Abstract
Cells grow, move, expand, shrink and die in the process of generating the characteristic shapes of organisms. Although the structures generated during development of the social amoeba Dictyostelium discoideum look nothing like the structures seen in metazoan embryogenesis, some of the morphogenetic processes used in their making are surprisingly similar. Recent advances in understanding the molecular basis for directed cell migration, cell type specific sorting, differential adhesion, secretion of matrix components, pattern formation, regulation and terminal differentiation are reviewed. Genes involved in Dictyostelium aggregation, slug formation, and culmination of fruiting bodies are discussed.
Keywords: G protein coupled receptors (GPCR), ultrasensitived LEGI, RasGTP, multicellular morphogenesis, development, molecular genetics, homologs, cell type divergence, proportioning
Advanced microscopy of cells tagged with fluorescent proteins has allowed many of the pathways of embryogenesis to be traced in great detail. However, understanding how properly positioned structures are sculpted from groups of cells to generate specializations at the head and tail, belly and back, is still a major challenge. Searching for the underlying forces at the level of tissues and specialized cells only increases the complexity of the problem. Sometimes it is more efficient to analyze the processes in simpler organisms with fewer cell types with the aim of recognizing universal principles of multicellular morphogenesis.
Recent progress in culturing patient derived stem cells and directing their differentiation down specific pathways has raised hopes that, some day soon, it will be possible to grow whole organs to replace those failing in the patient. Using structured 3D matrices to guide the growth of stem cells has shown much promise in generating complex living shapes such as tubules and alveoli (Fleig and Humphreys, 2014; Balestrini and Niklason, 2014). However, only a small proportion of the cells enter into functional structures and often there are serious abnormalities. It is imperative that we understand the principles of morphogenesis before relying on laboratory grown replacement organs.
The refinement of molecular biological techniques over the last half century accelerated comparative studies using model systems such as Dictyostelium, yeast, Hydra, C. elegans, Drosophila, sea urchins, zebra fish and mice (Gilbert, 2013). The genomes were sequenced and individual genes were translated, analyzed, compared and cataloged. Many of the proteins showed significant sequence similarity to proteins in other organisms including humans. Genes present in disparate organisms that had clearly descended from a common ancestoral gene were placed in clusters of orthologs. Biochemical characterization of one member of a cluster would suggest possible functions for all the members of the cluster. During this time, efforts were also underway to use genetics to modify every gene that controlled morphogenesis. Different techniques were used in different organisms depending on what worked best. Transposon and plasmid insertion was highly effective in Drosophila melanogaster and Dictyostelium discoideum, while genome-wide RNAi worked efficiently in C. elegans and zebra fish. Homologous recombination had to be used in mice and chemically modified antisense nucleic acids called morpholinos had to be used to modify gene expression during early embryogenesis of sea urchins. The lists of vital genes and the lists of morphological genes continue to grow. Already, it is clear that there is a conserved core of related proteins that are used in controlling morphogenesis as well as sets of idiosyncratic proteins unique to a given species. It is also clear that studies on certain aspects of development are much easier in some organisms that others. Mating type switching was worked out in the yeast Saccharomyces cerevisiae and found to give insight into dimorphism in distantly related yeast and fungi. Positional patterning has been studied in Hydra, C. elegans, and Drosophila, while organ formation has been delineated in sea urchins, zebra fish and mice.
The social amoeba Dictyostelium discoideum presents a convenient test system in which to explore such processes as directed cell movement, cell sorting, the role of an extracellular matrix, and terminal differentiation. This organism alternates between growing as single cells that are amenable to microbial style genetics and developing as a multicellular organism after chemotactic aggregation. Thereafter, two cell types differentiate that can be distinguished by the genes they express. The cell types are initially found at random positions within each aggregate but then sort out to the front or back of slug shaped structures containing about 105 cells that are surround by an extracellular matrix. After a period during which the slugs can migrate phototactically to the surface of the forest floor, the anterior cells differentiate into stalk cells and the posterior cells differentiate into spores. Together they build a fruiting body in which the spores are held up by a cellular stalk several millimeters long. The whole developmental process takes about 24 hrs and is mediated by several hundred morphogenetic genes (Loomis, 1975; 1978).
The life cycle of D. discoideum has a clear separation of growth and differentiation since there is no significant chromosomal DNA synthesis after development is initiated by the removal of all nutrients (Shaulsky and Loomis, 1995). Therefore, we can define morphogenetic genes as those in which mutations visibly affect structures at some stage of development but do not significantly affect growth. An effort to collect as many mutants as possible with aberrant or weird morphology has uncovered several hundred morphogenetic genes that are available at dictyBase [http://dictybase.org/Downloads/allmutants.html]. Most of the mutants were generated by plasmid insertion using Restriction Enzyme Mediated Insertion (REMI) (Kuspa and Loomis, 1992) but some were found by homologous recombination into candidate genes. Since the full genome sequence has been manually annotated and carefully curated (see Dictybase.org), the likely function of most genes can be inferred from comparison to orthologs in other organisms (Eichlinger et al., 2005). The presence of paralogs and multigene families can be readily seen in the genome, where they indicate that reverse genetics should be used to generate complex genotypes to test for specific roles in morphogenesis. While there have been several excellent reviews of developmental genes and morphogenesis in Dictyostelium (Chisholm and Firtel, 2004; Swaney, Huang and Devreotes, 2010; Sucgang et al., 2011; Kortholt et al., 2013), recent advances in understanding developmental genes and pathways in this organism can be related to similar processes in other multicellular organisms.
cAMP Waves
Unlike metazoans where fertilization of an egg by a sperm marks the beginning of embryogenesis, there is no unique cellular event that indicates that development has been initiated in Dictyostelium. Each individual cell responds to starvation clues by gradually altering its physiology such that it can cooperate with others to form multicellular structures. Synchronous development is initiated in the laboratory by separating the cells from their external source of nutrients in a centrifuge. washing them in buffer and incubating them as dense monolayers on solid moist supports. Nitrogen starvation signals the end of growth and the start of transcription of the genes encoding adenylyl cyclase (acaA), a secreted cAMP phophodiesterase (pdsA), and a seven transmembrane G protein coupled receptor (GPCR) that is specific for cAMP (carA) (see Table 1). The receptor is coupled to a trimeric G protein with the Gα2 subunit. The gene encoding Gα2, gpaB, is also induced immediately after the cells sense that they are nitrogen limited (Iranfar, Fuller and Loomis 2003). Within a few hours these mRNAs are translated and the cells can produce cAMP, secrete it using the preexisting 12 transmembrane transporter AbcB3 (Miranda et al., 2015), and respond to it when it binds to the Car1 receptor, triggering the release of Gα2 from Gβγ.
Table 1.
Aggregation Genes
| Gene | Product | Period of mRNA increase | Reference |
|---|---|---|---|
| acaA | adenylyl cyclase | 0 - 8 hr | Pitt et al., 1992 |
| carA | cAMP receptor | 0 - 4 hr | Klein et al., 1988 |
| pdsA | cAMP phosphodiesterase | 0 - 4 hr | Sucgang et al., 1997 |
| gpaB | G alpha 2 | 0 - 4 hr | Oyama et al., 1991 |
| rasC | Ras C | 0 - 4 hr | Lim et al., 2005 |
| rasG | Ras G | 0 - 2 hr | Thiery et al., 1992 |
| nfaA | RasGAP | 4 - 12 hr | Zhang et al., 2008 |
| abcB3 | ATP binding cassette B3 (cAMP) | 4 - 8 hr | Miranda et al., 2015 |
| pikA | Phosphatidyl inositol 3 kinase | 4 - 20 hr | Buczynski et al., 1997 |
| pten | Phosphatase and TEN | 0 - 12 hr | Iijima et al., 2002 |
| pkaC | Protein kinase A catalytic | 0 - 8 hr | Harwood et al., 1992 |
| pkaR | Protein kinase A regulatory | 0 - 8 hr | Firtel et al., 1990 |
| regA | cAMP phosphodiesterase | 0 - 4 hr | Shaulsky et al., 1996 |
| erkB | MAP kinase | 0 - 12 hr | Segall et al., 1995 |
| gtaC | GATA C | 0 - 4 hr | Keller et al., 2008 |
| rapA | Rap1 | 0 - 8 hr | Rebstein et al., 1997 |
| rapGAP | Rap1 GAP | 0 - 8 hr | Jeon et al., 2007 |
| piaA | TORC2 subunit | 0 - 12 hr | Chen et al., 1997 |
| pkbA | Protein kinase B (AKT) | 0 - 2 hr | Tang et al., 2011 |
| pkgB | Protein kinase B related | 0 - 8 hr | Meili et al., 2000 |
| cadA | gp24 | 0 - 4 hr | Knecht et al., 1987 |
| csaA | gp80 | 4 - 10 hr | Noegel et al., 1986 |
| tgrB/C | gp150 | 4 - 10 hr | Wang et al., 2000 |
One of the responses to extracellular cAMP is the rapid accumulation of active Ras protein on the cell membrane. Like other small GTPases, Ras is found in both the GDP bound form and the GTP bound form and it is the GTP form that is active. This molecular switch is activated by association with a specific GTP Exchange Factor, RasGEF, which facilates release of bound GDP and its rapid replacement with GTP. Ras has intrinsic GTPase activity but it is very weak until it is stimulated by a specific GTPase Activating Protein, RasGAP. The GTPase activity then converts GTP to GDP and turns off the Ras switch. When cAMP binds Car1, the dissociated subunits of the trimeric G protein activate both RasGEF and RasGAP (Takeda et al., 2012). RasGEF is both more rapidly activated than RasGAP and more rapidly inactivated. As a result, there is an initial surge in membrane associated RasGTP a few seconds after addition of cAMP to cells that have been developing for a few hours. The response adapts within 30 seconds and the level of membrane associated RasGTP returns to its initial value as the activity of RasGAP increases and surpasses that of RasGEF.
RasGTP stimulates the activity of the lipid kinase PI3K which converts phosphatidyl inositol phosphate PIP2 to PIP3. Various proteins bind through their PH-domains to membrane patches enriched in PIP3. One of these PH-domain proteins, CRAC, activates the adenylyl cyclase ACA when it binds to the membrane (Insall et al., 1994). As a result cells synthesize and secrete cAMP in response to an increase in exogenous cAMP such that the signal is relayed outward as a non-dissipating wave. Extracellular cAMP is then broken down by the secreted phosphodiesterase, PdsA, and the field of cells is ready for the next wave of cAMP.
For the first few hours of development of Dictyostelium there is not much to see in the way of morphogenesis. The cells just rest on the support and cringe slightly when a wave of cAMP passes over them. Between 4 and 8 hours of development, successive waves of cAMP spread out over the cells every 6 or 7 minutes. Dark field microscopy amplifies cellular differences that result in alterations in light scattering. When viewed this way, dark waves of cringing cells can be seen to spread across a lawn of developing cells covering the bottom of a petri dish. The waves are often concentric, giving bull's-eye target-like patterns, but small temporal or spatial perturbations can rapidly convert them into spiral waves. When two spirals intersect, they annihilate each other indicating that the cells are acting as an excitable medium entrained by extracellular signals. For an hour or so spiral waves spread across the the dish with hypnotic regularity but there is very little net movement of the cells. This is a form of morphogenesis but an unusual one. The closest parallel in mammalian cells is probably the periodic contractions that can be seen in confluent cells cultured from embryonic heart primordium. When cells fated to become heart muscle are dissociated and cultured as monolayers, they initially just grow and divide. However, after a few days they will spontaneously start to synchronously contract and continue pulsing for some time (Mitcheson, Hancox, and Levi, 1998; Nakajima et al., 2009; Majkut et al., 2013). This is a definitive characteristic of heart muscle cells and clearly involves a regular oscillator.
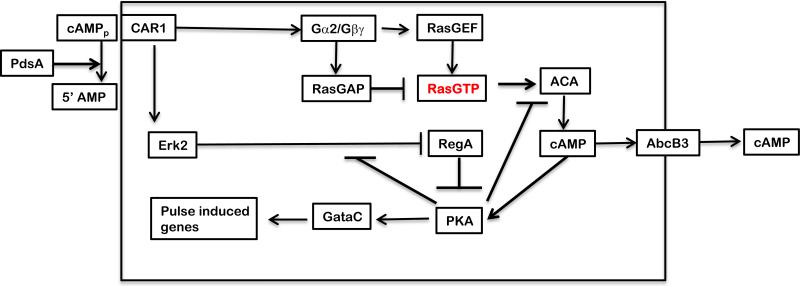
The circuit that generates the 6 minute periodicity in Dictyostelium is fairly well known (Laub and Loomis, 1998; Maeda et al., 2004). cAMP binding to the Car1 receptor not only stimulates ACA but also inhibits the intracellular phosphodiesterase RegA by acting through the protein kinase Erk2 (Figure 1). This two-pronged signal transduction results in rapid build up of cAMP, most of which is secreted. However, some stays within the cells where it activates protein kinase A (PKA). This enzyme has two catalytic subunits associated with two regulatory subunits. As such it is inactive. But, when cAMP binds to the regulatory subunits, they dissociate from the catalytic subunits allowing them to be fully active. This circuit oscillates because PKA activity blocks stimulation of ACA and the inhibition of RegA, such that cAMP levels rapidly return to their basal levels. PKA activity then diminishes until the next wave of exogenous cAMP comes by. Numerical simulations of this circuit showed it to be robust when multiple cells are entrained to the same period. Null mutations in the genes encoding any of the indicated components result in cells that fail to periodically generate pulses of cAMP or proceed through develoment normally. By these criteria these are morphogenetic genes.
Figure 1.
Oscillatory circuit. Binding of cAMP to the surface receptor (CAR1) activates adenylyl cyclase (ACA) via RasGTP. It also inhibits the cAMP phosphodiesterase (RegA) by activating the MAP kinase (Erk2). Both of these pathways are inhibited when the activity of cAMP dependent protein kinase (PKA) increases. Coupled differential equations of the kinetics indicate that PKA activity and the concentration of cAMP will oscillate with a 6 minute periodicity. PKA also stimulates the transcription factor GataC to induce aggregation stage genes. CAR1 is a GPCR that stimulates both RasGEF and RasGAP which form an incoherent feedforward loop to regulate RasGTP (Laub and Loomis, 1998; Takeda et al., 2012).
Similar circuits have been discovered in heart cells and the budding yeast Saccharomyces cerevisiae. Human aortic smooth muscle cells express a cAMP phosphodiesterase 4D5 which can be phosphorylated by PKA (Baillie, MacKenzie and Houslay, 2001). Just as in Dictyostelium, PKA activity overcomes inhibition of the phosphodiesterase resulting from phosphorylation by Erk. Complex cross-talk links the Erk and cAMP signaling pathways in both of these systems. Under conditions of stable adenylyl cyclase activity, the feedback of PKA activity on the enzyme that destroys its activator, cAMP, would be expected to result in oscillations in cAMP.
At moderate levels of stress yeast cells exhibit oscillations of the transcription factor Msn2 between the cytoplasm and the nucleus with a periodicity of a few minutes (Jacquet et al., 2003). This zinc-finger DNA binding protein mediates the responses to stress including nutritional limitation. PKA phosphorylates the Nuclear Localization Signal (NLS) of Msn2 such that it exits the nucleus and can no longer regulate transcription. Just as in aggregation stage Dictyostelium cells, yeast show oscillations in cAMP that result from negative feedback on adenylyl cyclase mediated by PKA. PKA activity also shows oscillations since this protein kinase stimulates cAMP phosphodiesterase activity which reduces the level of cAMP and PKA activation. Other connecting components of the PKA oscillation circuits in yeast and Dictyostelium differ to some extent but adenylyl cyclase is driven by the alternation in RasGDP and RasGTP in both (Broek et al., 1987). In yeast, PKA may stimulate RasGAP either directly or indirectly which would reduce the level of RasGTP and adenylyl cyclase activation. In the absence of PKA, Msn2 is not phosphorylated and stays in the nucleus (Garmendia-Torres, Goldbeter and Jacquet, 2007).
The oscillatory circuit in Dictyostelium not only regulates the synthesis and release of cAMP but also controls the activity of the transcription factor GataC (Cai et al., 2014). This DNA binding factor is found in the nucleus but is inactive until it is phosphorylated by PKA. Although phosphorylation activates GataC, it also leads to its exit from the nucleus after a minute or so. Much like the yeast transcription factor, GataC can return to the nucleus when it has been dephosphorylated in the cytoplasm. But, unlike Msn2, GataC is only active for a brief period when it is phosphorylated and still in the nucleus. This behavior helps explain why transcription of more than 85 Dictyostelium genes, including the four that are initially transcribed in the absence of cAMP signals, are stimulated by pulses of cAMP but not by constant high levels of cAMP (Iranfar, Fuller and Loomis, 2003).
Chemotactic Aggregation
After a series of waves passes over them, cells start to move up the gradient. In a field sparsely filled with cells, the individual cells can be seen to respond to incoming waves by lurching forward and then stopping as the wave passes by. The cells file in towards the center to form an aggregate. The pattern is so striking that it initially appears to be an entropy paradox - order seems to be generated out of nothing. In fact, each cell is metabolizing its stores of glycogen to generate the necessary energy to relay the signal and move. The cells are phenomenally sensitive to shallow gradients of cAMP, responding to differences of cAMP concentration that can be as little as 5% across the cell by migrating almost directly up the gradient (Fuller et al., 2010). At low concentrations of cAMP, this means that a front-back difference of less than a few hundred molecules over a background of ten thousand molecules is sufficient for establishing chemotactic directionality. How can such a small difference be amplified to give the observed accuracy of motility?
The Car1 receptor and its trimeric G protein, Gα2Gβγ are uniformly distributed over the surface of cells after 6 hours of development and do not become localized in response to cAMP gradients, so it is not a matter that a cell only looks forward (Jin et al., 2000; Janetopolis, Jin and Devreotes, 2001). As can be seen in Figure 1, Car1 initiates an incoherent feedforward loop in which both the Ras activator, RasGEF, and the Ras inhibitor, RasGAP, are stimulated (Takeda et al., 2012). If the activator, RasGEF, is localized to the membrane, its pattern of activity over the surface of the cell will be an accurate representation of the cAMP in the environment around the cell. If, on the other hand, the inhibitor, RasGAP, is a freely diffusing cytoplasmic protein, then its activity at any point in the cell will be an average of the cAMP in the environment around the cell. If the levels of RasGEF and RasGAP are balanced, then RasGEF will be higher than RasGAP at the front and lower at the back in a gradient of cAMP. This Local Excitation Global Inhibition (LEGI) model explains how most Ras will be in the GTP form at the front and in the GDP form elsewhere (Parent and Devreotes, 1999). Ultrasensitivity of the LEGI model resulting from the opposing activities of RasGEF and RasGAP can account for the dramatic amplification of the chemotactic gradient into the control of the direction of motility (Skoge et al., 2014).
Ultrasensitive LEGI can also explain why cells do not respond to the reversed slope of the cAMP gradient in the back of the wave. As the concentration of cAMP decreases in the back of the wave, global RasGAP activity decays more slowly than local RasGEF activity and so RasGAP is greater than RasGEF and little or no RasGTP can accumulate anywhere in the cell. In support of this model, RasGTP has been observed to rapidly disappear as soon as the concentration of cAMP begins to decrease (Skoge et al., 2014; Nakajima et al., 2014).
After about 5 hours of development, the cells change their behavior in the back of the waves. Rather than just stopping and going nowhere, they maintain their polarity and continue to move in the original direction for several minutes such that in a cAMP wave with a 6 minute periodicity the cells move continuously (Skoge et al., 2014). A biphasic memory module appears to have been added to the ultrasensitive LEGI mechanism which allows the cells to maintain their direction of movement for several minutes.
Aggregation competent cells respond to a gradient of cAMP by rapidly accumulating activated Ras in a patch on the surface closest to the source. RasGTP patches are correlated with increases in localized polymerization of actin. Pressure of the dendritic actin fibers on the plasma membrane generates a pseudopodal extension in the direction of the gradient. Understanding how surface patches of RasGTP direct pseduopod formation has to include the complexity of the signal transduction pathways leading from Ras to cytoskeletal control (Swaney, Huang and Devreotes, 2010).
Two members of the Ras family, RasG and RasC, are activated when Car1 binds cAMP and both appear to be involved in regulating chemotactic migration. The anterior patch of RasG - GTP activates PI3K which leads to the accumulation of PIP3 at the front where the protein kinase PKB can bind through its PH-domain (Figure 2). PIP3 is hydrolyzed to form PIP2 by the lipid phosphatase PTEN which rapidly dissociates from the surface membrane when Car1 binds cAMP but then reassociates with the membrane over the sides and back of the cell. Concentrating PTEN at the back further localizes PIP3 to the most anterior portion of the cell surface.
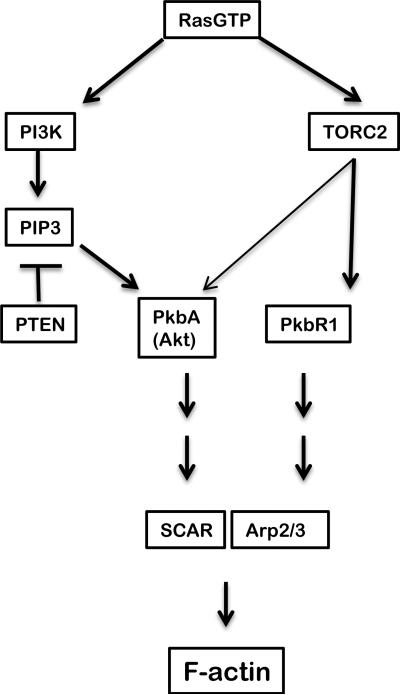
Figure 2.
Regulation of the actin cytoskeleton. The ultrasensitive LEGI model of RasGEF and RasGAP activation accounts for localization of RasGTP to the part of a cell closest to the source of cAMP. Phosphatidyl inositol triphosphate (PIP3) marks the front of the cell as the result of activation of PI3K by RasG-GTP. The PH domain of PKB localizes it to the anterior patch of PIP3 where it can be activated by the TORC2 complex which is stimulated by RasC-GTP. PKB and a related protein kinase (PkbR1) together indirectly stimulate the actin binding proteins SCAR and Arp2/3 leading to the polymerization of dendritic F-actin (modified from Swaney, Huang and Devreotes, 2010).
RasC is also activated at the anterior where it stimulates the kinase activity of the complex that includes Target of Rapamycin (TOR), Pia (Rictor), RIP3 (Sin1) and Lst8. This TORC2 complex phosphorylates and activates PKB (Figure 2). TORC2 also activates the related protein kinase PkbR1 which together with PKB stimulates intermediates that lead to the activation of the F-actin binding complexes SCAR and Arp2/3 such that they initiate polymerization of dendritic F-actin. Null mutations in many of these genes compromise chemotaxis (Swaney, Huang and Devreotes, 2010) (see Table 1). By these criteria they are morphogenetic genes. However, under certain conditions, such as steep gradients of fairly high concentrations of cAMP, many of these genes are dispensible. For instance, a strain in which all known genes encoding PI3K have been disrupted is still able to chemotax in steep gradients (Hoeller and Kay, 2007). It seems there are other minor pathways that synthesize PIP3.
Homologs of many of these genes are found in mammalian neutrophils that patrol the body looking for bacterial infections. These cells rapidly leave the circulatory system and move through the body in response to the presence of bacteria within tissues. They arrive at the site of bacterial infection by chemotaxis to small bacterial peptides. These cells are about the same size and shape as Dictyostelium cells and they move at the same rapid rate. Most surprisingly, many of the mechanisms of chemotaxis appear to have been conserved ever since they shared a common ancestor almost a billion years ago (Bagorda and Parent, 2008; Artemenko, Lampert and Devreotes, 2014).
Both Dictyostelium and neutrophils use GPCRs that are uniformly distributed over the surface to monitor the level of chemoattractants in the environment. RasGTP accumulates on the membrane shortly after the addition of chemoattractant in both cell types. In neutrophils, H-RasGTP is bound to PI3Kγ which is found at the front. PIP3 accumulates at the front in both cell types where it localizes PKB (referred to as AKT in mammalian cells) by presenting a docking site for the PH-domain (Servant et al., 2000). SCAR (referred to as WAVE in mammalian cells) is found at the front of Dictyostelium and neutrophils in gradients of chemoattractants where it facilitates the rapid polymerization of actin to form a dense mesh of cortical filaments.
Sustained forward movement requires that cells retract their rear ends. This requires that attachment to the substratum be weakened specifically at the back and that actomyosin fibers contract locally. While the cytoskeletal details are not clear in either Dictyostelium or neutrophils, dynamic phosphorylation of the myosin subunits appears to be essential for tail retraction (Bosgraaf et al., 2002). Likewise, it is not clear in either system how the localized association with the substratum is regulated. It may involve some of the same processes that regulate movement in response to hydrodynamic pressure (Zhu, Bouffanais, and Yue, 2014). Substratum adhesion in Dictyostelium clearly does not involve integrins since genes encoding homologs of such mammalian adhesion molecules are not present in the genome (Loomis et al. 2012). Since Dictyostelium cells can bind equally well to hydrophobic, hydrophilic or clean glass as well as to plastic surfaces, it is likely that they are using van der Waals attraction between their surface glycoproteins and the substratum (Loomis et al., 2012). There are reasons to think that neutrophils may also use van der Waals attractions for substrate adhesion.
Group behavior: Streaming
Multicellularity has evolved independently among eukaryotes several times in the last billion years (Eichinger et al., 2005). Plants, animals and Dictyostelium all gave rise to multicellular organisms by increasing the adhesion between cells but they used very different mechanisms to reach this end. Plants fused their cell walls, animals selected for strong cell-cell adhesion between embryonic cells, and Dictyostelium selected for developmentally regulated cell-adhesion proteins that could hold cells together when they aggregated chemotactically. While the proteins responsible for cell-cell adhesion in animals and Dictyostelium play similar roles, there is no evidence that they are derived from a common ancestoral set of proteins. Animals often use cadherins for cell-cell adhesion. Cadherins are single-pass membrane proteins from a large family of calcium dependent proteins that hold cells together by homotypic interactions. One of the cell-cell adhesion proteins of Dictyostelium, gp24, is also a single-pass membrane protein but shows no significant similarity in primary sequence of amino acids to cadherins (Knecht, Fuller and Loomis, 1987; Wong et al., 1996). Unlike cadherins, gp24 has a low affinity to calcium ions and its 3D structure is not significantly affected by calcium (Lin et al., 2006). Somewhat unfortunately, the locus encoding gp24 was named cadA in honor of cadherins.
gp24 was recognized using an approach pioneered by Gunther Gerisch (Beug et al., 1970). An assay for cell-cell adhesion was developed in which the number of cells dissociated from clumps by gentle shaking was counted microscopically (Knecht, Fuller and Loomis, 1987). The number decreased as the cells became more adhessive. Antibodies were raised to the surface proteins of cells just after they had started to develop and tested for those that could block cell-cell adhesion. The components of the cell surface were then fractionated and tested for the ability to neutralize the activity of adhesion-blocking antibodies. In this way gp24 was purified and a cDNA clone isolated. The mRNA from cadA was found to be very low in growing cells and to increase rapidly during early development (Table 1).
Gerisch (1968) was able to distinguish two separate cell-cell adhesion mechanisms on the basis of their sensitivity to addition of 10 mM EDTA. The adhesion mechanism that appeared first during development was sensitive to EDTA and termed Contact Sites B which was followed by an adhesion mechanism that was resistant to EDTA which he termed Contact Sites A. Adhesion mediated by gp24 was found to be EDTA sensitive and so was responsible for Contact Sites B. Its sensitivity to the calcium chelator EDTA lead to the idea that gp24 might be calcium dependent, much like cadherins. However, inhibition of Contact Sites B requires at least 10 times more EDTA than is necessary to reduce free calcium below the Kd. Early adhesion is not affected by 1 mM EDTA and so appears to be calcium independent. 10 mM EDTA is needed to inhibit Contact Sites B and must be doing so in a manner other than calcium chelation.
The surface protein that is responsible for Contact Sites A was purified based on its ability to neutralize antibodies that blocked the EDTA resistant adhesion (Ochia et al., 1982; Noegel et al., 1986). The Contact Sites A protein was found to be glycosylated, phopshorylated and sulfated and to carry a phospholipid tail that tethers it to the membrane (Schmidt and Loomis, 1982; Loomis, 1988; Barth et al., 1994). It mediates cell-cell adhesion by homophilic interaction between identical membrane proteins (Siu, Cho and Choi, 1987).
After about 8 hours of development almost all the cells are moving rapidly to the chemotactic signals emanating from the center of an aggregate. As some pass by a group of other cells going the same way, they veer towards them and together they form a stream. The cells appear to be held together in streams by both contact sites A and B since streams of wild type cells can exclude attenuated cells, but streams of mutant cells that are delayed in expressing contact site B and are missing gp80 let the attenuated cells enter into the streams (Xu et al., 1996). Single mutant cells lacking either contact sites A or B are still able to exclude the attenuated cells. The attenuated cells used in these experiments were lacking myosin heavy chain (mhcA-) and had weakened cortices.
Cells enter streams by pushing between cells and attaching to the posterior of the cell ahead. Many of the chemotactic components that are localized to the front of freely migrating single cells accumulate to high levels in the front of cells within streams although this seems to be the consequence of cell contact rather than chemotaxis (Dormann et al., 2002; Weijer, 2009). Time lapse movies show that cells surge forward when the cell ahead of them leaves a space. Otherwise, they are close packed and moving as a steady stream. They will continue into the center of the aggregate where they will continue to move rapidly by forming large rotating swirls (Seigert and Weijer, 1995). The cells move around the mound in either a clockwise or a counterclockwise fashion for several hours. This concerted movement does not depend on relay of the chemotactic signal cAMP since mutant cells in which the gene encoding the aggregation stage adenylyl cyclase, acaA, is disrupted swirl normally if PKA is constitutively active (Nicol et al., 1999). These cells do not form aggregates by chemotaxis but rather by accretion as cells in a dense population bump into each other by chance and then stick together (Wang and Kuspa, 1997). They then swirl around each other until a slug is formed.
Another EDTA resistant cell-cell adhesion system can be seen to act in cells lacking contact sites A. This mechanism depends on the gp150 surface protein that is encoded by tgrC1 (initially called lagC) (Geltosky et al., 1979; Dynes et al., 1994; Wang et al., 2000). It associates in a heterotypic manner to hold cells together and also acts as an intercellular signaling system to indicate the presence of close kin (Hirose et al., 2011). Mutants lacking TgrC1 are able to aggregate into loose mounds but fail to form tight mounds. Instead, the cells disperse from the mounds before trying once again to form a mound. Transcriptional progression through the developmental stages stalls and the cells fail to form either spores or stalk cells (Iranfar, Fuller and Loomis, 2006).
The receptor for TgrC1 is a closely related surface protein referred to as TgrB1 (Benabentos et al., 2011). Both tgrC1 and tgrB1 are highly polymorphic among wild populations of D. discoideum such that populations that have not shared a recent common ancestor are likely to have incompatible alleles. Only when TgrC and TgrB fit together can integrated mounds be constructed. When TgrB/C do not fit, the cells fail to form mixed mounds and go their separate ways (Hirose et al., 2011). Among other things, this kin recognition system protects each population from unrelated cells that do not make their fair share of stalk cells (Ho et al., 2013).
Divergence and Sorting of Cell Types
Some of the genes that are transcribed soon after TgrB/C heterodimers are formed between adjacent cells are cell type specific (Iranfar, Fuller and Loomis, 2006). Their products have been tagged with the green fluorescent protein GFP and followed from when they first appear in tight mounds to finding their final positions within either the prespore or the prestalk region of slugs (Figure 3). Individual cells could be followed by live cell imaging and shown to express either prespore or prestalk specific genes but not both. Moreover, cells that expressed prespore specific genes continued to express prespore specific genes and were never found to express prestalk specific genes; likewise, cells expressing prestalk specific genes did not express prespore specific genes unless the slug was forced to regulate the cell types.
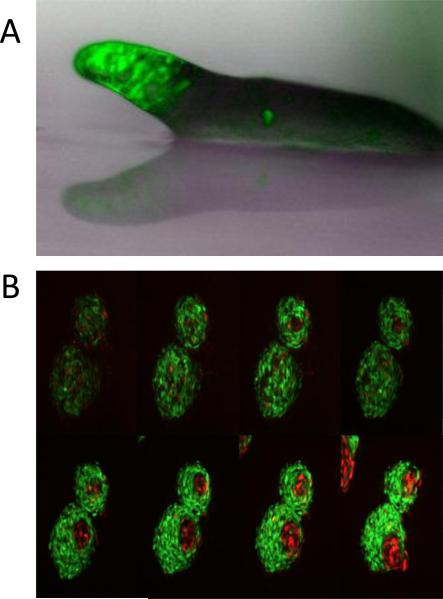
Figure 3.
Localization of prestalk cells. A. Cells expressing the prestalk marker ecmA::GFP (green) can be seen at the anterior of a migrating slug. Prespore cells in the back do not express this marker and are not fluorescent. B. Cells expressing the prestalk marker ecmA::RFP (red) and the prespore marker cotB::GFP (green) were developed on agar for 8 hours, dissociated and developed while constrained by a 5 micron ceiling. Images were taken at: 60; 101; 114; 120; 159; 190; 199; 229 minutes (photos by Albert Bae).
Cell type specific differentiation is often thought to occur either in a position dependent manner or in a spatially random manner followed by sorting out. In the first mechanism, positional information in the form of concentration differences in one or more morphogens is thought to distinguish the various regions and direct the appropriate differentiation (Wolpert 1969; 2011). In this manner distinct tissues can arise in a spatial pattern closely related to function. On the other hand, when cells differentiate in a salt and pepper pattern, they have to subsequently sort out by differential cell adhesion or chemotaxis. A clear example of spatially random differentiation followed by sorting out occurs at an early stage of mammalian embryogenesis. Cells of the inner cell mass respond heterogeneously to Fibroblast Growth Factor (FGF) such that some differentiate into primary endoderm and others into epiblast cells (Yamanaka et al., 2010). Initially these cells are interspersed but they subsequently sort out to form coherent tissues.
In Dictyostelium, expression of cell type specific genes has clearly shown that prespore and prestalk cells arise in a position-independent manner in mounds and then sort out to the anterior and posterior of slugs (Jermyn, Duffy and Williams, 1989; Fosnaugh and Loomis, 1993). Cells expressing prespore specific genes can be seen to appear slightly earlier in development than cells expressing prestalk specific genes. Both populations slowly increase until almost all cells express one or the other of the cell type markers. About twice as many cells express prespore specific genes as express prestalk specific genes. Unfortunately, it is still not known what biases a cell to differentiate one way or the other, nor how the proportions are subsequently adjusted.
One source of variability is the position of each cell in the cell cycle at the time when development is initiated. It has been shown that cells in early G2 phase will preferentially express prestalk genes while cells in late G2 will preferentially express prespore genes (Weijer, Duschl and David, 1984; Gomer and Firtel, 1987). This cell cycle connection is uncoupled in mutant strains lacking a small protein, RtoA, that affects control of cytosolic pH and calcium levels (Wood et al., 1996; Azhar et al., 2001). Nevertheless, rtoA- null mutants generate both prespore and prestalk cells and form fruiting bodies with spores and stalk cells in the proper proportions. Likewise, wild type cells collected in G1 were found to develop well without progressing further through the cell cycle (Chen and Kuspa, 2005). It appears that cells do not need to be distributed around the cell cycle for cell type divergence.
Cells that have been grown in liquid media containing glucose (G+) have much more stored glycogen than cells grown in the same medium without added sugar (G-) (Leach et al., 1973). When G+ and G- cells are mixed in equal numbers and allowed to develop together, the G+ cells preferentially become spores while G- cells preferentially become stalk cells. The G+ cells are not only better endowed with glycogen than G- cells, they are also somewhat larger. They may generate higher levels of ketoacids from glycogen metabolism under the conditions of nitrogen limitation. If the rate of differentiation is regulated by the levels of ketoacids, then prespore cells would be expected to differentiate first. Growth in the presence of glucose also reduces the levels of RasD-GTP which might bias them toward prespore specific differentiation (Chattwood et al., 2013). In any case, it seems to be selectively advantageous to recruit the largest, fattest cells to become prespore cells to help the next generation off to the best start.
If cells are allowed to swirl around for several hours while vertically constrained, the prestalk cells will spontaneously separate and swirl independently of prespore cells (Figure 3). More and more prestalk cells will form separate groups which may suddenly take off for the edge of the flattened pancake of cells and lead the other cells away (Nicol et al., 1999). Mounds that form on top of a moist support are not vertically constrained and develop a tip at their apeces. Prestalk cells appear at random and then move together to form a cluster. The cluster moves up towards the tip in a process that appears to use at least two distinct chemoattractants (Doolittle, Reddy, and McNally, 1995; Clow et al., 2000). The sorting out of prestalk cells to the tip of mounds has been computationally modelled and shown to require chemotaxis as well as differential adhesion (Jiang, Levine and Glazier, 1998). While some have assumed that chemotaxis to cAMP gradients directs prestalk cells to the tip, this is unlikely because a strain in which adenylyl cyclase, acaA, is disrupted and PKA is constitutively active is able to make nice slugs which give rise to both spores and stalk cells (Wang and Kuspa, 1997). These cells do not secrete measurable levels of cAMP and so cannot be using external cAMP as a chemotactic signal. Sorting out of prestalk cells in this strain may involve chemotaxis to some other compound.
Differential adhesion has also been considered as a mechanism that could lead to sorting out of the cell types (Lam et al., 1981; Kellerman and McNally, 1999). Disruption of the cadA gene had several consequences besides loss of gp24, including reduced sorting out of prespore and prestalk cells in the slugs (Wong et al., 2002). However, loss of the early adhesion mechanism also resulted in precocious appearance of the adhesion mechanism mediated by gp80 such that mutant cells were more adhesive rather than less adhesive than wild type cells. While these studies implicate cell-cell adhesion in sorting, they do not establish a specific mode since both gp24 and gp80 were affected by loss of cadA and other pleiotropic effects may be significant.
A mutant strain was isolated which had trouble transitioning from the mound to slug stage and formed small twisted fruiting bodies (Parkinson et al., 2009). The mutation was found to disrupt a gene encoding RapGAP which normally controls the activation of Rap1. Rap1GTP is involved in regulation of the cytoskeleton (Rebstein et al., 1997). Following addition of cAMP, Rap1GTP remains elevated longer in the RapGAP mutant than in wild type cells. While there are defects in sorting out of the prestalk sub-types PstO and PstA cells, prespore and prestalk cells sort out normally in the RapGAP mutant.
The TgrB/C adhesion system is clearly necessary for sorting out since null mutants are blocked at the loose aggregate stage and disperse shortly thereafter (Dynes et al., 1994). However, TgrB/C is also a signaling system essential for expression of the prespore and prestalk specific marker genes, so prespore and prestalk cells cannot be recognized in the loose aggregates. Mixing tgrC1- cells tagged with the prestalk marker ecmA: RFP or the prespore marker cotC::GFP together with excess wild type cells induces cell type specific gene expression in the mutant cells which can then be seen to sort out properly (Dynes et al., 1994). On the other hand, antibodies to TgrC1 are able to inhibit sorting of prestalk and prespore cells when they are dissociated from wild type slugs and allowed to reaggregate (Siu, Des Roches and Lam, 1983). These results could be interpreted to indicate that TgrC1 is playing an essential role in cell type sorting but could also be interpreted to indicate that coating the cell surfaces with antibodies is detrimental to sorting.
A majority of the cells in a mound express the prespore marker cotB:RFP before prestalk cells start to express ecmA:GFP. The number of prestalk cells continues to increase such that by the time that prestalk cells have formed a cluster near the top of each mound, they make up about 20% of all cells. This proportion is independent of the total number of cells in the aggregate and is maintained throughout the remainder of development (Raper, 1940; Bonner, 1952). Prestalk cells are found predominately at the anterior of migrating slugs and prespore cells are located in the posterior (Figure 4). If the proportions are experimentally modified by surgically isolating the anterior fifth of a slug or killing prespore cells with ricin, some prestalk cells transdifferentiate into prespore cells within 4 hours to reestablish the proportions of prespore to prestalk cells at 4 to 1 (Shaulsky and Loomis, 1993). A relatively simple model has been proposed to account for size invariant proportioning and regulation (Loomis, 1993; Soderbom and Loomis, 1998). In this model an extracellular regulator (R) is secreted by prespore cells but not by prestalk cells nor by cells which have not yet started to differentiate in a cell type specific manner. The regulator inhibits prespore differentiation in uncommitted cells and is broken down by all cells. The rate of accumulation of the regulator would then be the difference between the rate of synthesis (K1) times the number of prespore cells (Npsp) and the rate of degradation (K2) times the total number of cells (Nt) : dR/dt = K1 Npsp - K2 Nt. At steady state where the concentration of R is constant, K1 Npsp = K2 Nt. This predicts that the proportion of prespore cells Npsp/ Nt will be size invariant and determined by the ratio of K2 / K1. If the proportions are modified by removing almost all the prespore cells, the prestalk cells will regulate their proportions in the new smaller slug by generating new prespore cells.
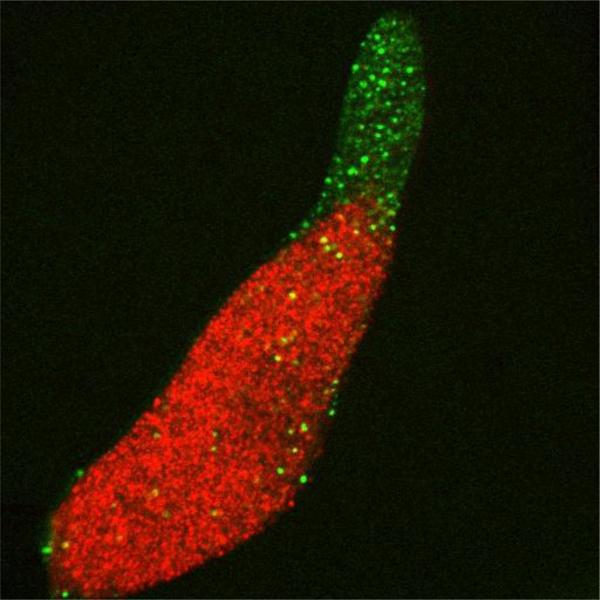
Figure 4.
Proportions of prestalk and prespore cells. Cells expressing nuclear localized GFP (green) and RFP (red) driven by the prestalk specific gene ecmA and the prespore specific gene cotB, respectively, were developed to the slug stage. Most green cells are in the anterior prestalk region but a few can be seen in the posterior; these are the anterior-like cells. Prespore cells make up 75 to 80% of the total number of cells (photo by Albert Bae).
The concentration of the regulator increases as the proportion of prespore cells increases until a threshold is reached where uncommitted cells are inhibited from differentiating into prespore cells and have to differentiate into prestalk cells. The competition to become prespore cells can be thought of as a race. The largest, fattest cells may have an advantage in this race. Unfortunately, the model cannot be directly tested until the identity of the regulator is known.
Mutations in a gene, tagA, are known to result in excess prestalk cells that fail to sort out properly (Good et al., 2003). They are also known to cause a slow down in transcription of developmental genes between 2 and 6 hours of development. While it has been proposed that TagA is required for the specification of the initial population of prespore cells, it seems equally likely that the developmental aberrations are the consequences of the temporary halt in the race. TagA is a 6 transmembrane ATP dependent transporter of the ABC B family with a protease domain fused to the N-terminus (Good et al., 2003). This protein might process proteins or be involved in secretion of intercellular signals (Cabral et al., 2006). After the lag, tagA- cells catch up such that the transcripional patterns in mutant and wild type cells are indistinguishable by 16 hours of development. However, by this time several of the cell type specific genes have been expressed promiscuously in the mutant cells and the slugs have ectopic patches of prestalk cells. Clearly, loss of tagA has multiple pleiotropic effects.
The slug as a multicellular organism
When mounds are fully formed, cellulose fibers and a set of cellulose binding proteins are secreted to form a sheath (Freeze and Loomis, 1977; Blanton et al., 2000; Ti et al., 1995). The sheath covers the whole mound and keeps out late comers. The major proteins of the extracellular matrix are encoded by ecmA, ecmB, ecmC, and ecmD that are first expressed at 12 hours of development and their mRNAs are found preferentially in prestalk cells (Table 2) (Parikh et al., 2010). These strongly related proteins form disulfide linked dimers and trimers among themselves and associate strongly with cellulose fibers to hold the whole mass of cells together as it converts into a slug. Mutant strains that cannot synthesize cellulose as the result of disruption of the gene encoding the catalytic subunit of cellulose synthetase still make slugs, but they are fragile and easily disrupted (Blanton et al., 2000). The slug fits all the definitions of a multicellular organism: it is visible to the naked eye and can be seen to migrate; the whole slug is surrounded by an extracellular matrix which delineates it from surrounding cells; specialized cell types are sequestered into distinct locales within the mass of cells and they can be considered somatic cells and germ cells since only spores can germinate to produce the next generation. Moreover, slugs are phototactic and migrate accurately towards light at the top of the soil (Raper, 1935; Poff and Loomis, 1973). They appear to function with purpose.
Table 2.
Slug Genes
| Gene | Product | Period of mRNA increase | Reference |
|---|---|---|---|
| ecmA | ST430 | 12 - 22 hr | Ceccarelli et al., 1987 |
| ecmB | ST310 | 12 - 24 hr | Ceccarelli et al., 1987 |
| ecmC | sheathin C | 12 - 20 hr | Ti et al., 1995 |
| ecmD | sheathin D | 12 - 16 hr | Ti et al., 1995 |
| dcsA | cellulose synthetase | 4 - 20 hr | Blanton et al., 2000 |
| mhcA | myosin heavy chain | 0 - 4 hr | Knecht and Loomis, 1987 |
| mlcR | myosin light chain | 0 - 4 hr | Chen et al., 1994 (X) |
| pkgB | protein kinase B | 0 - 8 hr | Meili et al., 2000 |
| limB | cytoskeletal protein | 0 - 8 hr | Chien et al., 2000 |
| tipD | atg16 related - ubiquitin-like conjugator | 0 - 8 hr | Stege et al., 1999 |
| atgl | autophagy protein kinase | 0 - 4 hr | Otto et al., 2004 |
| atg5 | ubiquitin-like conjugator | 0 - 8 hr | Otto et al., 2003 |
| atg6 | PI3K complex component | 0 - 8 hr | Otto et al., 2004 |
| atg7 | autophagy El-like | 0 - 8 hr | Otto et al., 2003 |
| atg8 | ubiquitin-like autophagy protein | 0 - 8 hr | Otto et al., 2003 |
| atg9 | transmembrane autophagy protein | 0 - 20 hr | Calvo-Garrido et al., 2010 |
| vmp1 | vacuolar membrane protein | 4 - 20 hr | Calvo-Garrido et al., 2010 |
| tipA | protein phosphatase-like | 0 - 4 hr | Stege et al., 1997 |
| tipB | unknown 41 kDa | 4 - 8 hr | Stege et al., 1999 |
| ubcB | ubiquitin-conjugating enzyme E2 | 0 - 4 hr | Clark et al., 1997 |
| mkkA | MEK kinase/ F box | 0 - 4 hr | Chung et al., 1998 |
| fbxA | F box/ WD40 | 4 - 16 hr | Nelson et al., 2000 |
| amdA | adenosine monophosphate deaminase | 0 - 16 hr | Chae et al., 2001 |
| chdC | DNA binding domain | 0 - 4 hr | Platt et al., 2013 |
A tip forms at the apex of mounds as the prestalk cells push up at the top. Cells lacking either myosin heavy chain or myosin light chain are weakened and do not form a tip (Knecht and Loomis, 1987; DeLozanne and Spudich, 1987; Chen et al., 1994). Likewise, cells with mutations in genes that affect F-actin polymerization, pkgB or limB, do not form a tip (Meili et al., 2000; Chien et al., 2000). The prestalk cells are left as a column of cells just under the apex in these strains and no slugs are formed. It appears that the actomyosin cortex has to be able to force the cluster of prestalk cells above the prespore cells.
Sorting of prestalk cells to a tip also requires the machinery of autophagy (Calvo-Garrido et al., 2010). Autophagy can mobilize degradation of portions of the cytoplasm to remove specific components or just metabolize it to generate subunits. About 16 dedicated genes are required to initiate and extend internal double membranes to make autophagic cups. Most cargo is trafficked to lysosomes but some is secreted from the cell. Mutations in 7 of these genes not only affect autophagy but also result in developmental arrest at the mound stage (Stege et al., 1999; Otto et al., 2003; 2004; Calvo-Garrido et al., 2010). It appears that coalescence of prestalk cells in a single tip requires the energy generated by autophagic degradation of cytoplasm or removal of specific proteins by selective autophagy.
There are several other genes that can give the multi-tipped phenotype when mutated (Table 2). The products of tipA and tipB appear to have partially overlapping functions; the phenotype of double mutants is not additive as it is for other combinations of genes affecting tip formation (Stege et al., 1997; 1999). However, their function is unknown.
Mutations in ubcB also result in developmental arrest before the formation of a dominant tip (Clark et al., 1997). The product of this gene is a clear homolog of ubiquitin-conjugating proteins that mark other proteins for degradation in the proteosome. There is evidence that one of the targets of UbcB is a putative MEK kinase component of a MAP kinase cascade involved in cell-type differentiation and sorting (Chung et al., 1998). Removing this protein kinase, MkkA, may be required for sorting out of prestalk cells to form a single tip.
Chromatin remodeling proteins physically change the interactions between DNA and nucleosomes and can affect access of transcriptional regulators to specific genes. Loss of one of these remodelers, ChdC, has pleiotropic effects on the transcriptional patterns of Dictyostelium during growth and development (Platt et al., 2013). Cells lacking ChdC grow more slowly than wild type cells but are able to aggregate normally and form mounds. However, further development is blocked and tips are not made. Loss of this remodeler in yeast, Drosophila, zebrafish, mice or humans also results in pleiotropic effects on growth and organ formation. It appears that cells which are sick at the start of development often arrest at the mound stage in Dictyostelium. It may be a difficult stage transition.
Once prestalk cells have sorted out and moved to the top of the mound, they extend upwards until they fall over and lie flat on the underlying support. In this horizontal position the tipped mound looks much like a small worm or slug as it moves toward light and heat. The slug is surrounded by an extracellular matrix that is extended at the front and left behind on the support as a collapsed tube (Raper, 1935; 1940; Shaffer, 1965). The sheath determines the direction of migration since it is only distensible at the front (Francis, 1964; Loomis, 1972).
While dissecting the regulatory region that controls transcription of the prestalk specific gene, ecmA, Jermyn et al. (1989) found that the proximal enhancer drove expression only in cells at the extreme tip which they named PstA cells. A more distal portion of the regulatory region drove expression in cells behind the PstA region that form a collar around the slug. They named these cells PstO cells. The relative proportions of these prestalk subtypes were found to be affected by mutations in several different genes. Mutations in the gene encoding adenosine monophosphate deaminaase, amdA, resulted in twice the number of PstA cells (Chae, Fuller and Loomis, 2001) while mutations in the F-box genes mkkA and fbxA affected the proportions of PstO cells (Chung et al., 1998; Nelson et al., 2000). These mutations act in a cell autonomous manner ruling out any role in production of an intercellular signal but consistent with affecting the response to such signals.
Phototaxis depends on focusing light across the tip of slugs such that cells on the side opposite the light are stimulated to move more rapidly than cells on the lit side. As the result of differential cell movement the slug turns towards the light. The action spectrum for phototaxis indicates that light may be absorbed by a heme-protein (Poff, Loomis and Butler, 1973; Poff and Butler, 1974). Genetic loci have been found which are essential for phototaxis but it has not been possible to isolated the affected genes because the strains were generated by chemical mutagenesis long before the techniques of plasmid insertion were discovered (Loomis, 1970). Some of these behavioral genes are likely to affect cellular movement while others are likely to affect properties of the sheath. Light may delay maturation of the sheath such that it can be stretched forward slightly more on the side away from the light where the light is focused. This would allow the slug to turn towards the light. Some of the mutations affecting phototactic directionality could be modifying components involved in sheath formation and maturation.
Culmination and terminal differentiation
The shape of slugs changes dramatically when they stop migrating and start to construct fruiting bodies. Prespore cells move under the tip until it is pointing up. Cellulose fibers are then deposited between the prestalk cells at the top to form a stalk tube. The tube is pulled down through the underlying cells until it hits the substratum. As the tube descends, it drags adjacent cells along such that a flat shelf forms around the anterior tip and the whole structure resembles a Mexican Hat. About an hour later, the stalk starts to rapidly elongate as new cellulose fibers are added at the top. Prestalk cells move into the stalk and increase several fold in volume by taking up water in a central vacuole (George, Hohl and Raper, 1972; Loomis, 1975). Once inside the tube, cells surround themselves with thick cellulosic walls that strengthen the stalk and allow it to hold the bulk of the cells on their way to the top where they will encapsulate into spores.
Early genetic studies of chemically mutagenized cells showed that several genes were required to initiate culmination and choreograph the relative movements of prespore and prestalk cells (Yanagisawa, Sussman and Loomis, 1967; Loomis, 1975). However, there was no way to isolate these genes or know what proteins they might encode. With the advent of efficient plasmid insertion mutagenesis (Kuspa and Loomis, 1992), a new set of mutations were isolated and their flanking regions sequenced (Table 3).
Table 3.
Culmination Genes
| Gene | Product | Period of mRNA increase | Reference |
|---|---|---|---|
| pkaR | PKA regulatory subunit | 0 - 8 hr | Mutzel et al., 1987 |
| regA | cAMP phosphodiesterase | 0 - 4 hr | Shaulsky et al., 1996 |
| rdeA | H2 phosphodiesterase | 0 - 8 hr | Chang et al., 1998 |
| yelA | eIF-4G related protein | 0 - 16 hr | Osherov et al., 1997 |
| aarA | aardvark (β-catenin) | 0 - 8 hr | Grimson et al., 2000 |
| cotA | spore coat protein SP96 | 4 - 16 hr | Fosnaugh et al., 1994 |
| cotB | spore coat protein SP70 | 4 - 16 hr | Fosnaugh et al., 1994 |
| cotC | spore coat protein SP60 | 4 - 16 hr | Fosnaugh et al., 1994 |
| PgtB | UDPgal transferase | 4 - 16 hr | West et al., 2009 |
| spiA | spore coat protein | 16 - 24 hr | Richardson and Loomis, 1992 |
| dgcA | diguanylate cyclase | 4 - 20 hr | Chen and Schaap, 2012 |
| acbA | SDF-2 precursor | 12 - 20 hr | Anjard and Loomis, 2005 |
| dhkA | receptor histidine kinase | 4 - 16 hr | Wang et al., 1996 |
| dhkB | receptor histidine kinase | 0 - 20 hr | Anjard and Loomis, 2008 |
| iptA | isopentenyltransferase | 20 - 24 hr | Anjard and Loomis, 2008 |
| stkA | stalky GATA factor | 4 - 20 hr | Chang et al., 1996 |
A series of rapid developing strains were isolated and their mutated genes shown to affect either the regulatory subunit of PKA, the internal cAMP phosphodiesterase, RegA, or the small H2 protein that is an essential component of the phosphotransfer system that activates RegA (Abe and Yanagisawa, 1983 ; Mutzel et al., 1987; Shaulsky, Escalante and Loomis, 1996; Chang et al. 1998). These strains were shown to initiate culmination under conditions where wild type cells were still waiting for an environmental signal. They also constitutively released intercellular signals that triggered terminal differentiation of spores and stalk cells (Anjard and Loomis, 2005; 2006; 2008; Anjard, Su and Loomis, 2009). Many of these signals also activated the cAMP/PKA pathway (Loomis, 2014). It seems that the responsibility for integrating prestalk and prespore terminal differentiation for optimal fruiting body formation is relegated to a large extent to PKA.
There is another gene that controls precocious spore formation, yelA (Osherov, Wang and Loomis, 1997). The product of this gene shows no significant homology to proteins of known function and does not seem to directly affect the cAMP/PKA pathway. Null mutations in yelA result in morphological arrest at the mound stage such that they never reach culmination. However, the surface of the mound gets rough and the cells encapsulate into unstable spores about 6 hours sooner than wild type cells. The bright yellow pigment that is usually only seen in sori of fruiting bodies accumulates in these mounds (Osherov, Wang and Loomis, 1997).
Mutant strains were also isolated that were unable to make fruiting bodies and only succeeded in making amorphous structures resembling snowmen that melted soon after being built. Sequencing of the affected gene, dcsA, indicated that is was likely to encode the catalytic subunit of cellulose synthetase (Blanton et al., 2000). Biochemical assays confirmed this assignment. Strains in which dcsA is disrupted are unable to make cellulose at any stage in development and lack both spores and stalks. The slugs are fragile and the culminants have little or no rigidity. Cellulose is commonly associated with plants but clearly also functions in development of Dictyostelium. It is also found in certain marine chordates such as tunicates which are enclosed in a tough tunic composed of proteins, complex polysaccharides and cellulose. Phylogenetic analyses of the gene for cellulose synthetase in plants, amoebozoa and animals showed that the ability to make cellulose was inherited from a common eukaryotic ancestor (Blanton et al., 2000). It appears to have been lost in most animals.
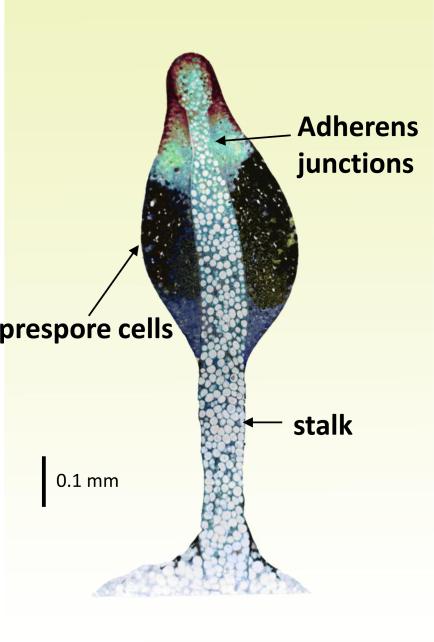
As the stalk rises, it is constricted near the top to make sure that it fits the size of the fruiting body (Figure 5). β-catenin generates adherens junctions between cells in a ring around the stalk such that they can squeeze the tube using aligned actin filaments (Grimson et al., 2000). Adherens junctions are common in vertebrates where they hold epithelial cells in sheets, but they have only been observed in Dictyostelium during culmination. Disruption of the gene encoding β-catenin, aarA, results in mechanically weak fruiting bodies which collapse onto the substratum, demonstrating the importance of tailoring the stalk to the overall size.
Figure 5.
Section through a culminant in which about half of the prestalk cells have entered the stalk tube and vacuolized (large white cells). Lower cup cells (blue) cradle the mass of prespore cells (dark blue). Adherens junctions were seen between prestalk cells that were next to the stalk tube just above the mass of prespore cells (Grimson et al., 2000).
As soon as the stalk starts to rise off the substratum, the prespore cells crawl up it. When the stalk is almost fully extended and the prespore cells are more than half way up, cells that are just beneath the prestalk cells near the top begin to encapsulate by fusing specialized Prespore Vesicles (PVs) with their surface membrane. Spore coat proteins and a galactose-rich polysacchardide synthesized by PgtB are stored in PVs during the slug stage and released during late culmination to generate the tough 0.1 Pm thick spore coat (Devine, Bergmann and Loomis, 1983; West et al., 2009). Mutants in which the genes encoding the 3 most prevalent spore coat proteins, SP60, SP70 and SP96, are disrupted form spores that are more porous than wild type spores as evidenced by entry of a labelled lectin (Fosnaugh, Fuller and Loomis, 1994). Likewise, mutations in the gene encoding UDPgal polysaccharide transferase, pgtB, reduce the integrity of spores (West et al., 2009).
Late in sporulation the cells express a spore specific gene, spiA, that encodes a protein added to the inner face of the spore coat (Richardson and Loomis, 1992). Mutation in spiA result in unstable spores that rapidly take up water and die rather than remaining dormant spores. It is clear that the spore coat not only determines the shape of spores but also their survival under adverse conditions.
Culmination is a highly cooperative venture in which the timing of terminal differentiation of both prespore and prestalk cells must be integrated so as to avoid premature encapsulation. One of the integrating signals is cyclic-di-GMP which is synthesized by prestalk cells (Chen and Schaap, 2012). Mutations that inactivate the enzyme that synthesizes cyclic-di-GMP, dgcA, result in arrest at the slug stage and failure to make either spores or stalk cells. Addition of μ1 μM cyclic-di- GMP restores the ability to form stalks, spores, and complete fruiting bodies.
Timing is also controlled by two peptide signals that are released during culmination (Anjard and Loomis, 2005; Anjard, Su and Loomis, 2011). One of these peptides, SDF-1, activates the late adenylyl cyclase ACG while the other, SDF-2, converts DhkA from a protein kinase to a protein phosphatase which inactivates the internal cAMP phosphodiesterase, RegA. As mentioned above, both of these signals activate the cAMP/PKA pathway to control timing. Mutations in the genes responsible for these pathways result in reduced efficiency of sporulation and stalk aberrations. Sporulation is also stimulated by a cytokinin that is synthesized very late in fruiting body construction (Anjard and Loomis, 2008). Strains lacking the enzyme isopentenyltransferase which synthesizes the precursor for the cytokinin discadenine are defective in sporulation (Table 3). Likewise, strains lacking the cytokinin receptor DhkB make few viable spores (Anjard and Loomis, 2008).
A member of the GATA family of DNA binding proteins mediates transcriptional responses essential for encapsulation of prespore cells (Chang et al., 1996). Cells carrying mutations in the gene encoding this zinc-finger protein, stkA, make stalky fruiting bodies in which all prespore cells enter the stalk tube and differentiate into mature stalk cells (Morrissey, Farnsworth and Loomis, 1981). The tall, thin stalks rise so high that they can lift the lid off a petri dish. These mutants also make it very clear that none of the prespore differentiations, such as accumulation of spore coat proteins or heteropolysaccharide in PVs, preclude the massive vacuolization that characterizes stalk cell terminal differentiation.
There are many unanswered morphological questions in culmination of Dictyostelium such as, what signals the tip to stop leading the slug and start culmination, what determines the shape of the tip as it moves up the stalk, what directs prestalk cells into the funnel at the top of the stalk, what tapers the stalk tube as it rises? Genetic control of these process may become apparent as more mutant strains are characterized in molecular detail.
Discussion
High throughput genetic techniques can potentially saturate all morphogenetic genes with mutations. However, high throughput often leads to overwhelming information that can result in less than complete attention to individual genes. Genes encoding components of previously characterized complexes or networks may be adequately annotated, but genes with novel or subtle roles may not be fully appreciated. Initial efforts to saturate developmental genes in eukaryotic organisms had to rely on chemical mutagenesis because molecular tagging was only possible after genomic sequencing. Mutated genes with morphological phenotypes could be mapped, but subsequent cloning and sequencing was always laborious and the collection of known morphological genes grew slowly. By the turn of the century, novel molecular techniques had been developed that permitted rapid recovery of genes of interest.
In Dictyostelium the approach consisted of selecting for cells that had randomly inserted a plasmid carrying a drug resistance gene. A critical part of the technique involved introducing a restriction enzyme at the same time as the plasmid to greatly increase the frequency of recovering transformants (Kuspa and Loomis, 1992). Insertion of the plasmid not only provided drug resistance but also disrupted the gene it happened to fall into. The random nature of these insertions tagged most genes dispensible for growth. This shot-gun approach gave unbiased entry into structural and biochemical processes that might otherwise have escaped attention. Working models were constructed that made predictions that could be tested by reverse genetics using homologous recombination. Validated models soon defined mechanisms that could account for matters of size and shape.
Variations on these techniques were used in other model systems to help establish a set of genetic networks involved one way or another in shaping tissues and organs across the animal kingdom. It is conceivable that there are morphogenetic modules that snap together like Lego pieces to generate a wide variety of structures in all sorts of different organisms, however, the number of possible complexes makes it impossible to predict how any given tissue or organ is fashioned. For now, most aspects of embryogenesis will have to be studied on an individual basis in each separate species.
Ultrasensitive incoherent feedforward loop
A common mechanism for regulating the activity of all sorts of proteins involves reversible phoshorylation. If the levels of kinase and phosphatase activity are close to each other and separately controlled, the regulation of the target protein may become ultrasensitive such that it all becomes active or all becomes inactive depending on slight changes in regulatory signals (Goldbeter and Koshland, 1981; 1984). When the kinase predominates, almost all the protein will be phosphorylated, but when the phosphatase predominates, very little of the protein will be phosphorylated. Such a sensitive balance can amplify the response dramatically. Likewise, when the GEF activity is greater than the GAP activity, almost all of the target GTPase protein will be in the active GTP form, while most will be in the inactive GDP form when GAP predominates. In this manner a wide range of small GTPases can act as ultrasensitive switches. Many different small GTPases are used to regulate different aspects of morphogenesis.
RasGTP plays a critical role in the chemotactic response of Dictyostelium to cAMP. The level of RasGTP is determined by the relative activities of a GEF and a GAP that are both activated following ligand binding to the cAMP receptor. This incoherent feedforward loop makes the cells exquisitely responsive to temporal changes in external cAMP while the ultrasensitivity amplifies the signal enormously. Responses such as these can be coupled to determination of size and shape of cells as well the structure of groups of cells.
Gradient sensing and concomitant accumulation of RasGTP at the front of a chemotaxing cell appears to result from the difference in the rate of diffusion of RasGEF and RasGAP. Being a membrane protein, RasGEF diffuses more slowly than RasGAP which is a cytosolic protein. As a result, GEF activity exceeds GAP activity at the front but not at the back, even when the external signal is only a few percent higher at the front than at the back. It is likely that such ultrasensitivity is used in many situations to give cells the ability to respond to subtle spatial differences. The only obvious drawback to ultrasensitive switches is that they may go off in error. Robustness can be built in downstream of an ultrasensitive switch by addition of feedback loops or cross talk.
Oscillatory Circuits
Regular oscillations add a temporal dimension that cells can use to tune their responses. Periodic responses on a time scale of seconds to minutes are often encountered in organisms ranging from bacteria to mammals (Winfree, 1975; 1977; Berg, 1990). Moreover, periodic phenomena are the delight of physicists because they can be subjected to precise mathematical treatment. The non-equilibrium dynamics of cAMP relay and response in Dictyostelium has held the interest of outstanding theoretical physicists for many years (Levine, 2014). Their input over the last 20 years has significantly raised the level of precision and sophistication of studies on aggregation and sorting out.
Computer assisted explorations of molecular circuits that could potentially account for the 6 minute periodicity of cAMP pulses led to specific proposals for functional connections between the surface receptors of cAMP and known internal activities (Laub and Loomis, 1998). The consequences of loss of one or more of these connections was measured and compared to the computer predictions as the model was refined (Maeda et al., 2004). A circuit with 6 regulated nodes was found to be sufficient for a broad range of behaviors. Similar circuits have been explored that might account for periodicity in bacterial chemotaxis, the cell cycle and brain activity (Mello and Tu, 2007; Nurse, Masui and Hartwell, 1998; Jensen et al., 2006; Bray, 2014a, b; Jadi and Sejnowski, 2014).
One of the clearest cases for periodic patterns in vertebrates can be seen in the rhythmic formation of somites during embryogenesis. These condensations of mesenchymal tissue go on to generate the spine, ribcage and associated muscles, nerves, blood vessels, cartilage and skin. Pairs of somites are formed along the anterior-posterior axis starting from the front. Rhythmic condensation is governed by a segmentation clock which is characterized by a period ranging from 30 minutes to a few hours depending on the organism (Goldbeter and Pourquie, 2008). In zebrafish, chicks, and mice feedback loops in presomitic cells lead to oscillations in the Wnt, Notch and FGF pathways (Krol et al., 2011). Together these pathways regulate transcription of a large number of genes. The abundance of mRNA from 24 zebrafish genes, 56 mouse genes, and 182 chick genes was found to oscillate in a cyclic manner in the presomitic tissues suggesting that they were controlled by the segmentation clock. Surprisingly only two genes, Hes1 and Hes5, encoding transcriptional represssors were found to be oscillatory in presomitic tissues of all three species. While somitic morphogenesis appears to be very similar among these organisms, the actual genes products involved in timing, sizing and specifying new somites appear to be quite different. Perhaps there is no unique circuit that produces the periodic events of vertebrate segmentation (Krol et al., 2011).
Just as segments are delineated by an extracellular matrix as they condense from the presomitic tissue, aggregates of Dictyostelium are surrounded by an extracellular matrix that keeps out latecomers and ensures that prespore and prestalk cells stay together (Raper, 1940; Freeze and Loomis, 1977 a, b). Unlike the basement membranes of animal species, the Dictyostelium sheath does not include fibronectin, collagen or other protein fibers recognized by integrins, but does contain a set of disulfide cross-linked cellulose binding proteins and cellulose fibers (Wang et al., 2001; Blanton et al., 2000). When an aggregate falls over and starts to migrate as a slug, the sheath is left behind as a collapsed tube. New sheath is continuously formed at the anterior tip where it can be stretched to allow the slug to turn and advance (Loomis, 1972). Individual cells gain traction on surrounding cells and move forward when space opens up ahead of them. The force is transmitted to the substratum through the sheath. As a consequence, only the peripheral cells that are in contact with the sheath power slug movement.
Most metazoan cells stick to components of extra cellular matrices by interaction of transmembrane proteins named integrins (Julich et al., 2009). These highly conserved proteins act as heterodimers which have different affinities to matrix proteins such as fibronectin, laminin, collagen, or vitronectin. Dictyostelium does not have the genes for these matrix proteins or for integrins and relies mostly on van der Waals forces to gain traction on its surroundings (Loomis et al., 2012; Tarantola et al., 2014). These innate non-specific interactions may be sufficient for movement of the cells within the sheath. Whether or not such interactions also function in metazoan tissues has yet to be determined.
The tip as the organizer
The results from grafting experiments led Raper (1940) to compare the tip of a slug with the classical “organizer” region described in amphibian embryos by Spemann and Mangold (1924). When Raper isolated tips from migrating slugs and grafted them onto the sides of other slugs, he found that the tips implanted and subsequently guided host cells away to form a separate slug. These and similar experiments by Rubin and Robertson (1975) showed that only tips had the ability to organize surrounding cells and that nearby tips would compete. These attributes are reminisent of those of the organizer found in the dorsal lip of the blastopore in amphibian embryos. However, it is known that the classical organizer acts by releasing signaling proteins that counteract the effects of protein signals released by ventral cells (Smith and Harland, 1992; De Robertis, 2006) and there is no evidence that such a complex interplay of signals occurs in the establishment of polarity in Dictyostelium slugs. It seems more likely that the distensible sheath at the anterior of the grafted tip allows cells half way down the slug to push out and form an independent slug.
Almost as soon as cells have formed aggregates the levels of adenylyl cyclase and cAMP phosphodiesterase start to decrease and by the time that slugs are formed only cells at the most anterior tip have retained these activities (Hall et al., 1993; Verkerke-van Wijk et al., 2001). While nanomolar pulses of cAMP might still be relayed among tip cells, they would not be relayed between prespore or PST O cells which have very low levels of ACA and PdsA. Moreover, cells lacking aggregation stage adenylyl cyclase as the result of deletion of acaA but constitutively expressing PKA are still able to form migrating slugs that culminate to form fairly normal fruiting bodies (Wang and Kuspa, 1997). Any cAMP synthesized in these mutant cells must be made by the late adenylyl cyclases ACR or ACG that are not activated by cAMP binding to CAR1. These enzymes may produce cAMP at a low steady rate and some of it might be released into the interstitial spaces from where it could bind to the late cAMP surface receptors CAR 2 and CAR 4. However, these enzymes cannot generate propagating waves of cAMP that were once thought to direct and coordinate cell movement throughout the slugs in the latter stages of development (Maree, Panfilov and Hogeweg, 1999; Dormann and Weijer, 2001; 2006).
Molecular markers of differentiation
The ability to visualize the presence of specific mRNAs by in situ hydbridization of cells in intact aggregates and slugs has allowed several different cell types to be distinguished by their patterns of gene expression (Escalante and Loomis, 1995; Maeda et al., 2003; Maruo et al., 2004). About 100 genes are found almost exclusively in the posterior three quarters of migrating slugs. These prespore genes are present in about the same abundance throughout the posterior region, indicating that prespore cells are essentially homogeneous. Another 100 genes are absent in prespore cells but are present in prestalk cells. Some are much more strongly expressed in PstA cells at the tip of slugs than in PstO cells just behind them, while others show the reverse pattern, being stronger in PstO cells than in PstA cells. There are also genes that are initially expressed in one of the prestalk subtypes and then expressed in another. This dynamic variation in transcriptional patterns indicates that there are multiple prestalk subtypes. However, it is not clear whether or not these subtypes play different roles in morphogenesis nor how important the variations in the temporal pattern might be for fruiting body formation. Perhaps it is not critical for certain genes to be expressed simultaneously in a given subtype of cell as long as the genes are sufficiently expressed.
Gene expression in the prestalk subtypes is unlikely to be regulated by positional information because the cells in the tip rapidly rotate around the long axis of each slug as it migrates. They stay at the front but go around every 5 minutes or so. Concentration differences in diffusible morphogens would not be able to indicate distance from other cell types because they would be quickly homogenized by the relative movement of the cells. Some other mechanism must regulate specific prestalk gene expression such as signaling through direct contact of the cell types.
In situ hybridization also showed that some genes are expressed early in development in all cells but are expressed only in prespore cells or in prestalk cells after 12 hours of development. Specalization of function can result from turning off a critical gene in a given cell type as well as from turning on an important gene. Preferential retention of gene expression can result in a specialized cell type fairly quickly.
The extracellular matrix in fruiting body formation
Many of the gymnastic moves of culmination that result in the elegantly tapering stalk holding up a ball of spores can be accounted for by changes in the properties of the extracellular matrix. The slime sheath restricts the direction of movement during migration because it can only be stretched at the front where it is newly made (Loomis, 1972). As a slug migrates, it moves over the sheath which is stationary on the substratum and left behind as a collapsed tube. Just behind the tip, the sheath matures into a strong, non-deformable covering which keeps the cells in line. If the sheath over the tip also becomes a non-deformable covering, then cells at the tip can no longer move forward and must reverse their direction and penetrate the cells behind them to keep moving. Shrinkage of prespore cells as they prepare for encapsulation may reduce the effective pressure in the back allowing the prestalk cells to push down through them like a reverse fountain. Prestalk cells construct a cellulosic stalk tube as they descend and start to build heavy cell walls. When the stalk tube hits bottom, further expansion of prestalk cells will result in upward elongation of the stalk. The stalk will push on the rigid sheath and lift the whole mass off the substratum until all the prestalk cells have entered the stalk tube, expanded and become enclosed in wooden coffins.
Previously it had been found that culmination could be simulated in a mathematical model driven by differential adhesion of the different cell types to each other and to the extracellular matrix (Maree and Hogeweg, 2001). Chemotactic responses to propagating waves of cAMP were considered to drive cellular movements. However, as mentioned above, the activatable adenylyl cyclase, the extracellular cAMP phosphodiesterase and the cAMP receptor CAR1 are removed from prespore cells as well as PstO cells and retained only in PstA cells (Hall et al., 1993; Verkerke-van Wijk et al., 2001). Relay of cAMP waves is dependent on these activities and so would be restricted to the PstA cells at the tip and cannot be directly involved in culmination. While the cell types are likely to have differences in their adhesive properties, such differences are unlikely to account for all the relative cell movements in the formation of a fruiting body. It is more likely that cells move whenever they can. As a result tip cells move into the stalk tube where they expand and extend the stalk. Prespore cells climb the growing stalk until they reach the top and encapsultate. The fruiting body is finished.
Summary
Insertional mutagenesis, homologous recombination and strong selective regimes to isolate suppressor mutations have collected genes responsible for multicellularity, cell sorting, coordinated movement and terminal differentiation. Well-characterized genes involved in Dictyostelium aggregation, slug formation or culmination are presented in Tables 1, 2, and 3 and discussed in the context of their morphogenetic pathways and processes. Their roles were compared to those of homologs in mammalian cells where there was often surprising similarity. For instance, oscillations in cAMP and PKA activity in both aggregating Dictyostelium and human heart cells was found to depend on the inhibition of cAMP phosphodiesterase by the protein kinase ERK that can be overcome by PKA in a negative feedback loop. Likewise, the major cell types arise at random in both the Dictyostelium mound and the embryonic inner cell mass, followed in both cases by sorting out. Directed movement in both Dictyostelium cells and human neutrophils results from extension of pseudopods in the direction of the chemoattractant. The signal is recognized by surface receptors that are members of the GPCR family and transduced via PI3K and and AKT to the activation of the Arp2/3 complex which initiates polymerization of actin to form dendritic networks in pseudopods in both systems.
While some of the morphogenetic mechanisms that function during Dictyostelium development are likely to be species specific, others will be found to be conserved or only slightly modified in metazoa. Clearly, it is more efficient to work out the basic mechanisms in a model system such as Dictyostelium and then confirm them in mammalian embryogenesis rather than the other way around. It is likely that most of the genes that are dispensible for growth but necessary for development of Dictyostelium have already been encountered in the saturation screens that have recently been carried out. Those with major roles have been highlighted while those with more subtle or redundant roles are still under study. The prospects for rapidly filling in the major gaps in knowledge have never been better.
Acknowledgements
I would like to thank Margarita Behrens and Adam Kuspa for constructive comments. I apologize to those whose work was not covered in depth due to constraints on the length of this review which are already strained.
References
- Abe K, Yanagisawa K. A new class of rapidly developing mutants in Dictyostelium discoideum: Implications for cyclic AMP metabolism and cell differentiation. Dev. Biol. 1983;95:200–210. doi: 10.1016/0012-1606(83)90018-0. [DOI] [PubMed] [Google Scholar]
- Anjard C, Loomis WF. Peptide signaling during terminal differentiation of Dictyostelium. Proc. Natl. Acad. Sci. USA. 2005;102:7607–7611. doi: 10.1073/pnas.0501820102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjard C, Loomis WF. GABA induces terminal differentiation of Dictyostelium through a GABA(B) receptor. Development. 2006;133:2253–2261. doi: 10.1242/dev.02399. [DOI] [PubMed] [Google Scholar]
- Anjard C, Loomis WF. Cytokinins induce sporulation in Dictyostelium. Development. 2008;135:819–827. doi: 10.1242/dev.018051. [DOI] [PubMed] [Google Scholar]
- Anjard C, Su Y, Loomis WF. Steroids initiate a signaling cascade that triggers rapid sporulation in Dictyostelium. Development. 2009;136:803–812. doi: 10.1242/dev.032607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjard C, Su Y, Loomis WF. The polyketide MPBD initiates the SDF-1 signaling cascade that coordinates terminal differentiation in Dictyostelium. Eukaryot Cell. 2011;10:956–63. doi: 10.1128/EC.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artemenko Y, Lampert TJ, Devreotes PN. Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell Mol Life Sci. 2014;71:3711–47. doi: 10.1007/s00018-014-1638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar M, Kennady PK, Pande G, Espiritu M, Holloman W, Brazill D, Gomer RH, Nanjundiah V. Cell cycle phase, cellular Ca2+ and development in Dictyostelium discoideum. Int. J. Dev. Biol. 2001;45:405–414. [PubMed] [Google Scholar]
- Bagorda A, Parent CA. Eukaryotic chemotaxis at a glance. J Cell Sci. 2008;121:2621–2624. doi: 10.1242/jcs.018077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie G, MacKenzie SJ, Houslay MD. Phorbol 12-myristate 13-acetate triggers the protein kinase A-mediated phosphorylation and activation of the PDE4D5 cAMP phosphodiesterase in human aortic smooth muscle cells through a route involving extracellular signal regulated kinase (ERK). Mol Pharmacol. 2001;60:1100–11. doi: 10.1124/mol.60.5.1100. [DOI] [PubMed] [Google Scholar]
- Balestrini JL, Niklason LE. Extracellular Matrix as a Driver for Lung Regeneration. Ann Biomed Eng. 2014 doi: 10.1007/s10439-014-1167-5. (DOI: 10.1007/s10439-014-1167-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A, Muller-Taubenberger A, Taranto P, Gerisch G. Replacement of the phospholipid-anchor in the contact site A glycoprotein of D. discoideum by a transmembrane region does not impede cell adhesion but reduces residence time on the cell surface. J. Cell Biol. 1994;124:205–215. doi: 10.1083/jcb.124.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabentos R, Hirose S, Sucgang R, Curk T, Katoh M, Ostrowski EA, Strassmann JE, Queller DC, Zupan B, Shaulsky G, Kuspa A. Polymorphic members of the lag gene family mediate kin discrimination in Dictyostelium. Curr Biol. 2009;19:567–572. doi: 10.1016/j.cub.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg HC. Bacterial microprocessing. Cold Spring Harbor Symp. Quant. Biol. 1990;55:539–545. doi: 10.1101/sqb.1990.055.01.052. [DOI] [PubMed] [Google Scholar]
- Blanton RL, Fuller D, Iranfar N, Grimson MJ, Loomis WF. The cellulose synthase gene of Dictyostelium. Proc. Natl. Acad. Sci. USA. 2000;97:2391–2396. doi: 10.1073/pnas.040565697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JT. The pattern of differentiation in amoeboid slime molds. Am. Naturalist. 1952;86:79–89. [Google Scholar]
- Bonner JT. The cellular slime molds. Princeton Univ. Press; Princeton, NJ.: 1959. [Google Scholar]
- Bonner JT. Induction of stalk cell differentiation by cyclic AMP in the cellular slime mold Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA. 1970;65:110–113. doi: 10.1073/pnas.65.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgraaf L, Russcher H, Smith JL, Wessels D, Soll DR, van Haastert PJM. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 2002;21:4560–4570. doi: 10.1093/emboj/cdf438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Limits of computational biology. Silico Biol. 2014 doi: 10.3233/ISB-140461. 10.3233/ISB-140461 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Intrinsic activity in cells and the brain. Mol Biol Cell. 2014;25:737–8. doi: 10.1091/mbc.E13-12-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D, Toda T, Michaeli T, Levin L, Birchmeier C, Zoller M, Powers S, Wigler M. The S. cerevisiae CDC25 gene product regulates the RAS/adenylate cyclase pathway. Cell. 1987;48:789–99. doi: 10.1016/0092-8674(87)90076-6. [DOI] [PubMed] [Google Scholar]
- Buczynski G, Grove B, Nomura A, Kleve M, Bush J, Firtel RA, Cardelli J. Inactivation of two Dictyostelium discoideum genes, DdPIK1 and DdPIK2, encoding proteins related to mammalian phosphatidylinositide 3-kinases, results in defects in endocytosis, lysosome to postlysosome transport, and actin cytoskeleton organization. J. Cell Biol. 1997;136:1271–1286. doi: 10.1083/jcb.136.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral M, Anjard C, Loomis WF, Kuspa A. Genetic evidence that the acyl coenzyme a binding protein acba and the serine protease/abc transporter tagA function together in Dictyostelium discoideum cell differentiation. Euk. Cell. 2006;5:2024–2032. doi: 10.1128/EC.00287-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Katoh-Kurasawa M, Muramoto T, Santhanam B, Long Y, Li L, Ueda M, Iglesias P, Shaulsky G, Devreotes P. Nucleocytoplasmic shuttling of a GATA transcription factor functions as a development timer. Science. 2014;343:1249531. doi: 10.1126/science.1249531. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Garrido J, Carilla-Latorre S, Kubohara Y, Santos-Rodrigo N, Mesquita A, Soldati T, Golstein P, Escalante R. Autophagy in Dictyostelium: genes and pathways, cell death and infection. Autophagy. 2010;6:686–701. doi: 10.4161/auto.6.6.12513. [DOI] [PubMed] [Google Scholar]
- Chae SC, Fuller D, Loomis WF. Altered cell-type proportioning in Dictyostelium lacking adenosine monophosphate deaminase. Dev. Biol. 2001;241:183–194. doi: 10.1006/dbio.2001.0491. [DOI] [PubMed] [Google Scholar]
- Chang WT, Newell PC, Gross JD. Identification of the cell fate gene stalky in Dictyostelium. Cell. 1996;87:471–481. doi: 10.1016/s0092-8674(00)81367-7. [DOI] [PubMed] [Google Scholar]
- Chang WT, Thomason PA, Gross JD, Newell PC. Evidence that the RdeA protein is a component of a multistep phosphorelay modulating rate of development in Dictyostelium. EMBO J. 1998;17:2809–2816. doi: 10.1093/emboj/17.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattwood A, Nagayama K, Bolourani P, Harkin L, Kamjoo M, Weeks G, Thompson CR. Developmental lineage priming in Dictyostelium by heterogeneous Ras activation. Elife. 2013;2:e01067. doi: 10.7554/eLife.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kuspa A. Prespore cell fate bias in G1 phase of the cell cycle in Dictyostelium discoideum. Eukaryot Cell. 2005;4:1755–64. doi: 10.1128/EC.4.10.1755-1764.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MY, Long Y, Devreotes PN. A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Devel. 1997;11:3218–3231. doi: 10.1101/gad.11.23.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PX, Ostrow BD, Tafuri SR, Chisholm RL. Targeted disruption of the Dictyostelium RMLC gene produces cells defective in cytokinesis and development. J. Cell Biol. 1994;127:1933–1944. doi: 10.1083/jcb.127.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Schaap P. The prokaryote messenger c-di-GMP triggers stalk cell differentiation in Dictyostelium. Nature. 2012;488:680–683. doi: 10.1038/nature11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S, Chung CY, Sukumaran S, Osborne N, Lee S, Ellsworth C, McNally JG, Firtel RA. The Dictyostelium LIM domain-containing protein LIM2 is essential for proper chemotaxis and morphogenesis. Mol. Biol. Cell. 2000;11:1275–1291. doi: 10.1091/mbc.11.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm RL, Firtel RA. Insights into morphogenesis from a simple developmental system. Nature Rev. Mol. Cell Biol. 2004;5:531–541. doi: 10.1038/nrm1427. [DOI] [PubMed] [Google Scholar]
- Chung CY, Reddy TBK, Zhou KM, Firtel RA. A novel, putative MEK kinase controls developmental timing and spatial patterning in Dictyostelium and is regulated by ubiquitin-mediated protein degradation. Genes Devel. 1998;12:3564–3578. doi: 10.1101/gad.12.22.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Nomura A, Mohanty S, Firtel RA. A ubiquitin-conjugating enzyme is essential for developmental transitions in Dictyostelium. Mol. Biol. Cell. 1997;8:1989–2002. doi: 10.1091/mbc.8.10.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clow PA, Chen TLL, Chisholm RL, McNally JG. Three-dimensional in vivo analysis of Dictyostelium mounds reveals directional sorting of prestalk cells and defines a role for the myosin II regulatory light chain in prestalk cell sorting and tip protrusion. Development. 2000;127:2715–2728. doi: 10.1242/dev.127.12.2715. [DOI] [PubMed] [Google Scholar]
- De Lozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Spemann's organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine KM, Bergmann JE, Loomis WF. Spore coat proteins of Dictyostelium discoideum are packaged in prespore vesicles. Dev. Biol. 1983;99:437–446. doi: 10.1016/0012-1606(83)90293-2. [DOI] [PubMed] [Google Scholar]
- Doolittle KW, Reddy I, McNally JG. 3D analysis of cell movement during normal and myosin-II-null cell morphogenesis in Dictyostelium. Dev. Biol. 1995;167:118–129. doi: 10.1006/dbio.1995.1011. [DOI] [PubMed] [Google Scholar]
- Dormann D, Weijer CJ. Propagating chemoattractant waves coordinate periodic cell movement in Dictyostelium slugs. Development. 2001;128:4535–4543. doi: 10.1242/dev.128.22.4535. [DOI] [PubMed] [Google Scholar]
- Dormann D, Weijer CJ. Chemotactic cell movement during Dictyostelium development and gastrulation. Curr Opin Genet Dev. 2006;16:367–73. doi: 10.1016/j.gde.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Dormann D, Weijer G, Parent CA, Devreotes PN, Weijer CJ. Visualizing PI3 kinase-mediated cell-cell signaling during Dictyostelium development. Curr. Biol. 2002;12:1178–1188. doi: 10.1016/s0960-9822(02)00950-8. [DOI] [PubMed] [Google Scholar]
- Dynes JL, Clark AM, Shaulsky G, Kuspa A, Loomis WF, Firtel RA. LagC is required for cell-cell interactions that are essential for cell-type differentiation in Dictyostelium. Genes Devel. 1994;8:948–958. doi: 10.1101/gad.8.8.948. [DOI] [PubMed] [Google Scholar]
- Eichinger L, Pachebat JA, Glockner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, Tunggal B, Kummerfeld S, Madera M, Konfortov BA, Rivero F, Bankier AT, Lehmann R, Hamlin N, Davies R, Gaudet P, Fey P, Pilcher K, Chen G, Saunders D, Sodergren E, Davis P, Kerhornou A, Nie X, Hall N, Anjard C, Hemphill L, Bason N, Farbrother P, Desany B, Just E, Morio T, Rost R, Churcher C, Cooper J, Haydock S, vanDriessche N, Cronin A, Goodhead I, Muzny D, Mourier T, Pain A, Lu M, Harper D, Lindsay R. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante R, Loomis WF. Whole-mount in situ hybridization of cell-type-specific mRNAs in Dictyostelium. Dev. Biol. 1995;171:262–266. doi: 10.1006/dbio.1995.1278. [DOI] [PubMed] [Google Scholar]
- Firtel RA, Chapman AL. Role for cAMP-dependent protein kinase-A in early Dictyostelium development. Genes Devel. 1990;4:18–28. doi: 10.1101/gad.4.1.18. [DOI] [PubMed] [Google Scholar]
- Fleig SV, Humphreys BD. Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin Pract. 2014;127:75–80. doi: 10.1159/000363680. [DOI] [PubMed] [Google Scholar]
- Fosnaugh K, Fuller D, Loomis WF. Structural roles of the spore coat proteins in Dictyostelium discoideum. Dev. Biol. 1994;166:823–825. doi: 10.1006/dbio.1994.1362. [DOI] [PubMed] [Google Scholar]
- Fosnaugh KL, Loomis WF. Enhancer regions responsible for temporal and cell-type-specific expression of a spore coat gene in Dictyostelium. Dev. Biol. 1993;157:38–48. doi: 10.1006/dbio.1993.1110. [DOI] [PubMed] [Google Scholar]
- Francis DW. Some studies on phototaxis of Dictyostelium. J. Cell. Compar. Physiol. 1964;64:131–138. doi: 10.1002/jcp.1030640113. [DOI] [PubMed] [Google Scholar]
- Freeze H, Loomis W. Isolation and characterization of a component of the surface sheath of Dictyostelium discoideum. J. Biol. Chem. 1977;252:820–824. [PubMed] [Google Scholar]
- Freeze H, Loomis WF. The role of the fibrillar component of the surface sheath in the morphogenesis of Dictyostelium discoideum. Dev. Biol. 1977;56:184–194. doi: 10.1016/0012-1606(77)90161-0. [DOI] [PubMed] [Google Scholar]
- Fuller D, Chen W, Adler M, Groisman A, Levine H, Rappel WJ, Loomis WF. External and internal constraints on eukaryotic chemotaxis. Proc Natl Acad Sci U S A. 2010;107:9656–9. doi: 10.1073/pnas.0911178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmendia-Torres C, Goldbeter A, Jacquet M. Nucleocytoplasmic oscillations of the yeast transcription factor Msn2: evidence for periodic PKA activation. Curr Biol. 2007;17:1044–9. doi: 10.1016/j.cub.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Garrod DR, Ashworth JM. Effect of growth conditions on development of the cellular slime mould, Dictyostelium discoideum. J. Embryol. Exp. Morphol. 1972;28:463–479. [PubMed] [Google Scholar]
- Geltosky JE, Weseman J, Bakke A, Lerner RA. Identification of a cell surface glycoprotein involved in cell aggregation in D. discoideum. Cell. 1979;18:391–398. doi: 10.1016/0092-8674(79)90058-8. [DOI] [PubMed] [Google Scholar]
- George RP, Hohl HR, Raper KB. Ultrastructural development of stalk-producing cells in Dictyostelium discoideum, a cellular slime mould. J. Gen. Microbiol. 1972;70:477–489. doi: 10.1099/00221287-70-3-477. [DOI] [PubMed] [Google Scholar]
- Gerisch G. Cell aggregation and differentiation in Dictyostelium. In: Moscona AA, Monroy A, editors. Current Topics in Developmental Biology. Ac. Press; New York: 1968. pp. 157–197. [DOI] [PubMed] [Google Scholar]
- Gilbert S. Developmental Biology. Sinauer Associates; Sunderland, MA.: 2013. [Google Scholar]
- Goldbeter A, Koshland DE., Jr. An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci U S A. 1981;78:6840–4. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A, Koshland DE., Jr. Ultrasensitivity in biochemical systems controlled by covalent modification. Interplay between zero-order and multistep effects. J Biol Chem. 1984;259:14441–7. [PubMed] [Google Scholar]
- Goldbeter A, Pourquie O. Modeling the segmentation clock as a network of coupled oscillations in the Notch, Wnt and FGF signaling pathways. J Theor Biol. 2008;252:574–85. doi: 10.1016/j.jtbi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gomer RH, Firtel RA. Cell-autonomous determination of cell-type choice in Dictyostelium development by cell-cycle phase. Science. 1987;237:758–762. doi: 10.1126/science.3039657. [DOI] [PubMed] [Google Scholar]
- Good JR, Cabral M, Sharma S, Yang J, Van Driessche N, Shaw CA, Shaulsky G, Kuspa A. TagA, a putative serine protease/ABC transporter of Dictyostelium that is required for cell fate determination at the onset of development. Development. 2003;130:2953–2965. doi: 10.1242/dev.00523. [DOI] [PubMed] [Google Scholar]
- Grimson MJ, Coates JC, Reynolds JP, Shipman M, Blanton RL, Harwood AJ. Adherens junctions and beta-catenin-mediated cell signalling in a non-metazoan organism. Nature. 2000;408:727–731. doi: 10.1038/35047099. [DOI] [PubMed] [Google Scholar]
- Hall AL, Franke J, Faure M, Kessin RH. The role of the cyclic nucleotide phosphodiesterase of Dictyostelium discoideum during growth, aggregation, and morphogenesis: overexpression and localization studies with the separate promoters of the pde. Dev. Biol. 1993;157:73–84. doi: 10.1006/dbio.1993.1113. [DOI] [PubMed] [Google Scholar]
- Harwood AJ, Hopper NA, Simon MN, Driscoll DM, Veron M, Williams JG. Culmination in Dictyostelium is regulated by the cAMP-dependent protein kinase. Cell. 1992;69:615–624. doi: 10.1016/0092-8674(92)90225-2. [DOI] [PubMed] [Google Scholar]
- Hirose S, Benabentos R, Ho HI, Kuspa A, Shaulsky G. Self-recognition in social amoebae is mediated by allelic pairs of tiger genes. Science. 2011;333:467–70. doi: 10.1126/science.1203903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HI, Hirose S, Kuspa A, Shaulsky G. Kin recognition protects cooperators against cheaters. Curr Biol. 2013;23:1590–5. doi: 10.1016/j.cub.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller O, Kay RR. Chemotaxis in the absence of PIP3 gradients. Curr. Biol. 2007;17:813–817. doi: 10.1016/j.cub.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Humphreys BD. Kidney structures differentiated from stem cells. Nat Cell Biol. 2014;16:19–21. doi: 10.1038/ncb2904. [DOI] [PubMed] [Google Scholar]
- Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Insall R, Kuspa A, Lilly PJ, Shaulsky G, Levin LR, Loomis WF, Devreotes P. CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol. 1994;126:1537–1545. doi: 10.1083/jcb.126.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranfar N, Fuller D, Loomis WF. Genome-wide expression analyses of gene regulation during early development of Dictyostelium discoideum. Euk. Cell. 2003;2:664–670. doi: 10.1128/EC.2.4.664-670.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranfar N, Fuller D, Loomis WF. Transcriptional regulation of post-aggregation genes in Dictyostelium by a feed-forward loop involving GBF and LagC. Dev. Biol. 2006;290:460–469. doi: 10.1016/j.ydbio.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Jacquet M, Renault G, Lallet S, De Mey J, Goldbeter A. Oscillatory nucleocytoplasmic shuttling of the general stress response transcriptional activators Msn2 and Msn4 in Saccharomyces cerevisiae. J Cell Biol. 2003;161:497–505. doi: 10.1083/jcb.200303030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadi MP, Sejnowski TJ. Cortical oscillations arise from contextual interactions that regulate sparse coding. Proc Natl Acad Sci U S A. 2014;111:6780–5. doi: 10.1073/pnas.1405300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Jensen TS, de Lichtenberg U, Brunak S, Bork P. Co-evolution of transcriptional and post-translational cell-cycle regulation. Nature. 2006;443:594–7. doi: 10.1038/nature05186. [DOI] [PubMed] [Google Scholar]
- Jeon TJ, Lee DJ, Lee S, Weeks G, Firtel RA. Regulation of Rap1 activity by RapGap1 controls cell adhesion at the front of chemotaxing cells. J. Cell Biol. 2007;179:833–843. doi: 10.1083/jcb.200705068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermyn KA, Duffy KT, Williams JG. A new anatomy of the prestalk zone in Dictyostelium. Nature. 1989;340:144–146. doi: 10.1038/340144a0. [DOI] [PubMed] [Google Scholar]
- Jermyn KA, Williams JG. An analysis of culmination in Dictyostelium using prestalk and stalk-specific cell autonomous markers. Development. 1991;111:779–787. doi: 10.1242/dev.111.3.779. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Levine H, Glazier J. Possible cooperation of differential adhesion and chemotaxis in mound formation of Dictyostelium. Biophys. J. 1998;75:2615–2625. doi: 10.1016/S0006-3495(98)77707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Zhang N, Long Y, Parent CA, Devreotes PN. Localization of the G protein betagamma complex in living cells during chemotaxis. Science. 2000;287:1034–1036. doi: 10.1126/science.287.5455.1034. [DOI] [PubMed] [Google Scholar]
- Julich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–21. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- Keller T, Thompson CR. Cell type specificity of a diffusible inducer is determined by a GATA family transcription factor. Development. 2008;135:1635–1645. doi: 10.1242/dev.020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman KA, McNally JG. Mound-cell movement and morphogenesis in Dictyostelium. Dev. Biol. 1999;208:416–429. doi: 10.1006/dbio.1999.9208. [DOI] [PubMed] [Google Scholar]
- Klein PS, Sun TJ, Saxe CL, III, Kimmel AR, Johnson RL, Devreotes PN. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988;241:1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Knecht DA, Fuller DL, Loomis WF. Surface glycoprotein, gp24, involved in early adhesion of Dictyostelium discoideum. Dev. Biol. 1987;121:277–283. doi: 10.1016/0012-1606(87)90160-6. [DOI] [PubMed] [Google Scholar]
- Knecht DA, Loomis WF. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987;236:1081–1085. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Kortholt A, Keizer-Gunnink I, Kataria R, Van Haastert PJ. Ras activation and symmetry breaking during Dictyostelium chemotaxis. J Cell Sci. 2013;126:4502–13. doi: 10.1242/jcs.132340. [DOI] [PubMed] [Google Scholar]
- Krol AJ, Roellig D, Dequeant ML, Tassy O, Glynn E, Hattem G, Mushegian A, Oates AC, Pourquie O. Evolutionary plasticity of segmentation clock networks. Development. 2011;138:2783–92. doi: 10.1242/dev.063834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuspa A, Loomis WF. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc. Natl. Acad. Sci. USA. 1992;89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TY, Pickering G, Geltosky J, Siu CH. Differential cell cohesiveness expressed by prespore and prestalk cells of Dictyostelium discoideum. Differentiation. 1981;20:22–28. doi: 10.1111/j.1432-0436.1981.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Laub MT, Loomis WF. A molecular network that produces spontaneous oscillations in excitable cells of Dictyostelium. Mol. Biol. Cell. 1998;9:3521–3532. doi: 10.1091/mbc.9.12.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach CK, Ashworth JM, Garrod DR. Cell sorting out during the differentiation of mixtures of metabolically distinct populations of Dictyostelium discoideum. J. Embryol. Exp. Morphol. 1973;29:647–661. [PubMed] [Google Scholar]
- Levine H. Learning physics of living systems from Dictyostelium. Phys Biol. 2014;11:053011. doi: 10.1088/1478-3975/11/5/053011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CJ, Zawadzki KA, Khosla M, Secko DM, Spiegelman GB, Weeks G. Loss of the Dictyostelium RasC protein alters vegetative cell size, motility and endocytosis. Exp. Cell Res. 2005;306:47–55. doi: 10.1016/j.yexcr.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lin Z, Sriskanthadevan S, Huang H, Siu CH, Yang D. Solution structures of the adhesion molecule DdCAD-1 reveal new insights into Ca(2+)-dependent cell-cell adhesion. Nature Struct. Mol. Biol. 2006;13:1016–1022. doi: 10.1038/nsmb1162. [DOI] [PubMed] [Google Scholar]
- Loomis WF. Mutants in phototaxis of Dictyostelium discoideum. Nature. 1970;227:745–746. doi: 10.1038/227745a0. [DOI] [PubMed] [Google Scholar]
- Loomis WF. Role of the surface sheath in the control of morphogenesis in Dictyostelium discoideum. Nature New Biol. 1972;240:6–9. doi: 10.1038/newbio240006a0. [DOI] [PubMed] [Google Scholar]
- Loomis WF. Dictyostelium discoideum. A developmental system. Ac. Press; New York: 1975. [Google Scholar]
- Loomis WF. The number of developmental genes in Dictyostelium. Birth Defects: Original Article Series. 1978;14(2):497–505. [PubMed] [Google Scholar]
- Loomis WF. The development of Dictyostelium discoideum. Ac. Press; New York: 1982. [Google Scholar]
- Loomis WF. Cell-cell adhesion in Dictyostelium discoideum. Dev. Genet. 1988;9:549–559. doi: 10.1002/dvg.1020090431. [DOI] [PubMed] [Google Scholar]
- Loomis WF. Lateral inhibition and pattern formation in Dictyostelium. Curr. Topics Dev. Biol. 1993;28:1–46. doi: 10.1016/s0070-2153(08)60208-2. [DOI] [PubMed] [Google Scholar]
- Loomis WF. Role of PKA in the timing of developmental events in Dictyostelium cells. Microbiol. Mol. Biol. Rev. 1998;62:684. doi: 10.1128/mmbr.62.3.684-694.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WF. Cell signaling during development of Dictyostelium. Dev Biol. 2014;391:1–16. doi: 10.1016/j.ydbio.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WF, Fuller D, Gutierrez E, Groisman A, Rappel WJ. Innate non-specific cell substratum adhesion. PLoS One. 2012;7:e42033. doi: 10.1371/journal.pone.0042033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WF, White S, R., D. A sequence of dependent stages in the development of Dictyostelium discoideum. Devel. Biol. 1976;53:171–177. doi: 10.1016/0012-1606(76)90221-9. [DOI] [PubMed] [Google Scholar]
- Maeda M, Lu SJ, Shaulsky G, Miyazaki Y, Kuwayama H, Tanaka Y, Kuspa A, Loomis WF. Periodic signaling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA. Science. 2004;304:875–878. doi: 10.1126/science.1094647. [DOI] [PubMed] [Google Scholar]
- Maeda M, Sakamoto H, Iranfar N, Fuller D, Maruo T, Ogihara S, Morio T, Urushihara H, Tanaka Y, Loomis WF. Changing patterns of gene expression in Dictyostelium prestalk cell subtypes recognized by in situ hybridization with genes from microarray analyses. Euk. Cell. 2003;2:627–637. doi: 10.1128/EC.2.3.627-637.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majkut S, Idema T, Swift J, Krieger C, Liu A, Discher DE. Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr Biol. 2013;23:2434–9. doi: 10.1016/j.cub.2013.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann SKO, Firtel RA. Cyclic AMP regulation of early gene expression in Dictyostelium discoideum: Mediation via the cell surface cyclic AMP receptor. Mol. Cell. Biol. 1987;7:458–469. doi: 10.1128/mcb.7.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maree AF, Hogeweg P. How amoeboids self-organize into a fruiting body: multicellular coordination in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 2001;98:3879–83. doi: 10.1073/pnas.061535198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maree AFM, Panfilov AV, Hogeweg P. Phototaxis during the slug stage of Dictyostelium discoideum: a model study. Proc. R. Soc. Lond. B. 1999;266:1351–1360. [Google Scholar]
- Meili R, Ellsworth C, Firtel RA. A novel Akt/PKB-related kinase is essential for morphogenesis in Dictyostelium. Curr. Biol. 2000;10:708–717. doi: 10.1016/s0960-9822(00)00536-4. [DOI] [PubMed] [Google Scholar]
- Mello BA, Tu Y. Effects of adaptation in maintaining high sensitivity over a wide range of backgrounds for Escherichia coli chemotaxis. Biophys J. 2007;92:2329–37. doi: 10.1529/biophysj.106.097808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda E, Nam E, Kuspa A, Shaulsky G. The ABC transporter, AbcB3, mediates cAMP export in D. discoideum development. Devel. Biol. 2015;397:203–211. doi: 10.1016/j.ydbio.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- Morris HR, Taylor GW, Masento MS, Jermyn KA, Kay RR. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature. 1987;328:811–814. doi: 10.1038/328811a0. [DOI] [PubMed] [Google Scholar]
- Morrissey JH, Farnsworth PA, Loomis WF. Pattern formation in Dictyostelium discoideum: an analysis of mutants altered in cell proportioning. Dev. Biol. 1981;83:1–8. doi: 10.1016/s0012-1606(81)80002-4. [DOI] [PubMed] [Google Scholar]
- Mutzel R, Lacombe ML, Simon MN, de Gunzburg J, Veron M. Cloning and cDNA sequence of the regulatory subunit of cAMP-dependent protein kinase from Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA. 1987;84:6–10. doi: 10.1073/pnas.84.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Ishihara S, Imoto D, Sawai S. Rectified directional sensing in long-range cell migration. Nat Commun. 2014;5:5367. doi: 10.1038/ncomms6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Sakabe M, Matsui H, Sakata H, Yanagawa N, Yamagishi T. Heart development before beating. Anat Sci Int. 2009;84:67–76. doi: 10.1007/s12565-009-0025-2. [DOI] [PubMed] [Google Scholar]
- Nelson MK, Clark A, Abe T, Nomura A, Yadava N, Funair CJ, Jermyn KA, Mohanty S, Firtel RA, Williams JG. An F-Box/WD40 repeat-containing protein important for Dictyostelium cell-type proportioning, slug behaviour, and culmination. Dev. Biol. 2000;224:42–59. doi: 10.1006/dbio.2000.9793. [DOI] [PubMed] [Google Scholar]
- Nicol A, Rappel WJ, Levine H, Loomis WF. Cell-sorting in aggregates of Dictyostelium discoideum. J. Cell Sci. 1999;112:3923–3929. doi: 10.1242/jcs.112.22.3923. [DOI] [PubMed] [Google Scholar]
- Noegel A, Gerisch G, Stadler J, Westphal M. Complete sequence and transcript regulation of a cell adhesion protein from aggregating Dictyostelium cells. EMBO J. 1986;5:1473–1476. doi: 10.1002/j.1460-2075.1986.tb04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P, Masui Y, Hartwell L. Understanding the cell cycle. Nat Med. 1998;4:1103–6. doi: 10.1038/2594. [DOI] [PubMed] [Google Scholar]
- Ochiai H, Schwartz H, Merkl R, Wagle G, Gerisch G. Stage-specific antigens reacting with monoclonal antibodies against contact site A, a cell-surface glycoprotein of Dictyostelium discoideum. Cell Differ. 1982;11:1–13. doi: 10.1016/0045-6039(82)90011-2. [DOI] [PubMed] [Google Scholar]
- Osherov N, Wang N, Loomis WF. Precocious sporulation and developmental lethality in yelA null mutants of Dictyostelium. Dev. Genet. 1997;20:307–319. doi: 10.1002/(SICI)1520-6408(1997)20:4<307::AID-DVG2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Otto GP, Wu MY, Kazgan N, Anderson OR, Kessin RH. Macroautophagy is required for multicellular development of the social amoeba Dictyostelium discoideum. J. Biol. Chem. 2003;278:17636–17645. doi: 10.1074/jbc.M212467200. [DOI] [PubMed] [Google Scholar]
- Otto GP, Wu MY, Kazgan N, Anderson OR, Kessin RH. Dictyostelium macroautophagy mutants vary in the severity of their developmental defects. J. Biol. Chem. 2004;279:15621–15629. doi: 10.1074/jbc.M311139200. [DOI] [PubMed] [Google Scholar]
- Oyama M, Kubota K, Okamoto K. Role of a guanine nucleotide binding protein, Galpha 2, in regulation of adenylate cyclase in Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 1991;175:55–60. doi: 10.1016/s0006-291x(05)81199-4. [DOI] [PubMed] [Google Scholar]
- Parent CA, Devreotes PN. A cell's sense of direction. Science. 1999;284:765–770. doi: 10.1126/science.284.5415.765. [DOI] [PubMed] [Google Scholar]
- Parikh A, Miranda ER, Katoh-Kurasawa M, Fuller D, Rot G, Zagar L, Curk T, Sucgang R, Chen R, Zupan B, Loomis WF, Kuspa A, Shaulsky G. Conserved developmental transcriptomes in evolutionarily divergent species. Genome Biol. 2010;11:R35. doi: 10.1186/gb-2010-11-3-r35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson K, Bolourani P, Traynor D, Aldren NL, Kay RR, Weeks G, Thompson CR. Regulation of Rap1 activity is required for differential adhesion, cell-type patterning and morphogenesis in Dictyostelium. J Cell Sci. 2009;122:335–344. doi: 10.1242/jcs.036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt GS, Milona N, Borleis J, Lin KC, Reed RR, Devreotes PN. Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell. 1992;69:305–315. doi: 10.1016/0092-8674(92)90411-5. [DOI] [PubMed] [Google Scholar]
- Platt JL, Rogers BJ, Rogers KC, Harwood AJ, Kimmel AR. Different CHD chromatin remodelers are required for expression of distinct gene sets and specific stages during development of Dictyostelium discoideum. Development. 2013;140:4926–36. doi: 10.1242/dev.099879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poff KL, Butler WL. Spectral characteristics of the photoreceptor pigment of phototaxis in Dictyostelium discoideum. Photochem. Photobiol. 1974;20:241–244. doi: 10.1111/j.1751-1097.1974.tb06573.x. [DOI] [PubMed] [Google Scholar]
- Poff KL, Butler WL, Loomis WF. Light-induced absorbance changes associated with phototaxis in Dictyostelium. Proc. Natl. Acad. Sci. USA. 1973;70:813–816. doi: 10.1073/pnas.70.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper KB. Dictyostelium discoideum, a new species of slime mold from decaying forest leaves. J. Agr. Res. 1935;50:135–147. [Google Scholar]
- Raper KB. Pseudoplasmodium formation and organization in Dictyostelium discoideum. J. Elisha Mitchell Sci. Soc. 1940;56:241–282. [Google Scholar]
- Rebstein PJ, Cardelli J, Weeks G, Spiegelman GB. Mutational analysis of the role of Rap1 in regulating cytoskeletal function in Dictyostelium. Exp. Cell Res. 1997;231:276–283. doi: 10.1006/excr.1996.3466. [DOI] [PubMed] [Google Scholar]
- Richardson DL, Loomis WF. Disruption of the sporulation-specific gene spiA in Dictyostelium discoideum leads to spore instability. Genes Devel. 1992;6:1058–1070. doi: 10.1101/gad.6.6.1058. [DOI] [PubMed] [Google Scholar]
- Richardson DL, Loomis WF, Kimmel AR. Progression of an inductive signal activates sporulation in Dictyostelium discoideum. Development. 1994;120:2891–2900. doi: 10.1242/dev.120.10.2891. [DOI] [PubMed] [Google Scholar]
- Rubin J, Robertson A. The tip of the Dictyostelium discoideum pseudoplasmodium as an organizer. J. Embryol. Exp. Morphol. 1975;33:227–241. [PubMed] [Google Scholar]
- Saito T, Kato A, Kay RR. DIF-1 induces the basal disc of the Dictyostelium fruiting body. Dev Biol. 2008;317:444–453. doi: 10.1016/j.ydbio.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JA, Loomis WF. Phosphorylation of the contact site A glycoprotein (gp80) of Dictyostelium discoideum. Dev. Biol. 1982;91:296–304. doi: 10.1016/0012-1606(82)90036-7. [DOI] [PubMed] [Google Scholar]
- Segall JE, Kuspa A, Shaulsky G, Ecke M, Maeda M, Gaskins C, Firtel RA. A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol. 1995;128:405–413. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G, Weiner O, Herzmark P, Balla T, Sedat J, Bourne H. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer BM. Cell movement within aggregates of the slime mould Dictyostelium discoideum revealed by surface markers. J. Embryol. Exp. Morphol. 1965;13:97–117. [PubMed] [Google Scholar]
- Shaulsky G, Escalante R, Loomis WF. Developmental signal transduction pathways uncovered by genetic suppressors. Proc. Natl. Acad. Sci. USA. 1996;93:15260–15265. doi: 10.1073/pnas.93.26.15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G, Loomis WF. Cell type regulation in response to expression of ricin-A in Dictyostelium. Dev. Biol. 1993;160:85–98. doi: 10.1006/dbio.1993.1288. [DOI] [PubMed] [Google Scholar]
- Shaulsky G, Loomis WF. Mitochondrial DNA replication but no nuclear DNA replication during development of Dictyostelium. Proc. Natl. Acad. Sci. USA. 1995;92:5660–5663. doi: 10.1073/pnas.92.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert F, Weijer CJ. Spiral and concentric waves organize multicellular Dictyostelium mounds. Curr. Biol. 1995;5:937–943. doi: 10.1016/s0960-9822(95)00184-9. [DOI] [PubMed] [Google Scholar]
- Siu C-H, Cho A, Choi HC. The contact site A glycoprotein mediates cell-cell adhesion by homophilic binding in Dictyostelium discoideum. J. Cell Biol. 1987;105:2523–2533. doi: 10.1083/jcb.105.6.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu CH, Des Roches B, Lam TY. Involvement of a cell-surface glycoprotein in the cell-sorting process of Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA. 1983;80:6596–6600. doi: 10.1073/pnas.80.21.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoge M, Yue H, Erickstad M, Bae A, Levine H, Groisman A, Loomis WF, Rappel W-J. Cellular memory in eukaryotic chemotaxis. Proc. Natl. Acad. Sci. USA. 2014;111:14448–14453. doi: 10.1073/pnas.1412197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–40. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Soderbom F, Loomis WF. Cell-cell signaling during Dictyostelium development. Trends Microbiol. 1998;6:402–406. doi: 10.1016/s0966-842x(98)01348-1. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. Archiv für Mikroskopische Anatomie und Entwicklungsmechanik. 1924;100:599–638. [Google Scholar]
- Stege JT, Laub MT, Loomis WF. tip genes act in parallel pathways of early Dictyostelium development. Dev. Genet. 1999;25:64–77. doi: 10.1002/(SICI)1520-6408(1999)25:1<64::AID-DVG7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Stege JT, Shaulsky G, Loomis WF. Sorting of the initial cell types in Dictyostelium is dependent on the tipA gene. Dev. Biol. 1997;185:34–41. doi: 10.1006/dbio.1997.8538. [DOI] [PubMed] [Google Scholar]
- Sucgang R, Kuo A, Tian X, Salerno W, Parikh A, Feasley CL, Dalin E, Tu H, Huang E, Barry K, Lindquist E, Shapiro H, Bruce D, Schmutz J, Salamov A, Fey P, Gaudet P, Anjard C, Babu MM, Basu S, Bushmanova Y, van der Wel H, Katoh-Kurasawa M, Dinh C, Coutinho PM, Saito T, Elias M, Schaap P, Kay RR, Henrissat B, Eichinger L, Rivero F, Putnam NH, West CM, Loomis WF, Chisholm RL, Shaulsky G, Strassmann JE, Queller DC, Kuspa A, Grigoriev IV. Comparative genomics of the social amoebae Dictyostelium discoideum and Dictyostelium purpureum. Genome Biol. 2011;12:R20. doi: 10.1186/gb-2011-12-2-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucgang R, Weijer CJ, Siegert F, Franke J, Kessin RH. Null mutations of the Dictyostelium cyclic nucleotide phosphodiesterase gene block chemotactic cell movement in developing aggregates. Dev. Biol. 1997;192:181–192. doi: 10.1006/dbio.1997.8720. [DOI] [PubMed] [Google Scholar]
- Swaney KF, Huang CH, Devreotes PN. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu Rev Biophys. 2010;39:265–89. doi: 10.1146/annurev.biophys.093008.131228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Shao D, Adler M, Charest P, Loomis WF, Levine H, Groisman A, Rappel W-J, Firtel RA. Incoherent feedforward control governs adaptation of activated Ras in a eukaryotic chemotaxis pathway. Science Signaling. 2012;5:ra2. doi: 10.1126/scisignal.2002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Iijima M, Kamimura Y, Chen L, Long Y, Devreotes P. Disruption of PKB signaling restores polarity to cells lacking tumor suppressor PTEN. Mol Biol Cell. 2011;22:437–47. doi: 10.1091/mbc.E10-06-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantola M, Bae A, Fuller D, Bodenschatz E, Rappel WJ, Loomis WF. Cell substratum adhesion during early development of Dictyostelium discoideum. PLoS One. 2014;9:e106574. doi: 10.1371/journal.pone.0106574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery R, Robbins S, Khosla M, Spiegelman GB, Weeks G. The effects of expression of an activated rasG mutation on the differentiation of Dictyostelium. Biochem. Cell Biol. 1992;70:1193–1199. doi: 10.1139/o92-165. [DOI] [PubMed] [Google Scholar]
- Ti ZC, Wilkins MR, Vardy PH, Gooley AA, Williams KL. Glycoprotein complexes interacting with cellulose in the “cell print” zones of the Dictyostelium discoideum extracellular matrix. Dev. Biol. 1995;168:332–341. doi: 10.1006/dbio.1995.1084. [DOI] [PubMed] [Google Scholar]
- Tomchik KJ, Devreotes PN. Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: A demonstration by isotope dilution-fluorography technique. Science. 1981;212:443–446. doi: 10.1126/science.6259734. [DOI] [PubMed] [Google Scholar]
- Verkerke-van Wijk I, Fukuzawa M, Devreotes PN, Schaap P. Adenylyl cyclase A expression is tip-specific in Dictyostelium slugs and directs StatA nuclear translocation and CudA gene expression. Dev. Biol. 2001;234:151–160. doi: 10.1006/dbio.2001.0232. [DOI] [PubMed] [Google Scholar]
- Wang B, Kuspa A. Dictyostelium development in the absence of cAMP. Science. 1997;277:251–254. doi: 10.1126/science.277.5323.251. [DOI] [PubMed] [Google Scholar]
- Wang J, Hou LS, Awrey D, Loomis WF, Firtel RA, Siu CH. The membrane glycoprotein gp150 is encoded by the lagC gene and mediates cell-cell adhesion by heterophilic binding during Dictyostelium development. Dev. Biol. 2000;227:734–745. doi: 10.1006/dbio.2000.9881. [DOI] [PubMed] [Google Scholar]
- Wang J, Hou LS, Awrey D, Loomis WF, Firtel RA, Siu CH. The membrane glycoprotein gp150 is encoded by the lagC gene and mediates cell-cell adhesion by heterophilic binding during Dictyostelium development. Dev. Biol. 2000;227:734–745. doi: 10.1006/dbio.2000.9881. [DOI] [PubMed] [Google Scholar]
- Wang N, Shaulsky G, Escalante R, Loomis WF. A two-component histidine kinase gene that functions in Dictyostelium development. EMBO J. 1996;15:3890–3898. [PMC free article] [PubMed] [Google Scholar]
- Wang N, Soderbom F, Anjard C, Shaulsky G, Loomis WF. SDF-2 induction of terminal differentiation in Dictyostelium discoideum is mediated by the membrane-spanning sensor kinase DhkA. Mol. Cell. Biol. 1999;19:4750–4756. doi: 10.1128/mcb.19.7.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, Slade MB, Gooley AA, Atwell BJ, Williams KL. Cellulose-binding modules from extracellular matrix proteins of Dictyostelium discoideum stalk and sheath. Eur. J. Biochem. 2001;268:4334–4345. doi: 10.1046/j.1432-1327.2001.02354.x. [DOI] [PubMed] [Google Scholar]
- Weijer CJ. Dictyostellium morphogenesis. Curr. Opin. Genet. Devel. 2004;14:392–398. doi: 10.1016/j.gde.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Weijer CJ, Duschl G, David CN. Dependence of cell-type proportioning and sorting on cell cycle phase in Dictyostelium discoideum. J. Cell Sci. 1984;70:133–145. doi: 10.1242/jcs.70.1.133. [DOI] [PubMed] [Google Scholar]
- West CM, Nguyen P, van der Wel H, Metcalf T, Sweeney KR, Blader IJ, Erdos GW. Dependence of stress resistance on a spore coat heteropolysaccharide in Dictyostelium. Euk Cell. 2009;8:27–36. doi: 10.1128/EC.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfree A. Unclocklike behaviour of biological clocks. Nature. 1975;253:315–319. doi: 10.1038/253315a0. [DOI] [PubMed] [Google Scholar]
- Winfree A. Phase control of neural pacemakers. Science. 1977;197:761–763. doi: 10.1126/science.887919. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and patterning revisited. J Theor Biol. 2011;269:359–65. doi: 10.1016/j.jtbi.2010.10.034. [DOI] [PubMed] [Google Scholar]
- Wong E, Yang CZ, Wang J, Fuller D, Loomis WF, Siu CH. Disruption of the gene encoding the cell adhesion molecule DdCAD-1 leads to aberrant cell sorting and cell-type proportioning during Dictyostelium development. Development. 2002;129:3839–3850. doi: 10.1242/dev.129.16.3839. [DOI] [PubMed] [Google Scholar]
- Wong EFS, Brar SK, Sesaki H, Yang CZ, Siu CH. Molecular cloning and characterization of DdCAD-1, a Ca2+-dependent cell-cell adhesion molecule, in Dictyostelium discoideum. J. Biol. Chem. 1996;271:16399–16408. doi: 10.1074/jbc.271.27.16399. [DOI] [PubMed] [Google Scholar]
- Wood SA, Ammann RR, Brock DA, Li L, Spann T, Gomer RH. RtoA links initial cell type choice to the cell cycle in Dictyostelium. Development. 1996;122:3677–3685. doi: 10.1242/dev.122.11.3677. [DOI] [PubMed] [Google Scholar]
- Xu XXS, Kuspa A, Fuller D, Loomis WF, Knecht DA. Cell-cell adhesion prevents mutant cells lacking myosin II from penetrating aggregation streams of Dictyostelium. Dev. Biol. 1996;175:218–226. doi: 10.1006/dbio.1996.0109. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Lanner F, Rossant J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development. 2010;137:715–24. doi: 10.1242/dev.043471. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Loomis WF, Sussman M. Developmental regulation of the enzyme UDP-galactose polysaccharide transferase. Exp. Cell Res. 1967;46:328–334. doi: 10.1016/0014-4827(67)90070-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Charest PG, Firtel RA. Spatiotemporal regulation of Ras activity provides directional sensing. Curr Biol. 2008;18:1587–1593. doi: 10.1016/j.cub.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bouffanais R, Yue DK. Persistent cellular motion control and trapping using mechanotactic signaling. PLoS One. 2014;9:e105406. doi: 10.1371/journal.pone.0105406. [DOI] [PMC free article] [PubMed] [Google Scholar]