Abstract
Objective:
Memory has been examined in subjects with imaging markers of cerebrovascular disease, but learning has been less well studied. We examined the relationship among subclinical cerebrovascular disease, cerebral volumes, and verbal learning in an ethnically and racially diverse community sample.
Methods:
A clinically stroke-free subset of Northern Manhattan Study participants underwent cognitive testing and brain MRI with quantification of white matter hyperintensity volume (WMHV) and total cerebral volume (TCV) using semiautomated segmentation. We used generalized linear regression and mixed models to examine the association between imaging findings and verbal learning.
Results:
There were 1,272 participants (61% women, mean age 70 ± 9 years). Participants with greater WMHV and smaller TCV remembered fewer total words on a list-learning task (β = −0.83 per SD change in WMHV, 95% confidence interval [CI] = −1.22 to −0.45, p < 0.0001; and β = 0.48 per SD change in TCV, 95% CI = 0.05 to 0.90, p = 0.03, respectively). Subclinical brain infarction (SBI) was not associated with total words learned (β = −0.04, 95% CI = −1.08 to 1.00, p = 0.94). Those with greater WMHV had increased odds of a flatter learning slope. After excluding participants with SBI, the association between total words learned and WMHV remained significant. All measurements were adjusted for age, education, race/ethnicity, medical insurance status, and the presence of SBI.
Conclusions:
White matter hyperintensities, a marker of cerebral small vessel disease, may have an impact on learning slope. This suggests that verbal learning performance can be incorporated into neuropsychological measures for vascular cognitive impairment and that cerebrovascular disease discovered on imaging affects the ability to learn new information.
White matter hyperintensities and “involutional changes” due to aging, vascular, or neurodegenerative causes are frequently found on the MRI scans of asymptomatic individuals. The contribution of such damage to changes in cognitive function is not completely understood.
Memory is made up of multiple processes, including registration, consolidation, storage, and retrieval, each of which may be differentially affected by various brain abnormalities, including executive dysfunction despite an intact temporal lobe.1 While some studies have focused on general memory performance, often with composite or summary scores,2–5 few studies have examined the effects of these brain markers on the learning process specifically. We examined the learning component of memory, and its relationship to vascular disease and brain volume measured on imaging studies.
The Northern Manhattan Study (NOMAS) is a community-based sample of participants, clinically stroke-free with a low prevalence of cognitive impairment and dementia, who underwent brain imaging and cognitive testing. Word-list learning tasks allow quantification of registration, learning slope, and immediate recall, all components of memory. We hypothesized that MRI markers of cerebrovascular damage would affect aspects of memory that depend on frontal lobe networks and cortical-subcortical connections6 that may be disrupted by these lesions.
METHODS
The NOMAS is a population-based cohort study that identified 3,298 stroke-free participants with random digit dialing using dual-frame sampling to find published and unpublished telephone numbers. Participants were eligible if they were older than 40 years, had never been diagnosed with stroke, and had lived in Northern Manhattan in a household with a telephone as described previously.7 Data were collected between 1993 and 2001 through interviews with trained bilingual research assistants using standardized data-collection instruments, a review of medical records, and physical and neurologic examinations by study physicians. Race and ethnicity were based on self-identification, and other demographic variables were obtained as described previously.7 Medical insurance status (Medicare or private insurance vs Medicaid or uninsured) was used as a proxy of socioeconomic status.
Standard protocol approvals, registrations, and patient consents.
Participants provided written informed consent and were recruited between 2003 and 2008. The study was approved by the institutional review boards of Columbia University and the University of Miami.
MRI examination.
Participants were enrolled in an MRI substudy during annual telephone follow-up beginning in 2003 using the following criteria: (1) age older than 55 years; (2) no contraindications to MRI; and (3) clinically stroke-free.
Imaging was performed on a 1.5-tesla MRI system (Philips Medical Systems, Best, the Netherlands) at the Columbia University Hatch Research Center. Images were transferred electronically to UC Davis for morphometric analysis of total cerebral volume (TCV) and white matter hyperintensity volume (WMHV) as previously described.8 Briefly, nonbrain elements were manually removed from the image by operator-guided tracing of the dura mater within the cranial vault, including the middle cranial fossa and excluding the posterior fossa and cerebellum. The resulting measure of the cranial vault was defined as the total intracranial volume. TCV was computed as the sum of voxels designated as whole brain volume by the segmentation process. WMHV was calculated as the sum of the voxels ≥3.5 SDs above the mean intensity of the image and multiplied by voxel dimensions and section thickness. Interrater reliabilities for the MRI measures of intracranial volume (0.97), brain volume (0.97), and WMHV (0.99) from this study were high. Brain infarction was determined according to a visual rating protocol using the size, location, and imaging characteristics of the lesion as described previously.9
Terminology.
The segmentation process included hyperintensities in the white matter and subcortical gray matter (but not the brainstem or cerebellum), but for consistency with prior publications we do not use the term “subcortical hyperintensities” from the Standards for Reporting Vascular Changes on Neuroimaging and continue to use the term “white matter hyperintensities.”10 We use the term subclinical brain infarction (SBI) because our readings included both large and small MRI-defined infarcts, both cortical and subcortical. We could not use the term “brain atrophy” as described in the STRIVE criteria because we did not exclude brain atrophy thought to be due to a focal injury, including infarction.
Cognitive testing.
On the day of the MRI, a cognitive battery was administered in a quiet room in either English or Spanish, based on the language spoken by the participant at home, by bilingual trained research assistants as previously detailed.11 The verbal learning test was a modified version of the California Verbal Learning Test–II and was administered by playing audio recordings of 12 semantically related and semantically unrelated words to the participant. Participants were asked to recall as many words as possible immediately after listening to the stimuli, and this process was repeated for a total of 5 learning trials.
Statistical analyses.
Both of the MRI markers, WMHV and TCV, were expressed as a proportion of total intracranial volume to correct for differences in head size. Words recalled on all 5 trials were summed to create the total number of words learned.
We used several approaches to characterize the relationship of the MRI markers with verbal learning and memory. First, we used a generalized linear regression model to examine the relationship of the MRI markers to memory as indicated by total number of words learned on the verbal learning test. We then used mixed models to evaluate the association of the MRI markers, characterized as either continuous or categorized in quartiles, with the number of words learned on each trial. Finally, we conducted group-based, mixed-model analyses to identify the distinct verbal learning patterns over the 5 verbal learning trials using SAS PROC TRAJ macro,12 and built multinomial logistic regression models to examine the markers as predictors of group (or pattern) membership after adjusting for the same set of covariates and initial performance on the first trial. Sensitivity analysis was performed to exclude patients with SBI to address concerns that SBI may mediate the associations between the variables. All data analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
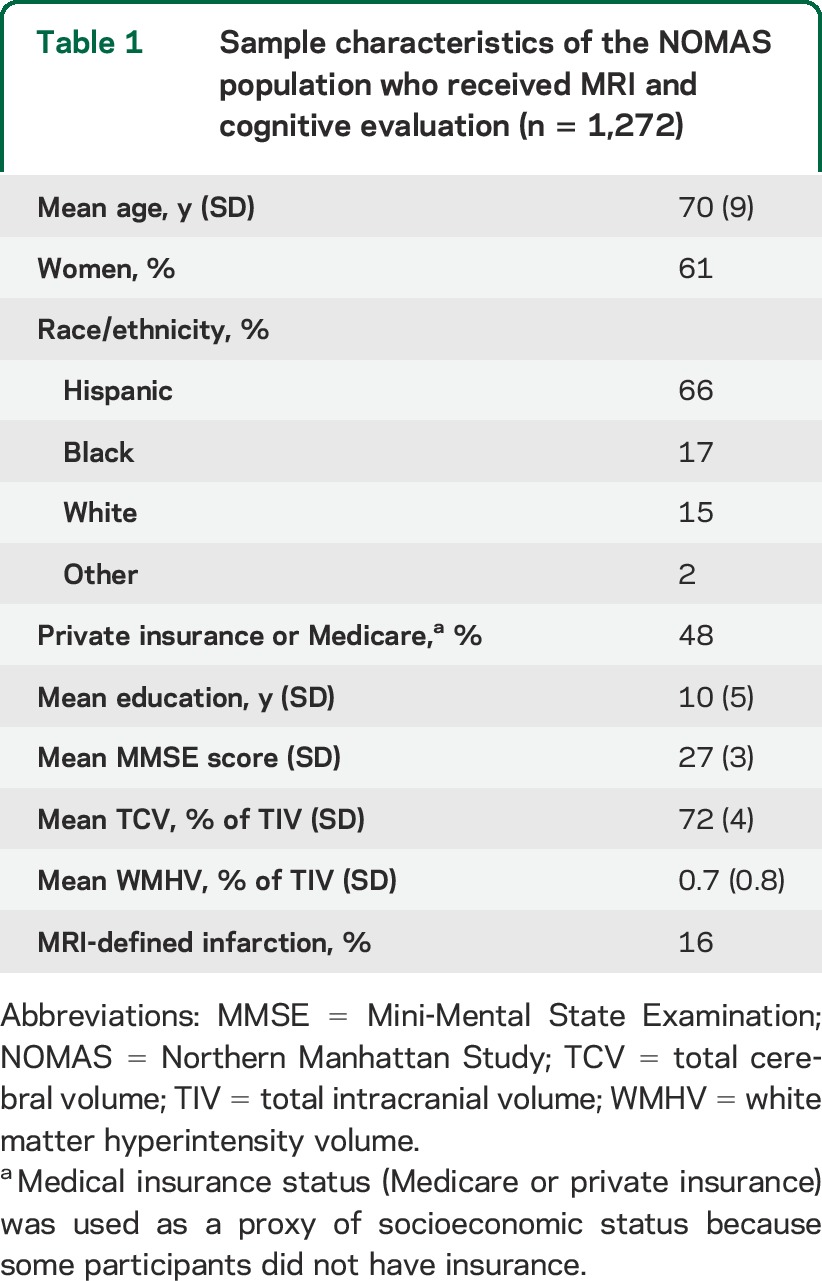
There were 1,272 NOMAS participants with MRI and cognitive data available (98% of the MRI subcohort). The characteristics of the sample are shown in table 1. Participants in the MRI cohort were older, more often Hispanic, and slightly healthier compared with the parent NOMAS cohort. The mean Mini-Mental State Examination score (±SD) was 27 ± 3, and the prevalence of MRI-defined brain infarcts was 16%.
Table 1.
Sample characteristics of the NOMAS population who received MRI and cognitive evaluation (n = 1,272)
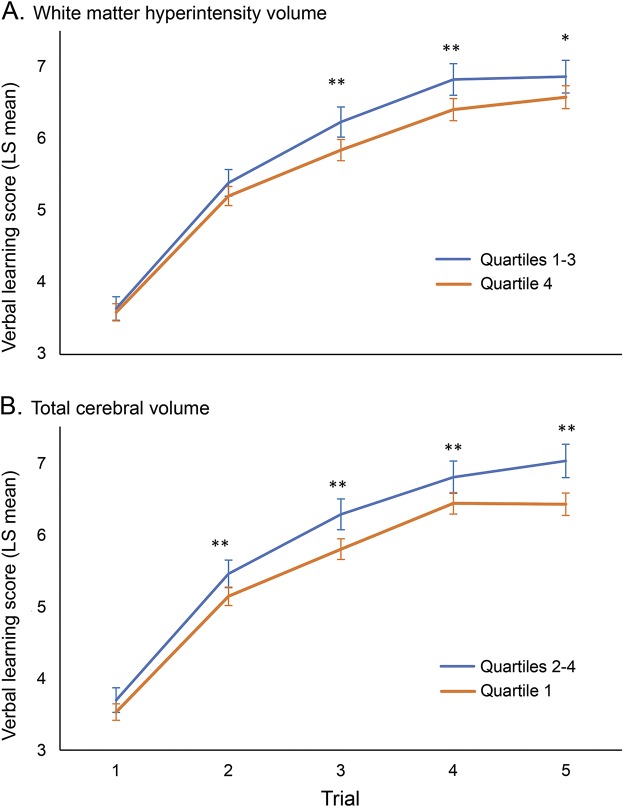
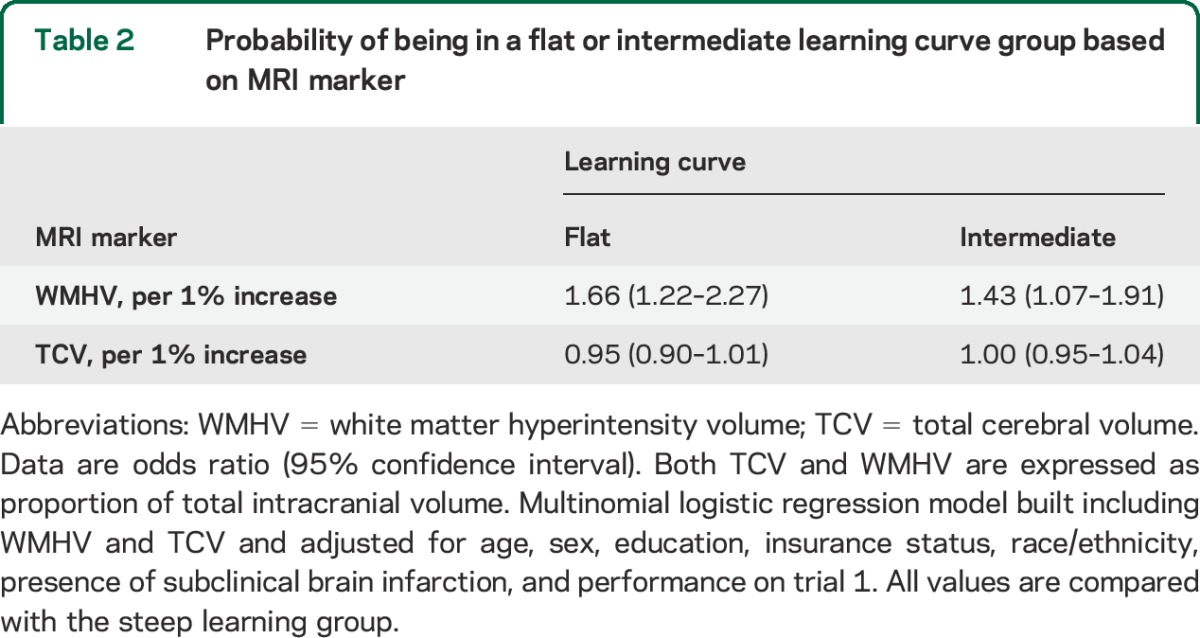
We first examined the association between WMHV and verbal learning and found a significant inverse association between WMHV and the total number of words learned across the 5 verbal learning trials after adjusting for sociodemographic factors and the presence of SBI and TCV (β = −0.83 per SD change in WMHV, 95% confidence interval [CI] = −1.22 to −0.45, p < 0.0001). We then examined whether white matter hyperintensity load affected the way participants learned words during the verbal learning test. While trial 1 performance was not significantly different between participants in the highest quartile of WMHV compared with the lower 3 quartiles, participants in the highest quartile of WMHV learned significantly fewer words on later trials (figure 1). When we examined WMHV as a continuous variable, we also found no significant difference in the number of words learned on the first trial, but on subsequent trials, those with greater WMHV learned significantly fewer words compared with the first trial (table e-1 on the Neurology® Web site at Neurology.org). We then used group-based trajectory modeling to determine whether WMHV affected the learning slope across all 5 verbal learning trials. The group-based trajectory model fit the sample into 3 learning patterns we referred to as flat (n = 341, 27%), intermediate (n = 670, 53%), and steep (n = 261, 20%) (see figure e-1). We found that participants with larger WMHV had greater odds of being in the flat (odds ratio [OR] = 1.7 per 1% greater WMHV, 95% CI = 1.2–2.3) or intermediate (OR = 1.4, 95% CI = 1.1–1.9) learning group than in the steep group (table 2).
Figure 1. Verbal learning plots based on quartiles of white matter hyperintensity and cerebral volume.
Plots are derived from least squares (LS) means in a mixed model after adjusting for age, sex, education, insurance status, race/ethnicity, and presence of subclinical brain infarction. Patients are divided by (A) top quartile of white matter hyperintensity volume (quartile 4) vs the lowest 3 quartiles (quartiles 1–3), and (B) bottom quartile of total cerebral volume (quartile 1) vs the top 3 quartiles (quartiles 2–4). *p < 0.05, **p < 0.01.
Table 2.
Probability of being in a flat or intermediate learning curve group based on MRI marker
Examining cerebral volumes continuously, greater TCV was positively associated with total words learned across the 5 learning trials (β = 0.48 per SD change in TCV, 95% CI = 0.05–0.90, p = 0.03) after adjusting for sociodemographic factors and the presence of SBI and WMHV. While trial 1 performance was not significantly different between participants in the lowest quartile of TCV compared with the upper 3 quartiles, participants in the lowest quartile of TCV learned significantly fewer words on all other trials (figure 1). TCV was also examined as a continuous variable, and there was no significant difference in the number of words learned on the first trial, while on subsequent trials, those with greater TCV learned significantly more words compared with the first trial (table e-1). We then examined learning patterns and found that those with greater TCV did not have significantly different odds of being in the flat or intermediate learning group compared with those with lower TCV (OR = 0.95 per 1% increase in TCV, 95% CI = 0.90–1.01, p = 0.08).
Participants with SBI did not learn fewer words across all trials than those without SBI (β = −0.04, 95% CI = −1.08 to 1.00, p = 0.94). We then performed a sensitivity analysis to examine the associations among WMHV, TCV, and total words learned in participants without SBI. The association between WMHV and total words learned remained significant (β = −0.67 per SD change in WMHV, 95% CI = −1.18 to −0.16, p = 0.01), but the association between TCV and total words learned did not (β = 0.28 per SD change in TCV, 95% CI = −0.19 to 0.76, p = 0.24).
DISCUSSION
Here, we demonstrate that white matter hyperintensities and total brain volumes are associated with worse learning in older community-dwelling adults. Participants with more white matter hyperintensities had different learning patterns than those with smaller brain volumes, suggesting these measures of brain damage each have effects on verbal learning.
Both greater WMHV and smaller cerebral volumes were associated with fewer total words learned over 5 trials, but white matter hyperintensity load, and not lower cerebral volume, was associated with flatter learning slopes. Lower TCV has been associated with aging, neurodegenerative pathology,13 and ischemic damage,10 and may impair attention, working memory, as well as the encoding and consolidation processes involved in learning new information. In our study, smaller brain volumes and WMHV both affected the number of words learned as trials progressed. An initial learning trial engages different cognitive processes than subsequent trials, as initial trial performance is more dependent on attention and working memory, whereas performance on subsequent trials involves multiple other processes including encoding and consolidation.14 Since neither TCV nor WMHV was associated with the number of words learned on the first trial, we suspect that some of these other processes are affected by these cumulative lesions. While our data do not provide a clear explanation, they do suggest that brain atrophy and cerebral small vessel disease may have distinct effects on learning. Multimodal studies that include regional measures of volume, diffusion tensor imaging, and functional connectivity could help to clarify these findings.
In this study, white matter hyperintensities were associated with flatter learning slopes, whereas smaller cerebral volumes were not, suggesting that encoding, consolidation, and retrieval may be differentially affected by white matter hyperintensities. Rate of learning a word list is directly associated with the process of encoding, which depends on attention and registration like early trial performance, but also the ability to semantically organize words.15 Consolidation may also affect performance on later learning trials, and therefore learning slope, and requires neurons to fire repetitively, thereby strengthening neural networks.16 White matter hyperintensities often affect long-tract connections between mesial temporal lobe structures and the frontal and parietal lobes.17,18 Our study suggests that white matter hyperintensities, in addition to their effect on attention, working memory, and information processing speed,19,20 can have an effect on learning via impaired organization and consolidation, which is likely not mediated by temporal lobe function alone.
Similar to the frontal lobe impairment seen with white matter lesions,21 subcortical infarctions may disconnect the frontal lobes from other cortical and subcortical regions, resulting in impaired executive function and semantic clustering,3,22 a strategy often used by participants to remember groups of words.23 Subclinical brain infarcts have been associated with lower verbal memory performance in patients without dementia,3,5 but SBI was not associated with total number of words learned in our study. However, power to detect an association may have been limited given that SBI was analyzed as a binary variable. Heterogeneity of SBI location also complicates such analyses, and future work with larger samples is needed. Although the prevalence of SBI in our study was 16%, our sensitivity analysis suggests that the lower learning performance associated with smaller TCV was dependent on the presence of SBI. Thus, small brain volumes may have an additive effect with SBI on the learning process. In contrast, WMHV was associated with impaired learning independent of SBI. In fact, the sensitivity analysis excluding those with SBI may have underestimated the association between WMHV and learning ability since white matter hyperintensities and brain infarctions frequently coexist.23 We propose that cerebral small vessel disease in the form of white matter hyperintensities (and by extension white matter lesions) independently impair encoding and consolidation processes, but studies designed to examine these processes more directly are needed.
One of the strengths of our study was the large number of clinically stroke-free participants who underwent imaging and cognitive testing. They came from diverse race/ethnic and socioeconomic groups that are representative of some urban populations in the United States. Our cohort was generally older but healthier than the larger NOMAS population because of a survival effect, as well as their ability to come to our center and undergo brain imaging. Participants with a history of stroke were carefully excluded from the sample through a detailed surveillance process that tracks stroke incidence. These factors would all tend to underestimate the associations of vascular disease and brain volume with cognitive performance in our study.
Several limitations should be noted. The cross-sectional design of this study precludes conclusions about causal associations between imaging findings and some of the cognitive performance measures. However, there is a prospective component to this study as well, since brain changes were present before the list-learning task. Because neither WMHV nor TCV was associated with performance on the first trial of the verbal learning test, our data suggest that these markers had an effect on learning and not registration. However, we had limited capability to examine each aspect of learning, such as storage, consolidation, and retrieval, because (1) we did not have many participants that forgot words on subsequent trials, which would be a marker of consolidation, and (2) we did not administer verbal cues, which would be a tool to measure retrieval ability.16 Some other limitations also exist. Although we found associations with verbal learning and memory, we were unable to relate these findings to cognitive disorders or dementia since we have not adjudicated these outcomes in the sample. Nonetheless, estimates based on sample-specific age- and education-adjusted norms suggest a prevalence below 5% of cognitive impairment at the time of the cognitive assessment used for the current analysis. Finally, we do not have data on white matter hyperintensity or infarction size or location in relation to verbal learning, and different lesion locations may have distinct effects. This will be interesting to examine in future studies.
We studied the effect of asymptomatic imaging findings on the learning process. Asymptomatic cerebrovascular disease can have a significant effect on learning in patients who do not complain of cognitive symptoms and may have intact temporal lobe function. In a similar study, impaired learning performance was associated with late-life depression.6 Although a minority of our participants complained of cognitive dysfunction, the extent to which slowed learning may subtly affect the lives of those with cerebrovascular damage remains to be determined. Our findings suggest that memory tests should be interpreted in the context of the specific memory process being examined, and that learning depends in large part on brain network connections and executive function. Our findings may be translated into neuropsychological models of vascular cognitive impairment and suggest that learning is impaired in people with asymptomatic cerebrovascular disease.
Supplementary Material
GLOSSARY
- CI
confidence interval
- NOMAS
Northern Manhattan Study
- OR
odds ratio
- SBI
subclinical brain infarction
- TCV
total cerebral volume
- WMHV
white matter hyperintensity volume
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
H.G.: principal author, drafting/revising the manuscript for content, study concept and design, analysis and interpretation of data. C. Dong: author, study concept and design, analysis and interpretation of data, statistical analysis. M.Y.: drafting/revising the manuscript for content. T.R.: drafting/revising the manuscript for content, study concept and design, obtaining funding. M.S.V.E.: drafting/revising the manuscript for content, obtaining funding. R.L.S.: drafting/revising the manuscript for content, obtaining funding. C. DeCarli: drafting/revising the manuscript for content, study supervision and coordination. Y.S.: drafting/revising the manuscript for content, study concept and design. C.B.W.: study concept and design, drafting/revising the manuscript for content, study supervision and coordination, obtaining funding.
STUDY FUNDING
Study supported by grants from the National Institute of Neurological Disorders and Stroke (R37 NS 29993; K02 NS 059729). Evelyn McKnight Brain Research Foundation funds the Evelyn F. McKnight Brain Institute, work affiliation of H.G., C. Dong, T.R., R.L.S., C.B.W.
DISCLOSURE
H. Glazer, C. Dong, M. Yoshita, T. Rundek, and M. Elkind report no disclosures. R. Sacco reports federal grant and foundation support for this project. C. DeCarli receives grant support from P30 AG 010129. Y. Stern is a consultant for GeneTech, and receives grant support from the NIH. C. Wright receives royalties from UpToDate for 2 chapters on vascular dementia, and receives grant support from federal (K02 NS 059729, R01 HL 108623) and private (American Heart Association Bugher Center project 14BFSC17690003) sources. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Wright CB, Zonderman AB. What can memory tests predict about the aging brain? The freedom to recall. Neurology 2013;80:1274–1275. [DOI] [PubMed] [Google Scholar]
- 2.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128:2034–2041. [DOI] [PubMed] [Google Scholar]
- 3.Blum S, Luchsinger JA, Manly JJ, et al. Memory after silent stroke: hippocampus and infarcts both matter. Neurology 2012;78:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Groot JC, de Leeuw F, Oudkerk M, Van Gijn J. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 2000;47:145–151. [DOI] [PubMed] [Google Scholar]
- 5.Vermeer SE, Prins ND, Den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 6.Lamar M, Charlton R, Zhang A, Kumar A. Differential associations between types of verbal memory and prefrontal brain structure in healthy aging and late life depression. Neuropsychologia 2012;50:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke 2007;38:3121–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 9.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI Twin Study. Stroke 1999;30:529–536. [DOI] [PubMed] [Google Scholar]
- 10.Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MSV, Wright CB. Construct validity of cognitive reserve in a multiethnic cohort: the Northern Manhattan Study. J Int Neuropsychol Soc 2009;15:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res 2001;29:374–393. [Google Scholar]
- 12.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur B, Himali JJ, Seshadri S, et al. Association between neuropathology and brain volume in the Framingham Heart Study. Alzheimer Dis Assoc Disord 2014;28:219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtiss G, Vanderploeg RD, Spencer J, Salazar AM. Patterns of verbal learning and memory in traumatic brain injury. J Int Neuropsychol Soc 2001;7:574–585. [DOI] [PubMed] [Google Scholar]
- 15.Vanderploeg RD, Crowell TA, Curtiss G. Verbal learning and memory deficits in traumatic brain injury: encoding, consolidation, and retrieval. J Clin Exp Neuropsychol 2010;23:37–41. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Morris RGM. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 2010;61:49–79. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher PC, Shallice T, Dolan RJ. The functional roles of prefrontal cortex in episodic memory: I: encoding. Brain 1998;121:1239–1248. [DOI] [PubMed] [Google Scholar]
- 18.DeCarli C, Murphy DGM, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995;45:2077–2084. [DOI] [PubMed] [Google Scholar]
- 19.Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke 2008;39:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Reed BR, Jagust WJ. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia 2005;43:1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004;63:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfe N, Linn R, Babikian VL, Knoefel JE, Albert ML. Frontal systems impairment following multiple lacunar infarcts. Arch Neurol 1990;47:129–132. [DOI] [PubMed] [Google Scholar]
- 23.Rost NS, Rahman RM, Biffi A, et al. White matter hyperintensity volume is increased in small vessel stroke subtypes. Neurology 2010;75:1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.