Synopsis
This phase I trial reports the first use of intra arterial temozolomide via isolated limb infusion for patients with advanced extremity melanoma. There was minimal toxicity and the maximum tolerated dose was determined.
Keywords: in-transit melanoma, regional chemotherapy
Background
Regional chemotherapy in the form of hyperthermic isolated limb perfusion (HILP) or the minimally invasive counterpart isolated limb infusion (ILI), allows delivery of a drug dose to tumor that is 10-15 times greater than can be achieved with systemic therapy. Although several different drugs have been evaluated (1-5), none has produced the same degree of response, both in magnitude and duration, compared to L-phenylalanine mustard (LPAM) for the treatment of unresectable in-transit melanoma (6-10). While LPAM HILP has been reported to have complete response (CR) rates in the 50-80% range, a multi-center retrospective study of LPAM via ILI found the CR rate to be approximately 30% (6-9). However the rate of severe toxicity requiring amputation (0.3%) from ILI was seven fold less than historically reported with HILP (2%)(8,9). While the 30% CR rate observed after ILI is generally higher than can be achieved with other therapies, the majority of patients will require additional therapy in attempts to eradicate persistent disease. Therefore, there is a need for more effective and durable therapies that can be administered to appropriately selected patients with the goal to maximize response.
Temozolomide (TMZ) is an oral DNA-methylating agent that has been used systemically to treat metastatic melanoma with relatively little toxicity but with clinical response rates of only 15-20% (11-13). In preclinical studies using an animal model of advanced extremity melanoma for several melanoma xenografts, we demonstrated that regional chemotherapy with an intra arterial formulation of TMZ was more effective than systemic TMZ and comparable to regional LPAM (14). Further analysis suggested that regional therapy with TMZ was more effective when compared with LPAM for xenografts with the low O(6)-methylguanine-DNA methyltransferase (MGMT) activity, whereas LPAM was more effective than TMZ in xenografts with high MGMT activity (15). This observation suggested that MGMT activity may be useful in predicting the efficacy of TMZ-based regional therapy for advanced extremity melanoma tumors. The primary objectives of this trial were to determine the dose limiting and non-dose limiting toxicities of intra-arterial TMZ treatment and to determine the maximum tolerated dose (MTD) of intra-arterial administration of TMZ during ILI that can be carried forward into a phase II trial.
Methods
Patient Eligibility
Patients were eligible for study if they were ≥ 18 years of age, had histologically confirmed recurrent American Joint Committee on Cancer stage IIIB, IIIC, or IV extremity melanoma, failed or recurred after a previous LPAM based regional chemotherapy (ILI or HILP), directly measurable cutaneous disease distal to planned tourniquet placement, Eastern Cooperative Oncology Group performance status of 0 or 1, and a palpable pulse in the affected extremity. All patients underwent whole body positron emission tomography/computed tomography within 6 weeks of ILI to evaluate presence of macroscopic disease which would make the patient ineligible. Patients were required to give informed consent, and the institutional review boards of the participating institutions approved the study.
Study Design
This trial was an open-label, single-arm, multicenter phase I study. Dose level selection proceeded according to a modified accelerated titration design. The starting dose of TMZ was 200 mg/m2 × 0.09 body surface area for the upper extremity and 200 mg/m2 × 0.18 BSA for the lower extremity. ILI was performed as previously described using a rapid infusion of TMZ (2-5 minutes) into the arterial portion of the circuit after the extremity had been warmed to at least 37°C. We planned to enroll one-patient cohorts with dose doubling between cohorts until the first 1st occurrence of a Grade 2 adverse event according to CTCAE (Common Terminology Criteria for Adverse Events v4.03). A dose-limiting toxicity was defined as all limb related, systemic related, non-hematologic drug or procedure related adverse events of at least Grade 3 in severity that occur up to 15 days following the ILI. We planned to enroll approximately 15 patients into the Phase I dose escalation portion of this trial with the possible addition of 10 more patients to achieve a goal of having 20 patients in the MTD.
Assessment of Tumor Response and Toxicity
Tumor response was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria modified for cutaneous lesions at one time point 3 months post-ILI. Toxicity was assessed by using CTCAE v4.03.
Pharmacokinetic Evaluations
High-performance liquid chromatography was used to measure TMZ and the active metabolite, 4-amino-5-imidazole-carboxamide (AIC) infusion circuit and systemic concentrations (16, 17).
Quantitative Polymerase Chain Reaction
Tumor biopsies were homogenized by using Lysing Matrix A (MP Bio-medicals, Solon, OH) and a mini bead-beater (Biospec Products, Bartlesville, OK). RNA was isolated, cDNA was synthesized, and quantitative polymerase chain reaction (qPCR) was performed as described previously. The QPCR Human Reference Total RNA is a high-quality control for quantitative PCR gene-expression analysis. QPCR Human Reference Total RNA is composed of total RNA from 10 human cell lines with quantities of RNA from the individual cell lines optimized to maximize representation of gene transcripts present in low, medium, and high abundance.
MGMT Methylation Analysis
Genomic DNA (800 ng) was treated with sodium bisulfite using the Zymo EZ DNA Methylation Kit (Zymo Research, Irvine, CA), which converts all unmethylated cytosines to uracils. Methylated cytosine is protected from this conversion. Two μl of the bisulfite modified DNA underwent PCR amplification in a 25 μl volume to produce a 253 bp product from a region on chromosome 10 approximately 400 bp usptream of the MGMT transcription start site using a predesigned assay (PyroMark CpG Assay # PM00149702; Qiagen; Valencia, CA) and the PyroMark PCR Kit (Qiagen). Methylation was averaged across the seven CpG sites and the average of the replicate runs was used for analysis. The average standard deviation across replicate runs was 0.26 (range, 0.09-0.68).
Definitions
The MTD was defined as the dose level below the maximally administered dose at which 2 or more patients experienced DLTs. Time to in-field extremity progression was defined as the time from the date of ILI to the date of in-field extremity progression; the time to progression distribution was compared to that of our historical LPAM treated patients with the log-rank test.
Results
Twenty-eight patients completed treatment at three institutions over 2.5 years. Patient characteristics and procedural variables are shown in Tables 1 and 2. Notably, once the MTD was determined, an amendment allowed patients who had not yet undergone any regional chemotherapy treatments to be treated. ILI was performed succesfully in all but one patient in whom ILI catheters could not be placed due to small venous vessel size related to a previous deep vein thrombosis from a prior HILP. One patient underwent temozolomide ILI but was subsequently removed from the study after determination that the patient had not met study inclusion criteria.
Table 1.
Patient Characteristics
| Variable | Total N=28 |
|---|---|
| Age (years) | Median 65, range 46-85 |
| Gender | 12 males (43%) |
| Stage IIIB, IIIC, IV | 11 (39%), 16 (57%), 1 (4%) |
| Lower Extremity/Upper Extremity | 23 (82%), 5 (18%) |
| Previous LPAM based RC | 25 (89%) |
Table 2.
Procedure variables LPAM corrected for IBW vs TMZ
| Variable | LPAM Mean,(N) | TMZ mean, (N=28) |
|---|---|---|
| Base excess 25 min | -8.53 (112) | -13.01 (27) |
| Pa02 25 min (mm Hg) | 6.94 (113) | 6.54 (28) |
| pH 25 min | 7.16 (113) | 7.00 (28) |
| Base excess 30 min | -9.31 (113) | -13.79 (28) |
| Pa02 30 min (mm Hg) | 7.40 (114) | 5.96 (28) |
| pH 30 min | 7.14 (114) | 6.99 (28) |
| Ischemic time (min) | 73.4 (116) | 60.9 (28) |
| Circulated volume (mL) | 1183 (61) | 1430 (23) |
| Peak temp (°C) | 38.6 (114) | 39.0 (28) |
| Peak CPK (U/L) * | 1911 (117) | 2622 (27) |
| Day peak CPK | 3.7 (112) | 1.1 (28) |
| Length of stay (days) | 7.7 (113) | 4.6 (28) |
Removing an extreme outlier from the TMZ group with a CK 23,379 U/L who had only a grade 1 clinical toxicity.
Four of the initial five enrolled patients completed ILI treatment with TMZ with successive dose doubling (200 mg/m2, 400 mg/m2, 800 mg/m2, and 1600 mg/m2 multiplied by 0.09 upper extremity or 0.18 lower extremity). Two patients at the 3200 mg/m2 dose had grade 2 clinical toxicities while 1 patient also had a grade 4 elevation in CPK. At the discretion of the principal investigator, the 3200 mg/m2 cohort was further expanded to a total of six patients. All of the next four patients at the 3200 mg/m2 had less than grade 2 clinical toxicities while two patients had grade 4 elevations in CPK. The CPK elevations suggested to the principal investigator that the MTD would likely be closer to 3200 mg/m2. As such, a formal amendment was obtained which stated that if the 3200 mg/m2 dose cohort was expanded to six patients without a DLT, the trial design would change to a traditional 3+3 design and future increases of 400 mg (or a 12.5% increase of the 3200mg/m2 dose) from previous dose level would be made thereafter until the occurrence of a DLT. The next five patients were treated at a dose of 3600 mg/m2 with three of the five patients having minimal toxicity. However, the second and fifth patient at the 3600 mg/m2 had DLTs comprised of compartment syndrome requiring fasciotomies with one patient having evidence of soft tissue necrosis requiring multiple debridements. With two of five patients experiencing DLTs, the 3600 mg/m2 dose was defined as the maximum administered dose and the 3200 mg/m2 dose the MTD. An additional thirteen patients were treated at the 3200 mg/m2 dose. Common toxicities for all cohorts are summarized in Table 3.
Table 3.
Clinical Toxicities
| Clinical Toxicities | Grade I toxicities No. (%) | Grade II and III toxicities No. (%) | Grade IV toxicities No. (%) |
|---|---|---|---|
| Cohort 1-4 (n=4) | |||
| Pain | 4 (100%) | ||
| Lymphedema | 3 (75%) | ||
| Erythoderma | 3 (75%) | ||
| Nausea | 1 (25%) | ||
| Maculopapular rash | 1 (25%) | ||
| Motor neuropathy | 1 (25%) | ||
| Cohort 5 (n=19) MTD | |||
| Pain | 8 (40%) | 3 (16%) | |
| Lymphedema | 8 (40%) | 5 (26%) | |
| Erythoderma | 7 (35%) | 3 (16%) | |
| Nausea | 6 (30%) | ||
| Maculopapular rash | 5 (25%) | ||
| Motor neuropathy | 4 (20%) | 1 (5%) | |
| CPK elevation* | 4 (20%) | 2 (10%) | 6 (32%) |
| Cohort 6 (n=5) MAD | |||
| Pain | 2 (33%) | 1 (17%) | |
| Lymphedema | 2 (33%) | ||
| Erythoderma | 3 (50%) | ||
| Nausea | |||
| Maculopapular rash | |||
| Motor neuropathy | 1 (17%) | 1 (17%) | |
| Muscle necrosis | 1 (37%) | 1 (37%) | |
| CPK elevation* | 1 (17%) | 3 (50%) |
serologic toxicity, a grade IV CPK lasting less than 7 days was not considered a DLT
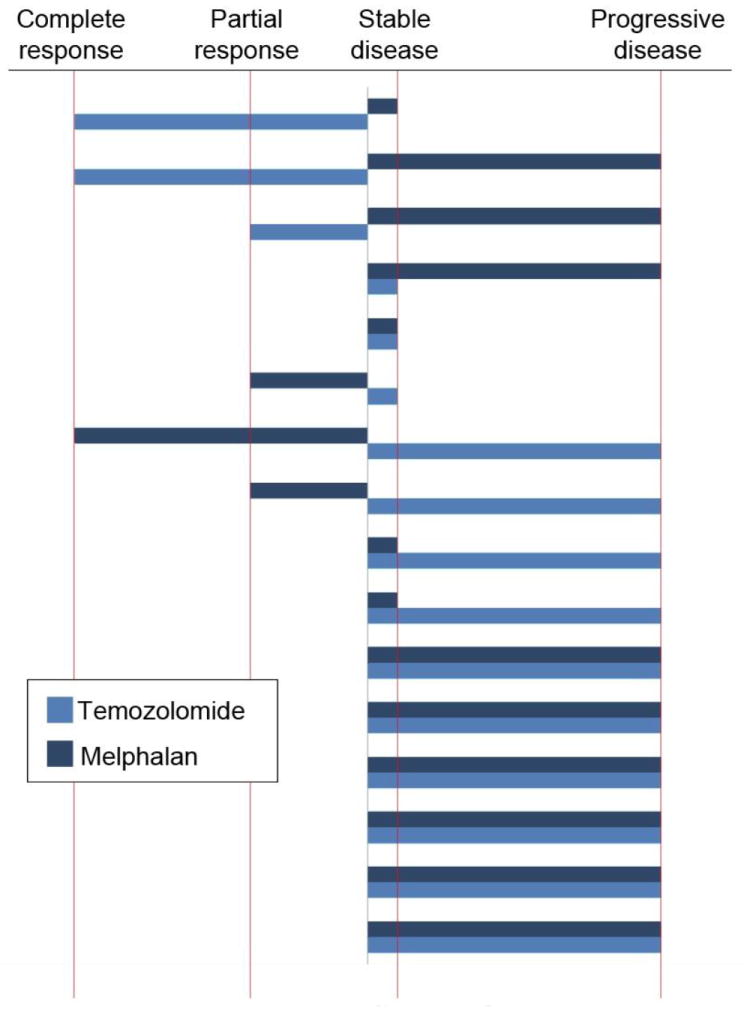
Response by cohort based on measured size changes to target lesions is shown in Figure 1. Although optimal response is defined at three months, patients with evidence of tumor growth or new histologically confirmed lesions could have a response determination before 3 months and be taken off study. Of the nineteen patients at the MTD, 2 patients had CR (10.5%), 1 patient had PR (5.3%), 3 patients had SD (15.8%), and 13 patients had PD (68.4%). At the maximum administered dose (3600 mg/m2), 1 patient had a PD at six weeks while four other patients had SD at the three month time point. For the 2 patients achieving a CR, 1 patient was free from extremity disease for 18 months before a solitary regional recurrence occurred that was resected. The other CR patient continues to be free of extremity disease at 18 months. Both CRs are free of distant metastatic disease at 18 months. Median time to extremity disease progression for nineteen patients at the MTD was 91 days compared to a median time to progression of 111 days (p<0.008) for a group of our historical LPAM treated patients (n=88) (Figure 2A). Sixteen patients at the MTD in this trial had undergone prior LPAM based regional chemotherapy; individual patient response to TMZ was different from response to LPAM in 9/16 (56%) of patients as shown in Figure 2B.
Figure 1. Change in tumor SOLD after temozolomide ILI, stratified by dose level.
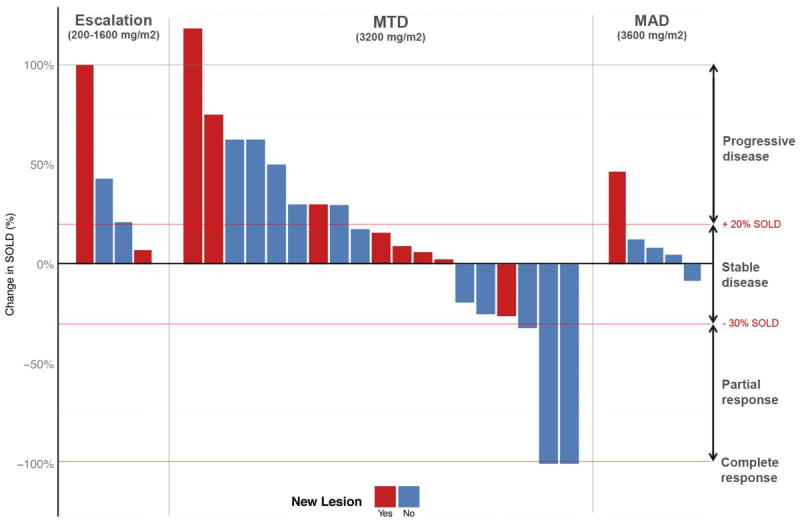
Percent change in tumor sum of longest diameter (SOLD) at 3 months post ILI with TMZ stratified by dose level. Red indicated appearance of new lesion, blue indicates no new lesions.
Figure 2.
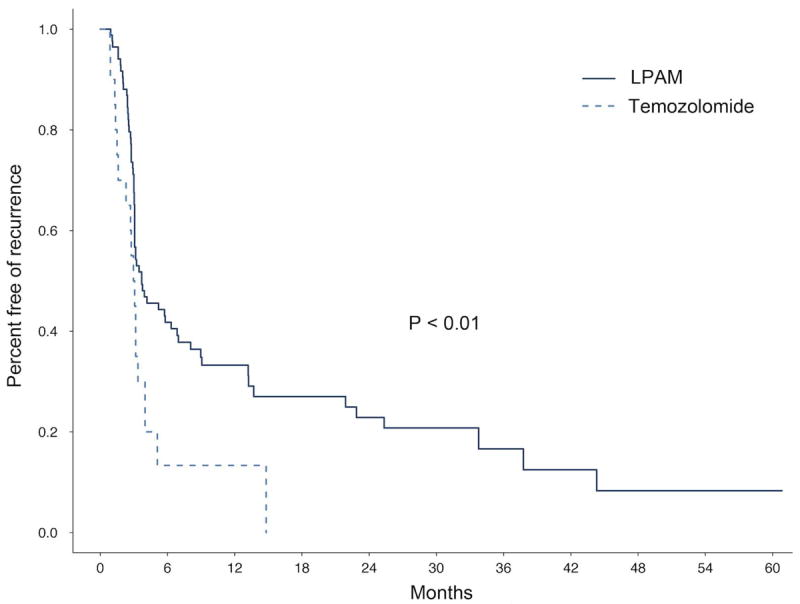
A. Time to in-field extremity progression, TMZ vs. LPAM
A: Kaplan Meier curve showing time to in-field extremity progression of disease after ILI with LPAM (dark blue) compared to ILI with TMZ (light blue).
B. RECIST response to ILI by patient, TMZ vs. LPAM
B: RECIST response by chemotherapy treatment. Each pair of bars represents 1 patient. The dark blue shows the patient’s response to ILI with LPAM, the light blue shows the patient’s response to ILI with TMZ. This figure should be published online only.
Pharmacokinetic analysis showed higher peak concentrations of TMZ and AIC (metabolite of TMZ) at increasing dose levels as expected (Figure 3). Neither peak TMZ concentration during ILI, peak AIC concentration, area under the time versus concentration curve for TMZ (data not shown), nor the area under the time versus concentration curve for AIC appeared to correlate with response or toxicity (Figure 3).
Figure 3. PK concentrations stratified by TMZ ILI dose level.
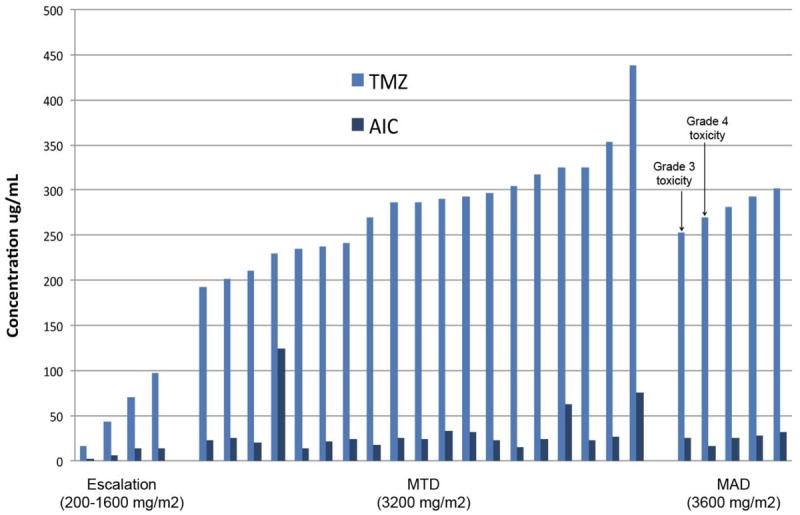
A: Peak concentrations of TMZ and AIC during ILI with TMZ by dose level. This figure should be published online only.
B: Peak concentrations of TMZ and AIC during ILI by response to treatment (n=28).
Figure 4 shows MGMT promoter methylation status and qPCR MGMT expression from pre-ILI tumor tissue for patients at the MTD (n=18) by response. There was no obvious correlation between MGMT promoter methylation status and either MGMT RNA expression or patient response. Likewise, while we did see some variability between patient MGMT RNA expression levels, we did not see a consistent relationship between MGMT levels and tumor response to treatment. Figure 5 shows MGMT expression by response for patients (n=11) at the MTD who had tumor biopsies obtained at multiple time points. MGMT levels were generally stable or decreased when comparing pre-ILI levels to those levels in tumors observed 24 hours post ILI or six weeks post ILI (Figure 5).
Figure 4. MGMT expression, stratified by RECIST response.
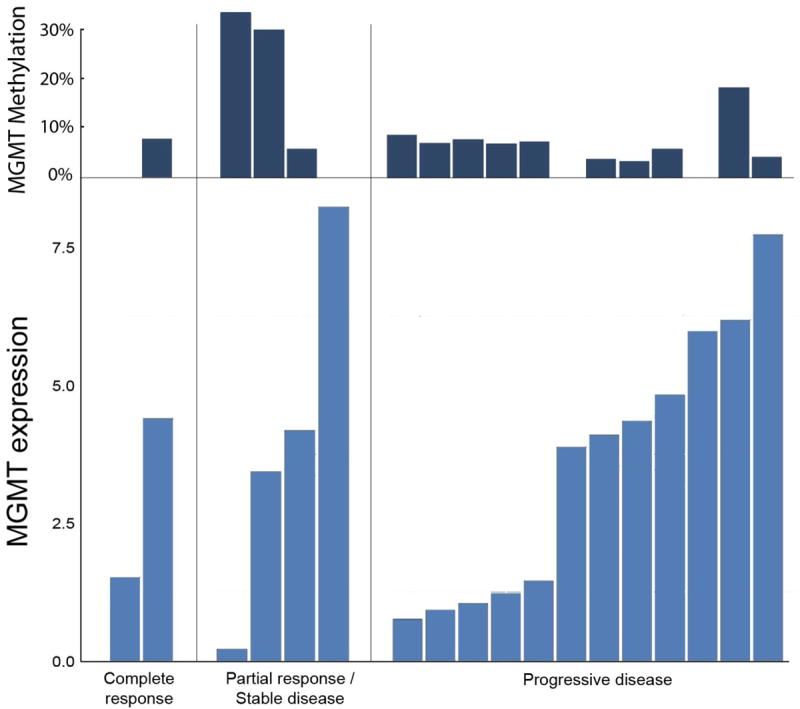
MGMT methylation (top dark blue) and MGMT qpcr expression (bottom light blue) on pre-ILI tumor for patients treated at the MTD (n=18).
Figure 5. Change in MGMT expression after exposure to TMZ.
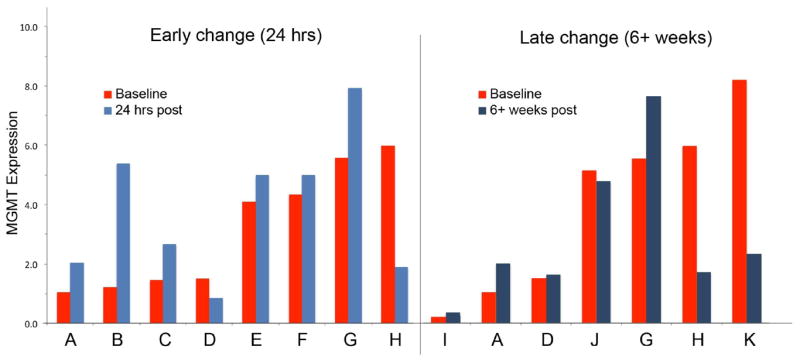
Change in MGMT expression in tumor tissue over time for 11 patients in whom sequential tumor biopsies were obtained. Red=baseline (pre treatment tumor biopsy), light blue= 24 hour post treatment tumor biopsy, dark blue= 6 weeks post treatment tumor biopsy.
Discussion
This is the first trial to use intra-arterial TMZ based regional chemotherapy for the treatment of advanced extremity melanoma. The MTD for future use in ILI was determined to be 3200 mg/m2 times 0.18 for the lower extremity and 0.09 for the upper extremity and a preliminary CR rate of 10.5% in 19 patients treated at the MTD was observed. Based upon genomic analysis of patients not responding to LPAM ILI using a TMZ gene signature that included MGMT, we anticipated about 40% of this group would be sensitive to TMZ ILI (18). While we did have two patients with CR, several factors may have lowered response. Notably, conversion of TMZ to the active metabolite is favored by an alkaline environment; the limb during infusion becomes progressive acidotic which could have affected drug delivery (19).
Not suprisingly, several patients in this study responded differently to TMZ ILI compared to LPAM ILI (Figure 2B) which we also demonstrated in in our preclinical work. Further work is needed as reliable biomarkers that predict patient response have yet to be identified for either LPAM ILI or TMZ (20-24). Here pharmacokinetics of temozolomide and the active metabolite were not predictive of toxicity or response MGMT expression and MGMT promoter methylation status were explored as a potential predictor of response with no obvious correlations found. In glioblastomas, MGMT promoter methylation status has been found to be a more sensitive measure of MGMT activity and subsequent responsiveness to alkylating agents (25, 26). Here MGMT promoter methylation did not correlate with tumor response. Finding correlations was potentially limited by a smaller number of patients (n=18) and the modest response rate.
By inducing tumor cell death, regional chemotherapy can potentially reduce the ability of the tumor to suppress immune responses and simultaneously prime and activate antigen-specific immune cells for tumor cell killing (27). TMZ results in the translocation and expression of calreticulin, a signal that encourages dendritic cells to phagocytose and cross-prime tumor antigen-specific T cells (28). While monitoring these effects is difficult, the immune response may ultimately explain the differential tumor responses. Type III TGF-β receptor expression is down regulated during progression in many human cancers (29). Recently, our group demonstrated that plasma type III TGF-β receptor levels were higher in patients responding to ILI with LPAM and had significantly improved overall survival (29). As we start to evaluate the ability of a regional treatment to generate a systemic immune response, combination strategies using newly approved drugs for melanoma will become important. Trials are currently underway or planned using ILI plus checkpoint blockade inhibitors (30, 31).
Despite the modest objective response rate in this group of predominately pretreated patients, the minimal toxicity of TMZ via ILI allows for additonal studies designed to improve the treatments efficacy. Twenty one percent of our historic LPAM via ILI treated patients (n=122) experienced a ≥ grade 3 limb toxicity (6). In this trial, no patient who receieved the MTD had more than a grade 2 clinical toxicity and only 7.1% (2/28) of all patients in the trial had ≥ grade three limb toxicity. Some institutions perform prophylactic fasciotomies at the time regional chemotherapy to avoid severe toxicity which may further reduce toxicity especially in limb perfusion. Another strategy given the low toxicity is to explore the use of repeat ILIs with TMZ. Repeat regional treatments with LPAM have been associated with improved response rates but carries increased toxicity, a side effect that may not be as pronounced with TMZ ILI (32, 33). A second strategy would be to try TMZ use in HILP. HILP appears to be a more effective way to deliver LPAM as compared to ILI but the HILP technique is also associated with a slightly higher toxicity profile (9). A third approach to improve TMZ ILI response rates might be strategies that involve MGMT inhibiton or other pathways involved in drug resistance(34).
Compared to prior experience with LPAM ILI, TMZ was well tolerated. TMZ should be continued to be explored for use in regional chemotherapy treatments with the goals of exploring how to better individualize treatment, optimize regional responses, and determining if this chemotherapeutic can help facilitate the generation of a systemic immune response to regional therapy.
Footnotes
Presented in part at the Society of Surgical Oncology’s 67th Annual Cancer Symposium. March 13-15, 2014. Phoenix, Arizona
A video of the presentation of the data in this article at the 67th Annual Society of Surgical Oncology Cancer Symposium is available at www.surgonc.org/vm.
Disclosures: Merck provided funding and drug (temozolomide, Temodar ®) for this phase I clinical trial.
References
- 1.Benckhuijsen C, Kroon BBR, van Geel AN, et al. Regional perfusion treatment with melphalan for melanoma in a limb: An evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157. [PubMed] [Google Scholar]
- 2.Krementz ET, Ryan RF. Chemotherapy of Melanoma of the Extremities by Perfusion: Fourteen years Clinical Experience. Ann Surg. 1972;175:900. doi: 10.1097/00000658-197206010-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson JF, Gianoutsos MP. Isolated Limb Perfusion for Melanoma: Effectiveness and Toxicity of Cisplatin Compared with that of Melphalan and Other Drugs. World J Surg. 1992;16:227. doi: 10.1007/BF02071525. [DOI] [PubMed] [Google Scholar]
- 4.Lejeune FJ, Ghanem GE. A Simple and Accurate New Method for Cytostatic Dosimetry in Isolation Perfusion of the Limbs Based on Exchangeable Blood Volume Determination. Cancer Res. 1987;47:639. [PubMed] [Google Scholar]
- 5.Briele HA, Djuric M, Jung DT, et al. Pharmacokinetics of Melphalan in Clinical Isolation Perfusion of the Extremities. Cancer Res. 1985;45:1885–9. [PubMed] [Google Scholar]
- 6.Raymond AK, Beasley GM, Broadwater G, et al. Current Trends in Regional Therapy for Melanoma: Lessons Learned from 225 Regional Chemotherapy Treatments between 1995 and 2010 at a Single Institution. J Am Coll Surg. 2012;214(2):245. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanki A, Kam PC, Thompson JF. Long-term results of hyperthermic, isolated limb perfusion for melanoma: a reflection of tumor biology. Ann Surg. 2007;245(4):591–6. doi: 10.1097/01.sla.0000251746.02764.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornett WR, Mccall LM, Petersen, et al. Randomized Multicenter Trial of Hyperthermic Isolated Limb Perfusion With Melphalan Alone Compared With Melphalan Plus Tumor Necrosis Factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol. 2010;24(25):4196–4201. doi: 10.1200/JCO.2005.05.5152. [DOI] [PubMed] [Google Scholar]
- 9.Beasley GM, Caudle A, Petersen RP, et al. A Multi-Institutional Experience of Isolated Limb Infusion: Defining Response and Toxicity in the US. J Am Coll Surg. 2009;208(5):706–15. doi: 10.1016/j.jamcollsurg.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Kroon HM, Moncrieff M, Kam PCA, et al. Outcomes Following Isolated Limb Infusion for Melanoma. A 14-Year Experience. Ann Surg Onc. 2008;15(11):3033–13. doi: 10.1245/s10434-008-9954-6. [DOI] [PubMed] [Google Scholar]
- 11.Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5:144–151. doi: 10.1634/theoncologist.5-2-144. [DOI] [PubMed] [Google Scholar]
- 12.Bleehen NM, Newlands ES, Lee SM, et al. Cancer Research Campaign phase II trial of temozolomide in metastatic melanoma. J Clin Oncol. 1995;13:910–913. doi: 10.1200/JCO.1995.13.4.910. [DOI] [PubMed] [Google Scholar]
- 13.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–16. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 14.Ueno T, Ko SH, Grubbs E, et al. Temozolomide is a novel regional infusion agent for the treatment of advanced extremity melanoma. Am J Surg. 2004;188(5):532–7. doi: 10.1016/j.amjsurg.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimoto Y, Augustine CK, Yoo JS, et al. Defining regional infusion treatment strategies for extremity melanoma: comparative analysis of melphalan and temozolomide as regional chemotherapeutic agents. Mol Cancer Ther. 2007;6(5):1492–500. doi: 10.1158/1535-7163.MCT-06-0718. [DOI] [PubMed] [Google Scholar]
- 16.Schering-Plaugh Research Institute (Protocol # LC/MS/MS 073.100)
- 17.Chowdhury SK, Laudicina D, Blumekrantz N, et al. An LC/MS/MS method for the quantitation of MTIC (5-(3-N-methyltriazen-1-yl)-imidazole-4-carboxamide), a bioconversion product of temozolomide, in rat and dog plasma. J Pharmaceutical and Biomedical Analysis. 1995;19(5):659–668. doi: 10.1016/s0731-7085(98)00198-8. [DOI] [PubMed] [Google Scholar]
- 18.Augustine CK, Yoo JS, Potti AN, et al. Genomic and molecular profiling predicts response to temozolomide in melanoma. Clinical Cancer Research. 2009;15:502–510. doi: 10.1158/1078-0432.CCR-08-1916. Figure correction in Clinical Cancer Research 15: 3240, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Stevens M, Bradshaw T. Temozolomide: mechanisms of action, repair and resistance. Curr Mol Phramcol. 2012 Jan;5(1):102–14. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 20.Beasley GM, Riboh JC, Augustine CK, et al. A Prospective Multi-Center Phase II Trial of Systemic ADH-1 in Combination with Melphalan via Isolated Limb Infusion (M-ILI) in Patients with Advanced Extremity Melanoma. J Clin Oncol. 2011;29(9):1210–1215. doi: 10.1200/JCO.2010.32.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beasley GM, Coleman AP, McMahon N, et al. A Phase I Multi-Institutional Study of Systemic Sorafenib in Conjunction with Regional Melphalan for In-Transit Melanoma of the Extremity. Ann Surg Onc. 2012;19(12):3896–905. doi: 10.1245/s10434-012-2373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beasley GM, Shetty G, Sparks S, et al. Plasma Cytokine Analysis in Patients with Advanced Extremity Melanoma Undergoing Isolated Limb Infusion. Ann Surg Onc. 2013;20(4):1128–35. doi: 10.1245/s10434-012-2785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon N, Cheng TY, Beasley GM, et al. Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol. 2009;16(4):953–961. doi: 10.1245/s10434-008-0288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santillan AA, Delman KA, Beasley GM, et al. Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion: a multi-institutional analysis. Ann Surg Onc. 2009;16(9):2570–0. doi: 10.1245/s10434-009-0563-9. [DOI] [PubMed] [Google Scholar]
- 25.Kishida Y, Nastume A, Toda H. Correlation between quantified promoter methylation and enzymatic activity of O6-methylguanine-DNA methyltransferase in glioblastomas. Tumor Biol. 2012;33(2):373–81. doi: 10.1007/s13277-012-0319-1. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi H, Yazawa T, Okudela K, et al. Inactivation of 06-methylguanine DNA methyltransferase in human lung adenocarncinoma relates to high grade histology and worse prognosis among smokers. Jpn J Cancer Res. 2002;93(2):184–9. doi: 10.1111/j.1349-7006.2002.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donepudi M, Jovasevic VM, Raychaudhuri P, et al. Melphalan induced up regulation of B7-1 surface expression of normal splenic B cells. Cancer Immunother. 2003;52:162–170. doi: 10.1007/s00262-002-0345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 29.Hanks BA, Holtzhausen A, Evans KS, et al. Type III TGF-β receptor downregulation generates an immunotolerant tumor microenvironment. J Clin Invest. 2013;8 doi: 10.1172/JCI65745. Epub aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Personal communication Mary S. Brady, Charlotte E. Ariyan, MD, PhD. Phase II Trial Of The Addition Of Ipilimumab (MDX-010) To Isolated Limb Infusion (ILI) With Standard Melphalan And Dactinomycin In The Treatment Of Advanced Unresectable Melanoma Of The Extremity
- 31.A Phase II trial of Neoadjuvant Ipilimumab followed by Melphalan via Isolated Limb Infusion for Patients with Unresectable In-transit Extremity Melanoma (AJCC Stage IIIB/IIIC) is currently before the IRB at Duke University, Douglas Tyler, PI.
- 32.A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Chai CY, Deneve JL, Beasley GM, et al. Ann Surg Oncol. 2012 May;19(5):1637–43. doi: 10.1245/s10434-011-2151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Kroon HM, Lin DY, Kam PC, Thompson JF. Cancer. 2009 May 1;115(9):1932–40. doi: 10.1002/cncr.24220. [DOI] [PubMed] [Google Scholar]
- 34.Ueno T, Ko SH, Yashimoto Y, et al. A novel regional strategy for extremity melanoma using modulation of chemotherapy resistance: intra-arterial temozolomide plus systemic O6-benzylguanine. Molecular Cancer Therapeutics. 2006;5:732–738. doi: 10.1158/1535-7163.MCT-05-0098. [DOI] [PubMed] [Google Scholar]


