Abstract
Increased mean platelet volume (MPV) is a marker of platelet activation. Platelet activation with cocaine use is not well studied. We wanted to investigate MPV levels in patients with cocaine-associated chest pain (CACP) as a marker of platelet activation. Retrospectively, MPV of 82 consecutive patients with CACP (group 1) with positive urine drug screen (UDS), without acute myocardial infarction (AMI) (group 1A) and with AMI with elevated troponin (group 1B), were included in the study. The control group (group 2) consisted of 89 consecutive patients admitted during the same time period with acute chest pain (ACP) who had negative UDS and negative cardiac markers with a normal cardiac stress test or normal coronary angiogram. Analysis showed no statistically significant difference of MPV between group 1, 8.46 ± 1.06 fL, versus group 2, 8.7 ± 1.07 fL; p = 0.142; and between group 1A, 8.46 ± 1.05 fL, and group 1B, 8.46 ± 1.09 fL; p = 0.983. By multiple linear regression analysis, MPV was not influenced by cocaine abuse (R = 0.269, R2 = 0.072, adjusted R2 = −0.009, p = 0.562). MPV is not elevated in patients with cocaine use even when they had AMI. Further studies may be necessary to investigate the role of platelet activation in patients with cocaine use and chest pain.
Keywords: cocaine use, mean platelet volume, platelet
Introduction
Cocaine is a naturally occurring substance found in South America’s Erythroxylum coca plant that has been in use for more than 5000 years [1]. Cocaine, one of the most commonly used illicit drugs in United States, is associated with a wide spectrum of cardiovascular complications including myocardial infarction first reported in 1982 [2]. Every year, more than 500,000 patients present to hospital emergency department with cocaine-associated cardiovascular complications, most commonly chest pain [3]. Many cases of myocardial infarction have been reported in cocaine users who have normal coronaries [4]. The exact pathogenesis of cocaine-associated chest pain (CACP) and myocardial infarction is not clear in users with normal coronary anatomy but has been hypothesized to be due to coronary vasoconstriction, focal coronary vasospasm, increased oxygen demand, and transient coronary thrombosis [5]. Cocaine can also promote endothelial dysfunction in subjects with significant coronary artery disease (CAD) and facilitate thrombus formation in diseased vessels [5]. In patients with cocaine-induced myocardial infarction, platelet-rich coronary thrombi were observed which suggested activation of platelets [6]. Increased platelet factor 4, β-thromboglobulin in nasal cocaine users [7], and a delayed increase in platelet P-selectin expression in conscious dogs with cocaine infusion [8] also suggest platelet activation with cocaine. Despite these findings, the role of platelet activation in patients with CACP remains unclear.
Mean platelet volume (MPV) has been shown to be an indicator of platelet activity. Higher MPV values are seen in patients with myocardial infarction and unstable angina [9]. Larger platelets contain more dense granules, produce more thromboxane A2, and express more glycoprotein Ib and IIb/IIIa receptors [10]. However, there are no published studies on the effect of cocaine use on MPV. We hypothesized that, in patients with CACP, especially with acute coronary syndrome, MPV will be increased, and this may reflect platelet activation in CACP.
Materials and Methods
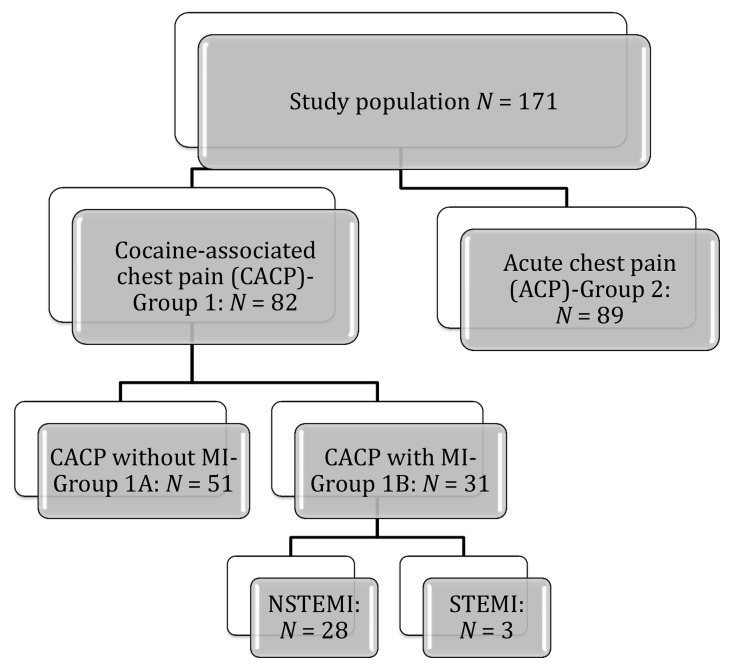
The study was a retrospective study. All patients seen in an urban university hospital between 2002 and 2010 were identified by cross-referencing the International Classification of Diseases, Ninth Revision (ICD-9), 970.81, 304.20, and 304.21 diagnostic codes in medical records’ search for cocaine use and chest pain/acute coronary syndromes. Universal definition for myocardial infarction is used to define AMI [11]. Positive cardiac marker is defined as troponin level with at least one value above the 99th percentile of the upper reference limit. Patients were included in the study if they had a documented history of cocaine use with urine or blood toxicology screen that revealed cocaine or cocaine metabolites and chest pain (Fig. 1). We included 82 consecutive cocaine-associated chest pain (CACP) patients (group 1) without myocardial infarction (group 1A, n = 31) and with myocardial infarction (group 1B, n = 51) who had positive blood or urine toxicology screen for cocaine in the study. We also reviewed the medical records of 89 consecutive patients with no known history of coronary artery disease and admitted during the same time period with acute chest pain (ACP), who had negative urine drug screen (UDS) and negative cardiac markers with a normal cardiac stress test and/or a normal coronary angiogram (group 2). Patients with CACP with no drug screen or negative drug screen and ACP patients with no drug screen were excluded in this study. In group 2, three patients had ST-elevation myocardial infarction; the rest had non-ST-elevation myocardial infarction. In this group, 24 patients had cardiac catheterization, and 9 of them required percutaneous intervention for obstructive CAD. Data collected included demographics, medical and cocaine use history, presenting characteristics, and diagnostic tests. Laboratory data were obtained from the time of admission by using the computerized database including platelet indices, platelet count, and white blood cell (WBC) count. As per the hospital’s policy, blood samples were drawn by vein puncture and collected in standard sterile dipotassium EDTA tubes. Complete blood count analyses were performed in the same Coulter analyzer model LH within 1 h as a standard in our institution, and analyzer was routinely checked for quality control. The reference value for MPV ranged between 6.5 and 10.9 fL. The hospital’s institutional review board approved this study.
Fig. 1.
Flow chart showing study design; cocaine-associated chest pain (CACP), acute chest pain (ACP), myocardial infarction (MI), non-ST elevation myocardial infarction (NSTEMI), ST elevation myocardial infarction (STEMI), N: number of patients
Statistical analysis
Data was analyzed with Statistical Package for Social Sciences version 10 (SPSS for Windows 17, Inc. Chicago, IL, USA). A p value of <0.05 was considered for statistical significance. The data was analyzed using an independent sample t-test, chi-square, and multiple linear regression analysis depending on the variables.
Results
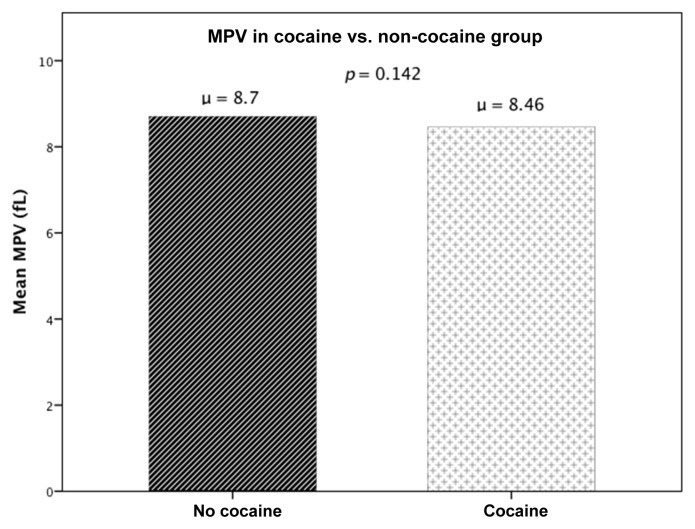
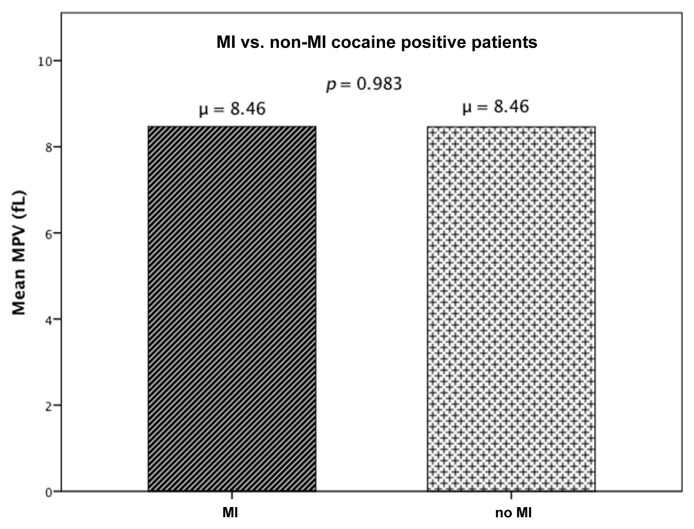
The study population consisted of 171 patients, out of whom the CACP group (group 1) had 82 patients and 89 patients in the control group (group 2). The demographic and medical treatment characteristics of the two groups were studied and compared as shown in Table I, CACP versus control group. An independent sample t‑test revealed that there was no statistically significant difference in MPV between CACP (group 1), 8.46 ± 1.06 fL, and control (group 2), 8.7 ± 1.07 fL; p = 0.142 (Fig. 2). Since the two groups had significantly different composition in terms of their demographic and clinical characteristics, to define independent factors influencing MPV, multiple linear regression analysis was used with MPV being the dependent variable for the equation and Cocaine group, age, gender, race, hypertension, diabetes, hyperlipidemia, congestive heart failure, cerebrovascular accident, end stage renal disease, smoking, and alcohol as the independent variables. MPV was not influenced by cocaine abuse (R = 0.269, R2 = 0.072, adjusted R2 = −0.009, p = 0.562), despite controlling for the possible confounding factors. To study MPV levels within cocaine users with and without AMI, an independent sample t-test was done comparing MPV levels in group 1A (CACP but no AMI) and group 1B (CACP with AMI). There was no statistically significant difference in MPV between the groups (group 1A: 8.46 ± 1.05 fL, group 1B: 8.46 ± 1.09 fL; p = 0.983) (Fig. 3). The medications that the patients started in emergency room were also compared between the groups; except β-blockers (p < 0.001), there was no difference between group 1 and group 2 (Table II).
Table I.
Clinical characteristics of the study population
| Total, N = 171 |
p | |||||||
|---|---|---|---|---|---|---|---|---|
| CACP, group 1 (n = 82) |
ACP, group 2 (n = 89) |
|||||||
| Mean ± SD | Count | Column, n % | Mean ± SD | Count | Column, n % | |||
| Age | 48 ± 7.92 | 52 ± 11.32 | 0.018* | |||||
| Sex | Female | 22 | 26.8 | 47 | 52.8 | 0.001* | ||
| Male | 60 | 73.2 | 42 | 47.2 | ||||
| Race | Non-Black | 13 | 15.9 | 36 | 40.4 | <0.001** | ||
| Black | 69 | 84.1 | 53 | 59.6 | ||||
| Hypertension | No | 22 | 27.2 | 19 | 21.3 | 0.376 | ||
| Yes | 59 | 72.8 | 70 | 78.7 | ||||
| Diabetes | No | 66 | 81.5 | 55 | 61.8 | 0.005* | ||
| Yes | 15 | 18.5 | 34 | 38.2 | ||||
| Dyslipidemia | No | 51 | 63.0 | 47 | 52.8 | 0.181 | ||
| Yes | 30 | 37.0 | 42 | 47.2 | ||||
| Obesity | No | 65 | 84.4 | 57 | 64.0 | 0.003* | ||
| Yes | 12 | 15.6 | 32 | 36.0 | ||||
| Stroke | No | 74 | 91.4 | 78 | 87.6 | 0.431 | ||
| Yes | 7 | 8.6 | 11 | 12.4 | ||||
| Peripheral arterial disease | No | 78 | 96.3 | 88 | 98.9 | 0.268 | ||
| Yes | 3 | 3.7 | 1 | 1.1 | ||||
| End-stage renal disease | No | 76 | 95.0 | 89 | 100.0 | 0.048* | ||
| Yes | 4 | 5.0 | 0 | 0.0 | ||||
| Smoking | No | 9 | 11.1 | 66 | 74.2 | <0.001** | ||
| Yes | 72 | 88.9 | 23 | 25.8 | ||||
| Alcohol | No | 22 | 27.2 | 60 | 67.4 | <0.001** | ||
| Yes | 59 | 72.8 | 29 | 32.6 | ||||
| Platelet count | 250.05 ± 98.94 | 254.84 ± 89.80 | 0.742 | |||||
| Hemoglobin | 13.66 ± 2.52 | 14.03 ± 2.20 | 0.313 | |||||
| CACP = cocaine-associated chest pain, ACP = acute chest painData are expressed as mean ± SD, as number of percentagep value is comparing the group 1 with group 2*p < 0.05**p < 0.001 | ||||||||
Fig. 2.
Showing comparison of MPV between cocaine-associated chest pain and control group
Fig. 3.
MPV levels in cocaine-associated chest pain (CACP) but no acute myocardial infarction (AMI) and CACP with AMI
Table II.
Medical treatment characteristics of both groups
| CACP, group 1 |
ACP, group 2 |
p | ||||
|---|---|---|---|---|---|---|
| Count | Column, n % | Count | Column, n % | |||
| Aspirin | No | 12 | 14.8 | 8 | 9.0 | 0.239 |
| Yes | 69 | 85.2 | 81 | 91.0 | ||
| P2y12 inhibitor | No | 47 | 58.0 | 56 | 62.9 | 0.514 |
| Yes | 34 | 42.0 | 33 | 37.1 | ||
| ACE inhibitor | No | 18 | 22.2 | 14 | 15.7 | 0.276 |
| Yes | 63 | 77.8 | 75 | 84.3 | ||
| Beta blocker | No | 61 | 75.3 | 21 | 23.6 | <0.001 |
| Yes | 20 | 24.7 | 68 | 76.4 | ||
| Statins | No | 27 | 33.3 | 19 | 21.3 | 0.079 |
| Yes | 54 | 66.7 | 70 | 78.7 | ||
| p value is comparing the group 1 with group 2CACP = cocaine-associated chest pain, ACP = acute chest painData are expressed as count, as number of percentageStatistically significant p < 0.05 | ||||||
Discussion
Mean platelet volume is a simple method of platelet function easily available on the routine complete blood count panel. The change in MPV occurs quickly in response to platelet activation. Because of these features, MPV has been studied as an indicator of platelet activation, and increased MPV has been reported in patients with known CAD risk factors such as smoking, diabetes mellitus, obesity, hypertension, and increased cholesterol compared to healthy controls. Patients with unstable angina have also been reported to have increased MPV compared to patients with stable angina [12].
Cocaine causes acute increase in heart rate, systolic and diastolic blood pressure [13], vasoconstriction in coronary arteries [10], accelerated coronary atherosclerosis [15], and direct myocardial injury [16]. All of these can contribute to cocaine-associated chest pain and also may affect platelets directly or indirectly. Despite that, in our study, we did not find any significant difference in MPV between patients with CACP and patients presenting with ACP who were not cocaine users and had negative UDS for cocaine. MPV levels were also not elevated in 51 of 82 patients with CACP who had AMI compared to ACP group. In fact, although there was no statistical significance, the mean MPV value in CACP patients was lower than the ACP group (8.46 ± 1.06 fL versus 8.7 ± 1.07 fL; p = 0.142). Of note, smoking and alcohol use were more common in the CACP group (Table I); we know that smoking can increase MPV, but the effect of alcohol on MPV level is not well known. The medical treatment used was also similar in both groups except β-blockers which are less commonly used in CACP group (Table II). In a recent study of hypertensive patients, 15–30 days after treatment with either selective or nonselective β-blockers, an increase in MPV with contraction of spleen has been reported [17]. Even then, in our study, MPV was lower in CACP group despite less β-blocker use.
Prognostic value of MPV also has been investigated in several studies. Patients with acute myocardial infarction (AMI) with an elevated MPV had a significantly higher risk of death compared to those with a normal MPV in these studies [9, 18, 19]. Mean platelet volume of more than 9 fL has also been shown to be associated with higher cardiovascular event rate in patients with coronary artery ectasia [20]. We do not know if lack of elevation in MPV in CACP patients even in those with AMI suggests a favorable outcome. More prospective studies will be needed for evaluation of prognostic significance of MPV levels in patients with cocaine-induced AMI.
Despite several studies, it is not very clear if MPV can be a used as a biomarker of platelet activity in an individual patient in clinical practice. There are methodological variables that may affect the MPV results, in addition to method of venipuncture, the degree of accuracy of filling, how the sampling tubes mixed, choice of anticoagulant used in tubes, and temperature at which MPV is analyzed [21]. MPV even differs in freshly produced platelets and in aging platelets [21]. All of this information brings up the question if the lack of difference in MPV levels in our study could be secondary to MPV being not a particularly sensitive marker of platelet activation. Perhaps other measures of overall platelet activation would have found a difference since there are some other studies suggestive of platelet activation with cocaine use. In a study of 14 healthy volunteers, Heesch et al. have shown an increase in platelet factor 4 and β-thromboglobulin 120 min after receiving intranasal cocaine [7]. Siegel et al. have shown elevated CRP, vWF, and fibrinogen in 10 cocaine-dependent users (6–20 uses of cocaine by inhalation per week over prior year; mean age, 41 + 6 years), compared to normal levels in 10 cocaine abusers (2–6 intranasal uses of cocaine per month over prior year; mean age, 26 + 4) with negative drug screen [22]. These studies were done in small number young volunteers without any symptoms. In contrast, our study population is older with significant symptoms and needed to be admitted to hospital.
Our study had limitations; it was a retrospective, single-center study, and the number of patients was limited. Body mass index can influence MPV, and this was not included in our analysis. MPV is known to increase over time in EDTA-anticoagulated samples, and the time delay between sample collection and laboratory analysis can affect MPV measurement. The recommended optimal measurement time for MPV is 120 min after the vein puncture. In our laboratory, the time interval is less than an hour, and this would have minimized the EDTA-induced platelet swelling effect in our study.
The control group did not consist of totally “healthy” people which is also a limitation of our study. In our study as a control group, we needed to include patients admitted at the same time interval who had negative urine drug screen (UDS), and to get as healthy as possible, we included patients with no known history of coronary artery disease and negative cardiac markers with a normal cardiac stress test and/or a normal coronary angiogram. As seen in Table I, smoking and alcohol were also statistically less consumed in control group compared to our study group.
Conclusions
MPV is not elevated in patients admitted with CACP whether they have AMI or not. Further studies may be necessary to investigate the role of platelet activation with cocaine use and associated chest pain since MPV may not be a sensitive marker of platelet activation in this population.
Funding Statement
Funding sources: None.
Footnotes
Authors’ contribution: NIA: designed the study, wrote and revised the manuscript; SR: collected data, wrote part of the manuscript, designed the study; AV: collected the data; AP: collected the data; AD: col-lected the data, made the tables; SS: did statistical analysis, made the tables and figures; PCR: revised the manuscript. All authors had full access to all data in the study and we all take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: The authors declare no conflict of interest.
Contributor Information
Nuri Ilker Akkus,
Saurabh Rajpal,
Andres Vargas,
Anderson Penuela,
Ashish Dwary,
Shivang H. Shah,
Pratap C. Reddy,
References
- 1.Gold M. Cocaine. New York: Plenum Publishing Corporation; 1993. pp. 11–24. [Google Scholar]
- 2.Coleman DL, Ross TF, Naughton JL. Myocardial ischemia and infarction related to recreational cocaine use. West J Med. 1982;136:444–446. [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel JB, Marhefka GD. Rethinking cocaine-associated chest pain and acute coronary syndromes. Mayo Clin Proc. 2011;86:1198–1207. doi: 10.4065/mcp.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minor RL, Scott DS, Brown DD, Winniford MD. Cocaine-induced myocardial infarction in patients with normal coronary arteries. Ann Intern Med. 1991;115:797–806. doi: 10.7326/0003-4819-115-10-797. [DOI] [PubMed] [Google Scholar]
- 5.Kloner RA, Hale S, Alker K, Rezkalla S. The effects of acute and chronic cocaine use on the heart. Circulation. 1992;85:407–419. doi: 10.1161/01.cir.85.2.407. [DOI] [PubMed] [Google Scholar]
- 6.Simpson RW, Edwards RD. Pathogenesis of cocaine-induced ischemic heart disease. Arch Pathol Lab Med. 1986;110:479–484. [PubMed] [Google Scholar]
- 7.Heesch CM, Wilhelm CR, Ristich J, Adnane J, Bontempo FA, Wagner WRY. Cocaine activates platelets and increases the formation of circulating platelet containing microaggregates in humans. Heart. 2000;83:688–695. doi: 10.1136/heart.83.6.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kugelmass AD, Shannon RP, Yeo EL, Ware JA. Intravenous cocaine induces platelet activation in the conscious dog. Circulation. 1995;91:1336–1340. doi: 10.1161/01.cir.91.5.1336. [DOI] [PubMed] [Google Scholar]
- 9.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. Epub 2009 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles H, Smith REA, Martin JF. Platelet glycoprotein IIb–IIIa and size are increased in acute myocardial infarction. Eur J Clin Invest. 1994;24:69–72. doi: 10.1111/j.1365-2362.1994.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction: universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. see also: Circulation 116, 2634–2653 (2007); J Am Coll Cardiol 50, 2173–2195 (2007) [DOI] [PubMed] [Google Scholar]
- 12.Boos CJ, Lip GY. Assessment of mean platelet volume in coronary artery disease – what does it mean? Thromb Res. 2007;120:11–13. doi: 10.1016/j.thromres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Foltin RW, Ward AS, Haney M, Hart CL, Collins ED. The effects of escalating doses of smoked cocaine in humans. Drug Alcohol Depend. 2003;70:149–157. doi: 10.1016/s0376-8716(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 14.Molitemo DJ, Willard JE, Lange RA. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N Engl J Med. 1994;330:454–459. doi: 10.1056/NEJM199402173300702. [DOI] [PubMed] [Google Scholar]
- 15.Dressier FA, Malekzadeh S, Roberts WC. Quantitative analysis of amounts of coronary arterial narrowing in cocaine addicts. Am J Cardiol. 1990;65:303–308. doi: 10.1016/0002-9149(90)90292-9. [DOI] [PubMed] [Google Scholar]
- 16.Peng SK, French WJ, Pelikan PC. Direct cocaine cardiotoxicity demonstrated by endomyocardial biopsy. Arch Pathol Lab Med. 1989;113:842–845. [PubMed] [Google Scholar]
- 17.Bakovic D, Pivac N, Eterovic D, Palada I, Valic Z, Paukovic-Sekulic B, Dujic Z. Changes in platelet size and spleen volume in response to selective and non-selective β-adrenoceptor blockade in hypertensive patients. Clin Exp Pharmacol Physiol. 2009;36:441–446. doi: 10.1111/j.1440-1681.2008.05090.x. [DOI] [PubMed] [Google Scholar]
- 18.Burr ML, Holliday RM, Fehily AM, Whitehead PJ. Haematological prognostic indices after myocardial infarction: evidence from the diet and reinfarction trial (DART) Eur Heart J. 1992;13:166–170. doi: 10.1093/oxfordjournals.eurheartj.a060141. [DOI] [PubMed] [Google Scholar]
- 19.Pabón Osuna P, Nieto Ballesteros F, Morinigo Muñoz JL, Sánchez Fernández PL, Arribas Jiménez A, Diego Domínguez M, Martín Luengo C. The effect of the mean platelet volume on the short-term prognosis of acute myocardial infarct. Rev Esp Cardiol. 1998;51:816–822. [PubMed] [Google Scholar]
- 20.Varol E, Uysal BA, Dogan A, Ozaydin M, Erdogan D. Mean platelet volume has a prognostic value in patients with coronary artery ectasia. Clin Appl Thromb Hemost. 2012;18:387–392. doi: 10.1177/1076029611427441. [DOI] [PubMed] [Google Scholar]
- 21.Lancé MD, Sloep M, Henskens YM, Marcus MA. Mean platelet volume as a diagnostic marker for cardiovascular disease: drawbacks of preanalytical conditions and measuring techniques. Clin Appl Thromb Hemost. 2012;18:561–568. doi: 10.1177/1076029612458147. [DOI] [PubMed] [Google Scholar]
- 22.Siegel AJ, Mendelson JH, Sholar MB, McDonald JC, Lewandrowski KB, Lewandrowski EL, Lipinska I, Ridker PM, Tofler GH. Effect of cocaine usage on C-reactive protein, von Willebrand factor, and fibrinogen. Am J Cardiol. 2002;89:1133–1135. doi: 10.1016/s0002-9149(02)02289-0. [DOI] [PubMed] [Google Scholar]