightly increased in the control group. Both C-reactive protein level and gas exchange improved by day 6 (both p<0.05) and there was a reduced need for continuous renal replacement therapy (p<0.05) in the patients receiving fish oil.
In contrast to the generally positive results of the previous studies which were conducted primarily in critically ill surgical patients, a randomized, double-blind, controlled trial in critically ill medical patients did not find clear medical benefit to the provision of omega-3 FAs in parenteral lipid emulsions.79 A total of 166 medical ICU patients were randomized to receive a mixture of medium and long-chain triglycerides (Lipofundin MCT®; Braun Medical, Melsungen, Germany) or a combination or Omegaven® and Lipofundin MCT® for 7 days. The primary endpoint a was more rapid reduction in plasma IL-6 and greater monocyte expression of HLR-DR (a marker of immune competence) in the group receiving omega-3 FAs. No differences were found between the groups in IL-6 or HLA-DR, nor did they detect difference in mortality, duration of mechanical ventilation, length of ICU stay, number of infections, additional immune and inflammatory markers, or bleeding events. The authors speculated that one possible reason an effect was not seen in this study was that the intervention may have been initiated after the inflammatory process was fully activated in these severely ill patients, as compared to surgical patients where fish oil supplementation has been most beneficial when administered before surgery.80 The study may also have been underpowered. In addition, this study used a control lipid emulsion that was lower in omega-6 FAs, due to the presence of MCT, than the standard lipid emulsion, thus reducing the average daily dose of linoleic acid and potentially resulting in a control formula that was less inflammatory.79
No adverse effects have been reported with the administration of lipid emulsions fortified with fish oil, suggesting they are safe to use in critically ill patients.71 The prior trials of omega-3 rich parenteral lipid emulsions are summarized in Table 3. Because the research available to date provides conflicting data on the effect of omega-3 FA fortified lipid emulsions in critically ill patients, its influence on inflammatory processes and clinical outcomes remains unclear.
Table 3.
Summary of Trials Studying Omega-3 Rich Parenteral Lipid Emulsion
| References | Population | Design | Emulsion | Findings |
|---|---|---|---|---|
| Mayer et al. | Sepsis; n=21 | P, R, C | O vs. L | Increased plasma concentrations of omega-3 FAs, increased incorporation of EPA and DHA over AA into mononuclear leukocyte cell membranes. Serum cytokine levels did not differ between groups. |
| Mayer et al.. | Septic shock; n=10 | P, R, C | O vs. L | CRP and leukocyte levels decreased in Omega-3 group and increased in Omega-6 group. LTB5 increased in the Omega-3 group. |
| Heller et al. | Critically ill; n=661 | P | O at different dosing levels | ICU and hospital length of stay, as well as mortality, were significantly reduced in patients receiving higher fish oil dose levels. Antibiotic use increased in patients receiving lower fish oil dose levels. |
| Wang et al. | Pancreatitis; n=40 | P, R, C, DB | 20% O+80% L vs. L alone | Patients in the Omega-3 group had higher serum EPA, lower CRP levels and better oxygenation. No differences were seen in WBC count, organ dysfunction and ICU length of stay. |
| Friesecke et al. | Medical ICU; n=166 | P, R, C, DB | Lip MCT vs. O+Lip MCT | No differences were found in IL-6 or other inflammatory markers, mortality, duration of mechanical ventilation, ICU length of stay, infectious complications or bleeding events. |
P, prospective; R, randomized; C, controlled; DB, double blind; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid; FA, fatty acid, O, Omegaven®; L, Lipoven®; Lip MCT, Lipofundin-MCT®, WBC, white blood cell; CRP, C-reactive protein; IL-6, interleukin-6; ICU, intensive care unit.
CONCLUSION
The clinical use of omega-3 fatty acids in critically ill patients appears to be safe. However, with the research that has been completed to date, it is not possible to definitively determine whether enteral or parenteral supplementation with omega-3 FAs has a positive effect on the inflammatory response, immune function, or clinical outcomes in critically ill patients with sepsis or acute lung injury. Although much is known about the mechanism of action of omega-3 FAs and their effect on cytokines and inflammatory mediators from research with animals and on healthy subjects, it remains unclear if omega-3 fatty acids delivered after initiation of severe stress is beneficial. Much of the available data is contradictory, or at least inconclusive, because studies are small and single-center, intention-to-treat analyses are not done, varying enteral or parenteral formulas are used, and patients are heterogeneous.
Studies involving nutritional support and pharmaconutrient therapy in critically ill patients are particularly difficult to conduct and interpret for multiple reasons. First, most critical care research is difficult due to issues with surrogate consent and a short enrollment window of time (e.g. most trials require enrollment within 24–48 hours after ICU admission to initiate treatment early in the course of critical illness). Second, critically ill patients are a very heterogeneous population and present with a variety of severe medical problems. Due to this heterogeneity, large numbers of patients are required to demonstrate an effect.81 Third, the delivery of enteral nutrition and pharmaconutrients is often interrupted or poorly tolerated in patients with severe illness. Fourth, complicating the analysis of an effect of omega-3 FAs is that fatty acids in many studies are often added to existing enteral nutrition formulas or lipid emulsions which contain macronutrients and other pharmaconutrients, thus rendering interpretation of the results and discerning which agent produced the effect difficult. Fifth, choice of control group agent in many trials is also in issue, with patients often receiving higher than usual doses of omega-6 fatty acids. Sixth, when conducting large clinical trials, it is difficult to obtain many biologic specimens at multiple clinical sites, thus making mechanistic studies more difficult. Finally, dosing data and pharmacokinetics studies of both enteral and parenteral omega-3 fatty acids in critically ill patients is virtually nonexistent.
All of the research showing a positive effect with enteral administration of omega-3 FAs in patients with sepsis and ALI involved the use of one enteral formula fortified with EPA, DHA, GLA and antioxidants and a high fat control formula that is rarely used in routine clinical care.52–54 The studies of parenteral omega-3 FA supplementation which suggest a positive effect have been conducted using a particular fish oil lipid emulsion (Omegaven®) while controls have received a soybean oil-based emulsion high in potentially inflammatory omega-6 FAs.75–79 New research should focus on determining the pharmacokinetics and optimal dosing of omega-3 FA in critically ill patients. Additional randomized, double-blinded, controlled clinical trials in which the omega-3 FAs are administered separately from standard enteral feeding formulas that are devoid of other pharmaconutrients, and in which the control agent is truly inert, are needed to definitively inform patient care.
Contributor Information
Julie M. Martin, Department of Medicine, University of Vermont College of Medicine, Burlington, VT, U.S.A..
Renee D. Stapleton, Department of Medicine, University of Vermont College of Medicine, Burlington, VT, U.S.A..
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 5.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 6.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES, Steinberg KP. Causes and timing of death in patients with ARDS. Chest. 2005;128:525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 8.Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–352. doi: 10.1007/s11745-003-1068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560S–569S. doi: 10.1093/ajcn/70.3.560s. [DOI] [PubMed] [Google Scholar]
- 10.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 12.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 13.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Parsons PE. Mediators and mechanisms of acute lung injury. Clin Chest Med. 2000;21:467–476. doi: 10.1016/s0272-5231(05)70159-3. [DOI] [PubMed] [Google Scholar]
- 15.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 16.Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, Eisner MD. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007;35:1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulger EM, Maier RV. Lipid mediators in the pathophysiology of critical illness. Crit Care Med. 2000;28:N27–N36. doi: 10.1097/00003246-200004001-00004. [DOI] [PubMed] [Google Scholar]
- 19.Palombo JD, Lydon EE, Chen PL, Bistrian BR, Forse RA. Fatty acid composition of lung, macrophage and surfactant phospholipids after short-term enteral feeding with n-3 lipids. Lipids. 1994;29:643–649. doi: 10.1007/BF02536099. [DOI] [PubMed] [Google Scholar]
- 20.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 22.Petrak RA, Balk RA, Bone RC. Prostaglandins, cyclo-oxygenase inhibitors, and thromboxane synthetase inhibitors in the pathogenesis of multiple systems organ failure. Crit Care Clin. 1989;5:303–314. [PubMed] [Google Scholar]
- 23.Marangoni F, Angeli MT, Colli S, et al. Changes of n-3 and n-6 fatty acids in plasma and circulating cells of normal subjects, after prolonged administration of 20:5 (EPA) and 22:6 (DHA) ethyl esters and prolonged washout. Biochim Biophys Acta. 1993;1210:55–62. doi: 10.1016/0005-2760(93)90049-f. [DOI] [PubMed] [Google Scholar]
- 24.Lee TH, Menica-Huerta JM, Shih C, Corey EJ, Lewis RA, Austen KF. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem. 1984;259:2383–2389. [PubMed] [Google Scholar]
- 25.Golman DWPW, Goetzl EJ. Human neutrophil chemotactic and degranulating activites of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983;117:282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- 26.Lee TH, Hoover RL, Williams JD, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985;312:1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- 27.Whelan J, Broughton KS, Kinsella JE. The comparative effects of dietary alpha-linolenic acid and fish oil on 4- and 5-series leukotriene formation in vivo. Lipids. 1991;26:119–126. doi: 10.1007/BF02544005. [DOI] [PubMed] [Google Scholar]
- 28.Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984;779:89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 29.Murphy MG. Dietary fatty acids and membrane protein function. J Nutr Biochem. 1990;1:68–79. doi: 10.1016/0955-2863(90)90052-m. [DOI] [PubMed] [Google Scholar]
- 30.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–108. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in T cell signalling and disease. Semin Cell Dev Biol. 2007;18:608–615. doi: 10.1016/j.semcdb.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 35.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 36.Lo CJ, Chiu KC, Fu M, Lo R, Helton S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NF kappa B activity. J Surg Res. 1999;82:216–221. doi: 10.1006/jsre.1998.5524. [DOI] [PubMed] [Google Scholar]
- 37.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega −3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- 39.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res. 2008;52:885–897. doi: 10.1002/mnfr.200700289. [DOI] [PubMed] [Google Scholar]
- 40.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer K, Seeger W. Fish oil in critical illness. Curr Opin Clin Nutr Metab Care. 2008;11:121–127. doi: 10.1097/MCO.0b013e3282f4cdc6. [DOI] [PubMed] [Google Scholar]
- 49.Singer P, Shapiro H. Enteral omega-3 in acute respiratory distress syndrome. Current opinion in clinical nutrition and metabolic care. 2009;12:123–128. doi: 10.1097/MCO.0b013e328322e70f. [DOI] [PubMed] [Google Scholar]
- 50.Pontes-Arruda A, Demichele S, Seth A, Singer P. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. JPEN J Parenter Enteral Nutr. 2008;32:596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- 51.Mancuso P, Whelan J, DeMichele SJ, Snider CC, Guszcza JA, Karlstad MD. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Crit Care Med. 1997;25:1198–1206. doi: 10.1097/00003246-199707000-00023. [DOI] [PubMed] [Google Scholar]
- 52.Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in patients with acute respiratory distress syndrome Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 54.Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- 55.Palombo JD, DeMichele SJ, Lydon EE, et al. Rapid modulation of lung and liver macrophage phospholipid fatty acids in endotoxemic rats by continuous enteral feeding with n-3 and gamma-linolenic fatty acids. Am J Clin Nutr. 1996;63:208–219. doi: 10.1093/ajcn/63.2.208. [DOI] [PubMed] [Google Scholar]
- 56.Mancuso P, Whelan J, DeMichele SJ, et al. Effects of eicosapentaenoic and gamma-linolenic acid on lung permeability and alveolar macrophage eicosanoid synthesis in endotoxic rats. Crit Care Med. 1997;25:523–532. doi: 10.1097/00003246-199703000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Palombo JD, DeMichele SJ, Lydon E, Bistrian BR. Cyclic vs continuous enteral feeding with omega-3 and gamma-linolenic fatty acids: effects on modulation of phospholipid fatty acids in rat lung and liver immune cells. JPEN J Parenter Enteral Nutr. 1997;21:123–132. doi: 10.1177/0148607197021003123. [DOI] [PubMed] [Google Scholar]
- 58.Palombo JD, DeMichele SJ, Boyce PJ, Noursalehi M, Forse RA, Bistrian BR. Metabolism of dietary alpha-linolenic acid vs eicosapentaenoic acid in rat immune cell phospholipids during endotoxemia. Lipids. 1998;33:1099–1105. doi: 10.1007/s11745-998-0311-x. [DOI] [PubMed] [Google Scholar]
- 59.Palombo JD, DeMichele SJ, Boyce PJ, et al. Effect of short-term enteral feeding with eicosapentaenoic and gamma-linolenic acids on alveolar macrophage eicosanoid synthesis and bactericidal function in rats. Crit Care Med. 1999;27:1908–1915. doi: 10.1097/00003246-199909000-00032. [DOI] [PubMed] [Google Scholar]
- 60.Elamin M, Hughes LF, Drew D. Effect of enteral nutrition with eicosapentaenoic acid (EPA), gamma-linolenic acid (GLA), and antioxidants reduced alveolar inflammatory mediators and proteins influx in patients with acute respiratory distress syndrome (ARDS) Chest. 2005;128:225S. [Google Scholar]
- 61.Moran V. Effect of an enteral feedin with eicosapentaenoic and gamma-linolenic acids on the outcome of mechanically ventilated critically ill septic patients. Crit Care Med. 2006;34:A70. [Google Scholar]
- 62.Heyland DK. Nutrition Clinical Practice Guidelines 4.1(b) [Accessed on August 26, 2009];Composition of enteral nutrition: fish oils 2009. Available at: http:www.criticalcarenutrition.com/docs/cpg/4.1bfish%20oils_FINAL.pdf.
- 63.Pacht ER, DeMichele SJ, Nelson JL, Hart J, Wennberg AK, Gadek JE. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- 64.Nelson JL, DeMichele SJ, Pacht ER, Wennberg AK. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants on antioxidant status in patients with acute respiratory distress syndrome. JPEN J Parenter Enteral Nutr. 2003;27:98–104. doi: 10.1177/014860710302700298. [DOI] [PubMed] [Google Scholar]
- 65.Calder PC. Immunonutrition in surgical and critically ill patients. Br J Nutr. 2007;(98 Suppl 1):S133–S139. doi: 10.1017/S0007114507832909. [DOI] [PubMed] [Google Scholar]
- 66.Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34:1980–1990. doi: 10.1007/s00134-008-1213-6. [DOI] [PubMed] [Google Scholar]
- 67.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–953. doi: 10.1001/jama.286.8.944. [DOI] [PubMed] [Google Scholar]
- 68.Rice T for the NHLBI ARDS Network. Trial of omega-3 fatty acid, gamma-linolenic acid and antioxidant supplemention in the management of acute lung injury (Omega); Paper presented at: American Thoracic Society International Conference; May, 18, 2009; San Diego, California. 2009. [Google Scholar]
- 69.Stapleton RD, Martin TR, Gundel SJ, et al. A phase II, randomized, double-blind, placebo-controlled, safety and efficacy study of fish oil (eicosapentaenoic acid and docosahexanoic acid) on lung and systemic inflammation in patients with acute lung injury. Am J Respir Crit Care Med. 2009;179:A2169. (Abstr.) [Google Scholar]
- 70.Suchner U, Katz DP, Furst P, et al. Impact of sepsis, lung injury, and the role of lipid infusion on circulating prostacyclin and thromboxane A(2) Intensive Care Med. 2002;28:122–129. doi: 10.1007/s00134-001-1192-3. [DOI] [PubMed] [Google Scholar]
- 71.Calder PC. Rationale for using new lipid emulsions in parenteral nutrition and a review of the trials performed in adults. Proc Nutr Soc. 2009:1–9. doi: 10.1017/S0029665109001268. [DOI] [PubMed] [Google Scholar]
- 72.McCowen KC, Friel C, Sternberg J, et al. Hypocaloric total parenteral nutrition: effectiveness in prevention of hyperglycemia and infectious complications--a randomized clinical trial. Crit Care Med. 2000;28:3606–3611. doi: 10.1097/00003246-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Battistella FD, Widergren JT, Anderson JT, Siepler JK, Weber JC, MacColl K. A prospective, randomized trial of intravenous fat emulsion administration in trauma victims requiring total parenteral nutrition. J Trauma. 1997;43:52–58. doi: 10.1097/00005373-199707000-00013. discussion 58–60. [DOI] [PubMed] [Google Scholar]
- 74.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 75.Mayer K, Gokorsch S, Fegbeutel C, et al. Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am J Respir Crit Care Med. 2003;167:1321–1328. doi: 10.1164/rccm.200207-674OC. [DOI] [PubMed] [Google Scholar]
- 76.Mayer K, Fegbeutel C, Hattar K, Omega-3 vs, et al. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive Care Med. 2003;29:1472–1481. doi: 10.1007/s00134-003-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Heller AR, Rossler S, Litz RJ, et al. Omega-3 fatty acids improve the diagnosis-related clinical outcome. Crit Care Med. 2006;34:972–979. doi: 10.1097/01.CCM.0000206309.83570.45. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Li W, Li N, Li J. Omega-3 fatty acids-supplemented parenteral nutrition decreases hyperinflammatory response and attenuates systemic disease sequelae in severe acute pancreatitis: a randomized and controlled study. JPEN J Parenter Enteral Nutr. 2008;32:236–241. doi: 10.1177/0148607108316189. [DOI] [PubMed] [Google Scholar]
- 79.Friesecke S, Lotze C, Kohler J, Heinrich A, Felix SB, Abel P. Fish oil supplementation in the parenteral nutrition of critically ill medical patients: a randomised controlled trial. Intensive Care Med. 2008;34:1411–1420. doi: 10.1007/s00134-008-1072-1. [DOI] [PubMed] [Google Scholar]
- 80.Tsekos E, Reuter C, Stehle P, Boeden G. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin Nutr. 2004;23:325–330. doi: 10.1016/j.clnu.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Preiser JC, Chiolero R, Wernerman J. Nutritional papers in ICU patients: what lies between the lines? Intensive Care Med. 2003;29:156–166. doi: 10.1007/s00134-002-1581-2. [DOI] [PubMed] [Google Scholar]