Abstract
Objective:
To describe the time elapsed from onset of pediatric convulsive status epilepticus (SE) to administration of antiepileptic drug (AED).
Methods:
This was a prospective observational cohort study performed from June 2011 to June 2013. Pediatric patients (1 month–21 years) with convulsive SE were enrolled. In order to study timing of AED administration during all stages of SE, we restricted our study population to patients who failed 2 or more AED classes or needed continuous infusions to terminate convulsive SE.
Results:
We enrolled 81 patients (44 male) with a median age of 3.6 years. The first, second, and third AED doses were administered at a median (p25–p75) time of 28 (6–67) minutes, 40 (20–85) minutes, and 59 (30–120) minutes after SE onset. Considering AED classes, the initial AED was a benzodiazepine in 78 (96.3%) patients and 2 (2–3) doses of benzodiazepines were administered before switching to nonbenzodiazepine AEDs. The first and second doses of nonbenzodiazepine AEDs were administered at 69 (40–120) minutes and 120 (75–296) minutes. In the 64 patients with out-of-hospital SE onset, 40 (62.5%) patients did not receive any AED before hospital arrival. In the hospital setting, the first and second in-hospital AED doses were given at 8 (5–15) minutes and 16 (10–40) minutes after SE onset (for patients with in-hospital SE onset) or after hospital arrival (for patients with out-of-hospital SE onset).
Conclusions:
The time elapsed from SE onset to AED administration and escalation from one class of AED to another is delayed, both in the prehospital and in-hospital settings.
Status epilepticus (SE) is one of the most common pediatric neurologic emergencies.1 It has a mortality of 0%–3%2–7 and morbidity that includes cognitive and neurodevelopmental impairments, epilepsy, and recurrent SE.2,8–10 SE is often refractory to the initial antiepileptic drugs (AEDs),11,12 and refractory SE is associated with poor outcome.12 Patient age, etiology, and SE duration all affect outcome,5,9,13 but only SE duration is a potentially modifiable factor by rapid AED treatment. By convention, the treatment of convulsive SE is a sequence of AEDs, typically using a benzodiazepine (BZD) first, followed by other classes of AEDs.14,15 Data on the time elapsed from the onset of SE to administration of the successive AEDs are limited.
Although no evidence-based AED timeline or optimal time window exists for this AED sequence, most current SE treatment protocols recommend that the first AED be administered within 5 minutes of seizure onset. If seizures persist, moving to the next AED class in the sequence should be done by 10 minutes and, if repeated AED doses do not control SE, the initiation of anesthetic dosing via continuous infusions should be started by 30–70 minutes of seizure onset.14,16 Timely AED administration and rapidly moving along the sequence of AED classes are intended to stop seizures as quickly as possible. Basic and clinical research suggest that longer-duration seizures are more treatment-resistant and are associated with a worse outcome.17–20 However, few data are available regarding the time elapsed from SE onset to AED administration in either the prehospital or in-hospital settings.
We aim to address this gap in knowledge by describing the timing and escalation of AED administration in pediatric convulsive SE both in the prehospital and in-hospital settings.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the institutional review board at each institution. Written informed consent was obtained from parents or guardians.
Study design.
The pediatric Status Epilepticus Research Group (pSERG) undertook this descriptive prospective observational study of health care delivery at 9 tertiary pediatric hospitals in the United States.21 The purpose of this study was to describe the timing and escalation of AED administration during all stages of AED administration in the prehospital and in-hospital settings in patients with refractory convulsive status epilepticus (RCSE).
Eligibility criteria included (1) admission between June 1, 2011, and June 30, 2013; (2) age from 1 month to 21 years; (3) focal or generalized convulsive epileptic seizures at onset; and (4) failure of ≥2 AEDs to terminate seizures or the initiation of a continuous infusion of AEDs for seizure control. We only considered seizures with a convulsive onset and considered the time to cessation of convulsive SE even if the patient continued to seize electrographically. Clinical presentation, EEG findings, and follow-up data were not consistent with psychogenic nonepileptic seizures in any of our patients. For the purposes of the inclusion criteria, different BZDs given as a nonanesthetic bolus dose (e.g., rectal diazepam followed by IV lorazepam) were counted as 1 drug, and were differentiated from BZDs administered by continuous infusion at anesthetic doses (e.g., midazolam), which were counted as a continuous infusion.
Exclusion criteria were (1) nonconvulsive SE detected on EEG without convulsive seizures at onset and (2) nonconvulsive SE with motor manifestations limited to infrequent myoclonic jerks. Only patients with complete clinical information at time of AED administration were eligible. If more than one episode of RCSE occurred during the study period, only the first episode was included. RCSE was classified as continuous if there was a single continuous clinical seizure and as intermittent if there were repeated clinical seizures without return to baseline in between. Although the type of AEDs used generally followed published guidelines, these were not binding for treating physicians, and therefore variability may have occurred based on incomplete protocol adherence at each site and on a case-by-case clinical assessment by individual physicians.
Assessments.
The primary descriptive measure was the time elapsed from seizure onset to administration of the first 3 AED doses. Times were assessed based on family and Emergency Medical Services (EMS) information for out-of-hospital onset and provider information and hospital records once in the hospital. However, we verified this information with both families and EMS when available to reduce and eliminate potential bias. Secondary descriptive measures were the time of administration by AED category and by setting of AED administration. Data were collected with a standardized data acquisition tool and entered into an electronic database hosted by Cincinnati Children's Hospital Medical Center. Details of the pSERG consortium can be found elsewhere.21
Statistical analysis.
Demographic and clinical characteristics were summarized with descriptive statistics. Time to administration of AEDs was described in a time-to-event analysis. Subgroup comparisons were performed with the Wilcoxon test. Unless stated otherwise, times were calculated from seizure onset. All statistical analyses were performed with STATA 12 (Stata Corp., College Station, TX).
RESULTS
Study population.
A total of 86 patients were enrolled. Five were excluded because of incomplete information, leaving 81 patients (44 male) with a median (p25–p75) age of 3.6 (1.2–8.8) years included in the analysis. Table 1 summarizes the main demographic characteristics and clinical features of the episodes.
Table 1.
Demographic and clinical characteristics


The initial AED was a BZD in 78 (96.3%) patients. In the remaining 3 patients the initial AED was levetiracetam (n = 2) or phenobarbital (n = 1). A total of 65/78 (83.3%) patients received at least 2 doses of BZDs and 31/78 (39.7%) patients received at least 3 doses of BZDs prior to escalation to other AED classes. The median (p25–p75) time until cessation of convulsive SE was 137 (80–300) minutes.
AED administration.
The median (p25–p75) time until administration of the first, second, and third AED doses were 28 (6–67) minutes, 40 (20–85) minutes, and 59 (30–120) minutes (figure 1). Patients with in-hospital seizure onset received their first AED dose earlier than those with out-of-hospital seizure onset: 5 (4.5–80) vs 30 (12–60) minutes, p = 0.0326. Similarly, patients with in-hospital seizure onset had a tendency to receive their second AED dose earlier than those with out-of-hospital onset: 15 (10–91) vs 44 (26.5–84) minutes, p = 0.0547 (figure e-1 on the Neurology® Web site at Neurology.org).
Figure 1. Kaplan-Meier curves representing time from seizure onset to administration of the first AED (green), second AED (blue), and third AED (red).
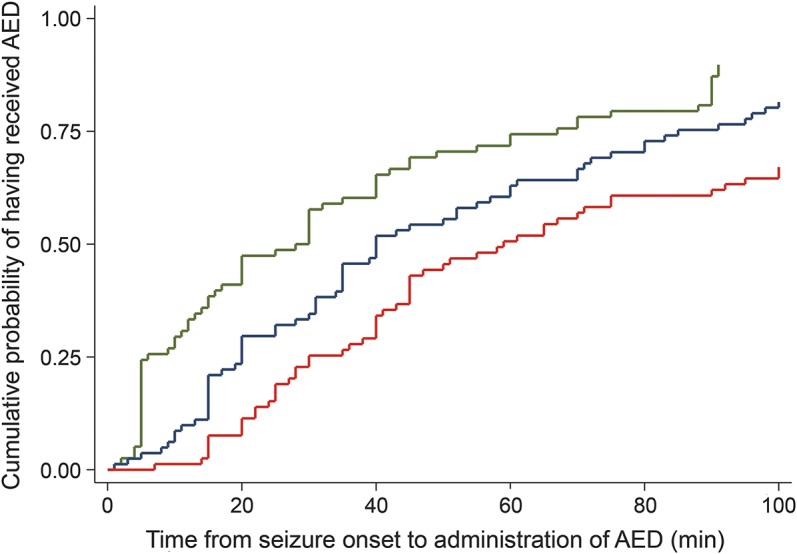
Time axis is truncated at 100 minutes. As an illustration of the delays in antiepileptic drug (AED) administration, 40 minutes after seizure onset, approximately 60% of patients had received their first AED, approximately 45% of patients had received a second AED, and approximately 25% of patients had received their third AED.
AED escalation: From BZDs to non-BZD AEDs to continuous infusions.
When considering AED classes, the first and second doses of BZDs were administered at a median (p25–p75) of 30 (6–70) minutes and 40 (20–95) minutes. The first and second doses of non-BZD AEDs were administered at 69 (40–120) minutes and 120 (75–296) minutes, respectively (figure 2). Thirty-two patients received at least one continuous infusion that was started at 180 (120–645) minutes. The median (p25–p75) number of administered doses of AED was 5 (4–6), of BZDs was 2 (2–3), and of non-BZD AEDs was 2 (2–3). When comparing patients who were eventually treated with a continuous infusion to those who were not, there were no differences in the number of doses of AEDs (5 [4–6] vs 5 [3–6], p = 0.84), number of doses of BZDs (3 [2–3] vs 2 [2–4], p = 0.55), or number of doses of non-BZD AEDs (2 [2–3] vs 2 [1–3], p = 0.64).
Figure 2. Kaplan-Meier curves representing time to benzodiazepine and non-benzodiazepine antiepileptic drug administration.

(A) Time from status epilepticus (SE) onset to benzodiazepine (BZD) administration. Time axis truncated at 100 minutes. Time to administration of first BZD (green) and second BZD (blue). As an illustration of the delays in BZD administration, 20 minutes after seizure onset, approximately 40% of patients had received their first BZD and approximately 25% of patients had received a second BZD. (B) Time from SE onset to non-BZD antiepileptic drug (AED) administration. Time axis truncated at 100 minutes. Time to administration of first non-BZD AED (green) and second non-BZD AED (blue). As an illustration of the delays in non-BZD AED administration, 40 minutes after seizure onset, approximately 25% of patients had received their first non-BZD AED and approximately 10% of patients had received a second non-BZD AED.
Prehospital AED administration.
In the subset of 64 patients with out-of-hospital seizure onset, only 16 (25%) had received the first AED dose by 20 minutes after seizure onset and only 24 (37.5%) received any AED prior to hospital arrival (figure 3).
Figure 3. Kaplan-Meier curves representing time to antiepileptic drug administration in the prehospital and in-hospital settings.
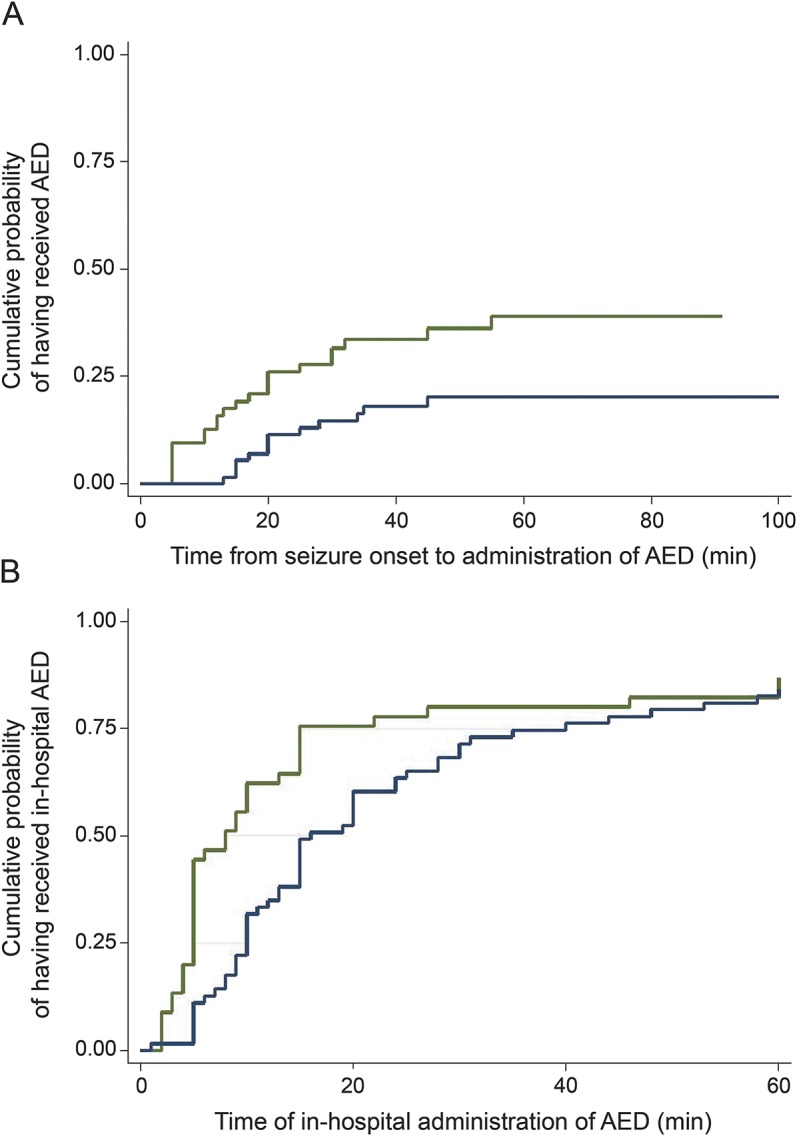
(A) Time from status epilepticus (SE) onset to out-of-hospital antiepileptic drug (AED) administration for patients with out-of-hospital SE onset (times were censored on hospital arrival). Time axis truncated at 100 minutes. Time to administration of first out-of-hospital AED (green) and second out-of-hospital AED (blue). As an illustration of the delays in the prehospital administration of AEDs, 20 minutes after seizure onset, approximately 25% of patients had received their first AED and approximately 10% of patients had received a second AED. (B) Time from hospital management (time 0 was time of SE onset for patients with in-hospital SE onset; time 0 was time of hospital arrival for patients with out-of-hospital SE onset) to in-hospital AED administration. Time axis truncated at 60 minutes. Time to administration of first in-hospital AED (green) and second in-hospital AED (blue). As an illustration of the delays in in-hospital administration of AEDs, 20 minutes after initiation of hospital management, approximately 75% of patients had received their first in-hospital AED and approximately 50% of patients had received a second in-hospital AED.
EMS administered at least an AED dose prior to hospital arrival in 23 (35.9%) patients, including the first BZD dose in 17 patients and a subsequent BZD dose after administration of a prior BZD dose by the family in 6 patients. EMS administered a non-BZD AED dose in 2 (IV fosphenytoin in both cases after prior administration of BZDs).
Among the 27 patients with out-of-hospital seizure onset and a prior diagnosis of epilepsy whose caregivers were assumed to have an acute treatment plan for prolonged seizures, only 12 (44.4%) received the first AED dose before arriving to the hospital. These AEDs were BZDs in all 12 patients, administered by the family in 7 patients and by EMS in 5. Considering only the patients with one or more prior episodes of SE whose families should have been familiar with diazepam rectal administration, only 3 of 9 patients (33.3%) received their first AED dose from their families.
In-hospital AED administration.
The first and second in-hospital AED doses were given at a median (p25–p75) of 8 (5–15) minutes and 16 (10–40) minutes, after seizure onset (for in-hospital seizure onset) or after hospital arrival (for out-of-hospital seizure onset) (figure 3). In the hospital, patients received 4 (4–6) total doses of AEDs, divided into 2 (2–3) doses of BZDs and 2 (2–3) doses of non-BZD AEDs. When comparing in-hospital and out-of-hospital onset, there were no differences in the number of total doses of AEDs (4 [4–6] vs 4 [4–6], p = 0.39), number of doses of BZDs (2 [1.5–3] vs 2 [2–3], p = 0.23), or number of doses of non-BZD AEDs (2 [2–3] vs 2 [2–3], p = 0.17). Even those receiving repeated BZD doses out of the hospital received several more BZD doses once in the hospital.
Time to treatment in different subgroups.
We compared patients with continuous vs intermittent SE and there were no statistically significant differences in the overall, prehospital, or in-hospital times to administration of the first 2 AED doses. We compared patients with vs without a prior diagnosis of epilepsy and there were no statistically significant differences in the overall, prehospital, or in-hospital times to administration of AED doses. Among patients with out-of-hospital onset, there were no differences in time to administration of AEDs when comparing patients with initial referral to an outside hospital vs initial referral to a pSERG hospital. For in-hospital AED administration, there were no differences in time to administration of AEDs when comparing patients with in-hospital vs out-of-hospital SE onset.
Relationship between timeliness of AED administration and duration of RCSE.
The duration of convulsive SE positively correlated with the time to administration of the first AED dose (Spearman correlation coefficient [SCC] = 0.3107, p = 0.0053), time to administration of the second AED dose (SCC = 0.4211, p = 0.0001), and time to administration of the third AED dose (SCC = 0.3876, p = 0.0004).
Other clinical characteristics.
Seventy-nine of 81 (98%) patients were admitted to an intensive care unit (ICU). During the SE episode, 61/81 (75%) patients were intubated. The median (p25–p75) ICU stay was 3 (2–10) days. ICU stay was shorter in patients who received the second non-BZD AED earlier, but there were no differences in ICU stay when comparing timing of administration of first BZD and first non-BZD AED (appendix e-1). Based on clinical assessment, at hospital discharge, approximately two-thirds of patients returned to their functional baseline (appendix e-1). Four (4.9%) patients died before hospital discharge. Table e-1 details the characteristics associated with AED administration. Appendix e-1 provides results on subgroup analyses.
DISCUSSION
Our data demonstrate that the time from seizure onset to the administration of AED doses and escalation between AED classes were delayed (table 2). Few patients received BZDs in the field, although many of our patients already had a diagnosis of epilepsy or prior SE episodes. Once in the hospital, patients received repeated doses of BZDs before moving to other AED classes even if the SE episode was ongoing for a prolonged period of time with previous BZDs having failed to control SE.
Table 2.
Recommended timeframes for AED administration, times found in our series, and strategies that may potentially reduce time to administration of AEDs
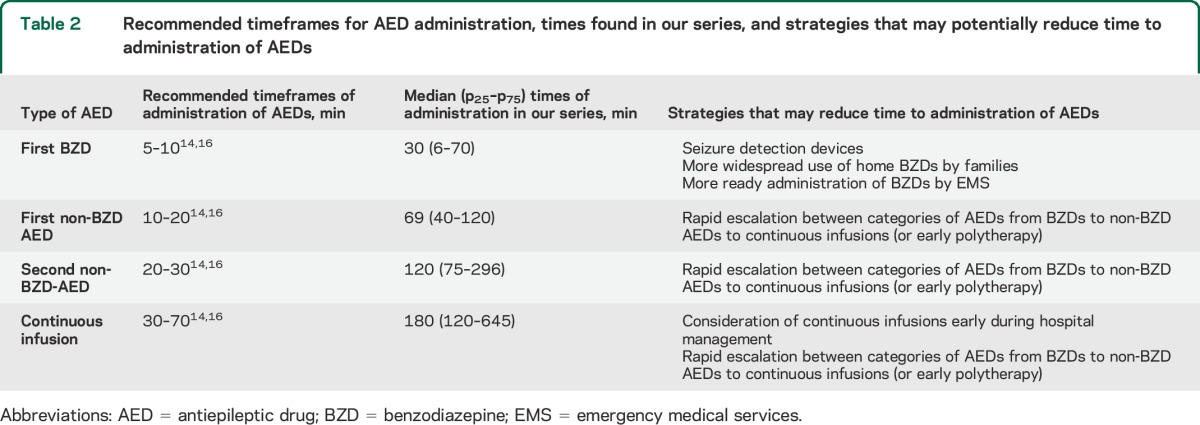
Current SE treatment protocols recommend a timely administration of AED doses and a rapid escalation between different classes of AEDs (table 2).14,15,22 The rationale for this recommendation includes (1) results from clinical studies suggesting better seizure control and reduction of brain injury with earlier AED administration23 and (2) results from animal models showing that prolonged SE causes brain damage24 and the response to BZDs decreases with seizure duration.19,20 In a series of 182 children with convulsive SE, for each minute delay from seizure onset to arrival at the emergency department there was a 5% cumulative increase in the risk of the SE episode lasting more than 60 minutes.25 In a series of 45 episodes of generalized convulsive SE in children, the duration of SE was shorter (32 vs 60 minutes) and the risk of recurrent seizures was lower (58% vs 85%) in the 19 episodes treated with prehospital diazepam than in the 26 episodes without prehospital treatment.17 In a study of 157 children with SE, a delay of more than 30 minutes in administering the first AED was associated with worse response to treatment.18 Our series also demonstrates that later administration of the first 3 AED doses correlates with a longer SE duration.
While these data suggest the importance of rapid AED administration in SE, there is limited literature on the timeliness of AED administration in clinical practice. Paucity of data can be partially attributed to lack of management of most seizures that stop after a first AED in reference hospitals, and even in prolonged seizures it is difficult to measure the time elapsed from seizure onset to administration of AEDs. In a retrospective study of 889 patients (625 adults and 264 children) with SE, approximately 60% of the patients received their first AED dose after 30 minutes and approximately 25% after 60 minutes.26 In a retrospective multicenter study of 542 episodes of convulsive seizures of 10 or more minutes duration in children, the median (p25–p75) time from hospital arrival until administration of a non-BZD AED was 24 (15–36) minutes.27 In a series of 199 children with febrile status epilepticus, the median time from seizure onset to administration of the first AED was 30 minutes.28 The limited information reported on time of AED administration in adults also points towards significant delays in drug administration.29–32 However, there are no series that have systematically studied the time of AED administration at all stages of SE treatment.
Our study addresses this gap in knowledge. In the prehospital setting, more than half of our patients did not receive any AED until hospital arrival. Surprisingly, lack of prehospital AED administration also occurred in patients with a prior diagnosis of epilepsy or prior SE episodes, a group in whom a plan should have been devised. Our study could not detect patients who received a rescue AED at home with successful seizure control, likely a majority of children. However, our data are consistent with a low rate of AED administration in the prehospital environment found in the prospective North London series both in patients with and without prior epilepsy.33 Families administered rectal diazepam in a minority of patients and midazolam in only one occasion. Furthermore, EMS did not administer or were not instructed to administer any AED dose before hospital arrival in more than 60% of cases. Despite the challenges and time associated with obtaining IV access in an out-of-hospital setting, EMS administered BZDs mostly by the IV route without taking full advantage of, at times, faster routes such as rectal, IM, or sublingual/buccal BZDs.22 Most patients were enrolled before the RAMPART trial was published,34 so EMS may consider other administration routes more frequently now. Escalation from BZDs to non-BZD AEDs occurred in a tiny minority of cases before hospital arrival, although it is probable that not all EMS are allowed to administer non-BZD AEDs. In-hospital care was characterized by repeated administration of BZDs, even in patients with out-of-hospital onset who received BZDs prior to hospital arrival.
The findings of this prospective observational study have identified several areas for improvement including earlier detection and treatment of seizures, more widespread use of home rescue BZDs, and rapid escalation of AED treatment or early polypharmacotherapy (table 2). We do not have data on what causes these delays in AED administration. Future studies of our consortium plan to assess the presumed causes of delay, such as preparation and delivery of AEDs through the pharmacy, difficulties recognizing ongoing seizures in patients with intermittent convulsive seizures, and recognition of SE as a time-sensitive emergency, similar to stroke or cardiorespiratory arrest. Our results support the implementation of policies that optimize timing and escalation of AED administration for seizures at the family, EMS, and hospital levels.
In this descriptive study, we did not account for multiple comparisons. However, our preplanned data analysis showed consistent delays when compared to recommended protocols. Limited sample size prevented a more complete subgroup analysis by site or by use of continuous infusions. Our population is not representative of all children with seizures or with SE, but only of children who did not respond to initial AEDs. This selection bias limits the generalizability of results. However, the purpose of this study was to evaluate timeliness of AED administration at all stages of SE treatment. As in prior literature, we observed that delayed administration of AEDs correlated with prolonged seizure duration but a causal relationship cannot be directly concluded. The question of whether treatment delay is the primary factor that differentiates a seizure aborted early during its course and an ongoing seizure that becomes SE remains unanswered. However, the literature suggests that treatment delay is a relevant factor associated with prolonged and refractory seizures and worse outcome.17,18,25 Although this diagnosis never can be excluded, the clinical presentation, EEG findings, and follow-up data were not consistent with psychogenic nonepileptic seizures in any of our patients.
It is unknown if AEDs were administered late because of the clinical impression that the episode was spontaneously resolving or because there was a delay between the decision to administer an AED and its actual administration (e.g., operational capacity such as availability of AEDs, pharmacy processing). We described the time elapsed from seizure onset to AED administration in pediatric SE and our results set the ground to implement health care policies that optimize timing of AED administration and future studies that determine if improved timing of AED administration improves outcomes. While the timing of administration of AEDs left room for improvement, the type of AEDs administered was uniform despite the lack of a common treatment protocol.
Our study provides a detailed description of all the stages of health care delivery for pediatric SE, firmly establishes that delays in AED administration and escalation do occur, and identifies specific opportunities for improvement both in the prehospital and in-hospital settings.
Supplementary Material
GLOSSARY
- AED
antiepileptic drug
- BZD
benzodiazepine
- EMS
Emergency Medical Services
- ICU
intensive care unit
- pSERG
pediatric Status Epilepticus Research Group
- RCSE
refractory convulsive status epilepticus
- SCC
Spearman correlation coefficient
- SE
status epilepticus
Footnotes
Supplemental data at Neurology.org
Editorial, page 2296
Contributor Information
And the Pediatric Status Epilepticus Research Group (pSERG):
Michele Jackson, Jacquelyn Klehm, Robert Faist, Melissa Sacco, Robin Kelley, Joshua Goldstein, Kristi Schmidt, Mark Wainwright, Rajit Basu, Karen Cornett, Roha Khalid, Kathryn Xixis, Christie Milleson, David Goldstein, Erin Heinzen, Lindsay Johnson, Nicole Walley, David Turner, Abeer Hani, Robert McNeil, Geetanjali Rathore, Angus Wilfong, Yi-Chen Lai, Christina Lopez, Alexis Topjian, Korwyn Williams, John Condie, Penny Overgaard, Nathan Dean, Tesfaye Zelleke, Tewodros Kebede, and Steven Weinstein
AUTHOR CONTRIBUTIONS
Iván Sánchez Fernández participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, acquisition of data, statistical analysis, and study supervision or coordination. Nicholas S. Abend participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, acquisition of data, and study supervision or coordination. Satish Agadi participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Sookee An participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Ravindra Arya participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. James Nicholas Brenton participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Jessica L. Carpenter participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Kevin E. Chapman participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. William D. Gaillard participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Tracy A. Glauser participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Howard P. Goodkin participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Kush Kapur participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Mohamad A. Mikati participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Katrina Peariso participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Margie Ream participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. James Riviello Jr. participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Robert C. Tasker participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, and acquisition of data. Tobias Loddenkemper participated in drafting and revising the manuscript for content, including medical writing for content, study concept and design, analysis and interpretation of data, acquisition of data, statistical analysis, and study supervision or coordination.
STUDY FUNDING
This study and consortium was funded by the Epilepsy Foundation of America (EF-213583, Targeted Initiative for Health Outcomes) and by the American Epilepsy Society/Epilepsy Foundation of America Infrastructure Award.
DISCLOSURE
I. Sánchez Fernández is funded by a grant for the study of epileptic encephalopathies from “Fundación Alfonso Martín Escudero” and by the HHV6 Foundation. N. Abend reports that he is an Epilepsia editorial board member, 2013–present, and Seizure editorial board member, 2012–present. He receives publishing royalties for Pediatric Neurocritical Care, Demos, 2013. He has grant funding from NIH (National Institute of Neurological Disorders and Stroke), K23NS076550, PI, 2011–present, and Epilepsy Foundation of America. S. Agadi and S. An report no disclosures relevant to the manuscript. R. Arya reports that he has research support from National Institute of Neurological Disorders and Stroke, NIH, 2UO1, NS045911 (PI: Tracy A. Glauser), 9/1/11–8/31/14, Impact of Initial Therapy And Response On Long Term Outcome In Children With CAE: coinvestigator (10% effort). J. Brenton, J. Carpenter, and K. Chapman report no disclosures relevant to the manuscript. W. Gaillard's income derives from clinical revenue generated for CNMC clinical care. Federal support provided by National Institute of Neurological Disorders and Stroke 1P30HD40677-01, 2K12NS052159-06A1 NIMH RO1 MH084961, 1R21MH092615, NSF 095998, CDC 1UO1DP003255, DOD/USAMRAA W81XWH-11-2-0198, and PICORE 527. Foundation support from Epilepsy Foundation of America, American Epilepsy Society, Infantile Epilepsy Research Foundation (Lundbeck), and CURE. The department conducts industry-supported trials from which no salary support is derived including Ovation Pharmaceuticals, King Pharmaceuticals, and PRA International/Eisai. Stock (held with spouse): Johnson & Johnson, Lily, GlaxoSmithKline, Pfizer, Siemens, and General Electric. Editorial board of Epilepsia and Epilepsy Research. T. Glauser and H. Goodkin report no disclosures relevant to the manuscript. K. Kapur has received support for statistical work on the grants from the NIH, National Institute of Neurological Disorders and Stroke, Guthy-Jackson Charitable Foundation, International Rett Syndrome Foundation, Simons Foundation, Nancy Lurie Marks Foundation, and Lundbeck and Danny Did Foundation. M. Mikati, K. Peariso, M. Ream, J. Riviello Jr., and R. Tasker report no disclosures relevant to the manuscript. T. Loddenkemper serves on the Laboratory Accreditation Board for Long Term (Epilepsy and Intensive Care Unit) Monitoring, on the Council of the American Clinical Neurophysiology Society, on the American Board of Clinical Neurophysiology, as an Associate Editor for Seizure, as Contributing Editor for Epilepsy Currents, and as an Associate Editor for Wyllie's Treatment of Epilepsy, 6th edition. He is part of pending patent applications to detect seizures and to diagnose epilepsy. He receives research support from the American Epilepsy Society, the Epilepsy Foundation of America, the Epilepsy Therapy Project, PCORI, the Pediatric Epilepsy Research Foundation, Cure, Danny-Did Foundation, HHV-6 Foundation, Lundbeck, Eisai, and Upsher-Smith. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Loddenkemper T, Goodkin HP. Treatment of pediatric status epilepticus. Curr Treat Options Neurol 2011;13:560–573. [DOI] [PubMed] [Google Scholar]
- 2.Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet 2006;368:222–229. [DOI] [PubMed] [Google Scholar]
- 3.DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46:1029–1035. [DOI] [PubMed] [Google Scholar]
- 4.Loddenkemper T, Syed TU, Ramgopal S, et al. Risk factors associated with death in in-hospital pediatric convulsive status epilepticus. PLoS One 2012;7:e47474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maytal J, Shinnar S, Moshe SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics 1989;83:323–331. [PubMed] [Google Scholar]
- 6.Singh RK, Stephens S, Berl MM, et al. Prospective study of new-onset seizures presenting as status epilepticus in childhood. Neurology 2010;74:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YW, Shek DW, Garcia PA, Zhao S, Johnston SC. Incidence and mortality of generalized convulsive status epilepticus in California. Neurology 2002;58:1070–1076. [DOI] [PubMed] [Google Scholar]
- 8.Martinos MM, Yoong M, Patil S, et al. Early developmental outcomes in children following convulsive status epilepticus: a longitudinal study. Epilepsia 2013;54:1012–1019. [DOI] [PubMed] [Google Scholar]
- 9.Raspall-Chaure M, Chin RF, Neville BG, Scott RC. Outcome of paediatric convulsive status epilepticus: a systematic review. Lancet Neurol 2006;5:769–779. [DOI] [PubMed] [Google Scholar]
- 10.Roy H, Lippe S, Lussier F, et al. Developmental outcome after a single episode of status epilepticus. Epilepsy Behav 2011;21:430–436. [DOI] [PubMed] [Google Scholar]
- 11.Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 2002;59:205–210. [DOI] [PubMed] [Google Scholar]
- 12.Sahin M, Menache CC, Holmes GL, Riviello JJ. Outcome of severe refractory status epilepticus in children. Epilepsia 2001;42:1461–1467. [DOI] [PubMed] [Google Scholar]
- 13.Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long-term mortality after a first episode of status epilepticus. Neurology 2002;58:537–541. [DOI] [PubMed] [Google Scholar]
- 14.Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 15.Riviello JJ, Jr, Claassen J, LaRoche SM, et al. Treatment of status epilepticus: an international survey of experts. Neurocrit Care 2013;18:193–200. [DOI] [PubMed] [Google Scholar]
- 16.Wilkes R, Tasker RC. Pediatric intensive care treatment of uncontrolled status epilepticus. Crit Care Clin 2013;29:239–257. [DOI] [PubMed] [Google Scholar]
- 17.Alldredge BK, Wall DB, Ferriero DM. Effect of prehospital treatment on the outcome of status epilepticus in children. Pediatr Neurol 1995;12:213–216. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson K, Metsaranta P, Huhtala H, Auvinen A, Kuusela AL, Koivikko M. Treatment delay and the risk of prolonged status epilepticus. Neurology 2005;65:1316–1318. [DOI] [PubMed] [Google Scholar]
- 19.Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci 2005;25:5511–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005;25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez Fernández I, Abend NS, Agadi S, et al. Gaps and opportunities in refractory status epilepticus research in children: a multi-center approach by the Pediatric Status Epilepticus Research Group (pSERG). Seizure 2014;23:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capovilla G, Beccaria F, Beghi E, Minicucci F, Sartori S, Vecchi M. Treatment of convulsive status epilepticus in childhood: recommendations of the Italian League against Epilepsy. Epilepsia 2013;54(suppl 7):23–34. [DOI] [PubMed] [Google Scholar]
- 23.Epilepsy Foundation of America's working group on status epilepticus. Treatment of convulsive status epilepticus: recommendations of the Epilepsy Foundation of America's Working Group on Status Epilepticus. JAMA 1993;270:854–859. [PubMed] [Google Scholar]
- 24.Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology 1990;40:13–23. [PubMed] [Google Scholar]
- 25.Chin RF, Neville BG, Peckham C, Wade A, Bedford H, Scott RC. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol 2008;7:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellock JM, Marmarou A, DeLorenzo R. Time to treatment in prolonged seizure episodes. Epilepsy Behav 2004;5:192–196. [DOI] [PubMed] [Google Scholar]
- 27.Lewena S, Pennington V, Acworth J, et al. Emergency management of pediatric convulsive status epilepticus: a multicenter study of 542 patients. Pediatr Emerg Care 2009;25:83–87. [DOI] [PubMed] [Google Scholar]
- 28.Seinfeld S, Shinnar S, Sun S, et al. Emergency management of febrile status epilepticus: results of the FEBSTAT study. Epilepsia 2014;55:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 2001;345:631–637. [DOI] [PubMed] [Google Scholar]
- 30.Aranda A, Foucart G, Ducasse JL, Grolleau S, McGonigal A, Valton L. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia 2010;51:2159–2167. [DOI] [PubMed] [Google Scholar]
- 31.Jordan KG. Status epilepticus: a perspective from the neuroscience intensive care unit. Neurosurg Clin N Am 1994;5:671–686. [PubMed] [Google Scholar]
- 32.Kämppi L, Mustonen H, Soinila S. Analysis of the delay components in the treatment of status epilepticus. Neurocrit Care 2013;19:10–18. [DOI] [PubMed] [Google Scholar]
- 33.Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC. Out-of-hospital treatment for convulsive status epilepticus (CSE) in childhood. Presented at the annual meeting of the American Epilepsy Society; 2004: 89.
- 34.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 2012;366:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


