Abstract
Background
HER2 is a well-established prognostic and predictive factor in invasive breast cancer. The role of HER2 in ductal breast carcinoma in situ (DCIS) is debated and recent data have suggested that HER2 is mainly related to in situ recurrences. Our aim was to study HER2 as a prognostic factor in a large population based cohort of DCIS with long-term follow-up.
Methods
All 458 patients diagnosed with a primary DCIS 1986–2004 in two Swedish counties were included. Silver-enhanced in situ hybridisation (SISH) was used for detection of HER2 gene amplification and protein expression was assessed by immunohistochemistry (IHC) in tissue microarrays. HER2 positivity was defined as amplified HER2 gene and/or HER2 3+ by IHC. HER2 status in relation to new ipsilateral events (IBE) and Invasive Breast Cancer Recurrences, local or distant (IBCR) was assessed by Kaplan-Meier survival analyses and Cox proportional hazards regression models.
Results
Primary DCIS was screening-detected in 75.5 % of cases. Breast conserving surgery (BCS) was performed in 78.6 % of whom 44.0 % received postoperative radiotherapy. No patients received adjuvant endocrine- or chemotherapy. The majority of DCIS could be HER2 classified (N = 420 (91.7 %)); 132 HER2 positive (31 %) and 288 HER2 negative (69 %)). HER2 positivity was related to large tumor size (P = 0.002), high grade (P < 0.001) and ER- and PR negativity (P < 0.001 for both). During follow-up (mean 184 months), 106 IBCRs and 105 IBEs were identified among all 458 cases corresponding to 54 in situ and 51 invasive recurrences. Eighteen women died from breast cancer and another 114 had died from other causes. The risk of IBCR was statistically significantly lower subsequent to a HER2 positive DCIS compared to a HER2 negative DCIS, (Log-Rank P = 0.03, (HR) 0.60 (95 % CI 0.38–0.94)). Remarkably, the curves did not separate until after 10 years. In ER-stratified analyses, HER2 positive DCIS was associated with lower risk of IBCR among women with ER negative DCIS (Log-Rank P = 0.003), but not for women with ER positive DCIS.
Conclusions
Improved prognostic tools for DCIS patients are warranted to tailor adjuvant therapy. Here, we demonstrate that HER2 positive disease in the primary DCIS is associated with lower risk of recurrent invasive breast cancer.
Keywords: Ductal carcinoma in situ (DCIS), HER2, Prognostic marker, Breast cancer
Background
In general, patients with ductal breast carcinoma in situ (DCIS) have an excellent prognosis in terms of survival [1, 2]. However, the rate of new ipsilateral events (IBE) is high, even higher than following invasive breast cancer [2]. Almost half of all IBEs subsequent to a DCIS are invasive cancer and survival after an invasive IBE has, not surprisingly, been reported to be worse than after a non-invasive recurrence [3]. The knowledge about clinical and pathological predictors of recurrence following DCIS is currently limited [4, 5]. Certain sub-types of DCIS might be more prone to recur as invasive cancer and new biological markers may provide additional prognostic and/or treatment predictive value [6–9]. Recently, genomic-based data from a study using the Oncotype DX® indicated that it was possible to predict the local relapse risk for a primary DCIS independently from classical factors [10].
HER2 is an established negative prognostic factor in invasive breast cancer [11]. The prognostic significance of HER2 status in DCIS is, however, less clear [7, 12]. Both the relation of HER2 to risk of recurrence and its role in the progression from in situ to invasive cancer have been debated [12]. HER2 over-expression is reported to be more frequent in DCIS than in invasive cancer [13]. This may seem counterintuitive as HER2 is proposed to play a role in tumour progression. Some studies report an even higher proportion of HER2 positivity in micro-invasive cancer [14, 15] and, in pre-operative tumour biopsies displaying DCIS, HER2 over-expression has been related to a co-existing invasive component in the surgical specimen [16, 17]. Furthermore, HER2 is associated with high histopathological grade both in invasive cancer and in DCIS [18].
HER2 amplification is an important factor in the classification of molecular subtypes of invasive breast cancer [19]. An approximation of the gene expression based subtypes using immunohistochemical (IHC) detection of HER2, estrogen receptor- (ER) and progesterone receptor (PR) expression has been suggested for invasive breast cancer [20]. DCIS has been divided into the same surrogate molecular subtypes as invasive breast cancer [6, 21–23] but the prognostic value of these subgroups has not been convincing in DCIS [8, 24].
We have previously studied the surrogate molecular subgroups in this population-based DCIS cohort [8, 24]. Those studies indicated a lower risk of invasive recurrence in HER2-positive DCIS, although not statistical significant. We therefore conducted this updated analysis on the same patient population with further extended follow-up time and pooled HER2 data focusing on the role of HER2 status in DCIS and its relation to prognosis.
Methods
Patients
All patients diagnosed with a primary DCIS between 1986 and 2004 in two Swedish counties (Uppland and Västmanland) were included in this DCIS cohort (n = 458). The majority underwent breast-conserving surgery (79 %), and clear surgical margins were obtained in 88 % among all cases irrespective of surgical intervention. All cases were histopathologically re-examined. Tumour tissue biopsies of 1.0 mm in duplicate from paraffin blocks of samples taken as part of standard care were used to construct tissue microarrays (TMA). Follow up was complete up to December 15th, 2013. This study was approved by the Ethics Committee at Uppsala University, Sweden (Dnr 2005:118) and no informed consent was needed.
Silver-enhanced In Situ Hybridization (SISH) and IHC
Scoring and IHC protocols are previously described in detail [21, 25]. SISH was performed on the automated instrument, Ventana Benchmark (Ventana Medical Systems, Tucson, AZ), as per the manufacturer’s protocols for the INFORM HER2 DNA probe and chromosome 17 probes. Testing for the HER2 gene and chromosome 17 was performed on sequential sections. Both probes are labelled with dinitrophenol and denaturation occurred on the instrument with enzyme digestion in protease 3 for eight minutes. The detection system used a multimer labelled with goat anti-rabbit antibody horseradish peroxidase as the linking step. Visualization occurred with the sequential addition of silver acetate as the source of ionic silver, hydroquinone, and hydrogen peroxide to give black metallic silver precipitate at the probe site. Counterstaining was performed with hematoxylin II on the instrument. The time taken for the complete run was 6.5 h. Both HER2 and chromosome 17-detection were performed on the same slide run. Gene amplification was assessed using the American Society of Clinical Oncology/College of American Pathologists guideline and Australian HER2 Advisory Board criteria for single HER2 probe testing (diploid, 1 to 2.5 copies/nucleus; polysemy >2.5 to 4 copies/nucleus; equivocal, >4 to 6 copies/nucleus; low-level amplification, >6 to 10 copies/ nucleus; and high-level amplification >10 copies/nucleus) and for dual HER2/CHR17 probe testing (non-amplified ratio <1.8; equivocal ratio, 1.8 to 2.2; gene amplification, >2.2) [26]. The HER2 status was predominantly relying on the SISH data. For those cases in which SISH failed, HER2 status was based on the IHC data and cases were considered HER2 positive if the IHC score was 3+, using the HercepTest©. As for hormone receptors (estrogen receptor alpha (ER) and progesterone receptor (PR)), tumours with 10 % or more nuclei stained were considered ER and PR positive, respectively. Staining intensity was not taken into consideration.
Statistic analyses
Baseline characteristics among women with different HER2 status were compared by Chi-square test for categorical variables or analysis of variance for continuous variables. Survival analyses were performed using Kaplan-Meier curves, including the Log-Rank test. Cox proportional hazards regression models were used to generate hazard ratios (HRs) with 95 % confidence intervals (CIs), with adjustment for radiotherapy, age at diagnosis (continuous), tumour size (two categories: ≤ 25 mm; >25 mm or multifocal), and ER status. Stratification analyses were performed by mode of detection, type of surgery and ER in different multivariate models. All statistical tests were two-sided, and p-values less than 0.05 were considered significant. Data were analysed using the SPSS Statistics, version 19 (IBM, Chicago, IL, USA) and SAS 9.3 (Cary, NC, USA).
Primary endpoints were Ipsilateral Breast cancer Events (IBE) and Invasive Breast Cancer Recurrences (IBCR). IBEs were divided in new in situ events and invasive IBEs. As for IBCR, an event was defined as an invasive IBE, a regional recurrence, a contralateral invasive breast cancer or distant metastasis. IBCR free survival was calculated based on the time from primary surgery to the date of any invasive breast cancer event, date of death or date of last follow-up as by December 15th 2013. For an event to be classified as IBE or IBCR, a minimum of 3 months had to pass after the primary surgery.
Results
Patient and tumour characteristics
Baseline characteristics by HER2 status are presented in Table 1. The mean age at diagnosis was 58.6 years, ranging from 30 to 90 years of age. A total of 202 women were diagnosed in Västerås, whereas the remaining 256 patients were diagnosed in Uppsala. In most cases, the DCIS was detected by mammographic screening (75.5 %). All patients except one underwent surgery with the majority of patients operated with breast conserving surgery (BCS) (78.6 %). Surgery was followed by postoperative radiotherapy (RT) in 35.2 % of all cases, although primarily for patients operated with BCS of whom 44.0 % received RT (Table 1). None of the patients received hormonal therapy or chemotherapy according to Swedish clinical guidelines.
Table 1.
Baseline characteristics by HER2 status in 458 women with a primary DCIS
| Characteristics | All | HER2 positive | HER2 negative | HER2 missing | |
|---|---|---|---|---|---|
| (n = 458) | (n = 132) | (n = 288) | (n = 38) | ||
| n (%) | n (%) | n (%) | P-valuea | n (%) | |
| Age (years) mean (n = 458) | 58.6 | 57.4 | 59.2 | 0.13 | 58.8 |
| Detection mode (n = 457) | |||||
| Screening | 345 (75.5 %) | 108 (81.8 %) | 210 (73.2 %) | 27 (71.1 %) | |
| Clinically | 112 (24.5 %) | 24 (18.2 %) | 77 (26.8 %) | 0.05 | 11 (28.9 %) |
| Type of Surgery (n = 457) | |||||
| BCS | 359 (78.6 %) | 98 (74.2 %) | 233 (81.2 %) | 28 (73.7 %) | |
| Mastectomy | 98 (21.4 %) | 34 (25.8 %) | 54 (18.8 %) | 0.11 | 10 (26.3 %) |
| Radiotherapy (n = 359) | |||||
| Yes | 158 (44.0 %) | 49 (50.0 %) | 100 (42.9 %) | 9 (32.1 %) | |
| No | 201 (56.0 %) | 49 (50.0 %) | 133 (57.1 %) | 0.32 | 19 (67.9 %) |
| Tumor size (mm) (n = 409) | |||||
| ≤ 25 | 294 (71.9 %) | 68 (61.3 %) | 203 (77.2 %) | 23 (65.7 %) | |
| > 25 or multifocal | 115 (28.1 %) | 43 (38.7 %) | 60 (22.8 %) | 0.002 | 12 (34.3 %) |
| Nuclear grade (n = 458) | |||||
| Grade 1 | 42 (9.2 %) | 1 (0.8 %) | 34 (11.8 %) | 7 (18.4 %) | |
| Grade 2 | 176 (38.4 %) | 20 (15.1 %) | 145 (50.3 %) | 11 (28.9 %) | |
| Grade 3 | 240 (52.4 %) | 111 (84.1 %) | 109 (37.8 %) | <0.001 | 20 (52.6 %) |
| ER status (n = 419) | |||||
| Positive | 307 (73.3 %) | 59 (45.7 %) | 243 (86.5 %) | 5 (55.6 %) | |
| Negative | 112 (26.7 %) | 70 (54.3 %) | 38 (13.5 %) | <0.001 | 4 (44.4 %) |
| PR status (n = 409) | |||||
| Positive | 213 (52.1 %) | 35 (28.2 %) | 175 (62.9 %) | 4 (57.1 %) | |
| Negative | 196 (47.93 %) | 89 (71.8 %) | 103 (37.1 %) | <0.001 | 3 (42.9 %) |
BCS breast conserving surgery, RT postoperative radiotherapy
aComparisons between HER2-negative and HER2-positive DCIS were made by χ2-test, except for age and size that was compared by T-test
Follow-up data
The mean follow up time was 183.5 months (range 3–329 months). A total of 105 IBEs were identified of which 54 were a new in situ and 51 an invasive IBE in the entire cohort of 458 patients. Among women treated with BCS (N = 324), recurrences were detected among 95 women comprising 49 in situ and 46 invasive cases. Eleven of the in situ IBEs were followed by a subsequent invasive IBE. One hundred and six IBCR were identified. The first invasive event was an invasive IBE in 50 cases, regional axillary metastasis in four, loco-regional together with distant metastases or distant metastasis only in twelve cases and a contralateral invasive cancer in 40 cases. In total, 32 patients developed generalized disease; fifteen subsequently to an invasive IBE, eight after an invasive contralateral cancer and nine had no reported prior local or regional recurrence. Eighteen women died from breast cancer and another 114 had died from other causes.
HER2 status and patient – and tumor characteristics
Of all 458 women, 420 could be HER2 classified using available SISH or IHC data; 132 were classified as HER2 positive (31.4 %) and 288 as HER2 negative. Of 344 cases with available SISH data, 118 (34.3 %) were HER2 positive and of 408 cases with IHC data 103 (25.2 %) were classified as HER2 positive (i.e. 3+). In comparison, SISH and IHC data showed concordance in 296 of 332 (89.2 %) cases with available data for both SISH and IHC. Twenty-nine of those 332 cases were classified as HER2 2+ by IHC, 21 of those 29 (72.4 %) were SISH positive and eight (27.6 %) were SISH negative. In 38 cases, data on HER2 status was missing all together. HER2 status was significantly related to tumor size, nuclear grade (NG) and hormone receptor status, and the HER2 positive tumours tended to be detected by screening more often than HER2 negative tumors. HER2 status was not associated with age at diagnosis, type of surgery or RT (Table 1). In analyses assessing risk of recurrences according to established patient-and tumor characteristics, clinically detected DCIS were more prone to recur locally (HR 1.78 (1.04–3.07), larger DCIS lesions were correlated to a borderline significant increased risk of in situ IBEs but not invasive IBEs (HR 1.88 (0.97–3.62) and 0.64 (0.30–1.34)), respectively. Other assessed factors were not associated with recurrences (Table 2).
Table 2.
Patient- and tumor characteristics of the primary DCIS in relation to risk of a breast cancer event. Risk of an ipsilateral breast cancer event (IBE) including risk of in situ IBEs and invasive ipsilateral recurrence, respectively, and any invasive recurrence (IBCR) (univariate Cox regression analyses)
| Characteristics | Ipsilateral Breast Events (IBE) | in situ IBEs | invasive IBEs | Invasive Breast Cancer Recurrence (IBCR) |
|---|---|---|---|---|
| Among BCS (n = 359) | BCS | BCS | Among all (n = 458) | |
| HR (95 % CI) | HR (95 % CI) | HR (95 % CI) | HR (95 % CI) | |
| Age (years) | ||||
| < 50 | Reference | Reference | Reference | Reference |
| 50–65 | 0.77 (0.44–1.31) | 0.92 (0.41–2.05) | 0.63 (0.30–1.32) | 0.68 (0.41–1.12) |
| > 65 | 1.19 (0.67–2.12) | 1.31 (0.55–3.11) | 1.09 (0.50–2.37) | 0.98 (0.57–1.66) |
| Detection mode | ||||
| Screening | Reference | Reference | Reference | Reference |
| Clinically | 1.78 (1.04–3.07) | 1.78 (0.81–3.91) | 2.09 (0.98–4.42) | 1.47 (0.93–2.35) |
| Type of Surgery | ||||
| BCS | - | - | - | Reference |
| Mastectomy | - | - | - | 0.55 (0.29–1.03) |
| RT after BCS | ||||
| No | Reference | Reference | Reference | Reference |
| Yes | 0.53 (0.33–0.86) | 0.50 (0.25–1.01) | 0.51 (0.27–0.98) | 0.91 (0.59–1.41) |
| Tumor size (mm) | ||||
| ≤ 25 | Reference | Reference | Reference | Reference |
| > 25 / multifocal | 1.14 (0.71–1.83) | 1.88 (0.97–3.62) | 0.64 (0.30–1.34) | 0.89 (0.55–1.45) |
| Nuclear grade | ||||
| Grade 1 | Reference | Reference | Reference | Reference |
| Grade 2 | 0.90 (0.41–1.96) | 1.05 (0.30–3.63) | 0.73 (0.27–1.99) | 0.82 (0.38–1.77) |
| Grade 3 | 0.83 (0.39–1.79) | 1.03 (0.30–3.49) | 0.63 (0.23–1.69) | 0.64 (0.30–1.37) |
| ER status | ||||
| Negative | Reference | Reference | Reference | Reference |
| Positive | 0.90 (0.52–1.55) | 0.80 (0.38–1.71) | 1.02 (0.47–2.22) | 1.16 (0.70–1.93) |
| PR status | ||||
| Negative | Reference | Reference | Reference | Reference |
| Positive | 0.83 (0.52–1.32) | 1.04 (0.53–2.02) | 0.66 (0.35–1.27) | 1.06 (0.69–1.63) |
RT radiotherapy, BCS breast conserving surgery, IBE ipsilateral breast events, IBCR invasive breast cancer recurrence
HER2 and survival analyses
Among the 420 women with available HER2 status, a total of 102 women experienced recurrences during follow-up. HER2 positivity in the primary DCIS was not a risk factor for IBE in women undergoing BCS (Log-Rank P = 0.40, HR 1.20 (95 % CI, 0.78–1.85)), (Fig. 1a and Table 3). Interestingly, divided by type of IBE, HER2 positivity showed a borderline statistically significant increased risk of in situ IBEs (Log-Rank P = 0.09, HR 1.63 (95 % CI, 0.92–2.89), and no association with risk of invasive IBEs (Log-Rank p = 0.48, HR 0.78 (95 % CI, 0.40–1.55)), (Fig. 1b–c and Table 3). Results from the multivariate analyses did not differ substantially from the univariate analyses (Table 3).
Fig. 1.
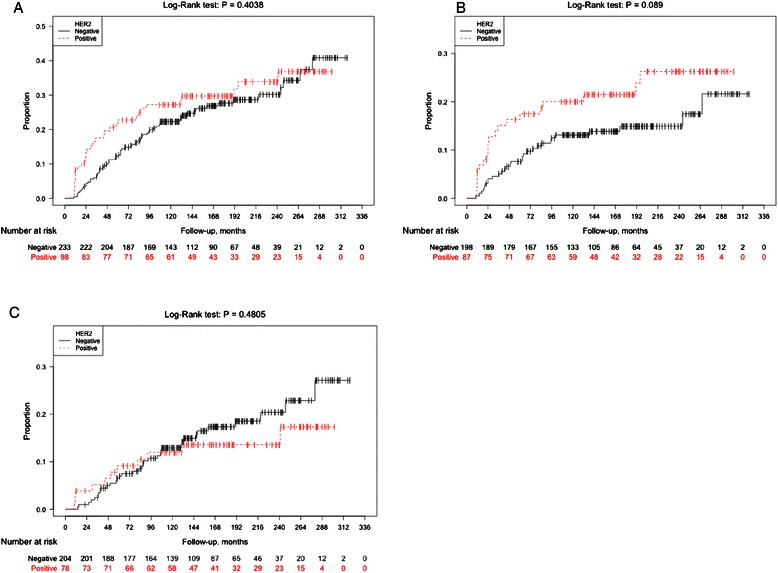
Ipsilateral Breast cancer Events (IBE) according to HER2 status of the primary DCIS. Kaplan-Meier plots showing ipsilateral recurrence-free survival analyses (IBE) among women with DCIS treated with breast conserving surgery (BCS) with respect to HER2 status of the primary DCIS regarding all ipsilateral events (a), ipsilateral in situ events (b), and ipsilateral invasive events (c)
Table 3.
Cox regression analyses by HER2 status in 458 women with a primary DCIS
| DCIS | ||
|---|---|---|
| HER2 positive | HER2 negative | |
| HR (95 % CI) | HR (95 % CI) | |
| Ipsilateral Breast Events (IBE) | ||
| BCS (n = 331) (events = 95) | ||
| Univariate HR (95 % CI) | 1.20 (0.78–1.85) | (Ref) |
| aAdjusted HR (95 % CI) | 1.27 (0.83–1.96) | (Ref) |
| bAdjusted HR (95 % CI) | 1.17 (0.75–1.83) | (Ref) |
| cAdjusted HR (95 % CI) | 1.22 (0.76–1.95) | (Ref) |
| BCS, in situ IBEs (events = 49) | ||
| Univariate HR (95 % CI) | 1.63 (0.92–2.89) | (Ref) |
| aAdjusted HR (95 % CI) | 1.76 (0.99–3.11) | (Ref) |
| bAdjusted HR (95 % CI) | 1.40 (0.77–2.54) | (Ref) |
| cAdjusted HR (95 % CI) | 1.65 (0.88–3.11) | (Ref) |
| BCS, invasive IBEs (events = 46) | ||
| Univariate HR (95 % CI) | 0.78 (0.40–1.55) | (Ref) |
| aAdjusted HR (95 % CI) | 0.83 (0.42–1.64) | (Ref) |
| bAdjusted HR (95 % CI) | 0.87 (0.43–1.75) | (Ref) |
| cAdjusted HR (95 % CI) | 0.80 (0.38–1.66) | (Ref) |
| Invasive Breast Cancer Recurrence (IBCR) | ||
| All patients (n = 420) (Events = 102) | ||
| Univariate HR (95 % CI) | 0.60 (0.38–0.94) | (Ref) |
| aAdjusted HR (95 % CI) | 0.58 (0.38–0.95) | (Ref) |
| bAdjusted HR (95 % CI) | 0.59 (0.37–0.95) | (Ref) |
| cAdjusted HR (95 % CI) | 0.59 (0.36–0.98) | (Ref) |
BCS breast conserving surgery
aAdjusted for radiotherapy
bAdjusted for radiotherapy, age at diagnosis (continuous) and size
cAdjusted for radiotherapy, age at diagnosis and ER
The risk of IBCRs was statistically significantly lower subsequent to a HER2 positive DCIS compared to a HER2 negative primary DCIS (Log-Rank P = 0.03, HR 0.60 (95 % CI, 0.38–0.94)) (Fig. 2 and Table 3). Remarkably, the curves in the Kaplan-Meier plot did not separate until after almost 10 years (Fig. 2). In the multivariate analyses, HRs after adjustments were very similar to the crude analyses (Table 3).
Fig. 2.
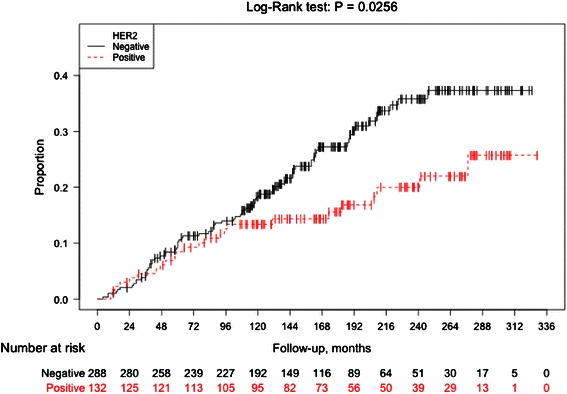
Invasive recurrence-free-survival according to HER2 status of the primary DCIS. Kaplan-Meier plot showing invasive recurrence-free survival analyses (IBCR) among women with a DCIS with respect to HER2 status of the primary DCIS
The survival analyses were stratified for ER-status, and for patients with an ER negative DCIS, HER2 positivity predicted a significantly lower risk of IBCR (Log-Rank, P = 0.003), (Fig. 3a), which was not the case for ER positive DCIS patients (Log-Rank, P = 0.76), (Fig. 3b). In analyses restricted to women who undergoing BCS, ER status remained an effect modifier for the association between HER2 status and IBCR.
Fig. 3.
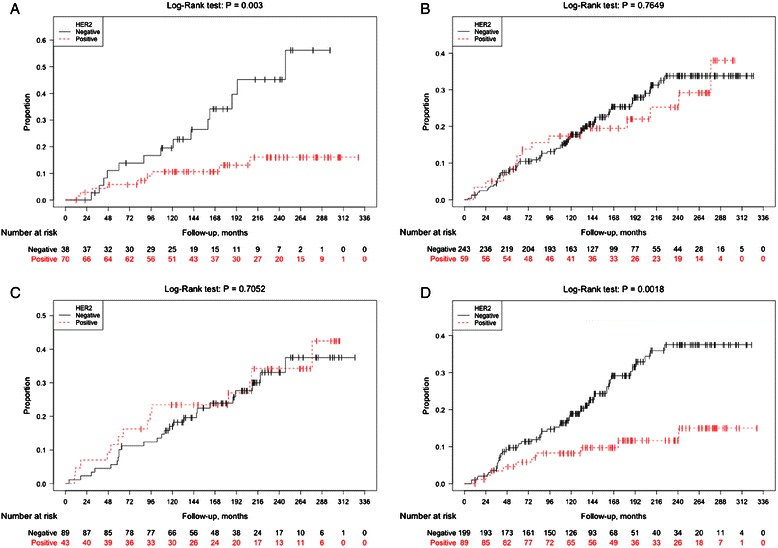
Invasive recurrence-free-survival according to HER2 of the primary DCIS, stratified for ER status and age, respectively. Kaplan-Meier analyses stratified for ER status of the primary DCIS, demonstrating invasive recurrence-free survival (IBCR) with respect to HER2 status of the primary DCIS among ER negative (a) and ER positive DCIS patients (b). Additional Kaplan-Meier curves show analyses stratified for age at diagnosis (≤50 years/> 50 years) displaying invasive recurrence-free survival (IBCR) according to HER2 status of the primary DCIS among young patients (≤50 years at diagnosis), (c) and older patients with age 50 years or above at diagnosis (d)
The prognostic value of age was studied in survival analysis using age as at dichotomous variable of ≤ 50 years (N = 141, 31 %) or > 50 years (N = 317, 69 %). Age was not proven to be a prognostic indicator of neither invasive nor in situ recurrence (data not shown). However, age added substantial information to the prognostic value of HER2 status. For presumably premenopausal women aged 50 or below, HER2 expression in the DCIS revealed no prognostic information (Fig. 3c). In contrast, for women who were older than 50 years of age at diagnosis, HER2 positivity was associated with a markedly improved recurrence-free survival for invasive disease (Log-Rank, P = 0.002), (Fig. 3d). The age-stratified analyses for in situ IBEs did not reveal any significant results.
Discussion
In this long-term follow-up DCIS cohort, positive HER2 status in the primary lesion predicted lower risk of late invasive breast cancer recurrence compared to negative HER2 status in the primary DCIS. HER2 positivity did on the other hand indicate a non-significant higher risk of local in situ IBEs.
DCIS is by definition a non-invasive disease exerting a rather high risk of local recurrence but an especially low risk of lethal outcome from the disease [27]. However, an increasing rate of early-stage disease, including DCIS, is observed due to mammography screening [28]. The ideal screening method aims at fractioning indolent disease from cancers that will ultimately cause harm if undetected at an early stage. Current mammography methods are incapable of characterising the aggressiveness of a given breast tumor, and tissue based assessment of biological entities is warranted for improved clinical decision-making [1, 29, 30]. DCIS includes a plethora of diseases ranging from conditions where active surveillance might be adequate, to conditions where tumors should be surgically removed followed by adjuvant treatment. Prediction of recurrences is thus a high priority; both prognostic and treatment predictive factors should be identified [29].
Previous studies have shown increased risk of DCIS recurrences among patients with HER2 positive disease in the primary DCIS [31, 32]. These findings were vaguely indicated but not statistically significantly confirmed in this study. More importantly, HER2 status was predictive of invasive recurrences in this study showing that HER2 positive tumors were less reluctant to recur as an invasive tumor. The positive prognostic value of HER2 in DCIS demonstrated here might seem counterintuitive given the data showing HER2 to be positively related to tumor size, multifocality and nuclear grade and may reflect an independent role of HER2 in DCIS as a long-term prognostic biomarker. This study includes an extensive long-term follow-up, which enabled the detection of the lower incidence of late-recurrences among patients with HER2 positive DCIS. The Kaplan-Meier curves for invasive recurrences were concordant for several years, and not until 10 years after the primary DCIS, did the curves separate. The discordances between our results and the results generated by Kerlikowske et al. [32] may well be explained by the differences in follow-up time. More importantly, the endpoints applied in this study are not identical to previous studies among which any ipsilateral recurrence regardless of type has served as endpoint. Our results stating that HER2 positive DCIS is a beneficial prognostic marker for invasive recurrent disease highlights the need for studying several different endpoints to understand the prognostic value of a given biological marker. Presumably the key endpoint should be invasive recurrence representing the actual threat to the patient’s life as discussed in the review by Benson et al. stating the main goals of DCIS treatment to be prevention of recurrent invasive disease, minimised treatment-related morbidity, and optimised cosmetics [29].
In a previous publication, we evaluated the prognostic impact of molecular subtypes defined by the immunohistochemical surrogate classification [24]. The subtypes were based on the proposed classification for invasive breast cancer according to the St Gallen consensus meeting in 2011 [20]. HER2 cases were thus presented in two subtypes, i.e. Luminal B(HER2+) and HER2 + (ER-/PR-) leaving few cases in each subtype, which might explain the non-significant data generated in that study. Herein, we report significant results for HER2 as a prognostic marker for recurrent invasive disease, presumable prone to the facts that, in this study, all HER2 positive DCIS were analysed together and moreover, the follow-up period has been extended.
The significantly reduced risk estimates for patients with HER2 positive DCIS might help identifying a low-risk group for whom adjuvant treatment after surgical excision could safely be omitted. To keep overtreatment to an innocuous minimum is essential for DCIS-patients among whom a substantial amount of the lesions may be of limited clinical importance [28].
This study has several strengths, such as its relatively large sample size and confirmation of all events by a physician. Another important strength was the long-term follow-up of more than 15 years (mean). During follow-up invasive recurrences were identified in 23 % (106/458) of the women with a previous DCIS. From a biological point of view, the identified regional or distant metastases have most likely evolved from an invasive foci in the breast, which could have occurred at any time between the DCIS diagnosis and the diagnosis of metastatic disease, although not detected and therefore not possible to report in this study. The number of invasive recurrences does, however, correspond with numbers from previous publications on DCIS patients showing a recurrence rate of 25 % within 10 years of follow-up, 50 % of these being invasive recurrences [33], and 18 % during 5 years of follow-up [34]. Unequal recurrence-estimates may be explained be differences in follow-up length and the fact that no systemic endocrine treatment is recommended Swedish DCIS patients.
Some considerations are worth discussing for the interpretation of our results. All evaluated tumor biomarkers were investigated on TMAs, which may have limited the assessment of heterogeneous expression compared to whole sections. Previous validation studies of TMA-based biomarker assessment, however, show accurate concordance between TMAs and whole sections motivating use of TMAs for routine breast biomarker (ER, PR, HER2) analyses in the clinical setting [35]. A previous publication including a subset of this study population, showed an overall concordance of 80 % between TMAs and whole sections for IHC assessment of ER, PR, and HER2, and notably the prognostic value of these markers was similar irrespective of assessment based on TMA or whole sections [36]. This study is predominantly based on SISH regarding HER2 status with an anticipated high accuracy compared to IHC [37]. Breast conserving surgery represents the surgical intervention predominantly used in our cohort. Compared to mastectomy, the outcome following this surgical approach requires higher accuracy in pre-surgical imaging of the tumor extend. No data on pre-surgical imaging was however available for this study and differences could not be addressed further, The challenge of surgical margins differ depending of surgical method as evidenced in this cohort with clear margins obtained in 94 % of cases undergoing mastectomy compared to 86 % among the women who underwent breast conserving surgery. However, no association between surgical method and HER2 status was found, and the risk of confounding by surgical method is considered small.
HER2 positive disease is a strong predictor for impaired recurrence-free survival in invasive breast cancer, opposing the results on DCIS in this study. This controversy may reflect the diverse expression of HER2 in invasive lesions and adjacent DCIS components as recently discussed in a review by Cowell et al. [30]. The tentative explanations given are that HER2 amplification may either have been lost in the progression from ductal carcinoma in situ to invasive breast cancer, or that the invasive component has arisen from a DCIS clone without HER2 amplification [30]. Biologically, the striking issue is why progression to invasive disease would imply loss of HER2 amplification, which warrants further attention.
Although this study implies, that patients with HER2 positive DCIS are less likely to experience recurrent invasive disease, the HER2 positive DCIS disease has substantial clinical interest as a targetable early-stage disease. This has been acknowledged by recently conducted clinical trials testing the efficacy of HER2 targeting therapy with lapatinib in DCIS patients (ClinicalTrials.gov NCT00555152 and NCT00857714, respectively). The results of these trials are currently not published, however the trial results by Estevés et al. assessing lapatinib in the pre-surgical setting reported significant inhibition of HER2 signalling and reduced tumor size following 4 weeks of treatment in HER2 positive DCIS patients [38]. Further, other biomarker trials have assessed the effects of trastuzumab in DCIS patients, among which, one trial has reported the results, showing no significant tumor effects in terms of proliferation and apoptosis after a single-dose monotherapy trastuzumab pre-surgically [39]. None of these studies have addressed the long-term benefits of HER2 targeted therapy in DCIS. However, a currently recruiting phase III randomized trial prescribing trastuzumab versus placebo concomitantly with radiotherapy, may shed light over the long-term effects (ClinicalTrials NCT00769379).
Conclusions
Clinical decision making for DCIS patients is still challenged by the inability to predict recurrence or, most importantly, progression to invasive disease. In line with the models developed for invasive breast cancer, such as Adjuvant! [40], a comprehensive set of solid biomarkers for DCIS prognosis and treatment prediction has repeatedly been requested. In this large DCIS cohort with extensive follow-up, we demonstrate significantly improved long-term invasive disease-free survival for patients with HER2 positive disease in the primary DCIS. These results indicate that HER2 status could add significant information in future development of prognostic and predictive DCIS models.
Acknowledgement
We acknowledge the funding obtained from the Swedish Research Council; the Swedish Cancer Society; the Skåne University Hospital donation funds; the Governmental Funding of Clinical Research within the National Health Services, and the Mrs Berta Kamprad foundation for Cancer Research.
Abbreviations
- BCS
Breast conserving surgery
- CI
Confidence interval
- DCIS
Ductal carcinoma in situ of the breast
- ER
Estrogen receptor
- HER2
Human epidermal growth factor receptor 2
- HR
Hazard ratio
- IHC
Immunohistochemical
- IBCR
Invasive breast cancer recurrences (invasive ipsilateral/regional/contralateral recurrence or distant metastasis)
- IBE
Ipsilateral breast cancer event
- PR
Progesterone receptor
- RT
Postoperative radiotherapy
- SISH
Silver-enhanced in situ hybridization
- TMA
Tissue microarray
Footnotes
Competing interests
S. Borgquist declares that she has previously received lecturer salary from Roche. The other authors declare that they have no competing interests.
Authors’ contributions
SBo and WZ were responsible for data analyses, and manuscript preparation and editing. KJ was involved in the pathology review and construction of tissue microarrays. RMA was responsible for HER2 scoring of stains and SISH together with WZ. TS and SBu up-dated the follow up in the cohort. CB and TS helped with the interpretation of the results and with drafting the manuscript. FW designed the overall study, coordinated the study, and helped draft and finalize the manuscript. All authors contributed in the preparation of the manuscript and all authors read and approved the final manuscript.
Contributor Information
Signe Borgquist, Email: signe.borgquist@med.lu.se.
Wenjing Zhou, Email: wenjing.zh@gmail.com.
Karin Jirström, Email: karin.jirstrom@med.lu.se.
Rose-Marie Amini, Email: Rose-Marie.Amini@igp.uu.se.
Thomas Sollie, Email: thomas.sollie@orebroll.se.
Therese Sørlie, Email: Therese.Sorlie@rr-research.no.
Carl Blomqvist, Email: Carl.Blomqvist@helsinki.fi.
Salma Butt, Email: salma.butt@med.lu.se.
Fredrik Wärnberg, Email: fredrik.warnberg@surgsci.uu.se.
References
- 1.Esserman LJ, Thompson IM, Jr, Reid B. Overdiagnosis and overtreatment in cancer: an opportunity for improvement. JAMA. 2013;310:797–8. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 2.Correa C, McGale P, Taylor C, Wang Y, Clarke M, Davies C, Peto R, Bijker N, Solin L, Darby S. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–77. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee LA, Silverstein MJ, Chung CT, Macdonald H, Sanghavi P, Epstein M, Holmes DR, Silberman H, Ye W, Lagios MD. Breast cancer-specific mortality after invasive local recurrence in patients with ductal carcinoma-in-situ of the breast. Am J Surg. 2006;192:416–9. doi: 10.1016/j.amjsurg.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Ringberg A, Nordgren H, Thorstensson S, Idvall I, Garmo H, Granstrand B, Arnesson LG, Sandelin K, Wallgren A, Anderson H, et al. Histopathological risk factors for ipsilateral breast events after breast conserving treatment for ductal carcinoma in situ of the breast–results from the Swedish randomised trial. Eur J Cancer. 2007;43:291–8. doi: 10.1016/j.ejca.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Yi M, Meric-Bernstam F, Kuerer HM, Mittendorf EA, Bedrosian I, Lucci A, Hwang RF, Crow JR, Luo S, Hunt KK. Evaluation of a breast cancer nomogram for predicting risk of ipsilateral breast tumor recurrences in patients with ductal carcinoma in situ after local excision. J Clin Oncol. 2012;30:600–7. doi: 10.1200/JCO.2011.36.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark SE, Warwick J, Carpenter R, Bowen RL, Duffy SW, Jones JL. Molecular subtyping of DCIS: heterogeneity of breast cancer reflected in pre-invasive disease. Br J Cancer. 2011;104:120–7. doi: 10.1038/sj.bjc.6606021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lari SA, Kuerer HM. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–61. doi: 10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W, Jirstrom K, Johansson C, Amini RM, Blomqvist C, Agbaje O, Warnberg F. Long-term survival of women with basal-like ductal carcinoma in situ of the breast: a population-based cohort study. BMC Cancer. 2010;10:653. doi: 10.1186/1471-2407-10-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou W, Johansson C, Jirstrom K, Ringberg A, Blomqvist C, Amini RM, Fjallskog ML, Warnberg F. A comparison of tumor biology in primary ductal carcinoma in situ recurring as invasive carcinoma versus a New in situ. Int J Breast Cancer. 2013;2013:582134. doi: 10.1155/2013/582134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW, Jr, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701–10. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohrisch C, Piccart M. An overview of HER2. Semin Oncol. 2001;28:3–11. doi: 10.1016/S0093-7754(01)90103-4. [DOI] [PubMed] [Google Scholar]
- 12.Latta EK, Tjan S, Parkes RK, O’Malley FP. The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol. 2002;15:1318–25. doi: 10.1097/01.MP.0000038462.62634.B1. [DOI] [PubMed] [Google Scholar]
- 13.Allred DC, Clark GM, Tandon AK, Molina R, Tormey DC, Osborne CK, Gilchrist KW, Mansour EG, Abeloff M, Eudey L, et al. HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol. 1992;10:599–605. doi: 10.1200/JCO.1992.10.4.599. [DOI] [PubMed] [Google Scholar]
- 14.Yu KD, Wu LM, Liu GY, Wu J, Di GH, Shen ZZ, Shao ZM. Different distribution of breast cancer subtypes in breast ductal carcinoma in situ (DCIS), DCIS with microinvasion, and DCIS with invasion component. Ann Surg Oncol. 2011;18:1342–8. doi: 10.1245/s10434-010-1407-3. [DOI] [PubMed] [Google Scholar]
- 15.Horimoto Y, Tokuda E, Arakawa A, Kosaka T, Saito M, Kasumi F. Significance of HER2 protein examination in ductal carcinoma in situ. J Surg Res. 2011;167:e205–10. doi: 10.1016/j.jss.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Liao N, Zhang GC, Liu YH, Li XR, Yao M, Xu FP, Li L, Wu YL. HER2-positive status is an independent predictor for coexisting invasion of ductal carcinoma in situ of the breast presenting extensive DCIS component. Pathol Res Pract. 2011;207:1–7. doi: 10.1016/j.prp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Roses RE, Paulson EC, Sharma A, Schueller JE, Nisenbaum H, Weinstein S, Fox KR, Zhang PJ, Czerniecki BJ. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1386–9. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warnberg F, Nordgren H, Bergkvist L, Holmberg L. Tumour markers in breast carcinoma correlate with grade rather than with invasiveness. Br J Cancer. 2001;85:869–74. doi: 10.1054/bjoc.2001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livasy CA, Perou CM, Karaca G, Cowan DW, Maia D, Jackson S, Tse CK, Nyante S, Millikan RC. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum Pathol. 2007;38:197–204. doi: 10.1016/j.humpath.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Muggerud AA, Hallett M, Johnsen H, Kleivi K, Zhou W, Tahmasebpoor S, Amini RM, Botling J, Borresen-Dale AL, Sorlie T, Warnberg F. Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol Oncol. 2010;4:357–68. doi: 10.1016/j.molonc.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannemann J, Velds A, Halfwerk JB, Kreike B, Peterse JL, van de Vijver MJ. Classification of ductal carcinoma in situ by gene expression profiling. Breast Cancer Res. 2006;8:R61. doi: 10.1186/bcr1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W, Jirstrom K, Amini RM, Fjallskog ML, Sollie T, Lindman H, Sorlie T, Blomqvist C, Warnberg F. Molecular subtypes in ductal carcinoma in situ of the breast and their relation to prognosis: a population-based cohort study. BMC Cancer. 2013;13:512. doi: 10.1186/1471-2407-13-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou W, Jirstrom K, Johansson C, Amini RM, Blomqvist C, Agbaje O, Warnberg F. Long-term survival of women with basal-like ductal carcinoma in situ of the breast: a population-based cohort study. BMC Cancer. 2010;10:653. [DOI] [PMC free article] [PubMed]
- 26.Francis GD, Jones MA, Beadle GF, Stein SR. Bright-field in situ hybridization for HER2 gene amplification in breast cancer using tissue microarrays: correlation between chromogenic (CISH) and automated silver-enhanced (SISH) methods with patient outcome. Diagn Mol Pathol. 2009;18:88–95. doi: 10.1097/PDM.0b013e31816f6374. [DOI] [PubMed] [Google Scholar]
- 27.Silverstein MJ, Lagios MD, Martino S, Lewinsky BS, Craig PH, Beron PJ, Gamagami P, Waisman JR. Outcome after invasive local recurrence in patients with ductal carcinoma in situ of the breast. J Clin Oncol. 1998;16:1367–73. doi: 10.1200/JCO.1998.16.4.1367. [DOI] [PubMed] [Google Scholar]
- 28.Independent UK Panel on Breast Cancer Screening, Department of Health/United Kingdom, England: The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–86. [DOI] [PubMed]
- 29.Benson JR, Wishart GC. Predictors of recurrence for ductal carcinoma in situ after breast-conserving surgery. Lancet Oncol. 2013;14:e348–57. doi: 10.1016/S1470-2045(13)70135-9. [DOI] [PubMed] [Google Scholar]
- 30.Cowell CF, Weigelt B, Sakr RA, Ng CK, Hicks J, King TA, Reis-Filho JS. Progression from ductal carcinoma in situ to invasive breast cancer: revisited. Mol Oncol. 2013;7:859–69. doi: 10.1016/j.molonc.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakovitch E, Nofech-Mozes S, Hanna W, Narod S, Thiruchelvam D, Saskin R, Spayne J, Taylor C, Paszat L. HER2/neu and Ki-67 expression predict non-invasive recurrence following breast-conserving therapy for ductal carcinoma in situ. Br J Cancer. 2012;106:1160–5. doi: 10.1038/bjc.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, Sanchez H, Jimenez C, Stewart K, Chew K, et al. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102:627–37. doi: 10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allred DC, Anderson SJ, Paik S, Wickerham DL, Nagtegaal ID, Swain SM, Mamounas EP, Julian TB, Geyer CE, Jr, Costantino JP, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–73. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams KE, Barnes NL, Cramer A, Johnson R, Cheema K, Morris J, Howe M, Bundred NJ. Molecular phenotypes of DCIS predict overall and invasive recurrencedagger. Ann Oncol. 2015;26(5):1019-25. doi:10.1093/anninc/mdv062. [DOI] [PubMed]
- 35.Thomson TA, Zhou C, Ceballos K, Knight B. Tissue microarray for routine clinical breast biomarker analysis. The British Columbia Cancer Agency 2008 experience. Am J Clin Pathol. 2010;133:909–14. doi: 10.1309/AJCP1IU7BTSAQGYZ. [DOI] [PubMed] [Google Scholar]
- 36.Warnberg F, Amini RM, Goldman M, Jirstrom K. Quality aspects of the tissue microarray technique in a population-based cohort with ductal carcinoma in situ of the breast. Histopathology. 2008;53:642–9. doi: 10.1111/j.1365-2559.2008.03156.x. [DOI] [PubMed] [Google Scholar]
- 37.Arnould L, Roger P, Macgrogan G, Chenard MP, Balaton A, Beauclair S, Penault-Llorca F. Accuracy of HER2 status determination on breast core-needle biopsies (immunohistochemistry, FISH, CISH and SISH vs FISH) Mod Pathol. 2012;25:675–82. doi: 10.1038/modpathol.2011.201. [DOI] [PubMed] [Google Scholar]
- 38.Estevez LG, Suarez-Gauthier A, Garcia E, Miro C, Calvo I, Fernandez-Abad M, Herrero M, Marcos M, Marquez C, Lopez Rios F, et al. Molecular effects of lapatinib in patients with HER2 positive ductal carcinoma in situ. Breast Cancer Res. 2014;16:R76. doi: 10.1186/bcr3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuerer HM, Buzdar AU, Mittendorf EA, Esteva FJ, Lucci A, Vence LM, Radvanyi L, Meric-Bernstam F, Hunt KK, Symmans WF. Biologic and immunologic effects of preoperative trastuzumab for ductal carcinoma in situ of the breast. Cancer. 2011;117:39–47. doi: 10.1002/cncr.25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivotto IA, Bajdik CD, Ravdin PM, Speers CH, Coldman AJ, Norris BD, Davis GJ, Chia SK, Gelmon KA. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–25. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]


