Abstract
Cardiovascular disease is a leading cause of death worldwide, necessitating the development of effective treatment strategies. A myocardial infarction involves the blockage of a coronary artery leading to depletion of nutrient and oxygen supply to cardiomyocytes and massive cell death in a region of the myocardium. Cardiac tissue engineering is the growth of functional cardiac tissue in vitro on biomaterial scaffolds for regenerative medicine application. This strategy relies on the optimization of the complex relationship between cell networks and biomaterial properties. In this review, we discuss important biomaterial properties for cardiac tissue engineering applications, such as elasticity, degradation, and induced host response, and their relationship to engineered cardiac cell environments. With these properties in mind, we also emphasize in vitro use of cardiac tissues for high-throughput drug screening and disease modelling.
1 Introduction
Cardiovascular health issues, including myocardial infarctions (MI), cause over 2.5 million deaths in the United States each year [1]. An MI involves the partial or complete blockage of a coronary artery, inducing cardiomyocyte death [2–4]. The healing process post-MI involves an inflammatory response, the removal of dead cardiomyocytes (CMs), and rigid non-contractile scar tissue formation [5]. Pathological dilation of the afflicted ventricle results in adverse cardiac remodelling and inefficient pumping. This causes permanent impairment to the patient, and without treatment over 50% of patients experience heart failure after five years [6]. Current treatments focus on left ventricular assist device (LVAD) implantation or heart transplantation, which are limited by a number of factors including the number of available donors and host immune rejection.
Tissue engineering uses living cells to help maintain, improve, or enhance the function of human organs and tissues [2, 7, 8]. Biomaterial scaffolds are used to support and direct the growth of engineered tissues comprised of expanded populations of human cells in vitro. Cardiac tissue engineering (CTE) uses these strategies to assess, maintain, or improve human cardiac tissue [2, 9]. Although reconstructing an entire functional human organ in vitro remains to be achieved, the field continues to progress and will reduce the need for invasive organ transplantation and assist clinicians in the goal of improving human quality of life [2, 7, 8]. CTE requires the precise control over the nano-, micro- and macro-scale tissue structure using biomaterials, cells, and bioreactors. CTE has also been used as a tool for increasing knowledge in the fields of developmental and cell biology, and in vitro cardiotoxicity drug screening [5, 10].
This review discusses the important role of biomaterials in CTE, and notable applications of engineered cardiac tissues. We highlight key properties of biomaterials used for CTE scaffolds, their advantages and limitations. Furthermore, cell populations and cardiac tissue maturation used in CTE are outlined. Emphasis will be placed on these solutions in the context of applications, most notably the use of CTE in drug discovery.
2 Biomaterials for cardiac tissue engineering
The wide range of physical properties in different human tissues necessitates a variety of polymers within the field of tissue engineering [11]. Polymers can be derived from natural sources or produced synthetically; initial work with polymer biomaterials involved the implantation of natural polymers, such as collagen [5, 6, 12]. Synthetic components are an attractive alternative to biologically derived polymers. With use of biologically derived polymers, chemical modifications are both difficult and often change the bulk properties of the material [4, 13, 14]. A high degree of product variability is also of major concern with the use of biologically derived polymers.
In CTE, biomaterials serve predominately as scaffolds for tissue formation and vehicles for the delivery of engineered tissues [3, 5–7, 12, 15]. Scaffolds for CTE require a number of criteria to be carefully considered to allow for optimal tissue function including: physical properties of the polymer (e.g. strength and elasticity), degradation rates, and host response [6]. Furthermore, these properties help to dictate the body’s elicited immune response.
To satisfy the dynamic nature of heart function and myocardial remodelling post-MI, the ideal cardiac biomaterial should account for several design parameters. Matching the mechanical properties of the myocardium is an important cardiac biomaterial property [16]. The Young’s modulus of the adult human myocardium ranges non-linearly from 10–20kPa (start of diastole) to 200–500kPa (end of diastole) [17–20]. A rigid and inelastic patch placed on the heart will impede contraction. A scaffold should not be constructed too soft as pathological cardiac dilation can be reduced by mechanically reinforcing the myocardium [21]. In addition, materials capable of achieving tissue-like compliance (e.g. hydrogels) must allow for easy handling/suturing. An ideal biomaterial should also comply with the changes in strain experienced by the myocardium of approximately ±15% [22–24]. The anisotropic (directionally dependent) stiffness of the heart tissue [25–29] is an important design parameter that the scaffold should replicate. Mathematical modelling and in vivo experiments on dogs demonstrated that the patches with anisotropic mechanical properties (Dacron with slits), placed onto an infarcted myocardium resulted in improved functionality compared to isotropic patches [30, 31]. The cyclic contraction of the myocardium necessitates a biomaterial that is elastomeric. The patch should biodegrade over the desired period, matching the remodelling process of the heart post MI, that usually takes 6–8 weeks, to avoid fibrous capsule formation and a chronic inflammatory response in the myocardium. Ensuring that the material degrades at the same rate as the heart heals is crucial for success [32].
Comprehending the immune response to an implanted biomaterial is required to determine the clinical relevance. The immediate response of the host to implantation of a foreign material is known as the tissue response [4, 8, 33, 34]. Upon implantation, host proteins adhere to the implanted biomaterial surface. These proteins signal monocytes and other leukocytes to begin the foreign body cascade [4, 35, 36]. Macrophage recruitment and differentiation dictates the formation of foreign body giant cells and fibrous capsule formation [4, 35, 36]. The way a biomaterial influences macrophage polarization has been found to influence the host immune response to implanted devices significantly. Specifically, M1 macrophages induce an inflammatory response in the body, while M2 macrophages encourage tissue repair [4, 35, 36].
Material design looks to minimize the inflammatory and immune response as well as the fibrous capsule formation. Formation of a fibrous capsule inhibits the efficacy of a tissue scaffold, as cells are not able to integrate and interact with the surrounding cells, and the apparent stiffness of the material is increased [2]. Furthermore, this limits vascularization, which is important as it facilitates growth through delivery of blood, as well as ensures the viability of exogenously applied cells [37]. Many designs of cardiac biomaterials aim to encourage the polarization of macrophages to the M2 phenotype, in order to minimize fibrous capsule formation and promote incorporation into host tissue [4, 35, 38]. Minimization of fibrous capsule formation, as well as reduction in the foreign body reaction and chronic inflammation, are considered benchmarks to biocompatibility assessment [5, 8, 33, 39].
Finally, selecting an appropriate animal model to assess the effectiveness of the designed therapy based on cardiac tissue engineering remains critical. A number of small rodent cardiac infarct models have been examined to assess the ability of engineered tissues to treat cardiac diseases [38, 40–43]. Coronary artery ligation and cryo-injury to the ventricle wall are common methods employed to induce MI in animal models [38, 42–44]. Cell seeded scaffolds have proven to successfully improve left ventricle function in late stages of cardiac failure [40, 42, 44], however cell injection therapies have also found success in reducing myocardial damage after acute MI in rat models [45]. Serious considerations must be taken when deciding when to administer treatments, as incorrectly timed administration could potentially exacerbate the existing condition [6], despite of how well-designed the biomaterial used for the therapeutic approach is. Application of a material in early stages of remodelling can improve healing but potentially increase inflammatory response, where as treatment following the initial immune response allows for the development of scar tissue [6]. In vivo studies can be performed on a variety of immuno-compromised animal species, in cases where human pluripotent stem cells (hPSCs) or embryonic stem cells (hESC) are used as a source of CMs, either seeded on the biomaterial scaffold or injected with it. One example is athymic rats that lack adaptive immunity [46].
Rodent models have also been used to study integration of hESC derived CMs in the myocardium [47–49], although they have been scrutinized as unsuitable due to the large difference in heart rates between human CMs (60–120bpm) and rodent ventricular myocytes (350–600bpm). Shiba and colleagues have recently used guinea pigs as a more suitable model (200–250bpm) to demonstrate conclusively that hESC-CMs can electrically couple and minimize arrhythmias in cryo-induced MI hearts [50]. However, in the most recent non-human primate model, transplantation of hESC derived CM resulted in transient arrhythmia [51]. Below, we will highlight the most common natural and synthetic biomaterials used for cardiac tissue engineering in vitro and in vivo.
2.1 Naturally derived biomaterials
Natural biomaterials include polymers that are derived from biological sources. In CTE the most notable materials are collagen and gelatin (derived from mammalian extracellular matrix (ECM)) and alginate [52]. ECM derived materials were amongst the first to be used in tissue engineering due to the composition and structural properties similar to the in vivo tissues and organs [53]. When solubilized into individual components from an ECM structure and without postprocessing, collagen and gelatin materials do not exhibit sufficient mechanical properties for the formation of scaffolds, limiting their applications to injectable formulations when used as a homopolymer [6]. Zimmerman et al demonstrated for the first time that an engineered heart tissue (EHT) implanted onto infarcted rat hearts can halt the deterioration of the left ventricle after MI, restore cardiac function, and promote neovascularization (Figure 1B) [40]. They seeded neonatal rat heart cells into a collagen hydrogel, conditioned the constructs with mechanical stimulation and implanted them 14 days post MI onto the epicardial surface of syngeneic rats.
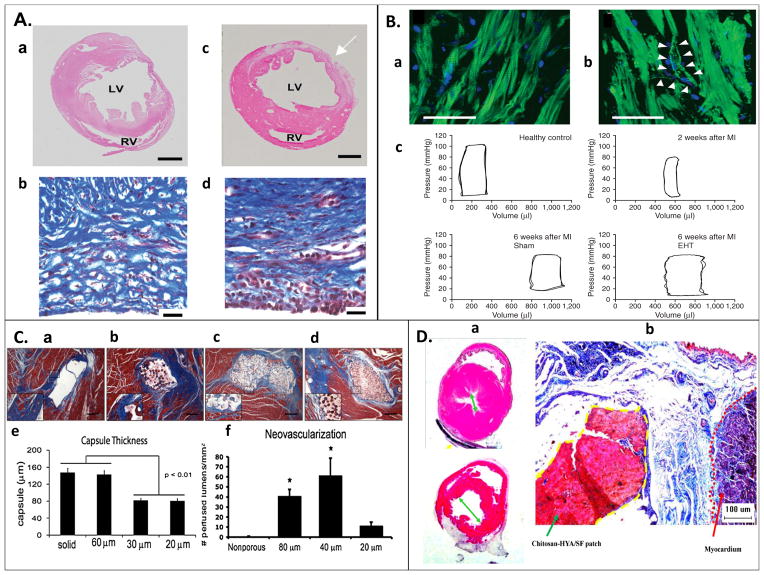
Figure 1. Examples of in vivo studies.
(A) Sections of left ventricle 3 weeks post MI (a) without and (b) with CDC graft, suggesting improved tissue repair with CDC graft. Masson’s Trichome staining of heart wall indicating collagen deposition (blue), and viable muscle cells (red). (d) Specimens with implanted grafts indicated more aligned collagen deposition and higher percentage of surviving muscle cells than (c) non-implanted specimens [43]. (B) (a) Sarcomeric organization in engineered heart tissue (EHT) four weeks post-implantation, stained for actin filaments (green) and nulcei (blue). (b) Putative blood vessel formation observed, with both endothelial and smooth muscle cells. (c) Pressure volume loop of healthy, infarcted, and grafted rat hearts suggest, hearts implanted with an EHT showed less left ventricle dilation and more similar function to normal hearts six weeks post-MI [40]. (C) Poly(2-hydroxyethyl methacrylate-co-methacrylic acid) hydrogel patch surrounded by collagen capsule (blue) and host myocardium (red) of a nude rat at 28 days after patch implantation. (a) Nonporous and (b) 60μm porous constructs had thicker and denser fibrous capsule than constructs with (c) 30μm and (d) 20μm pores, (e) this thickness was quantified. Neovascularization was more prevalent in the (f) 40μm pore construct, quantified by the presence of rat endothelial cell marker (RECA-1+) on lumen structures [38]. (D) (a) Hematoxylin and eosin stain of heart post MI (b) treated with chitosan-hyaluronan/silk fibronin patch compared to a post MI control presents reduction of left ventricle dilation with the patch treatment. (b) Masson’s Trichome stained heart wall at infarct zone; the dotted yellow line indicates the patch, and the dotted red line indicates the myocardium. The patch adhered strongly to the epicardial surface, and reduced fibrosis in the infarct zone [44].
The use of natural materials has been expanded through incorporation into co-polymer structures with synthetic biomaterials, such as polycapralactone and poly-L-lactic acid [53], or other natural biomaterials such as chitosan for improvement of scaffold or hydrogel properties [54]. Chitosan-hyaluronan/silk fibronin patches have been successfully implanted onto the hearts of infarcted rats and helped reduce the left ventricle diameter post MI, maintain ventricular wall thickness and enhance fractional shortening 8 weeks post-MI compared to the MI only controls, while eliciting a minimal immune response (Figure 1D) [44].
Alginate is a biopolymer derived from brown algae that has been applied in many tissue engineering applications due to its simple extraction procedure and abundance [11]. Although the poor mechanical properties of alginate limit its use, it has often been incorporated into scaffold co-polymers and injectables to tune their application in a similar manner to collagen and gelatin [11, 55]. Landa et al used alginate hydrogels to develop support for cardiac repair, relying on high concentrations of calcium in the ECM to cause alginate gelation, demonstrating maintenance of ventricular dimensions and wall thickness, as well as preservation of fractional shortening compared to MI-only controls. [56].
Scaffolds and hydrogels obtained from decellularized organs have been applied in cardiac tissue engineering as aspects of their molecular domains are utilized for cell attachment, including maintained scaffold architecture [4, 57]. However, the procedure of decellularization is complicated, and ECM composition changes while reduction in biological activity may often be observed with proteolytic digestion [58]. Through use of detergents, Ott et al showed the ability to maintain the substrate architecture when removing dead cellular content and developing new cardiac tissue in vitro, demonstrating a small degree of pump function (~2% of native) 8 days after seeding of neonatal rat CMs onto the decellularized rat heart ECM [59]. Singelyn et al used decellularized porcine myocardium as an injectable gel scaffold, resulting in the smooth muscle and endothelial cell migration in vivo and in turn the potential for cardiac regeneration applications [60]. Similarly, Sarig et al used a novel technique for the decellularlization of thick porcine myocardium ECM, which served as a scaffold for seeded mesenchymal stem cells (MSC) [61]. Furthermore, Sief-Naraghi et al injected porcine ECM-derived hydrogels into the hearts of late-stage infarcted pigs (2 weeks post MI); three months after implantation, cardiac function, ventricular volumes, and global wall movement increased compared to the control specimens [62].
Although these methods present an effective solution to the biomaterial requirement of CTE, the use of natural materials constrains the adaptability of material properties. Natural materials can allow for a reduced host response, however synthetic materials allow for tuneable mechanical properties [14].
2.2 Synthetic biomaterials - polyesters
Synthetic biomaterials provide an attractive alternative to natural materials. With the ability to control the entire synthesis procedure, the mechanical properties, topography and structure of a material can be modified to reflect the specifics of an application. These materials can be classified on a high level by their ability to biodegrade. In vitro applications rely on non-degradable materials to interact with cells and tissues, providing the desired mechanical properties without cytotoxic effects [6]. For in vivo applications of CTE, biodegradable polymers are preferred to allow for the engineered tissue to integrate and minimize adverse host response. This aspect of review focuses on polyesters, well-researched degradable biomaterials, and their important properties when considering them for CTE applications. The importance of non-degradable materials is discussed in conjunction with in vitro applications.
Polyesters are the most researched type of synthetic biomaterial to date, partly because they present range of mechanical diversity [11, 63]. The most notable polyesters are FDA-approved polycapralactone, poly-L-lactic acid, and poly(lactic-co-glycolic acid), which have been applied in many tissue engineering applications [2, 6, 8, 12–14, 33, 64]. They are used in different forms, often mixed into co-polymers to allow for the control of degradation rate [6]. These materials often serve as a benchmark when studying the characteristics of new materials [6, 39, 65].
Polyesters degrade mainly through hydrolysis making their degradation largely dependent on properties that dictate water penetration [33]. In vitro polyester degradation studies are preformed under conditions of excess water and reveal how readily the substance undergoes polymer chain scission through hydrolysis. Differences in the rates of hydrolysis are related to the linkages within the polymer chains, and their respective susceptibility to hydrolysis. Polyesters undergo hydrolysis due to susceptibility of the ester linkage to nucleophilic attack [11]. The degradation is autocatalyzed by the formation of carboxylic end groups, increasing acidity of the surrounding solution and increasing the strength of water as a nucleophile (Figure 2) [33, 66].
Figure 2. Mechanism of hydrolysis for polyester polymer backbone.
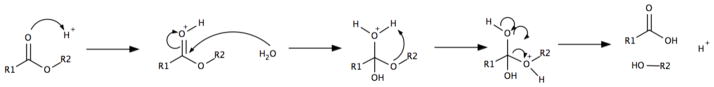
Water acts as a nucleophile, attacking and cleaving the ester linkage in the polymer backbone. Degradation is accelerated under acidic conditions.
When placed in vivo, the degradation properties can change significantly depending on a variety of factors including water penetration rate, scaffold geometry, susceptibility to hydrolysis and specific body conditions [11, 33, 64]. Ideally, polymers for cardiac cell delivery should survive in vivo for at least one week and fully degrade by eight weeks (in rat models) to allow for cells to have initial support as well as proper integration into the host tissue [6]. This time-frame is additionally motivated by the fact that it takes approximately six weeks (in rat models) for the remodelling process to complete upon an MI. Future application in human clinical trails would necessitate adaptation in degradation properties, as the remodelling process takes place over a three month period [67].
As the majority of polyester materials degrade primarily through bulk erosion, scaffolds hold their shape until a critical point before breaking apart. This is attributed to uniform degradation throughout the material. Some applications desire degradation through surface erosion, where the material is broken down from the outside in, while maintaining many of its physical properties with interior polymer chains shielded from degradation. This is characteristic of enzyme-assisted degradation, which is common with materials such as poly(1,3-trimethylene carbonate) [68]. One of the most cited drawbacks of polyester degradation is the creation of an acidic environment locally that might influence host response [69]. As polyester science continues to develop, new materials have been tailored to adapt these degradation properties. Poly(glycerol sebacate) presented a degradation profile that suggests surface erosion, improving its in vivo applicability [70]. Materials often combine polyester linkages with others’ hydrolytically stable linkages, which present different degradation properties in an effort to alter the rate and characteristics of polymer breakdown [71].
The use of synthetic materials has been widespread in CTE applications. Wagner and colleagues developed a synthetic hydrogel copolymer (N-isopropyla-crylamide, 2-hydroxyethyl methacrylate, and degradable methacrylate polylactide) loaded with basic fibroblast growth factor (bFGF) and insulin-like growth factor 1 (IGF1) that both reduced left ventricle dilation post MI and encouraged M2 macrophage recruitment in infarcted rat hearts when implanted 2 weeks post infarct [72]. Kim et al successfully implanted a nanopatterned polyethylene glycol based hydrogel matrix seeded with cardiosphere-derived cells (CDCs) onto infarcted rat hearts. Twenty-one days after engraftment they observed significant tissue repair in both the cell-seeded and pure scaffold samples, as well as more organized deposition of collagen and high myocardial cell survival (Figure 1A) [43]. Furthermore, Matsubayashi et al developed a porous poly(ε-caprolactone-co-L-lactide) cardiac patch seeded with vascular smooth muscle cells for left-ventricular aneurysm repair, designed to prevent ventricular dilation post MI [42]. Patches were surgically implanted four weeks after left anterior descending coronary artery (LAD) ligation in syngeneic rats, using a modified endoventricular circular patch plasty (EVCPP) repair of the infarct area. Eight weeks post-implantation they observed the patch had integrated with host tissue, and that systolic function (developed pressure) increased significantly in hearts grafted with cell-seeded patches compared to control MI groups and the cell-free patches [42].
Recent research has focused on decreasing immune response through the design of modified polymers, referred to as immunomodulating biomaterials [4]. Using surface modification and incorporation of biologically active molecules, such as growth factors and peptides, undesired protein adhesion can be prevented, and cellular adhesion can be promoted [4]. Furthermore, these factors can be used to encourage vascularization, and incorporation of the scaffold into the native tissue [6, 7, 37]. Similarly, microscopic physical adaptation to scaffold topology has been shown to improve cellular adhesion and vascularization [6, 12, 73]. These methods improve biocompatibility without compromising desirable physical properties. For example, porous poly(2-hydroxyethyl methacrylate-co-methacrylic acid) hydrogel scaffolds mimicking the structure and organization of native myocardium have been fabricated to deliver CMs to diseased rat hearts (Figure 1C) [38]. The effect of pore size on fibrous encapsulation and neovascularization was investigated. In vivo results indicated the ability to control inflammatory response through the scaffold architecture and induce prohealing pathways with porosity, while maximizing neovascularization and minimizing fibrosis. This work also enabled the design of cardiac scaffolds that encouraged CM alignment and developed cell density similar to the native tissue [38].
The advancement in the field of CTE with synthetic materials has been considerable, but further work is needed to design a material for the appropriate properties for specific applications. Limitations in these popular materials have been related to their elastomeric, degradation, and biocompatibility properties highlighting the importance of appropriately tuning these properties in the development of new biomaterials.
3 Cell environment in engineered cardiac tissue
CTE involves a complex relationship between biomaterial scaffolds and cell populations. CMs, the contractile cells of cardiac cell matrix, operate in unison to create a powerful contractile force to pump blood throughout the body. As discussed previously, the properties of biomaterials dictate the efficacy of a scaffold in supporting the growth and coupling of a cardiac cell network. This section of the review will discuss the important considerations on a cellular level when developing engineered cardiac tissue.
3.1 Cell sources
As human CMs do not regenerate within the post-natal myocardium, in vitro development must rely on differentiation from pluripotent stem cell sources. We will not discuss all cell sources for CTE in detail, as they have been reviewed extensively [15, 52, 74, 75]. The majority of work outlined in this review focuses on the use of hPSCs as a starting point for cell regeneration [74]. These cells can generally be categorized into hESCs or human induced pluripotent stem cells (hiPSC) and they can give rise to millions of contractile CMs [76]. hiPSCs are of particular interest as they can be derived from human somatic cells, improving their availability and minimizing ethical considerations [77]. In CTE applications, hPSCs are differentiated into CMs for generation of functional cardiac tissue in vitro [74].
Many clinical trials in the area of cardiac cell replacement have focused on the use of bone marrow MSCs, peripheral blood mononuclear cells, or resident cardiac cells. These cell types have not shown the ability to provide a replacement for CMs, by e.g. trans-differentiation [78], but instead to improve heart function post-MI through paracrine effects. The relative simplicity of this treatment strategy has led to expansive clinical trials, but recent work has brought into question the efficacy of this method. Meta-analysis of clinical trials that employed intracoronary cell injection post-acute MI presented a small increase (3%) in left ventricular ejection fraction (LVEF), decrease in infarct size (−5.6%) as well as end systolic volume (−7.4mL) [79]. Overall, it is difficult to conclude the degree of functional improvement of such treatments due to the discrepancies in clinical trials [80, 81].
Direct transdifferentiation of fibroblasts to CMs has also been attempted by transfection of cardiac transcription factors. In work by Srivastava and colleagues, delivery of Gata4, Mef2c and Tbx5 through viral infection in vivo to murine cardiac fibroblasts post-MI presented CM-like cells three days post-infection[82]. The ability to reprogram murine fibroblasts derived from adult mouse tail-tip to express cardiac genes through the application of GATAH, HAND2, MEC2C and TBX5 has also been demonstrated in vitro [83]. Transfection of these factors in vivo post-MI into non-CM dividing cells of the myocardium resulted in a decrease of the pathological remodelling [83]. This work has been further applied to human foreskin fibroblast cells, by application of four human cardiac transcription factors, including GATA binding protein 4, Hand2, T-box5, myocardin, and two microRNAs, miR-1 andmiR-133, resulting in human CM-like cells in vitro starting from human fibroblasts, as verified through cardiac gene expression [84]. This group continues to work to simplify the approach, improving its reproducibility through the development of a standard protocol for in vitro transdifferentiation [85].
3.2 Multiple cell populations
Native tissues are comprised of multiple cell types. Therefore, the presence of different cell types found in a tissue is essential for recreating a realistic engineered tissue microenvironment. In order to engineer accurate cardiac models, the native myocardium structure and composition serves as a template. The native human myocardium is comprised of multiple cells types including CMs (atrial and ventricular), cardiac fibroblasts, smooth muscle cells, endothelial cells, pacemaker cells, epicardial cells, endocardial cells, and Purkinje cells. Although contractile force is primarily generated by CMs, support from non-myocytes (primarily cardiac fibroblasts and ECs) is critical for proper function.
Nearly every CM bundle in the myocardium is located adjacent to a capillary[86] and EC-CM cross-talk is well known as obligatory in cardiac development and function[87, 88]. Three-dimensional (3D) co-culture of ECs and CMs improved the spatial organization, function, and survival[89]. Engineered cardiac tissue cultured on an in vitro bed of vasculature yielded improved electrical properties and tissue structure compared to cardiac tissue grown with no pre-vasculature [90].
The role of cardiac fibroblasts in maintaining proper cardiac function is multifaceted. Cardiac fibroblasts deposit ECM [91], assist in the mechanical transfer of contraction to the ECM, release paracrine signals, and facilitate electro-mechanical coupling of the myocardium [92, 93]. Cardiac fibroblasts are also central to many cardiac pathologies [92]. The presence of a 3D ECM is critical for the creation of a cellular niche. Iyer et al showed that conditioning the microenvironment with the presence of both ECs and NIH 3T3 fibroblasts or applying their conditioned media resulted in cardiac organoids with improved contractility, organization, and survival [94]. Further work revealed that Connexin-43 (Cx-43) levels in engineered cardiac tissues were enhanced by the presence of vascular endothelial growth factor secreting fibroblasts and ECs [95]. However, the ratio of cell types is important because the simultaneous culture of ECs, fibroblasts, and CMs resulted in non-contractile cardiac organoids. It was also demonstrated that the optimal ratio of NKX2.5+CMs to Cluster of Differentiation (CD) 90+ nonmyocytes was 3:1 in formation of hESC-based contractile cardiac microtissues [96]. Cultivating the two cell types in the described ratio improved the tissue remodelling and spatial homogeneity of contractile proteins resulting in enhanced function, and increased the expression of maturation-associated cardiac genes compared to the other tested ratios. Increased integrin expression and paracrine signalling from the non-myocytes was responsible for the improved tissue assembly. The highly organized spatial arrangement of these cells types in myocardial tissue is crucial for proper function. Thus, engineered cardiac models should also aim to reproduce a similar level of tissue organization.
3.3 Tissue formation strategies
Serving as models to discover novel therapeutics or drug toxicities in vitro, single CMs cultured in vitro have been well-characterized by various methods, such as immunostaining and patch-clamping [97–99]. However, single CMs are not necessarily an appropriate model for all toxicology studies, as they cannot directly mimic synchronous contractions of a syncytium. Therefore, two-dimensional (2D) and 3D tissue constructs have been developed to better recapitulate the higher functions of tissues containing a multitude of CMs.
Cell-cell coupling by gap junctions, plays an important role in the action potential propagation in a cardiac tissue. Moreover, the alignment and cell-cell interaction in the monolayer could be controlled by micro-patterning methods. Karp et al. patterned photocrosslinkable chitosan on glass and tissue culture polystyrene to create cell-repellent regions. The CMs on the cell-adhesive regions were able to beat spontaneously after one week [100]. Badie et al cultured CMs following micro- and macroscopic guidance of fibronectin coating and recapitulated the directions of native cardiac fibres as characterized by high-resolution diffusion tensor magnetic resonance imaging [101]. Microelectrode arrays (MEA) have been developed to culture CM monolayers and provide insight on cardiac field potentials by impedance measurements [102–104]. Natarajan et al created CM monolayers aligned with MEAs by patterning fibronectin to guide cellular attachment thus controlling action potential propagation [105] (Figure 3A). Creating topographical cues on culture substrates guides the alignment of CMs, which results in the desired characteristics of anisotropy and improved physiological properties [106, 107]. Contractile CM thin films on elastomers have also been produced to measure contractile forces combined with a quantification of action potential propagation (Figure 3B) [108].
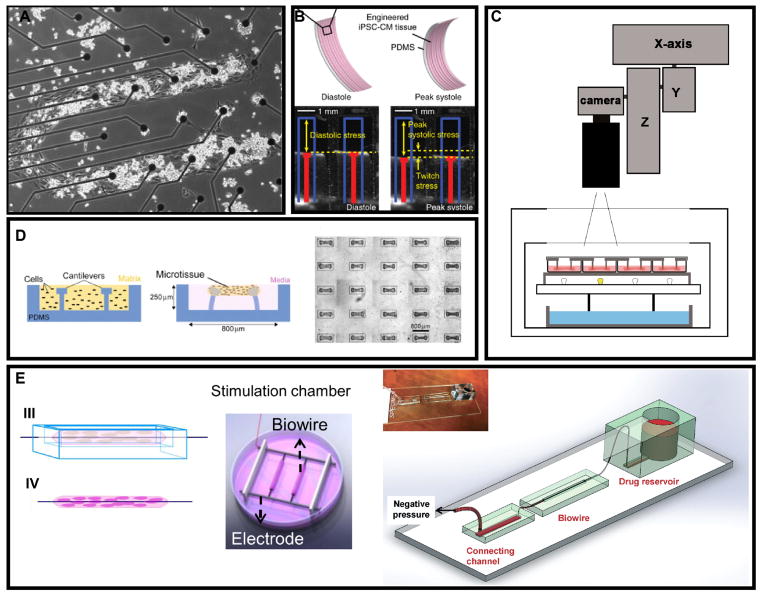
Figure 3. Strategies for generating 2D and 3D cardiac tissue in vitro.
(A) CM monolayers cultured on patterned MEA for guided action potential propagation [105]. (B) CM monolayers cultured on flexible elastomer films for contractile force measurement [108]. (C) High-throughput platform for monitoring contractile activity of an array of fibrin-based engineered heart tissues [113]. (D) Cardiac micro-tissues around micro-cantilevers to measure contractile properties [114]. (E) Cardiac biowire set-up with a suspended template to guide tissue formation and cell alignment. The phenotype of the CM was matured under external electrical stimulation and the drug candidates were applied by perfusion through micro-tubing within the biowire, providing improved physiological relevance [115].
Native cardiac tissue has a 3D structure that works in unison to generate force against a load. This structure is replete with ECM and cellular interactions on all geometric faces of CMs, which 2D substrates cannot easily replicate. 3D scaffolds made of natural or synthetic polymers can create an environment, including appropriate topographical cues and stiffness, on which cardiac cells can be seeded and cultivated to make dense cardiac tissue such that the biophysical properties comparable to the native myocardium are achieved [109]. More recently, technologies including stereolithography and femtosecond laser scanning have been applied to precisely control the geometry and structure of scaffolds. For example, Ma et al seeded CMs derived from long QT syndrome type 3 (LQT3) IPS-CMs onto synthetic filamentous matrices fabricated by femtosecond laser and studied the cardiac contractility in response to a panel of drugs [110].
The use of gel-compaction method is exemplified in self-assembled cardiac tissue construction where increased contraction force is observed in relation to greater cell density [111]. Importantly, placing microfabricated constraints to guide the gel compaction process can control the geometry of the final cardiac microtissue. During cultivation, the cells develop cell-cell and cell-matrix interactions to compact the cell-gel mixture and cell alignment is then mediated by the self-generated stress guided by the resistance of constraints. Cell alignment may be attributed to the alignment of ECM fibrils [112].
Several recent studies produced engineered cardiac micro-tissues relying on the gel-compaction method. Schaaf et al developed a technique to construct a large series of fibrin-based miniature engineered heart tissues with a high yield and reproducibility (Figure 3C) [113]. Ramade et al described the protocol to generate arrays of cardiac microtissues (Figure 3D) using a small cell number per construct with potential in high-throughput drug screening [114]. The biowire platform (Figure 3E) used suspended suture templates to create longitudinal microtissues that remained stable for weeks and generated more mature cardiac tissues with electrical stimulation after the gel compaction stage [115]. Xiao et al further developed this platform by replacing the suture template with micro-tubing to mimic the capillaries in cardiac bundles. This created a novel method to introduce pharmaceutical agents to cardiac tissue by perfusion and the negative inotropic effect of nitric oxide has been demonstrated as an example [116].
3.4 Electrical maturation of cardiac tissues
Applying electrical stimulation while culturing cells and tissues has proven to be essential for CM maturation [117]. Current and electrical fields are endogenous in embryonic development, which provides justification for electrical stimulation in the biomimetic approach to tissue engineering. This is especially true for cardiac tissue, which is the body’s largest bioelectrical source [118].
Electrical field stimulation can be used in 2D or 3D cultures and helps cells and tissue to better achieve structural and phenotypical properties that are characteristic of the mature native cardiac tissue. Tissue formation results are consistently improved for certain cell types grown with electrical stimulation [118–120]. The generalized setup for applying electric field stimulation in culture requires an anode and a cathode, usually two carbon rods, which are connected to an electrical source [118, 119]. It is important that these electrodes are aligned in parallel and that the wires connecting the electrodes to the electrical source oppose each other on opposite electrodes. Wire placement balances electrode resistance, and alignment ensures a uniform electric field gradient at all points between the electrodes [118]. With these considerations, cells tend to grow and arrange themselves along the electric field between the two rods [119].
When located closer to the anodic or cathodic rod, cells whose ends are aligned with the electric field achieve greater polarization, which results in the generation of an action potential once a critical threshold is reached. The resulting synchronous contractions enhance the structural protein organization and ultimately promote gap junction formation between the ends of cells, which enhances propagation of electrical signals. These features are important in driving organization of sarcomeres [119], an essential cell apparatus for contractile behaviour, and a hallmark of CM maturity [117].
Success of electrical stimulation is dependent on its initiation time point [119]. Three days of pre-culture before application of field stimulation is enough time for excitation-contraction coupling machinery within the cells to develop the ability to transmit electric signals and contract in response to pacing. Optimization of cytoskeletal and electrical properties is evident when electric field stimulation is applied at the correct time within a maturation protocol.
The conditions of electrical stimulation must also be properly calibrated during setup. For example, it has been found that symmetric biphasic square pulses at a low frequency yield a lower excitation threshold, higher cell density, and higher levels of Cx-43 than do monophasic pulses or non-stimulated tissues [121]. Biphasic pulses may be better for inducing action potential excitation because the two pulses act synergistically leading up to excitation [122]. The stimulus frequency can also control contractile behaviour and amplitudes, where increased frequency decreases contractile amplitude until a chaotic behaviour is reached at frequencies above the maximum capture rate [123]. However, the controlled ramp-up in stimulation frequency (1 to 3Hz and 1 to 6Hz) over a period of one week, has demonstrated beneficial effects on tissue maturation[115].
Materials that are suitable for the application of cardiac tissue engineering with electrical stimulation are ideally as conductive as the native tissue. Native myocardium has a direct current conductivity of 0.1 S/m [74]. Polymer materials such as polypyrrole (PPy), polyaniline (PANi), polythiophene (PT), and poly(3,4-ethylenedioxythiophene) (PEDOT) have conductive properties that are useful for scaffolds for cardiac tissue engineering [124]. However, other non-conductive biomaterials may be impregnated with conductive nanoparticles to provide the electrical properties. Gold nanoparticles have been used with electrospun coils and alginate to produce a scaffold with enhanced conductivity, resulting in improved cardiac excitability, cellular attachment, and Cx-43 levels [125, 126]. Another option is to dope the scaffold material with carbon nanotubes (cNTs). cNTs have been paired with several scaffold materials such as porous gelatin, polylactic acid, crosslinked methacrylated gelatin and macroporous chitosan [74, 127–129]. Incorporation of cNTs in the matrix improves conductivity and provides good mechanical properties [74]. The cNTs mimic a nanofibrous structure of the extracellular matrix (ECM) of the heart [127].
CMs can be observed for evidence of proper maturation on the molecular, cellular, ultrastructural, and functional levels. Electrical stimulation during culture yields several benefits [119] that can be comprehensively assessed. On the molecular level, gene expression, protein levels and their distribution may be measured and assessed, often using the polymerase chain reaction, immunohistochemical analyses, and electron microscopy [117, 119]. For example, signs of maturation in human CMs include diminishing levels of atrial natriuretic factor (ANF), increase of β-myosin heavy chain (MHC) relative to α-MHC, increase of cardiac α-actin relative to skeletal α-actin and decrease in SERCA2α [130].
Ultrastructural level assessments include cell morphology, as well as the abundance and organization of specific features such as sarcomeres, gap junctions, desmosomes, T-tubules etc. At this level, electrical stimulation yields better cell alignment and coupling, increased mitochondrial content and activity [117], sarcomere alignment [120], myofibril organization [115], and a larger cell size. Cells that are larger in size are more capable of handling impulse propagation, achieving better rates of action potential depolarization, and higher total contractile force [117]. These observations are similar for cell shape effects [120, 131].
The overall functional features improved by electrical stimulation are contraction amplitude, excitation threshold, maximum capture rate, transmembrane potential, conduction velocity, and action potential duration [117, 119, 132]. Generally, it can be concluded that electric field stimulation both quantitatively and qualitatively helps engineered cardiac tissue develop features that resemble mature CMs when compared to non-stimulated cell culture. Similarity to native tissue is essential for implantation or use as a surrogate for in-vitro testing [132].
4 Applications of cardiac tissue engineering in disease modelling and cardiotoxicity screening
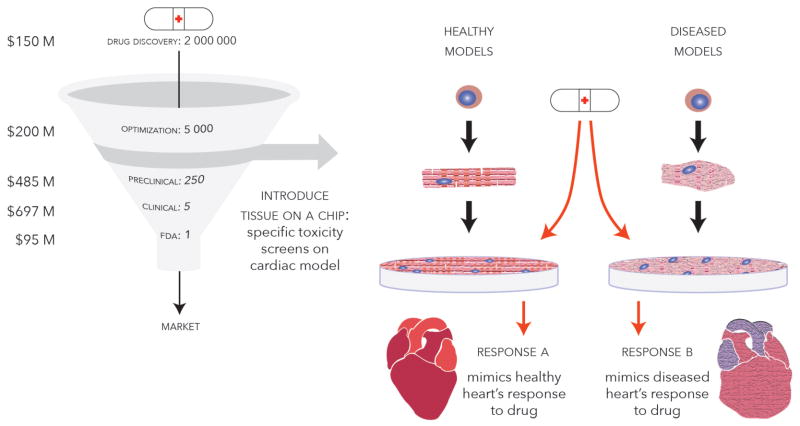
Drug discovery programs include screening of a large number of molecules in cells, tissues and in vivo systems (Figure 4). In general, promotion or inhibition of certain cell signalling pathways can be triggered to achieve a desired therapeutic effect. To perform a search for an appropriate therapeutic agent, high throughput chemical and biological assays are specifically designed for screening large libraries of different chemicals, proteins and RNAs, which typically include millions of compounds. Once therapeutic targets are identified, they must be optimized for desired properties while altering other aspects, such as affinity to proteins and hydrophobicity, through minor changes of structure. These stages focus on developing efficacy of the drug [133].
Figure 4. Tissue-on-a-chip models.
potentially allow for high-throughput screening of pharmaceuticals in vitro on human cell-based substrates in a relatively cost effective manner when compared to typical testing methods that involve extensive testing on explanted animal heart tissue slices and pre-clinical studies. These platforms can be tailored to specific diseases using cell lines from the affected patients, and exhibit the tissue response properties of the native cardiac tissue.
Subsequently, in vitro toxicity screening on cells followed by in vivo pre-clinical and clinical trials are commonly used to test the drug’s safety and efficacy. Drug development cost was recently estimated at $2.6 billion per drug, a 145% increase from a 2003 estimation [134]. Despite the heavy investment of time and money, pharmaceutical companies face their greatest losses when a drug fails in the later stages of development and post market. Drug toxicities and adverse effects that pass through pre-clinical and clinical trials may result in patient deaths and civil law suits.
Cardiovascular related toxicity is one of the leading causes of drug withdrawal post market [135]. The US Food and Drug Administration’s (FDA) reports that 19% of drugs pulled post market had cardiovascular safety issues, such as Tegaserod (Zelnorm), Sibutramine (Reductil/Meridia), Propoxyphene (Darvocet/Darvon) and Rosiglitazone (Avandia) [136]. The FDA requires additional safety measurements related to human ether-a-gogo related gene (hERG) channel electrophysiology in order to improve drug safety. A significant portion of withdrawn drugs with cardiovascular side effects have a high affinity for the hERG channel and are linked to the prolonged QT syndrome (LQTS), causing arrhythmia or Torsade(s) de Pointes (TdP) [97]. Currently, CHO and HEK cell lines are engineered to have stable expression of hERG channel for cardiotoxicity studies [137]. However, disturbances of other potassium currents, such as delayed rectifier current (IK), IKs(slow) and IKr(rapid) function, may also contribute to LQTS and cardiac arrhythmia [138]. Similarly, blockage of the L-type calcium channels can significantly influence the duration of the AP and cause insufficient cardiac output [139]. Moreover, kinase pathways are often targeted in oncology research, which frequently induce serious cardiovascular damage. This damage is potentially caused by mitochondrial perturbation [140, 141] and irregular adenosine monophosphate activated protein kinase activity [142].
Performing electrophysiological measurements in functional 3D tissues, without cell-dissociation, is difficult. Conventional patch clamping in single cells can provide ion channel conductance, but not cell-to-cell conduction throughout the tissue. Current technology focuses on MEAs to detect field potential and assess impulse propagation in monolayer and self-assembled aggregates[143, 144]. Current studies use fibronectin to grow monolayers or aggregates on a planar surface, which allows for sufficient contact between the cells and MEAs for measurements of spatial AP propagation [143–145]. Typical parameters retrieved from this assessment are AP duration and field potential propagation velocity across the tissue. Clements and Thomas used hESC-CMs in a 48-well MEA plate for generating multi-parameter profiles on the effects of 21 well known compounds targeting key cardiac ion channels, successfully demonstrating the sensitivity of this multi-parameter toxicity screening technique [144].
Materials used for MEAs are limited to electrically conductive and non-degradable materials to ensure long-term system stability. Silicon incorporated with titanium nitride-gold contact electrodes is commonly used in high-resolution systems [146, 147]. Polydimethylsiloxane (PDMS), a soft, easily castable elastomer, is used in MEAs to enhance the biocompatibility and stretchability [148]. Indium Tin Oxide (ITO), a transparent and biocompatible substrate possesses electrical properties that can facilitate both electrical sensing and electrical stimulation when used with micropatterning techniques [149]. With elastomeric material (i.e. PDMS), and gold/ITO micropatterning, MEAs have potential to incorporate electrical and mechanical stimulation compartments in a medium-high throughput platform to enhance cardiac tissue maturation.
As many signalling pathways and biomarkers are related to drug induced cardiotoxicity, the development of a more integrated human-cell based protocol to assess cardiac function is critically important [150]. Recent successes in stem cell cardiogenic differentiation have provided means to tackle this issue. The ability to achieve close to 100% cardiogenic differentiation significantly enhances the availability of human CMs [151]. hiPSC can potentially be used to optimize a personal therapeutic plan corresponding to patient-specific responses to drugs [152, 153]. For example, hiPSCs were derived from heart failure patients, differentiated into CMs, tested for drug responses and engrafted into a rat heart [154].
Many cardiac disorders have underlying genetic causes. Patients with type 1 (gene KCNQ1, IKs)[138] and type 2 (gene hERG + MiRP1, IKr) LQTS have mutations in potassium ion channels, which result in prolonged action potentials and high risk of arrhythmia [155]. LQTS type 3 (SCN5A, INa) shows mutation in sodium ion channels [156]. Timothy syndrome (LQT 8, gene CACNA1C, ICa,L) causes mutated L-type calcium channel Ca(v)1.2, which results in irregular calcium transients and abnormal APs [157]. Patients suffering from LEOPARD syndrome (gene PTPN11) show abnormalities in the RAS-mitogen-activated protein kinase signalling pathway and present clinical pathological hypotrophy [158]. Patients with familial dilated cardiomyopathy (gene R173W) carry a mutation in cardiac troponin T protein expression and abnormal sarcomeric alpha-actinin distribution [159]. These are a few of many examples of genetic cardiac conditions that have been investigated using hiPSC derived CMs in vitro.
hiPSC from these patients can help generate in vitro disease models to study cellular and tissue level pathological mechanisms and in turn develop new therapeutic agents for target diseases. Patient–specific hiPSC lines have been differentiated into CMs in various studies [155–159]. For example, Wang et al used patient-derived hiPSC-CMs to investigate the mitochondrial cardiomyopathy underlying Barth syndrome [160]. hiPSC derived CM from patients suffering from LQTS type 3 demonstrated a significantly longer AP duration, and persistent late Na+ current [156]. These signature abnormalities can be reversed by application of a sodium blocker, Mexiletine [156]. With similar approaches, Timothy syndrome [157], LEOPARD syndromes [158] and arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) [161], have been investigated to show relevant pathological phenotypes with respect to cellular morphology, intercellular structures, calcium transients, electrophysiology and contractile behaviours. Moreover, Liang et al studied different drug related toxicity profiles on hiPSC-derived CMs from both healthy patients and patients with hereditary LQTS, familial hypertrophic cardiomyopathy and familial dilated cardiomyopathy [99].
It is well accepted that 3D tissue culture has greater physiological relevance compared to conventional 2D culture, including gene and protein expression, cell-cell signalling pathways, and cell-ECM interactions [162–165]. Hence, in order to best mimic in vivo conditions, a 3D tissue culture platform is necessary for in-depth investigation as it can enable the assessment of both contractile force and relaxation characteristics. These systems must provide efficient functional readouts while maintaining optimum culture conditions for tissue maturation.
To carry out more reliable cardiotoxicity screening, several requirements must be met. First, human CMs must be cultured in a 3D environment and subjected to chemical and physical stimuli for maturation before testing; the maturity of the tissue correlates to the reliability of data output. Furthermore, high throughput production of qualified tissues is needed to satisfy the demand of drug testing. Hence, reliable tissue growth requires automation and miniaturization. Production of the tissue should be performed in large quantities with reproducible standard dimensions and physiological properties to minimize sample variation. Also, the drug testing device itself should not interact with the applied drugs (e.g. drug absorption commonly encountered in PDMS based platforms) and produce functional readouts. Finally, it is preferred that the vasculature be incorporated in these tissues to capture important interactions between the cardiac tissue and the vascular cells.
The 3D micro-tissue platforms outlined above (3.3), prepared through self-aggregation or template guided gel compaction, are used towards this goal. The resulting tissues have cell densities similar to the natural tissue and possess the potential to simulate in vivo cell cross-talk and ECM accumulation [166]. With greater similarity between artificial tissues and native cardiac tissues comes improved accuracy of predictive data from in vitro models. This necessitates the incorporation of biochemical and physical stimulation components for maturation processes as well as long-term automated observation methods. Contractile force transients the tissue develops in 3D systems are sensitive to application of various drugs [167]. It is an important functional outcome, as it correlates with clinical measurements of cardiac function, such as preload, afterload and ejection fraction [40, 168, 169]. The evaluation of contractile behaviour involves measurement of force amplitude, beat frequency and duration, and upstroke (contraction)/relaxation slope and duration. Arrays of flexible PDMS-based posts are often used as cantilevers to measure these forces [170]. Using beam bending theory, force can be calculated when the elastic modulus of PDMS and dimensions of the posts are known. Eschenhagen et al used this technique in a 24-well format with fibrin enhanced ECM and successfully demonstrated the sensitivity of platform to digoxin, chromanol 293B, quinidine, and erythromycin [171]. Boudou et al performed a similar experiment, but were able to reduce the post size to a hundred micron-scale, significantly reducing the required cell quantity (<5000 cells per tissue) [167]. These tissues responded well to isoprotenerol treatment, which was consistent with literature.
Force sensing platforms should utilize materials with similar elastomeric properties to native cardiac tissue while also providing adequate stress between anchor points to enable cell/tissue alignment. The materials should be biocompatible, non-degradable, and inert to both culture and drug testing environment to ensure stability of the system. Furthermore, the material needs to be easily combined with other compartments, such as electrical stimulation circuits, and/or cyclic stretching apparatus.
PDMS is still the most common material used in these applications due to high adaptability of fabrication procedures. [172–174]. However, PDMS is also highly hydrophobic, causing it to absorb hydrophobic drugs, therefore it has been certified as a drug release vehicle in many studies [172–174]. This absorption capability is problematic for in vitro drug testing applications. In addition, the high Young’s modulus of PDMS (1.32–2.97 MPa [175]) can potentially trigger pathological signalling pathways in culture [176]. Clear, flexible and castable polyurethane materials developed by the Ingber lab have the potential to replace PDMS while eliminating these undesirable properties [177]. Furthermore, commonly used materials such as polystyrene, polyesters and polypropylene that are inert to most drugs and biological agents could be used in these platforms.
5 Conclusions
In this review, we have discussed the aspects of biomaterial based cardiac tissue engineering and its applications. Biomaterials serve as a basis to these tissue engineering solutions, as properties such as elasticity and degradation can be tuned, which helps to minimize the host response, organize cardiac tissue, and dictate the similarity of scaffolds to the native tissue. The complex relationship between the cell matrix developed in vitro and these materials allows for development of cardiac tissue. Although some success has been seen in vivo, major developments in CTE have been in in vitro disease models, which may allow for high throughput testing of therapeutics. These platforms could allow pharmaceutical companies to discover new therapeutic targets, increasing the potential of finding a therapeutic solution.
Acknowledgments
This work is funded by the Heart and Stoke Foundation GIA T6946, the Canadian Institutes of Health Research (CIHR) Operating Grant (MOP-126027), NSERC Discovery Grant (RGPIN 326982-10), and National Institutes of Health grant 2R01 HL076485. Milica Radisic is supported by Canada Research Chair (Tier 2) and Steacie Fellowship.
References
- 1.Chiu LL, Radisic M. Controlled release of thymosin beta4 using collagen-chitosan composite hydrogels promotes epicardial cell migration and angiogenesis. Journal of controlled release : official journal of the Controlled Release Society. 2011;155:376–85. doi: 10.1016/j.jconrel.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295:1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 3.Furth ME, Atala A, Van Dyke ME. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials. 2007;28:5068–73. doi: 10.1016/j.biomaterials.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 4.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 5.Bouten CV, Dankers PY, Driessen-Mol A, Pedron S, Brizard AM, Baaijens FP. Substrates for cardiovascular tissue engineering. Advanced drug delivery reviews. 2011;63:221–41. doi: 10.1016/j.addr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Reis LA, Chiu LL, Feric N, Fu L, Radisic M. Biomaterials in myocardial tissue engineering. Journal of tissue engineering and regenerative medicine. 2014 doi: 10.1002/term.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Feric NT, Thavandiran N, Nunes SS, Radisic M. The role of tissue engineering and biomaterials in cardiac regenerative medicine. Canadian Journal of Cardiology. 2014 doi: 10.1016/j.cjca.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Liang S, Thouas GA. Elastomeric biomaterials for tissue engineering. Progress in Polymer Science. 2013;38:584–671. [Google Scholar]
- 9.Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circulation research. 2014;114:354–67. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Xiao Y, Hsieh A, Thavandiran N, Radisic M. Micro- and nanotechnology in cardiovascular tissue engineering. Nanotechnology. 2011;22:494003. doi: 10.1088/0957-4484/22/49/494003. [DOI] [PubMed] [Google Scholar]
- 11.Ulery BD, Nair LS, Laurencin CT. Biomedical Applications of Biodegradable Polymers. Journal of polymer science Part B, Polymer physics. 2011;49:832–64. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2008;17(Suppl 4):467–79. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian H, Tang Z, Zhuang X, Chen X, Jing X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Progress in Polymer Science. 2012;37:237–80. [Google Scholar]
- 14.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 15.Naderi H, Matin MM, Bahrami AR. Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. Journal of biomaterials applications. 2011;26:383–417. doi: 10.1177/0885328211408946. [DOI] [PubMed] [Google Scholar]
- 16.Radisic M, Park H, Gerecht S, Cannizzaro C, Langer R, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1357–68. doi: 10.1098/rstb.2007.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–62. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 18.Weis SM, Emery JL, Becker KD, McBride DJ, Jr, Omens JH, McCulloch AD. Myocardial mechanics and collagen structure in the osteogenesis imperfecta murine (oim) Circ Res. 2000;87:663–9. doi: 10.1161/01.res.87.8.663. [DOI] [PubMed] [Google Scholar]
- 19.Coirault C, Samuel JL, Chemla D, Pourny JC, Lambert F, Marotte F, et al. Increased compliance in diaphragm muscle of the cardiomyopathic Syrian hamster. J Appl Physiol (1985) 1998;85:1762–9. doi: 10.1152/jappl.1998.85.5.1762. [DOI] [PubMed] [Google Scholar]
- 20.Omens JH. Stress and strain as regulators of myocardial growth. Progress in biophysics and molecular biology. 1998;69:559–72. doi: 10.1016/s0079-6107(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 21.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomater. 2011;7:1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuong CJ, Sacks MS, Templeton G, Schwiep F, Johnson RL., Jr Regional deformation and contractile function in canine right ventricular free wall. The American journal of physiology. 1991;260:H1224–35. doi: 10.1152/ajpheart.1991.260.4.H1224. [DOI] [PubMed] [Google Scholar]
- 23.Rappaport D, Adam D, Lysyansky P, Riesner S. Assessment of myocardial regional strain and strain rate by tissue tracking in B-mode echocardiograms. Ultrasound Med Biol. 2006;32:1181–92. doi: 10.1016/j.ultrasmedbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Sacks MS, Chuong CJ. Biaxial mechanical properties of passive right ventricular free wall myocardium. Journal of biomechanical engineering. 1993;115:202–5. doi: 10.1115/1.2894122. [DOI] [PubMed] [Google Scholar]
- 25.Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nature materials. 2008;7:1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes JW, Borg TK, Covell JW. STRUCTURE AND MECHANICS OF HEALING MYOCARDIAL INFARCTS. Annual Review of Biomedical Engineering. 2005;7:223–53. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 27.Chuong C, Sacks M, Templeton G, Schwiep F, Johnson R. Regional deformation and contractile function in canine right ventricular free wall. American Journal of Physiology-Heart and Circulatory Physiology. 1991;260:H1224–H35. doi: 10.1152/ajpheart.1991.260.4.H1224. [DOI] [PubMed] [Google Scholar]
- 28.Rappaport D, Adam D, Lysyansky P, Riesner S. Assessment of myocardial regional strain and strain rate by tissue tracking in B-mode echocardiograms. Ultrasound in medicine & biology. 2006;32:1181–92. doi: 10.1016/j.ultrasmedbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Demer LL, Yin FC. Passive biaxial mechanical properties of isolated canine myocardium. The Journal of physiology. 1983;339:615–30. doi: 10.1113/jphysiol.1983.sp014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fomovsky GM, Macadangdang JR, Ailawadi G, Holmes JW. Model-based design of mechanical therapies for myocardial infarction. Journal of cardiovascular translational research. 2011;4:82–91. doi: 10.1007/s12265-010-9241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fomovsky GM, Clark SA, Parker KM, Ailawadi G, Holmes JW. Anisotropic reinforcement of acute anteroapical infarcts improves pump function. Circulation Heart failure. 2012;5:515–22. doi: 10.1161/CIRCHEARTFAILURE.111.965731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jawad H, Lyon AR, Harding SE, Ali NN, Boccaccini AR. Myocardial tissue engineering. British medical bulletin. 2008;87:31–47. doi: 10.1093/bmb/ldn026. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced drug delivery reviews. 2012;64:72–82. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JM. BIOLOGICAL RESPONSES TO MATERIALS. Annual Review of Materials Research. 2001;31:81–110. [Google Scholar]
- 35.Palmer JA, Abberton KM, Mitchell GM, Morrison WA. Macrophage phenotype in response to implanted synthetic scaffolds: an immunohistochemical study in the rat. Cells, tissues, organs. 2014;199:169–83. doi: 10.1159/000363693. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Seminars in immunology. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery M, Zhang B, Radisic M. Cardiac Tissue Vascularization: From Angiogenesis to Microfluidic Blood Vessels. Journal of cardiovascular pharmacology and therapeutics. 2014;19:382–93. doi: 10.1177/1074248414528576. [DOI] [PubMed] [Google Scholar]
- 38.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proceedings of the National Academy of Sciences. 2010;107:15211–6. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran RT, Thevenot P, Gyawali D, Chiao JC, Tang L, Yang J. Synthesis and characterization of a biodegradable elastomer featuring a dual crosslinking mechanism. Soft matter. 2010;6:2449–61. doi: 10.1039/C001605E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature medicine. 2006;12:452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann WH, Didie M, Doker S, Melnychenko I, Naito H, Rogge C, et al. Heart muscle engineering: an update on cardiac muscle replacement therapy. Cardiovascular research. 2006;71:419–29. doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 42.Matsubayashi K, Fedak PW, Mickle DA, Weisel RD, Ozawa T, Li RK. Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation. 2003;108(Suppl 1):II219–25. doi: 10.1161/01.cir.0000087450.34497.9a. [DOI] [PubMed] [Google Scholar]
- 43.Kim DH, Kshitiz, Smith RR, Kim P, Ahn EH, Kim HN, et al. Nanopatterned cardiac cell patches promote stem cell niche formation and myocardial regeneration. Integrative biology : quantitative biosciences from nano to macro. 2012;4:1019–33. doi: 10.1039/c2ib20067h. [DOI] [PubMed] [Google Scholar]
- 44.Chi NH, Yang MC, Chung TW, Chou NK, Wang SS. Cardiac repair using chitosan-hyaluronan/silk fibroin patches in a rat heart model with myocardial infarction. Carbohydrate polymers. 2013;92:591–7. doi: 10.1016/j.carbpol.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. American journal of physiology Heart and circulatory physiology. 2006;290:H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 46.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circulation research. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nature biotechnology. 2004;22:1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 48.Gepstein L, Ding C, Rahmutula D, Wilson EE, Yankelson L, Caspi O, et al. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells. 2010;28:2151–61. doi: 10.1002/stem.545. [DOI] [PubMed] [Google Scholar]
- 49.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, et al. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 50.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012 doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacology & therapeutics. 2005;105:151–63. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Prabhakaran MP, Venugopal J, Kai D, Ramakrishna S. Biomimetic material strategies for cardiac tissue engineering. Materials Science and Engineering: C. 2011;31:503–13. [Google Scholar]
- 54.Reis LA, Chiu LL, Liang Y, Hyunh K, Momen A, Radisic M. A peptide-modified chitosan-collagen hydrogel for cardiac cell culture and delivery. Acta biomaterialia. 2012;8:1022–36. doi: 10.1016/j.actbio.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 55.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, et al. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. Journal of the American College of Cardiology. 2009;54:1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 57.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. Journal of the American College of Cardiology. 2012;59:751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freytes DO, Stoner RM, Badylak SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. Journal of biomedical materials research Part B, Applied biomaterials. 2008;84:408–14. doi: 10.1002/jbm.b.30885. [DOI] [PubMed] [Google Scholar]
- 59.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nature medicine. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 60.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarig U, Au-Yeung GC, Wang Y, Bronshtein T, Dahan N, Boey FY, et al. Thick acellular heart extracellular matrix with inherent vasculature: a potential platform for myocardial tissue regeneration. Tissue Eng Part A. 2012;18:2125–37. doi: 10.1089/ten.tea.2011.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, et al. Safety and Efficacy of an Injectable Extracellular Matrix Hydrogel for Treating Myocardial Infarction. Science Translational Medicine. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barrett DG, Yousaf MN. Thermosets synthesized by thermal polyesterification for tissue engineering applications. Soft matter. 2010;6:5026. [Google Scholar]
- 64.Burkersroda Fv, Schedl L, Göpferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23:4221–31. doi: 10.1016/s0142-9612(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 65.Barrett DG, Luo W, Yousaf MN. Aliphatic polyester elastomers derived from erythritol and ?α,ω-diacids. Polymer Chemistry. 2010;1:296. [Google Scholar]
- 66.Vieira AC, Guedes RM, Marques AT. Development of ligament tissue biodegradable devices: a review. Journal of biomechanics. 2009;42:2421–30. doi: 10.1016/j.jbiomech.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Guan J. Hydrogels for Cardiac Tissue Engineering. Polymers. 2011;3:740–61. [Google Scholar]
- 68.Vyner MC, Li A, Amsden BG. The effect of poly(trimethylene carbonate) molecular weight on macrophage behavior and enzyme adsorption and conformation. Biomaterials. 2014;35:9041–8. doi: 10.1016/j.biomaterials.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 69.Li S. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. Journal of Biomedical Materials Research. 1999;48:342–53. doi: 10.1002/(sici)1097-4636(1999)48:3<342::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate) Journal of Biomedical Materials Research Part A. 2003;66A:192–7. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 71.Mukherjee S, Gualandi C, Focarete ML, Ravichandran R, Venugopal JR, Raghunath M, et al. Elastomeric electrospun scaffolds of poly(L-lactide-co-trimethylene carbonate) for myocardial tissue engineering. Journal of materials science Materials in medicine. 2011;22:1689–99. doi: 10.1007/s10856-011-4351-2. [DOI] [PubMed] [Google Scholar]
- 72.Nelson DM, Hashizume R, Yoshizumi T, Blakney AK, Ma Z, Wagner WR. Intramyocardial injection of a synthetic hydrogel with delivery of bFGF and IGF1 in a rat model of ischemic cardiomyopathy. Biomacromolecules. 2014;15:1–11. doi: 10.1021/bm4010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoshi RA, Behl S, Ameer GA. Nanoporous Biodegradable Elastomers. Advanced Materials. 2009;21:188–92. [Google Scholar]
- 74.Pahnke A, Montgomery M, Radisic M. Spatial and Electrical Factors Regulating Cardiac Regeneration and Assembly. 2015:71–92. [Google Scholar]
- 75.Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. Myocardial tissue engineering: a review. Journal of tissue engineering and regenerative medicine. 2007;1:327–42. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- 76.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences. 2012;109:E1848–E57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 78.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circulation research. 2013;113:810–34. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–7. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 80.Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun-Shin MJ, Jones S, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. Bmj. 2014;348:g2688. doi: 10.1136/bmj.g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, et al. Stem cell treatment for acute myocardial infarction. The Cochrane Library; 2012. [DOI] [PubMed] [Google Scholar]
- 82.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–8. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nam Y-J, Song K, Luo X, Daniel E, Lambeth K, West K, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proceedings of the National Academy of Sciences. 2013;110:5588–93. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qian L, Berry EC, Fu JD, Ieda M, Srivastava D. Reprogramming of mouse fibroblasts into cardiomyocyte-like cells in vitro. Nature protocols. 2013;8:1204–15. doi: 10.1038/nprot.2013.067. [DOI] [PubMed] [Google Scholar]
- 86.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]
- 87.Brutsaert DL, Fransen P, Andries LJ, De Keulenaer GW, Sys SU. Cardiac endothelium and myocardial function. Cardiovasc Res. 1998;38:281–90. doi: 10.1016/s0008-6363(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 88.Giordano FJ, Gerber HP, Williams SP, VanBruggen N, Bunting S, Ruiz-Lozano P, et al. A cardiac myocyte vascular endothelial growth factor paracrine pathway is required to maintain cardiac function. P Natl Acad Sci USA. 2001;98:5780–5. doi: 10.1073/pnas.091415198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation. 2004;110:962–8. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiu LL, Montgomery M, Liang Y, Liu H, Radisic M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc Natl Acad Sci U S A. 2012;109:E3414–23. doi: 10.1073/pnas.1210580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–63. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 92.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–26. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 93.Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Interactive and Integrative Cardiology. 2006;1080:76–84. doi: 10.1196/annals.1380.007. [DOI] [PubMed] [Google Scholar]
- 94.Iyer RK, Chiu LL, Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J Biomed Mater Res A. 2009;89:616–31. doi: 10.1002/jbm.a.32014. [DOI] [PubMed] [Google Scholar]
- 95.Iyer RK, Odedra D, Chiu LL, Vunjak-Novakovic G, Radisic M. Vascular endothelial growth factor secretion by nonmyocytes modulates Connexin-43 levels in cardiac organoids. Tissue Eng Part A. 2012;18:1771–83. doi: 10.1089/ten.tea.2011.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thavandiran N, Dubois N, Mikryukov A, Massé S, Beca B, Simmons CA, et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proceedings of the National Academy of Sciences. 2013;110:E4698–E707. doi: 10.1073/pnas.1311120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caspi O, Itzhaki I, Kehat I, Gepstein A, Arbel G, Huber I, et al. In vitro electrophysiological drug testing using human embryonic stem cell derived cardiomyocytes. Stem Cells Dev. 2009;18:161–72. doi: 10.1089/scd.2007.0280. [DOI] [PubMed] [Google Scholar]
- 98.Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–9. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 99.Liang P, Lan F, Lee AS, Gong T, Sanchez-Freire V, Wang Y, et al. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 2013;127:1677–91. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karp JM, Yeo Y, Geng W, Cannizarro C, Yan K, Kohane DS, et al. A photolithographic method to create cellular micropatterns. Biomaterials. 2006;27:4755–64. doi: 10.1016/j.biomaterials.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 101.Badie N, Bursac N. Novel micropatterned cardiac cell cultures with realistic ventricular microstructure. Biophys J. 2009;96:3873–85. doi: 10.1016/j.bpj.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guo L, Abrams RM, Babiarz JE, Cohen JD, Kameoka S, Sanders MJ, et al. Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci. 2011;123:281–9. doi: 10.1093/toxsci/kfr158. [DOI] [PubMed] [Google Scholar]
- 103.Guo L, Coyle L, Abrams RM, Kemper R, Chiao ET, Kolaja KL. Refining the human iPSC- cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 2013;136:581–94. doi: 10.1093/toxsci/kft205. [DOI] [PubMed] [Google Scholar]
- 104.Lamore SD, Kamendi HW, Scott CW, Dragan YP, Peters MF. Cellular impedance assays for predictive preclinical drug screening of kinase inhibitor cardiovascular toxicity. Toxicol Sci. 2013;135:402–13. doi: 10.1093/toxsci/kft167. [DOI] [PubMed] [Google Scholar]
- 105.Natarajan A, Stancescu M, Dhir V, Armstrong C, Sommerhage F, Hickman JJ, et al. Patterned cardiomyocytes on microelectrode arrays as a functional, high information content drug screening platform. Biomaterials. 2011;32:4267–74. doi: 10.1016/j.biomaterials.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang J, Chen A, Lieu DK, Karakikes I, Chen G, Keung W, et al. Effect of engineered anisotropy on the susceptibility of human pluripotent stem cell-derived ventricular cardiomyocytes to arrhythmias. Biomaterials. 2013;34:8878–86. doi: 10.1016/j.biomaterials.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 107.Chen A, Lee E, Tu R, Santiago K, Grosberg A, Fowlkes C, et al. Integrated platform for functional monitoring of biomimetic heart sheets derived from human pluripotent stem cells. Biomaterials. 2014;35:675–83. doi: 10.1016/j.biomaterials.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip. 2011;11:4165–73. doi: 10.1039/c1lc20557a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song H, Zandstra PW, Radisic M. Engineered heart tissue model of diabetic myocardium. Tissue Eng Part A. 2011;17:1869–78. doi: 10.1089/ten.tea.2010.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma Z, Koo S, Finnegan MA, Loskill P, Huebsch N, Marks NC, et al. Three-dimensional filamentous human diseased cardiac tissue model. Biomaterials. 2014;35:1367–77. doi: 10.1016/j.biomaterials.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baar K, Birla R, Boluyt MO, Borschel GH, Arruda EM, Dennis RG. Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J. 2005;19:275–7. doi: 10.1096/fj.04-2034fje. [DOI] [PubMed] [Google Scholar]
- 112.Barocas VH, Tranquillo RT. An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. Journal of biomechanical engineering. 1997;119:137–45. doi: 10.1115/1.2796072. [DOI] [PubMed] [Google Scholar]
- 113.Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PloS one. 2011;6:e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramade A, Legant WR, Picart C, Chen CS, Boudou T. Microfabrication of a platform to measure and manipulate the mechanics of engineered microtissues. Methods in cell biology. 2014;121:191–211. doi: 10.1016/B978-0-12-800281-0.00013-0. [DOI] [PubMed] [Google Scholar]
- 115.Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nature methods. 2013;10:781–7. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke AQ, et al. Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab on a Chip. 2013;14:869–82. doi: 10.1039/c3lc51123e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circulation research. 2014;114:511–23. doi: 10.1161/CIRCRESAHA.114.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, et al. Electrical stimulation systems for cardiac tissue engineering. Nature protocols. 2009;4:155–73. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lasher RA, Pahnke AQ, Johnson JM, Sachse FB, Hitchcock RW. Electrical stimulation directs engineered cardiac tissue to an age-matched native phenotype. Journal of tissue engineering. 2012;3:2041731412455354. doi: 10.1177/2041731412455354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiu LLY, Iyer RK, King J-P, Radisic M. Biphasic Electrical Field Stimulation Aids in Tissue Engineering of Multicell-Type Cardiac Organoids. 2011 doi: 10.1089/ten.tea.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tung L, Borderies JR. Analysis of electric field stimulation of single cardiac muscle cells. Biophysical Journal. 1992;63:371–86. doi: 10.1016/S0006-3495(92)81632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liau B, Zhang D, Bursac N. Functional cardiac tissue engineering. Regenerative medicine. 2012;7:187–206. doi: 10.2217/rme.11.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kai D, Prabhakaran MP, Jin G, Ramakrishna S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. Journal of biomedical materials research Part A. 2011;99:376–85. doi: 10.1002/jbm.a.33200. [DOI] [PubMed] [Google Scholar]
- 125.Fleischer S, Shevach M, Feiner R, Dvir T. Coiled fiber scaffolds embedded with gold nanoparticles improve the performance of engineered cardiac tissues. Nanoscale. 2014;6:9410–4. doi: 10.1039/c4nr00300d. [DOI] [PubMed] [Google Scholar]
- 126.Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, et al. Nanowired three-dimensional cardiac patches. Nature Nanotechnology. 2011;6:720–5. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim Sb, et al. Carbon-Nanotube-Embedded Hydrogel Sheets for Engineering Cardiac Constructs and Bioactuators. ACS Nano. 2013;7:2369–80. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mooney E, Mackle JN, Blond DJ, O’Cearbhaill E, Shaw G, Blau WJ, et al. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33:6132–9. doi: 10.1016/j.biomaterials.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 129.Martins AM, Eng G, Caridade SG, Mano JF, Reis RL, Vunjak-Novakovic G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules. 2014;15:635–43. doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–43. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 131.Ahadian S, Ostrovidov S, Hosseini V, Kaji H, Ramalingam M, Bae H, et al. Electrical stimulation as a biomimicry tool for regulating muscle cell behavior. Organogenesis. 2013;9:87–92. doi: 10.4161/org.25121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chan YC, Ting S, Lee YK, Ng KM, Zhang J, Chen Z, et al. Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. Journal of cardiovascular translational research. 2013;6:989–99. doi: 10.1007/s12265-013-9510-z. [DOI] [PubMed] [Google Scholar]
- 133.Sukardi H, Chng HT, Chan EC, Gong Z, Lam SH. Zebrafish for drug toxicity screening: bridging the in vitro cell-based models and in vivo mammalian models. Expert Opin Drug Metab Toxicol. 2011;7:579–89. doi: 10.1517/17425255.2011.562197. [DOI] [PubMed] [Google Scholar]
- 134.New drug costs soar to $2.6 billion. Nat Biotechnol. 2014;32:1176. [Google Scholar]
- 135.Dykens JA, Marroquin LD, Will Y. Strategies to reduce late-stage drug attrition due to mitochondrial toxicity. Expert Rev Mol Diagn. 2007;7:161–75. doi: 10.1586/14737159.7.2.161. [DOI] [PubMed] [Google Scholar]
- 136.Piccini JP, Whellan DJ, Berridge BR, Finkle JK, Pettit SD, Stockbridge N, et al. Current challenges in the evaluation of cardiac safety during drug development: translational medicine meets the Critical Path Initiative. Am Heart J. 2009;158:317–26. doi: 10.1016/j.ahj.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 137.Chen MX, Sandow SL, Doceul V, Chen YH, Harper H, Hamilton B, et al. Improved functional expression of recombinant human ether-a-go-go (hERG) K+ channels by cultivation at reduced temperature. BMC Biotechnol. 2007;7:93. doi: 10.1186/1472-6750-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363:1397–409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 139.Mahajan A, Sato D, Shiferaw Y, Baher A, Xie LH, Peralta R, et al. Modifying L-type calcium current kinetics: consequences for cardiac excitation and arrhythmia dynamics. Biophys J. 2008;94:411–23. doi: 10.1529/biophysj.106.98590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Will Y, Dykens JA, Nadanaciva S, Hirakawa B, Jamieson J, Marroquin LD, et al. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol Sci. 2008;106:153–61. doi: 10.1093/toxsci/kfn157. [DOI] [PubMed] [Google Scholar]
- 141.Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci. 2007;97:539–47. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
- 142.Mellor HR, Bell AR, Valentin JP, Roberts RR. Cardiotoxicity associated with targeting kinase pathways in cancer. Toxicol Sci. 2011;120:14–32. doi: 10.1093/toxsci/kfq378. [DOI] [PubMed] [Google Scholar]
- 143.Braam SR, Tertoolen L, van de Stolpe A, Meyer T, Passier R, Mummery CL. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:107–16. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 144.Clements M, Thomas N. High-Throughput Multi-Parameter Profiling of Electrophysiological Drug Effects in Human Embryonic Stem Cell Derived Cardiomyocytes Using Multi-Electrode Arrays. Toxicol Sci. 2014 doi: 10.1093/toxsci/kfu084. [DOI] [PubMed] [Google Scholar]
- 145.Kaneko T, Nomura F, Hamada T, Abe Y, Takamori H, Sakakura T, et al. On-chip in vitro cell-network pre-clinical cardiac toxicity using spatiotemporal human cardiomyocyte measurement on a chip. Sci Rep. 2014;4:4670. doi: 10.1038/srep04670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002;91:659–61. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 147.Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circulation research. 2010;107:186–99. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 148.Liang G, Guvanasen GS, Xi L, Tuthill C, Nichols TR, DeWeerth SP. A PDMS-based integrated stretchable microelectrode array (isMEA) for neural and muscular surface interfacing. IEEE Trans Biomed Circuits Syst. 2013;7:1–10. doi: 10.1109/TBCAS.2012.2192932. [DOI] [PubMed] [Google Scholar]
- 149.Tandon N, Marsano A, Maidhof R, Numata K, Montouri-Sorrentino C, Cannizzaro C, et al. Surface- patterned electrode bioreactor for electrical stimulation. Lab Chip. 2010;10:692–700. doi: 10.1039/b917743d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–4. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 151.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E1848–57. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yulin X, Lizhen L, Lifei Z, Shan F, Ru L, Kaimin H, et al. Efficient generation of induced pluripotent stem cells from human bone marrow mesenchymal stem cells. Folia Biol (Praha) 2012;58:221–30. doi: 10.14712/fb2012058060221. [DOI] [PubMed] [Google Scholar]
- 153.Frobel J, Hemeda H, Lenz M, Abagnale G, Joussen S, Denecke B, et al. Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Reports. 2014;3:414–22. doi: 10.1016/j.stemcr.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zwi-Dantsis L, Huber I, Habib M, Winterstern A, Gepstein A, Arbel G, et al. Derivation and cardiomyocyte differentiation of induced pluripotent stem cells from heart failure patients. European heart journal. 2013;34:1575–86. doi: 10.1093/eurheartj/ehs096. [DOI] [PubMed] [Google Scholar]
- 155.Levine E, Rosero SZ, Budzikowski AS, Moss AJ, Zareba W, Daubert JP. Congenital long QT syndrome: considerations for primary care physicians. Cleve Clin J Med. 2008;75:591–600. doi: 10.3949/ccjm.75.8.591. [DOI] [PubMed] [Google Scholar]
- 156.Ma D, Wei H, Zhao Y, Lu J, Li G, Sahib NB, et al. Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. International journal of cardiology. 2013;168:5277–86. doi: 10.1016/j.ijcard.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 157.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–4. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–12. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Science translational medicine. 2012;4:130ra47. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616–23. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature. 2013;494:105–10. doi: 10.1038/nature11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chitcholtan K, Asselin E, Parent S, Sykes PH, Evans JJ. Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp Cell Res. 2013;319:75–87. doi: 10.1016/j.yexcr.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 163.Bellis AD, Bernabe BP, Weiss MS, Shin S, Weng S, Broadbelt LJ, et al. Dynamic transcription factor activity profiling in 2D and 3D cell cultures. Biotechnol Bioeng. 2013;110:563–72. doi: 10.1002/bit.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maltman DJ, Przyborski SA. Developments in three-dimensional cell culture technology aimed at improving the accuracy of in vitro analyses. Biochem Soc Trans. 2010;38:1072–5. doi: 10.1042/BST0381072. [DOI] [PubMed] [Google Scholar]
- 165.Lovitt CJ, Shelper TB, Avery VM. Advanced cell culture techniques for cancer drug discovery. Biology (Basel) 2014;3:345–67. doi: 10.3390/biology3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnology and bioengineering. 2003;82:403–14. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 167.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue engineering Part A. 2012;18:910–9. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Riegler J, Gillich A, Shen Q, Gold JD, Wu JC. Cardiac tissue slice transplantation as a model to assess tissue-engineered graft thickness, survival, and function. Circulation. 2014;130:S77–86. doi: 10.1161/CIRCULATIONAHA.113.007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–30. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 170.Sniadecki NJ, Chen CS. Microfabricated silicone elastomeric post arrays for measuring traction forces of adherent cells. Methods Cell Biol. 2007;83:313–28. doi: 10.1016/S0091-679X(07)83013-5. [DOI] [PubMed] [Google Scholar]
- 171.Eschenhagen T, Zimmermann WH, Kleber AG. Electrical coupling of cardiac myocyte cell sheets to the heart. Circulation research. 2006;98:573–5. doi: 10.1161/01.RES.0000215627.13049.5d. [DOI] [PubMed] [Google Scholar]
- 172.Maeda H, Ohashi E, Sano A, Kawasaki H, Kurosaki Y. Investigation of the release behavior of a covered-rod-type formulation using silicone. J Control Release. 2003;90:59–70. doi: 10.1016/s0168-3659(03)00158-5. [DOI] [PubMed] [Google Scholar]
- 173.Lee JH, Pidaparti RM, Atkinson GM, Moorthy RS. Design of an implantable device for ocular drug delivery. J Drug Deliv. 2012;2012:527516. doi: 10.1155/2012/527516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kimura T. Studies on the clinical application of medical grade silicone rubber to maxillofacial prosthetics--bacteriological analysis of the surface adherent substance and the prevention of its attachment. Kokubyo Gakkai Zasshi. 1985;52:558–86. doi: 10.5357/koubyou.52.558. [DOI] [PubMed] [Google Scholar]
- 175.Johnston ID, McCluskey DK, Tan CKL, Tracey MC. Mechanical characterization of bulk Sylgard 184 for microfluidics and microengineering. Journal of Micromechanics and Microengineering. 2014;24 [Google Scholar]
- 176.Marsano A, Maidhof R, Wan LQ, Wang Y, Gao J, Tandon N, et al. Scaffold stiffness affects the contractile function of three-dimensional engineered cardiac constructs. Biotechnol Prog. 2010;26:1382–90. doi: 10.1002/btpr.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Domansky K, Leslie DC, McKinney J, Fraser JP, Sliz JD, Hamkins-Indik T, et al. Clear castable polyurethane elastomer for fabrication of microfluidic devices. Lab Chip. 2013;13:3956–64. doi: 10.1039/c3lc50558h. [DOI] [PMC free article] [PubMed] [Google Scholar]