Fingolimod (FTY720, Gilenya, Novartis Pharma AG, Basel, Switzerland) is a sphingosin-1-phosphate (S1P) receptor modulator with immunomodulatory properties that traps naive and central memory T cells in lymph nodes, leading to reduced numbers of peripheral blood lymphocytes, and is the first licensed oral drug for multiple sclerosis (MS).1,2 We report a case of a near fatal herpes simplex virus 1 (HSV-1) encephalitis under the licensed dose of fingolimod in a patient with MS.
Case report.
A 38-year-old man was diagnosed with relapsing-remitting MS in 2007. In 2007, he entered the TRANSFORMS phase 3 study comparing fingolimod (0.5 or 1.5 mg daily) with interferon-β-1a (Avonex, Biogen Idec, Cambridge, UK) and placebo. Since August 2008, he participated in the TRANSFORMS extension study and received fingolimod (1.25 or 0.5 mg), and from April 2010 he continued with 0.5 mg (Umbrella). In March 2013, he had minor deficits with an Expanded Disability Status Scale score (EDSS3) of 2.5 (which ranges from 0 to 10, with higher scores indicating greater disability and 10 = death from MS). From 2007–2013, the patient only had 1 MS relapse and showed EDSS worsening from 1.5 to 2.5.
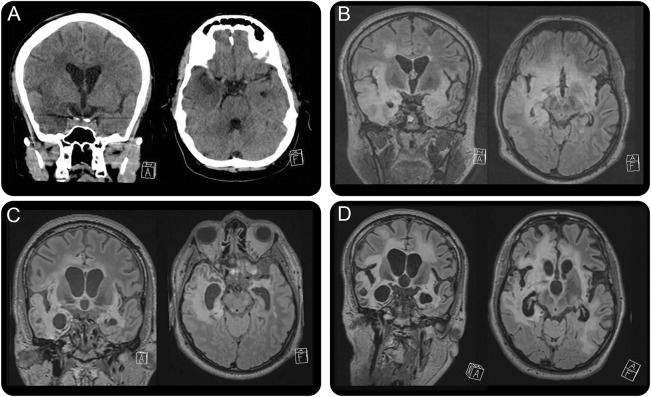
In April 2014, he was admitted to the emergency department with loss of consciousness, fever, and epileptic seizures. Cranial CT showed a hypodense area in the right temporal lobe (figure, A). Diagnosis of HSV-1 encephalitis was made by PCR from CSF showing 216.721 HSV-1 DNA copies/mL, 10 cells/µL, and total protein of 0.519 g/L. Blood count showed lymphopenia of 410 cells/µL. Anti-HSV-1 immunoglobulin G antibodies were already positive at presentation in serum and CSF, while anti-HSV-1 immunoglobulin M and ani-HSV-2 antibodies remained negative. The medical history mentioned a previous herpes labialis. Antiviral therapy with IV acyclovir was initiated immediately at the day of presentation at the standard dose. Cranial MRI showed signs of nonhemorrhagic encephalitis of archecortical areas of both hemispheres (figure, B).
Figure. Neuroimaging.
(A) Cranial CT scan at the day of admission (coronal slices left, axial slices right) shows a hypodense area of the right temporal lobe. (B) MRI scan of the brain (coronal slices left, axial slices right; fluid-attenuated inversion recovery [FLAIR] sequences are depicted) shows hypodense areas of archecortical structures and the right temporal lobe. The follow-up MRI scans at 4 months (C) and 9 months (D) show progressive and widespread leukoencephalopathy. Depicted are coronal (left) and axial (right) slices of FLAIR sequences. The positioning of the head in the scanner differs, partially related to agitation of the patient during the examination. All scans were performed and provided by the Department of Neuroradiology, University Hospital Zurich, Switzerland.
After 34 days, the patient was referred to a neurologic rehabilitation center. At discharge, he was alert, showed signs of right dominant tetraparesis, and was unable to speak due to a tracheal cannula, resulting in an EDSS of 9.5, indicating severe disability. Follow-up examinations in the next 9 months showed clinical worsening and progressive brain atrophy accentuated in the postencephalitic regions (figure, C and D).
Discussion.
Despite its potent immunomodulatory effects leading to reduced circulating lymphocyte counts, fingolimod did not lead to increased rates of severe infection in clinical phase 3 studies.1,2,4 Only immunity against herpesviruses with CNS latency, especially varicella-zoster virus (VZV) and HSV, shows subtle impairments. This has been shown by 2 patients who died from fatal infections during 1.25 mg fingolimod and additional course of corticosteroids for MS relapse treatment in 1 phase 3 study.2 Furthermore, a second phase 3 study showed an increased rate of VZV infections,4 whereas no increase in herpesvirus infections was seen in a third phase 3 study.1 Our patient had not received corticosteroids or other immunomodulatory treatments apart from fingolimod; the only comedication was bupropion (Wellbutrin, GlaxoSmithKline AG, Münchenbuchsee, Switzerland) and occasionally methylphenidate (Ritalin, Novartis Pharma AG, Basel, Switzerland). Prior to onset of HSV-1 encephalitis, he had not shown any evidence of immunologically relevant comorbidities during continuous follow-up in our MS outpatient clinic since 2007. Besides these rare and fatal cases, a recent study reported that fingolimod treatment of MS lowers VZV-specific immunity and suggested that subclinical VZV reactivation, demonstrated by PCR detection of VZV DNA in the saliva, is higher among patients treated with fingolimod compared with healthy controls.5
Different from other immunomodulatory drugs that can compromise specific aspects of immune control, fingolimod treatment may lead to a subtle, but in some cases clinically relevant compromise of immune responses against herpesviruses that stay in their latent stages in the nervous system compartment, i.e., VZV and HSV-1, while immune responses against those that reside in immune organs (cytomegalovirus and Epstein-Barr virus) appear unaffected. In order to try to avoid serious adverse events, it will be important to understand these interactions better.
By reporting this case of HSV-1 encephalitis in a patient taking 0.5 mg fingolimod, we want to stress the importance of further studies on how fingolimod impairs immunity against HSV-1 and VZV to prevent such severe complications in the future.
Footnotes
Author contributions: Dr. Pfender, Dr. Jelcic, Dr. Linnebank, Dr. Schwarz, and Dr. Martin contributed in clinical management of the patient and writing of the manuscript.
Study funding: No targeted funding reported.
Disclosure: N. Pfender reports no disclosures relevant to the manuscript. I. Jelcic has received speaker honoraria or unrestricted grants from Biogen Idec and Novartis. M. Linnebank has received grants, honoraria, or funding from Bayer, Biogen Idec, Genzyme, Merck, Novartis, and Teva. U. Schwarz reports no disclosures relevant to the manuscript. R. Martin has served as advisor or member of speaker's boards for Biogen Idec, Novartis, and Genzyme. The group of R. Martin has received unrestricted grants from Biogen Idec and Novartis. Go to Neurology.org for full disclosures.
References
- 1.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–415. [DOI] [PubMed] [Google Scholar]
- 3.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 4.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:545–556. [DOI] [PubMed] [Google Scholar]
- 5.Ricklin ME, Lorscheider J, Waschbisch A, et al. T-cell response against varicella-zoster virus in fingolimod-treated MS patients. Neurology 2013;81:174–181. [DOI] [PubMed] [Google Scholar]