Abstract
Anopheles albimanus is a major malaria mosquito vector in Colombia. In the present study, wing variability (size and shape) in An. albimanus populations from Colombian Maracaibo and Chocó bio-geographical eco-regions and the relationship of these phenotypic traits with environmental factors were evaluated. Microsatellite and morphometric data facilitated a comparison of the genetic and phenetic structure of this species. Wing size was influenced by elevation and relative humidity, whereas wing shape was affected by these two variables and also by rainfall, latitude, temperature and eco-region. Significant differences in mean shape between populations and eco-regions were detected, but they were smaller than those at the intra-population level. Correct assignment based on wing shape was low at the population level (<58%) and only slightly higher (>70%) at the eco-regional level, supporting the low population structure inferred from microsatellite data. Wing size was similar among populations with no significant differences between eco-regions. Population relationships in the genetic tree did not agree with those from the morphometric data; however, both datasets consistently reinforced a panmictic population of An. albimanus. Overall, site-specific population differentiation is not strongly supported by wing traits or genotypic data. We hypothesize that the metapopulation structure of An. albimanus throughout these Colombian eco-regions is favoring plasticity in wing traits, a relevant characteristic of species living under variable environmental conditions and colonizing new habitats.
Keywords: Anopheles albimanus, Microevolution, Geometric morphometrics, Microsatellites, Environment, Phenotype
1. Introduction
Malaria is the most important parasitic vector-borne disease and it is also the third-pathogen-specific cause of human deaths per year worldwide (Hill et al., 2005). Among Latin American malaria vector mosquitoes, Anopheles albimanus Wiedemann, 1820 is a dominant species (Sinka et al., 2010) and it constitutes one of the main Colombian vectors (Gutiérrez et al., 2008; Montoya-Lerma et al., 2011; Quiñones et al., 1987) typically found in low coastal lands under 400 m (Fleming, 1986), but it has also been recorded at high altitudes (995–1941 m) (González and Martínez, 2006; Martínez-Palacios and Pletsch, 1963). It is a generalist species (Fuller et al., 2012); immature stages develop in a wide variety of breeding sites and can tolerate a wide range of water conditions such as salinity, turbidity and pollution (Faran, 1980; Henderson, 1948). Adult females exhibit plasticity in blood-feeding behavior, biting humans or animals, indoors or outdoors (Olano et al., 1997), and can fly up to 32 km (Breeland, 1972). Additionally, their populations differ in Plasmodium susceptibility, longevity, and insecticide resistance (Cáceres et al., 2011; Collins et al., 1976; Frederickson, 1993; Grieco et al., 2005; Warren et al., 1977).
In Colombia, An. albimanus is mainly distributed in the Pacific and Caribbean Coasts that are part of Chocó (CBP) and Maracaibo (MBP) bio-geographic provinces, respectively (Morrone, 2006). Both regions present differences in topography, vegetation cover, forest types, land use and weather conditions (IDEAM, 2001). The Pacific humid tropical forest is one of the rainiest regions globally, characterized by high relative humidity and precipitation. In contrast, the Caribbean dry tropical forest is drier and has higher temperatures (IDEAM, 2001; IGAC, 2002). In these two regions, An. albimanus plays a different role in malaria transmission (Gutiérrez et al., 2008). All current evidence supports single taxon status for An. albimanus throughout its geographic distribution, with low population genetic structuring and little genetic differentiation among Colombian populations (De Merida et al., 1999; Gutiérrez et al., 2009; Loaiza et al., 2010a,b).
For infected anophelines, flight performance plays a major role in host seeking dispersal, maneuverability and injection of Plasmodium sporozoites into the vertebrate host (Dudley, 2000). Therefore, wing morphology (size and shape) is expected to be adapted for an optimum flight performance under different environmental conditions (Norberg, 1995). However, these wing traits not only depend on the genetics but also on the environment, selective pressures and random events at the molecular level (Fusco and Minelli, 2010). Therefore, the exploration of phenotypic and genetic differences in An. albimanus populations could shed light on malaria transmission dynamics.
Geometric morphometrics (GM) can provide valuable information on phenotypic variability and population structure with the advantage of being a low-cost and rapid tool (Dujardin, 2011); but despite its proven utility for insect wing analysis there have been very few studies of Anopheles species (Dujardin and Garros, 2013). In GM, the size and shape of the wing are analyzed independently for heritability and environmental sensitivity (Klingenberg, 2010). Different from size, shape provides more reliable indirect information about genetic variation of natural populations exhibiting high and stable heritability (Bitner-Mathe and Klaczko, 1999; Dujardin, 2008; Falconer and Mackay, 1996; Moraes et al., 2004a,b). Consequently, phenotypic data are of interest, especially when analyzed in relation to genetic markers.
We hypothesized that Colombian An. albimanus populations vary at the bio-geographical eco-regional level and that some environmental variables (temperature, relative humidity, rainfall, longitude, latitude and elevation) affect wing size and shape. To test this hypothesis, the phenetic structure of An. albimanus was evaluated using morphometric traits, and the environmental effect on wing size and shape was determined. Furthermore, wing shape variation was compared to microsatellite data (Gutiérrez et al., 2009), to test for congruence between the phenetic and genetic structure.
2. Materials and methods
2.1. Source of specimens
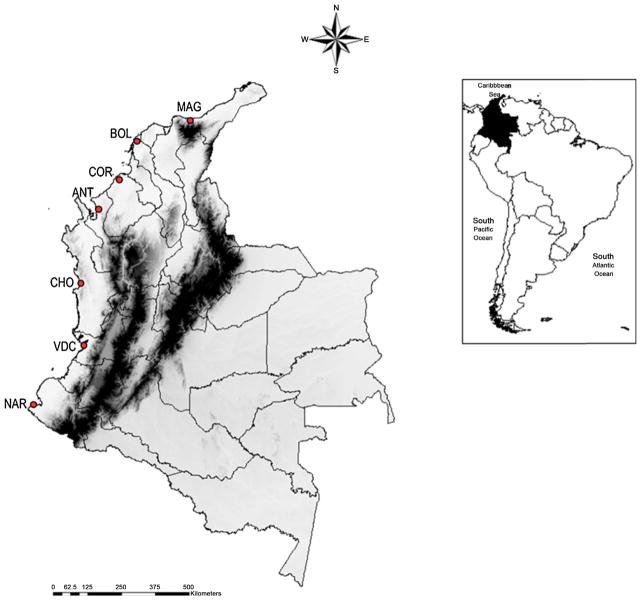
Specimens were from the Molecular Microbiology Laboratory collection, Universidad de Antioquia; details of collection procedures, identification and bioethics have been published (Gutiérrez et al., 2008, 2009). Specimens represented seven municipalities, four in MBP and three in CBP (Fig. 1 and Table 1). Distances among localities measured applying a straight line, found that the most distant municipalities were Tumaco, Nariño Department (NAR, CBP) and Santa Marta, Magdalena Department (MAG, MBP), separated by approximately 1112 km and the nearest were Moñitos, Córdoba Department (COR) and Turbo, Antioquia Department (ANT), both in MBP, located approximately 142 km apart. Molecular species confirmation of 11% randomly selected specimens used in the morphometric analysis matched the expected restriction pattern for An. albimanus (Cienfuegos et al., 2008; Zapata et al., 2007), ensuring accurate taxonomic status.
Fig. 1.
Collection sites for An. albimanus. In the Colombian map are depicted the Departments (letter codes) and municipalities (circles) for collection sites. MAG: Magdalena (Santa Marta), BOL: Bolívar (Santa Rosa de Lima), COR: Córdoba (Moñitos), ANT: Antioquia (Turbo), CHO: Chocó (Nuquí), VDC: Valle del Cauca (Buenaventura), NAR: Nariño (Tumaco).
Table 1.
Data for Colombian Anopheles albimanus analyzed in this study.
| Bio-geographic provincea/coast | Department | Municipality (locality sampled) | n | Time of collection month/year | Longitude | Latitude | Elevationb (m) | T (°C)c | RH (%)c | Rainfall (mm)c |
|---|---|---|---|---|---|---|---|---|---|---|
| Chocó/Pacific | Nariño (NAR) | Tumaco (Alto Guandipa) | 36 | 12/2005 | 2°29′ N | 78°26′ W | 2 | 25.60 | 86 | 1.49 |
| Valle del Cauca (VDC) | Buenaventura (Puerto España) | 37 | 08/2006 | 4°02′ N | 77°26′ W | 11 | – | – | – | |
| Chocó (CHO) | Nuquí (Nuquí, Panguí) | 40 | 6, 11/2005 | 5°42′ N | 77°16′ W | 5 | 24.77 | 93 | 33 | |
| Maracaibo/Caribbean | Antioquia (ANT) | Turbo (Camerún) | 34 | 11/2007 | 8°08′ N | 76°43′ W | 3 | 27.55 | 88 | 3.09 |
| Córdoba (COR) | Moñitos (Peri-urban areas) | 25 | 09/2005 | 9°15′ N | 76°06′ W | 46 | 27.86 | 84 | 4.64 | |
| Bolivar (BOL) | Santa Rosa de Lima (Ciénaga, El Hatillo) | 38 | 7, 9, 10/2005 03, 06/2006 |
10°26′ N | 75°21′ W | 53 | – | – | 3.87 | |
| Magdalena (MAG) | Santa Marta (Los Achiotes) | 35 | 08/2005 02/2006 |
11°15′ N | 73°36′ W | 119 | – | – | – |
Bio-geographic province as described by Morrone (2006).
Elevation data from Google Earth®.
Average values of the month prior to the collection date.
2.2. Environmental data
Environmental data (temperature, relative humidity and rainfall) for sites in Nariño (NAR), Chocó (CHO), Antioquia (ANT) and Córdoba (COR) were obtained from nearest weather stations of the Instituto de Hidrología, Meteorología y Estudios Ambientales-IDEAM, Colombia; data used corresponded to the previous month to a collection (Barrera et al., 1999). Latitude, longitude and elevation were recorded using GPS or Google Earth®.
2.3. Wing geometric morphometric analysis
2.3.1. Sample preparation, landmark collection and repeatability
The wings of An. albimanus females were mounted on microscope slides with coverslips using commercial glue. To avoid possible intra-individual variation interference in the GM analysis (Zelditch et al., 2004), only the right wing was analyzed, photographed using a digital camera (Moticam 2500, Motic China Group Co., Ltd.), coupled to an Olympus® Trinocular Microscope SZ61. Thirteen wing type I landmarks (Bookstein, 1991) located at vein intersections were digitized for each specimen (Fig. 2). Landmark repeatability was tested in 30 wings selected randomly, digitized twice by the same person (GFG); the measurement error was computed as 1-R, with R as the repeatability index (Arnqvist and Märtensson, 1998). The mounted wing slides were deposited in a collection of the Laboratorio de Microbiología Molecular, Universidad de Antioquia, Colombia, and the wing images were deposited in the CLIC image bank (http://mome-clic.com/clic-bank/).
Fig. 2.
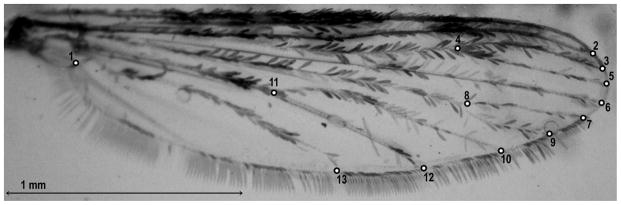
Right wing of An. albimanus. Numbers represent the position of landmarks. Scale = 1 mm.
2.3.2. Morphometrics data acquisition
For digitized landmarks, raw coordinates were superimposed using a generalized least-squares algorithm. This procedure separates shape information (partial warps) from variation in size, position and orientation (Zelditch et al., 2004). To reduce the dimensionality of wing shape data, partial warps were first subjected to principal component analysis (PCA), and only the principal components (relative warps, RW) accounting for 95% of the total shape variation were used for further analyses. To test whether the projection onto tangent space was a good approximation of the fitted coordinate configurations in Kendall’s shape space, the correlation between Procrustes and Euclidean distances was computed.
2.3.3. Size analysis
The size variable was the isometric estimator known as centroid size (CS), defined as the square root of the sum of the squared distances of the landmarks from the center of gravity of a configuration (Bookstein, 1991). CS was used as a measure of overall wing size. Since CS data violated the assumption of normality and homoscedasticity, variation in CS was tested among populations using a nonparametric Kruskal–Wallis test based on 1000 permutations. Post hoc pairwise comparisons following a significant Kruskal–Wallis test were performed using the Bonferroni correction. In addition, we compared CS variances using a non-parametric test based on 1000 permutations (Caro-Riaño et al., 2009). The relationship between CS and the environmental variables was explored using a Spearman correlation for the four populations for which data were available. The level of statistical significance was set at p < 0.05. Several additional linear regressions were performed to infer size variation across the longitude, latitude and elevation to explore possible clinal variation related to CS. The statistical significance of geographical distances and CS was evaluated by a Mantel test. A generalized linear mixed model (GLMM) was applied to the wing CS data. The R2 (coefficient of partial determination) values for single-factor models were calculated, followed by the partial R2 for each environmental variable.
2.3.4. Allometry
The allometric effect or the change in shape associated with size differences (Klingenberg, 1998) was evaluated with a multivariate regression of shape variables onto size. The statistical significance was estimated by non-parametric permutation tests using 1000-runs (Good, 2000). Since a significant allometric effect was found in the dataset, a multivariate analysis of covariance (MANCOVA) was performed to compare allometric trends among populations. The slopes did not differ significantly; therefore, the allometric effect was controlled by computing allometry-free variables.
2.3.5. Shape analysis
A multivariate analysis of variance (MANOVA; 1000 permutations) and pairwise tests were carried out on the landmark data to compare wing shape among populations. To assess wing shape differences between populations, the allometry-free variables were used as input for a Canonical Variate Analysis (CVA), and to obtain Procrustes and Mahalanobis distances. CVA determines whether the pre-defined seven populations can be statistically distinguished based on the relative warps matrix. The success of the CVA in assigning specimens to populations was determined using a cross-validation procedure in which each specimen is omitted from the initial calculation of the CV axes and used as a test set. The omitted specimen is then treated as an unknown and assigned to the population whose mean is closest using the CV axes (Webster and Sheets, 2010). The shape means for the seven populations were plotted along the two canonical variate axes based on the Procrustes distance matrix.
The statistical significance of the Mahalanobis distances was computed by a nonparametric permutation test (1000 runs) with Bonferroni correction. Correlation among geographic and Mahalanobis distances was examined by a Mantel test (10,000 permutations) using straight line geographic distances between collection sites. The Procrustes distance matrix was used to build an unrooted Neighbor-Joining (NJ) tree. Individual MANOVAs were used to test the effect of environmental variables on An. albimanus wing shape. Finally, QST values, a quantitative trait analog for FST values (Miller et al., 2008), were calculated to measure the phenotypic divergence for CS and wing shape variables.
2.4. Combining phenotypic, genotypic and spatial data
Microsatellite genotypes and allele frequencies for each population (Gutiérrez et al., 2009) were used to compute pairwise genetic distances among seven populations with Nei’s genetic distances. A NJ tree was built using the Phylip package with 1000 bootstrap replicates (Felsenstein, 2005).
Georeferenced phenotypic and genetic data were integrated with an approach based on a statistical model to decipher the intraspecific structure of Colombian An. albimanus populations. This model assumes the existence of several clusters that display homogeneity in terms of genetic and phenotypic variation (Guillot et al., 2012). The dataset, consisting of 280 specimens genotyped at four microsatellite loci (Gutiérrez et al., 2009) and 245 with morphometric data, was analyzed using data combinations of phenotypic (P), genetic (G) and spatial (S) matrices, as follows: (i) the phenotypic data under the spatial model PS, (ii) the genetic data under the spatial model GS and (iii) phenotypic and genetic data under the spatial model PGS. For each scenario, 10 independent Markov chain Monte Carlo runs of 100,000 iterations were performed, discarding the first 10,000 iterations as burn-in (Guillot et al., 2012). The correlation between the phenotypic (Mahalanobis distances) and genetic distances (FST) was estimated as the coefficient of determination using a linear regression.
2.5. Software
The digitization of landmarks, Procrustes superimposition, and shape and size analyses were performed using the CLIC package (Dujardin, 2008). PAST software was also used for CS comparison through a nonparametric ANOVA, detection of possible outliers and Mantel test (Hammer et al., 2001). The validation of the tangent space was tested in tpsSmall by Jim Rohlf (http://life.bio.sunysb.edu/ee/rohlf/software.html). Pairwise geographical distances between collection sites were calculated using the GPS coordinates in DIVA-GIS (Hijmans et al., 2001). The Phylip package was used to construct the unrooted NJ trees (Felsenstein, 2005). Collection sites were plotted according to geographic coordinates on a map using ESRI ArcGIS® software version 10. MANOVAs were performed in SAS® version 9.0.2 software (SAS Institute Inc., Cary, NC, USA). Additional regressions were computed in PAST. The integration of phenotypic and genetic data under several models was done in Geneland (Guillot et al., 2012).
3. Results
3.1. Geometric morphometrics
3.1.1. Repeatability
Comparison of two digitization sets for the same specimens showed good repeatability for x, y coordinates (R ≈ 0.90), centroid size (R = 0.99) and the relative warps (R = 0.92).
3.1.2. Wing size
The morphometric analyses of 245 An. albimanus showed a significant difference in CS among populations (Kruskal–Wallis test, p < 0.0001), with overlap in their distribution; however, the median CS for the ANT population was slightly significantly larger (Fig. 3 and Table 2). The CHO–BOL comparison was also significant. Pairwise comparison pooling CS data for all MBP and CBP populations did not reveal any statistically significant difference (Mann–Whitney U test, p = 0.30). Variance analysis of CS did not detect significant differences between the populations, except for the MAG–NAR pair (Table 2). There was a low but statistically significant correlation between CS and elevation (Spearman rank correlation; r = −0.27, p < 0.0001), and CS and relative humidity (Spearman rank correlation; r = 0.32, p = 0.0002). The GLMM analysis including these two variables revealed that only approximately 11.6% of the variance in CS is accounted for by their linear combination.
Fig. 3.
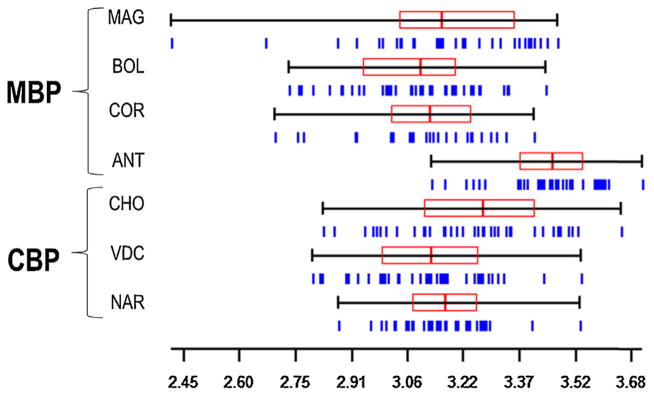
Wing centroid size distribution for Anopheles albimanus females by sampling site. Vertical lines under the quantiles represent specimens. Each box denotes the median as a line across the middle and the quartiles (25th and 75th percentiles) at its ends. Scale is in mm.
Table 2.
Wing size comparisons between Colombian An. albimanus populations.
| MAG | BOL | COR | ANT | CHO | VDC | NAR | |
|---|---|---|---|---|---|---|---|
| MAG | – | 0.176 | 0.324 | 0.010 | 0.467 | 0.147 | 0.001 |
| BOL | 0.064 | – | 0.879 | 0.141 | 0.499 | 0.882 | 0.060 |
| COR | 0.305 | 0.559 | – | 0.154 | 0.667 | 0.786 | 0.075 |
| ANT | 0.000 | 0.000 | 0.000 | – | 0.034 | 0.176 | 0.718 |
| CHO | 0.131 | 0.000 | 0.008 | 0.000 | – | 0.413 | 0.011 |
| VDC | 0.360 | 0.289 | 0.719 | 0.000 | 0.007 | – | 0.085 |
| NAR | 0.942 | 0.021 | 0.129 | 0.000 | 0.051 | 0.210 | – |
Comparison of p values of variances (above diagonal) and means (below diagonal).
Results in bold denote significance after Bonferroni correction (p-value < 0.0024).
A linear regression analysis showed that elevation was significant (p = 0.001) and negatively related to CS; An. albimanus had larger wing size at lower elevation. In contrast, the linear regressions did not show significant trends of CS with latitude (p = 0.081) or longitude (p = 0.524). Therefore, Bergmann’s rule (Shelomi, 2012) was violated.
3.1.3. Wing shape
Regression of Procrustes distances onto the tangent space proved to be a good approximation of the fitted coordinate configurations. The slope of the regression was 0.999 with a correlation of 1. Therefore, the interpretation of results from relative warp morphospace is accurate. The analysis indicated that 22 RWs accounted for the total wing shape variation. RW1 and RW2 overlapped in morphospace and together explained 40.07% of the variation. The first 12 RWs together accounted for 95% of the variation; therefore, they were used for subsequent analysis. Variation in wing shape was depicted as a landmark movement from a consensus configuration using deformation grids (Fig. 4). RW1 axis detected the greatest movement of landmarks (4 and 8) along the medial area of the wing; albeit other landmarks on the edge of the wing and landmark 1 showed movement, and RW2 showed the greatest movement with landmarks 1, 4, 8 and 13.
Fig. 4.
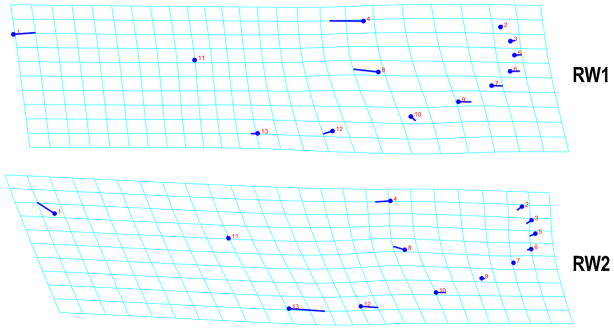
Deformation grids showing the wing shape variation. Visualization of landmark movement using deformation grids for the PCA positive extremes of the RW1 and RW2 axes; there is no magnification.
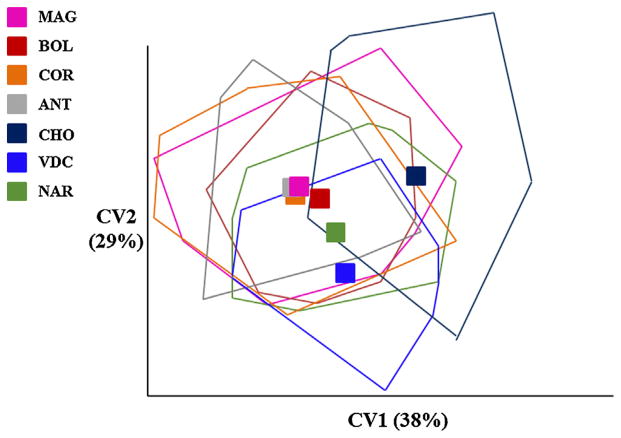
MANOVA detected significant differences in shape variables among populations (Wilk’s lambda: 0.3358, F: 3.827, p < 0.001) with all mean wing shape pairwise comparisons producing statistically significant differences (p < 0.001), as did all the populations pooled by eco-region (i.e., MBP or CBP) (Wilk’s lambda: 0.8050, F: 2.4227, p < 0.001). Discrimination among populations in the morphospace defined by the CV axes showed that the first two CV axes accounted for 67% of the total shape variation. They were projected without evident separation of populations, largely overlapping, however, the CHO locality specimens remained the most distinct (Table 3 and Fig. 5). The majority of pairwise population comparisons using the Mahalanobis distances were statistically significant different (p < 0.05), with some exceptions, i.e., population pairs MAG–COR, MAG–BOL, COR–BOL, COR–ANT, all in MBP. Also, NAR (CBP)–COR (MBP) populations were not significantly different. In addition, Procrustes distances comparison yielded similar but non significant results for population pairs COR–BOL and COR–ANT, but differed with respect to comparisons of MAG–BOL and MAG–NAR, also non-significant. Of the above phenetic distance analyses (i.e., Mahalanobis or Procrustes distances) (Table 3), the CHO population was the most divergent. The NJ tree based on Procrustes distances (Fig. 6A), clustered these geographical populations into two main groups: (i) ANT–COR and VDC–NAR, and (ii) BOL–MAG, with CHO separate from both clusters. There was no significant relationship between Mahalanobis and geographic distances (Mantel test, p = 0.939), indicating that wing shape is not influenced by distance.
Table 3.
Mahalanobis and Procrustes distances among mean wing shape configurations of An. albimanus populations.
| MAG | BOL | COR | ANT | CHO | VDC | NAR | |
|---|---|---|---|---|---|---|---|
| MAG | – | 0.0055 | 0.0091 | 0.0098 | 0.0142 | 0.0113 | 0.0076 |
| BOL | 1.0034 | – | 0.0072 | 0.0083 | 0.0140 | 0.0101 | 0.0092 |
| COR | 1.2266 | 1.0211 | – | 0.0048 | 0.0144 | 0.0104 | 0.0097 |
| ANT | 1.5480 | 1.5213 | 0.8596 | – | 0.0142 | 0.0119 | 0.0098 |
| CHO | 1.8890 | 1.8824 | 2.1039 | 2.1825 | – | 0.0174 | 0.0147 |
| VDC | 1.9817 | 1.6651 | 1.7995 | 1.9795 | 2.1413 | – | 0.0090 |
| NAR | 1.3443 | 1.4088 | 1.4483 | 1.5727 | 1.8890 | 1.3360 | – |
Above diagonal: Procrustes distances; below diagonal: Mahalanobis distances derived from CVA scores computed from 12 RWs. Significant values after Bonferroni correction are in bold.
Fig. 5.
Canonical Variate Analysis (CVA) for wing shape of An. albimanus. Scatter plot from canonical variate analysis of wing shape for seven An. albimanus populations.
Fig. 6.
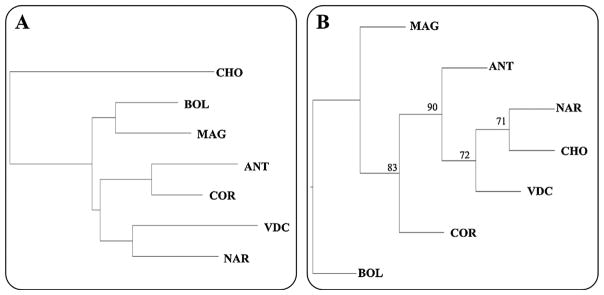
Neighbor-Joining trees. NJ trees obtained from: (A) morphometric [Procrustes’ distances] dataset and (B) genetic [Nei’s distances]. Numbers at the branches (B) refer to bootstrap values (resampling method available only for genetic data).
In general, discriminant analysis based on Mahalanobis distances resulted in a poor assignment of An. albimanus populations, with the highest correct assignment being for CHO specimens (57.5%), and the lowest for BOL specimens (13.16%) (Table 4). However, pooling the specimens by eco-regions in the supervised classification resulted in correct assignment >70%. Individual MANOVAs revealed a significant effect of all environmental variables on An. albimanus wing shape except longitude (Table 5).
Table 4.
CVA classification matrix for An. albimanus with a Jackknife cross-validation.
| A priori assignments |
A posteriori assignments
|
||||||
|---|---|---|---|---|---|---|---|
| MAG | BOL | COR | ANT | CHO | VDC | NAR | |
| MAG | 31.43 (11) | 20 (7) | 5.71 (2 | 11.43 (4) | 17.14 (6) | 5.71 (2) | 8.57 (3) |
| BOL | 23.68 (9) | 13.16 (5) | 15.79 (6) | 10.53 (4) | 18.42 (7) | 13.16 (5) | 5.26 (2) |
| COR | 12 (3) | 16 (4) | 16 (4) | 28 (7) | 12 (3) | 8 (2) | 8 (2) |
| ANT | 5.88 (2) | 8.82 (3) | 26.47 (9) | 32.35 (11) | 8.82 (3) | 8.82 (3) | 8.82 (3) |
| CHO | 2.5 (1) | 10 (4) | 7.5 (3) | 2.5 (1) | 57.5 (23) | 2.5 (1) | 17.5 (7) |
| VDC | 10.81 (4) | 10.81 (4) | 5.41 (2) | 8.11 (3) | 5.41 (2) | 43.24 (16) | 16.22 (6) |
| NAR | 13.89 (5) | 11.11 (4) | 5.56 (2) | 5.56 (2) | 8.33 (3) | 22.22 (8) | 33.33 (12) |
Percentages and number of specimens (in parentheses) assigned according to populations using the first 12 RWs derived from allometry-free variables. Percentages of correctly classified specimens are in bold.
Table 5.
Results of MANOVAs testing the effect of environmental factors and eco-regions on wing shape of An. albimanus.
| Factor | Wilks’ lambda | F statistics | DF1 | DF2 | p value |
|---|---|---|---|---|---|
| Elevation | 0.1967 | 3.1 | 132 | 1269.8 | <.0001 |
| Latitude | 0.0217 | 1.46 | 594 | 3446.8 | <.0001 |
| Longitude | 0.1568 | 1.12 | 330 | 2298.5 | 0.0826 |
| Temperature | 0.1539 | 2.98 | 88 | 433.49 | <.0001 |
| Relative humidity | 0.1539 | 2.98 | 88 | 433.49 | <.0001 |
| Rainfall | 0.1539 | 2.98 | 88 | 433.49 | <.0001 |
| Eco-region | 0.4227 | 3.33 | 66 | 657.84 | <.0001 |
DF: degrees of freedom. Statistically significant values (p < 0.05) are in bold.
3.2. Inferring An. albimanus population structure
Nei’s distances derived from genetic data clustered the CBP population (NAR, VDC, CHO) separately from the others (Fig. 6B). The ANT population was more related to the CBP cluster than to the MBP populations (ANT, COR, BOL, MAG). Furthermore, MBP populations did not form a unique cluster in the NJ tree.
The dataset of georeferenced genetic and phenotypic markers showed good congruence in the several independent MCMC runs. The analyses, using data combinations in Geneland, produced different numbers of clusters (Fig. 7). The PS and PGS models inferred a single An. albimanus population in MBP (Fig. 7A and C), whereas the GS model assigned the ANT population to a different and unique cluster (Fig. 7B). The pattern for CBP populations under the GS and PGS models (Fig. 7B and C) was similar, grouping the CHO and NAR An. albimanus populations together and assigning the VDC population to a different cluster. In general, the FST values among the three clusters identified with the PGS model (Fig. 7C) showed little differentiation (MBP populations vs. NAR–CHO: FST = 0.045; MBP populations vs. VDC: FST = 0.030; VDC vs. NAR–CHO: FST = 0.022). The overall genetic distance FST was 0.033, and was not related to geographical distance (Mantel test, p = 0.56) (Table 6). The quantitative phenotypic divergence or QST value derived from CS was 0.86, and for all shape variables it was 0.52. In addition, linear regression revealed that genetic distances were not correlated with phenotypic distances (p = 0.52).
Fig. 7.
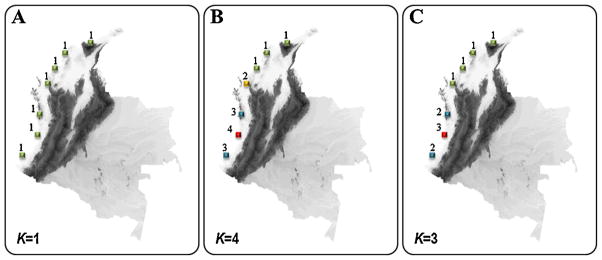
Patterns of population structure inferred using Geneland software. Numbers depict populations assigned to a specific cluster (different colors for the color version). (A) Phenotypic data under the spatial model – PS; (B) genetic data under the spatial model – GS; (C) phenotypic and genetic data under the spatial model – PGS. K: number of clusters. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 6.
Pairwise FST values (upper diagonal)derived from microsatellite analysis and geographic distances in km (lower diagonal) for An. albimanus populations.
| MAG | BOL | COR | ANT | CHO | VDC | NAR | |
|---|---|---|---|---|---|---|---|
| MAG | – | 0.008 | 0.016 | 0.036 | 0.081 | 0.054 | 0.084 |
| BOL | 211.8 | – | 0.009 | 0.013 | 0.056 | 0.042 | 0.063 |
| COR | 352.9 | 155.3 | – | 0.019 | 0.049 | 0.032 | 0.060 |
| ANT | 487.1 | 296.8 | 141.6 | – | 0.010 | 0.022 | 0.024 |
| CHO | 734.7 | 564.1 | 412.0 | 273.9 | – | 0.027 | 0.005 |
| VDC | 907.6 | 748.5 | 599.0 | 463.2 | 190.1 | – | 0.025 |
| NAR | 1112.1 | 948.2 | 796.3 | 657.0 | 384.2 | 205.2 | – |
Statistically significant pairwise FST values are in bold for p < 0.05 after 1000 permutations.
4. Discussion
This study found (i) an overall wing size and shape similarity among An. albimanus populations, (ii) a significant effect of environmental factors on wing traits, (iii) a lack of population differentiation using genetic and wing shape data, and (iv) incongruence between morphometric and genetic patterns, not related to geography.
Centroid size was used as a measure of overall wing size differences among An. albimanus populations. The slight variation in wing size among populations suggests that either this trait is not a target of strong differential selection or that wing size in An. albimanus is well suited to changing environmental conditions (Whitman and Agrawal, 2009). Particularly, the wing size variations detected could result from environmental changes experienced by immature stages during their development in breeding sites (Falconer and Mackay, 1996; Lynch and Walsh, 1998; Okech et al., 2007). For example, food availability and larval competition have been reported as influencing CS variation in adult mosquitoes (da Silva Araújo et al., 2012; Jirakanjanakit et al., 2007; Kweka et al., 2012; Paaijmans et al., 2009; Schneider et al., 2004).
The ANT An. albimanus had considerably larger wings than the other populations. Previous reports do not provide a clear consensus on a positive relationship between wing size and body size (Koella and Lyimo, 1996); however, it has been suggested that larger anopheline females have life history traits that in general confer fitness, and in some cases influence the ability to transmit the malaria parasite successfully. Among these traits, higher fecundity (Lyimo and Takken, 1993), longer lifespan (Takken et al., 1998), and an increase their chances of mating (Okanda et al., 2002). Larger females also have the ability to generate an effective immune response (Suwanchaichinda and Paskewitz, 1998) and to express insecticide resistance (Oliver and Brooke, 2013). In this study, the reasons for and implications of the ANT population having larger wings was not determined. Further, for An. albimanus, the association of wing CS variation with malaria transmission has not been studied. A complex species-specific pattern of larger mosquitoes related to higher malaria parasite infection has been observed in Anopheles gambiae; in contrast, in Anopheles stephensi this correlation was not detected (Takken et al., 2013).
Although mean wing shape comparisons revealed significant differences between populations, clear site-specific population differentiation is not evident in this species. However, an eco-regional pattern in wing shape variation was detected when populations were pooled, improving their correct assignment (>70%). At the population level, the morphospace of the first two canonical variables showed that there was no clear-cut separation between the groups, which is in agreement with a high proportion of misassignments and supports the findings of low population structure, as demonstrated by Geneland analysis using phenotypic and spatial data (one cluster). Coincidentally, wing shape changes in An. albimanus were in the medial area that was also the most variable area in Anopheles artroparvus (Vicente et al., 2011) and Anopheles superpictus (Aytekin et al., 2009). Overall, most populations in MBP (ANT, BOL or MAG) did not differ significantly in mean wing shape, with the exception of COR. Previous studies of genetic variation in An. albimanus (Gutiérrez et al., 2009) and other Colombian Anopheles species have found contemporary gene flow among various MBP populations (Gutiérrez et al., 2010; Jaramillo et al., 2011; Rosero et al., 2012). Gene flow tends to homogenize the genetic background of populations, including those genes involved in phenotypic traits (Hartl and Clark, 2007), therefore, and as reported (Fusco and Minelli, 2010), the slight wing shape differences detected may be the result of phenotypic plasticity.
Given the complexity and number of environmental factors having an effect on wing traits, results show that the variables evaluated provided relevant information for the Colombian An. albimanus populations. Our data indicated that 11.6% of the variance in CS was explained by relative humidity (RH) and elevation. The effect of RH on size, as determined by CS, has been reported for other mosquito models (Morales Vargas et al., 2010; Kennington et al., 2003). Among the other variables, rainfall and mean air temperature did not show any significant effect on CS. This was expected since wing size seems to be more affected by the aquatic microclimate of the immature stage (Loetti et al., 2011; Stephens and Juliano, 2012; Sutcliffe and Benedict, 2012).
Environmental gradients produced by variables such as altitude or latitude are well known to impose spatially varying selective pressures on populations distributed along the gradient (Cheng et al., 2012), as observed for several Dipteran species (Hoffmann and Weeks, 2007; Huey et al., 2000). Thus, the relationship between latitude and CS (Bergmann’s rule) was explored, with no findings of the effect of this rule or its inverse, i.e., the countergradient variation pattern (Blanckenhorn and Demont, 2004), acting on An. albimanus populations CS. Although in this study the populations spanned the distribution of An. albimanus in Colombia, perhaps the latitudinal effect should be reevaluated for a larger geographic area across Latin America. In contrast, a significant negative tendency between CS and elevation was detected, i.e., the An. albimanus population with the largest mean wing size was from the ANT collection site located 3 m above sea level.
All the environmental variables except longitude showed a significant effect on wing shape variation. Similarly, significant results were found for An. funestus in Africa for elevation, rainfall and temperature and other variables (Ayala et al., 2011). Although the relationship between wing shape and environment remains unclear, one possible explanation is that this phenotypic trait, controlled by numerous genetic factors (Birdsall et al., 2000; Zimmerman et al., 2000), might be sensitive to environmental stressors during development (Klingenberg, 2010). Additionally, seasonal and temporal variations can affect the wing size and shape of insects (Francuski et al., 2011; Schachter-Broide et al., 2009; Vidal et al., 2012). Because the mosquitoes analyzed in this study were caught over a three year period, covering dry (December–February and June–August) and rainy (March–May and September–November) seasons, the effect of time and seasonality cannot be discarded. However, an additional analysis of BOL population specimens collected during both seasons did not show significant differences on wing shape (data not shown), which suggest that perhaps seasonality is not affecting this trait. On the other hand, the temporal influence on wing size and shape variation for An. albimanus remains to be tested.
Differential population histories could have influenced wing morphological evolution in An. albimanus. Mitochondrial marker COI detected signals of differential population histories for both MBP and CBP, i.e., MBP was more related to Central American than to CBP populations (Gutiérrez et al., 2009; Loaiza et al., 2010b). Congruently, a higher correct classification was obtained for specimens at the eco-regional level (MBP or CBP) than for geographical populations, at the collection site level, indicating that there are slight intraspecific differences in wing shape between these eco-regions. It is worth noting that the different models employed to combine phenotypic, genetic and spatial data did not agree in their clustering pattern. This could be explained because phenotypic traits commonly represent direct targets of natural selection, providing information not obtained with neutral molecular markers. Current evidence supports an An. albimanus metapopulation with gene flow among demes (De Merida et al., 1999; Gutiérrez et al., 2009; Loaiza et al., 2010b), i.e., the expectation is that plasticity is favored over local specialization under a broad range of conditions (Sultan and Spencer, 2002). The low FST values between the clusters suggest that slight wing morphology differences could be reflecting plastic responses more than genetic divergence in An. albimanus. These results may imply that An. albimanus exhibits plasticity to successfully colonize novel environments, as suggested for other species (Fierst, 2011; Loaiza et al., 2011).
In this study, population relationships inferred from genetic and morphometric data did not agree for several possible reasons. For example, and contrary to the neutral character of microsatellite data, the phenetic pattern could be affected by factors such as differential selection, non-additive genetic variation, differential mutation rates, environmental effects, and regulatory variation (Reed and Frankham, 2001). The finding that QST > FST implies that the degree of differentiation in wing traits exceeds what is expected by genetic drift alone, with natural selection favoring different phenotypes (i.e., wing shape) in the different populations (Pujol et al., 2008; Whitlock, 2008). This is expected because variation in wing traits could modify flight performance and related functions such as the dispersion and success in finding mates in Anopheles (Sanford et al., 2011). Further research using common garden experiments will be required to quantify the roles of selection and plasticity on these traits during development (Porcher et al., 2004). The lack of a significant relationship among geographic, genetic and phenetic distances suggests that geographical distance cannot explain the slight wing shape differentiation pattern among An. albimanus populations. It is possible that the short flight range of An. albimanus (Hobbs et al., 1974) and similar environmental conditions among some localities favor wing shape similarity.
5. Conclusions
In this study, Colombian An. albimanus populations showed slight variability in wing size and shape. This is likely a result of current gene flow that favors plasticity of wing traits. Taken together, wing traits and microsatellite data consistently support An. albimanus as a panmictic population, despite environmental heterogeneity. However, the effect of environment on wing traits needs further evaluation to elucidate patterns of environmental gradients acting upon An. albimanus across its Neotropical distribution. Further, the geometric morphometric approach constitutes a complementary tool to genetic data that may provide relevant insights into the phenetic structure of medically important vector species such as An. albimanus. Integrative approaches, as the one used here, are needed to better understand the complex phenomena that underlie anopheline differentiation processes. Finally, the results of our study expand knowledge of the Colombian An. albimanus population structure, information useful to guide malaria control strategies.
Acknowledgments
This work was supported by Comité para el Desarrollo de la Investigación-CODI, Grant No. 8700-689 to MMC and Estrategia para la Sostenibilidad de Grupos 2013–2014, Universidad de Antioquia Grant No. E01719. GFG received financial support for his doctoral studies from Departamento Administrativo de Ciencia, Tecnología e Innovación-COLCIENCIAS, Colombia, Concovatoria 511, 2010. We thank Nelson Naranjo at Laboratorio de Microbiologia Molecular-UdeA for microsatellite genotyping, Sara Bickersmith and William Lainhart at the Wadsworth Center’s Griffin Laboratory for reading and critical comments on earlier versions of the manuscript. We are grateful to three anonymous reviewers that made valuable comments on the manuscript.
Contributor Information
Giovan F. Gómez, Email: giovan19@gmail.com.
Edna J. Márquez, Email: ejmarque@unal.edu.co.
Lina A. Gutiérrez, Email: liangutibui@gmail.com.
Jan E. Conn, Email: jconn@wadsworth.org.
Margarita M. Correa, Email: margaritcorrea@gmail.com, mcorrea@quimbaya.udea.edu.co.
References
- Arnqvist G, Märtensson T. Measurement error in geometric morphometrics: empirical strategies to assess and reduce its impact on measure of shape. Acta Zool Acad Sci Hung. 1998;44:73–96. [Google Scholar]
- Ayala D, Caro-Riano H, Dujardin JP, Rahola N, Simard F, Fontenille D. Chromosomal and environmental determinants of morphometric variation in natural populations of the malaria vector Anopheles funestus in Cameroon. Infect Genet Evol. 2011;11:940–947. doi: 10.1016/j.meegid.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytekin S, Aytekin AM, Alten B. Effect of different larval rearing temperatures on the productivity (Ro) and morphology of the malaria vector Anopheles superpictus Grassi (Diptera: Culicidae) using geometric morphometrics. J Vector Ecol. 2009;34:32–42. doi: 10.1111/j.1948-7134.2009.00005.x. [DOI] [PubMed] [Google Scholar]
- Barrera R, Grillet ME, Rangel Y, Berti J, Aché A. Temporal and spatial patterns of malaria reinfection in northeastern Venezuela. Am J Trop Med Hyg. 1999;61:784–790. doi: 10.4269/ajtmh.1999.61.784. [DOI] [PubMed] [Google Scholar]
- Birdsall K, Zimmerman E, Teeter K, Gibson G. Genetic variation for the positioning of wing veins in Drosophila melanogaster. Evol Dev. 2000;2:16–24. doi: 10.1046/j.1525-142x.2000.00034.x. [DOI] [PubMed] [Google Scholar]
- Bitner-Mathe BC, Klaczko LB. Heritability, phenotypic and genetic correlations of size and shape of Drosophila mediopunctata wings. Heredity (Edinb) 1999;83(Pt 6):688–696. doi: 10.1046/j.1365-2540.1999.00606.x. [DOI] [PubMed] [Google Scholar]
- Blanckenhorn WU, Demont M. Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol. 2004;44:413–424. doi: 10.1093/icb/44.6.413. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge University; Cambridge: 1991. [Google Scholar]
- Breeland SG. Studies on the ecology of Anopheles albimanus. Am J Trop Med Hyg. 1972;21:751–754. doi: 10.4269/ajtmh.1972.21.751. [DOI] [PubMed] [Google Scholar]
- Cáceres L, Rovira J, Garcia A, Torres R. Determination of the resistance to organophosphate, carbamate, and pyrethroid insecticides in Panamanian Anopheles albimanus (Diptera: Culicidae) mosquitoes. Biomedica. 2011;31:419–427. doi: 10.1590/S0120-41572011000300014. [DOI] [PubMed] [Google Scholar]
- Caro-Riaño H, Jaramillo N, Dujardin JP. Growth changes in Rhodnius pallescens under simulated domestic and sylvatic conditions. Infect Genet Evol. 2009;9:162–168. doi: 10.1016/j.meegid.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Cheng C, White BJ, Kamdem C, Mockaitis K, Costantini C, Hahn MW, Besansky NJ. Ecological genomics of Anopheles gambiae along a latitudinal cline: a population-resequencing approach. Genetics. 2012;190:1417–1432. doi: 10.1534/genetics.111.137794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cienfuegos A, Córdoba L, Gómez GF, Luckhart S, Conn J, Correa M. Diseño y evaluación de metodologías basadas en PCR-RFLP de ITS2 para la identificación molecular de mosquitos Anopheles spp. (Diptera: Culicidae) de la Costa Pacífica de Colombia. Rev Bioméd. 2008;19:35–44. [Google Scholar]
- Collins WE, Skinner JC, Warren M, Richardson B. Studies on human malaria in Aotus monkey. VII Comparative infectivity of two strains of Plasmodium vivax to Anopheles freeborni, A maculatus, and for strains of A albimanus. J Parasitol. 1976;62:190–194. [PubMed] [Google Scholar]
- da Silva Araújo M, Gil LH, de Almeida e Silva A. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar J. 2012;11:261. doi: 10.1186/1475-2875-11-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Merida AM, Palmieri M, Yurrita M, Molina A, Molina E, Black WCt. Mitochondrial DNA variation among Anopheles albimanus populations. Am J Trop Med Hyg. 1999;61:230–239. doi: 10.4269/ajtmh.1999.61.230. [DOI] [PubMed] [Google Scholar]
- Dudley R. The Biomechanics of Insect Flight: Form, Function, Evolution. 1. Princeton University Press; Princeton, New Jersey: 2000. [Google Scholar]
- Dujardin JP. Morphometrics applied to medical entomology. Infect Genet Evol. 2008;8:875–890. doi: 10.1016/j.meegid.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Dujardin JP. Modern morphometrics of medically important insects. In: Tibayrenc M, editor. Genetics and Evolution of Infectious Diseases. Elsevier; Burlington: 2011. pp. 473–501. [Google Scholar]
- Dujardin JP, Garros C. Genetic and phenetic approaches to Anopheles systematics. In: Manguin S, editor. Anopheles Mosquitoes – New insights into Malaria Vectors. InTech; Croatia: 2013. pp. 81–105. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Prentice Hall; London, Longman: 1996. [Google Scholar]
- Faran ME. A revision of the Albimanus Section of the subgenus Nyssorhynchus of Anopheles. Contrib Am Entomol Inst. 1980;15:1–215. [Google Scholar]
- Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington; Seattle: 2005. [Google Scholar]
- Fierst JL. A history of phenotypic plasticity accelerates adaptation to a new environment. J Evol Biol. 2011;24:1992–2001. doi: 10.1111/j.1420-9101.2011.02333.x. [DOI] [PubMed] [Google Scholar]
- Fleming G. Biología y ecología de los vectores de malaria. OPS; Washington, DC: 1986. [Google Scholar]
- Francuski L, Matic I, Ludoski J, Milankov V. Temporal patterns of genetic and phenotypic variation in the epidemiologically important drone fly, Eristalis tenax. Med Vet Entomol. 2011;25:135–147. doi: 10.1111/j.1365-2915.2011.00956.x. [DOI] [PubMed] [Google Scholar]
- Frederickson EC. Bionomics and Control of Anopheles albimanus. Pan American Health Organization, Pan American Sanitary Bureau Regional Office of the World Health Organization; Washington, DC: 1993. Technical paper No. 34. [Google Scholar]
- Fuller DO, Ahumada ML, Quinones ML, Herrera S, Beier JC. Near-present and future distribution of Anopheles albimanus in Mesoamerica and the Caribbean Basin modeled with climate and topographic data. Int J Health Geogr. 2012;11:13. doi: 10.1186/1476-072X-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco G, Minelli A. Phenotypic plasticity in development and evolution: facts and concepts. Introduction Philos Trans R Soc Lond B: Biol Sci. 2010;365:547–556. doi: 10.1098/rstb.2009.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González R, Martínez LM. Nuevo registro de distribución altitudinal de Anopheles albimanus Wiedemman (Diptera: Culicidae) en Colombia. Bol Museo Entomol Univ Valle. 2006;7:19–23. [Google Scholar]
- Good P. Permutation Tests: A Practical Guide to Resampling Methods for Testing Hypotheses. Springer; New York: 2000. [Google Scholar]
- Grieco JP, Achee NL, Roberts DR, Andre RG. Comparative susceptibility of three species of Anopheles from Belize, Central America, to Plasmodium falciparum (NF-54) J Am Mosq Control Assoc. 2005;21:279–290. doi: 10.2987/8756-971X(2005)21[279:CSOTSO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Guillot G, Renaud S, Ledevin R, Michaux J, Claude J. A unifying model for the analysis of phenotypic, genetic, and geographic data. Syst Biol. 2012;61:897–911. doi: 10.1093/sysbio/sys038. [DOI] [PubMed] [Google Scholar]
- Gutiérrez LA, Gómez GF, Gonzalez JJ, Castro MI, Luckhart S, Conn JE, Correa MM. Microgeographic genetic variation of the malaria vector Anopheles darlingi root (Diptera: Culicidae) from Cordoba and Antioquia, Colombia. Am J Trop Med Hyg. 2010;83:38–47. doi: 10.4269/ajtmh.2010.09-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez LA, Naranjo N, Jaramillo LM, Muskus C, Luckhart S, Conn JE, Correa MM. Natural infectivity of Anopheles species from the Pacific and Atlantic Regions of Colombia. Acta Trop. 2008;107:99–105. doi: 10.1016/j.actatropica.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Gutiérrez LA, Naranjo NJ, Cienfuegos AV, Muskus CE, Luckhart S, Conn JE, Correa MM. Population structure analyses and demographic history of the malaria vector Anopheles albimanus from the Caribbean and the Pacific regions of Colombia. Malar J. 2009;8:259. doi: 10.1186/1475-2875-8-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer O, Harper DAT, Ryan PD. PAST: Paleontological Statistics software package for education and data analysis. Palaentol Electrón. 2001;4:9. [Google Scholar]
- Hartl DL, Clark AG. Principles of Population Genetics. 4. Sinauer Associates; Sunderland, MA: 2007. [Google Scholar]
- Henderson JM. The eradication of Anopheles albimanus in Puerto Rico. An ecology discussion. Part I. Mosq News. 1948;43:456–459. [Google Scholar]
- Hijmans RJ, Guarino L, Cruz M, Rojas E. Computer tools for spatial analysis of plant genetic resources data: DIVA-GIS. Plant Genet Resour Newsl. 2001;127:15–19. [Google Scholar]
- Hill CA, Kafatos FC, Stansfield SK, Collins FH. Arthropod-borne diseases: vector control in the genomics era. Nat Rev Microbiol. 2005;3:262–268. doi: 10.1038/nrmicro1101. [DOI] [PubMed] [Google Scholar]
- Hobbs J, Lowe R, Schereck C. Studies on fight range and survival of Anopheles albimanus Wiedemann in El Salvador. I Dispersal and survival during the dry season. Mosq News. 1974;34:389–393. [Google Scholar]
- Hoffmann AA, Weeks AR. Climatic selection on genes and traits after a 100 year-old invasion: a critical look at the temperate-tropical clines in Drosophila melanogaster from eastern Australia. Genetica. 2007;129:133–147. doi: 10.1007/s10709-006-9010-z. [DOI] [PubMed] [Google Scholar]
- Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L. Rapid evolution of a geographic cline in size in an introduced fly. Science. 2000;287:308–309. doi: 10.1126/science.287.5451.308. [DOI] [PubMed] [Google Scholar]
- IDEAM. El medio ambiente en Colombia. 2. Instituto de Hidrología, Meteorología y Estudios Ambientales, IDEAM. Ministerio del Medio Ambiente; República de Colombia, Bogotá, DC: 2001. [Google Scholar]
- IGAC. Atlas de Colombia. 6. Instituto Geográfico Agustín Codazzi; IGAC, Bogotá, DC: 2002. [Google Scholar]
- Jaramillo LM, Gutiérrez LA, Luckhart S, Conn JE, Correa MM. Molecular evidence for a single taxon, Anopheles nuneztovari s.l., from two endemic malaria regions in Colombia. Mem Inst Oswaldo Cruz. 2011;106:1017–1023. doi: 10.1590/s0074-02762011000800020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirakanjanakit N, Leemingsawat S, Thongrungkiat S, Apiwathnasorn C, Singhaniyom S, Bellec C, Dujardin JP. Influence of larval density or food variation on the geometry of the wing of Aedes (Stegomyia) aegypti. Trop Med Int Health. 2007;12:1354–1360. doi: 10.1111/j.1365-3156.2007.01919.x. [DOI] [PubMed] [Google Scholar]
- Kennington WJ, Killeen JR, Goldstein DB, Partridge L. Rapid laboratory evolution of adult wing area in Drosophila melanogaster in response to humidity. Evolution. 2003;57:932–936. doi: 10.1111/j.0014-3820.2003.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biol Rev Camb Philos Soc. 1998;73:79–123. doi: 10.1017/s000632319800512x. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP. Evolution and development of shape: integrating quantitative approaches. Nat Rev Genet. 2010;11:623–635. doi: 10.1038/nrg2829. [DOI] [PubMed] [Google Scholar]
- Koella JC, Lyimo EO. Variability in the relationship between weight and wing length of Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 1996;33:261–264. doi: 10.1093/jmedent/33.2.261. [DOI] [PubMed] [Google Scholar]
- Kweka EJ, Zhou G, Beilhe LB, Dixit A, Afrane Y, Gilbreath TM, 3rd, Munga S, Nyindo M, Githeko AK, Yan G. Effects of co-habitation between Anopheles gambiae s.s and Culex quinquefasciatus aquatic stages on life history traits. Parasit Vectors. 2012;5:33. doi: 10.1186/1756-3305-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza JR, Scott ME, Bermingham E, Rovira J, Conn JE. Evidence for Pleistocene population divergence and expansion of Anopheles albimanus in Southern Central America. Am J Trop Med Hyg. 2010a;82:156–164. doi: 10.4269/ajtmh.2010.09-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza JR, Scott ME, Bermingham E, Sanjur OI, Wilkerson R, Rovira J, Gutiérrez LA, Correa MM, Grijalva MJ, Birnberg L, Bickersmith S, Conn JE. Late Pleistocene environmental changes lead to unstable demography and population divergence of Anopheles albimanus in the northern Neotropics. Mol Phylogenet Evol. 2010b;57:1341–1346. doi: 10.1016/j.ympev.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza JR, Bermingham E, Sanjur OI, Scott ME, Bickersmith SA, Conn JE. Review of genetic diversity in malaria vectors (Culicidae Anophelinae) Infect Genet Evol. 2011;12:1–12. doi: 10.1016/j.meegid.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Loetti V, Schweigmann N, Burroni N. Development rates, larval survivorship and wing length of Culex pipiens (Diptera: Culicidae) at constant temperatures. J Nat Hist. 2011;45:2207–2217. [Google Scholar]
- Lyimo EO, Takken W. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Med Vet Entomol. 1993;7:328–332. doi: 10.1111/j.1365-2915.1993.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. 1. Sinauer Associates; Sunderland, MA: 1998. [Google Scholar]
- Martínez-Palacios A, Pletsch DJ. Highlights of mosquito investigation and control in Mexico: past, present and future. Proceedings of the Fiftieth Annual Meeting of the New Jersey Mosquito Extermination Association; 1963. pp. 72–80. [Google Scholar]
- Miller JR, Wood BP, Hamilton MB. FST and QST under neutrality. Genetics. 2008;180:1023–1037. doi: 10.1534/genetics.108.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya-Lerma J, Solarte YA, Giraldo-Calderon GI, Quinones ML, Ruiz-Lopez F, Wilkerson RC, González R. Malaria vector species in Colombia: a review. Mem Inst Oswaldo Cruz. 2011;106:223–238. doi: 10.1590/s0074-02762011000900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes EM, Manfrin MH, Laus AC, Rosada RS, Bomfin SC, Sene FM. Wing shape heritability and morphological divergence of the sibling species Drosophila mercatorum and Drosophila paranaensis. Heredity (Edinb) 2004a;92:466–473. doi: 10.1038/sj.hdy.6800442. [DOI] [PubMed] [Google Scholar]
- Moraes EM, Manfrin MH, Laus AC, Rosada RS, Bomfin SC, Sene FM. Wing shape heritability and morphological divergence of the sibling species Drosophila mercatorum and Drosophila paranaensis. Heredity (Edinb) 2004b;92:466–473. doi: 10.1038/sj.hdy.6800442. [DOI] [PubMed] [Google Scholar]
- Morales Vargas RE, Ya-Umphan P, Phumala-Morales N, Komalamisra N, Dujardin JP. Climate associated size and shape changes in Aedes aegypti (Diptera Culicidae) populations from Thailand. Infect Genet Evol. 2010;10:580–585. doi: 10.1016/j.meegid.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Morrone JJ. Biogeographic areas and transition zones of Latin America and the Caribbean islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu Rev Entomol. 2006;51:467–494. doi: 10.1146/annurev.ento.50.071803.130447. [DOI] [PubMed] [Google Scholar]
- Norberg UM. How a long tail and changes in mass and wing shape affect the cost for flight in animals. Funct Ecol. 1995;9:48–54. [Google Scholar]
- Okanda FM, Dao A, Njiru BN, Arija J, Akelo HA, Toure Y, Odulaja A, Beier JC, Githure JI, Yan G, Gouagna LC, Knols BG, Killeen GF. Behavioural determinants of gene flow in malaria vector populations: Anopheles gambiae males select large females as mates. Malar J. 2002;1:10. doi: 10.1186/1475-2875-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okech BA, Gouagna LC, Yan G, Githure JI, Beier JC. Larval habitats of Anopheles gambiae s.s (Diptera: Culicidae) influences vector competence to Plasmodium falciparum parasites. Malar J. 2007;6:50. doi: 10.1186/1475-2875-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olano V, Carrasquilla G, Mendez F. Transmission of urban malaria in Buenaventura, Colombia: entomological features. Rev Panam Salud Publica. 1997;1:287–294. [PubMed] [Google Scholar]
- Oliver SV, Brooke BD. The effect of larval nutritional deprivation on the life history and DDT resistance phenotype in laboratory strains of the malaria vector Anopheles arabiensis. Malar J. 2013;12:44. doi: 10.1186/1475-2875-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Huijben S, Githeko AK, Takken W. Competitive interactions between larvae of the malaria mosquitoes Anopheles arabiensis and Anopheles gambiae under semi-field conditions in western Kenya. Acta Trop. 2009;109:124–130. doi: 10.1016/j.actatropica.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Porcher E, Giraud T, Goldringer I, Lavigne C. Experimental demonstration of a causal relationship between heterogeneity of selection and genetic differentiation in quantitative traits. Evolution. 2004;58:1434–1445. doi: 10.1111/j.0014-3820.2004.tb01725.x. [DOI] [PubMed] [Google Scholar]
- Pujol B, Wilson AJ, Ross RI, Pannell JR. Are Q(ST)–F(ST) comparisons for natural populations meaningful? Mol Ecol. 2008;17:4782–4785. doi: 10.1111/j.1365-294X.2008.03958.x. [DOI] [PubMed] [Google Scholar]
- Quiñones ML, Suárez MF, Fleming G. Distribución y bionomía de los anofelinos de la Costa Pacífica de Colombia. Colomb Méd. 1987;18:19–24. [Google Scholar]
- Reed DH, Frankham R. How closely correlated are molecular and quantitative measures of genetic variation? A meta-analysis. Evolution. 2001;55:1095–1103. doi: 10.1111/j.0014-3820.2001.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Rosero DA, Jaramillo LM, Gutiérrez LA, Conn JE, Correa MM. Genetic diversity of Anopheles triannulatus s.l (Diptera Culicidae) from northwestern and southeastern Colombia. Am J Trop Med Hyg. 2012;87:910–920. doi: 10.4269/ajtmh.2012.12-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford MR, Demirci B, Marsden CD, Lee Y, Cornel AJ, Lanzaro GC. Morphological differentiation may mediate mate-choice between incipient species of Anopheles gambiae s.s. PLoS ONE. 2011;6:e27920. doi: 10.1371/journal.pone.0027920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter-Broide J, Gurtler RE, Kitron U, Dujardin JP. Temporal variations of wing size and shape of Triatoma infestans (Hemiptera: Reduviidae) populations from northwestern Argentina using geometric morphometry. J Med Entomol. 2009;46:994–1000. doi: 10.1603/033.046.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JR, Morrison AC, Astete H, Scott TW, Wilson ML. Adult size and distribution of Aedes aegypti (Diptera: Culicidae) associated with larval habitats in Iquitos, Peru. J Med Entomol. 2004;41:634–642. doi: 10.1603/0022-2585-41.4.634. [DOI] [PubMed] [Google Scholar]
- Shelomi M. Where are we now? Bergmann’s rule sensu lato in insects. Am Nat. 2012;180:511–519. doi: 10.1086/667595. [DOI] [PubMed] [Google Scholar]
- Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE, Hay SI. The dominant Anopheles vectors of human malaria in the Americas: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:72. doi: 10.1186/1756-3305-3-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens CR, Juliano SA. Wing shape as an indicator of larval rearing conditions for Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2012;49:927–938. doi: 10.1603/me12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan SE, Spencer HG. Metapopulation structure favors plasticity over local adaptation. Am Nat. 2002;160:271–283. doi: 10.1086/341015. [DOI] [PubMed] [Google Scholar]
- Sutcliffe AC, Benedict MQ. Effective larval Foraging in Large, Low-Diet Environments by Anopheles gambiae. Psyche. 2012;2012:8. [Google Scholar]
- Suwanchaichinda C, Paskewitz SM. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize sephadex beads. J Med Entomol. 1998;35:157–161. doi: 10.1093/jmedent/35.2.157. [DOI] [PubMed] [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Takken W, Smallegange RC, Vigneau AJ, Johnston V, Brown M, Mordue-Luntz AJ, Billingsley PF. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasit Vectors. 2013;6:345. doi: 10.1186/1756-3305-6-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente JL, Sousa CA, Alten B, Caglar SS, Falcuta E, Latorre JM, Toty C, Barre H, Demirci B, Di Luca M, Toma L, Alves R, Salgueiro P, Silva TL, Bargues MD, Mas-Coma S, Boccolini D, Romi R, Nicolescu G, do Rosario VE, Ozer N, Fontenille D, Pinto J. Genetic and phenotypic variation of the malaria vector Anopheles atroparvus in southern Europe. Malar J. 2011;10:5. doi: 10.1186/1475-2875-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal PO, Carvalho E, Suesdek L. Temporal variation of wing geometry in Aedes albopictus. Mem Inst Oswaldo Cruz. 2012;107:1030–1034. doi: 10.1590/s0074-02762012000800011. [DOI] [PubMed] [Google Scholar]
- Warren M, Collins WE, Richardson BB, Skinner JC. Morphologic variants of Anopheles albimanus and susceptibility to Plasmodium vivax and P. falciparum. Am J Trop Med Hyg. 1977;26:607–611. doi: 10.4269/ajtmh.1977.26.607. [DOI] [PubMed] [Google Scholar]
- Webster M, Sheets D. A practical introduction to landmark-based geometric morphometrics. In: Alroy J, Hunt G, editors. Quantitative Methods in Paleobiology. The Paleontological Society; USA: 2010. pp. 163–188. [Google Scholar]
- Whitlock MC. Evolutionary inference from QST. Mol Ecol. 2008;17:1885–1896. doi: 10.1111/j.1365-294X.2008.03712.x. [DOI] [PubMed] [Google Scholar]
- Whitman DW, Agrawal AA. What is phenotypic plasticity and why is it important? In: Whitman DW, Ananthakrishnan TN, editors. Phenotypic Plasticity of Insects: Mechanisms and Consequences. Science Publishers; Enfield: 2009. pp. 1–63. [Google Scholar]
- Zapata MA, Cienfuegos AV, Quiros OI, Quinones ML, Luckhart S, Correa MM. Discrimination of seven Anopheles species from San Pedro de Uraba, Antioquia, Colombia, by Polymerase Chain Reaction-Restriction Fragment Length Polymorphism analysis of ITS sequences. Am J Trop Med Hyg. 2007;77:67–72. [PubMed] [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets D, Fink WL. Geometric Morphometrics for Biologist: A Primer. Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- Zimmerman E, Palsson A, Gibson G. Quantitative trait loci affecting components of wing shape in Drosophila melanogaster. Genetics. 2000;155:671–683. doi: 10.1093/genetics/155.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]