Abstract
Incentives have been successfully used to reduce smoking in hard-to-treat (HTT) smokers by progressively reinforcing lower levels of breath carbon monoxide (CO). When compared to schedules only providing incentives for smoking abstinence, using a progressive (percentile) criterion facilitates longer periods of smoking abstinence. However, participants receiving incentives for lower breath CO levels on percentile schedules typically earn more for their first abstinent breath CO sample, relative to participants receiving incentives only for smoking abstinence. Many studies show that larger incentive magnitude increases abstinence rates. The present study tested the effects of different incentive schedules on rates of abstinence maintenance while holding the initial incentive magnitude constant for 93 HTT smokers to eliminate initial abstinence incentive magnitude as a potential confound. Smokers were randomized to percentile, fixed criterion, or random incentive schedules. The incentive magnitude for the first abstinent breath CO sample (< 3 ppm) was $5.00 for percentile and fixed criterion incentive participants, and then increased by $0.50 for each consecutive abstinent breath CO sample. All groups had similar patterns of meeting the abstinence criterion for at least one visit. However, once this abstinence criterion was met, abstinence was more likely to be maintained by fixed criterion incentive participants. Unlike previous studies comparing percentile and fixed criterion schedules, percentile incentive schedules were not associated with longer periods of abstinence, relative to fixed criterion incentive schedules. Further studies that manipulate initial incentive magnitude are needed to test whether the difference between the current and previous studies was due to initial incentive magnitude.
Keywords: cigarettes, contingency management, hard-to-treat, shaping
Contingency management (CM) is a behavior modification procedure that provides incentives contingent on engaging in a target behavior (Higgins, Silverman, & Heil, 2008). For example, in a CM program for smoking cessation, a monetary incentive is available when a participant abstains from smoking for a certain period of time. Abstinence is verified by an objective test, such as breath carbon monoxide (CO). CM can reduce breath CO levels both in smokers not intending to quit (Romanowich & Lamb, 2013; Stitzer & Bigelow, 1982, 1984, Tidey, O’Neill & Higgins, 2002) and those with cessation plans (Dallery, Meredith & Glenn, 2008; Gilbert, Crauthers, Mooney, McClemon & Jensen, 1999; Meredith, Grabinski & Dallery, 2011; Rand, Stitzer, Bigelow & Mead, 1989; Shoptaw, Rotheram-Fuller, Yang, Frosch, Nahom, Jarvick et al., 2002).
CM smoking cessation treatments can be effective at decreasing smoking. However, how effective these treatments are, and for whom these treatments are effective, depends on the contingencies between the objective test of abstinence (in smoking cessation studies, this is most often breath CO) and incentive delivery. For instance, in a series of analog studies, Roll and co-workers showed that how the value of the incentive changes with increasing lengths of abstinence can be a determinant of how likely smokers are to be abstinent for an extended period. In particular, payment schedules that increase with each sequential abstinent sample delivery and reset to the original value with delivery of a non-abstinent sample are more effective at promoting longer periods of abstinence than schedules that non-contingently deliver incentives, schedules that deliver a fixed incentive amount and do not increase with increasing lengths of abstinence, and schedules that increase the available incentive with increasing abstinence lengths, but do not reset incentive value following the delivery of non-abstinent sample (Roll, Higgins, Badger, 1996; Roll & Higgins, 2000). However, these effects were apparent only in participants who initiated abstinence. Thus, in participants who initiate abstinence, escalating payment schedules with reset contingencies promote longer periods of meeting the abstinence target behavior.
Participants who do not readily initiate abstinence may be especially hard to treat. These hard to treat individuals may benefit from shaping. Shaping involves differentially reinforcing successive approximations of the target behavior (Catania 1988). Preston (Preston, Umbricht, Wong & Epstein, 2001) used a shaping procedure in cocaine abusers to promote abstinence from cocaine, and found that the shaping procedure resulted in more participants initiating abstinence. Participants in the shaping group could earn incentives for urine samples that showed a 25% reduction in cocaine metabolite levels from the previously collected sample, or for samples that were consistent with abstinence. A control group could only earn incentives for samples consistent with abstinence. These contingencies were in effect for 3 weeks, followed by 5 weeks during which both groups received incentives only for abstinent samples. During this second phase, shaping group participants were more likely to deliver an abstinent sample than control group participants. Thus, shaping appeared to improve abstinence likelihood in cocaine abusers.
Lamb and co-workers have also investigated using percentile schedules to shape abstinence in hard to treat smokers. Percentile schedules are a quantitative approach to shaping that increases contact with incentive contingencies (Galbicka, 1994). Percentile schedules provide an incentive for lower breath CO samples in a window of recent breath CO samples (Lamb, Morral, Kirby, Iguchi & Galbicka, 2004). This window refers to the number of previous samples included in the percentile calculation. For example, if the window is the last four breath CO samples, participants would only qualify for an incentive if their current breath CO was one of the lowest three of the last five samples, including the current breath CO sample (i.e., lower that the third best of the last four samples previously collected). This would define a 60th percentile incentive schedule. Additionally, abstinence is always reinforced. Thus, if abstinence is achieved through shaping of progressively lower breath CO samples, abstinence itself leads to an incentive. Percentile schedules have successfully reduced breath CO levels in smokers both intending to quit smoking (Lamb, Kirby, Morral, Galbicka & Iguchi, 2004; Lamb, Kirby, Morral, Galbicka & Iguchi, 2010) and smokers who do not have immediate cessation plans (Lamb, Kirby, et al., 2004; Lamb, Morral, Galbicka, Kirby & Iguchi, 2005; Lamb, Morral, Kirby, Javors, Galbicka & Iguchi, 2007). Of particular note, Lamb et al (2010) showed that shaping with a percentile schedule increased the number of positive outcomes among hard to treat smokers who did not readily initiate abstinence.
However, both the study by Preston et al (2001) and the studies by Lamb and co-workers used shaping procedures in conjunction with an escalating payment schedule. Combining these two procedures frequently resulted in shaping group participants earning a larger incentive for the first abstinent sample, relative to control group participants. For abstinence only participants, the available incentive could only increase for meeting the abstinence criterion. However, for shaping group participants abstinence was not required to receive an incentive; the magnitude for each successive criterion incentive could increase before abstinence was achieved. As a result of shaping group participants meeting the shaping criterion multiple consecutive times before achieving abstinence, the mean incentive earned for the first abstinent breath CO sample between the shaping and abstinence criterion groups was $8.47 and $2.50, respectively (Lamb et al., 2010). Higher potential earnings for delivering the first abstinent breath CO sample rather than shaping may explain the increased abstinence for shaping group participants. Research has consistently shown that larger potential incentives result in larger target behavior change during CM treatments (Lamb, Kirby et al., 2004; Romanowich & Lamb, 2010; Stitzer & Bigelow, 1983; Silverman, Chutuape, Bigelow & Stitzer, 1999). Thus, the current experiment was designed to test percentile schedules against an abstinence criterion incentive CM intervention for HTT smokers when incentive magnitudes for initial abstinence were equated. Given the large amount of CM research supporting the effect of incentive magnitude on abstinence rates, we hypothesized that equating the incentive magnitudes for the first abstinent breath CO sample would eliminate the difference in abstinence initiation between participants in the percentile and fixed criterion incentive conditions.
Methods
Participants
We recruited 94 participants working at or near University of Texas Health Science Center at San Antonio who smoked at least 15 cigarettes per day, smoked regularly for at least one year, and were planning to quit smoking within the next month. All participants were ≥ 18 years old at intake and produced an intake breath CO ≥ 15 ppm. Participants were expected to report to the research site and deliver a breath CO sample each workday (Monday – Friday), unless an absence was arranged ahead of time. Participants were paid $1.00 for each visit, regardless of breath CO level. Participants were also told what their breath CO criterion was for their next visit. This entire procedure generally took less than 5 minutes. Visits for most participants were scheduled in the morning between 7:30 a.m. and 10:00 a.m. Visits for workers on evening or night shifts coincided with the beginning of their shift, so that these visits were equivalent to the morning for individuals working more typical shifts.
Procedure
Vitalograph CO monitors (Vitalograph Inc. Lenexa, KS) were used to take breath CO samples. Participants were required to take a deep breath, hold it for 20 sec, and then to expire over 20 sec into the disposable mouthpiece of the CO monitor. The peak breath CO reading was taken as the participant’s breath CO level. Breath CO levels increase with increasing cigarette consumption and decline with abstention (Henningfield, Stitzer & Griffiths, 1980). We used an abstinence breath CO criterion of < 3 ppm based on our previous smoking cessation studies (Javors, Hatch & Lamb, 2005). During each visit participants completed a form inquiring about medication use to aid in smoking cessation in the past day and how much they had smoked. Participants returned these forms after receiving any earned payments, and were told that their answers to these questions did not affect earned payments.
All participants were classified as HTT based on their performance during a 5-visit abstinence trial. This abstinence trial began the day after intake was completed. During the abstinence trial, participants could receive $5.00 for each breath CO sample < 3 ppm (up to $25.00 total for the 5-visit trial). Breath CO samples ≥ 3 ppm did not earn an incentive. Participants that failed to deliver a single breath CO sample < 3 ppm during the 5-visit trial were classified as HTT. Participants producing at least one breath CO sample < 3 ppm were considered early successes and enrolled in an alternative CM smoking cessation study. Previous research has shown participants not able to deliver a single abstinent breath CO sample during an abstinence trial do more poorly during intervention, relative to participants delivering at least one abstinent sample (Lamb, Morral, et al., 2004).
Immediately after the 5-visit abstinence trial, HTT participants were randomly assigned to one of three groups: percentile, fixed criterion, or random incentives. Random assignment to one of the three groups was accomplished by randomly assigning 2 participants to the percentile group, 2 to the fixed criterion group and 1 to the random incentives group from each group of 5 participants completing intake. Approximately 40 participants were assigned to the percentile and fixed criterion groups, and 20 participants were assigned to the random incentives group. Randomization was stratified by intent to use medication to help them stop smoking, and by order of study entry.
Ninety-four participants completed intake, were classified as HTT based on their performance during the 5-visit abstinence trial, and were randomized to one of the three incentive groups. Table 1 shows demographic information for all 94 participants randomized by group. Participants in the three groups were statistically similar on all intake measures, as judged by t and probability tests, except for the proportion of participants attending Vocational/Technical School/Community College. A higher proportion of percentile incentive participants achieved this level of education, relative to fixed criterion participants (χ2 = 8.46, p < 0.01). After randomization, participants were expected to submit a breath CO sample every workday (Monday – Friday) for 60 visits, which lasted approximately 12 weeks.
Table 1.
| Condition
|
|||
|---|---|---|---|
| Percentile | Fixed Criterion | Random | |
| Number | 36 | 40 | 18 |
| Female (%) | 12 (33) | 12 (30) | 7 (39) |
| Age [M (SD)] | 44.9 (12.2) | 43.8 (12.7) | 43.1 (11.1) |
| Caucasian (%) | 27 (75) | 22 (55) | 12 (67) |
| Marital Status | |||
| Single (%) | 15 (42) | 15 (38) | 5 (28) |
| Married (%) | 11 (31) | 12 (30) | 5 (28) |
| Other (%) | 10 (28) | 13 (33) | 8 (44) |
| Income (US$) | |||
| < 15,000 (%) | 13 (36) | 12 (30) | 9 (50) |
| 15–24,999 (%) | 6 (17) | 6 (15) | 4 (22) |
| 25–34,999 (%) | 8 (22) | 8 (20) | 1 (6) |
| >35,000 (%) | 9 (25) | 14 (35) | 4 (22) |
| Employment | |||
| Full time (%) | 17 (47) | 25 (63) | 6 (33) |
| Education | |||
| GED or HS (%) | 10 (28) | 19 (48) | 7 (39) |
| Vo tech or AA (%) | 19 (53) | 9 (23) | 7 (39) |
| Bachelors + (%) | 7 (19) | 12 (30) | 4 (22) |
| Parent(s) smoked | |||
| Yes (%) | 33 (92) | 34 (85) | 14 (78) |
| Mom only (%) | 2 (6) | 5 (15) | 4 (29) |
| Dad only (%) | 12 (36) | 11 (32) | 3 (21) |
| Both (%) | 19 (58) | 18 (53) | 7 (50) |
| Age of | |||
| First cigarette [M (SD)] | 14.4 (3.4) | 15.7 (6.6) | 17.1 (5.2) |
| Regular smoker [M (SD)] | 17.2 (4.3) | 18.5 (6.3) | 19.7 (5.9) |
| Lives with a smoker | |||
| Yes (%) | 20 (56) | 23 (58) | 11 (61) |
| Previous Quit Attempt? | |||
| Yes (%) | 29 (81) | 35 (88) | 15 (83) |
| Average | |||
| Cigarettes per day [M (SD)] | 24.2 (8.5) | 24.2 (7.4) | 27.1 (9.4) |
| Intake Breath CO ppm [M (SD)] | 24.0 (7.9) | 24.2 (8.7) | 26.1 (9.0) |
Participants in the percentile group earned incentives by meeting a criterion set by a percentile schedule. The percentile schedule reinforced breath CO samples in the best 60th percentile of breath CO samples, and breath CO samples < 3 ppm (i.e., abstinence). A window of 4-visits was used. If the breath CO sample was < 3 ppm or the breath CO sample was lower than the third best sample relative to last four breath CO samples delivered, then that breath CO sample earned an incentive. For example, if the previous four breath CO samples were 9, 14, 12, 6 ppm, respectively, then a breath CO sample of < 12 ppm was required to earn the next incentive. Thus, each breath CO sample needed to be one of the three lowest from the past five delivered, or < 3 ppm, to qualify for an incentive. We used a 60th percentile schedule based on previous data showing lower breath CO levels for HTT smokers with less stringent (i.e., higher percentiles) percentile schedules (Lamb, Kirby, et al, 2004). In addition, we used a 4-visit window based on previous data showing lower breath CO levels in participants not seeking to quit smoking, relative to a 9-visit window (Lamb, Morral et al., 2005). Participants in the fixed criterion group only earned incentives for each breath CO sample < 3 ppm.
The value of the incentive for participants in both groups only increased with breath CO samples < 3 ppm. For participants in both the percentile and the fixed criterion groups, the value of the incentive started at $5.00 and increased by $0.50 with the delivery of each breath CO sample < 3 ppm. Thus, the second consecutive sample with a breath CO < 3 ppm earned $5.50, the third $6.00, and so on. The value of the incentive reset to $5.00 with either a missed visit or delivery of a breath CO sample ≥ 3 ppm. Delivery of 5 sequential breath CO samples < 3 ppm reinstated the highest incentive value obtained by that participant. Breath CO samples ≥ 3 ppm earned participants in the percentile group $5.00 when these samples met criteria (i.e., were in the 60th percentile). Hence, the incentive value earned for the first breath CO < 3 ppm was the same for participants in both groups: $5.00.
There was no breath CO level contingency for participants in the random incentives group. Participants in the random incentives group had a 60% chance of receiving an incentive on any given visit, contingent only on submitting a breath CO sample. The probability of receiving an incentive for each visit was independent of other visits. The 60% probability was chosen to equal the percentile value. Incentive value increased in the same manner as above, but was only dependent on attendance, not breath CO level. The value of the incentive increased with all attended visits despite the fact that on approximately 40% of these visits no incentive was delivered. For example, on the first visit a participant might not earn an incentive, but on the second sequential visit the participant could earn $5.50. For all three groups a maximum of $1185.00 was available during the 60-visit incentive period.
Immediately after randomization all participants were given a brief description of the incentive contingency that they were randomized into after the 5-visit abstinence trial. Percentile incentive participants were told that the value of the breath CO changed to help them smoke less. Fixed criterion incentive participants were told that they could only earn incentives for not smoking. Random incentive participants were told that they would receive incentives only for attendance; incentives were independent of amount smoked.
Data Analysis
Because participants were enrolled based on their inability to meet the breath CO < 3 ppm criterion during the 5-visit abstinence trial, the data were not assumed to be normally distributed. Therefore, we used Kruskal-Wallis non-parametric tests to estimate differences between the three groups. Based on our hypothesis, the most important comparison was between participants in the percentile and fixed criterion incentive groups. However, the number of participants in the random incentive group was much smaller than either of the other two groups. Thus, even if there was no difference between all three groups from the Kruskal-Wallis test, we still preformed Mann-Whitney tests for differences between participants in the percentile and fixed criterion incentive groups. We used chi-square tests for all proportional differences between groups. We included sex as a covariate in all analyses. Prior research has shown that women are less likely to quit smoking than men (Perkins & Scott, 2008; Piper, et al., 2007; Torchalla, Okoli, Hemsing & Greaves, 2011).
Results
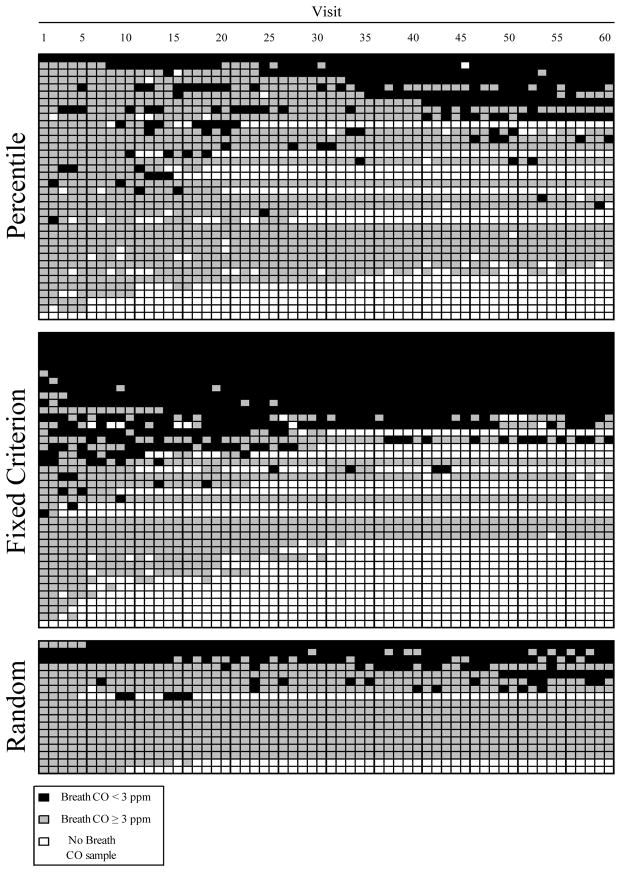
Figure 1 is an event record showing each participant’s (y-axis) breath CO sample as a function of study visit (x-axis) for each study group. During the 60-visit treatment phase 64% (23 of 36) and 63% (25 of 40) of percentile and fixed criterion incentive participants submitted at least one abstinent breath CO sample (< 3 ppm), respectively. Forty-four percent (8 of 18) of random incentive participants submitted at least one abstinence breath CO sample. There was no difference between any of the groups, or between sexes in terms of the proportion of participants able to provide at least one breath CO < 3 ppm (all χ2 tests, p > 0.05).
Figure 1.
Event records for percentile, fixed criterion and random incentive participants. An individual participant constitutes one row on the ordinate. Visit number is shown on the abscissa. Black areas represent visits with breath CO samples < 3 ppm. Gray areas represent visits with breath CO samples ≥ 3 ppm. White areas represent missed visits.
For those participants who were able to meet the breath CO criterion at least once, the number of visits before they produced a breath CO < 3 ppm differed between groups (H = 16.68, df (2), p < 0.01). Sex was not a significant covariate for time to first breath CO < 3 ppm (F = 1.67, df (1), p = 0.20). On average participants produced their first breath CO sample < 3 ppm on visit 19, 4 and 14 for percentile, fixed criterion and random incentive participants, respectively. Specifically, percentile incentive participants took longer to produce a breath CO sample < 3 ppm than fixed criterion incentive participants (z = 4.01, p < 0.01). For those participants that submitted at least one breath CO sample < 3 ppm, the total number of criterion breath CO samples submitted was not statistically different between the three groups (H = 5.89, df (2), p = 0.053). Sex was not a significant covariate for total number of submitted criterion breath CO samples (F = 0.03, df (1), p = 0.87). The average number of criterion breath CO samples was 9 (SD = 15), 40 (26), and 11 (19) for percentile, fixed criterion and random incentive participants, respectively. However, there was a significant difference between percentile and fixed criterion incentive participants for total number of criterion breath CO samples (z = 2.17, p = 0.03). On average, fixed criterion incentive participants produced more criterion breath CO samples than percentile incentive participants, given that they had produced at least one abstinent breath CO sample.
Figure 1 also shows the daily attendance of participants, in addition to delivered breath CO samples. The average number of delivered breath CO samples per participant (out of 60) was 42 (SD = 22), 40 (24), and 52 (19) for the percentile, fixed criterion and random incentive groups, respectively. The number of delivered breath CO samples was different between groups (H = 8.57, df (2), p < 0.02). However, there was no difference in the number of delivered breath CO samples between percentile and fixed criterion incentive participants (z = −0.10, p = 0.92). Sex was not a significant covariate for the total number of delivered breath CO samples (F = 1.09, df (1), p = 0.30).
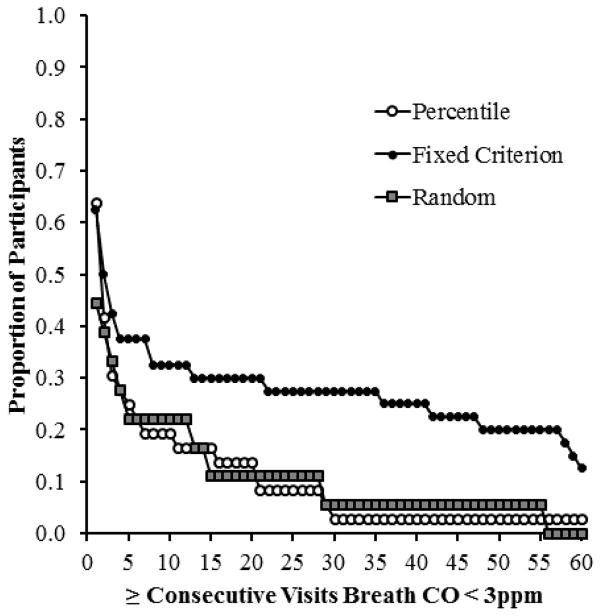
The ability to maintain breath CO levels < 3 ppm, once started was not different between groups (H = 4.65, df (2), p = 0.10). Sex was not a significant covariate for the ability to maintain breath CO levels < 3 ppm (F = 0.04, df (1), p = 0.84). There was a significant difference between percentile and fixed criterion incentive participants (z = 2.07, p = 0.04), with fixed criterion incentive participants maintaining a longer duration of breath CO levels < 3 ppm. Figure 2 shows this difference by plotting the proportions of participants delivering at least a given consecutive number of breath CO samples < 3ppm by group. The proportion of participants able to maintain breath CO levels < 3 ppm in the percentile and fixed criterion incentive groups began to diverge after about three consecutive breath CO samples < 3 ppm, with fixed criterion incentive participants better able to maintain longer periods of breath CO levels < 3 ppm relative to percentile incentive participants (0.43 v. 0.31). Conversely, by three consecutive breath CO samples < 3 ppm, the ability to maintain criterion breath CO levels was similar between percentile and random incentive participants (0.31 v. 0.33).
Figure 2.
Proportion of participants who achieved at least a given number of consecutive breath CO samples < 3 ppm across each group. Open circles, closed circles and gray squares represent the percentile, fixed criterion, and random incentive groups, respectively.
The ability to follow the first breath CO < 3 ppm with a second consecutive breath CO < 3 ppm was also different between percentile and fixed criterion participants. Of the 23 percentile incentive participants that had at least one breath CO sample < 3 ppm, only 6 had a breath CO sample < 3 ppm on the next visit. Conversely, of the 25 fixed criterion participants with at least one breath CO sample < 3 ppm, 16 had a breath CO sample < 3 ppm on the next visit. This difference was statistically significant (χ2 = 6.94; p < 0.02). However, for those 17 percentile incentive participants unable to produce a second consecutive breath CO < 3 ppm after the first one, 8 earned an incentive on the next visit for a breath CO ≥ 3 ppm. That is, on consecutive visits these participants earned $5.00 for both a breath CO < 3 ppm and a breath CO ≥ 3 ppm.
Fixed criterion incentive participants generally earned more than percentile incentive participants, although this difference was not statistically significant (z = −1.92, p = 0.055). On average, percentile incentive participants earned $181.63 (SD = $212.71), while fixed criterion incentive participants earned $323.01 ($479.83). Random incentive participants earned on average $639.64 ($283.06), but were not included in the statistical test because their probability for payments on any given visit was equivalent to the percentile incentive group (0.60). Given that a breath CO sample was delivered, the proportion of visits in which an incentive was earned was higher for percentile incentive participants, relative to fixed criterion incentive participants (z = −2.13, p = 0.03). The mean proportion of visits resulting in an incentive was 0.63, 0.39, and 0.65 for the percentile, fixed criterion and random incentive groups, respectively. These proportions were a direct result of the percentile and probability schedules for the percentile and random incentive groups.
Discussion
When the incentive for the first breath CO sample < 3 ppm was held constant for HTT smokers, fixed criterion incentive participants were faster to meet the abstinence criterion and produced more consecutive criterion breath CO samples than percentile incentive participants. This occurred even though percentile incentive participants were more likely to receive a contingent incentive for lowering their breath CO levels than fixed criterion incentive participants. These results suggest that using a shaping procedure without an escalating schedule for meeting the shaping requirements can be problematic. We hypothesize that these problematic effects result at least in part from shaping participants being exposed to two contingencies 1) delivering a second consecutive breath CO sample < 3 ppm and earning $5.50 and 2) delivering a breath CO sample meeting the shaping contingency to earn $5.00. Fixed criterion participants were only exposed to a contingency of delivering a breath CO sample < 3 ppm and earning $5.50, or otherwise not earning an incentive. For shaping participants, this $0.50 difference between continuing to abstain and continuing to smoke at a low rate seems to be less effective at promoting continued delivery of samples < 3 ppm than the fixed criterion participant’s $5.50 differential. This rationale is described in more detail below.
Percentile incentive participants frequently contacted the incentive contingency (approximately 63% of breath CO samples resulted in an incentive), yet infrequently delivered breath CO samples < 3 ppm, relative to fixed criterion participants. If a relative incentive increase ($0.50) was not large enough to motivate continued large changes in smoking, then percentile incentive participants should have alternated between decreasing and increasing their smoking when the costs of decreasing smoking were not sufficient based on the incentive available. This alternating pattern was frequently observed for percentile incentive participants. For instance, percentile incentive participants were less likely than fixed criterion participants to follow their first breath sample CO < 3 ppm with a second consecutive breath CO sample < 3 ppm. Yet, almost half of the percentile incentive participants that did not replicate their first breath CO sample < 3 ppm earned $5.00 for not replicating that abstinent breath CO level.
Conversely, fixed criterion participants were never faced with a choice of whether or not to decrease their breath CO by a small amount for an incentive – incentives were only earned for large changes in breath CO levels (< 3 ppm). That is, small changes in smoking resulted in $0, whereas large changes in smoking resulted in $5.00. It may be the case that percentile incentive participants learned more about decreasing smoking than they did about eliminating smoking. Perhaps more participants would have maintained criterion breath CO levels if the incentive difference between decreasing and the criterion breath CO level was larger (e.g., $2.50 difference instead of a $0.50 difference) for percentile incentive participants. While this idea has not explicitly been tested in HTT smokers, it leads directly from the percentile contingency in Lamb et al (2010). On average, percentile incentive participants received $8.47 for their first abstinent breath CO sample, while only receiving $2.50 for their first percentile criterion breath CO sample. Perhaps more importantly, a failed breath CO sample would decrease the next incentive for a percentile criterion breath CO sample to $2.50. In the current study, this reset contingency decreased the next percentile criterion breath CO sample to $5.00.
While incentive magnitude was not manipulated in this study, other studies have shown that initial incentive magnitude may not be an important factor in abstinence rates. For example, in two experiments (Correia, Sigmon, Silverman, Bigelow & Stitzer, 2005; Silverman, Wong, Umbricht-Schneiter, Montoya, Shuster & Preston, 1998) $50 start-up bonuses did not increase either brief (Correia et al., 2005) or sustained cocaine abstinence (Silverman et al., 1998), relative to escalating-only incentive participants. More recently, Higgins et al. (2014) demonstrated that increasing the incentive amounts during the initial weeks of an escalating schedule produced no better outcomes in pregnant women’s cessation attempts. More importantly, the increased incentive amounts were no better than a non-contingent incentive condition at increasing fetal growth. Participants that only experienced an escalating schedule did show increased fetal growth, relative to both the increased initial incentive and non-contingent incentive participants. Thus, simply increasing incentive amounts during the beginning of an escalating incentive schedule does not increase abstinence, and in some cases may decrease outcome effects. This is also generally consistent with the current results, in that an escalating incentive schedule with a reset contingency is better than an incentive schedule with additional modifications (i.e., shaping or early incentive magnitude) at maintaining abstinence.
There were no sex differences for any of the measured dependent variables. While prior research has found that females are less likely to abstain from smoking during smoking cessation pharmacotherapies (Perkins & Scott, 2008; Piper, et al., 2007; Torchalla, et al., 2011), sex differences for contingency management have, when reported, generally not been statistically significant. In one of the largest incentive-based randomized control smoking cessation trials to date, Volpp et al. (2010) did not show a significant effect of sex on long-term (9- or 12-months post-treatment) smoking cessation. If this finding is consistently replicated, incentive-based smoking cessation treatments could have an advantage for female participants over pharmacotherapies. However, at this point it is premature to conclude that the effects of contingency management for smoking cessation are relatively equal between sexes. Additional research is necessary to determine how reliable this finding is.
The current study also has weaknesses, some of which are related to the points made above. First, we did not include a percentile incentive group that earned escalating incentives for each percentile criterion breath CO sample. Including this group and replicating the previous results would have strengthened our conclusion that the combination of an escalating incentive contingency and an increased magnitude for abstinence initiation produced the findings in Lamb et al. (2010) with HTT smokers. Also, although we tried to make sure that all of the participants understood the incentive contingencies, we did not include any systematic way of measuring whether or not participants fully comprehended the incentive contingency that they were randomized into.
In general, our results show the importance of incentive schedules on subsequent patterns of behavior. It is likely that the incentive contingencies immediately after the first abstinent breath CO sample led to the group differences in sequential abstinent breath CO samples. This is where there was the largest relative difference in potential incentives between groups, as described earlier. However, future studies will need to manipulate the initial incentive magnitude to determine how important initial incentive magnitude is for explaining the differences between the current results and previous experiments (Lamb et al., 2010; Preston et al., 2001).
Acknowledgments
The research reported in this paper was supported by grant DA013304 to R. J. Lamb.
References
- Catania AC. Learning. Upper Saddle River, NJ: Prentice Hall; 1998. [Google Scholar]
- Correia CJ, Sigmon SC, Silverman K, Bigelow G, Stitzer ML. A comparison of voucher-delivery schedules for the initiation of cocaine abstinence. Experimental and Clinical Psychopharmacology. 2005;13:253–258. doi: 10.1037/1064-1297.13.3.253. [DOI] [PubMed] [Google Scholar]
- Dallery J, Meredith S, Glenn IM. A deposit contract method to deliver abstinence reinforcement for cigarette smoking. Journal of Applied Behavior Analysis. 2008;41:609–615. doi: 10.1901/jaba.2008.41-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbicka G. Shaping in the 21st century: moving percentile schedules into applied settings. Journal of Applied Behavior Analysis. 1994;27:739–760. doi: 10.1901/jaba.1994.27-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA. Effects of monetary contingencies on smoking relapse: Influences of trait depression, personality, and habitual nicotine intake. Experimental and Clinical Psychopharmacology. 1999;7:174–181. doi: 10.1037//1064-1297.7.2.174. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stitzer ML, Griffiths RR. Expired air carbon monoxide accumulation and elimination as a function of number of cigarettes smoked. Addictive Behaviors. 1980;5:265–272. doi: 10.1016/0306-4603(80)90049-0. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH, editors. Contingency management in substance abuse. New York: Guilford Press; 2008. [Google Scholar]
- Higgins ST, Washio Y, Lopez AA, Heil SH, Solomon MEL, Hanson JD. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Preventative Medicine. 2014 doi: 10.1016/j.ypmed.2014.03.024. http://dx.doi.org/10.1016/j.ypmed.2014.03.024. [DOI] [PMC free article] [PubMed]
- Javors MA, Hatch JP, Lamb RJ. Evaluation of cut-off levels for breath carbon monoxide as a marker for cigarette smoking over the past 24 hours. Addiction. 2005;100:159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Improving contingency management programs for addiction. Addictive Behaviors. 2004;29:507–523. doi: 10.1016/j.addbeh.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Shaping smoking cessation in hard-to-treat smokers. Journal of Consulting and Clinical Psychology. 2010;78:62–71. doi: 10.1037/a0018323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Galbicka G, Kirby KC, Iguchi MY. Shaping reduced smoking in smokers without cessation plans. Experimental and Clinical Psychopharmacology. 2005;13:83–92. doi: 10.1037/1064-1297.13.2.83. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Iguchi MY, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence. 2004;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Javors MA, Galbicka G, Iguchi MY. Contingencies for change in complacent smokers. Experimental and Clinical Psychopharmacology. 2007;15:245–255. doi: 10.1037/1064-1297.15.3.245. [DOI] [PubMed] [Google Scholar]
- Meredith SE, Grabinski MJ, Dallery J. Internet-based group contingency management to promote abstinence from cigarette smoking: a feasibility study. Drug and Alcohol Dependence. 2011;118:23–30. doi: 10.1016/j.drugalcdep.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine & Tobacco Research. 2008;10:1245–1251. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Efficacy of buproprion alone and in combination with nicotine gum. Nicotine & Tobacco Research. 2007;9:947–954. doi: 10.1080/14622200701540820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Umbricht A, Wong CJ, Epstein DH. Shaping cocaine abstinence by successive approximation. Journal of Consulting and Clinical Psychology. 2001;69:643–654. doi: 10.1037//0022-006x.69.4.643. [DOI] [PubMed] [Google Scholar]
- Rand CS, Stitzer ML, Bigelow GE, Mead AM. The effects of contingent payment and frequent workplace monitoring on smoking abstinence. Addictive Behavior. 1989;14:121–128. doi: 10.1016/0306-4603(89)90041-5. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST. A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug and Alcohol Dependence. 2000;58:103–109. doi: 10.1016/s0376-8716(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ. An experimental comparison of three difference schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis. 1996;29:495–505. doi: 10.1901/jaba.1996.29-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P, Lamb RJ. Effects of escalating and descending schedules of incentives in cigarette smoking in smokers without plans to quit. Journal of Applied Behavior Analysis. 2010;43:357–367. doi: 10.1901/jaba.2010.43-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowich P, Lamb RJ. The effect of framing incentives as either losses or gains with contingency management for smoking cessation. Addictive Behaviors. 2013;38:2084–2088. doi: 10.1016/j.addbeh.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvick ME, Rawson RA, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, Stitzer ML. Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology. 1999;146:128–138. doi: 10.1007/s002130051098. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. Journal of Consulting and Clinical Psychology. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced carbon monoxide levels in cigarette smokers. Addictive Behaviors. 1982;7:403–412. doi: 10.1016/0306-4603(82)90010-7. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent payment for carbon monoxide reduction: effects of pay amount. Behavior Therapy. 1983;14:647–656. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for carbon monoxide reduction: within-subject effects of pay amount. Journal of Applied Behavior Analysis. 1984;17:477–483. doi: 10.1901/jaba.1984.17-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, O’Neill SC, Higgins ST. Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine in outpatients with schizophrenia. Experimental and Clinical Psychopharmacology. 2002;10:241–247. doi: 10.1037//1064-1297.10.3.241. [DOI] [PubMed] [Google Scholar]
- Torchalla I, Okoli C, Hemsing N, Greaves L. Gender differences in smoking behavior and cessation. Journal of Smoking Cessation. 2011;6:9–16. [Google Scholar]
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine. 2010;360:699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]