Abstract
Purpose
To assess the three-dimensional repeatability of thickness measurements for epithelium, stroma, cornea, flap, and residual stromal bed using the Artemis very high-frequency (VHF) digital ultrasound arc-scanner (ArcScan Inc).
Methods
Five consecutive measurements were obtained for 10 eyes of 10 patients 1 year after LASIK using the Artemis VHF digital ultrasound arc-scanner across the central 10-mm diameter of the cornea. Repeatability analysis was performed for thickness measurements for each corneal layer—epithelium, stroma, cornea, flap, and residual stromal bed. The standard deviation of repeated measurements (point-repeatability) was calculated for each measurement location in 0.1-mm steps for the 10 × 10-mm matrix. The pooled standard deviation of the point-repeatability for each measurement location within the central 1-, 2-, and 3-mm radius was calculated (region-repeatability). The corneal thickness of the baseline scan set was compared to that of subsequent scan sets within the same session and plotted over time to assess any possible hydration effects of the immersion technique.
Results
The repeatability at the corneal vertex was 0.58 μm for epithelium, 1.78 μm for stroma, 1.68 μm for cornea, 1.68 μm for flap, and 2.27 μm for residual stromal bed. The region-repeatability within the central 1-mm radius was 1.01 μm for epithelium, 3.44 μm for stroma, 3.35 μm for cornea, 2.81 μm for flap, and 3.97 μm for residual stromal bed. The mean difference in corneal thickness from the baseline value was within 1.25 μm for each of the subsequent four scan sets over a 5-minute immersion period.
Conclusions
Layered pachymetry of the epithelium, stroma, cornea, flap, and residual stromal bed showed high repeatability with the Artemis VHF digital ultrasound arc-scanner. The high repeatability validates the use of the Artemis for in vivo layered pachymetry.
Very high-frequency (VHF) digital ultrasound has been shown by our group to provide sufficient resolution to determine the intra-corneal layers, including the epithelium, stroma, and the LASIK flap interface, with the first confirmed measurement of the corneal epithelium in vivo using a prototype rectilinear VHF digital ultrasound scanning system in 1993.1 We also reported the first high-precision VHF three-dimensional epithelial thickness mapping system.2,3 This system, acquiring a series of parallel, rectilinear B-scans, was capable of mapping the thickness of individual corneal layers within the central 3- to 4-mm diameter. By using digital signal processing techniques (the I-scan), a 2.0-μm reproducibility for epithelial thickness measurements was obtained.2,3 We further improved epithelial thickness measurement precision to 1.3 μm by increasing the fidelity of the digitized signal.4 This VHF digital ultrasound system has been used to characterize central corneal epithelial lenticular anatomy and to demonstrate that the power of the epithelium is not constant from eye to eye.5 We also examined the shape of Bowman's layer,6 the measurement of anterior corneal scars for planning therapeutic keratectomy,7-10 the quantitative analysis of corneal scarring (haze) after photorefractive keratectomy (PRK),8 the measurement of the depth of radial keratotomy incisions,11 the measurement of the depth of keratectomy,12 and epithelial and stromal changes after lamellar corneal surgery.4
Very high-frequency digital ultrasound technology has gradually improved both in precision and in area of acquisition. The other major improvement, in 1997, was introducing an arc-scan motion so that the transducer was kept near perpendicular to the corneal surface.13 This system increased the corneal acquisition area to a 10-mm zone. The repeatability of 10 consecutive examinations for 1 eye has been shown to be less than 1.3 μm within an 8-mm diameter, with a central repeatability of 0.5 μm for epithelial thickness and less than 8.0 μm within an 8-mm diameter, with a central repeatability of 1.5 μm for corneal thickness.14 This system was used to investigate postoperative refractive surgery complications,15-17 epithelial and stromal changes induced by Intacs (Addition Technology, Des Moines, Ill),18 evaluate excimer laser ablation depth and residual stromal thickness after LASIK,19,20 and to measure flap thickness reproducibility.21
From the prototype arc-scanner, a commercially approved prototype, referred to as the Artemis 1, was developed in 1999 where the major improvement was to invert the scanning system so that the patient could sit upright, which was more practical than the supine position. The Artemis 1 has been used to measure flap thickness reproducibility22,23; provide diagnoses for optical complications after corneal refractive surgery24-26; demonstrate epithelial changes after myopic LASIK,27 hyperopic LASIK,28 and orthokeratology29; investigate the sizing issues related to phakic intraocular lenses (IOLs)30-33; measurement of stromal change to evaluate excimer laser ablation depth34; describe the epithelial thickness35 and stromal thickness36 in a normal population; describe corneal, epithelial, and stromal thickness in a keratoconic population37; and using epithelial thickness profiles to screen for keratoconus.38,39 The Artemis 1 prototype was used for the present study.
Following the Artemis 1 commercial prototype, further changes were made to develop a more compact model called the Artemis 2 (ArcScan Inc, Morrison, Colo), which has been used by a number of investigators for scientific studies.40-44 The main difference is that Artemis 1 uses a polyvinydifluoride (PVDF) transducer whereas Artemis 2 uses a lithium niobate transducer (which is more sensitive). Both PVDF and lithium niobate probes produce the same accuracy of measurement, as accuracy varies according to frequency and bandwidth, not sensitivity. The digital signal processing technology and the arc described for anterior segment as well as corneal scanning are identical for both instruments.
The Artemis 2 was discontinued while development of a novel three-mode (rectilinear, telecentric, and sector) VHF digital ultrasound scanner (Artemis 3) is completed. The Artemis 3 will have all the functionality of the Artemis 1 for corneal scanning and biometry, but in addition will be able to scan and delineate the entire circumference of the human capsule around the lens. By measuring the capsular bag, it may be able to better determine the effective lens position of an IOL in cataract surgery and provide the possibility of haptic sizing for accommodative IOLs, which should increase the accuracy of the refractive result and decrease the necessity to use excimer laser corneal procedures after cataract surgery.
The purpose of this study was to report the repeatability of thickness measurements of the cornea, epithelium, stroma, flap, and residual stromal bed with the Artemis 1 VHF digital ultrasound arc-scanner.
Patients and Methods
Patient Population
This was a prospective study including 10 patients chosen at random from a subset of another larger study investigating the accuracy of flap thickness with the VisuMax femtosecond laser (Carl Zeiss Meditec, Jena, Germany).23 The inclusion criteria for the parent study were volunteer patients suitable for LASIK with a myopic spherical equivalent refraction up to −6.50 diopters. Myopic LASIK was performed by one of the authors (D.Z.R.) at the London Vision Clinic, London, United Kingdom, with the MEL 80 excimer laser (Carl Zeiss Meditec) and the VisuMax femtosecond laser (Carl Zeiss Meditec).
Procedure
Each patient was scanned with the Artemis VHF digital ultrasound arc-scanner, as described below, 1 year after LASIK. All patients were scanned by an expert user (D.Z.R.) and the scans were analyzed by a trained and experienced observer (T.J.A.). To test the repeatability of layered pachymetry in vivo, five three-dimensional scan sets were obtained consecutively for the left eye of each patient. The patient was asked to look away from the fixation light between each of the five scan sets. Informed consent was obtained from each patient and the study was performed in accordance with an institutional review board–approved protocol.
Artemis VHF Digital Ultrasound Arc-Scanning
Scanning System and Procedure
Artemis VHF digital ultrasound is carried out using an ultrasonic standoff medium, and so provides the advantages of immersion scanning (eg, the tear film is not incorporated into the corneal or epithelial thickness measurement, and there is no physical contact of the transducer with the cornea). The patient sits and positions the chin and forehead into a headrest while placing the eye in a soft rimmed eye-cup. Warm, sterile, normal saline (33°C) is filled into the darkened scanning chamber. The patient fixates on a narrowly focused aiming beam, which is coaxial with the infrared camera, the corneal vertex, and the center of rotation of the scanning system. The technician adjusts the center of rotation of the system until it is coaxial with the corneal vertex. In this manner, the position of each scan plane is maintained about a single point on the cornea and corneal mapping is therefore centered on the corneal vertex. A speculum is not required as patients find it comfortable to open the eye without blinking in the warm saline bath and voluntary elevation of the upper lid produces exposure of the central 10 mm of cornea in virtually all patients.
A broadband, 50-MHz, VHF-focused ultrasound transducer (bandwidth approximately 10 to 60 MHz) is swept in an arc such that the adjustable radius of curvature at the transducer focal plane matches that of any of the different curvatures within the globe (ie, cornea, iris plane, and retina) using a patented scan-arc adjustment mechanism to enable maximum perpendicularity (and signal-to-noise ratio) to be obtained. Each scan sweep takes approximately 0.25 seconds and consists of 128 scan lines or pulse-echo vectors. For three-dimensional scan sets, the scan sequence consisted of 4 meridional scans at 45° intervals. During the acquisition of each scan, data were converted (in near real-time) to a B-scan displayed on the control-computer screen. Each B-scan reveals information regarding centration, ranging, and eye movements that may have occurred during the scan sweep. The examiner either accepted or chose to repeat a particular meridional sweep before proceeding to the next. Performing a three-dimensional scan set with the Artemis 1 takes approximately 2 to 3 minutes per eye.
Data Analysis
Ultrasound data are digitized and stored for subsequent processing to B-scan images for visualization and I-scan traces for biometry using digital signal processing technology and software developed by our group at Weill-Cornell Medical College, New York, New York. Digital signal processing (deconvolution and determination of the signal envelope by analytic signal magnitude detection) to I-scans3 was used to detect corneal tissue interfaces along each pulse-echo vector. The time-based distances between I-scan peaks were converted to microns using the speed of sound constant for cornea of 1640 m/s.45 The resolution of the system is 21 μm, meaning that distinct echo-peaks can be seen on the I-scan for a corneal layer thicker than 21 μm.
Interfaces between tissues are detected at the location of the maximum change in acoustic impedance (the product of the density and the speed of sound). It was first demonstrated in 1993 that acoustic interfaces detected in the cornea were located spatially at the epithelial surface and at the interface between epithelial cells and the anterior surface of Bowman's layer.1 The posterior boundary of the stroma with VHF digital ultrasound is located at the interface between the endothelium and the aqueous, as this is the location of the maximum change in acoustic impedance. Therefore, stromal thickness with VHF digital ultrasound is measured from the front surface of Bowman's layer to the back surface of the endothelium.
Each of the four meridional B-scans were analyzed by an interactive, semi-automatic expert system to locate the interface peaks for the anterior corneal surface, Bowman's interface, the flap interface, and the posterior corneal surface along each scan line within the B-scan.46 The interface peaks found automatically by the expert system were double-checked manually and changes were made by the observer in places where the incorrect peak had been identified. The positional localization of each peak was hence achieved in a three-dimensional coordinate system according to the meridian of the B-scan, the scan line within the B-scan, and the axial (radial) location of the peak within the B-scan. Surface point coordinates from all interfaces were stored in matrix format and used for reconstruction of the acoustic interfaces in three-dimension. The thickness of each layer was then derived from the distance between surfaces in the radial direction (perpendicular to the back surface of the cornea).47 A linear polar/radial interpolation function was used to interpolate between scan meridians to produce a Cartesian matrix over a 10-mm diameter in 0.1-mm steps. The interpolation function also includes auto-correlation of back surface curvatures to center and align the meridional scans. This is our standard scanning protocol as it provides sufficiently high density of information in the central cornea with lower density of information in the periphery where it is less needed.
Repeatability Analysis
The following repeatability analysis was performed for each corneal layer including the full cornea, epithelium, stroma, flap, and residual stromal bed.
Within-Eye Point-Repeatability
The standard deviation of the five repeated measurements for each eye was calculated for every 0.1-mm step within the Cartesian matrix to represent the point-repeatability for each eye. The pooled standard deviation (square root of the mean variance)48 for the 10 eyes was calculated for each 0.1-mm step within the Cartesian matrix to represent the within-eye point-repeatability for the population (σw2). The within-eye point-repeatability and range of point-repeatability at the corneal vertex ([0, 0] coordinate) was reported. The coefficient of repeatability represents the range within which 95% of repeated measurements will be, calculated as 1.96 × σw2.48 The coefficient of variation is calculated as the ratio of the within-eye point-repeatability and the mean of the repeated measurements.49 The coefficient of repeatability and coefficient of variation were calculated for the within-eye point-repeatability at the corneal vertex. The intraclass correlation coefficient was calculated for thicknesses at the corneal vertex.50 The within-eye point-repeatability was plotted for the 10-mm diameter using surface fill plots X,Y,Z to display the data on a color scale (DeltaGraph v5.0; SPSS Inc, Richmond, California). A Cartesian 1-mm grid was superimposed with the origin centered at the corneal vertex.
Within-Eye Region-Repeatability
The pooled standard deviation of the point-repeatability data within the central 1-, 2-, and 3-mm radius was calculated to represent the region-repeatability for each eye. The pooled standard deviation of the region-repeatability for the 10 eyes was calculated for the central 1-, 2-, and 3-mm radius to represent the within-eye region-repeatability for the population. The within-eye region-repeatability and range of region-repeatability was reported.
Minimum Thickness Within-Eye Repeatability
The minimum corneal thickness and residual stromal bed thickness was found for each of the 5 repeated scan sets for each eye. The standard deviation was calculated for each eye to represent the repeatability of the minimum corneal thickness and residual stromal bed. The pooled standard deviation for the 10 eyes was calculated to represent the within-eye repeatability for the minimum corneal thickness and residual stromal bed. The within-eye repeatability and range of repeatability were reported for the minimum corneal thickness and residual stromal bed.
Longitudinal Analysis
Artemis VHF digital ultrasound is performed with the eye immersed in normal saline at 33°C; therefore, each eye in the present study required immersion for the time taken to obtain five scan sets. Hydration changes within the cornea while the eye is immersed could potentially cause changes in corneal layer thickness. Corneal tonicity could vary and theoretically some corneas may swell, whereas others may deturgess. To also investigate the effect of immersion on corneal pachymetric scanning, the minimum corneal thickness was analyzed longitudinally for the five consecutive scan sets. The minimum corneal thickness of the first scan set was taken as the baseline measurement. The minimum corneal thicknesses of the subsequent four scan sets were adjusted by subtracting the baseline measurement to find the difference from the baseline measurement. A positive difference would represent corneal thickening whereas a negative difference would represent corneal thinning. The timestamp from the creation of the data file was used to measure the time taken for each scan set.
Descriptive statistics, comparative statistics, and linear regression analysis were performed in Microsoft Excel 2003 (Microsoft Corp, Redmond, Washington).
Results
Ten eyes of 10 patients were included in the study. The population mean age was 31.6±7.5 years, median 30.0 years, ranging from 23.9 to 52.1 years. The median time point at which Artemis scans were obtained was 11.9 months after LASIK, ranging from 9.8 to 17.0 months with eight patients scanned between 11.7 and 13.2 months after LASIK.
Figure 1 demonstrates the raw (non-geometrically corrected) B-scan of the left cornea of patient 4 produced from the scan data 12 months after LASIK. The cornea appears relatively flat or straight from top to bottom of the image, indicating that the trajectory followed by the scanning arc of the transducer was nearly conformal to the corneal surface. Superimposed on the B-scan image is the I-scan trace derived by analysis of the digitized radiofrequency ultrasonic data, corresponding to the scan line indicated on the B-scan (horizontal line radial to the corneal cross-section). The peaks of the I-scan correspond to acoustic interfaces along the vector through the corneal B-scan image centrally. Figure 2 shows the geometrically corrected B-scan image of the same cornea as well as a red line trace of the interfaces detected using digital signal processing and automated analysis of the I-scan trace.
Figure 1.
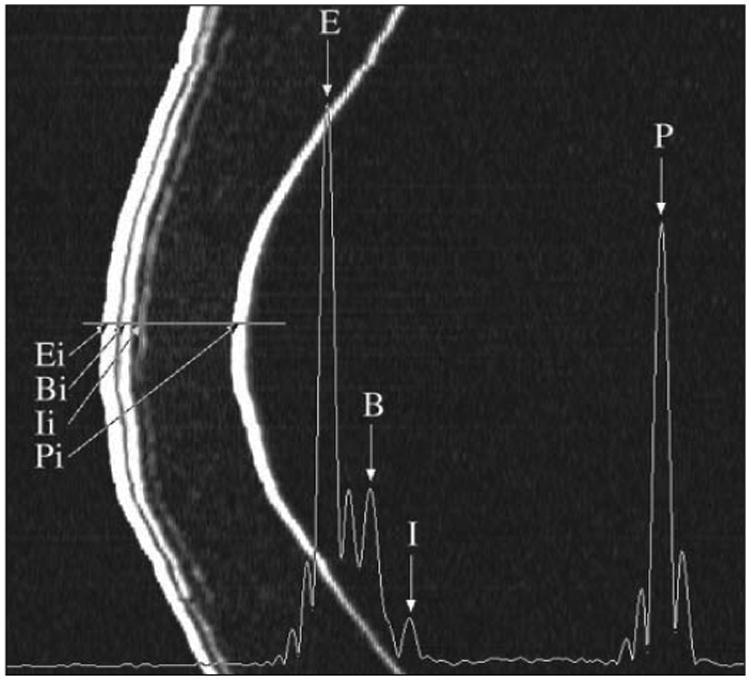
Non-geometrically corrected B-scan of a cornea 12 months after LASIK. The scan was performed in the visual axis in the horizontal plane. The B-scan is constructed of 128 pulse echo vectors stacked horizontally. There is a large z-axis zoom in scale (2 mm represented horizontally on the image) relative to the lateral distance (10 mm represented vertically on the image). The B-scan demonstrates interfaces detected ultrasonically for the epithelial surface (Ei), the surface of Bowman's layer (Bi), the lamellar interface (Ii), and the endothelial/posterior corneal surface (Pi). Superimposed on the figure is the I-scan derived by digital processing of the radio frequency ultrasonic B-scan data. The I-scan demonstrates sharp, distinct peaks representing the acoustic interfaces of saline-epitheium (E), epithelium-Bowman's (B), the lamellar interface (I), and the endothelial-aqueous interface (P).
Figure 2.
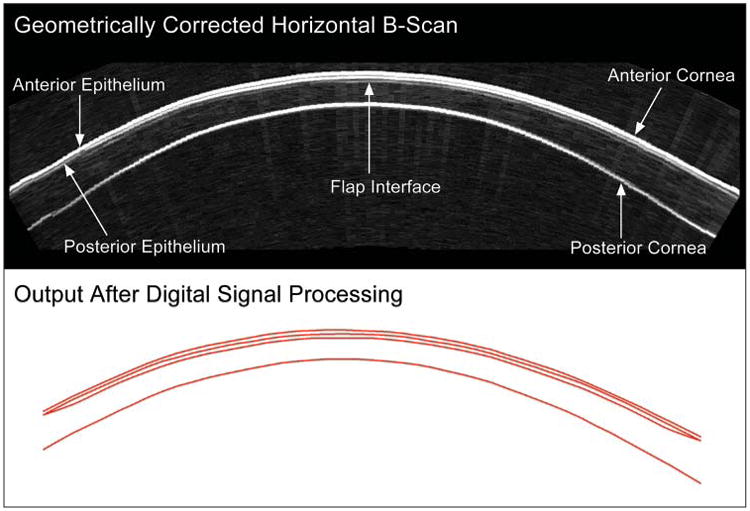
Geometrically corrected B-scan of a cornea 12 months after LASIK. Digital signal processing is performed on the B-scan signal and layer thickness measurements are obtained by a computer algorithm on the I-scan (see Figure 1), resulting in the red line image of the interfaces.
Table 1 shows the average and range of the point-repeatability, the coefficient of repeatability, coefficient of variation, intraclass correlation coefficient, and the average and range of region-repeatability for each layer including the cornea, epithelium, stroma, flap, and residual stromal bed as well as the minimum thickness repeatability for the cornea and residual stromal bed. Figure 3 shows the average point-repeatability plotted for the 10-mm diameter for the epithelium, stroma, cornea, flap, and residual stromal bed.
Table 1. Within-eye Point-repeatability and Region-repeatability of 10 Eyes That Underwent Artemis VHF Digital Ultrasound 1 Year After LASIK.
| Epithelium | Stroma | Cornea | Flap | Residual Stromal Bed | |
|---|---|---|---|---|---|
| Within-eye Point-repeatability – Corneal Vertex | |||||
| Within-eye point-repeatability (μm) | 0.58 [0.18, 0.99] | 1.78 [0.40, 2.63] | 1.68 [0.85, 2.32] | 1.68 [0.85, 2.74] | 2.27 [1.09, 4.28] |
| Coefficient of repeatability (μm) | 1.14 | 3.50 | 3.29 | 3.29 | 4.46 |
| Coefficient of variation (%) | 0.97 | 0.43 | 0.35 | 1.39 | 0.64 |
| Intraclass correlation coefficient | 0.973 | 0.999 | 0.999 | 0.969 | 0.998 |
| Within-eye Point-repeatability – Minimum Thickness | |||||
| Minimum thickness repeatability (μm) | 1.36 [0.67, 1.68] | 2.76 [0.49, 6.08] | |||
| Within-eye Region-repeatability (μm) | |||||
| Within 1-mm radius | 1.01 [0.73, 1.35] | 3.44 [2.32, 4.54] | 3.35 [2.03, 4.33] | 2.81 [2.01, 3.66] | 3.97 [2.66, 5.30] |
| Within 2-mm radius | 0.94 [0.70, 1.27] | 4.92 [3.27, 6.46] | 4.84 [3.00, 6.59] | 2.79 [2.12, 3.50] | 5.18 [3.50, 7.11] |
| Within 3-mm radius | 1.04 [0.75, 1.32] | 6.49 [4.25, 8.54] | 6.02 [3.85, 7.86] | 4.35 [2.94, 7.25] | 7.58 [5.04, 10.87] |
Note. The range for the study population is reported in brackets.
Figure 3.
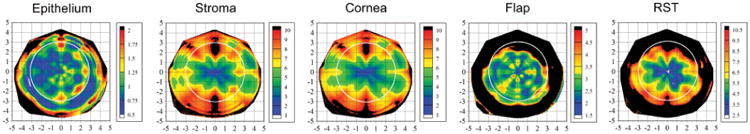
The average point-repeatability plotted for the 10-mm diameter for the epithelium, stroma, cornea, flap, and residual stromal bed (RST) based on 5 consecutive measurements from 10 left eyes. The color scale represents the point-repeatability in microns. A Cartesian 1-mm grid is superimposed with the origin at the corneal vertex. Positive x values represent the temporal cornea and negative values represent the nasal cornea. Positive y values represent the superior cornea and negative values represent the inferior cornea. A white circle is superimposed on the maps to identify a central region with 3-mm radius.
Epithelium
The repeatability of epithelial thickness at the corneal vertex was 0.58 μm. The repeatability of epithelial thickness was reasonably similar across the whole cornea with a repeatability between 0.43 and 1.36 μm in 90% of locations within the central 6-mm diameter.
Stroma, Cornea, and Residual Stromal Thickness
At the corneal vertex, the repeatability was 1.78 μm for stromal thickness, 1.68 μm for corneal thickness, and 2.27 μm for residual stromal thickness. The repeatability of stromal, corneal, and residual stromal thickness was highest centrally and progressively worse at greater radius from the corneal vertex. The repeatability of stromal, corneal, and residual stromal thickness was higher in a horizontal band within 1 mm above and below the horizontal meridian through the corneal vertex. The lowest stromal, corneal, and residual stromal thickness repeatability was found in the superior and inferior regions. The repeatability of stromal thickness was between 1.60 and 4.55 μm in 90% of locations within the central 2-mm diameter and between 1.60 and 6.70 μm in 90% of locations within the central 4-mm diameter. The repeatability of corneal thickness was between 1.53 and 4.40 μm in 90% of locations within the central 2-mm diameter and between 1.53 and 6.60 μm in 90% of locations within the central 4-mm diameter. The repeatability of residual stromal thickness was between 2.14 and 5.03 μm in 90% of locations within the central 2-mm diameter and between 2.14 and 6.90 μm in 90% of locations within the central 4-mm diameter. The highest repeatability for stromal, corneal, and residual stromal thickness was found in between each of the four scanned meridians at 45° intervals where the data had been interpolated between scan meridians.
Flap
The repeatability of flap thickness at the corneal vertex was 1.68 μm. The repeatability of flap thickness was similar across the whole cornea with a repeatability between 1.50 and 3.90 μm in 90% of locations within the central 6-mm diameter.
Longitudinal Analysis
The first scan set for each eye was obtained within the first minute of immersion. Each subsequent scan set was acquired within the next minute, meaning that the eye had been immersed for 5 minutes once the fifth scan set had been acquired. The mean difference between the minimum corneal thickness of the first scan set and each of the four subsequent scan sets is plotted in Figure 4. No statistically significant difference was noted in minimum corneal thickness between the first scan set and the second, third, or fourth scan sets. The second scan set was on average 0.7 μm thinner than the first scan set (P=.068). The third scan set was on average 0.6 μm thinner than the first scan set (P=.336). The fourth scan set was on average 0.4 μm thinner than the first scan set (P=.533). The fifth scan set was on average 1.2 μm thicker than the first scan set, which was a statistically significant difference (P=.034).
Figure 4.
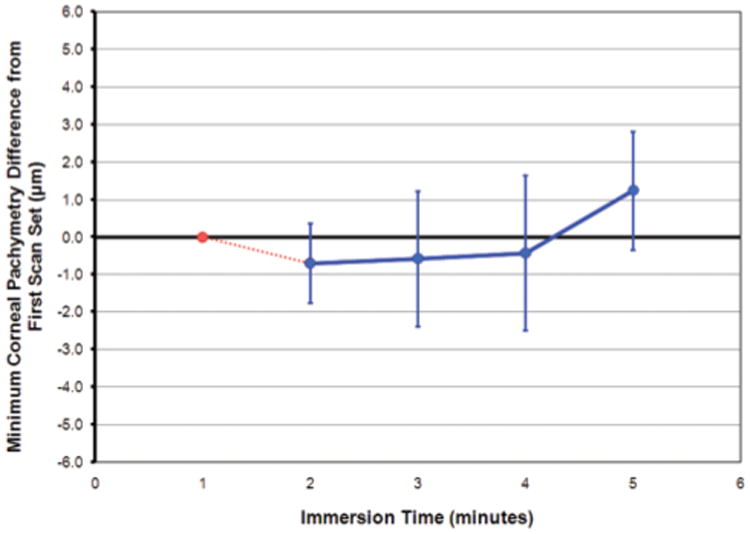
Line plot showing the mean difference between the minimum corneal thickness of the first scan set and each of the four subsequent scan sets. The first scan set is shown as a reference point in red. The blue data points are the mean difference from the first scan set. The error bars represent one standard deviation for each of the subsequent scan sets.
Discussion
We have shown very high repeatability in the range of 0.58 to 2.27 μm of three-dimensional wide-area pachymetry using the Artemis VHF digital ultrasound arc-scanner for the epithelium, stroma, cornea, flap, and residual stromal bed.
Accurate and repeatable corneal thickness measurements are important for the safe practice of corneal refractive surgery19,20 as well as for other applications such as the management of conditions including keratoconus, glaucoma, Fuchs' dystrophy, and others. There is a wide range of commercially available instruments capable of measuring corneal thickness, whereas optical slit-scanning pachymetry, Scheimpflug, and optical coherence tomography (OCT) also offer corneal thickness maps across the cornea. For comparison, a literature search was performed in Medline on December 11, 2008, using the keywords “repeatability cornea pachymetry.” Studies were included if the standard deviation of repeated measurements was reported or could be derived from the reported values. Table 2 provides the repeatability of central corneal thickness measurements for all currently published studies listed in ascending order of repeatability.51-66 For each study, the standard deviation of repeated measurements, the coefficient of repeatability (1.96 × σw2) and the coefficient of variation (σw2/mean) is reported either directly from the manuscript or calculated from the reported results. One study reported the repeatability of corneal thickness measurements with the Visante OCT and the Artemis 2 VHF digital ultrasound scanner.43 However, we excluded the results of this study from the table because of the unrealistic, perfect repeatability of 0.00 μm reported for the Visante.43 The repeatability of corneal thickness with the Artemis 2 was reported to be 2.22 μm.43
Table 2. Repeatability of Central Corneal Thickness Measurements.
| Author (Year) | Instrument | Method | n | m | σw2 (μm) | COR (μm) | CV (%) |
|---|---|---|---|---|---|---|---|
| Reinstein et al (2009) | Artemis 1 | VHF digital ultrasound | 10 | 5 | 1.68 | 3.29 | 0.35 |
| Barkana et al51 (2005) | OLCR | Low-coherence reflectometry | 4 | 10 | 1.82 | 3.56 | 0.33 |
| Mohamed et al52 (2007) | Visante | Optical coherence tomography | 27 | 2 | 2.36 | 4.63 | 0.44 |
| Spadea et al53 (2007) | OLCR | Low-coherence reflectometry | 48 | 2 | 2.76 | 5.4 | 0.51 |
| Lackneret al54 (2005) | SP-2000 | Ultrasound | 30 | 2 | 3.60 | 7.06 | 0.65 |
| Fam et al55 (2005) | Orbscan II | Slit-scanning tomography | 20 | 5 | 3.62 | 7.10 | 0.67 |
| Suzuki et al56 (2003) | SP-2000P | Non-contact specular microscopy | 20 | 2 | 3.63 | 7.11 | 0.70 |
| Wirbelauer et al57 (2004) | Pocket Pachymeter | Ultrasound | 16 | 6 | 3.70 | 7.25 | 0.68 |
| Muscat et al58 (2002) | Humphrey Zeiss OCT | Optical coherence tomography | 3 | 10 | 3.70 | 7.25 | 0.70 |
| Barkana et al51 (2005) | Not reported | Ultrasound | 4 | 10 | 3.91 | 7.65 | 0.71 |
| Wang et aI59 (2008) | Corneo-gage Plus | Ultrasound | 48 | 2 | 3.93 | 7.86 | 0.50 |
| Zhao et al60 (2007) | SP-2000P | Non-contact specular microscopy | 40 | 3 | 4.02 | 7.88 | 0.76 |
| Zhao et al60 (2007) | SP-3000 | Ultrasound | 40 | 3 | 4.26 | 8.35 | 0.78 |
| Jonuscheit & Doughty61 (2007) | Orbscan II | Slit-scanning tomography | 24 | 3 | 4.30 | 8.43 | 0.81 |
| Jonuscheit & Doughty61 (2007) | Echopach Pachymeter 3M | Ultrasound | 24 | 3 | 4.30 | 8.43 | 0.82 |
| Sacu et al66 (2005) | ACMaster | Partial coherence interferometry | 20 | 2 | 4.30 | 8.43 | 0.79 |
| Wirbelauer et al57 (2004) | OCPOnline | Optical coherence tomography | 16 | 6 | 4.30 | 8.43 | 0.80 |
| Suzuki et al56 (2003) | Orbscan | Slit-scanning tomography | 20 | 2 | 4.61 | 9.04 | 0.86 |
| Barkana et al51 (2005) | Pentacam | Rotating Scheimpflug camera | 4 | 10 | 4.62 | 9.06 | 0.84 |
| Spadea et al53 (2007) | Corneo-gage Plus | Ultrasound | 48 | 2 | 4.64 | 9.10 | 0.86 |
| Wang et al59 (2008) | OCPOnline | Optical coherence tomography | 48 | 2 | 4.74 | 9.47 | 0.66 |
| Suzuki et al56 (2003) | SP-2000 | Ultrasound | 20 | 2 | 4.88 | 9.56 | 0.89 |
| Li et al62 (2008) | Visante | Optical coherence tomography | 25 | 3 | 4.90 | 9.60 | 0.90 |
| Wirbelauer & Pham64 (2004) | SL-OCT | Optical coherence tomography | 25 | 3 | 4.90 | 9.60 | 0.95 |
| Sin & Simpson63 (2006) | Humphrey Zeiss OCT | Optical coherence tomography | 18 | 3 | 5.43 | 10.64 | 1.01 |
| Li et al62 (2008) | SL-OCT | Optical coherence tomography | 25 | 3 | 5.80 | 11.40 | 1.00 |
| Lackner et al54 (2005) | Pentacam | Rotating Scheimpflug camera | 30 | 2 | 6.10 | 11.96 | 1.13 |
| User Manual65 (2007) | Visante | Optical coherence tomography | 78 | 3 | 6.70 | 13.13 | 1.22 |
| Lackner et al54 (2005) | Orbscan | Slit-scanning tomography | 30 | 2 | 6.90 | 13.52 | 1.30 |
Values printed in light gray are derived from other reported values.
n = number of eyes included in the study, m = number of repeated measurements, σw2 = standard deviation (SD) of m repeated measurements, COR = coefficient of repeatability (1.96 * SD), CV = coefficient of variation (ratio of SD over the mean of the measurements)
OCPOnline, SL-OCT (Heidelberg Engineering GmbH, Heidelberg, Germany); Corneo-gage Plus (Sonogage Inc, Cleveland, Ohio); Artemis 1 (ArcScan Inc, Morrison, Colorado); SP-3000, SP-2000 (Tomey Ltd, Nagoya, Japan); Visante, Humphrey Zeiss OCT, ACMaster (Carl Zeiss Meditec, Jena, Germany); SP-2000P (Topcon Ltd, Tokyo, Japan); OLCR (Haag Streit, Bern, Switzerland); Orbscan, Orbscan II (Bausch & Lomb, Rochester, New York); Echopach Pachymeter 3M (Phakosystems, Toronto, Canada); Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany); Pocket Pachymeter (Quantel Medical, Clermont-Ferrand, France)
Accuracy of corneal thickness measurements was not performed in this study because we did not have access to an in vivo equivalent corneal phantom of known thickness. However, we previously published an error analysis on VHF digital ultrasound measurements.14 The potential error of VHF digital ultrasound measurements is based on the known speed of sound in cornea (1640 m/s) and the maximum theoretical limits of this speed of sound. Given that saline possesses a speed of sound of 1532 m/s45 and that our group has demonstrated a lower limit for the speed of sound of 1616 m/s in ex vivo bovine corneas,67 it is unlikely that the human cornea could possess a speed of sound of less than 1610 m/s at the lower extreme. Given that cartilage possesses a speed of sound of approximately 1668 m/s (at 50 MHz; extrapolated from 1568 m/s at 25 MHz),68 it is unlikely that the epithelium could possess a speed of sound of more than 1670 m/s at the upper extreme. Given these range limits for the speed of sound, a theoretical error analysis predicts an absolute maximum potential error of 1.8% for the accuracy of VHF digital ultrasound measurements.
Artemis VHF digital ultrasound corneal thickness measurements are obtained with the eye immersed in normal saline at 33°C. Any changes in hydration of the cornea during immersion could potentially introduce error into the measurement. The present study found that the minimum corneal thickness did not change for the first 4 minutes after immersion, and that by 5 minutes there was a 1.2-μm change (see Fig 4). Scan sets obtained within the first 4 minutes of immersion were all similar to the baseline measurement. At 5 minutes after immersion, the fifth scan set was found to be 1.2 μm thicker than baseline. This result suggests that there might be approximately 1 μm of corneal edema induced by immersion in normal saline at 33°C after 5 minutes. During routine scanning, Artemis three-dimensional corneal scan data are obtained within 3 minutes of immersion, thus we can expect thickness data to be true and accurate. Further study is required to assess corneal thickness changes over a longer immersion period to confirm whether the result of the fifth scan set is due to corneal hydration and determine what thickness changes there might be over a longer immersion period.
Repeatable epithelial thickness measurements are useful for a wide variety of applications including measuring epithelial thickness profile changes after refractive surgery,27 diagnosing optical complications due to an irregular stromal surface,4,15,24 and determining the minimum possible flap thickness to use for thin-flap LASIK to avoid a buttonhole. This is particularly useful when considering the possibility of creating a flap in an eye that has previously had PRK where the epithelium might have thickened to compensate for the tissue removed. We recently described the potential for using epithelial thickness profiles to screen for early keratoconus based on micronic changes in the epithelial thickness profile characteristic of compensation for an underlying stromal surface cone.38,39 The repeatability of <1 μm reported here shows that Artemis VHF digital ultrasound can be used to detect changes in epithelial thickness in the order of 1 μm, so it can be used to detect the earliest changes in the epithelium that occur in keratoconus, changes that will occur before changes in corneal topography.38,39
For comparison, a literature search was performed in Medline on December 11, 2008, using the keywords “repeatability epithelium cornea.” The epithelium can be imaged by OCT64,69 and confocal microscopy70-72; however, only two previous studies were identified where the repeatability of epithelial thickness measurements had been performed and the results of these studies are shown in Table 3.
Table 3. Repeatability of Central Epithelial Thickness Measurements.
| Author (Year) | Instrument | Method | n | m | σw2 (μm) | COR (μm) | CV (%) |
|---|---|---|---|---|---|---|---|
| Reinstein (2009) | Artemis 1 | VHF digital ultrasound | 10 | 5 | 0.58 | 1.14 | 0.97 |
| Wirbelauer & Pham64 (2004) | SL-OCT | OCT | 25 | 3 | 3.24 | 6.35 | 5.69 |
| Sin & Simpson63 (2006) | Humphrey Zeiss OCT | OCT | 18 | 3 | 3.33 | 6.53 | 6.41 |
Values printed in light gray are derived from other reported values.
n = number of eyes included in the study, m = number of repeated measurements, σw2 = standard deviation (SD) of m repeated measurements, COR = coefficient of repeatability (1.96 * SD), CV = coefficient of variation (ratio of SD over the mean of the measurements), OCT = optical coherence tomography Artemis 1 (ArcScan Inc, Morrison, Colorado); SL-OCT (Heidelberg Engineering GmbH, Heidelberg, Germany); Humphrey Zeiss OCT (Carl Zeiss Meditec, Jena, Germany)
The B-scan image presented in Figure 1 demonstrates the clarity and signal amplitude of the LASIK flap interface along its entire length in an Artemis scan 1 year after surgery. The I-scan trace demonstrates that this signal amplitude translates into a well-defined peak resulting in the high flap thickness repeatability of 1.68 μm reported here. The clarity and signal amplitude of the flap interface in the early postoperative period is greater, so one would expect the repeatability of flap thickness to be even higher for Artemis measurements obtained at an earlier time point. Although the clarity and signal amplitude diminishes over time, meaning that the peak on the I-scan trace becomes smaller, rounder, and less well-defined, the flap can often still be identified along its entire length with confidence for as long as a decade after LASIK. Figure 5 shows an example of an Artemis B-scan obtained for a patient 9 years after LASIK with an Automated Corneal Shaper microkeratome (Bausch & Lomb, Rochester, NY).
Figure 5.
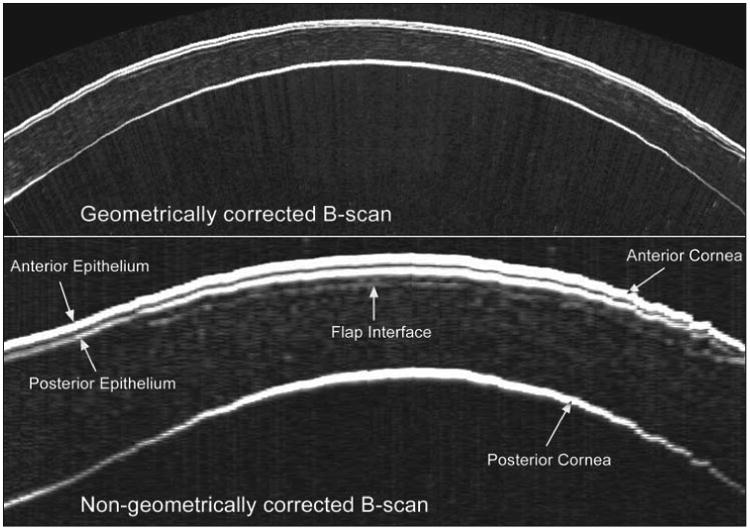
Geometrically corrected (top) and non-geometrically corrected (bottom) B-scan of a cornea 9 years after LASIK with an Automated Corneal Shaper microkeratome (Bausch & Lomb). The flap interface can still be clearly identified along its entire length 9 years postoperatively. The bottom scan has been zoomed to emphasize the interfaces, so that the image width represents 10 mm and the image height represents 1.2 mm.
The flap interface can also be imaged using OCT64,69,73-79 and confocal microscopy80-83; however, a Medline search on December 11, 2008, using the keywords “repeatability flap LASIK” revealed only 2 published studies that reported the repeatability of flap thickness measurements. Using a prototype version of the Visante OCT (Carl Zeiss Meditec) and a computer automated algorithm to analyze the flap thickness from the analytic signal for scans, the flap thickness was measured 3 times in 36 eyes treated with the Hansatome microkeratome (Bausch & Lomb, Salt Lake City, Utah) and 15 eyes treated with the IntraLase femtosecond laser (Abbott Medical Optics, Irvine, California).74 The measurements were obtained 1 week after LASIK. The repeatability of central measurements (within 2-mm diameter) was 5.6 μm for the Hansatome group and 4.8 μm for the IntraLase group.74 The repeatability for peri-central measurements (2- to 5-mm diameter) was 3.0 μm for the Hansatome group and 2.1 μm for the IntraLase group.74 Using the Visante OCT, two repeated measurements were obtained each for two observers at 1-month postoperative follow-up of flaps created using a Hansatome XP microkeratome (Bausch & Lomb).72 The standard deviation of repeated measurements was not reported; however, the standard deviation of the difference between the two measurements was 8.11 μm and 8.59 μm for the two observers.72 This translates to a maximum difference of approximately 23.8 μm (99% of values will lie within 2.77 standard deviations) between two repeated flap thickness measurements. In comparison, the central repeatability of flap thickness measurements of 1.68 μm reported in the present study for the Artemis translates to a maximum difference of 4.7 μm between two flap thickness measurements. The flap thickness repeatability is also reported in the Visante OCT User Manual for a study where three repeated measurements were performed in 78 individuals.65 The flap thickness repeatability measured 1 mm from the corneal vertex was reported to be 8.7 μm.65
As a general rule, given that 99% of measurements are within 2.77 standard deviations of the mean, the precision of the measuring tool should be at least one-third of the reproducibility of the data set to justify the validity of a reproducibility study. Knowledge of the actual repeatability of a pachymetric device is critical if this tool is to be used, eg, for the assessment of flap thickness reproducibility. For instance, one published study using a Visante OCT reports a flap thickness standard deviation of 5 μm for the IntraLase based on peripheral flap thickness measurements using manual location of the flap interface on a B-scan image.76 The reported flap thickness repeatability of the Visante OCT for manual measurements is more than 8 μm65 and the software flap tool used to make manual measurements can only obtain measurements in screen pixel increments of 12 μm. Therefore, the precision of the Visante OCT was not sufficient to distinguish IntraLase flap thicknesses to the required level, meaning that the reported flap thickness reproducibility of 5 μm76 cannot be taken as a reliable assessment of IntraLase flap thickness reproducibility. Using a standard 5 cm ruler to measure the width of a human hair (approximately 0.1 mm)84 is a simple example that demonstrates this concept; the ruler would only be able to measure the width of the hair to the nearest 0.5 mm, so the repeatability would appear to be very high as virtually every measurement would be recorded as 0.5 mm.
Optical coherence tomography appears to be a promising technology because of the convenience of obtaining measurements without fluid immersion; however, there appear to be a few weaknesses compared with the high repeatability achieved by VHF digital ultrasound. First, it is difficult to detect the flap interface centrally with OCT because of the signal clipping (saturation) generated by the corneal reflex and the perpendicularity of the stromal lamellae. This was demonstrated by the lower repeatability reported for central flap thickness measurements with the Visante OCT compared with peri-central measurements74 and the fact that the repeatability at the central point location has not been reported in any study. Unfortunately, a central flap thickness measurement is the most important location as the residual stromal bed will be thinnest centrally after myopic LASIK, as the cornea is thinnest and the ablation depth is deepest centrally. Second, internal corneal interfaces such as the flap interface are less detectable by optical means than by ultrasound. A flap interface will always represent an acoustic discontinuity required for ultrasound imaging, whereas optical imaging of a flap interface is more difficult as the optical discontinuity of the flap interface diminishes significantly over time.65 This is understandable given that severed corneal stromal lamellae are bathed in glycosaminoglycan tissue glue, which provides refractive index homogeneity and hence minimizes light scatter at the flap interface (which is, of course, desirable as increased light scatter at the lamellar interface would result in high levels of glare following LASIK). No studies have reported the flap thickness repeatability with the Visante OCT for eyes longer than 1 month after LASIK, whereas the flap thickness repeatability of the Artemis reported in the present study is based on eyes 1 year after LASIK.72,74 A direct comparison between ultrasound and optical is needed to settle these issues. Because optical measurement is based on refractive index assumptions, it may be possible to improve OCT accuracy by calibration with VHF digital ultrasound findings. Indeed, original prototypes of the Visante OCT were calibrated by taking a central handheld ultrasound corneal thickness measurement and using this information to calibrate the dewarping of the raw OCT scan image.85
Accurate and repeatable measurements of the residual stromal thickness are important for the safe practice of LASIK retreatments as we have described previously.19,20,22 The calculation of the predicted residual stromal thickness is affected by errors from corneal thickness measurement, variation in ablation depth, and most importantly, variations in flap thickness. Depending on the microkeratome or femtosecond laser used to create the flap, there is a risk that the actual residual stromal thickness is thinner than predicted. Therefore, a direct measurement of the residual stromal thickness is paramount before deciding whether to proceed with retreatment. Also, due to the possibility of a non-uniform flap,21 the ability to map the residual stromal bed across the whole cornea provides the advantage of finding the minimum thickness of the residual stromal bed, which may not be guaranteed using intraoperative, single-point, handheld ultrasound pachymetry. The ability to measure the residual stromal thickness profile in vivo also allows the surgeon to precisely plan any retreatment surgery, which might involve irregular ablation profiles such as topography-86 or wavefront-guided87 ablations; the postoperative retreatment residual stromal thickness can be calculated at the location of the deepest ablation so that the optimal treatment can be planned.
The repeatability of corneal thickness in the present study was highest centrally and progressively worse at greater radius from the corneal vertex, which is to be expected as corneal thickness increases radially, and would exaggerate any misalignment errors. In contrast, the repeatability of epithelial thickness and flap thickness was relatively homogeneous. The thickness of the epithelium and flap would be expected to be reasonably uniform across the cornea, and hence thickness measurements of the epithelium and flap will be less affected by misalignment errors. The lowest corneal thickness repeatability was found in the superior and inferior regions, most likely due to interference from the eyelid during scanning in some scan sets. The highest corneal thickness repeatability was found in the region where the data had been interpolated between the meridional scans at 45° intervals. At the scanned meridians, only one measurement is being used (the measurement from the scan itself), whereas between meridians, an average of the values from the two neighboring meridians is used, weighted for distance from each meridian. Therefore, the interpolated measurements between meridional scans would be expected to have a higher repeatability as they are derived from a weighted average of two measurements rather than a single measurement.
Artemis VHF digital ultrasound offers high repeatability for single point and wide area layered corneal pachymetry. Repeatable measurements of the thinnest cornea and residual stromal bed are crucial for the safety of corneal refractive surgery and LASIK retreatments. Repeatable measurements of the epithelium may also be useful for keratoconus screening.
Acknowledgments
Supported in part by NIH grant EB000238 and the Dyson Foundation, Millbrook, New York.
Footnotes
Drs Reinstein, Silverman, and Coleman have a proprietary interest in the Artemis technology (ArcScan Inc, Morrison, Colorado), and are the authors of patents related to VHF digital ultrasound administered by the Cornell Research Foundation, Ithaca, New York. The remaining authors have no proprietary or financial interest in the materials presented herein.
Preparation in part fulfillment of the requirements for the doctoral thesis, University of Cambridge, for Dr Reinstein.
Author Contributions: Study concept and design (D.Z.R., T.J.A., M.G., R.H.S., D.J.C.); data collection (T.J.A.); analysis and interpretation of data (D.Z.R., T.J.A.); drafting of the manuscript (D.Z.R., T.J.A.); critical revision of the manuscript (D.Z.R., M.G., R.H.S., D.J.C.); statistical expertise (T.J.A.)
References
- 1.Reinstein DZ, Silverman RH, Coleman DJ. High-frequency ultrasound measurement of the thickness of the corneal epithelium. Refract Corneal Surg. 1993;9(5):385–387. [PubMed] [Google Scholar]
- 2.Reinstein DZ, Silverman RH, Trokel SL, Coleman DJ. Corneal pachymetric topography. Ophthalmology. 1994;101(3):432–438. doi: 10.1016/s0161-6420(94)31314-5. [DOI] [PubMed] [Google Scholar]
- 3.Reinstein DZ, Silverman RH, Rondeau MJ, Coleman DJ. Epithelial and corneal thickness measurements by high-frequency ultrasound digital signal processing. Ophthalmology. 1994;101(1):140–146. doi: 10.1016/s0161-6420(94)31373-x. [DOI] [PubMed] [Google Scholar]
- 4.Reinstein DZ, Silverman RH, Sutton HF, Coleman DJ. Very high-frequency ultrasound corneal analysis identifies anatomic correlates of optical complications of lamellar refractive surgery: anatomic diagnosis in lamellar surgery. Ophthalmology. 1999;106(3):474–482. doi: 10.1016/S0161-6420(99)90105-7. [DOI] [PubMed] [Google Scholar]
- 5.Reinstein DZ, Patel S, Aslanides IM, Silverman RH, Coleman DJ. Epithelial lenticular types of human cornea: classification and analysis of influence on PRK. Ophthalmology. 1995;102(Suppl):156. [Google Scholar]
- 6.Patel S, Reinstein DZ, Silverman RH, Coleman DJ. The shape of Bowman's layer in the human cornea. J Refract Surg. 1998;14(6):636–640. doi: 10.3928/1081-597X-19981101-11. [DOI] [PubMed] [Google Scholar]
- 7.Reinstein DZ, Silverman RH, Trokel SL, Allemann N, Coleman DJ. High-frequency ultrasound digital signal processing for biometry of the cornea in planning phototherapeutic keratectomy. Arch Ophthalmol. 1993;111(4):430–431. doi: 10.1001/archopht.1993.01090040020013. [DOI] [PubMed] [Google Scholar]
- 8.Allemann N, Chamon W, Silverman RH, Azar DT, Reinstein DZ, Stark WJ, Coleman DJ. High-frequency ultrasound quantitative analyses of corneal scarring following excimer laser keratectomy. Arch Ophthalmol. 1993;111(7):968–973. doi: 10.1001/archopht.1993.01090070088025. [DOI] [PubMed] [Google Scholar]
- 9.Reinstein DZ, Aslanides IM, Silverman RH, Asbell PA, Coleman DJ. High-frequency ultrasound corneal pachymetry in the assessment of corneal scars for therapeutic planning. CLAO J. 1994;20(3):198–203. doi: 10.1097/00140068-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Aslanides IM, Reinstein DZ, Silverman RH, Lazzaro DR, Rondeau MJ, Rodriguez HS, Coleman DJ. High-frequency ultrasound spectral parameter imaging of anterior corneal scars. CLAO J. 1995;21(4):268–272. [PubMed] [Google Scholar]
- 11.Lazzaro DR, Aslanides IM, Belmont SC, Silverman RH, Reinstein DZ, Muller JW, Lloyd HO, Coleman DJ. High frequency ultrasound evaluation of radial keratotomy incisions. J Cataract Refract Surg. 1995;21(4):398–401. doi: 10.1016/s0886-3350(13)80527-5. [DOI] [PubMed] [Google Scholar]
- 12.Cusumano A, Coleman DJ, Silverman RH, Reinstein DZ, Rondeau MJ, Ursea R, Daly SM, Lloyd HO. Three-dimensional ultrasound imaging. Clinical applications. Ophthalmology. 1998;105(2):300–306. doi: 10.1016/s0161-6420(98)93211-0. [DOI] [PubMed] [Google Scholar]
- 13.Silverman RH, Reinstein DZ, Raevsky T, Coleman DJ. Improved system for sonographic imaging and biometry of the cornea. J Ultrasound Med. 1997;16(2):117–124. doi: 10.7863/jum.1997.16.2.117. [DOI] [PubMed] [Google Scholar]
- 14.Reinstein DZ, Silverman RH, Raevsky T, Simoni GJ, Lloyd HO, Najafi DJ, Rondeau MJ, Coleman DJ. Arc-scanning very high-frequency digital ultrasound for 3D pachymetric mapping of the corneal epithelium and stroma in laser in situ keratomileusis. J Refract Surg. 2000;16(4):414–430. doi: 10.3928/1081-597X-20000701-04. Erratum in J Refract Surg. 2001;17(1):4. [DOI] [PubMed] [Google Scholar]
- 15.Holland SP, Srivannaboon S, Reinstein DZ. Avoiding serious corneal complications of laser assisted in situ keratomileusis and photorefractive keratectomy. Ophthalmology. 2000;107(4):640–652. doi: 10.1016/s0161-6420(99)00131-1. [DOI] [PubMed] [Google Scholar]
- 16.Reinstein DZ. Consultation section. Refractive surgical problem. J Cataract Refract Surg. 2001;27(9):1350–1352. [PubMed] [Google Scholar]
- 17.Reinstein DZ, Ameline B, Puech M, Montefiore G, Laroche L. VHF digital ultrasound three-dimensional scanning in the diagnosis of myopic regression after corneal refractive surgery. J Refract Surg. 2005;21(5):480–484. doi: 10.3928/1081-597X-20050901-10. [DOI] [PubMed] [Google Scholar]
- 18.Reinstein DZ, Srivannaboon S, Holland SP. Epithelial and stromal changes induced by intacs examined by three-dimensional very high-frequency digital ultrasound. J Refract Surg. 2001;17(3):310–318. doi: 10.3928/1081-597X-20010501-04. [DOI] [PubMed] [Google Scholar]
- 19.Reinstein DZ, Srivannaboon S, Archer TJ, Silverman RH, Sutton H, Coleman DJ. Probability model of the inaccuracy of residual stromal thickness prediction to reduce the risk of ectasia after LASIK part I: quantifying individual risk. J Refract Surg. 2006;22(9):851–860. doi: 10.3928/1081-597X-20061101-04. [DOI] [PubMed] [Google Scholar]
- 20.Reinstein DZ, Srivannaboon S, Archer TJ, Silverman RH, Sutton H, Coleman DJ. Probability model of the inaccuracy of residual stromal thickness prediction to reduce the risk of ectasia after LASIK part II: quantifying population risk. J Refract Surg. 2006;22(9):861–870. doi: 10.3928/1081-597X-20061101-05. [DOI] [PubMed] [Google Scholar]
- 21.Reinstein DZ, Sutton HF, Srivannaboon S, Silverman RH, Archer TJ, Coleman DJ. Evaluating microkeratome efficacy by 3D corneal lamellar flap thickness accuracy and reproducibility using Artemis VHF digital ultrasound arc-scanning. J Refract Surg. 2006;22(5):431–440. doi: 10.3928/1081-597X-20060501-03. [DOI] [PubMed] [Google Scholar]
- 22.Reinstein DZ, Couch DG, Archer T. Direct residual stromal thickness measurement for assessing suitability for LASIK enhancement by Artemis 3D very high-frequency digital ultrasound arc scanning. J Cataract Refract Surg. 2006;32(11):1884–1888. doi: 10.1016/j.jcrs.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Reinstein DZ, Archer TJ, Gobbe M, Johnson N. Accuracy and reproducibility of artemis central flap thickness and visual outcomes of LASIK with the Carl Zeiss Meditec VisuMax femtosecond laser and MEL 80 excimer laser platforms. J Refract Surg. 2010;26(2):107–119. doi: 10.3928/1081597X-20100121-06. [DOI] [PubMed] [Google Scholar]
- 24.Reinstein DZ, Archer T. Combined Artemis very high-frequency digital ultrasound-assisted transepithelial phototherapeutic keratectomy and wavefront-guided treatment following multiple corneal refractive procedures. J Cataract Refract Surg. 2006;32(11):1870–1876. doi: 10.1016/j.jcrs.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Reinstein DZ, Rothman RC, Couch DG, Archer TJ. Artemis very high-frequency digital ultrasound-guided repositioning of a free cap after laser in situ keratomileusis. J Cataract Refract Surg. 2006;32(11):1877–1882. doi: 10.1016/j.jcrs.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Reinstein DZ, Archer TJ. Evaluation of irregular astigmatism with Artemis very high-frequency digital ultrasound scanning. In: Wang M, editor. Irregular Astigmatism: Diagnosis and Treatment. Thorofare, NJ: SLACK Inc; 2007. pp. 29–42. [Google Scholar]
- 27.Reinstein DZ, Srivannaboon S, Gobbe M, Archer T, Silverman R, Sutton H, Coleman DJ. Epithelial thickness profile changes induced by myopic LASIK as measured by Artemis very high-frequency digital ultrasound. J Refract Surg. 2009;25(5):444–450. doi: 10.3928/1081597x-20090422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinstein DZ, Archer T, Gobbe M, Silverman R, Coleman DJ. Epithelial thickness after hyperopic LASIK: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. doi: 10.3928/1081597X-20091105-02. published online ahead of print November 24, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinstein DZ, Gobbe M, Archer TJ, Couch D, Bloom B. Epithelial, stromal, and corneal pachymetry changes during orthokeratology. Optom Vis Sci. 2009;86(8):E1006–E1014. doi: 10.1097/OPX.0b013e3181b18219. [DOI] [PubMed] [Google Scholar]
- 30.Reinstein DZ, Archer TJ, Silverman RH, Coleman DJ. Accuracy, repeatability, and reproducibility of Artemis very high-frequency digital ultrasound arc-scan lateral dimension measurements. J Cataract Refract Surg. 2006;32(11):1799–1802. doi: 10.1016/j.jcrs.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rondeau MJ, Barcsay G, Silverman RH, Reinstein DZ, Krishnamurthy R, Chabi A, Du T, Coleman DJ. Very high frequency ultrasound biometry of the anterior and posterior chamber diameter. J Refract Surg. 2004;20(5):454–464. doi: 10.3928/1081-597X-20040901-08. [DOI] [PubMed] [Google Scholar]
- 32.Lovisolo CF, Reinstein DZ. Phakic intraocular lenses. Surv Ophthalmol. 2005;50(6):549–587. doi: 10.1016/j.survophthal.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Reinstein DZ, Archer TJ, Silverman RH, Rondeau MJ, Coleman DJ. Correlation of anterior chamber angle and ciliary sulcus diameters with white-to-white corneal diameter in high myopes using Artemis VHF digital ultrasound. J Refract Surg. 2009;25(2):185–194. doi: 10.3928/1081597x-20090201-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinstein DZ, Archer TJ, Gobbe M. Corneal ablation depth readout of the MEL 80 excimer laser compared to Artemis three-dimensional very high-frequency digital ultrasound stromal measurements. J Refract Surg. doi: 10.3928/1081597X-20100114-02. published online ahead of print January 28, 2010. [DOI] [PubMed] [Google Scholar]
- 35.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24(6):571–581. doi: 10.3928/1081597X-20080601-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reinstein D, Archer T, Gobbe M, Silverman R, Coleman DJ. Stromal thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2009;25(9):776–786. doi: 10.3928/1081597X-20090813-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial, stromal and total corneal thickness in keratoconus: three-dimensional display with artemis very high-frequency digital ultrasound. J Refract Surg. 2010;26(4):259–271. doi: 10.3928/1081597X-20100218-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25(7):604–610. doi: 10.3928/1081597X-20090610-06. [DOI] [PubMed] [Google Scholar]
- 39.Reinstein DZ, Archer TJ, Gobbe M. Stability of LASIK in topographically suspect keratoconus confirmed non-keratoconic by Artemis VHF digital ultrasound epithelial thickness mapping: 1-year follow-up. J Refract Surg. 2009;25(7):569–577. doi: 10.3928/1081597X-20090610-02. [DOI] [PubMed] [Google Scholar]
- 40.Paul T, Lim M, Starr CE, Lloyd HO, Coleman DJ, Silverman RH. Central corneal thickness measured by the Orbscan II system, contact ultrasound pachymetry, and the Artemis 2 system. J Cataract Refract Surg. 2008;34(11):1906–1912. doi: 10.1016/j.jcrs.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Werner L, Lovisolo C, Chew J, Tetz M, Müller M. Meridional differences in internal dimensions of the anterior segment in human eyes evaluated with 2 imaging systems. J Cataract Refract Surg. 2008;34(7):1125–1132. doi: 10.1016/j.jcrs.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 42.Krueger RR, Dupps WJ., Jr Biomechanical effects of femtosecond and microkeratome-based flap creation: prospective contralateral examination of two patients. J Refract Surg. 2007;23(8):800–807. doi: 10.3928/1081-597X-20071001-10. [DOI] [PubMed] [Google Scholar]
- 43.Piñero DP, Plaza AB, Alió JL. Anterior segment biometry with 2 imaging technologies: very-high-frequency ultrasound scanning versus optical coherence tomography. J Cataract Refract Surg. 2008;34(1):95–102. doi: 10.1016/j.jcrs.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 44.Werner L, Chew J, Mamalis N. Experimental evaluation of ophthalmic devices and solutions using rabbit models. Vet Ophthalmol. 2006;9(5):281–291. doi: 10.1111/j.1463-5224.2006.00495.x. [DOI] [PubMed] [Google Scholar]
- 45.Coleman DJ, Woods S, Rondeau MJ, Silverman RH. Ophthalmic ultrasonography. Radiol Clin North Am. 1992;30(5):1105–1114. [PubMed] [Google Scholar]
- 46.Najafi DJ, Reinstein DZ, Silverman RH, Coleman DJ. An expert system for corneal layer three dimensional pachymetry. Invest Ophthalmol Vis Sci. 1997;38(Suppl):S920. [Google Scholar]
- 47.Segall M, Reinstein DZ, Johnson NF. Visualizing VHF ultrasound of the human cornea. IEEE Computer Graphics Applications. 1999;19(4):74–82. [Google Scholar]
- 48.Bland JM, Altman DG. Measurement error. BMJ. 1996;313(7059):744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bland JM, Altman DG. Measurement error proportional to the mean. BMJ. 1996;313(7049):106. doi: 10.1136/bmj.313.7049.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bland JM, Altman DG. Measurement error and correlation coefficients. BMJ. 1996;313(7048):41–42. doi: 10.1136/bmj.313.7048.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barkana Y, Gerber Y, Elbaz U, Schwartz S, Ken-Dror G, Avni I, Zadok D. Central corneal thickness measurement with the Pentacam Scheimpflug system, optical low-coherence reflectometry pachymeter, and ultrasound pachymetry. J Cataract Refract Surg. 2005;31(9):1729–1735. doi: 10.1016/j.jcrs.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 52.Mohamed S, Lee GK, Rao SK, Wong AL, Cheng AC, Li EY, Chi SC, Lam DS. Repeatability and reproducibility of pachymetric mapping with Visante anterior segment-optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48(12):5499–5504. doi: 10.1167/iovs.07-0591. [DOI] [PubMed] [Google Scholar]
- 53.Spadea L, Giammaria D, Di Genova L, Fiasca A. Comparison of optical low coherence reflectometry and ultrasound pachymetry in the measurement of central corneal thickness before and after photorefractive keratectomy. J Refract Surg. 2007;23(7):661–666. doi: 10.3928/1081-597X-20070901-04. [DOI] [PubMed] [Google Scholar]
- 54.Lackner B, Schmidinger G, Pieh S, Funovics MA, Skorpik C. Repeatability and reproducibility of central corneal thickness measurement with Pentacam, Orbscan, and ultrasound. Optom Vis Sci. 2005;82(10):892–899. doi: 10.1097/01.opx.0000180817.46312.0a. [DOI] [PubMed] [Google Scholar]
- 55.Fam HB, Lim KL, Reinstein DZ. Orbscan global pachymetry: analysis of repeated measures. Optom Vis Sci. 2005;82(12):1047–1053. doi: 10.1097/01.opx.0000192348.37026.09. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki S, Oshika T, Oki K, Sakabe I, Iwase A, Amano S, Araie M. Corneal thickness measurements: scanning-slit corneal topography and noncontact specular microscopy versus ultrasonic pachymetry. J Cataract Refract Surg. 2003;29(7):1313–1318. doi: 10.1016/s0886-3350(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 57.Wirbelauer C, Aurich H, Jaroszewski J, Hartmann C, Pham DT. Experimental evaluation of online optical coherence pachymetry for corneal refractive surgery. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):24–30. doi: 10.1007/s00417-003-0700-2. [DOI] [PubMed] [Google Scholar]
- 58.Muscat S, McKay N, Parks S, Kemp E, Keating D. Repeatability and reproducibility of corneal thickness measurements by optical coherence tomography. Invest Ophthalmol Vis Sci. 2002;43(6):1791–1795. [PubMed] [Google Scholar]
- 59.Wang JC, Bunce C, Lee HM. Intraoperative corneal thickness measurement using optical coherence pachymetry and corneo-gage plus ultrasound pachymetry. J Refract Surg. 2008;24(6):610–614. doi: 10.3928/1081597X-20080601-10. [DOI] [PubMed] [Google Scholar]
- 60.Zhao MH, Zou J, Wang WQ, Li J. Comparison of central corneal thickness as measured by non-contact specular microscopy and ultrasound pachymetry before and post LASIK. Clin Experiment Ophthalmol. 2007;35(9):818–823. doi: 10.1111/j.1442-9071.2007.01633.x. [DOI] [PubMed] [Google Scholar]
- 61.Jonuscheit S, Doughty MJ. Regional repeatability measures of corneal thickness: Orbscan II and ultrasound. Optom Vis Sci. 2007;84(1):52–58. doi: 10.1097/01.opx.0000254045.62252.b4. [DOI] [PubMed] [Google Scholar]
- 62.Li H, Leung CK, Wong L, Cheung CY, Pang CP, Weinreb RN, Lam DS. Comparative study of central corneal thickness measurement with slit-lamp optical coherence tomography and visante optical coherence tomography. Ophthalmology. 2008;115(5):796–801. e2. doi: 10.1016/j.ophtha.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Sin S, Simpson TL. The repeatability of corneal and corneal epithelial thickness measurements using optical coherence tomography. Optom Vis Sci. 2006;83(6):360–365. doi: 10.1097/01.opx.0000221388.26031.23. [DOI] [PubMed] [Google Scholar]
- 64.Wirbelauer C, Pham DT. Monitoring corneal structures with slitlamp-adapted optical coherence tomography in laser in situ keratomileusis. J Cataract Refract Surg. 2004;30(9):1851–1860. doi: 10.1016/j.jcrs.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 65.Carl Zeiss Meditec. Visante OCT User's Manual, 2006
- 66.Sacu S, Findl O, Buehl W, Kiss B, Gleiss A, Drexler W. Optical biometry of the anterior eye segment: interexaminer and intraexaminer reliability of ACMaster. J Cataract Refract Surg. 2005;31(12):2334–2339. doi: 10.1016/j.jcrs.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 67.Silverman RH, Patel MS, Gal O, Sarup A, Deobhakta A, Dababneh H, Reinstein DZ, Feleppa EJ, Coleman DJ. Effect of corneal hydration on ultrasound velocity and backscatter. Ultrasound Med Biol. 2009;35(5):839–846. doi: 10.1016/j.ultrasmedbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Myers SL, Dines K, Brandt DA, Brandt KD, Albrecht ME. Experimental assessment by high frequency ultrasound of articular cartilage thickness and osteoarthritic changes. J Rheumatol. 1995;22(1):109–116. [PubMed] [Google Scholar]
- 69.Wang J, Thomas J, Cox I, Rollins A. Noncontact measurements of central corneal epithelial and flap thickness after laser in situ keratomileusis. Invest Ophthalmol Vis Sci. 2004;45(6):1812–1816. doi: 10.1167/iovs.03-1088. [DOI] [PubMed] [Google Scholar]
- 70.Erie JC, Patel SV, McLaren JW, Ramirez M, Hodge DO, Maguire LJ, Bourne WM. Effect of myopic laser in situ keratomileusis on epithelial and stromal thickness: a confocal microscopy study. Ophthalmology. 2002;109(8):1447–1452. doi: 10.1016/s0161-6420(02)01106-5. [DOI] [PubMed] [Google Scholar]
- 71.Erie JC, Hodge DO, Bourne WM. Confocal microscopy evaluation of stromal ablation depth after myopic laser in situ keratomileusis and photorefractive keratectomy. J Cataract Refract Surg. 2004;30(2):321–325. doi: 10.1016/j.jcrs.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 72.Hu MY, McCulley JP, Cavanagh HD, Bowman RW, Verity SM, Mootha VV, Petroll WM. Comparison of the corneal response to laser in situ keratomileusis with flap creation using the FS15 and FS30 femtosecond lasers: clinical and confocal microscopy findings. J Cataract Refract Surg. 2007;33(4):673–681. doi: 10.1016/j.jcrs.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 73.Maldonado MJ, Ruiz-Oblitas L, Munuera JM, Aliseda D, García-Layana A, Moreno-Montañés J. Optical coherence tomography evaluation of the corneal cap and stromal bed features after laser in situ keratomileusis for high myopia and astigmatism. Ophthalmology. 2000;107(1):81–87. doi: 10.1016/s0161-6420(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 74.Li Y, Netto MV, Shekhar R, Krueger RR, Huang D. A longitudinal study of LASIK flap and stromal thickness with high-speed optical coherence tomography. Ophthalmology. 2007;114(6):1124–1132. doi: 10.1016/j.ophtha.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 75.Thompson RW, Jr, Choi DM, Price MO, Potrezbowski L, Price FW., Jr Noncontact optical coherence tomography for measurement of corneal flap and residual stromal bed thickness after laser in situ keratomileusis. J Refract Surg. 2003;19(5):507–515. doi: 10.3928/1081-597X-20030901-05. [DOI] [PubMed] [Google Scholar]
- 76.Stahl JE, Durrie DS, Schwendeman FJ, Boghossian AJ. Anterior segment OCT analysis of thin IntraLase femtosecond flaps. J Refract Surg. 2007;23(6):555–558. doi: 10.3928/1081-597X-20070601-03. [DOI] [PubMed] [Google Scholar]
- 77.Peters NT. OCT analysis of flap thickness. J Refract Surg. 2008;24(2):117–119. doi: 10.3928/1081597X-20080201-01. [DOI] [PubMed] [Google Scholar]
- 78.Izquierdo L, Jr, Henriquez MA, Zakrzewski PA. Detection of an abnormally thick LASIK flap with anterior segment OCT imaging prior to planned LASIK retreatment surgery. J Refract Surg. 2008;24(2):197–199. doi: 10.3928/1081597X-20080201-11. [DOI] [PubMed] [Google Scholar]
- 79.Cheng AC, Ho T, Lau S, Wong AL, Leung C, Lam DS. Measurement of LASIK flap thickness with anterior segment optical coherence tomography. J Refract Surg. 2008;24(9):879–884. doi: 10.3928/1081597X-20081101-05. [DOI] [PubMed] [Google Scholar]
- 80.Gokmen F, Jester JV, Petroll WM, McCulley JP, Cavanagh HD. In vivo confocal microscopy through-focusing to measure corneal flap thickness after laser in situ keratomileusis. J Cataract Refract Surg. 2002;28(6):962–970. doi: 10.1016/s0886-3350(02)01275-0. [DOI] [PubMed] [Google Scholar]
- 81.Javaloy Estañ J, Vidal MT, Quinto A, De Rojas V, Alió JL. Quality assessment model of 3 different microkeratomes through confocal microscopy. J Cataract Refract Surg. 2004;30(6):1300–1309. doi: 10.1016/j.jcrs.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 82.Ramirez M, Hernandez-Quintela E, Naranjo-Tackman R. A comparative confocal microscopy analysis after LASIK with the IntraLase femtosecond laser vs Hansatome microkeratome. J Refract Surg. 2007;23(3):305–307. doi: 10.3928/1081-597X-20070301-15. [DOI] [PubMed] [Google Scholar]
- 83.Patel SV, Maguire LJ, McLaren JW, Hodge DO, Bourne WM. Femtosecond laser versus mechanical microkeratome for LASIK: a randomized controlled study. Ophthalmology. 2007;114(8):1482–1490. doi: 10.1016/j.ophtha.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 84.Ley B. Diameter of a human hair. Elert G, editor. [Accessed December 2008];The Physics Factbook. 1999 Available at: http://hypertextbook.com/facts/1999/BrianLey.shtml.
- 85.Baikoff G. Imaging techniques of anterior segment of the eye: applications of OCT in refractive surgery; Presented at: XXII Congress of the European Society of Cataract and Refractive Surgery; September 18-22, 2004; Paris, France. [Google Scholar]
- 86.Reinstein DZ, Archer TJ, Gobbe M. Combined corneal topography and corneal wavefront data in the treatment of corneal irregularity and refractive error in LASIK or PRK using the Carl Zeiss Meditec MEL80 and CRS Master. J Refract Surg. 2009;25(6):503–515. doi: 10.3928/1081597X-20090512-04. [DOI] [PubMed] [Google Scholar]
- 87.Reinstein DZ, Archer TJ, Couch D, Schroeder E, Wottke M. A new night vision disturbances parameter and contrast sensitivity as indicators of success in wavefront-guided enhancement. J Refract Surg. 2005;21(5):S535–S540. doi: 10.3928/1081-597X-20050901-23. [DOI] [PubMed] [Google Scholar]


