Abstract
Clinical trials investigating the analgesic efficacy of cannabinoids in multiple sclerosis have yielded mixed results, possibly due to psychotropic side effects mediated by cannabinoid CB1 receptors. We hypothesized that a CB2-specific agonist (JWH-133) would decrease hyperalgesia in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. 4 weeks after induction of experimental autoimmune encephalomyelitis, we found that intrathecal administration of JWH-133 (10–100 μg) dose-dependently reduced both mechanical and cold hypersensitivity without producing signs of sedation or ataxia. The anti-hyperalgesic effects of JWH-133 could be dose-dependently prevented by intrathecal co-administration of the CB2 antagonist, AM-630 (1–3 μg). Our results suggest that JWH-133 acts at CB2 receptors, most likely within the dorsal horn of the spinal cord, to suppress the hypersensitivity associated with experimental autoimmune encephalomyelitis. These are the first pre-clinical studies to directly promote CB2 as a promising target for the treatment of central pain in an animal model of multiple sclerosis.
Keywords: Pain, CB2 receptor, JWH-133, AM-630, multiple sclerosis
Introduction
Multiple sclerosis (MS) is an autoimmune-inflammatory neurodegenerative disease of the central nervous system that afflicts well over 2 million people worldwide. MS disrupts the function of the brain, spinal cord and optic nerves and is characterized by acute inflammation, demyelination and axonal and neuronal loss. Neuropathic pain is one of the most frequent symptoms of MS, reported by roughly half of all patients [12]. MS patients present not only with spontaneous pain, but also several forms of evoked pain including cutaneous mechanical and cold hypersensitivity in the distal extremities [31, 39]. Despite its heavy impact on patient quality of life, pain in MS is often neglected or undertreated. Conventional analgesics either show low efficacy or produce unwanted side effects [29].
Emerging data suggest that activation of endogenous cannabinoid receptors can suppress neuropathic pain [23, 33]. Cannabinoid receptor (CB) subtypes include CB1 and CB2. CB1 is predominately located in neurons within the central nervous system, while CB2 is localized primarily on immune and microglial cells [28]. CB2-immunoreactivity is significantly up-regulated at the lesion sites of post-mortem human spinal cord from patients with MS [47], as well as 4 weeks after MOG33–55-induction of EAE in mice [24, 32].
Double-blind randomized controlled trials on the analgesic efficacy of the CB1/CB2 agonist Sativex, an oromucosal spray of tetrahydrocannabinol and cannabidiol, have yielded mixed results [18, 41]. Other CB agonist preparations such as delta-9-tetrahydrocannabinol or nabilone consistently reduce MS pain, but at the expense of dizziness, headache, fatigue and/or impaired judgment [38, 43, 48]. Mixed results are likely due in part to the psychotropic side effects associated with CB1 activation, and point to the CB2 as an attractive target for chronic pain [2, 25]. To date, however, an exhaustive literature search indicates that no study has evaluated the efficacy of a CB2 selective agonist for pain in MS. Here we address this question in a validated experimental autoimmune encephalomyelitis (EAE) mouse model. EAE is associated with neurodegenerative pathology, and a behavioral phenotype that is reflective of MS in humans, including the development of cutaneous mechanical and cold hypersensitivity [30, 34]. We hypothesized that intrathecal administration of a CB2-selective agonist, JWH-133 [17, 46], would decrease mechanical and cold hypersensitivity without locomotor side effects, and that this would be blocked by the CB2-selective antagonist, AM-630.
Materials and Methods
EAE Model
Our EAE model was designed with reagent concentrations that produce a mild-to-moderate form of EAE, always yielding clinical scores of motor dysfunction below 3 and 4 over the 4 week testing period [34]. All animals in the current study exhibited neither paresis of both hindlimbs nor paralysis of one hindlimb, thus allowing nociceptive paw reflex testing. Briefly, myelin oligodendrocyte glycoprotein 35–55 (MOG33–55, AnaSpec Inc, Fremont, CA) was emulsified in a 1:1 solution of 1x PBS and complete Freund’s adjuvant (CFA). To minimize the hyperalgesia produced by CFA itself, we reduced its concentration to 3 mg/ml. We used female C57BL/6 mice (Charles River), aged 12–14 weeks, housed 4 to a cage, habituated for 1 week in a temperature and humidity controlled environment on a 14/10-h light/dark cycle. Food and water were available ad libitum. Procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky, protocol (#2010-0770). MOG was bilaterally injected (150 μg/100 μl s.c.) at the flanks under light isoflurane anesthesia on Days 0 and 6. Pertussis toxin (List Biological Laboratories, Campbell, CA) was injected (200 ng/200 μl, i.p.) on days 0 and 2 to facilitate opening of the blood brain barrier to T cells by histamine-induced vascular leakage and/or the priming of autoreactive T cells [16]. Control mice received CFA and pertussis toxin, but not MOG. Average weight of mice on week 4 was 21.8 ± 0.2 g.
Intrathecal (i.t.) Drug Administration
Intrathecal injection was performed in lightly restrained unanesthetized mice as previously described [11]. We injected 5 μl of drug [JWH-133 (Tocris, UK), AM-630 (Tocris, UK), or WIN55,212-2 (Cayman Chemical, Ann Arbor, MI) and/or vehicle [ethanol: alkamuls EL-620 (Rhodia, Cranbury, NJ): saline in a volume ratio of 1:1:8]. The data of Figure 1 includes animals that were injected twice (Days 24 and 28 after the first MOG injection) using a balanced crossover design. Animals receiving vehicle on day 24 received drug on day 28, and vice versa. Group means of either vehicle or drug on day 24 and day 28 did not differ, and so were combined for final analysis. The data of Figure 2 were obtained on day 28 after two consecutive i.t. injections, separated by 15 min: first AM-630 or vehicle and then JWH-133, followed by behavioral testing sessions.
Figure 1. Intrathecal JWH-133 suppresses mechanical and cold hypersensitivity in a dose-dependent manner.
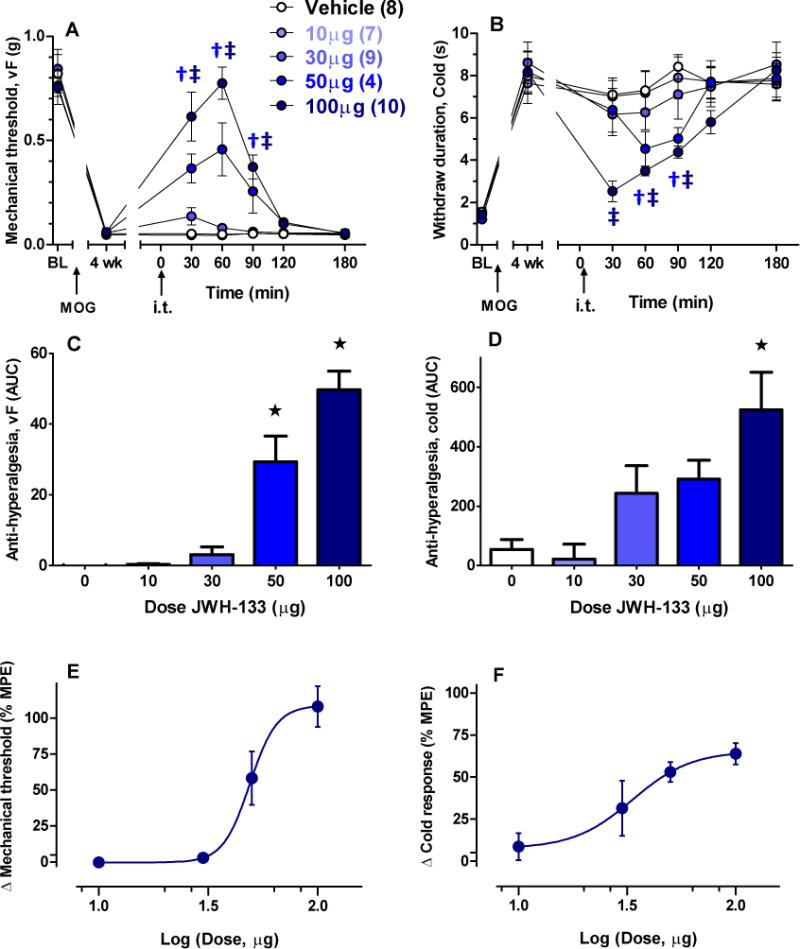
The CB2-selective agonist JWH-133 was intrathecally (i.t.) administered 24–28 d (4 wk) after induction of EAE in C57/B6 mice. Time course and area under the curve (AUC) analysis of mechanical (A,C) and cold (B,D) hypersensitivity after JWH-133. Dose-response analysis of the data at 60 min for mechanical (E) and cold hypersensitivity (F), respectively. MPE: maximum possible effect. Parentheses refer to number of animals per group. †p < 0.05 50 μg vs vehicle; ‡p < 0.05 100 μg vs vehicle; ★p < 0.05 vs vehicle.
Figure 2. CB2 antagonist blocks the anti-hyperalgesic effects of JWH-133.
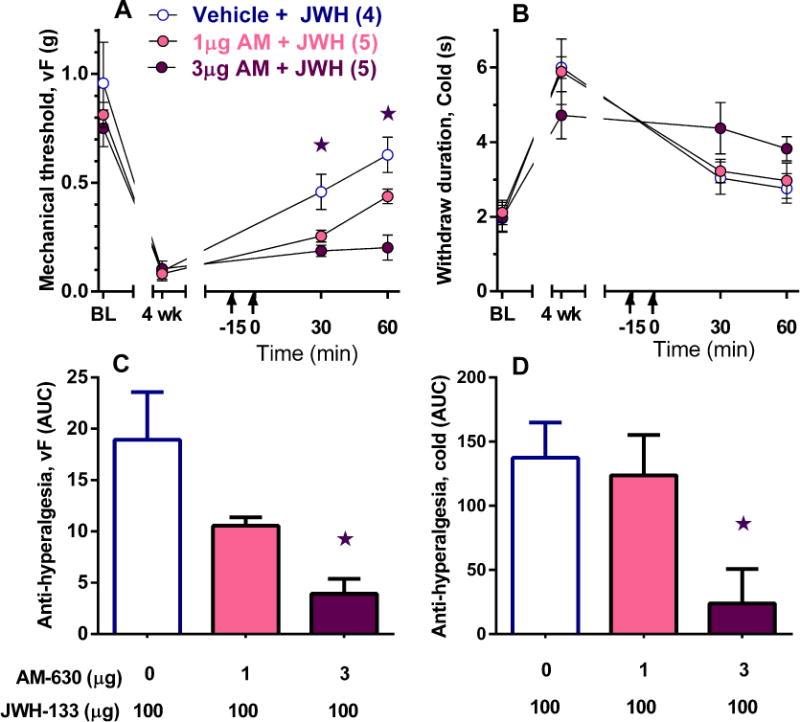
The CB2 antagonist AM-630 was i.t. administered 15 min prior to i.t. JWH-133 (100 μg). Time course and AUC analysis (0–60 min) indicate that AM-630 attenuated the anti-hyperalgesic effects of JWH-133 on mechanical (A, C) and cold (B, D) responses. ★different from vehicle. ★p < 0.05 vs vehicle.
Behavioral Testing
All animals were acclimated to a stainless steel grid within individual Plexiglas tubes for 30 to 60 min prior to behavioral testing. Somatosensory testing using mechanical (von Frey hair) and cold (plantar application of 5–8 μL acetone) stimuli was conducted as previously described [37]. All experiments were conducted by a female investigator (W.F.), blinded to model and identity of drugs.
Statistics
In Figs. 1A–B and 2A–B, differences between means were analyzed by two-way analysis of variance (ANOVA). Drug/Dose was a grouping factor and time was the repeated measure. If a significant interaction was found (p < 0.05), ANOVA was followed by post-hoc Bonferroni tests. Data were re-plotted as area under the curve, calculated using the trapezoidal method (Figs. 1C–D and 2C–D). Effects of Drug were analyzed by one-way ANOVA followed by post-hoc Dunnett multiple comparison test. The best fit line for dose-response curves (Figs. 1E–F) was generated following non-linear regression analysis based on the 60 min post-injection thresholds. % Maximum Possible Effect (MPE) was calculated as:
All data are presented as mean ± SEM. ★p < 0.05 was considered statistically significant.
Results
CB2 agonist JWH-133 reduced EAE hypersensitivity in a dose-dependent manner
We first tested the hypothesis that activation of spinal CB2 suppresses mechanical and cold hypersensitivity in EAE mice. As illustrated in Figs 1A–D, at doses based on previous analgesia studies in mice [6, 21, 46], JWH-133 dose-dependently reduced mechanical (1A, F4, 33 = 32.5, p <0.0001) and cold hypersensitivity (1B, F4, 33 = 2.8, p < 0.05). The anti-hyperalgesic effect of JWH-133 (100 μg) for mechanical and cold hypersensitivity peaked at 60 and 30 min respectively, with restoration of thresholds to baseline levels (0.77 ± 0.08 g, p > 0.05 vs baseline; 2.52 ± 0.05 s, p > 0.05 vs baseline, respectively) within 180 min. Area under the curve (AUC) analysis (0–180 min) illustrates the concentration-dependent actions of JWH-133 (1C–D, p < 0.01). As illustrated in Figs. 1E–F, dose-response curves yielded EC50 values of 49.0 μg and 33.5 μg for the mechanical and cold modalities, respectively. As illustrated in Supplementary Figure S1, JWH-133 (100 μg) did not change rotarod latency (p > 0.05).
CB2 antagonist AM-630 prevented the anti-hyperalgesic effects of JWH-133
To further evaluate CB2 as the target of JWH-133, we intrathecally administrated 100 μg JWH-133 followed by the highly selective CB2 antagonist AM-630 [35] at intrathecal doses in the low μg range [13, 19]. We did not include an AM-630 alone control group because these doses do not change sensory thresholds [9, 19]. AUC analysis illustrates that AM-630 dose-dependently attenuated the inhibitory effects of 100 μg JWH-133 on mechanical (2C, F2, 11 = 15.0, p < 0.001) and cold hypersensitivity (2D, F2, 11 = 4.2, p < 0.05).
Discussion
CB2 is an emerging target for pain relief as suggested by clinical trials and data from animal models of chronic pain [2, 5, 33]. For example, CB2 mRNA or protein levels were up-regulated in the spinal cord after peripheral nerve injury in rat [42, 44, 49]. Furthermore, intrathecal administration of CB2 selective agonists generally reduced hyperalgesia in rodent models of peripheral neuropathic pain (as reviewed in [33], but see [4]). These anti-hyperalgesic effects were abolished in CB2 knockout animals [46] or by co-administration of a CB2 selective antagonist [3, 13, 25], advancing a spinal CB2 site of activation. The current results extend these findings to the EAE model of multiple sclerosis pain. We found that the CB2 agonist JWH-133 reduced mechanical and cold hypersensitivity in EAE mice in a dose-dependent manner. CB2 deletion mutant mice exhibit more severe disease scores [26], while chronic systemic administration of CB2 agonists ameliorated disease progression in EAE animals [22, 32]; however, it is highly unlikely that a single injection of the CB2 agonist JWH-133 could impact disease progression within the 3 hr window of behavioral observation in the current study. Pre-treatment with the CB2 antagonist, AM-630, reversed the anti-hyperalgesic effects of JWH-133, suggesting a contribution of spinal CB2 to pain control in the EAE model. Consistent with this conclusion, MOG35–55 increased CB2 mRNA and protein levels in the spinal cord of EAE animals [24, 32], and CB2-immunoreactivity is significantly up-regulated at the lesion sites of postmortem human spinal cord from patients with MS [47]. Our results are consistent with the analgesic effects of oral cannabinoid-based medications in patients with spinal cord injury [14, 27] and of CB2 agonists in rodents with spinal cord injury [1, 15].
Previous studies in a mouse model of nerve injury reported that intrathecal administration of 31 μg of JWH-133 (approximately 1.5–2.0 mg/kg) reduced behavioral signs of peripheral neuropathic pain, suggesting a spinal site of action [46]. Similarly, in the current study we found that intrathecal doses of up to 100 μg of JWH-133 reduced behavioral signs of central neuropathic pain. Although we did not attempt injection of an equivalent systemic dose of JWH-133 (4.5 mg/kg, based on our average mouse weight of 22g), previous studies in the CFA model of inflammatory pain or the brachial plexus avulsion model of peripheral neuropathic pain indicate that systemic doses at or exceeding 10 mg/kg for JWH-133 are required to exert anti-hyperalgesic effects [7, 8]. Similarly, a 5 mg/kg i.p. dose of JWH-133 failed to reduce pain thresholds in the second phase of the formalin model of ongoing inflammatory pain [20]. These data support our conclusion that intrathecal injection of JWH-133 acts at spinal sites and does not diffuse out of the spinal cord at sufficient concentrations to exert its anti-hyperalgesic actions at peripheral CB2. Further supporting a spinal site of action, we found that intrathecal injection of a low dose (3 μg) of the CB2 antagonist AM-630 blocked the anti-hyperalgesic effects of intrathecal JWH-133. This low dose is equivalent to 0.135 mg/kg (based on our average mouse weight of 22g), which is much lower than the 3mg/kg i.p. dose of AM-630 that was used to block the effect of 10mg/kg JWH-133 [7, 8], and so is unlikely to produce systemic effects. However, we cannot entirely exclude a contribution of peripheral CB2 at the intrathecal doses of JWH-133 used in our study because JWH-133 (1–10 mg/kg i.p.) reduced weight bearing in rat models of cisplatin-induced neuropathic pain and carrageenan-induced inflammatory pain [10, 40].
Our rotarod data indicate that WIN55-212,2 produces ataxia, consistent with previous reports in rodents [25], and a consensus that activation of central CB1 causes psychotropic effects and therefore compromises the therapeutic efficacy of cannabinoids to reduce clinical chronic pain, including MS pain [33, 36]. By contrast, we found that JWH-133 reduced hypersensitivity at doses that do not produce ataxia. This is consistent with previous studies in other pain models indicating an absence of motor impairment or catalepsy following systemic injection of analgesic 20 mg/kg doses JWH-133 [45]. Although JWH-133 exhibits substantial selectivity (200 X) for CB2 over CB1 [17], we cannot exclude a contribution of CB1 receptors located in the DRG and spinal cord to its analgesic actions because we did not administer it in combination with a CB1 antagonist. Thus, our data suggest that selective targeting of spinal CB2 reduces behavioral signs of neuropathic pain in the EAE model without untoward side effects. Taken together with an emerging literature indicating that inhibitory effects of CB2 activation on the immune system may reduce the progression of neuromuscular dysfunction in the EAE model [22, 32], we conclude that the targeting of central CB2 poses strong therapeutic potential for the treatment of both motor dysfunction and pain in MS patients.
Supplementary Material
Highlights.
-
►
CB2 receptor activation with JWH-133 reduces central neuropathic pain in mice.
-
►
The site of anti-hyperalgesic action of JWH-133 includes the spinal cord.
-
►
JWH-133 reduces hypersensitivity at doses that do not produce ataxia.
-
►
First pre-clinical studies to promote CB2 for treatment of multiple sclerosis pain.
Acknowledgments
Supported by NIHR01NS62306 and University of Kentucky Start Up funds. We thank Mark Ingram with the University of Kentucky medical library for exhaustive literature searches. All authors have approved the final article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
There are no financial or other relationships that might lead to a conflict of interest. WF contributed in concept and study design, data acquisition and analysis, drafting and revising the manuscript. BKT contributed in obtaining funding for the project, concept and study design, data analysis, drafting and revising the manuscript.
References
- 1.Ahmed MM, Rajpal S, Sweeney C, Gerovac TA, Allcock B, McChesney S, Patel AU, Tilghman JI, Miranpuri GS, Resnick DK. Cannabinoid subtype-2 receptors modulate the antihyperalgesic effect of WIN 55,212-2 in rats with neuropathic spinal cord injury pain. The spine journal: official journal of the North American Spine Society. 2010;10:1049–1054. doi: 10.1016/j.spinee.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain research reviews. 2009;60:255–266. doi: 10.1016/j.brainresrev.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. The European journal of neuroscience. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 4.Brownjohn PW, Ashton JC. Spinal cannabinoid CB2 receptors as a target for neuropathic pain: an investigation using chronic constriction injury. Neuroscience. 2012;203:180–193. doi: 10.1016/j.neuroscience.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 5.Burston JJ, Sagar DR, Shao P, Bai M, King E, Brailsford L, Turner JM, Hathway GJ, Bennett AJ, Walsh DA, Kendall DA, Lichtman A, Chapman V. Cannabinoid CB2 receptors regulate central sensitization and pain responses associated with osteoarthritis of the knee joint. PloS one. 2013;8:e80440. doi: 10.1371/journal.pone.0080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curto-Reyes V, Boto T, Hidalgo A, Menendez L, Baamonde A. Antinociceptive effects induced through the stimulation of spinal cannabinoid type 2 receptors in chronically inflamed mice. European journal of pharmacology. 2011;668:184–189. doi: 10.1016/j.ejphar.2011.06.057. [DOI] [PubMed] [Google Scholar]
- 7.da Silva KA, Paszcuk AF, Passos GF, Silva ES, Bento AF, Meotti FC, Calixto JB. Activation of cannabinoid receptors by the pentacyclic triterpene alpha,beta-amyrin inhibits inflammatory and neuropathic persistent pain in mice. Pain. 2011;152:1872–1887. doi: 10.1016/j.pain.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Del Fabbro L, Borges Filho C, Cattelan Souza L, Savegnago L, Alves D, Henrique Schneider P, de Salles HD, Jesse CR. Effects of Se-phenyl thiazolidine-4-carboselenoate on mechanical and thermal hyperalgesia in brachial plexus avulsion in mice: mediation by cannabinoid CB1 and CB2 receptors. Brain research. 2012;1475:31–36. doi: 10.1016/j.brainres.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Desroches J, Charron S, Bouchard JF, Beaulieu P. Endocannabinoids decrease neuropathic pain-related behavior in mice through the activation of one or both peripheral CB(1) and CB(2) receptors. Neuropharmacology. 2014;77:441–452. doi: 10.1016/j.neuropharm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Elmes SJ, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, Chapman V. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Advanced drug delivery reviews. 2003;55:1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 12.Foley PL, Vesterinen HM, Laird BJ, Sena ES, Colvin LA, Chandran S, MacLeod MR, Fallon MT. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain. 2013;154:632–642. doi: 10.1016/j.pain.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Gadotti VM, You H, Petrov RR, Berger ND, Diaz P, Zamponi GW. Analgesic effect of a mixed T-type channel inhibitor/CB2 receptor agonist. Molecular pain. 2013;9:32. doi: 10.1186/1744-8069-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagenbach U, Luz S, Ghafoor N, Berger JM, Grotenhermen F, Brenneisen R, Mader M. The treatment of spasticity with Delta9-tetrahydrocannabinol in persons with spinal cord injury. Spinal cord. 2007;45:551–562. doi: 10.1038/sj.sc.3101982. [DOI] [PubMed] [Google Scholar]
- 15.Hama AT, Sagen J. Cannabinoid receptor-mediated antinociception with acetaminophen drug combinations in rats with neuropathic spinal cord injury pain. Neuropharmacology. 2010;58:758–766. doi: 10.1016/j.neuropharm.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofstetter HH, Shive CL, Forsthuber TG. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. Journal of immunology. 2002;169:117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- 17.Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, Martin BR. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorganic & medicinal chemistry. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- 18.Iannitti T, Kerr BJ, Taylor BK. Mechanisms and Pharmacology of Neuropathic Pain in Multiple Sclerosis. Current topics in behavioral neurosciences. 2014 doi: 10.1007/7854_2014_288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda H, Ikegami M, Kai M, Ohsawa M, Kamei J. Activation of spinal cannabinoid CB2 receptors inhibits neuropathic pain in streptozotocin-induced diabetic mice. Neuroscience. 2013;250:446–454. doi: 10.1016/j.neuroscience.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Jafari MR, Golmohammadi S, Ghiasvand F, Zarrindast MR, Djahanguiri B. Influence of nicotinic receptor modulators on CB2 cannabinoid receptor agonist (JWH133)-induced antinociception in mice. Behavioural pharmacology. 2007;18:691–697. doi: 10.1097/FBP.0b013e3282f00c10. [DOI] [PubMed] [Google Scholar]
- 21.Katsuyama S, Mizoguchi H, Komatsu T, Nagaoka K, Sakurada S, Sakurada T. The cannabinoid 1 receptor antagonist AM251 produces nocifensive behavior via activation of ERK signaling pathway. Neuropharmacology. 2010;59:534–541. doi: 10.1016/j.neuropharm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Kong W, Li H, Tuma RF, Ganea D. Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cellular immunology. 2014;287:1–17. doi: 10.1016/j.cellimm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landry RP, Martinez E, DeLeo JA, Romero-Sandoval EA. Spinal cannabinoid receptor type 2 agonist reduces mechanical allodynia and induces mitogen-activated protein kinase phosphatases in a rat model of neuropathic pain. The journal of pain: official journal of the American Pain Society. 2012;13:836–848. doi: 10.1016/j.jpain.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou ZY, Chen C, He Q, Zhao CB, Xiao BG. Targeting CB(2) receptor as a neuroinflammatory modulator in experimental autoimmune encephalomyelitis. Molecular immunology. 2011;49:453–461. doi: 10.1016/j.molimm.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Malan TP, Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: pain relief without psychoactive effects? Current opinion in pharmacology. 2003;3:62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 26.Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, Giovannoni G, Pertwee RG, Yamamura T, Buckley NE, Hillard CJ, Lutz B, Baker D, Dittel BN. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nature medicine. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- 27.Maurer M, Henn V, Dittrich A, Hofmann A. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. European archives of psychiatry and clinical neuroscience. 1990;240:1–4. doi: 10.1007/BF02190083. [DOI] [PubMed] [Google Scholar]
- 28.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 29.Nick ST, Roberts C, Billiodeaux S, Davis DE, Zamanifekri B, Sahraian MA, Alekseeva N, Munjampalli S, Roberts J, Minagar A. Multiple sclerosis and pain. Neurological research. 2012;34:829–841. doi: 10.1179/1743132812Y.0000000082. [DOI] [PubMed] [Google Scholar]
- 30.Olechowski CJ, Truong JJ, Kerr BJ. Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (EAE) Pain. 2009;141:156–164. doi: 10.1016/j.pain.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Osterberg A, Boivie J, Thuomas KA. Central pain in multiple sclerosis–prevalence and clinical characteristics. Eur J Pain. 2005;9:531–542. doi: 10.1016/j.ejpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Palazuelos J, Davoust N, Julien B, Hatterer E, Aguado T, Mechoulam R, Benito C, Romero J, Silva A, Guzman M, Nataf S, Galve-Roperh I. The CB(2) cannabinoid receptor controls myeloid progenitor trafficking: involvement in the pathogenesis of an animal model of multiple sclerosis. The Journal of biological chemistry. 2008;283:13320–13329. doi: 10.1074/jbc.M707960200. [DOI] [PubMed] [Google Scholar]
- 33.Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahn EJ, Iannitti T, Donahue RR, Taylor BK. Sex differences in a mouse model of multiple sclerosis: neuropathic pain behavior in females but not males and protection from neurological deficits during proestrus. Biology of sex differences. 2014;5:4. doi: 10.1186/2042-6410-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross RA, Brockie HC, Stevenson LA, Murphy VL, Templeton F, Makriyannis A, Pertwee RG. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. British journal of pharmacology. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PF. Cannabinoids in the treatment of pain and spasticity in multiple sclerosis. Curr Opin Investig Drugs. 2002;3:859–864. [PubMed] [Google Scholar]
- 37.Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7224–7229. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ. 2004;329:253. doi: 10.1136/bmj.38149.566979.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truini A, Barbanti P, Pozzilli C, Cruccu G. A mechanism-based classification of pain in multiple sclerosis. Journal of neurology. 2013;260:351–367. doi: 10.1007/s00415-012-6579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vera G, Cabezos PA, Martin MI, Abalo R. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacology, biochemistry, and behavior. 2013;105:205–212. doi: 10.1016/j.pbb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10:434–441. doi: 10.1191/1352458504ms1082oa. [DOI] [PubMed] [Google Scholar]
- 42.Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience. 2005;132:1093–1102. doi: 10.1016/j.neuroscience.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Wissel J, Haydn T, Muller J, Brenneis C, Berger T, Poewe W, Schelosky LD. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: a double-blind placebo-controlled cross-over trial. Journal of neurology. 2006;253:1337–1341. doi: 10.1007/s00415-006-0218-8. [DOI] [PubMed] [Google Scholar]
- 44.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 45.Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nature neuroscience. 2011;14:1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto W, Mikami T, Iwamura H. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. European journal of pharmacology. 2008;583:56–61. doi: 10.1016/j.ejphar.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC neurology. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. Journal of neurology, neurosurgery, and psychiatry. 2012;83:1125–1132. doi: 10.1136/jnnp-2012-302468. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. The European journal of neuroscience. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


