Abstract
Materials and Methods
Chemical composition was studied with the help of the scanning electron microscope with energy-dispersion spectrometer. Immunohistochemical reaction showed the p53 and Ki-67 receptors expression. The study of DNA fragmentation was performed in agarose gel.
Results
There was an interrelation between the accumulations of the trace elements with the degree of cancer malignancy. There were 85% of cases with positive reaction to Ki-67 and 40% cases with positive reaction to p53. We found a moderate correlation between the accumulation of microelements in the breast cancer tissue and the level of proliferative activity. We noted the combination of the increase of DNA fragmentation with the expression of p53 and Ki-67 receptors.
Conclusions
The trace elements can cause the initiation and the progression of the tumorous growth, which is expressed in the increased proliferation of tumor cells. This leads to the destabilization of the genetic material which can be expressed in the synthesis of mutant p53 protein. Finally, it leads to the block of apoptosis and regulatory effects of cells. This can cause the tumor progression and the destabilization of the genome, which is reflected in the increased DNA fragmentation.
Keywords: apoptosis, breast cancer, DNA fragmentation, heavy metal, proliferation
Introduction
Breast cancer (BC) occupies the first place among all localizations of women’s cancer. In Ukraine, a new case of disease is diagnosed every 35–37 min and every hour a woman dies from BC [1]. Hormonal disturbances, genetic predisposition, environmental factors, benign breast tumors, nutrition disorders, and viral contamination were described as etiological factors of BC [2, 3]. There is ample support for the claim that heavy metal salts influence BC development in many cases [4, 5]. Heavy metals influence the processes of apoptosis and proliferative activity, and this leads to the stimulation of malignant transformation and the initiation and progression of tumorous growth [6].
The study of tumor cells expression of proteins has been paramount in determining the prognosis of cancer in recent years. On the basis of the evidence which is currently available, it seems fair to suggest that the expression of p53 apoptosis receptor and Ki-67 proliferation serves as above-mentioned markers [7, 8]. The synthesis of p53 “mutant” type protein (p53 mt) leads to cells mimicry of the properties of p53 “wild” proapoptotic protein, and the cells avoid the influence of immune system. The study of Ki-67 protein (proliferation marker), which is expressed in all cells that have come out of G0 division phase, enables to reveal “hidden” proliferative potential of this tumor and assess the degree of malignancy and divide patients into groups with relatively favorable and unfavorable prognosis [9].
One of the indications of apoptosis is the fragmentation of DNA, which occurs in several stages. In the first stage, DNA is cleaved into large portions formed of several million base pairs (bp) in length. In the second stage, it is cleaved into 50,000–300,000 bp. The third stage represents single nucleosomes or polynucleosomes fragmentation (200–1000 bp) [10]. There are many reasons for such DNA damage, but the most important of them is direct DNA damage made by active oxygen forms or fast nucleases activation in the process of apoptosis [11].
The objectives of this paper are as follows: the investigation of the expression of p53 “mutant” protein and Ki-67 proliferative marker in normal breast tissue, in tumor breast tissues, and in distant metastases; the determination of the level of DNA fragmentation in the above-mentioned groups of tissues; and the identification of correlation between receptor expression and DNA fragmentation.
Materials and Methods
The tissue that had been received during the postoperative biopsy was kept in the refrigerator. It became the subject of the further investigation after the establishing of the histological diagnosis. We selected 20 cases of the infiltrative ductal carcinoma of the second and the third degree of malignancy according to Scarff–Bloom–Richardson, 5 cases of distant metastases, and 5 cases of normal breast tissue.
Paraffin sections (of 5 µm thickness) were subjected to dewaxing and applied on spectral pure graphite rods. Chemical composition was studied with the help of the scanning electron microscope with energy-dispersion spectrometer. Digital images of the specimen and the indicators of microelements content were identified with the help of Magellanes and VCU software.
The material for the immunohistochemical study was fixed in 10% neutral formalin for 24 h, and then paraffin blocks were made of it. Then sections with the thickness of 3–4 mm were made, and they were subjected to the standard process of dehydration in xylol and alcohols of rising concentration. Immunohistochemical reaction consisted of 2 stages. During the first stage, incubation with primary rabbit antibodies in dilution of 1:100 took place (SP5 clone was used for determination of the p53 receptors expression, and SP6 clone was used for determination of Ki-67 receptors expression). During the second stage, incubation with secondary antibodies (UltraVision ONE HRP Polymer) took place. The level of Ki-67 receptor expression was evaluated according to Fitzgibbons’ recommendations [12]. Tumors had P53 positive status provided that more than 5% of nuclei had intense staining or 10%–50% of nuclei had moderate coloration.
DNA was extracted from the tissue using a lysis buffer which consisted of 30 mM of hydroxymethylaminomethane (Tris), 10 mM ethylenediamine-tetraacetic acid (EDTA), 1% sodium dodecyl sulfate, and 5 mg proteinase K (pH 8.0). DNA was purified with phenol–chloroform extraction and the following precipitation in absolute ethanol. The study of DNA fragmentation was performed in 1% agarose gel based on electrophoretic buffer (Tris – 24.22 g, EDTA – 1.862 g, acetic acid – 8396 mL, H2O – 73.3 mL, pH 8.0 – after mixing, the components were 50 times diluted in distilled water). Electrophoretic product was visualized using luminescence in the ultraviolet spectrum (420 nm), after combining DNA with acridine orange. We used λ phage (~49,000 bp) as a marker of DNA.
Mathematical calculations were done using Microsoft Excel 2010 with 12.0.5 Attestat option. We found such indicators as chi-squared Pearson’s test, Student’s T-criterion, and Pearson’s correlation coefficient (with statistical significance p < 0.05).
Results
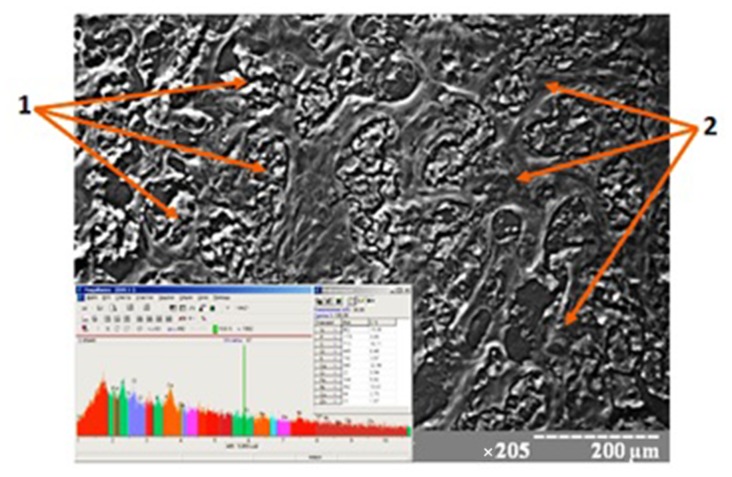
The study of the breast tissue chemical composition showed the accumulation of the chemical elements in the component parts both in unchanged mammary gland and in tumor tissue. Such macroelements as calcium, phosphorus, potassium, sulfur, and sodium are dominated among them. Such heavy metals as zinc, iron, copper, chromium, nickel, and lead are encountered in different proportions among other microelements. There is an interrelation (p < 0.05) between the accumulation of the above elements with the degree of cancer malignancy (Fig. 1). We observed the absence of the same amount of elements in distant metastases compared with the primary center (p > 0.05).
Fig. 1.
Scanning image and chemical composition of BC tissue. 1 – Tumorous parenchyma, 2 – tumorous stroma. Magnification: ×205
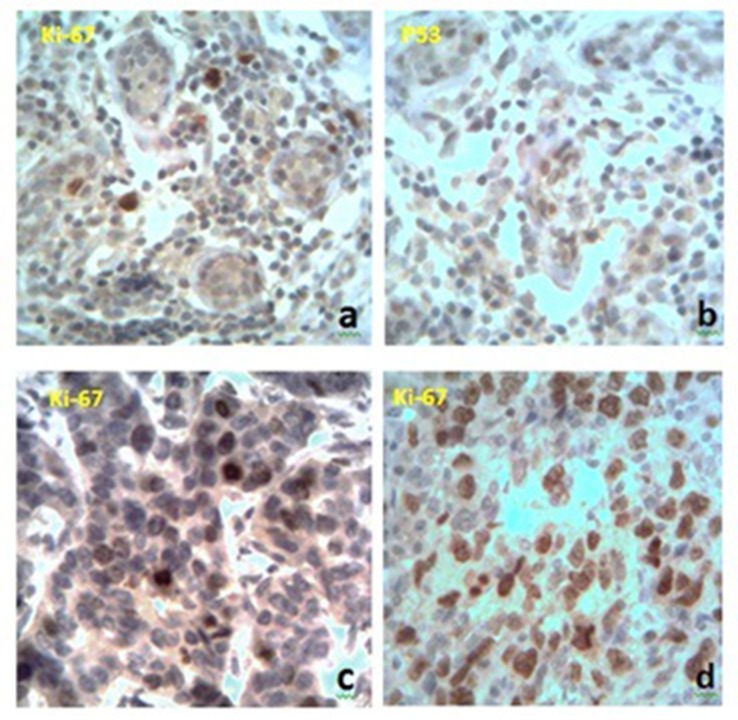
Immunohistochemical analysis has revealed different variants of receptors expression. In the intact breast tissue, we found some cells that were positive to Ki-67 receptors and had a mild expression of p53. The correlation between these two types of receptors has not been identified (Fig. 2a,b). When studying proapoptotic and proliferative markers in tumor tissue, we found different values of receptors expression. There were 85% of cases with positive reaction to Ki-67 and 40% cases with positive reaction to p53. We also traced a moderate correlation between these indicators (p < 0.05). The level of receptors expression in distant metastases was slightly higher than their value in primary tumors. We found a moderate correlation between the accumulation of microelements in the breast cancer tissue and the level of proliferative activity (Fig. 2c,d).
Fig. 2.
Immunohistochemical expression of Ki-67 and p53 receptors. a, b – Intact breast tissue, c – BC with less levels of trace elements, d – BC with a higher level of trace elements. Magnification: ×400
When studying DNA with the help of electrophoresis in agarose gel for 200 minutes, we found the following information: DNA of intact tissue was located near the starting line, indicating the absence of fragmentation of genetic material (Fig. 3, tracks 10 and 13), DNA of tumor tissue had different level of fragmentation (Fig. 3, tracks 2–4, 6–9, and 12), and in some cases, it was nearly completely fragmented (Fig. 3, tracks 2 and 12). Comparing the change in tumor DNA (Fig. 3, tracks 6 and 12) with changes in distant metastases (Fig. 3, tracks 5 and 11), we noticed that they were very similar and had a slightly more expressed character in tumor foci in lymph nodes.
Fig. 3.
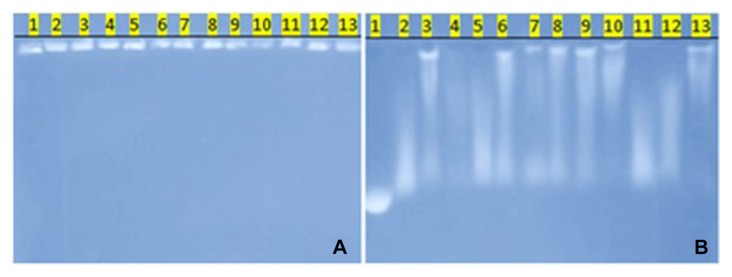
DNA electrophoresis in agarose gel. The black line is the line start. A – the beginning of electrophoresis, B – the finish of electrophoresis (200 min). 1 – DNA of λ phage
We noted the combination of the increase of DNA fragmentation with the expression of p53 and Ki-67 receptors.
Discussion
Undoubtedly, the process of carcinogenesis is a polyetiological disease in which heavy metals play a key role speaking about women who live in environmentally contaminated regions. On the one hand, the increased amount of trace elements in breast cancer tissue suggests their participation in the initiation of tumor growth; on the other hand, the combination of accumulation of elements with increasing malignancy indicates their role in the progression of carcinogenesis. If we compare distant metastases with the primary focus, we can say that the absence of the same amount of heavy metals in distant metastases says about their impact in the place of accumulation. In other places of their dissemination, neoplastic process continues to progress from the “farmed” tumor.
Some cells of normal breast tissue are in the process of constant division, as it is evidenced from single positively stained cells. Their coloration is connected with Ki-67 receptor. On the other hand, there are some cells that, due to the impact of endogenous or exogenous factors, die or need reparative effects. Trace elements play the role of exogenous factors. The p53 protein is the indicator of the process, which we have found in the normal tissue. Studying these parameters in malignant tumors, we found that cells had much more proliferative potential. This parameter was higher in the tumor tissue, which has accumulated more trace elements, and this indicated their influence on cell division. We think that the mechanism of this effect is in their influence on receptors for steroid hormones as “ecological” estrogens and provokes cells to division. As a result, they avoid the controlled influence of macroorganism that causes disorders in cell division and leads to different mutations of genetic material.
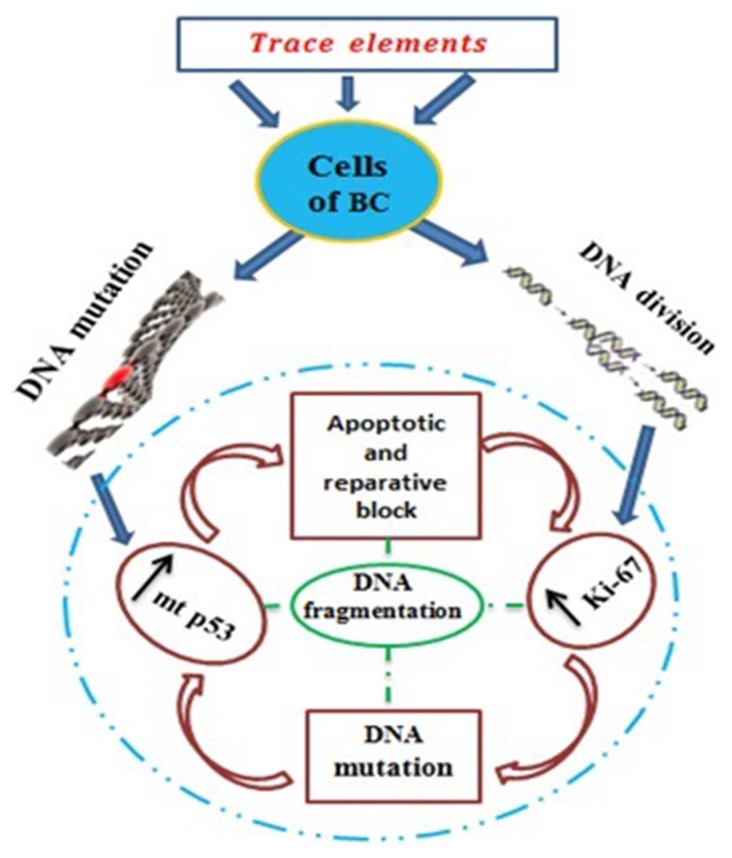
One of such mutant genes in breast tumors is the p53 gene, the product of which is mt p53 protein. This central “mutant” p53 protein which is responsible for apoptosis and transcriptional activity falls out of the control range of cell homeostasis. This causes the lack of block in the division of cancer cells and their reparation, which leads to the increased proliferative activity and the appearance of new mutations (Schema 1). The cycle is repeated again and again. In our research, we observed precisely this regularity: the increased expression of p53 proapoptotic protein was accompanied by overexpression of Ki-67 proliferative marker. The possibility to cell division increases and the number of mutant p53 in metastatic foci rises. All this suggests the origin of this cell from the parent tumor and the following progression of carcinogenesis.
Schema 1.
Pathogenetic links in the tumor process
The results of the DNA electrophoresis have revealed a clear regularity between the degree of tumor progression, cell proliferative activity, destabilization of programmed cell death, and the levels of DNA fragmentation. Objectively, this shows the degree of instability of the genetic material in the progression of malignant tumorous process. The latter arises in the unregulated division of tumor cells due to the lack of endogenous corrective actions (Schema 1).
Conclusions
Such trace elements as zinc, iron, copper, chromium, nickel, and lead can cause the initiation and the progression of the tumorous growth, which is expressed in the increased proliferation of tumor cells. This leads to the destabilization of the genetic material which can be expressed in the synthesis of mutant p53 protein although the process of the synthesis of the misleading p53 may be the primary process, too. Finally, it leads to the block of apoptosis and regulatory effects of cells. This can cause the tumor progression and the destabilization of the genome, which is reflected in the increased DNA fragmentation.
Funding Statement
Funding sources: No financial support was received for this study.
Footnotes
Authors’ contribution: RA: made the critical review, LM: prepared the article, prepared figures, MR: manuscript preparation, KYe: manuscript preparation, GO: literature review, LYu: prepared figures. All authors read and approved the final form.
Conflict of interest: The authors declare that they have no conflict of interest.
Contributor Information
Anatolii Romaniuk,
Mykola Lyndin,
Roman Moskalenko,
Yevhen Kuzenko,
Oksana Gladchenko,
Yuliia Lyndina,
References
- 1.Tavassoli FA, Devilee P. World Health Organization Classification of Tumours. Lyon: IARC Press; 2003. Tumor of the breast and female genital organs; p. 432. [Google Scholar]
- 2.Hirose K, Matsuo K, Iwata H, Tajima K. Dietary patterns and the risk of breast cancer in Japanese women. Cancer Sci. 2007;98(9):1431–1438. doi: 10.1111/j.1349-7006.2007.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS, Narod SA. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 5.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Hashemi M, Ghavami S, Eshraghi M, Booy EP, Los M. Cytotoxic effects of intra- and extracellular zinc chelation on human breast cancer cells. Eur J Pharmacol. 2007;557:9–19. doi: 10.1016/j.ejphar.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Bartley AN, Ross W. Validation of p53 immunohistochemistry as a prognostic factor in breast cancer in clinical practice. Arch Pathol Lab Med. 2002;126(4):456–458. doi: 10.5858/2002-126-0456-VOPIAA. [DOI] [PubMed] [Google Scholar]
- 8.Gerson R, Alban L, Martínez A, Villalobos A, Serrano A. KI67 in breast cancer: correlation between proliferation cellular and other prognostic factors. J Clin Oncol. 2009;27:86–92. [Google Scholar]
- 9.Jouat W, Arnold N. Is the Ki-67 labelling index ready for clinical use? Ann Oncol. 2011;22:500–502. doi: 10.1093/annonc/mdq732. [DOI] [PubMed] [Google Scholar]
- 10.Huang X, Halicka HD, Traganos F, Tanaka T, Kurose A, Darzynkiewicz Z. Cytometric assessment of DNA damage in relation to cell cycle phase and apoptosis. Cell Prolif. 2005;38(4):223–243. doi: 10.1111/j.1365-2184.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowis D, Makowski M, Stokłosa T, Legat M, Issat T, Gołąb J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochimica Polonica. 2005;2:339–352. [PubMed] [Google Scholar]
- 12.Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, Ruby SG, O’Malley F, Simpson JF, Connolly JL, Hayes DF, Edge SB, Lichter A, Schnitt SJ. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]