Abstract
Chronic myelogenous leukemia (CML) is a malignant disease characterized by expression of p210-BCR-ABL, the product of the Philadelphia chromosome. Survival of CML patients has been significantly improved with the introduction of tyrosine kinase inhibitors that induce long-term hematologic remissions. However, mounting evidence indicates that the use of a single tyrosine kinase inhibitor does not cure this disease due to the persistence of p210-BCR-ABL at the molecular level or the acquired resistance in the stem cell compartment to individual inhibitors. We have recently shown in a murine model that deficiency of the Rho GTPases Rac1 and Rac2 significantly reduces p210-BCR-ABL-mediated proliferation in vitro and myeloproliferative disease in vivo, suggesting Rac as a potential therapeutic target in p210-BCR-ABL-induced disease. This target has been further validated using a first-generation Rac-specific small molecule inhibitor. In this review we describe the role of Rac GTPases in p210-BCR-ABL-induced leukemogenesis and explore the possibility of combinatorial therapies that include tyrosine kinase inhibitor(s) and Rac GTPase inhibitors in the treatment of CML.
Keywords: Rac GTPases, chronic myelogenous leukemia, BCR-ABL, imatinib
Introduction
The p210-BCR-ABL fusion protein that is generated from a reciprocal translocation between the breakpoint-cluster region (BCR) gene on Chromosome 22 and the Abelson leukemia (ABL) gene on Chromosome 9 is necessary and sufficient for the development of chronic myelogenous leukemia (CML).1,2 Although allogeneic stem cell transplantation is a curative therapy for the treatment of CML, most patients lack suitable donors or are not eligible for transplant due to advanced age.3–6 The development of imatinib mesylate, a tyrosine kinase inhibitor that has been shown to induce complete hematologic and cytogenetic responses in many patients, has provided an effective means of treatment of CML and has rejuvenated the field of rationalized drug design.7 Imatinib targets the abnormal kinase activity of CML blasts and induces apoptosis of p210- BCR-ABL+ cells.8,9 However, in a proportion of patients the persistence of p210-BCR-ABL+ cells or the development of p210-BCR-ABL kinase mutants that confer resistance to imatinib have been demonstrated.10,11 While a second generation of tyrosine kinase inhibitors including nilotinib and dasatinib are effective at inhibiting the activities of most imatinib-resistant p210-BCR-ABL mutants, the use of sequential Abl kinase inhibitor therapy has been shown to select for compound mutations that confer resistance to both drugs and increase p210-BCR-ABL oncogenicity,12 suggesting that other signaling components downstream of p210-BCR-ABL should be considered as potential therapeutic targets.
The Rac subfamily of Rho GTPases comprising the highly related mammalian proteins Rac1, Rac2 and Rac3 has previously been implicated in p210-BCR-ABL-mediated transformation using cell lines and in acute myelogenous leukemia (AML) cell migration dependent on vascular endothelial growth factor paracrine stimulation.13 Rac1, in particular, has been identified as an important downstream component of BCR-ABL signaling, suggesting Rac GTPases as possible molecular targets for interrupting abnormal signaling in CML blasts.14–19 Our findings that the combinatorial loss of Rac1 and Rac2 significantly attenuates p210-BCR-ABL-induced proliferation in vitro and myeloproliferative disease (MPD) in vivo provide additional genetic evidence that the Rac GTPases may be attractive therapeutic targets in p210-BCR-ABL-mediated MPD.20 These genetic data were further substantiated experimentally by use of NSC23766, a first-generation small molecule inhibitor that specifically blocks activation of the Rac GTPases. While our data confirm that Rac GTPases are candidate therapeutic targets in p210-BCR-ABL-mediated disease, a number of questions remain regarding the role of Rac and other Rho GTPases in p210-BCR-ABL-induced leukemogenesis.
Relationship between Rac GTPases and p210-BCR-ABL in CML
The Rac subfamily of Rho GTPases has been implicated in a variety of different cellular functions, including adhesion, migration, actin assembly, transcription activation, cell cycle progression and cell survival (reviewed in Blanchard21). Similar to other Ras-related GTPases, Rac GTPases cycle between inactive, GDP-bound and active, GTP-bound conformations to transduce signals to effector proteins that mediate a multitude of cellular responses. Three structurally related proteins, Rac1, Rac2 and Rac3, have been identified. While Rac1 and Rac3 are ubiquitously expressed, expression of Rac2 is restricted to hematopoietic tissues. Thus, hematopoietic cells are unique in expressing all three Rac proteins. Our laboratory has previously shown that Rac1 and Rac2 are essential for the regulation of multiple hematopoietic stem cell functions with unique as well as overlapping roles, including adhesion, migration, proliferation and apoptosis.22 In effecting these responses, Rac GTPases have been shown to activate signaling molecules that coincide with known downstream targets of p210-BCR-ABL,18,19 such as the Ras/MAP kinases (ERK, p38 and JNK), phosphatidylinositol-3-kinase (PI3K)/Akt, Bcl-XL and focal adhesion kinase (FAK).
These earlier observations highlight a possible relationship between Rac GTPases and p210-BCR-ABL, although the specific role(s) of the individual Rac subfamily members in the development of disease in vivo have not previously been defined. Skorski et al.16 showed that activation of Rac GTPases is enhanced in 32Dcl3 myeloid precursor cells ectopically expressing p210-BCR-ABL. Additionally, survival of mice injected with 32Dcl3 cells co-expressing p210-BCR-ABL and a dominant-negative N17Rac mutant was markedly extended, compared to mice transplanted with 32Dcl3 cells expressing p210-BCR-ABL alone.16 Harnois et al.17 showed that the p210-BCR-ABL fusion protein forms a stable complex with Rac1, Rac2, and other RhoGTPases including RhoA and Cdc42 and may directly activate these Rho GTPases through the Dbl homology domain of Bcr. Conversely, these Rho GTPases may be activated by p210-BCR-ABL through the recruitment of Vav1,17 a hematopoietic-specific guanine nucleotide exchange factor (GEF) that is crucial for Rac activation in lymphoid23 and other hematopoietic cells. Sini et al.14 have described activation of Rac by Abl-induced tyrosine phosphorylation of Sos-1, which could be inhibited by genetic or pharmacological inhibition of Abl. Additionally, p210-BCR-ABL has previously been shown to display a Rac-dependent induction in transformation-associated changes in cytoskeletal functions such as actin assembly, migration and adhesion, all known functions of Rho GTPases, particularly Rac.18,19 Finally, Diaz-Blanco et al.24 have recently shown that Rac1 and Rac2 were significantly upregulated in CD34+ human chronic phase CML bone marrow (BM) cells. These and other data imply that p210-BCR-ABL interacts directly and/or indirectly with Rac, Rho and Cdc42 to activate these GTPases in cell lines.
In support of this postulated involvement of Rac GTPases in p210-BCR-ABL-mediated disease, we have demonstrated hyperactivation of Rac1 and Rac2 and, to a lesser extent, Rac3 in hematopoietic stem cells and progenitors (HSC/P) isolated from chronic phase CML patients. These data confirm that Rac GTPases are abnormally activated in chronic phase disease. Experimentally, Rac GTPases were also shown to be hyperactivated in primary murine BM cells expressing p210-BCR-ABL after retrovirus-mediated gene transfer.20 To determine the importance of the individual Rac GTPases in the development of p210-BCR-ABL-mediated CML, we employed an in vivo retroviral murine model of hematopoietic stem cell transformation combined with the use of BM cells from gene-targeted mice to effect deletion of Rac1 alone, Rac2 alone and Rac1 in combination with Rac2. As originally described,1 this model has demonstrated that expression of p210-BCR-ABL in murine HSC/P can induce an MPD, including the development of leukocytosis, splenomegaly, extramedullary hematopoiesis in the liver and pulmonary hemorrhage due to extensive granulocyte infiltration in the lung. In our reported studies, while the median survival of p210-BCR-ABL-expressing wild-type (WT) and Rac1-deficient mice was 23 and 22 days, respectively, the median survival of p210-BCR-ABL-expressing Rac2-deficient mice was significantly increased to 43 days, and the median survival of p210-BCR-ABL-expressing Rac1/Rac2-deficient mice was even more strikingly increased to 92 days. This result suggests that individual Rac GTPases play unique roles in p210-BCR-ABL-mediated leukemogenesis, as has been described for normal HSC/P functions.22,25
Using this genetic approach, we also monitored the disease phenotype of the p210-BCR-ABL-expressing WT and Rac-deficient animals. Expression of p210-BCR-ABL in WT, Rac1-and Rac2-deficient HSC/P led to the development of oligoclonal myeloid-lineage leukemias. Expression of p210-BCR-ABL in Rac1/Rac2-deficient HSC/P led to altered disease phenotype, with mice showing oligoclonal leukemias of myeloid, lymphoid or bi-lineage immunophenotypes, suggesting that Rac1 and Rac2 are critical for transformation and MPD development in vivo. The mechanism of these differences in disease phenotypes is still being investigated but could be related to alterations in downstream signaling pathways in the absence of Rac1 and Rac2 and/or compensatory alterations in the activity of Rac3.
Loss of Rac GTPases alters the signaling cascades activated by p210-BCR-ABL
The results presented above suggest that Rac1 and Rac2 play an important role in the development of p210-BCR-ABL-mediated MPD, but in the absence of these GTPases, expression of p210-BCR-ABL can lead to the eventual progression of phenotypically altered disease via unspecified downstream signaling components. What is the mechanism by which p210-BCR-ABL mediates disease in the absence of Rac1 and Rac2? To answer this question, we first analyzed the status of Rac3 activation in splenocytes harvested from leukemic Rac1/Rac2-deficient animals. Rac3 is the third member of the Rac subfamily of Rho GTPases that was originally discovered by screening the p210-BCR-ABL-expressing erythroid blastic-phase CML cell line K562.26 Rac3 activation has been demonstrated in p190-BCR-ABL-expressing malignant precursor B-lineage lymphoblasts27 and is associated with the invasive phenotype of breast carcinomas,28,29 suggesting that Rac3 hyperactivation could play a specific role in cancer development and invasiveness. We demonstrated that Rac3 was hyperactivated in p210-BCR-ABL-expressing leukemic animals in the absence of Rac1 and Rac2.20 These data, along with the observed differences in survival mediated by Rac1- versus Rac2-deficient HSC, support the hypothesis that individual Rac GTPases play unique roles in the development of p210-BCR-ABL-mediated disease. Studies are underway to further explore the specific roles of each Rac GTPase in disease evolution and phenotype.
Mice that express p210-BCR-ABL with a point mutation in the ATP-binding site of ABL do not develop leukemia.30 This indicates that ABL kinase activity is required for p210-BCR-ABL-induced transformation. However, the p210-BCR-ABL fusion protein is also composed of several structural domains that play distinct roles in cell signaling. Phosphorylation of Bcr at tyrosine 177 recruits Grb2/Gab2 and Sos, which results in Ras, ERK, JNK and p38 MAP kinase activation.31,32 Phosphorylation of Bcr at tyrosine 177 also leads to the recruitment of SHP2 and PI3K/Akt.33 Ras may be activated by two additional substrates of p210-BCR-ABL, the adapter molecules Shc and CrkL.34,35 The actin-binding domain and the C-terminal domain of ABL, while not necessary for p210-BCR-ABL-mediated leukemogenesis experimentally, may contribute to the malignant behavior of p210-BCR-ABL leukemic blasts.36 As mentioned previously, the role of a Dbl homology domain present in p210-BCR-ABL but not in a shorter form of BCR-ABL (p190-BCR-ABL) remains controversial. This domain may mediate Rac activation17 that has been demonstrated to be necessary for full Ras-mediated transformation.16 Finally, the Src homology domains SH2 and SH3 of p210-BCR-ABL bind to the Src family kinase member Hck and can phosphorylate signal transducer and activator of transcription 5 (STAT5) independently of Janus kinase activation.37 These known signaling functions of the fusion protein suggest that p210-BCR-ABL-mediated signals may converge on Rac through several pathways such as Ras/MAPK (ERK, p38 and JNK), PI3K/Akt, Bcl-xL and FAK to alter proliferation and survival.
We analyzed activation of ERK, JNK, p38, Akt, STAT5 and CrkL in splenocytes harvested from p210-BCR-ABL-expressing WT, Rac1-deficient, Rac2-deficient and Rac1/Rac2-deficient animals. Increased baseline phosphorylation of each of these signaling components was apparent in cells derived from leukemic WT animals, as well as Rac1-deficient leukemic animals. However, activation of downstream pathways including ERK, JNK, p38 and Akt was attenuated in Rac2-deficient leukemic cells and almost completely abrogated in the Rac1/Rac2-deficient cells, correlating with the overall survival that was observed in animals from each of these genotypes. The decreased activation of downstream pathways was not due to decreased ABL tyrosine kinase activity, as autophosphorylation of p210-BCR-ABL was still noted in these cells.20 STAT5 phosphorylation also was still detectable in leukemic cells regardless of the presence or absence of Rac1 and Rac2 GTPase activity. These data suggest that STAT5 may be the crucial signaling component for leukemia development in Rac1/Rac2-deficient HSC/P.
Surprisingly, activation of CrkL, which has been suggested to be an effector that binds directly to p210-BCR-ABL,34 was decreased in Rac2-deficient and practically abrogated in Rac1/Rac2-deficient leukemias. CrkL activation has recently been reported to be dependent on a large multimeric protein complex that contains at least PI3K, docking protein 2 (DOK2), CrkL, Vav and Rac.38,39 Thus, our data support a hypothesis that Rac participates in the activation of CrkL in the context of a multi-protein complex.
Putative role of STAT5 in the development of Rac3-mediated MPD
As mentioned previously, Rac3 was found to be hyperactivated in Rac1/Rac2-deficient leukemic animals, suggesting that this GTPase may be important in the eventual development of disease. These results imply that either the individual Rac GTPases play specific roles in the development of p210-BCR-ABL-mediated disease or the combinatorial loss of Rac expression modulates the disease phenotype. Interestingly, STAT5 activation was also apparent in Rac1/Rac2-deficient leukemic animals, suggesting that disease development also may be modulated by this protein.
STAT5 activation has been shown to be pivotal in myeloid differentiation40 and multiple groups have demonstrated a critical role of STAT5 in the pathogenesis of CML,41–45 but STAT proteins in leukemic transformation remain highly controversial. The physical interaction of p210-BCR-ABL and STAT5 was delineated by Nieborowska-Skorska et al.46 using retroviral expression of BCR-ABL mutants in 32Dc13 cells. They showed that deletion of the SH2 domain accompanied by a point mutation in the SH3 domain of p210-BCR-ABL abolished STAT5 activation, as did deletion of both the SH2 and SH3 domains. Nieborowska-Skorska et al.46 also demonstrated that cells expressing these STAT5 activation-deficient p210-BCR-ABL mutants were more apoptotic than cells expressing unmutated protein. Additionally, a constitutively active STAT5 mutant was able to rescue cells expressing STAT5-deficient BCR-ABL mutants from apoptosis while 32Dcl3 cells co-expressing a dominant-negative STAT5 mutant and BCR-ABL still underwent apoptosis, confirming the protective effect of STAT5.46
Sillaber et al.47 demonstrated that inducible expression of a truncated STAT5 protein (DSTAT5) could dimerize with endogenous STAT5 and inhibit STAT5-induced gene transcription and growth in p210-BCR-ABL-expressing Ba/F3 hematopoietic cells, suggesting that STAT5 activation was responsible for most of the cell growth induced by p210-BCR-ABL. Expression of ΔSTAT5 resulted in inhibition of STAT5-induced transcription and a significant reduction in cell growth due to decreased cell viability and greater cell sensitivity to cytotoxic agents such as hydroxyurea and cytarabine.47 Ba/F3 cells transfected with a vector expressing the Y177F mutant of BCR-ABL exhibited decreased tyrosine phosphorylation and activation of STAT1 and STAT5, compared with transfectants expressing wild-type BCR-ABL, suggesting that phosphorylation of Tyr-177 may be important for the activation of STAT signaling pathways by BCR-ABL. Tyrosine 177 of BCR-ABL has previously been shown as critical for binding to the adaptor protein GRB2, which mediates Ras/MAP kinase activation, suggesting that STAT5 activation may depend on Ras/Rac activation. Finally, STAT5 activation mediated by autocrine secretion of granulocyte-macrophage colony-stimulating factor has recently been shown to be responsible for the outgrowth of imatinib-resistant CML,48 implying that STAT5 may be an alternative escape pathway by which leukemic cells circumvent tyrosine kinase inhibition.
Conversely, other studies suggest that STAT5 is not important in the pathogenesis of CML. Specifically, lethally irradiated mice transplanted with p210-BCR-ABL-expressing STAT5a/b N-terminal deletion mutant (Stat5a/bΔN/ΔN) cells developed disease as rapidly as mice injected with p210-BCR-ABL-expressing WT cells, suggesting that STAT5 is not essential for the development of p210-BCR-ABL-mediated disease.49 Similar to our findings with Rac1-/Rac2-deficient BM, the majority of the p210-BCR-ABL-expressing WT mice developed myeloid lineage leukemias, while the p210-BCR-ABL-expressing STAT5a/b N-terminal deletion mutant mice had either myeloid, lymphoid or bilineage leukemias.49 Interestingly, when the entire Stat5a/b gene locus was deleted, p185-BCR-ABL-expressing Stat5a/bnull/null cells were resistant to transformation and did not induce lymphoid leukemia development in mice,50 suggesting that STAT5 is critical for the development of p185-BCR-ABL-mediated disease. Whether p210-BCR-ABL-expression in the absence of Stat5 (that is, in Stat5a/bnull/null cells) can modulate the development of MPD is unknown, but represents an intriguing question. Due to these discrepancies, the role of STAT5 in p210-BCR-ABL-mediated disease needs to be further characterized.
Compensatory hyperactivation of Rac3 in the absence of Rac1 and Rac2 may be responsible for the eventual disease development that is observed. The question now remains whether this Rac3-mediated disease is due to activation of the STAT5 signaling cascade.
Rac GTPases as targets for p210-BCR-ABL-mediated CML therapy
On the basis of these genetic data, we examined the effect of NSC23766 on p210-BCR-ABL-induced transformation. NSC23766 is a first-generation, Rac-specific small molecule inhibitor51 that was developed based on the GEF-Rac1 GTPase complex and computer-assisted virtual screening. NSC23766 was found to fit into a shallow surface groove of Rac1 that has been shown to be critical for GEF specification. In published studies, NSC23766 was shown to effectively inhibit Rac protein binding and activation by the Rac-specific GEFs TrioN or Tiam1 in a dose-dependent manner. In contrast, NSC23766 did not interfere with the binding or activation of Cdc42 or RhoA by their respective GEFs. In cells, NSC23766 effectively blocked serum- or platelet-derived growth factor-induced Rac1 activation and lamellipodia formation, but did not affect endogenous Cdc42 or RhoA activity. NSC23766 reduced growth stimulated by the Rac-GEFs Trio or Tiam1, but not proliferation stimulated by the promiscuous Rho/Cdc42 GEFs such as Dbl, Lbc, intersectin or a constitutively active Rac1 mutant. Importantly, NSC23766 suppressed Trio, Tiam1 or Ras-induced cell transformation and was shown to attenuate solid tumor cell line transformation and invasion. When human prostate cancer PC-3 cells were treated with NSC23766, Rac1 activity was down-regulated, and proliferation, anchorage independent growth and invasion phenotypes that require endogenous Rac1 activity were inhibited.51
We have previously shown that retention of murine HSC/P is a Rac-dependent function.22,25,52 On the basis of initial observations of NSC23766 inhibition of Rac activation, we subsequently demonstrated mobilization of HSC/P following a single in vivo dose of the compound.25 Compared with phosphate-buffered saline (PBS)-treated controls, the Rac inhibitor induced an ~2-fold increase in circulating progenitors at 6 h after injection. The mobilization of HSC/P by NSC23766 was dose-dependent and reversible, with the number of circulating HSC/P returning to normal values within 24 h post injection. To determine if the effect of the inhibitor was associated with specific inhibition of Rac1 and Rac2, we incubated BM lineage negative/c-kit+ cells with stromal-derived factor-1α and NSC23766. At a dose as low as 10 µm, activation of both Rac1 and Rac2 was inhibited.25 We further found that NSC23766 was effective in suppressing downstream Rac signaling, as measured by the effect on the phosphorylation status of p21-activated kinase-1, a known effector of Rac. The inhibitor appeared to be both reversible and nontoxic in vivo when administered at a dose of 2.5 mg per kg daily for a period of 65 days.25 Thus, NSC23766 constitutes a Rac-specific small molecule inhibitor that is capable of reversing cancer cell phenotypes associated with Rac deregulation and blocking Rac activation in HSC/P in vivo.
To validate Rac GTPases as antileukemic targets in p210-BCR-ABL-induced disease, we incubated p210-BCR-ABLexpressing murine cells with increasing concentrations of NSC23766.20 NSC23766 potently inhibited the growth of these cells and also suppressed proliferation of Rac1/Rac2-deficient cells harvested from p210-BCR-ABL-expressing mice, further supporting the role of Rac3 hyperactivation in disease development. In addition, NSC23766 inhibited proliferation of cells expressing the tyrosine-kinase inhibitor-resistant p210-BCR-ABL-T315I mutation. In the presence of both NSC23766 and imatinib, proliferation of these cells was inhibited by >90%. Treatment with NSC23766 also led to selective killing of human CML blast crisis HSC/P with limited toxicity on normal murine or human HSC/P in vitro.
We then determined the effect of NSC23766 in vivo using our retroviral transduction and transplantation model. Mice in which NSC23766 was continually administered survived significantly longer than PBS-treated mice. NSC23766 reversed the CML survival/growth in an in vivo model of human CML disease after transplantation of chronic-phase purified CD34+ cells in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.20 These results further validate Rac as a candidate target in p210-BCR-ABL-mediated disease.
Could targeting Rac GTPases be useful for inducing leukemia stem cell egression from the nurturing leukemic stem cell niche?
Recently, the ‘cancer stem cell’ hypothesis, which attempts to explain the presence of ‘residual’ therapy-resistant cancer cells in patients and in related animal models, has attracted much attention.53 The theory suggests that cancer may arise from a rare population of putative cancer stem cells. Leukemia stem cells (LSCs), which share characteristics with normal HSCs but initiate disease instead of supporting normal hematopoiesis, have been demonstrated in several leukemias, most prominently CML.54 Like normal HSCs, LSCs are thought to reside in the BM niche, although the nature of LSC interaction with the supporting BM microenvironment, where leukemia presumably arises, remains unclear.54 Recent studies in a human AML mouse model and a CML mouse model using anti-CD44 antibody to disrupt potential LSC interactions with the BM niche have provided strong evidence that LSCs depend upon interactions within a specific niche.55,56 The fact that targeting the LSC-expressed cell surface molecule CD44 by monoclonal antibodies effectively suppressed both AML and CML leukemia progression, and induced LSC differentiation raises the possibility that the LSC–niche interaction could be a valid drug target for more effective eradication of leukemia. Since Rac activities are dysregulated in CML and Rac is known to be a central regulator of HSC adhesion, migration and interaction with the BM niche,22,25 it is possible that Rac targeting by specific inhibitors could transiently mobilize LSCs for therapeutic benefits. As mentioned above, our group has shown previously that Rac1−/−;Rac2−/− HSCs demonstrate defective adhesion, migration and lodging in the BM endosteum, and administration of the Rac activation inhibitor NSC23766 induces stem cell mobilization in a dose-dependent manner. It is thus an attractive proposal that future strategies of Rac targeting may be adopted in a similar fashion to induce egression of CML stem cells from the BM niche.
What type of CML patients may benefit from Rac inhibitor therapies?
Since multiple pathways, directly dependent or independent of BCR-ABL expression, are activated in CML during the evolution of disease, we postulate that Rac inhibition in conjunction with ABL tyrosine kinase domain inhibitors may represent a novel method of combined therapy early in the disease.
Levels of BCR-ABL mRNA,57–59 protein60 or phosphoprotein61 increase during disease progression. This may be related to the higher levels of expression of BCR-ABL in CD34+ CML cells compared with more differentiated myeloid cells, and the fact that some patients even in complete cytogenetic response display a persistent population of HSC/P that express high levels of BCR-ABL.62 High levels of expression of BCR/ABL are probably responsible for the fact that intracellular drug levels may be insufficient to reach the degree of kinase inhibition required to induce cell death in HSC/P.63 Whether there is correlation between BAC-ABL expression and Rac activation is not yet known, but it is quite possible that a BCR-ABL gain of function translates into higher Rac activation. We found that Rac1, Rac2 and to a lesser extent, Rac3 are hyperactivated in CD34+ human chronic phase CML cells,20 and a Rac-specific small molecule inhibitor significantly reduced the clonogenicity of blastic phase CML granulo-macrophage progenitors, and in vivo it significantly reduced the leukemic burden in NOD/SCID mice transplanted with chronic-phase CML CD34+ cells. Altogether this suggests that Rac inhibition may indeed impair leukemic growth in very different phases of the disease. This is even more relevant when chronic phase CML is believed to derive from HSC with engraftment and self-renewal ability, while blastic phase CML is believed not to derive from HSC but from granulo-macrophage progenitors.64 The mechanisms of disease persistence in patients treated with tyrosine kinase inhibitors appear to be related to both BCR-ABL-dependent and -independent pathways.65 Among the BCR-ABL-dependent pathways, the appearance of mutations in the kinase domain that confer high resistance (reviewed in Melo and Barnes66), or even moderate degrees of resistance to tyrosine kinase inhibitors67 and the maintenance of high-expressing HSC/P even in patients in complete cytogenetic response have been shown to be responsible for disease persistence.
Among the BCR-ABL-independent pathways, the overexpression of drug transporters which are likely to influence intracellular levels of tyrosine kinase inhibitors and the simultaneous signaling through cytokine receptor-dependent pathways, in cells that still respond to cytokine stimulation, have been cited.65 We are unable to confirm whether NSC23766 chemical conformation51 is an adequate substrate for drug transporters, but since Rac activation is central in many of the key signaling pathways of HSC/P, Rac inhibition may also impair BCR-ABL-independent pathways and represent a valid adjuvant in the therapy of CML. In addition, since Rac appears likely to integrate multiple pathways downstream of BCR-ABL transforming activities, Rac inhibition combined with ABL tyrosine kinase domain inhibitors may represent a novel method of combined therapy early in the disease.
Summary and model of disease development
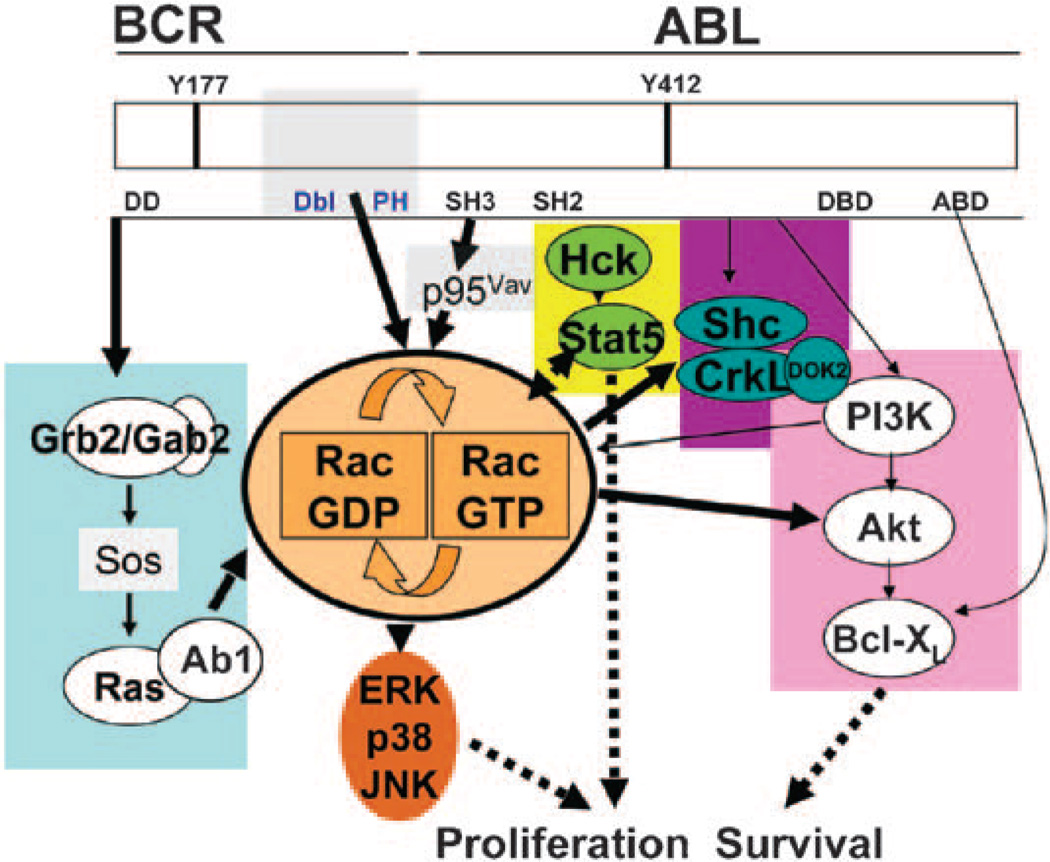
Rac GTPases appear to play a critical role in the development of leukemogenesis associated with p210-BCR-ABL expression and represent novel targets for therapeutic intervention, as depicted in the model shown in Figure 1. The p210-BCR-ABL fusion protein may activate Rac GTPases either directly through the Dbl homology domain of Bcr or via recruitment of the Vav1 GEF. In addition, loss of Rac expression may inhibit the formation of a large multimeric protein complex containing PI3K, DOK2, CrkL and Vav, thus inhibiting CrkL phosphorylation. Downregulation of Rac activation leads to almost complete abrogation of MAP kinase and PI3K signaling pathways, suggesting that Rac GTPases are required for activation of multiple p210-BCR-ABL-mediated signaling cascades.
Figure 1.
Model of activation of Rac GTPases in BCR-ABL-induced leukemogenesis. The various pathways of activation downstream of p210-BCR-ABL are indicated with different color codes. Gray-shaded areas indicate molecules or domains with guanine nucleotide exchange factor (GEF) activity. The Dbl and pleckstrin homology (Dbl and PH) domains, only present in p210-BCR-ABL, activate Rho GTPases directly. The SH3 domain in both p190- and p210-BCR-ABL activates p95Vav (Vav1). Y177/Y412, tyrosine residues that can be phosphorylated; Dbl, Rac GTPase exchange factor; SH2/SH3, Src homology domains; DD; dimerization domain; DBD, DNA-binding domain; ABD, actin-binding domain.
Our results also suggest that STAT5 activation is maintained in the absence of Rac1 and Rac2. We speculate that Rac1/Rac2 activation may be a key for p210-BCR-ABL-induced leukemogenesis in this setting. While Rac1 and Rac2 isoforms activate multiple pathways, compensatory Rac3 activation may be responsible for signaling downstream of p210-BCR-ABL and inducing leukemogenesis in the absence of Rac1 and Rac2.
NSC23766, an inhibitor of all three isoforms of Rac that are expressed by hematopoietic cells, induces significant regression of murine p210-BCR-ABL-induced leukemias and human CML in xenogeneic grafts, demonstrating that intervention of Rac activation is a new tool in treating p210-BCR-ABL-induced leukemias. The finding that a single dose of the Rac inhibitor induces mobilization of HSC/P25 raises the possibility that NSC23766 could also mobilize leukemic stem cells from their niche, thus inhibiting the stem cell properties characteristic of these cells. These results suggest that the Rac GTPases may prove to be useful therapeutically by targeting alternative signaling pathways, which may be responsible for resistance and relapse in CML.
Acknowledgements
This work was supported by the National Institute of Health grant numbers HL69974 and DK62757 (DAW), Leukemia Lymphoma Society grant 6152-06 (DAW), T32 HD046387 (EKT) and the Department of Defense New Investigator Award CM064050 (JAC). DAW and YZ may obtain royalties based on milestones set forth in a licensing agreement between Cincinnati Children’s Hospital Medical Center and Amgen related to the development of drug inhibitors of Rac GTPases.
References
- 1.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 2.Michor F, Iwasa Y, Nowak MA. The age incidence of chronic myeloid leukemia can be explained by a one-mutation model. Proc Natl Acad Sci USA. 2006;103:14931–14934. doi: 10.1073/pnas.0607006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 4.Goldman JM. Treatment of chronic myeloid leukaemia: some topical questions. Baillieres Clin Haematol. 1997;10:405–421. doi: 10.1016/s0950-3536(97)80015-7. [DOI] [PubMed] [Google Scholar]
- 5.Fausel C. Targeted chronic myeloid leukemia therapy: seeking a cure. J Manag Care Pharm. 2007;13(8) Suppl A:8–12. doi: 10.18553/jmcp.2007.13.s8-a.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savona M, Talpaz M. Chronic myeloid leukemia: changing the treatment paradigms. Oncology (Williston Park) 2006;20:707–711. discussion 712–4, 719, 724. [PubMed] [Google Scholar]
- 7.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 8.Druker BJ, O’Brien SG, Cortes J, Radich J. Chronic myelogenous leukemia. Hematology (Am Soc Hematol Educ Program) 2002;1:111–135. doi: 10.1182/asheducation-2002.1.111. [DOI] [PubMed] [Google Scholar]
- 9.Deininger MW, Goldman JM, Lydon N, Melo JV. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood. 1997;90:3691–3698. [PubMed] [Google Scholar]
- 10.Gorre ME, Ellwood-Yen K, Chiosis G, Rosen N, Sawyers CL. BCR-ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR-ABL chaperone heat shock protein 90. Blood. 2002;100:3041–3044. doi: 10.1182/blood-2002-05-1361. [DOI] [PubMed] [Google Scholar]
- 11.Lowenberg B. Minimal residual disease in chronic myeloid leukemia. N Engl J Med. 2003;349:1399–1401. doi: 10.1056/NEJMp038130. [DOI] [PubMed] [Google Scholar]
- 12.Shah NP, Skaggs BJ, Branford S, Hughes TP, Nicoll JM, Paquette RL, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J Clin Invest. 2007;117:2562–2569. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casalou C, Fragoso R, Nunes JF, Dias S. VEGF/PLGF induces leukemia cell migration via P38/ERK1/2 kinase pathway, resulting in Rho GTPases activation and caveolae formation. Leukemia. 2007;21:1590–1594. doi: 10.1038/sj.leu.2404668. [DOI] [PubMed] [Google Scholar]
- 14.Sini P, Cannas A, Koleske AJ, Di Fiore PP, Scita G. Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat Cell Biol. 2004;6:268–274. doi: 10.1038/ncb1096. [DOI] [PubMed] [Google Scholar]
- 15.Renshaw MW, Lea-Chou E, Wang JY. Rac is required for v-Abl tyrosine kinase to activate mitogenesis. Curr Biol. 1996;6:76–83. doi: 10.1016/s0960-9822(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 16.Skorski T, Wlodarski P, Daheron L, Salomoni P, Nieborowska-Skorska M, Majewski M, et al. BCR/ABL-mediated leukemogenesis requires the activity of the small GTP- binding protein Rac. Proc Natl Acad Sci USA. 1998;95:11858–11862. doi: 10.1073/pnas.95.20.11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harnois T, Constantin B, Rioux A, Grenioux E, Kitzis A, Bourmeyster N. Differential interaction and activation of Rho family GTPases by p210bcr-abl and p190bcr-abl. Oncogene. 2003;22:6445–6454. doi: 10.1038/sj.onc.1206626. [DOI] [PubMed] [Google Scholar]
- 18.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz M. Rho signalling at a glance. J Cell Sci. 2004;117(Part 23):5457–5458. doi: 10.1242/jcs.01582. [DOI] [PubMed] [Google Scholar]
- 20.Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, et al. Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell. 2007;12:467–478. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard JM. Small GTPases, adhesion, cell cycle control and proliferation. Pathol Biol (Paris) 2000;48:318–327. [PubMed] [Google Scholar]
- 22.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 23.Turner M, Billadeau DD. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 24.Diaz-Blanco E, Bruns I, Neumann F, Fischer JC, Graef T, Rosskopf M, et al. Molecular signature of CD34(+) hematopoietic stem and progenitor cells of patients with CML in chronic phase. Leukemia. 2007;21:494–504. doi: 10.1038/sj.leu.2404549. [DOI] [PubMed] [Google Scholar]
- 25.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 26.Haataja L, Groffen J, Heisterkamp N. Characterization of RAC3, a novel member of the Rho family. J Biol Chem. 1997;272:20384–20388. doi: 10.1074/jbc.272.33.20384. [DOI] [PubMed] [Google Scholar]
- 27.Cho YJ, Zhang B, Kaartinen V, Haataja L, de Curtis I, Groffen J, et al. Generation of rac3 null mutant mice: role of Rac3 in Bcr/Abl-caused lymphoblastic leukemia. Mol Cell Biol. 2005;25:5777–5785. doi: 10.1128/MCB.25.13.5777-5785.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mira JP, Benard V, Groffen J, Sanders LC, Knaus UG. Endogenous, hyperactive Rac3 controls proliferation of breast cancer cells by a p21-activated kinase-dependent pathway. Proc Natl Acad Sci USA. 2000;97:185–189. doi: 10.1073/pnas.97.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan AY, Coniglio SJ, Chuang YY, Michaelson D, Knaus UG, Philips MR, et al. Roles of the Rac1 and Rac3 GTPases in human tumor cell invasion. Oncogene. 2005;24:7821–7829. doi: 10.1038/sj.onc.1208909. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Ren R. Bcr-Abl efficiently induces a myeloproliferative disease and production of excess interleukin-3 and granulocyte-macrophage colony-stimulating factor in mice: a novel model for chronic myelogenous leukemia. Blood. 1998;92:3829–3840. [PubMed] [Google Scholar]
- 31.Pendergast AM, Quilliam LA, Cripe LD, Bassing CH, Dai Z, Li N, et al. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell. 1993;75:175–185. [PubMed] [Google Scholar]
- 32.Puil L, Liu J, Gish G, Mbamalu G, Bowtell D, Pelicci PG, et al. Bcr-Abl oncoproteins bind directly to activators of the Ras signalling pathway. EMBO J. 1994;13:764–773. doi: 10.1002/j.1460-2075.1994.tb06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sattler M, Mohi MG, Pride YB, Quinnan LR, Malouf NA, Podar K, et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell. 2002;1:479–492. doi: 10.1016/s1535-6108(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 34.Oda T, Heaney C, Hagopian JR, Okuda K, Griffin JD, Druker BJ. Crkl is the major tyrosine-phosphorylated protein in neutrophils from patients with chronic myelogenous leukemia. J Biol Chem. 1994;269:22925–22928. [PubMed] [Google Scholar]
- 35.Pelicci G, Lanfrancone L, Salcini AE, Romano A, Mele S, Grazia Borrello M, et al. Constitutive phosphorylation of Shc proteins in human tumors. Oncogene. 1995;11:899–907. [PubMed] [Google Scholar]
- 36.Wertheim JA, Perera SA, Hammer DA, Ren R, Boettiger D, Pear WS. Localization of BCR-ABL to F-actin regulates cell adhesion but does not attenuate CML development. Blood. 2003;102:2220–2228. doi: 10.1182/blood-2003-01-0062. [DOI] [PubMed] [Google Scholar]
- 37.Klejman A, Schreiner SJ, Nieborowska-Skorska M, Slupianek A, Wilson M, Smithgall TE, et al. The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells. EMBO J. 2002;21:5766–5774. doi: 10.1093/emboj/cdf562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishihara H, Maeda M, Oda A, Tsuda M, Sawa H, Nagashima K, et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100:3968–3974. doi: 10.1182/blood-2001-11-0032. [DOI] [PubMed] [Google Scholar]
- 39.Sattler M, Verma S, Pride YB, Salgia R, Rohrschneider LR, Griffin JD. SHIP1, an SH2 domain containing polyinositol-5-phosphatase, regulates migration through two critical tyrosine residues and forms a novel signaling complex with DOK1 and CRKL. J Biol Chem. 2001;276:2451–2458. doi: 10.1074/jbc.M006250200. [DOI] [PubMed] [Google Scholar]
- 40.Miranda MB, Johnson DE. Signal transduction pathways that contribute to myeloid differentiation. Leukemia. 2007;21:1363–1377. doi: 10.1038/sj.leu.2404690. [DOI] [PubMed] [Google Scholar]
- 41.Carlesso N, Frank DA, Griffin JD. Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J Exp Med. 1996;183:811–820. doi: 10.1084/jem.183.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilaria RL, Jr, Van Etten RA. P210 and P190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 43.Lin TS, Mahajan S, Frank DA. STAT signaling in the pathogenesis and treatment of leukemias. Oncogene. 2000;19:2496–2504. doi: 10.1038/sj.onc.1203486. [DOI] [PubMed] [Google Scholar]
- 44.Hoover RR, Gerlach MJ, Koh EY, Daley GQ. Cooperative and redundant effects of STAT5 and Ras signaling in BCR/ABL transformed hematopoietic cells. Oncogene. 2001;20:5826–5835. doi: 10.1038/sj.onc.1204549. [DOI] [PubMed] [Google Scholar]
- 45.Spiekermann K, Pau M, Schwab R, Schmieja K, Franzrahe S, Hiddemann W. Constitutive activation of STAT3 and STAT5 is induced by leukemic fusion proteins with protein tyrosine kinase activity and is sufficient for transformation of hematopoietic precursor cells. Exp Hematol. 2002;30:262–271. doi: 10.1016/s0301-472x(01)00787-1. [DOI] [PubMed] [Google Scholar]
- 46.Nieborowska-Skorska M, Wasik MA, Slupianek A, Salomoni P, Kitamura T, Calabretta B, et al. Signal transducer and activator of transcription (STAT)5 activation by BCR/ABL is dependent on intact Src homology (SH)3 and SH2 domains of BCR/ABL and is required for leukemogenesis. J Exp Med. 1999;189:1229–1242. doi: 10.1084/jem.189.8.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sillaber C, Gesbert F, Frank DA, Sattler M, Griffin JD. STAT5 activation contributes to growth and viability in Bcr/Abl-transformed cells. Blood. 2000;95:2118–2125. [PubMed] [Google Scholar]
- 48.Wang Y, Cai D, Brendel C, Barett C, Erben P, Manley PW, et al. Adaptive secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR/ABL+ progenitors via JAK-2/STAT-5 pathway activation. Blood. 2007;109:2147–2155. doi: 10.1182/blood-2006-08-040022. [DOI] [PubMed] [Google Scholar]
- 49.Sexl V, Piekorz R, Moriggl R, Rohrer J, Brown MP, Bunting KD, et al. Stat5a/b contribute to interleukin 7-induced B-cell precursor expansion, but abl- and bcr/abl-induced transformation are independent of stat5. Blood. 2000;96:2277–2283. [PubMed] [Google Scholar]
- 50.Hoelbl A, Kovacic B, Kerenyi MA, Simma O, Warsch W, Cui Y, et al. Clarifying the role of Stat5 in lymphoid development and Abelson-induced transformation. Blood. 2006;107:4898–4906. doi: 10.1182/blood-2005-09-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang FC, Atkinson SJ, Gu Y, Borneo JB, Roberts AW, Zheng Y, et al. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci USA. 2001;98:5614–5618. doi: 10.1073/pnas.101546898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 54.Williams DA, Cancelas JA. Leukaemia: niche retreats for stem cells. Nature. 2006;444:827–828. doi: 10.1038/444827a. [DOI] [PubMed] [Google Scholar]
- 55.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 56.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 57.Gaiger A, Henn T, Horth E, Geissler K, Mitterbauer G, Maier-Dobersberger T, et al. Increase of bcr-abl chimeric mRNA expression in tumor cells of patients with chronic myeloid leukemia precedes disease progression. Blood. 1995;86:2371–2378. [PubMed] [Google Scholar]
- 58.Lin CS, Lim SK, D’Agati V, Costantini F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 59.Elmaagacli AH, Beelen DW, Opalka B, Seeber S, Schaefer UW. The amount of BCR-ABL fusion transcripts detected by the real-time quantitative polymerase chain reaction method in patients with Philadelphia chromosome positive chronic myeloid leukemia correlates with the disease stage. Ann Hematol. 2000;79:424–431. doi: 10.1007/s002770000169. [DOI] [PubMed] [Google Scholar]
- 60.Guo JQ, Wang JY, Arlinghaus RB. Detection of BCR-ABL proteins in blood cells of benign phase chronic myelogenous leukemia patients. Cancer Res. 1991;51:3048–3051. [PubMed] [Google Scholar]
- 61.Schultheis B, Szydlo R, Mahon FX, Apperley JF, Melo JV. Analysis of total phosphotyrosine levels in CD34+ cells from CML patients to predict the response to imatinib mesylate treatment. Blood. 2005;105:4893–4894. doi: 10.1182/blood-2005-01-0210. [DOI] [PubMed] [Google Scholar]
- 62.Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 63.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 64.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 65.Deininger MW. Optimizing therapy of chronic myeloid leukemia. Exp Hematol. 2007;35(4) Suppl 1:144–154. doi: 10.1016/j.exphem.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441–453. doi: 10.1038/nrc2147. [DOI] [PubMed] [Google Scholar]
- 67.Chu S, Xu H, Shah NP, Snyder DS, Forman SJ, Sawyers CL, et al. Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood. 2005;105:2093–2098. doi: 10.1182/blood-2004-03-1114. [DOI] [PubMed] [Google Scholar]