Abstract
Objective:
To study the efficacy and safety of low-dose oral tetrahydrocannabinol (THC) in the treatment of dementia-related neuropsychiatric symptoms (NPS).
Methods:
This is a randomized, double-blind, placebo-controlled study. Patients with dementia and clinically relevant NPS were randomly assigned to receive THC 1.5 mg or matched placebo (1:1) 3 times daily for 3 weeks. Primary outcome was change in Neuropsychiatric Inventory (NPI), assessed at baseline and after 14 and 21 days. Analyses were based on intention-to-treat.
Results:
Twenty-four patients received THC and 26 received placebo. NPS were reduced during both treatment conditions. The difference in reduction from baseline between THC and placebo was not significant (mean difference NPItotal: 3.2, 95% confidence interval [CI] −3.6 to 10.0), nor were changes in scores for agitation (Cohen-Mansfield Agitation Inventory 4.6, 95% CI −3.0 to 12.2), quality of life (Quality of Life–Alzheimer's Disease −0.5, 95% CI −2.6 to 1.6), or activities of daily living (Barthel Index 0.6, 95% CI −0.8 to 1.9). The number of patients experiencing mild or moderate adverse events was similar (THC, n = 16; placebo, n = 14, p = 0.36). No effects on vital signs, weight, or episodic memory were observed.
Conclusions:
Oral THC of 4.5 mg daily showed no benefit in NPS, but was well-tolerated, which adds valuable knowledge to the scarce evidence on THC in dementia. The benign adverse event profile of this dosage allows study of whether higher doses are efficacious and equally well-tolerated.
Classification of evidence:
This study provides Class I evidence that for patients with dementia-related NPS, low-dose THC does not significantly reduce NPS at 21 days, though it is well-tolerated.
Most patients with dementia will experience neuropsychiatric symptoms (NPS) over the course of their disease.1 While nonpharmacologic interventions are preferred, data on their efficacy remains limited and the interventions are not easily applicable in clinical practice.2 Pharmacologic treatment is challenging, as currently available medications have important drawbacks concerning the benefit-to-risk ratio.3–6 This implicates a serious health care problem, as 62% of community-dwelling patients and up to 80% of nursing home residents have clinically relevant symptoms.7,8 Structured analgesic treatment has recently been demonstrated to be beneficial for dementia-related NPS and in particular agitation.9 Δ-9-Tetrahydrocannabinol (THC), the main constituent of cannabis, has both psychoactive and analgesic properties,10,11 and might therefore serve as an alternative pharmacologic treatment. Indeed, some preliminary studies suggested improvement in agitation and nocturnal motor activity in patients with Alzheimer disease (AD).12,13 The effect of THC on the endocannabinoid system is mediated by 2 cannabinoid receptors: CB1 receptors are expressed in several brain regions, especially the basal ganglia, cerebellum, hippocampus, amygdala, and hypothalamus; CB2 receptors are primarily found in cells and organs of the immune system. Therefore, THC probably has a wide range of CB1-mediated receptor interactions with the endocannabinoid system affecting emotion, cognition, and behavior. Moreover, psychotropic effects are also exerted through interaction with other receptors and neurotransmitters, such as acetylcholine, dopamine, serotonin, γ-aminobutyric acid, glutamate, norepinephrine, prostaglandins, and opioid peptides.14 Interestingly, several animal studies also suggest a neuroprotective effect of cannabinoids in the disease pathology of AD itself, which is primarily based on a reduction in the inflammatory response by microglia cells and the increase of amyloid-β clearance.15,16 Nonetheless, firm evidence of the efficacy and safety of THC or other cannabinoids in this vulnerable patient group is lacking and data on older patients in general are scarce.17 The current article reports the largest study carried out so far on evaluating the efficacy and safety of oral THC for behavioral disturbances in patients with dementia.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice, approved by a certified ethics committee of the Radboud university medical center (Radboudumc) and registered at www.clinicaltrials.org (NCT01608217). Assessments were done by researchers from the Department of Geriatric Medicine of Radboudumc (Nijmegen, the Netherlands) and the Department of Elderly of Vincent van Gogh Institute (psychiatric hospital, Venray, the Netherlands) from November 2012 to June 2014. Participants were recruited from 9 participating institutes throughout the southeast of the Netherlands, including geriatric outpatient clinics (n = 2 clinics), psychiatric clinics (n = 3), nursing homes (n = 3, including in total 6 locations), and a regional network of integrated care for community-dwelling patients with dementia. Written informed consent was provided at screening by the patient and closest involved proxy, the first only in case the patient was judged capable of consent.
Study design.
This was a randomized, double-blind, placebo-controlled, multicenter, phase II trial. Potential participants were screened for eligibility within 4 weeks prior to start of study medication, by assessment of somatic and cognitive status and severity of behavioral disturbances. Assessments were done at the outpatient clinic, nursing home, or at home, depending on patient preference. Study intervention was initiated after baseline. Efficacy assessments were scheduled after 14 ± 2 treatment days (phone call) and 21 ± 2 treatment days (visit). For the purpose of safety assessment and compliance, several phone calls were performed by the researchers during the intervention period (days 2, 7, and 14). Follow-up assessments by telephone were performed 2 weeks after study completion.
Participants.
Patients diagnosed with AD or vascular or mixed dementia according to National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association18 or National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche en l'Enseignement en Neurosciences19 criteria were eligible for participation if they had clinically relevant NPS (minimal Neuropsychiatric Inventory [NPI] score ≥10), with symptoms reported on agitation, aggression, or aberrant motor behavior, existing at least 1 month prior to screening. A caregiver had to be available who was in touch with the patient at least twice a week and supervised the patient's care. Exclusion criteria were current major psychiatric disorders and any severe or instable concomitant illness, in particular seizures, arrhythmias necessitating treatment other than a β-blocker or digoxin, severe heart failure, or any concomitant disease necessitating treatment changes. Other exclusion criteria were frequent falling due to orthostatic hypotension, a history or current alcohol or drug abuse, and use of tricyclic antidepressants, fluoxetine, or carbamazepine. Use of concurrent psychotropic medication was allowed, provided that the dose and frequency were kept stable within 2 weeks before and during trial conduction. Analgesic drugs had to be stopped prior to baseline assessments, although use of analgesic and psychotropic escape medication was allowed.
Changes to study protocol.
We initially recruited patients with behavioral disturbances as well as persistent pain complaints to secondarily assess the efficacy of THC on pain in patients with dementia. However, the number of eligible patients with both symptoms was much lower than predicted from the literature.20 After inclusion of the first 8 patients, the criterion of pain was omitted. In the amended study, pain assessments were still included, allowing secondary evaluation of the efficacy of THC in reducing pain-related behavior and pain intensity in a subgroup of patients, of which the methods and results are described in appendix e-1 and table e-1 on the Neurology® Web site at Neurology.org.
Intervention and randomization.
Active treatment consisted of 1.5 mg THC in tablet form (Namisol, Echo Pharmaceuticals, Weesp, the Netherlands) 3 times daily for a period of 3 weeks. This daily dose was based on preliminary positive results of previous trials in patients with severe AD.12,13,21 Control treatment consisted of matched placebo tablets. Additionally, patients received 1,000 mg acetaminophen 3 times daily in case of pain complaints, or of suspected pain in noncommunicative patients, based on physical examination at screening and information from the caregiver or physician. Study medication was administered at 9 am, 2 pm, and 8 pm by the primary caregiver or nursing home staff. Study medication was packed and distributed by the pharmacy of Radboudumc according to Good Manufacturing Practice. Randomization (allocation ratio 1:1) was performed by an independent statistician using a computer-generated randomization program, of which the algorithm was stratified per center and minimized22 for NPI score, dementia severity, sex, and current opioid use. Treatment allocation was strictly concealed from participants, caregivers, investigators, and all other personnel directly involved in the study and was not made available until study completion and database lock.
Outcome measures.
Primary outcome measure.
The primary outcome was change in NPS, measured with NPI.23 This questionnaire evaluates 12 behavioral domains, including agitation/aggression and aberrant motor behavior, which were the behavioral domains of interest. The frequency and severity of NPS were scored per domain by questioning a caregiver, which resulted in a total score ranging from 0 to 144 (a higher score indicating greater impairment). NPI was assessed at baseline, day 14 (by telephone interview), and day 21 by trained researchers.
Secondary efficacy outcome measures.
Secondary outcomes included assessment of agitated behavior and aggression (Cohen-Mansfield Agitation Inventory [CMAI]24), activities of daily living (Barthel Index25), and quality of life (Quality of Life–Alzheimer's Disease Scale [QoL-AD]26). These were all assessed at baseline and day 21. Overall change was assessed by the primary caregiver, using the Caregiver Clinical Global Impression of Change (CCGIC), a 7-point scale ranging from marked improvement to marked worsening from baseline.
Safety assessments.
Adverse events.
Adverse events (AEs) were solicited from patients and their caregivers at all visits and phone calls up to 2 weeks after study drug discontinuation, using clinical observation, open questions, and a set of questions on possible THC-related adverse symptoms, including the most frequently reported AEs in the phase I study with healthy elderly.27 AEs were coded following the classification of Medical Dictionary for Regulatory Activities. An AE was defined as serious if it was fatal or life-threatening, required or prolonged hospitalization, or resulted in persistent or significant disability or incapacity.
Other safety assessments.
Other safety assessments consisted of evaluation of blood pressure, heart rate, and weight, assessed at screening, baseline, and day 21, and ECG and biochemistry and hematology blood samples, assessed at screening and day 21. The Paired Associate Learning Wechsler Memory Scale–Revised (PAL WMS-R)28 was used for assessment of possible effects of THC on episodic memory function (baseline and day 21).
Statistical analysis.
The study sample size was estimated based on a clinically relevant difference of 4 points on NPI,29,30 a SD of 12 points,31,32 and an estimated correlation with baseline of 0.6 and interclass correlation coefficient of 0.6. Approximately 130 patients were required for a power of 80% (2-sided testing at 0.05). We were not able to enroll this number of subjects within the available time period, due to delay in getting formal approval for THC use at all sites from the Health Care Inspectorate. After trial ending, we performed an analysis to calculate the power to yield a statistically significant difference in favor of THC, in case we would have been able to extend the study to 130 subjects. This analysis is known as the calculation of conditional power. The analysis used 10,000 simulated extensions of the outcome data of the realized sample to the planned sample size, based on the real data that were acquired. Efficacy and safety analyses were based on the intention-to-treat principle and performed in accordance with a prespecified statistical analysis plan, finalized before unmasking of treatment assignment. The primary endpoint, mean difference (including 95% confidence interval [CI]) in NPI total score from baseline to 14 and 21 treatment days, was evaluated in a linear mixed model with participants as random factor and treatment, center, baseline NPI, Clinical Dementia Rating score, sex, current opioid use, and time as fixed factors. All assumptions for regression models were assessed by viewing plots of the residual values to check for linearity and homoscedasticity. Analysis was repeated for all NPI subdomain scores. In a post hoc analysis, we determined the efficacy for 2 subgroups: ambulatory patients and inpatients. Other secondary efficacy outcome measures, weight, and vital signs were assessed similarly to the primary analysis (without data on day 14, as these were not collected). Pearson correlation coefficients were calculated for change from baseline of NPI and CCGIC scores on day 14 and day 21. Due to the limited number of participants included in the PAL WMS-R assessments group, these differences were compared using Mann-Whitney U test. For analysis of AEs, the number of patients with at least 1 unique episode was tabulated per treatment group and group difference on incidence (using χ2) and severity of AEs (using Mann-Whitney U) was analyzed. Statistical analyses were done using SAS version 9.2 and SPSS version 20 for Windows.
Classification of evidence.
This interventional study provides Class I evidence that oral THC of 4.5 mg daily is not effective in reducing behavioral disturbances in patients with dementia (ΔNPItotal: 3.2, 95% CI −3.6 to 10.0) and is well-tolerated (occurrence of AEs THC vs placebo: 16 [66.7%] vs 14 [523.8%] patients, χ2, p = 0.36).
RESULTS
Study participants.
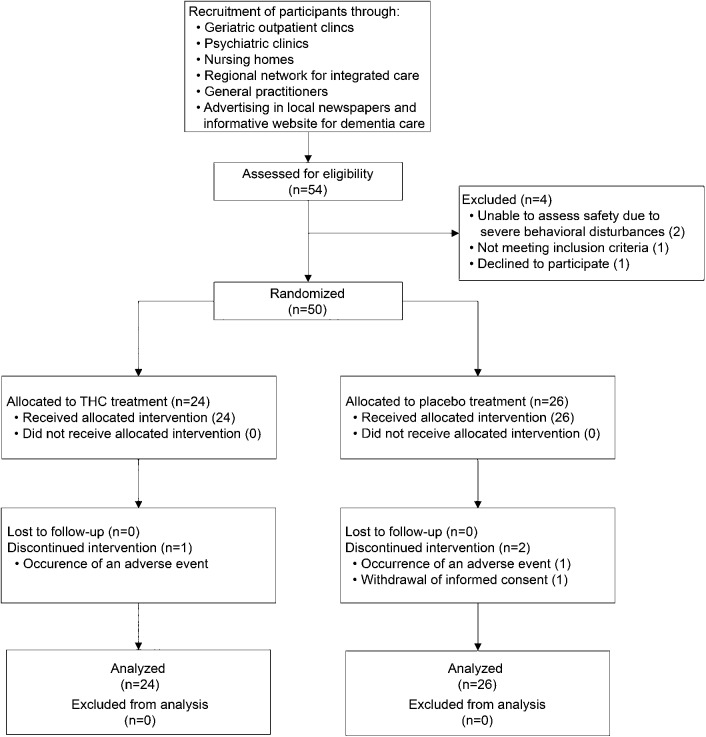
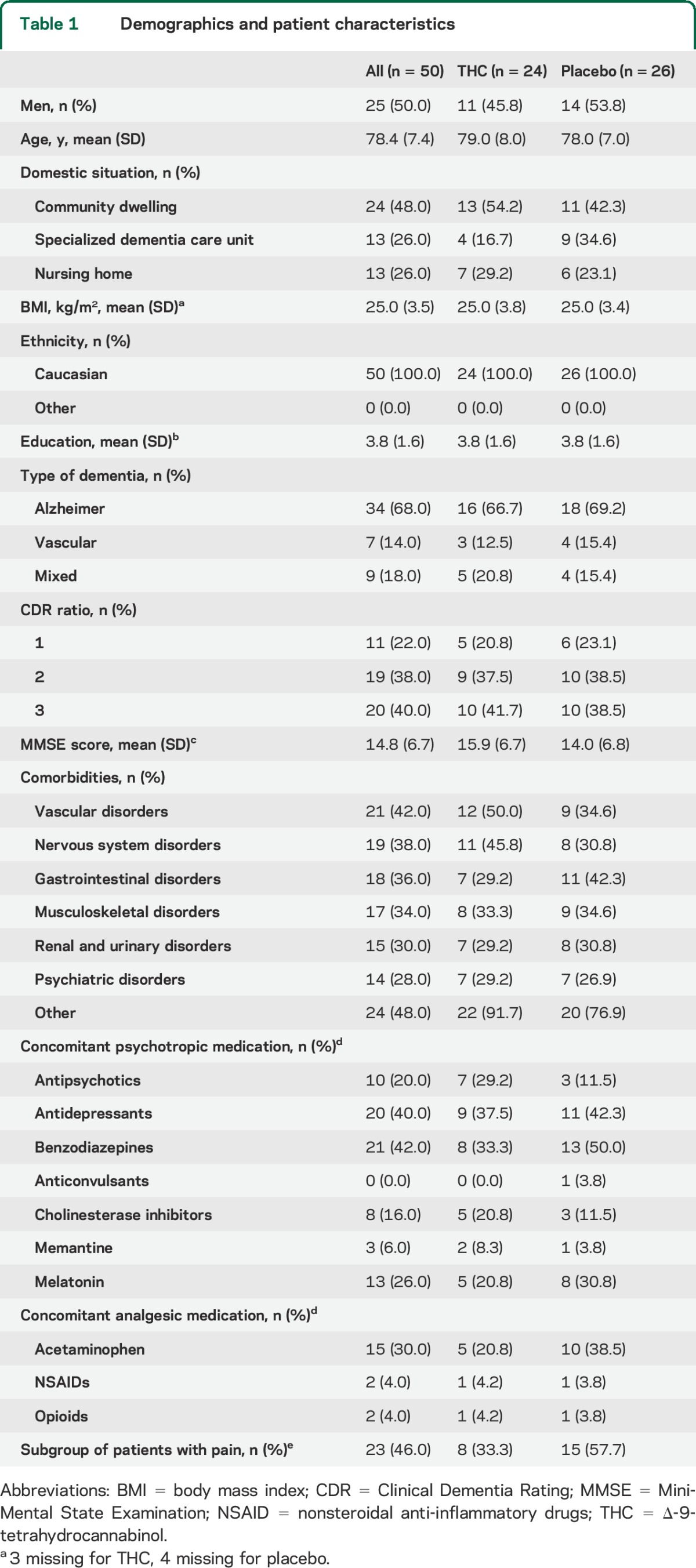
In total, 54 patients were assessed for eligibility, of whom 50 were randomized and received study medication (THC, n = 24; placebo, n = 26) (figure). Patient characteristics are presented in table 1. Overall, 47 patients (94%) completed the study, while 3 patients discontinued participation due to the occurrence of AEs (n = 2) and withdrawal of informed consent (n = 1).
Figure. CONSORT flowchart of recruitment and selection.
THC = tetrahydrocannabinol.
Table 1.
Demographics and patient characteristics

Treatment compliance and concurrent medication use.
Median treatment compliance, based on remaining pill count, was 98% (67%–100%) in the THC group and 100% (94%–100%) in the placebo group. Twenty-nine patients received acetaminophen (THC, n = 13; placebo, n = 16). Four patients (16.7%) in the THC group received escape medication, compared to 2 patients (7.7%, p = 0.33) in the placebo group, which consisted of benzodiazepines (oxazepam 5 mg, lorazepam 1 mg) and acetaminophen (500 mg).
Efficacy.
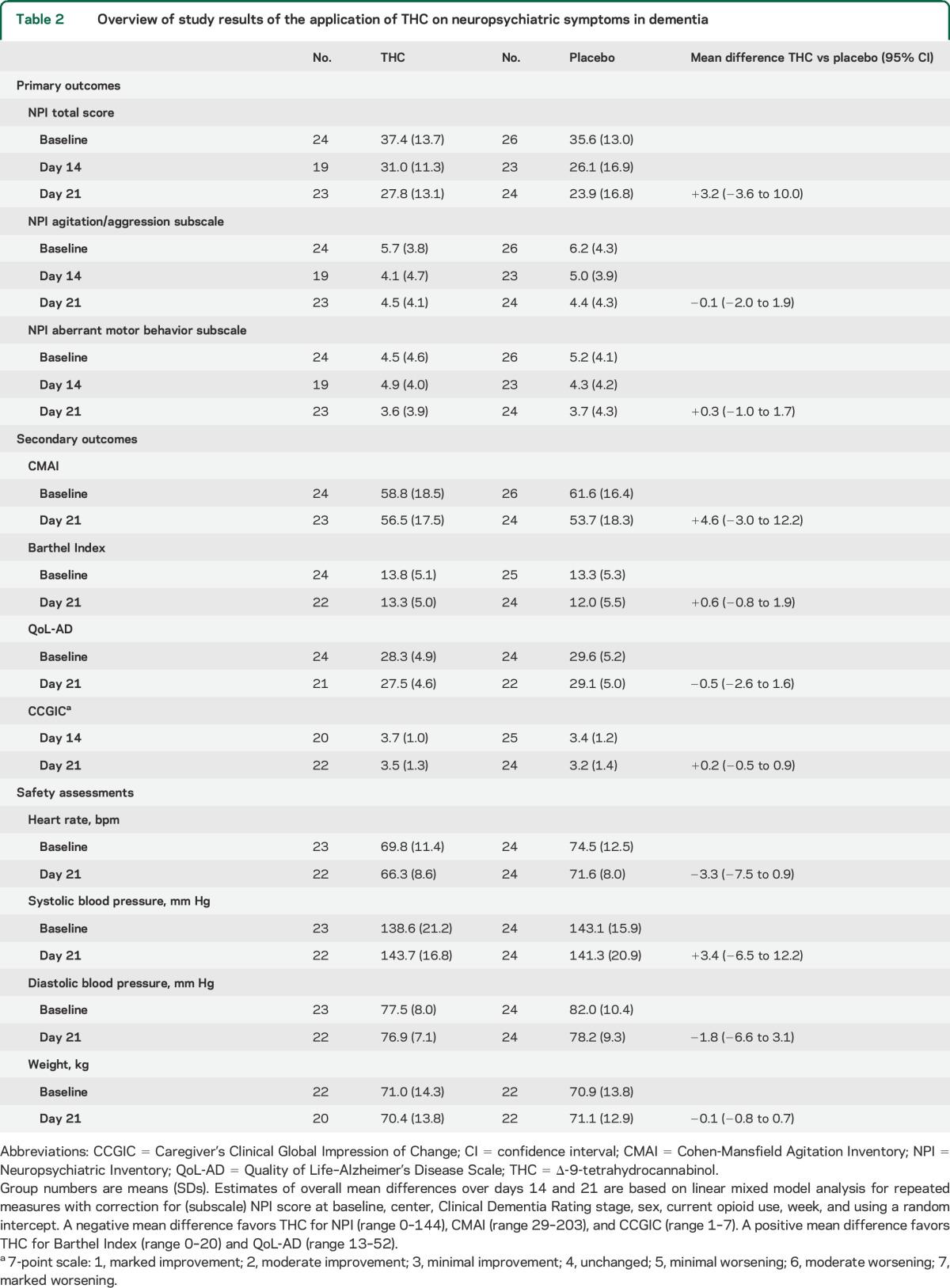
Study results are presented in table 2. NPI total score decreased in both treatment conditions after 14 days (THC, p = 0.002; placebo, p = 0.002) and 21 days (THC, p = 0.003; placebo, p = 0.001). There was no difference between THC and placebo over 21 treatment days (ΔNPItotal: 3.2, 95% CI −3.6 to 10.0). Additionally, no differences were observed on agitation (ΔNPIagitation: −0.1, 95% CI −2.0 to 1.9), aberrant motor behavior (ΔNPIaberrant motor behavior: 0.3, 95% CI −1.0 to 1.7), or other NPI subdomains (see table e-2), except for the domain “eating disorders” in favor of placebo (ΔNPIeating disorders: 1.0, 95% CI 0.0–1.92). Analysis per subgroup showed no benefit of THC in community-dwelling patients (ΔNPItotal: 5.0, 95% CI −1.8 to 11.7) or in inpatients (ΔNPItotal: 1.5, 95% CI −10.0 to 13.1). There were no significant differences between the intervention groups on CMAI, QoL-AD, and Barthel Index. CCGIC scores after 3 weeks showed that 8 (36.4%) patients in the THC group had minimal to marked improvement from baseline, which was not significantly different from 12 patients (50.0%) in the placebo group (χ2, p = 0.35). A strong correlation was observed between NPI and CCGIC scores (day 14: Pearson r = 0.65, p < 0.001; day 21: Pearson r = 0.73, p < 0.01). The conditional power to still detect a difference in NPI score of at least 4 points in favor of THC treatment, in case we would have been able to extend the trial from the actual number of subjects (n = 47, 23 on THC and 24 on placebo) to the initially planned number of subjects (130, 65 per treatment arm), was 5%.
Table 2.
Overview of study results of the application of THC on neuropsychiatric symptoms in dementia
Safety.
AEs.
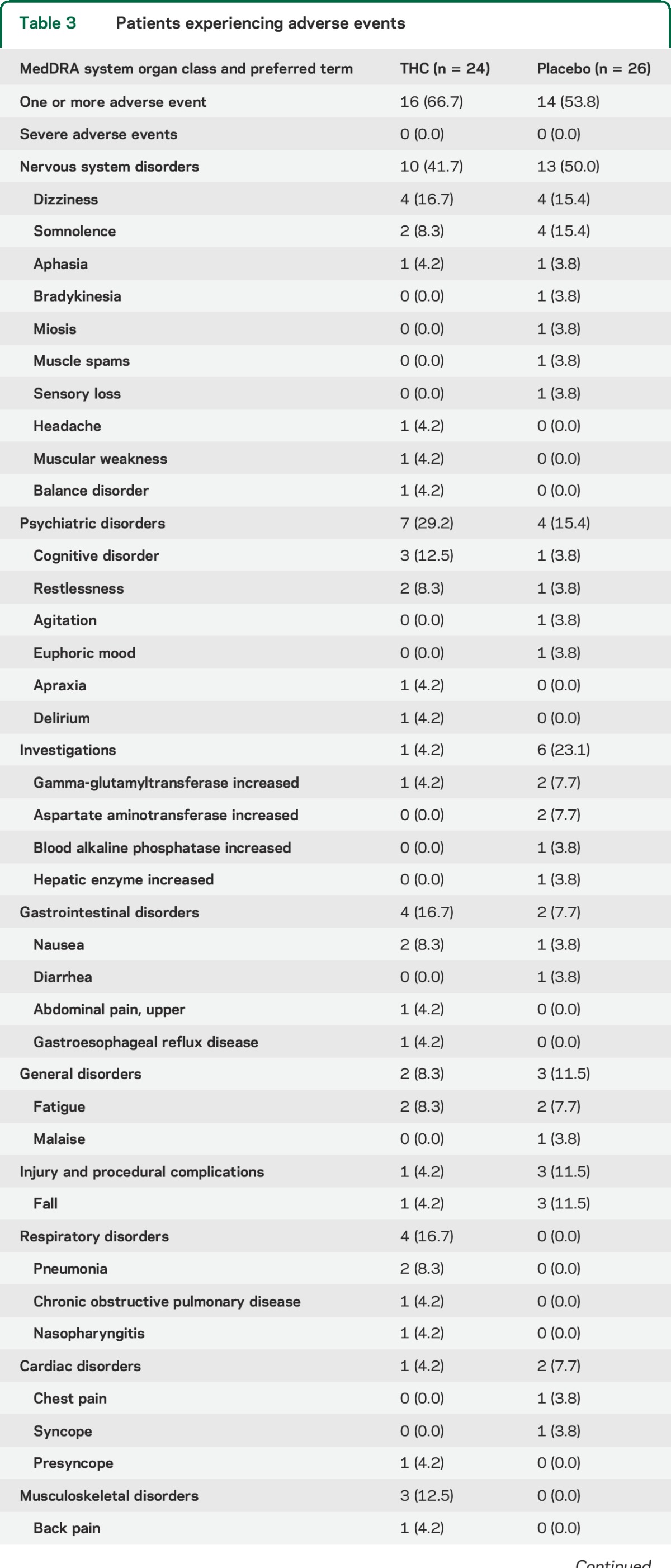
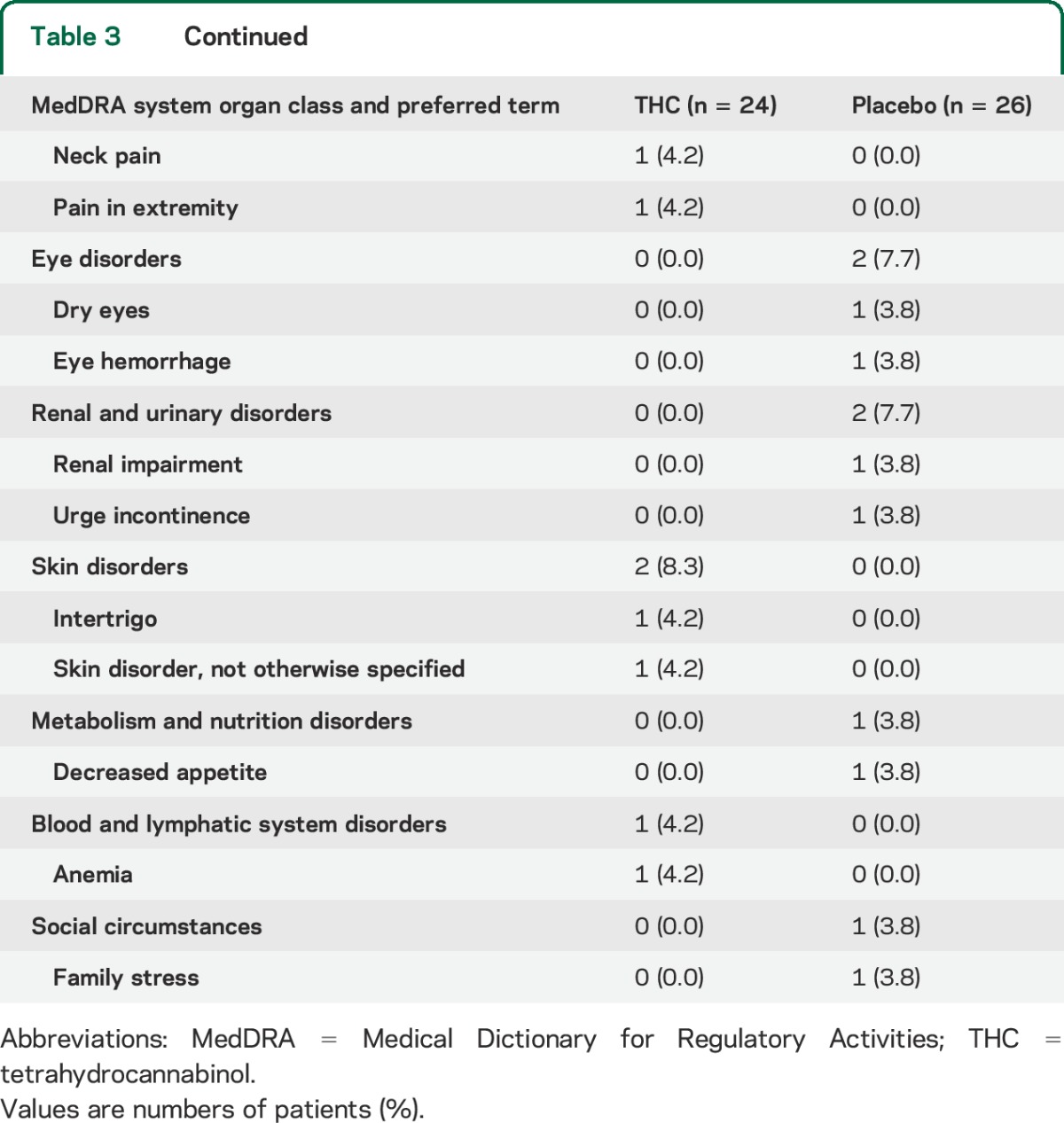
The occurrence of AEs was similarly divided along treatment groups (table 3). In the THC group, 16 patients (66.7%) experienced at least 1 AE, compared to 14 (53.8%) in the placebo group (χ2, p = 0.36). Two patients dropped out due to the occurrence of AEs; one patient developed pneumonia within 2 days after initiation of THC treatment, and one patient experienced persistent nausea on placebo. One serious AE occurred during placebo treatment, which was not related to study medication. This patient was admitted to a specialized dementia care unit due to high caregiver burden.
Table 3.
Patients experiencing adverse events
Other safety outcomes.
There were no changes between the groups concerning heart rate, blood pressure, and weight (table 2). Episodic memory scores were available for 18 patients with a mild dementia severity. PAL WMS-R scores decreased by 1.2 points in the THC group and 1.4 points in the placebo group, which was not significantly different (p = 1.0).
DISCUSSION
We found no benefit of 4.5 mg oral THC daily on behavioral disturbances in patients with dementia after 3 weeks of treatment. Additionally, there were no benefits for THC on quality of life, activities of daily living, or pain-related behavior and pain intensity (appendix e-1), while THC was safe and well-tolerated. The number of patients experiencing AEs was similar in both groups, while known THC-mediated AEs, such as dizziness, somnolence, and falls, were more frequently reported during placebo treatment. None of the participants reported a feeling “high,” nor was behaving “high” observed by caregivers or research staff. The current trial is the largest randomized controlled trial (RCT) so far studying oral THC in NPS in dementia, with valid and rigorous trial methods. The study sample was representative for the overall dementia population, in terms of age, dementia severity, and domestic situation. Patients with severe aggressive behavior could not be included, as the study's safety assessments cannot be adequately conducted in this group. Taking into account this limitation associated with this specific patient population, we have included a sample that is representative for the majority of the target population with clinically relevant NPS; the level of behavioral disturbances, assessed by NPI, was moderate and comparable to previous intervention trials.33–35 We observed an improvement in NPS in both groups over the duration of the study period, which has been reported before.34,35 The substantial degree of improvement in the placebo group is striking (table 2), and may be due to many factors including attention and support by the study team, expectations of patients and caregivers concerning THC, and training of nursing home personnel (together called the Hawthorne or in-study effect36). To correct for this substantial placebo response within individual patients, it might be worthwhile to implement an individually randomized crossover design in future studies. Despite the fact that we studied a vulnerable patient population, the attrition level was low (6%) and adherence high (98%–100%). This suggests a highly motivated group of participants and caregivers, in combination with the occurrence of only mild AEs. This study has some limitations. Most importantly, we failed to enroll the planned number of patients, despite comprehensive recruitment efforts throughout various health care settings. Rigorous national regulations on medical cannabinoids hindered implementation of the study in the participating clinics. Additionally, fewer than expected patients visiting the clinics had clinically relevant NPS as well as pain. Omitting the latter inclusion criterion significantly stimulated the recruitment. Despite this underenrollment, the conditional power of 5% emphasizes that it was very unlikely that exposure of more participants to the study interventions and assessments would have influenced our conclusion. Contrary to the current RCT, previous studies all reported positive effects of oral THC (2.5–7 mg daily) in patients with dementia.12,13,21,37 However, important methodologic factors significantly limit the robustness of these findings: inclusion of small number of patients (n = 2 and n = 15) and uncontrolled or retrospective study designs. In a previous randomized trial, we studied dosages up to 3 mg THC daily, and did not observe a significant reduction in NPS, nor any relevant AEs or effects on vital functions or mobility (unpublished data, 2014). Therefore, we used a dosage of 4.5 mg THC daily in this study.
Recent developments regarding the extended legalization of marijuana for medical purposes in over 30 US states has stimulated the discussion of the therapeutic potential and safety profile of cannabinoids for various indications.38,39 Momentarily, effective and safe treatments for NPS in patients with dementia are lacking.40 Several pharmacotherapeutic options have been explored, such as acetylcholinesterase inhibitors and antidepressants,33,34 but they often have a suboptimal benefit-risk profile. For example, while high-dose citalopram appears to effectively reduce agitation and overall behavioral disturbances, significant cardiac AEs limit its usefulness in this vulnerable population.34 Our current trial indicates that 4.5 mg THC daily can be safely administered to patients with dementia. The observation that there was no biological signal of AEs suggests that the dosage was too low, as a psychoactive drug is rarely effective without showing any side effects. Therefore, our results warrant further research using higher dosages of THC in the treatment of dementia-related NPS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the staff of the Department of Geriatric Medicine, Jeroen Bosch Ziekenhuis, 's Hertogenbosch; Vitalis WoonZorg Groep, Eindhoven; Liemerije, Zevenaar; Stichting LuciVer, Wijchen; Department of Elderly Care, GGNet, Apeldoorn; GGZ Breburg, Tilburg; and Hulp bij Dementie, Venray, for the recruitment of potential participants; the nursing home staff of Vincent van Gogh Institute, Liemerije, Vitalis WoonZorg Groep, and Stichting LuciVer; and their research assistants for their assistance in outcome assessments.
GLOSSARY
- AD
Alzheimer disease
- AE
adverse event
- CCGIC
Caregiver Clinical Global Impression of Change
- CI
confidence interval
- CMAI
Cohen-Mansfield Agitation Inventory
- NPI
Neuropsychiatric Inventory
- NPS
neuropsychiatric symptoms
- PAL WMS-R
Paired Associate Learning Wechsler Memory Scale–Revised
- QoL-AD
Quality of Life–Alzheimer's Disease Scale
- RCT
randomized controlled trial
- THC
Δ-9-tetrahydrocannabinol
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Geke A.H. van den Elsen: study concept and design, acquisition of data, analysis and interpretation of data, preparation of the manuscript, manuscript guarantor. Amir I.A. Ahmed: study concept and design, acquisition of data, critical review of the manuscript. Robbert-Jan Verkes: study concept and design, critical review of the manuscript. Cees Kramers: study concept and design, critical review of the manuscript. Ton Feuth: analysis and interpretation of data, critical review of the manuscript. Paul B. Rosenberg: interpretation of data, critical review of the manuscript. Marjolein A. van der Marck: study concept and design, study supervision, interpretation of data, critical review of the manuscript, manuscript guarantor. Marcel M.G. Olde Rikkert: study concept and design, study supervision, interpretation of data, critical review of the manuscript, manuscript guarantor.
STUDY FUNDING
Funded by the European Regional Development Fund and the Province of Gelderland. The Investigation Medical Product was provided by Echo Pharmaceuticals, Weesp, the Netherlands, which did not provide financial support for the study and had no role in study design, collection, analysis or interpretation of data, or writing the report.
DISCLOSURE
G. van den Elsen was supported by a grant from the European Regional Development Fund for the conduct of the study. A. Ahmed, R. Verkes, and C. Kramers report no disclosures relevant to the manuscript. T. Feuth was supported by a grant from the European Regional Development Fund for the conduct of the study. P. Rosenberg reports no disclosures relevant to the manuscript. M. van der Marck was supported by a grant from the European Regional Development Fund for the conduct of the study. M. Olde Rikkert reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry 2008;23:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G, Kelly L, Lewis-Holmes E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technol Assess 2014;18:1–226, v–vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA 2005;294:1934–1943. [DOI] [PubMed] [Google Scholar]
- 4.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry 2006;14:191–210. [DOI] [PubMed] [Google Scholar]
- 5.Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ 2005;330:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauthier S, Cummings J, Ballard C, et al. Management of behavioral problems in Alzheimer's disease. Int Psychogeriatr 2010;22:346–372. [DOI] [PubMed] [Google Scholar]
- 7.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002;288:1475–1483. [DOI] [PubMed] [Google Scholar]
- 8.Zuidema SU, Derksen E, Verhey FR, Koopmans RT. Prevalence of neuropsychiatric symptoms in a large sample of Dutch nursing home patients with dementia. Int J Geriatr Psychiatry 2007;22:632–638. [DOI] [PubMed] [Google Scholar]
- 9.Husebo BS, Ballard C, Sandvik R, Nilsen OB, Aarsland D. Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ 2011;343:d4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppel BS, Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014;82:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klumpers LE, Beumer TL, van Hasselt JG, et al. Novel Delta(9) -tetrahydrocannabinol formulation Namisol(R) has beneficial pharmacokinetics and promising pharmacodynamic effects. Br J Clin Pharmacol 2012;74:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walther S, Mahlberg R, Eichmann U, Kunz D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology 2006;185:524–528. [DOI] [PubMed] [Google Scholar]
- 13.Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer's disease. Int J Geriatr Psychiatry 1997;12:913–919. [PubMed] [Google Scholar]
- 14.Baker D, Pryce G, Giovannoni G, Thompson AJ. The therapeutic potential of cannabis. Lancet Neurol 2003;2:291–298. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci 2005;25:1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aso E, Sanchez-Pla A, Vegas-Lozano E, Maldonado R, Ferrer I. Cannabis-based medicine reduces multiple pathological processes in AbetaPP/PS1 mice. J Alzheimers Dis 2015;43:977–991. [DOI] [PubMed] [Google Scholar]
- 17.van den Elsen GA, Ahmed AI, Lammers M, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev 2014;14:56–64. [DOI] [PubMed] [Google Scholar]
- 18.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 20.Achterberg WP, Pieper MJ, van Dalen-Kok AH, et al. Pain management in patients with dementia. Clin Interv Aging 2013;8:1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry 2014;22:415–419. [DOI] [PubMed] [Google Scholar]
- 22.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials: a review. Control Clin Trials 2002;23:662–674. [DOI] [PubMed] [Google Scholar]
- 23.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 1997;48:S10–S16. [DOI] [PubMed] [Google Scholar]
- 24.Cohen-Mansfield J. Conceptualization of agitation: results based on the Cohen-Mansfield agitation inventory and the agitation behavior mapping instrument. Int Psychogeriatr 1996;8(suppl 3):309–315; discussion 351–354. [DOI] [PubMed] [Google Scholar]
- 25.Sainsbury A, Seebass G, Bansal A, Young JB. Reliability of the Barthel Index when used with older people. Age Ageing 2005;34:228–232. [DOI] [PubMed] [Google Scholar]
- 26.Thorgrimsen L, Selwood A, Spector A, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer's Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord 2003;17:201–208. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed AI, van den Elsen GA, Colbers A, et al. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. Eur Neuropsychopharmacol 2014;24:1475–1482. [DOI] [PubMed] [Google Scholar]
- 28.Wechsler D. Wechsler Memory Scale–Revised Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 29.Cummings JL. Neuropsychiatric Inventory (NPI): setting the standard for Alzheimer research. Available at: www.npitest.net.
- 30.Mega MS, Masterman DM, O'Connor SM, Barclay TR, Cummings JL. The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch Neurol 1999;56:1388–1393. [DOI] [PubMed] [Google Scholar]
- 31.Ihl R, Bachinskaya N, Korczyn AD, et al. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: a randomized controlled trial. Int J Geriatr Psychiatry 2011;26:1186–1194. [DOI] [PubMed] [Google Scholar]
- 32.Reese JP, Hessmann P, Seeberg G, et al. Cost and care of patients with Alzheimer's disease: clinical predictors in German health care settings. J Alzheimers Dis 2011;27:723–736. [DOI] [PubMed] [Google Scholar]
- 33.Fox C, Crugel M, Maidment I, et al. Efficacy of memantine for agitation in Alzheimer's dementia: a randomised double-blind placebo controlled trial. PLoS One 2012;7:e35185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014;311:682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med 2006;355:1525–1538. [DOI] [PubMed] [Google Scholar]
- 36.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walther S, Schupbach B, Seifritz E, Homan P, Strik W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J Clin Psychopharmacol 2011;31:256–258. [DOI] [PubMed] [Google Scholar]
- 38.Adler JN, Colbert JA. Clinical decisions: medicinal use of marijuana: polling results. N Engl J Med 2013;368:e30. [DOI] [PubMed] [Google Scholar]
- 39.Farrell M, Buchbinder R, Hall W. Should doctors prescribe cannabinoids? BMJ 2014;348:g2737. [DOI] [PubMed] [Google Scholar]
- 40.Kales HC, Gitlin LN, Lyketsos CG; Detroit Expert Panel on A, Management of Neuropsychiatric Symptoms of D. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc 2014;62:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.