Abstract
Fractures are the most common large-organ, traumatic injuries to humans. The repair of bone fractures is a postnatal regenerative process that recapitulates many of the ontological events of embryonic skeletal development. Although fracture repair usually restores the damaged skeletal organ to its pre-injury cellular composition, structure and biomechanical function, about 10% of fractures will not heal normally. This article reviews the developmental progression of fracture healing at the tissue, cellular and molecular levels. Innate and adaptive immune processes are discussed as a component of the injury response, as are environmental factors, such as the extent of injury to the bone and surrounding tissue, fixation and the contribution of vascular tissues. We also present strategies for fracture treatment that have been tested in animal models and in clinical trials or case series. The biophysical and biological basis of the molecular actions of various therapeutic approaches, including recombinant human bone morphogenetic proteins and parathyroid hormone therapy, are also discussed.
Introduction
Fracture healing and bone repair are postnatal processes that mirror many of the ontological events that take place during embryonic development of the skeleton and have been extensively reviewed elsewhere.1–5 The recapitulation of these ontological processes is believed to make fracture healing one of the few postnatal processes that is truly regenerative, restoring the damaged skeletal organ to its pre-injury cellular composition, structure and bio- mechanical function. Interestingly, a comparison of the transcriptome of mouse callus tissues across a 21-day period of fracture healing showed that about one-third of the mouse homologues of the genes expressed by human embryonic stem cells are preferentially induced. Many of the homeotic genes that control appendicular limb development also show increased expression during fracture healing.6 Finally, all the primary morphogenetic pathways that are active during embryonic skeletal development are expressed in fracture calluses,6 and have therefore become the focal point of efforts to develop new therapies. In this Review, we place these biological processes in the context of how trauma and the immune system, as a component of the injury response, are related to the developmental aspects of fracture healing. We then review the relationships between ontogeny and the recovery of skeletal function. Finally, we focus on specific biophysical, local and systemic therapies that have been used to promote fracture healing and on the various biological processes they promote.
Phases of fracture healing
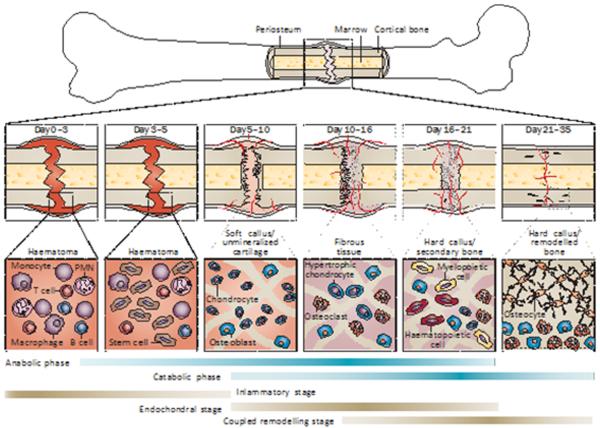
Fracture healing and skeletal tissue repair involve an initial anabolic phase characterized by an increase in tissue volume related to the de novo recruitment and differentiation of stem cells that form skeletal and vascular tissues. Immediately adjacent to the fracture line, a cartilaginous callus will form. Peripheral to this central region, at the edges of the new cartilage tissues, the periosteum swells and primary bone formation is initiated.7,8 Concurrent with cartilage tissue development, cells that will form the nascent blood vessels that supply the new bone are recruited and differentiate in the surrounding muscle sheath.9,10 The increases in the vascular bed that surrounds and then grows into the callus are further reflected by the increased blood flow into the area of tissue repair. As chondrocyte differentiation progresses, the cartilage extracellular matrix undergoes mineralization and the anabolic phase of fracture repair terminates with chondrocyte apoptosis.11,12 The histological and cellular progression of these events are shown in Figure 1. The anabolic phase is followed by a prolonged phase in which catabolic activities predominate, and is characterized by a reduction in the volume of the callus tissues. During this phase of predominately catabolic activity, such as cartilage resorption, specific anabolic processes continue to take place; secondary bone formation is initiated as the cartilage is resorbed and primary angiogenesis continues as the nascent bone tissues replace the cartilage. Subsequently, when bone remodelling begins, the first mineralized matrix produced during primary bone formation is resorbed by osteoclasts, and then the secondary bone laid down during the period of cartilage resorption is also resorbed. As the bony callus tissue continues to be resorbed, this prolonged period is characterized by coupled cycles of osteoblast and osteoclast activity in which the callus tissues are remodelled to the bone's original cortical structure (termed `coupled remodelling' here). During this period, the marrow space is re-established and the original marrow structure of haematopoietic tissue and bone is regenerated. In the final period of the catabolic phase, extensive vascular remodelling takes place in which the increased vascular bed regresses and the high vascular flow rate returns to its pre-injury level.13,14 Although these processes take place consecutively, they overlap substantially and are a continuum of changing cell populations and signalling processes within the regenerating tissue. A temporal overview of the biological events of fracture healing, and the cell types involved at each stage of fracture healing, are presented in Figure 2.
Figure 1.
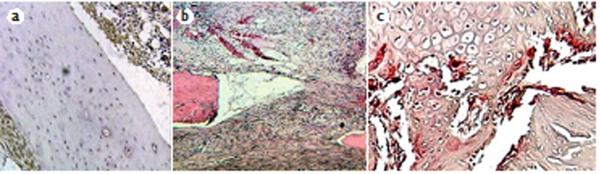
Histology of early stages of mouse femur fracture repair. a | The inflammatory stage of fracture repair 24 h after injury. Sections were immunoreacted with anti-TNF antibodies to show innate immune responses to the injury of both the periosteum and marrow cell populations (brown) with haematoxylin counterstaining (blue). b | Late inflammatory stage 3 days after injury, cellular streaming of undefined fibrous cells and early angiogenic cells forming small vessels are evident at the fracture site. Stained with haematoxylin and eosin. c | The late endochondral stage, 14 days after injury. The section was stained for tartrate-resistant acid phosphatase to show the recruitment of resorptive osteoclasts (bright red).
Figure 2.
Femur fracture repair. The major metabolic phases (blue bars) of fracture healing overlap with biological stages (brown bars). The primary metabolic phases (anabolic and catabolic) of fracture healing are presented in the context of three major biological stages (inflammatory, endochondral bone formation and coupled remodelling) that encompass these phases. The primary cell types that are found at each stage, and the time span of their prevalence in each stage, are denoted. The time scale of healing is equivalent to a mouse closed femur fracture fixed with an intramedullary rod. Abbreviations: BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; DKK1, Dickkopf-related protein 1; LRP, LDL-receptor-related protein; MSC, mesenchymal stem cell; PMN, polymorphonuclear leukocyte; PTH, parathyroid hormone; PTHrP, parathyroid-hormone-related protein; RANKL, receptor activator of nuclear factor κB ligand.
Control of fracture healing
Innate and adaptive immune functions
Both innate and adaptive immune processes are essential during the anabolic and catabolic phases of fracture healing. In the initial inflammatory stage after injury, specific cell-mediated immune functions remove necrotic tissues, promote angiogenesis and initiate repair.15–17 Interestingly, fracture leads to suppression of the immune system,18 with a local increase in the number of induced T regulatory (iTREG) cells that suppress active adaptive immune responses within the fracture callus.19 Studies have further shown that mesenchymal stem cells actively maintain a hypoimmunogenic state20,21 through the production of immunosuppressive paracrine factors,22–24 or through the direct actions of these cells on immune-cell populations, including T cells.25,26 Such effects suggest that these cells impart immune tolerance throughout the early stages of endochondral bone formation and provide protection to the developing tissues by suppressing allo-proliferation of T cells during stem-cell recruitment and cartilage formation.19 At different stages of fracture healing, cytokines with inflammatory and immune functions, including IL-1β,19 IL-6,27,28 IL-17F,19,29 IL-2319 and TNF,12,30 are variably expressed and have different effects. During the period of acute inflammation immediately after injury, TNF and IL-6 recruit cells needed for tissue regeneration and their complete absence has been shown to delay skeletogenic mesenchymal stem cell differentiation.12,27–30 On the other hand, if inflammation remains unresolved, such as with a bacterial infection at the injury site, healing can fail.31 In the context of chronic inflammation, such as in streptozotocin-induced diabetes mellitus in mice, the cartilage callus is prematurely removed during fracture healing,32,33 and in mouse models of systemic lupus erythematosus (SLE), osteoclast activity and bone turnover are high.19 In both of these disease models, less primary and secondary bone accumulation occurs during fracture healing than in control mice. Anti-inflammatory biologic agents and antiresorptive agents might therefore have therapeutic value in fracture healing for patients with either SLE or diabetes mellitus.34
Evidence for direct T-cell involvement in the control of fracture healing comes from studies that showed negative effects after lymphocyte depletion.35–38 Studies of fracture healing in Rag1−/− mice, which lack T cells and B cells, showed that although cartilage maturation and replacement was delayed and overall less mineralized tissue accumulated,29 these mice recovered mechanical function earlier than wild-type mice.39 γδ T cells, which can detect the products of stressed or damaged cells even in the absence of antigen presentation,40 are also involved in fracture repair. In other studies, mice deficient in γδ T cells have shorter times to fracture union and earlier development of mature fracture calluses than γδ-T-cell-sufficient mice.41 Finally, in a model of SLE, in which mice have defective Fas receptors (TNF receptor superfamily member 6), the percentage of iTREG cells was increased in both callus and bone tissues during the period of active cartilage formation, while at the same time the number of activated T cells in these tissues decreased, despite increased numbers of activated T cells in the spleen, indicative of an autoimmune condition.19 These results suggest that activated iTREG cells are chondroprotective and are consistent with studies showing that mesenchymal stem cells induce both the differentiation and immunosuppressive function of iTREG cells.42,43 Pharmacological factors that alter immune function and inflammation might have negative and positive effects on fracture healing and are important to consider in the context of specific comorbidities that affect regeneration.34
Stem cell origins
The extent of external soft-tissue and hard-tissue trauma,44,45 and the mechanical strain produced by therapeutic interventions, will each effect the origin of the stem cells that contribute to healing, the extent of cartilage-cell versus bone-cell differentiation and the progression of fracture healing.46–48 Results from transgenic lineage tracking have shown that the fracture callus is formed mostly of cells from the periosteum.49 Other studies showed that periosteal cells specifically respond to bone morphogenetic protein 2 (BMP-2) to promote both chondrogenesis and osteogenesis, whereas cells in the marrow space will form only bone in response to BMP-2.50 Mechanisms controlling cortical bone repair might, therefore, be different to those that repair and remodel trabecular bone, and those that take place in the medullary space.
The extent and type of trauma that accompanies a fracture will also affect the tissue source from which stem cells that contribute to the callus are derived. Transgenic mouse studies using a reporter gene conditionally activated in muscle stem cells showed that these cells do not contribute substantially to callus formation in a model of closed tibial fracture. By contrast, studies of open tibial fractures, in which the periosteum was surgically stripped from the bone with additional muscle fenestration, showed that almost half the cells in the callus were derived from the surrounding muscle.51 Thus, the extent of injury that can be repaired is limited; too much damage to the muscle and periosteum can overcome the supply of tissue-regenerative stem cells. Also important is to consider the extent to which the vascular tissues in the surrounding muscle sheath are compromised; failure of angiogenesis after fracture or osteotomy can lead to nonunion.44
Stability
The overall stability of the fixation and immobilization of the fracture will also affect the patterns of skeletogenic stem cell differentiation into chondrocytes or osteoblasts, with more-extensive cartilage tissue formation associated with less stability, and increasing stability with more bone tissue.48 Interestingly, when fractures are not stably fixed, angiogenesis is initially increased.52–54 Excessive interfragmentary instability, however, will impede cartilage replacement, diminish angiogenesis and prevent bone from bridging the fracture gap.54 Therefore, an optimal `window' of interfragmentary motion seems to be needed to enable normal calluses to develop and stably bridge a fracture. Together, these mouse studies have considerable bearing on the potential application of treatments, such as BMP therapy, to human fractures. The data indicate that the number of stem cells, the tissue source of these cells and their ability to be recruited are dependent on the extent of injury and the stability of the fracture union.
Mechanisms of ontogeny and postnatal healing
Both fracture healing and endochondral bone formation are directly regulated by BMPs,55,56 fibroblast growth factor 2 (FGF-2),57,58 hedgehog proteins,59,60 parathyroid hormone (PTH), PTH-related protein,61,62 transforming growth factor β (TGF-β),63 protein wingless morphogenetic factors, Wnt proteins and Wnt signalling antagonists.64,65 Several of these morphogenetic processes form interactive feedback loops, including coregulation of BMPs and Wnt signalling proteins;66,67 of PTH or PTH- related protein67 and FGF-2;69 and of PTH-related protein and Wnt proteins.67–70 Furthermore, some of these factors regulate interaction between different cell and tissue types during skeletal healing. Therefore, how therapeutic drugs coordinate the temporal and spatial interactions of different tissues of the skeletal organ (vascular, skeletal and haematopoietic) must be understood before their application in the treatment of delayed healing or nonunion of fractures.
Therapy
Several strategies have been developed to clinically enhance fracture healing. In general, fracture healing can be enhanced by either biophysical or biological means. With regard to biophysical enhancement, substantial research has been conducted on the use of electromagnetic fields and low-intensity pulsed ultrasonography. In the case of biological enhancement, strategies can be subdivided into local and systemic. Although a multitude of methods, materials and factors have been studied, this Review focuses on those strategies that have undergone rigorous scientific testing in preclinical animal studies as well as clinical trials or case series. A summary of experimentally tested approaches to promote fracture healing is presented in Box 1.
Biophysical enhancement
Electromagnetic fields have been investigated as a means of skeletal repair for more than 60 years.71 Primarily applied to the treatment of nonunited fractures,72 they have had up to 80% success rates in clinical studies.73,74 In one study that demonstrated success in the treatment of delayed union, however, the effects of the electromagnetic field on fresh fractures were unclear.75 In a meta-analysis of randomized controlled trials by Mollon et al.,76 2,546 citations were reviewed, 11 articles met the inclusion criteria: criteria were the use of a random allocation of treatments; inclusion of patients presenting with a long-bone lesion; a treatment arm receiving electromagnetism of any wave- form to affect bone-healing; a treatment arm receiving no active intervention; and report of the effect of electromagnetic stimulation on direct bone-healing. Evidence from four trials reporting on delayed or nonunited fractures demonstrated an overall nonsignificant pooled relative risk of 1.76 (95% CI, 0.8–3.8; P = 0.15; I= 60%) in favour of electromagnetic stimulation. The authors concluded that although the pooled analysis did not show a significant effect of electromagnetic stimulation on delayed unions or nonunited long-bone fractures, the methodological limitations and heterogeneity of studies creates uncertainty as to this conclusion.
Low-intensity pulsed ultrasonography has also been studied in clinical settings, including in patients with long- bone fractures of the upper and lower extremities,77,78 in patients having osteotomies, and in smokers with fractures (smoking is a negative risk factor for fracture healing). A meta-analysis of randomized controlled trials identified 13 reports, of which five investigated outcomes relevant to fracture healing.79 These five studies used conservative nonoperative approaches. Two of the studies were of moderate quality, one of which reported improved functional recovery after treatment of clavicle fracture, and the other reported no improvement in functional recovery after treatment of stress fractures; the other three trials were low quality and reported improved functional recovery after nonoperative treatment of long-bone fractures. Quality assessment was based on grades of recommendation according to Wright et al.80 The investigators concluded, and we agree with their assessment, that the effect of low-intensity pulsed ultrasonography on the healing of fractures is moderate to very low in quality and the data are conflicting.79
Local biological enhancement
Local strategies for the repair and regeneration of bone include the use of osteogenic materials, including autologous bone marrow, peptide signalling molecules (FGF-2 and platelet-derived growth factors [PDGFs]) and morphogenetic factors (BMPs and Wnt proteins). Although many other metabolites and proteins have been investigated, those discussed in this article are, we believe, the most extensively studied and have the great- est potential to be therapeutically targeted to enhance skeletal repair.
Bone marrow grafting
The field of biotechnology continues to investigate and develop new molecules for skeletal repair, such as the safe and effective method of percutaneous autologous bone-marrow grafting for the treatment of atrophic tibial diaphysis nonunions. In a prospective case series, Hernigou et al.81 used this method to treat 60 patients with uninfected aseptic nonunions. Patients underwent bone-marrow aspiration from both iliac crests. Samples were concentrated on a cell separator and then injected into the nonunion, resulting in fracture unions in 53 of the 60 patients. In vitro cellular analysis of aliquots taken prior to injection showed positive correlations between the number of colony-forming units detected and the volume of mineralized callus at 4 months. The seven patients with nonunited fractures also had fewer colony-forming units in their grafts than those patients whose fractures united. These results suggest that autologous bone marrow might be an effective material for the enhancement of skeletal repair, and that the quality of the harvesting technique and cell preparation might affect the efficacy of the graft.
FGF-2
Kawaguchi et al.82 investigated the use of recombinant human FGF-2 to enhance tibial-shaft fracture healing. 70 patients with a transverse or short-oblique fracture of the tibial shaft were randomized to one of three groups and were assessed for 24 weeks. Patients in each group were injected, into their fracture, with a placebo (gelatin hydro- gel), or with a low dose (0.8 mg) or high dose (2.4 mg) of FGF-2 hydrogel. The cumulative percentages of patients with radiographically proven fracture union were higher in the FGF-2-treated groups than in the placebo-treated group, and no differences between the high-dose and low- dose FGF-2 groups were reported. None of the patients underwent a secondary intervention, and no differences in the number or type of adverse events were detected between the three groups.
PDGF
Another peptide signalling molecule that has been extensively studied for the enhancement of skeletal repair is recombinant human homodimeric PDGF subunit B (PDGF-BB). DiGiovanni et al.83 enrolled 434 (with 397 completing the study) patients in a prospective rand- omized controlled (2:1) noninferiority trial of patients requiring hind foot or ankle arthrodesis. The investigators tested the hypothesis that PDGF-BB, combined with a β-tricalcium phosphate matrix (n = 260; 394 joints), would be safe and effective as an alternative to the current standard of care, an autologous bone graft from the iliac crest (n = 137; 203 joints). CT showed that bones from 159 patients (262 joints) in the PDGF group and 85 (127 joints) in the autograft group were fused at 6 months. The PDGF group had less pain and an improved safety profile. The investigators concluded that, in patients requiring hindfoot or ankle arthrodesis, treatment with PDGF has similar fusion rates, less pain and fewer adverse effects in comparison with autografting.
BMPs
BMPs are possibly the most extensively investigated candidates for the enhancement of skeletal repair, particularly recombinant human BMP-2 and BMP-7. Although these therapies were developed for similar purposes, their testing and regulation by the FDA has been different. Friedlaender et al.84 selected recombinant human BMP-7 to treat tibial nonunions in a prospective randomized con- trolled trial of 124 patients. All nonunions were at least 9 months old with no improvement in the 3 months prior to each patient enrolling in the study. 63 patients were treated with a statically locked intramedullary nail plus BMP-7 with type I collagen as a carrier. The 61 individuals in the control group were treated with an autologous bone graft. At 9 months, 81% and 75% of the BMP-7-treated patients and 85% and 85% of the autologous bone-graft- treated patients were `healed' (P = 0.524), as judged by lack of pain at the fracture site and radiographic assessment, respectively. Although the investigators concluded that BMP-7 treatment for tibial nonunion is safe and effective, the data showed no improvement compared with autologous bone grafting; therefore, the FDA did not provide premarket approval for this `device'. Composite recombinant human BMPs with their delivery vehicles, such as a type I collagen carrier, are regulated as a device. Instead, a `humanitarian device exemption' was issued,85 allowing a limited distribution to 4,000 patients per year. As part of this agreement, the institutions where the surgeries are performed must have an institutional review board to monitor use of the device.
A different clinical setting was selected for the study of recombinant human BMP-2; a prospective randomized controlled trial of open tibial-shaft fractures was con- ducted in which patients (n = 450) received initial irrigation and debridement by the surgeon as well as a statically locked intramedullary nail (standard of care) or standard of care plus, at the time of wound closure, either 0.75mg/kg or 1.50 mg/kg BMP-2 embedded in a type I collagen sponge.86 After 12 months, the risk of secondary interventions was reduced by 44% in the group treated with the high dose of BMP-2 compared with standard of care alone (P = 0.005). 58% of the BMP-2 group was `healed', as determined by radiographic evidence and lack of pain at the fracture site, compared with 38% of the group treated by usual care (P = 0.001). Compared with usual care, those patients treated with the high dose of BMP-2 had fewer failures of the nail, fewer infections and faster wound-healing. The FDA granted premarket approval for recombinant human BMP-2,85 and this treatment is now available in several countries for the treatment of fresh, open tibial fractures.
Since the FDA-approval of BMP therapy, several investigations have re-examined their clinical use and reported sobering results. In a double-blind, randomized, controlled phase II–III trial, the efficacy and safety of recombinant human BMP-2 for the treatment of closed tibial diaphysial fractures was studied in comparison with the standard of care alone.87 Co-primary endpoints of the study were the time to fracture union and the time to return to normal function. However, the study was terminated after 6 months when an interim analysis of the results from 180 patients revealed no shortening in the time to fracture union in the active groups of the study compared with standard of care. The median time to pain-free, full-weight bearing was also not substantially different between groups. The investigators concluded that the times to fracture union and full-weight bearing in patients with closed fractures treated with intra- medullary nail fixation were not substantially reduced by BMP-2 treatment. In a related study, the healing of open tibial fractures treated with intramedullary nail fixation was not substantially accelerated by the addition of an absorbable type I collagen sponge containing BMP-2.88
Although the focus of this article is fracture healing, as opposed to spinal arthrodesis, the findings of the Yale Open Data Access (YODA) project89 should be mentioned because they affect the use of BMP-2 in the field of musculoskeletal care. By performing a safety and effectiveness study of recombinant human BMP-2 for spinal fusion, a meta-analysis of individual participant data showed no evidence of clinically meaningful differences between treatment with BMP-2 and an iliac crest bone- graft. BMP-2 had similar complication rates to iliac bone grafting, but had higher rates during off-label procedures. The risk of cancer, for example, was slightly higher with the use of BMP-2 (relative risk 1.98; 95% CI 0.86–4.54). Both recombinant human BMP-2 and BMP-7 are available under various regulatory conditions, but the development of safer and more effective materials is an important goal.
Systemic biological enhancement
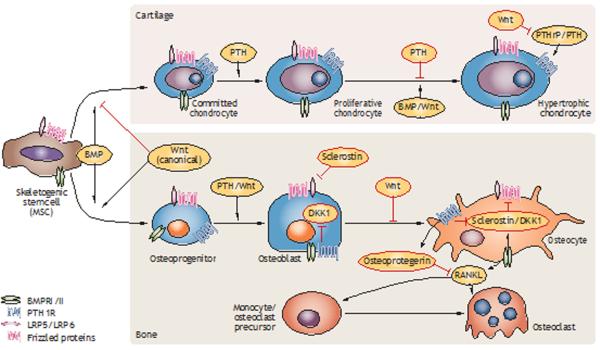
In the future, hopefully, a patient with a bone fracture will be treated with an injection or pill that would enhance, accelerate or otherwise augment skeletal healing as an adjunct to surgical treatment. Several strategies might achieve this goal. Candidate therapies include the use of PTH or humanized monoclonal anti-sclerostin or anti-Dickkopf-related protein 1 antibodies. The cross-talk of these therapies with the BMP pathway, their function in endochondral bone formation and intramembranous bone formation, and their production during coupled remodelling are presented in Figure 3.65 In addition, the common observation that patients with a sustained head or spinal cord injury can have enhanced skeletal healing suggests that a circulating factor, or perhaps a unique neurological mechanism, could be induced to enhance bone repair.
Figure 3.
Crosstalk between Wnt, PTH and BMP signalling in cartilage and bone cell lineages. Wnt ligands mediate canonical signalling through β-catenin and BMP signalling can be mediated by SMAD1, SMAD3 and SMAD5. The LRP5 and LRP6 antagonists sclerostin and DKK1 are a central focus of targeted antibody-based therapeutics. The primary stages in the lineage progression of cartilage and bone cells as they differentiate from skeletogenic stem cells are depicted. Major stimulatory and inhibitory effects on the differentiation and proliferation of the two lineages are denoted. Primary effects of the BMP, PTH and Wnt pathway on osteoclast differentiation are indirectly mediated by differing pathway activities that regulate paracrine factor expression in osteocytes, which in turn regulate osteoclast differentiation and function. Abbreviations: BMP, bone morphogenetic protein; BMPR, bone morphogenetic protein receptor; DKK1, Dickkopf-related protein 1; LRP, LDL receptor-related protein; MSC, mesenchymal stem cell; PTH, parathyroid hormone; PTH1R, parathyroid hormone 1 receptor; PTHrP, parathyroid-hormone-related protein; RANKL, receptor activator of nuclear factor κB ligand; SMAD, mothers against decapentaplegic homologue.
Parathyroid hormone
PTH is a naturally occurring hormone that modulates mineral homeostasis and has been developed as a drug for the treatment of osteoporosis. The enhancement of fracture healing using both the active-site portion of the molecule (amino acids 1–34, also known as teriparatide) and the full-length molecule (amino acids 1–84) have been studied. Alkhiary et al.70 investigated standard closed diaphysial femoral fractures in 270 male Sprague Dawley rats administered a daily (up to 35 days) subcutaneous injection of vehicle or of 5 μg/kg or 30 μg/kg PTH (1–34). By 21 days, calluses from the group treated with 30 μg of PTH (1–34) had significant (P <0.05) increases in torsional strength, stiffness, bone mineral content, bone mineral density and cartilage volume, compared with controls. No changes in osteoclast density were detected, suggesting that treatment with PTH (1–34) enhanced bone formation, but did not induce bone resorption. The investigators concluded that daily systemic administration of PTH (1–34) produces a sustained anabolic effect throughout the bone-modelling phase of fracture healing. These data support a clinical trial in which postmenopausal women with a distal radius fracture requiring closed reduction and immobilization, but not surgery, were randomly assigned to 8 weeks of once-daily injections of placebo, or of 20 μg or 40 μg PTH (1–34) within 10 days of fracture (n = 34 in each group).90 The estimated median time from fracture to first radiographic evidence of complete cortical bridging in three of four cortices was 9.1, 7.4, and 8.8 weeks in the three groups, respectively (overall P = 0.015). Statistically significant differences between the two doses of PTH (1–34) were not found, but the time to healing was shorter in the 20 μg PTH (1–34) group than in the placebo group (P = 0.006). The investigators concluded that fracture repair can be accelerated by 20 μg PTH (1–34), but that these results warrant further study. In a prospective randomized controlled study to evaluate the effects of PTH (1–84) on pelvic fracture healing and functional outcome in postmenopausal women, 65 patients had radiographic and CT examination of pelvic fractures.91 21 patients were treated with a once- daily injection of 100 μg of PTH (1–84), beginning 2 days after admission to hospital, and the other 44 patients were injected with saline. All patients were treated with 1,000 mg of calcium and 800 IU of vitamin D. CT was repeated at 4-week increments until radiographic evi- dence of cortical bridging at the fracture site was visible. The results showed a mean time to fracture healing of 7.8 weeks for the PTH (1–84) group compared with 12.6 weeks for the control group (P <0.001). The investigators concluded that, in postmenopausal patients with osteoporosis, the PTH (1–84) fragment accelerates pelvic fracture healing, and improves the functional outcome assessed with a visual analogue scale for pain and a `timed up and go' test.91
The Wnt family
The Wnt family of signalling molecules is only now beginning to be the focus of studies to enhance skeletal healing. Wnt proteins are a family of secreted proteins that regulate diverse developmental processes. Activation of Wnt signalling blocks preadipocyte differentiation and stimulates osteoblastogenesis. Data show that some Wnts, such as protein Wnt10b, shift cells towards the osteoblastic lineage by induction of the transcription factors runt-related transcription factor 2 (RUNX2), homeobox protein DLX5 and transcription factor Sp7 (also known as osterix), and also by suppression of adipogenic transcription factors.92
LDL receptor-related proteins are a family of cell- surface receptors involved in diverse biological processes, including lipid metabolism, retinoid uptake and neu- ronal migration. LDL receptor-related protein 5 (LRP5) is required for signalling of genes in the Wnt family by acting as a co-receptor. Activation of Wnt signalling in osteoblasts normally stimulates bone formation, and antagonism of Wnt signalling by secreted proteins from the Dickkopf family prevents formation of the active complex of LRP5 and thus modulates bone mass. Loss- of-function mutations in LRP5 also impair activation of Wnt signalling and reduce bone mass; LRP5 Gly171Val impairs the ability of Dickkopf-related proteins to antag- onize the Wnt pathway, and unopposed Wnt signalling leads to increased bone mass (Figure 3).93 Sclerostin, the SOST gene product expressed exclusively by osteocytes, inhibits LRP5 and thus inhibits the Wnt signalling pathway.94–96 Indeed, the sclerosing bone dysplasias van Buchem disease (also known as hyperostosis corticalis generalisata familiaris) and sclerosteosis are characterized by thick skulls, square jaws and finger abnormali- ties, and are associated with loss-of-function mutations in SOST.94,95 Several studies have demonstrated that anti-sclerostin antibody treatment enhances metaphysial bone-healing in rats97 and enhances proximal tibial defect healing in ovariectomized rats.97 Increased serum levels of sclerostin during human fracture healing have also been detected.98 Furthermore, a study of postmenopausal women with osteoporosis, although not a definitive clinical trial of fracture treatment, has shown that anti- sclerostin antibody (romosozumab) therapy can increase bone mineral density and bone formation.99 These studies suggest that members of the Wnt signalling pathway, and sclerostin in particular, might be therapeutic targets for the enhancement of skeletal repair.
Recovery of biological and physical function
Skeletal healing is defined functionally by the recovery of weight-bearing by fractured bone, and is dependent on structural and material features of the tissue. Because aspects of tissue structure and material properties of the tissue are variably affected by multiple biological processes, and each of these processes will be affected uniquely by a given therapy, each therapy will facilitate this recovery of function through differing processes. Therapeutics for fracture healing should therefore be developed and administered according to the biological processes they modify, how these actions affect tissue material composition and structure, and the relationships between composition and structure to the recovery of mechanical function. The use of specific therapeutics in a situation in which healing has been compromised should also be considered in the context of how comorbidties can affect different biological processes and impede healing. Importantly, some therapeutics can affect fracture healing by their actions on multiple biological processes. Therefore, when considering the use of a therapeutic one should consider which biological process would be compromised by a given comorbidity and which therapeutic might best modify the compromised state. Additionally, timing the use of a therapeutic to have its maximal effect on a specific biological process is important to improve the progression of healing. Two examples are presented to demonstrate these relationships and Table 1 summarizes how various therapeutics facilitates the recovery of function in comparison with their biological effects.
Table 1.
Timing and effects of therapies for fracture healing
| Therapy | Optimal therapeutic window | Biological effects | Structural effects | Mechanical effects | Accelerated fracture union? |
|---|---|---|---|---|---|
| PTH | Throughout all stages | Chondrocyte and osteoblast proliferation Delayed chondrocyte hypertophy Increased coupled remodelling | Increased callus size, bone mass and mineral content | Increased stiffness and strength | Yes |
| Anti-sclerostin or anti-DKK1 antibodies | Late endochondral phase Throughout bone remodelling | Osteprogenitor expansion Chondrocyte hypertrophy Delayed cartilage resorption Decreased coupled remodelling | Increased callus size, bone mass and mineral content | Increased stiffness and strength | Yes |
| BMP-2 | Inflammatory period | Stem cell commitment of chondrogenic and osteogenic lineages Chondrocyte hypertophy and coupled remodelling | Increased callus size | Increased stiffness | No |
Abbreviations: BMP-2, bone morphogenetic protein 2; DKK1, Dickkopf-related protein 1; PTH, parathyroid hormone.
In the first example, PTH improved healing in a mouse femoral allograft model by promoting external callus formation and cartilage development, thereby facilitating the bridging of the graft with the surrounding bone.100 Furthermore, PTH can promote intramedullary secondary remodelling of the graft, enabling new bone formation to replace the graft.62 Consistent with these studies are prior findings that daily systemic administration of PTH (1–34) enhanced fracture-healing both by promoting early callus formation through endochondral bone forma- tion as well as by producing a sustained anabolic effect throughout the remodelling period of fracture healing.70 Therefore, with different biological activities, increased cartilage formation and increased coupled remodelling, which separately increase both the cross-sectional area and the amount of mineralized tissue, PTH improves the two separate phases of fracture healing.
In a different study, of tibial metaphysial fracture healing in rabbits, treatment with both PTH and recombinant human BMP-7 increased bone volume at the site of injury, but neither treatment alone improved mechanical function.101 Combination treatment improved mechanical function, the quantity of bone tissue in the defect and integration between new and old bone tissues within the injury site and the surrounding tissues. Interestingly, histological examination showed that recombinant human BMP-7 seemed to be strictly anabolic and only facilitated cortical bone repair, whereas PTH functioned in the context of coupled remodelling within the underlying marrow space.101 Consistent with these results are data showing that BMPs selectively target periosteal stem-cell differentiation.42 Also of interest is that inhibition of BMP signalling, taking place constitutively with the homeostatic maintenance of bone, leads to increased bone mass; this effect is mediated by the loss of BMP regulation of expres- sion of SOST and Dickkopf-related proteins by osteogenic cells within the medullary space.66 Such divergent biologi- cal effects of both PTH and BMPs suggest, therefore, that careful consideration must be given to both the timing and duration of their clinical use and to the therapeutic applications for which they are most efficacious.
Conclusions
Optimizing conditions for the harvest, selection, expansion and formulation of osteogenic stem cell preparations is needed to advance the field of skeletal healing and to set the stage for developing new local and systemic therapies. We also need to develop better delivery systems for stem cells, growth factors and osteoinductive substances, and to explore systemic applications of osteogenic agents. Identification of appropriate experimental settings and measurable, meaningful clinical endpoints for human clinical trial design are also required. These objectives, if based on a strong foundation of basic science knowledge of skeletal healing, will lead to new methods to improve the care of patients with skeletal injuries.
Key points.
-
■
Fractures are the most common large-organ, traumatic injuries in humans and approximately 10% do not heal properly
-
■
Fracture healing involves an anabolic phase of increasing tissue volume, which forms new skeletal tissues, followed by a prolonged catabolic phase in which tissue is remodelled to the original structure
-
■
Fracture healing is regulated by the nature and extent of trauma, the stability of fracture fixation and biological processes, including immunological and developmental processes associated with skeletal ontology
-
■
Multiple strategies, involving biophysical, local and systemic cell-based systemic therapies that manipulate the morphogenetic processes that control skeletal development are used to promote healing
-
■
The two most widely examined therapies for biologically enhancing fracture healing are bone morphogenetic proteins, which act locally, and parathyroid hormone, which acts systemically
-
■
To advance the field of skeletal healing and to set the stage for developing new local and systemic therapies, conditions for skeletogenic stem cell recruitment and differentiation need to be optimized
Box 1 | Tested methods of enhancing fracture healing.
Biophysical enhancement
Electromagnetic field stimulation72–76
Low-intensity pulsed ultrasound stimulation77–79
Locally applied biological enhancement71
Osteogenic materials
Osteoconductive materials
-
■
Calcium phosphates
-
■
Calcium hydroxyapetites
-
■
Calcium sulphates
-
■
Calcium phosphate/collagen composites
-
■
Allogeneic bone
-
■
Demineralized bone matrix
-
■
Tissue repair factors
Fibroblast growth factors57,58,60,82
Platelet-derived growth factors83
Osteoinductive and morphogenetic factors
Systemic biological enhancement
Parathyroid hormone61,62,67,70
Anti-sclerostin antibodies64,96–98
Anti-Dickkopf-related protein 1 antibodies95
Footnotes
Author contributions
T.A.E. and L.C.G. contributed equally to researching data for the article, substantially contributing to discussion of the content, writing the article and to review/editing of the manuscript before submission.
Competing interests The authors declare no competing interests
References
- 1.Bolander ME. Regulation of fracture repair by growth factors. Proc. Soc. Exp. Biol. Med. 1992;200:165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- 2.Einhorn TA. The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 1998;355(Suppl):S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mech. Dev. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 4.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J. Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 5.Vortkamp A, et al. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech. Dev. 1998;71:65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- 6.Bais M, et al. Transcriptional analysis of fracture healing and the induction of embryonic stem cell-related genes. PLoS ONE. 2009;4:e5393. doi: 10.1371/journal.pone.0005393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36(Suppl. 3):55–57. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA, Einhorn TA, Bolander ME, Cruess RL, Bucholz RW, Heckman JD. Rockwood and Green's Fractures in Adults. Williams and Wilkins; Lippincott: 2001. pp. 245–271. [Google Scholar]
- 9.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29:560–564. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 10.Kurdy NM, Weiss JB, Bate A. Endothelial stimulating angiogenic factor in early fracture healing. Injury. 1996;27:143–145. doi: 10.1016/0020-1383(95)00169-7. [DOI] [PubMed] [Google Scholar]
- 11.Young LF, Choi YW, Behrens FF, DeFouw DO, Einhorn TA. Programmed removal of chondrocytes during endochondral fracture healing. J. Orthop. Res. 1998;6:144–149. doi: 10.1002/jor.1100160124. [DOI] [PubMed] [Google Scholar]
- 12.Gerstenfeld LC, et al. Impaired fracture healing in the absence of TNF-α signaling: the role of TNF-α in endochondral cartilage resorption. J. Bone Miner. Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 13.Melnyk M, Henke T, Claes L, Augat P. Revascularisation during fracture healing with soft tissue injury. Arch. Orthop. Trauma Surg. 2008;128:1159–1165. doi: 10.1007/s00402-007-0543-0. [DOI] [PubMed] [Google Scholar]
- 14.Holstein JH, et al. Endostatin inhibits callus remodeling during fracture healing in mice. J. Orthop. Res. 2013;31:1579–1584. doi: 10.1002/jor.22401. [DOI] [PubMed] [Google Scholar]
- 15.Kon T, et al. Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 16.Ozaki A, Tsunoda M, Kinoshita S, Saura R. Role of fracture hematoma and periosteum during fracture healing in rats: interaction of fracture hematoma and the periosteum in the initial step of the healing process. J. Orthop. Sci. 2005;5:64–70. doi: 10.1007/s007760050010. [DOI] [PubMed] [Google Scholar]
- 17.Timlin MD, et al. Fracture hematoma is a potent proinflammatory mediator of neutrophil function. J. Trauma. 2005;58:1223–1229. doi: 10.1097/01.ta.0000169866.88781.f1. [DOI] [PubMed] [Google Scholar]
- 18.Meert KL, Ofenstein JP, Sarnaik AP. Altered T cell cytokine production following mechanical trauma. Ann. Clin. Lab. Sci. 1998;28:283–288. [PubMed] [Google Scholar]
- 19.Al-Sebaei MO, et al. Role of Fas and TREG cells in fracture healing as characterized in the Fas-deficient (lpr) mouse model of lupus. J. Bone Miner. Res. 2014;29:1478–1491. doi: 10.1002/jbmr.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 21.Noël D, Djouad F, Bouffi C, Mrugala D, Jorgensen C. Multipotent mesenchymal stromal cells and immune tolerance. Leuk. Lymphoma. 2007;48:1283–1289. doi: 10.1080/10428190701361869. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo R, et al. A functional role for soluble HLA-G. antigens in immune modulation mediated by mesenchymal stromal cells. Cytotherapy. 2008;10:364–375. doi: 10.1080/14653240802105299. [DOI] [PubMed] [Google Scholar]
- 23.Morandi F, et al. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells. 2008;26:1275–1287. doi: 10.1634/stemcells.2007-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montespan F, Deschaseaux F, Sensébé L, Carosella ED, Rouas-Freiss N. Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G. and display immunomodulatory properties in HLA- mismatched settings: implications in bone repair therapy. J. Immunol. Res. 2014;2014:23034. doi: 10.1155/2014/230346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najar M, et al. Immune-related antigens, surface molecules and regulatory factors in human- derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev. 2012;8:1188–1198. doi: 10.1007/s12015-012-9408-1. [DOI] [PubMed] [Google Scholar]
- 26.Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–936. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallace A, Cooney TE, Englund R, Lubahn JD. Effects of interleukin-6 ablation on fracture healing in mice. J. Orthop. Res. 2011;29:1437–1442. doi: 10.1002/jor.21367. [DOI] [PubMed] [Google Scholar]
- 29.Nam D, et al. T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PLoS ONE. 2012;7:e40044. doi: 10.1371/journal.pone.0040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glass GE, et al. TNF-α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl Acad. Sci. USA. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motsitsi NS. Management of infected nonunion of long bones: the last decade (1996–2006) Injury. 2007;39:55–60. doi: 10.1016/j.injury.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 32.Alblowi J, et al. High levels of tumor necrosis factor-α contribute to accelerated loss of cartilage in diabetic fracture healing. Am. J. Pathol. 2009;175:1574–1585. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kayal J, et al. TNF-α mediates diabetes enhanced chondrocyte apoptosis during fracture healing and stimulates chondrocyte apoptosis through FOXO-1. J. Bone Miner. Res. 2010;25:1604–1615. doi: 10.1002/jbmr.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 35.Santavirta S, et al. Immunologic studies of nonunited fractures. Acta Orthop. Scand. 1992;63:579–586. [PubMed] [Google Scholar]
- 36.Askalonov AA. Changes in some indices of cellular immunity in patients with uncomplicated and complicated healing of bone fractures. J. Hyg. Epidemiol. Microbiol. Immunol. 1991;25:307–310. [PubMed] [Google Scholar]
- 37.Askalonov AA, Gordienko SM, Avdyunicheva OE, Bondarenko AV, Voronkov SF. The role of T system immunity in reparatory regeneration of the bone tissue in animals. J. Hyg. Epidemiol. Microbiol. Immunol. 1987;31:219–224. [PubMed] [Google Scholar]
- 38.Hauser CJ, et al. The immune microenvironment of human fracture/soft-tissue hematomas and its relationship to systemic immunity. J. Trauma. 1997;42:895–903. doi: 10.1097/00005373-199705000-00021. [DOI] [PubMed] [Google Scholar]
- 39.Toben D, et al. Fracture healing is accelerated in the absence of the adaptive immune system. J. Bone Miner. Res. 2011;26:113–124. doi: 10.1002/jbmr.185. [DOI] [PubMed] [Google Scholar]
- 40.Born WK, Reardon CL, O'Brien RL. The function of γδ T cells in innate immunity. Curr. Opin. Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Colburn NT, Zaal KJ, Wang F, Tuan R. A role for gamma delta T-cells in a mouse model of fracture healing. Arthritis Rheum. 2009;60:1694–1703. doi: 10.1002/art.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melief SM, et al. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- 43.Yan Z, Zhuansun Y, Chen R, Li J, Ran P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp. Cell Res. 2014;15:65–74. doi: 10.1016/j.yexcr.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Utvåg SE, Grundnes O, Rindal DB, Reikerås O. Influence of extensive muscle injury on fracture healing in rat tibia. J. Orthop. Trauma. 2003;17:430–435. doi: 10.1097/00005131-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Claes L, et al. The effect of both a thoracic trauma and a soft-tissue trauma on fracture healing in a rat model. Acta Orthop. 2011;82:223–227. doi: 10.3109/17453674.2011.570677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardner TN, Stoll T, Marks L, Mishra S, Knothe Tate M. The influence of mechanical stimulus on the pattern of tissue differentiation in a long bone fracture—an FEM study. J. Biomech. 2000;33:415–425. doi: 10.1016/s0021-9290(99)00189-x. [DOI] [PubMed] [Google Scholar]
- 47.Claes LE, Heigele CA. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J. Biomech. 1999;32:255–266. doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 48.Morgan EF, et al. Correlations between local strains and tissue phenotypes in an experimental model of skeletal healing. J. Biomech. 2010;43:2418–2424. doi: 10.1016/j.jbiomech.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu YY, Lieu S, Lu C, Colnot C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone. 2010;47:65–73. doi: 10.1016/j.bone.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu R, et al. Myogenic progenitors contribute to open but not closed fracture repair. BMC Musculoskelet. Disord. 2011;12:288. doi: 10.1186/1471-2474-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olerud S, Strömberg L. Intramedullary reaming and nailing: its early effects on cortical bone vascularization. Orthopedics. 1986;9:1204–1208. doi: 10.3928/0147-7447-19860901-06. [DOI] [PubMed] [Google Scholar]
- 53.McCarthy ID, Hughes SP. The vascular response to fracture micromovement. Clin. Orthop. Relat. Res. 1994;301:281–290. [PubMed] [Google Scholar]
- 54.Claes L, Eckert-Hübner K, Augat P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J. Orthop. Res. 2002;20:1099–1105. doi: 10.1016/S0736-0266(02)00044-X. [DOI] [PubMed] [Google Scholar]
- 55.Axelrad TW, Einhorn TA. Bone morphogenetic proteins in orthopaedic surgery. Cytokine Growth Factor Rev. 2009;20:481–488. doi: 10.1016/j.cytogfr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J. Clin. Invest. 2008;118:421–428. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du X, Xie Y, Xian CJ, Chen L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J. Cell Physiol. 2012;227:3731–3734. doi: 10.1002/jcp.24083. [DOI] [PubMed] [Google Scholar]
- 58.Fei Y, Gronowicz G, Hurley MM. Fibroblast growth factor-2, bone homeostasis and fracture repair. Curr. Pharm. Des. 2013;19:3354–3363. doi: 10.2174/1381612811319190002. [DOI] [PubMed] [Google Scholar]
- 59.Edwards PC, et al. Sonic hedgehog gene-enhanced tissue engineering for bone regeneration. Gene Ther. 2005;12:75–86. doi: 10.1038/sj.gt.3302386. [DOI] [PubMed] [Google Scholar]
- 60.Song K, Rao NJ, Chen ML, Huang ZJ, Cao YG. Enhanced bone regeneration with sequential delivery of basic fibroblast growth factor and sonic hedgehog. Injury. 2011;42:796–802. doi: 10.1016/j.injury.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Barnes GL, et al. Stimulation of fracture-healing with systemic intermittent parathyroid hormone treatment. J. Bone Joint Surg. Am. 2008;90(Suppl. 1):120–127. doi: 10.2106/JBJS.G.01443. [DOI] [PubMed] [Google Scholar]
- 62.Jørgensen NR, Schwarz P. Effects of anti- osteoporosis medications on fracture healing. Curr. Osteoporos. Rep. 2011;9:149–145. doi: 10.1007/s11914-011-0065-0. [DOI] [PubMed] [Google Scholar]
- 63.Patil AS, Sable RB, Kothari RM. An update on transforming growth factor-β (TGF-β): sources, types, functions and clinical applicability for cartilage/bone healing. J. Cell. Physiol. 2011;226:3094–3103. doi: 10.1002/jcp.22698. [DOI] [PubMed] [Google Scholar]
- 64.Virk MS, et al. Systemic administration ofsclerostin antibody enhances bone repair in a critical-sized femoral defect in a rat model. J. Bone Joint Surg. Am. 2013;95:694–701. doi: 10.2106/JBJS.L.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr. Rev. 2012;33:747–783. doi: 10.1210/er.2011-1060. [DOI] [PubMed] [Google Scholar]
- 66.Kamiya N. The role of BMPs in bone anabolism and their potential targets SOST and DKK1. Curr. Mol. Pharmacol. 2012;5:153–163. doi: 10.2174/1874467211205020153. [DOI] [PubMed] [Google Scholar]
- 67.Kakar S, et al. Enhanced chondrogenesis and Wnt signaling in PTH treated fractures. J. Bone Miner. Res. 2007;22:1903–1912. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 68.Baron R, Kneissel M. Wnt signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 69.Fei Y, Hurley MM. Role of fibroblast growth factor 2 and Wnt signaling in anabolic effects of parathyroid hormone on bone formation. J. Cell. Physiol. 2012;227:3539–3545. doi: 10.1002/jcp.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alkhiary YM, et al. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34) J. Bone Joint Surg. Am. 2005;87:731–741. doi: 10.2106/JBJS.D.02115. [DOI] [PubMed] [Google Scholar]
- 71.Yasuda I. Fundamental aspects of fracture treatment. J. Kyoto Med. Soc. 1953;4:395–406. [PubMed] [Google Scholar]
- 72.Brighton CT, et al. A multicenter study of the treatment of non-union with constant direct current. J. Bone Joint Surg. Am. 1981;63:2–13. [PubMed] [Google Scholar]
- 73.Brighton CT. Current concepts review. The treatment of non-unions with electricity. J. Bone Joint Surg. Am. 1981;63:847–851. [PubMed] [Google Scholar]
- 74.Scott G, King JB. A prospective, double-blind trial of electrical capacitive coupling in the treatment of non-union of long bones. J. Bone Joint Surg. Am. 1994;76:820–826. doi: 10.2106/00004623-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 75.Sharrard WJ. A double-blind trial of pulsed electromagnetic fields for delayed union of tibial fractures. J. Bone Joint Surg. Am. 1990;72:347–355. doi: 10.1302/0301-620X.72B3.2187877. [DOI] [PubMed] [Google Scholar]; Mollon B, da Silva V, Busse JW, Einhorn TA, Bhandari M. Electrical stimulation for long-bone fracture-healing: a meta-analysis of randomized controlled trials. J. Bone Joint Surg. Am. 2008;90:2322–2330. doi: 10.2106/JBJS.H.00111. [DOI] [PubMed] [Google Scholar]
- 76.Heckman J, Ryaby J, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture- healing by non-invasive, low-intensity pulsed ultrasound. J. Bone Joint Surg. Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Kristiansen TK. The effect of low power specifically programmed ultrasound on the healing time of fresh fractures using a Colles' model. J. Orthop. Trauma. 1990;4:227–228. [Google Scholar]
- 78.Busse JW, et al. Low intensity pulsed ultrasonography for fractures: systematic review of randomized controlled trials. BMJ. 2009;338:351–360. doi: 10.1136/bmj.b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wright JG, Einhorn TA, Heckman JD. Grades of recommendation. J. Bone Joint Surg. Am. 2005;87:1909–1910. doi: 10.2106/JBJS.8709.edit. [DOI] [PubMed] [Google Scholar]
- 80.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone- marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Joint Surg. Am. 2005;87:1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 81.Kawaguchi H, et al. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: a randomized, placebo- controlled trial. J. Bone Miner. Res. 2010;12:2735–2743. doi: 10.1002/jbmr.146. [DOI] [PubMed] [Google Scholar]
- 82.DiGiovanni CW, et al. Recombinant human platelet-derived growth factor-BB and β-tricalcium phosphate (rhPDGF-BB/β-TCP): an alternative to autogenous bone graft. North American Ortrhopedic Foot and Ankle Study Group. J. Bone Joint Surg. Am. 2013;95:1184–1192. doi: 10.2106/JBJS.K.01422. [DOI] [PubMed] [Google Scholar]
- 83.Friedlaender GE, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatement of tibial nonunions. J. Bone Joint Surg. Am. 2001;83(Suppl. 1):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 84.Blue Cross & Blue Shield of Mississippi Bone morphogenetic protein. 2014 [online], http://www.bcbsms.com/com/bcbsms/apps/policysearch/views/viewpolicy.php?&noprint= yes&path=%2Fpolicy %2Femed%2Fbone_morphogenetic_protein.html.
- 85.Govender S, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) study group. J. Bone Joint Surg. Am. 2002;84:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 86.Lyon T, et al. Efficacy and safety of recombinant human bone morphogenetic protein-2/calcium phosphate matrix for closed tibial diaphyseal fracture: a double-blind, randomized, controlled phase I–II/III trial. J. Bone Joint Surg. Am. 2013;95:2088–2096. doi: 10.2106/JBJS.L.01545. [DOI] [PubMed] [Google Scholar]
- 87.Aro HT, et al. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J. Bone Joint Surg. Am. 2011;93:801–808. doi: 10.2106/JBJS.I.01763. [DOI] [PubMed] [Google Scholar]
- 88.Simmonds MC, et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann. Intern. Med. 2013;158:877–879. doi: 10.7326/0003-4819-158-12-201306180-00005. [DOI] [PubMed] [Google Scholar]
- 89.Aspenberg P, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 2010;24:404–414. doi: 10.1359/jbmr.090731. [DOI] [PubMed] [Google Scholar]
- 90.Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1–84 accelerates fracture- healing in pubic bones of elderly osteoporotic women. J. Bone Joint Surg. Am. 2011;93:1583–1587. doi: 10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 91.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- 92.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 93.Staehling-Hampton K, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am. J. Med. Genet. 2002;110:144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- 94.Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin. Genet. 2003;63:192–197. doi: 10.1034/j.1399-0004.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 95.Agholme F, Li X, Isaksson H, Ke HZ, Aspenberg P. Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J. Bone Miner. Res. 2012;24:2412–2418. doi: 10.1002/jbmr.135. [DOI] [PubMed] [Google Scholar]
- 96.McDonald MM, et al. Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectromized rats. J. Orthop. Res. 2012;30:1541–1548. doi: 10.1002/jor.22109. [DOI] [PubMed] [Google Scholar]
- 97.Sarahrudi K, Thomas A, Albrecht C, Aharinejad S. Strongly enhanced levels of sclerostin during human fracture healing. J. Orthop. Res. 2012;30:1549–1155. doi: 10.1002/jor.22129. [DOI] [PubMed] [Google Scholar]
- 98.McClung MR. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 99.Takahata M, Schwarz EM, Chen T, O'Keefe RJ, Awad HA. Delayed short-course treatment with teriparatide (PTH(1–34)) improves femoral allograft healing by enhancing intramembranous bone formation at the graft-host junction. J. Bone Miner. Res. 2012;27:26–37. doi: 10.1002/jbmr.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morgan EF, et al. Combined effects of recombinant human BMP-7 (rhBMP-7) and parathyroid hormone (1–34) in metaphyseal bone healing. Bone. 2008;43:1031–1038. doi: 10.1016/j.bone.2008.07.251. [DOI] [PubMed] [Google Scholar]