Abstract
Endogenous activation of μ-opioid receptors (MORs) provides relief from acute pain. Recent studies have established that tissue inflammation produces latent pain sensitization (LS) that is masked by spinal MOR signaling for months, even after complete recovery from injury and re-establishment of normal pain thresholds. Disruption with MOR inverse agonists reinstates pain and precipitates cellular, somatic and aversive signs of physical withdrawal; this phenomenon requires N-methyl-D-aspartate receptor-mediated activation of calcium-sensitive adenylyl cyclase type 1 (AC1). In this review, we present a new conceptual model of the transition from acute to chronic pain, based on the delicate balance between LS and endogenous analgesia that develops after painful tissue injury. First, injury activates pain pathways. Second, the spinal cord establishes MOR constitutive activity (MORCA) as it attempts to control pain. Third, over time, the body becomes dependent on MORCA, which paradoxically sensitizes pain pathways. Stress or injury escalates opposing inhibitory and excitatory influences on nociceptive processing as a pathological consequence of increased endogenous opioid tone. Pain begets MORCA begets pain vulnerability in a vicious cycle. The final result is a silent insidious state characterized by the escalation of two opposing excitatory and inhibitory influences on pain transmission: LS mediated by AC1 (which maintains accelerator), and pain inhibition mediated by MORCA (which maintains the brake). This raises the prospect that opposing homeostatic interactions between MORCA analgesia and latent NMDAR–AC1-mediated pain sensitization create a lasting vulnerability to develop chronic pain. Thus, chronic pain syndromes may result from a failure in constitutive signaling of spinal MORs and a loss of endogenous analgesic control. An overarching long-term therapeutic goal of future research is to alleviate chronic pain by either: a) facilitating endogenous opioid analgesia, thus restricting LS within a state of remission; or b) extinguishing LS altogether.
Keywords: pain, adenylyl cyclase, NMDA, latent sensitization, opioid, analgesia, constitutive activity, dependence, addiction
2. Introduction
Serious tissue injury causes the acute pain that we are all familiar with. For elusive reasons, acute pain accelerates towards a state of incapacitating chronic pain in hundreds of millions of people around the world. For example, back injuries lead to unrelenting, rampant low back pain that destroys quality of life. Unfortunately, adequate therapeutic strategies to stop chronic pain do not exist. Even the most powerful opiate pain killers such as morphine and oxycodone produce severe adverse effects including cognitive impairment and respiratory depression, as well as societal issues with drug diversion. Side effects aside, chronic pain responds poorly to treatment, despite considerable efforts towards the development of efficacious analgesic drugs. A fundamental but somewhat neglected long-term goal in the field is to better understand the ability of the central nervous system (CNS) to intrinsically inhibit the mechanisms that accelerate pain. In this chapter we discuss a relatively new approach: to search for the mechanisms whereby mammals naturally recover from the pain of inflammation. This chapter focuses on the endogenous opioid system. During injury, opioid peptides are released at sites of pain modulation where they act at opioid receptors to put the brakes on the transmission of pain signals to the brain. Indeed, soon after tissue injury produces pain, compensatory analgesic systems involving μ, δ, and/or κ opioid receptors can be activated at multiple sites of pain modulation including the spinal cord and brain, and might temper the intensity of acute postoperative pain in humans (Levine et al., 1978). We describe recent data indicating that opioid receptors can acquire the potential to oppose chronic pain via a constitutive, ligand-independent, activation mechanism. An understanding of the body's own pain defenses within the CNS should provide valuable insight into new strategies to prevent the transition from acute to chronic pain.
3. Opioid Receptors and Endogenous Analgesia
3.1. Opioid Receptors
Cutaneous noxious stimuli drive ascending pain transmission through the spinal release of glutamate and peptide neurotransmitters from presynaptic terminals of primary sensory neurons (Basbaum et al., 2009). Opioid receptors include the μ (MOR), δ (DOR), and κ (KOR) types. Each are widely distributed throughout the nervous system, including key sites of pain modulation (Mansour et al., 1995, Erbs et al., 2014). In addition to expression in brain, peripheral nerve endings and dorsal root ganglia (DRG), opioid receptors decorate the central terminals of primary afferent neurons and second order neurons in the dorsal horn (DH) of the spinal cord (Besse et al., 1990, Kohno et al., 1999, Spike et al., 2002, Marker et al., 2005, Scherrer et al., 2009, Heinke et al., 2011). In specific, MORs and DORs produce their antinociceptive effects in molecularly and functionally distinct populations of sensory afferents terminating in the DH (Bardoni et al., 2014). This review will focus on the ability of spinally-located MORs to exert long-lasting inhibition of spinal pain transmission that is triggered by tissue injury.
Opioid receptor activation either by endogenous ligands or by exogenously administered agonists elicits powerful spinal antinociception (Yaksh, 1987, Yaksh et al., 1988). MORs are a vital presynaptic target, and their activation leads to reduction of neurotransmitter (e.g. glutamate) release from the central terminals of primary afferent neurons (Jessell and Iversen, 1977, Duggan and North, 1983, Yaksh et al., 1988, Chang et al., 1989, Hori et al., 1992, Suarez-Roca and Maixner, 1992, Glaum et al., 1994, Terman et al., 2001), ultimately leading to inhibition of spinal excitatory pain transduction (Yoshimura and North, 1983). MORs are also an important postsynaptic target, as they are found in a population of mostly excitatory neurons in laminae I and II, where they inhibit the firing of action potentials, presumably leading to inhibition of nociceptive transmission to the brain (Willcockson et al., 1984, Jeftinija, 1988, Schneider et al., 1998, Kohno et al., 1999, Aicher et al., 2000).
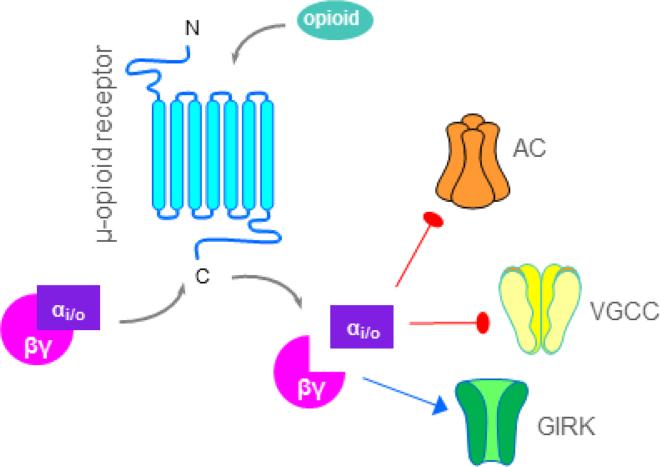
All opioid receptor subtypes are members of the heterotrimeric guanosine 5′-triphosphate–binding protein (G protein)–coupled receptor (GPCR) superfamily, Class A rhodopsin subfamily. Agonists dissociate Gαi/o which then inhibits adenylyl cyclase-mediated production of adenosine 3′,5′-cyclic monophosphate (cAMP), thus decreasing the opening of voltage-gated Ca2+ channels (VGCC) (Kohno et al., 1999, Kondo et al., 2005). The dissociated Gαβγ subunits promote the opening of G protein-coupled inwardly-rectifying potassium channels (GIRKs) to further hyperpolarize the neuron (Figure 1). This review will primarily focus on opioidergic inhibition/regulation of spinal adenylyl cyclases, specifically the calcium-sensitive adenylyl cyclase type 1.
Figure 1. Opioidergic signaling.
Opioid agonists bind to the extracellular binding pocket of opioid receptors to activate intracellular inhibitory G-proteins (Gαi/o—βγ). Dissociated G-proteins can reduce neuronal excitation and/or neurotransmitter release via inhibition of adenylyl cyclases (AC), voltage-gated calcium channels (VGCC), and activation of inward-rectifying potassium channels (GIRK). Red blunted lines indicate inhibition and blue arrows indicate activation.
3.2. Compensatory development of endogenous analgesia
Pain intensity and duration are regulated by numerous inhibitory systems, including spinally secreted opioid peptides and subsequent activation of opioid receptors (Basbaum and Fields, 1984) (Ossipov et al., 2010). During noxious stimulation or after severe tissue injury, opioid systems in the brain and spinal cord orchestrate an adaptive compensatory response to inhibit pain in humans under several circumstances. For example, opioidergic analgesia systems are thought to be activated when a patient undergoes surgery, when a soldier is wounded in battle, or when a runner runs a marathon. Positron emission tomography studies in humans indicate that sustained pain causes the release of endogenous substances acting at MORs in the brain (Zubieta et al., 2001) (presumably to regulate the sensory and affective components of the pain experience). In rodents, repetitive or sustained noxious stimuli increase concentrations and/or release of opioid peptides (e.g. endorphins, enkephalins, dynorphins and endomorphins) at sites of pain modulation within the rodent DH (Yaksh and Elde, 1981, Basbaum and Fields, 1984, Iadarola et al., 1986, Noguchi et al., 1992, Song and Marvizon, 2003a, Ossipov et al., 2010), which can induce MOR activation (Tambeli et al., 2009), though this has yet to be shown in human spinal cord. Synaptic mechanisms of endogenous opioid inhibition in the DH include modulation of neuronal excitability and fine-tuning of glutamatergic nociceptive transmission at both presynaptic (Hori et al., 1992, Terman et al., 2001) and post-synaptic neurons (Willcockson et al., 1984, Jeftinija, 1988, Aicher et al., 2000).
Numerous neuromodulatory systems other than the opioids promote endogenous analgesia (Millan, 2002). For example, conditional knockdown of neuropeptide tyrosine (NPY) before the initiation of inflammation extended the time course of mechanical and heat hyperalgesia (Solway et al., 2011). Similarly, dual blockade of cannabinoid CB1 and CB2 receptor signaling prevents the resolution of postoperative allodynia (Alkaitis et al., 2010). These studies indicate that endogenous NPY and endocannabinoids hasten the resolution of inflammatory and neuropathic pain. Therefore it is likely that endogenous MOR constitutive activity works in concert with numerous neuromodulatory systems to reduce nociception and aid in the resolution of pain.
3.3.Endogenous mu opioid receptor analgesia in the dorsal horn
The above mentioned studies indicate that noxious stimulation recruits pain inhibitory opioidergic systems. When the stimulus produces physical tissue or nerve injury, this compensatory response can be quite long-lasting. Animal studies suggest that tissue injury can increase opioid receptor density and opioid receptor intracellular signaling in the dorsal horn, even after inflammatory hyperalgesia has subsided.
MOR expression in the superficial spinal cord progressively increases over days to weeks during chronic inflammation states and is suggested to aid in the analgesic effectiveness of exogenously applied opiate drugs (Ji et al., 1995, Goff et al., 1998, Mousa et al., 2002). This can be long lasting: autoradiography studies demonstrate that opioid receptor density remains elevated for almost 3 months in a complete Freund's adjuvant (CFA) model of rheumatoid polyarthritis (Calza et al., 2000). We recently reported that injury-induced MOR signaling does not necessarily dissipate over time, and can be maintained long enough to oppose chronic pain. We reported that: 1) disruption of Gαi/o signaling with intrathecal pertussis toxin precipitated mechanical hyperalgesia in CFA-21d but not sham mice; 2) the antinociceptive effects (hotplate assay) of the MOR selective agonist DAMGO were potentiated in the post-hyperalgesia state, 21 days after intraplantar injection of CFA; and 3) DAMGO-stimulated GTPγS35 binding in lumbar spinal cord sections was increased (↑Emax) in CFA-21d mice compared to sham (Corder et al., 2013).
Augmentations in spinal MOR-G-protein coupling lasted for at least 3 weeks after the injury, even though the initial bout of allodynia had resolved. This is consistent with other studies showing that noxious stimulation can induce spinal MOR activation that outlasts the pain stimulus that initiated it (Tambeli et al., 2009). Taken together, these findings demonstrate that injury increases MOR-G-protein signal transduction in the DH that persists after the resolution of inflammatory hyperalgesia. Studies utilizing opioid receptor antagonists, discussed next, suggest that this long-lasting MOR signaling is poised to mediate endogenous antinociception. The remainder of this review discusses the evidence that this signaling can oppose the emergence and overt manifestations of chronic hyperalgesia.
3.4. Opioid receptor antagonists increase pain intensity
By turning on opioid receptor systems, the body can temper acute postoperative pain, allowing the soldier to escape from the battlefield and the marathon runner to finish the race. This system is mimicked therapeutically when morphine is given to relieve various forms of pain, such as postoperative pain. On the other hand, one can predict that interruption of the opioid system would worsen the pain after surgery, traumatic injury, or pain during a marathon. This prediction is likely to be true, as indicated by numerous studies showing that opioid receptor antagonists can increase pain intensity. For example, animal studies of ongoing or persistent inflammatory pain demonstrate that naloxone or naltrexone can increase mechanical and/or heat hypersensitivity (Millan et al., 1987, Herz and Millan, 1988, Hurley and Hammond, 2000, 2001, Schepers et al., 2008a). Furthermore, as summarized in Table 1, human studies demonstrate that naloxone enhances hyperalgesia in the acute pain setting (Buchsbaum et al., 1977, Levine et al., 1979, Frid et al., 1981, Jungkunz et al., 1983, Anderson et al., 2002). This literature suggest that endogenous opioid analgesia provides an intrinsic braking mechanism that exerts inhibitory control of acute pain intensity soon after tissue injury (Ossipov et al., 2010). But whether opioid receptor signaling is maintained for sufficient duration to counter long-lasting nociceptive mechanisms remained a mystery long after the initial demonstration of endogenous opioid inhibition of acute pain. Can the endogenous opioid system persistently (or indefinitely) oppose persistent pain? This question remained difficult to answer for several reasons. First, ethical issues confound opioid receptor antagonist studies in humans – one can imagine the difficulty in recruiting chronic pain patients who would submit to an intervention that would worsen their pain. Second, most animal models of chronic pain were developed to produce maximal allodynia and hyperalgesia, with the aim to evaluate the pain-reducing efficacy of pharmacological and other interventions. As a result, a floor effect would interfere with the ability to evaluate the ability of opioid receptor antagonist to further decrease nociceptive thresholds. To get around this problem, a small number of studies have utilized tissue or nerve injury models that produce either a relatively low incidence or a relatively low intensity of pain-like behavior. For example, naloxone produces allodynia in rodents with nerve injury that had initially failed to develop behavioral signs of neuropathic pain (Back et al., 2006). Also, opioids can mask the early incidence of pancreatic cancer pain (Sevcik et al., 2006). Third, endogenous opioid analgesia may mask the very existence of hyperalgesia, leading to the false impression that pain sensitization either: 1. never developed after the injury (Back et al., 2006, De Felice et al., 2011); or 2. completely resolved after an initial bout of pain-like behavior. This third idea can readily be tested with the administration of opioid receptor antagonists either during or after the apparent resolution of hyperalgesia (e.g. during the post-hyperalgesia state). For example, following the resolution of hyperalgesia after partial nerve ligation (Guan et al., 2010); or inflammation (Campillo et al., 2011, Corder et al., 2013), naloxone reinstated hypersensitivity. The remainder of this chapter focusses on this approach, which has helped us to understand in great detail the opposing mechanisms that determine latent central sensitization, and will ultimately help in the search to find interventions that prevent or alleviate chronic pain.
Table 1.
Representative human studies describing the effect of naloxone on experimental and postoperative pain. Overall these studies indicate that naloxone can increase pain, signifying the importance of endogenous opioid analgesic activity after injury.
| Nociceptive model | Naloxone dose | n | Pain report | Reference |
|---|---|---|---|---|
| electric shock | 0.8 mg | 5 | no change | El-Sobsky et al. 1976 |
| electric shock | 2 mg | 21 | ↑ pain | Buchsbaum et al. 1977 |
| electric shock plus cold water stressor | 0.8 mg | 32 | ↑ pain | Jungkunz et al. 1983 |
| electric shock and thermal probe | 20 mg | 24 | no change | Stacher et al. 1988 |
| Ischemia | 2 mg | 52 | ↑ pain | Frid et al. 1981 |
| ischemia | 2 mg | 12 | no change | Posner and Burke 1985 |
| post-operative pain | 9 mg | 26 | ↑ pain | Levine et al. 1978 |
| post-operative pain | 0.4 – 10 mg | 77 | ↑ pain | Levine et al. 1979 |
| post-operative pain | 4 mg | 6 | no change | Skjelbred and Lokken. 1983 |
| post-operative pain | 10 mg | 89 | ↑ pain | Gracely et al. 1983 |
| subcutaneous capsaicin | 140 μg/kg | 20 | no change | Benedetti et al. 1999 |
| topical capsaicin | 100 μg/kg | 12 | ↑ pain | Anderson et al. 2002 |
| 1st degree burn | 21 μg/kg | 22 | no change | Werner et al. 2013 |
4. Spinal mechanisms of persistent pain
4.1. Central Sensitization
Chronic pain is determined by facilitatory mechanisms such as long-term potentiation of synaptic strength in DH neurons (Ikeda et al., 2006, Latremoliere and Woolf, 2009, Ruscheweyh et al., 2011). Injury-induced spinal long-term potentiation may be a potential mechanism of a larger phenomenon that develops after severe tissue injury, known as central sensitization, which refers to an increased responsiveness of CNS nociceptive neurons to normal or sub-threshold afferent input. Central sensitization is an NMDAR-dependent phenomenon that is widely believed to contribute to chronic pain states (Latremoliere and Woolf, 2009). Less appreciated, however, are data suggesting that central sensitization can persist in the absence of behavioral signs of hypersensitivity (Reichling and Levine, 2009, Asiedu et al., 2011), in a silent form often referred to as “latent sensitization” (LS).
4.2. Latent Sensitization
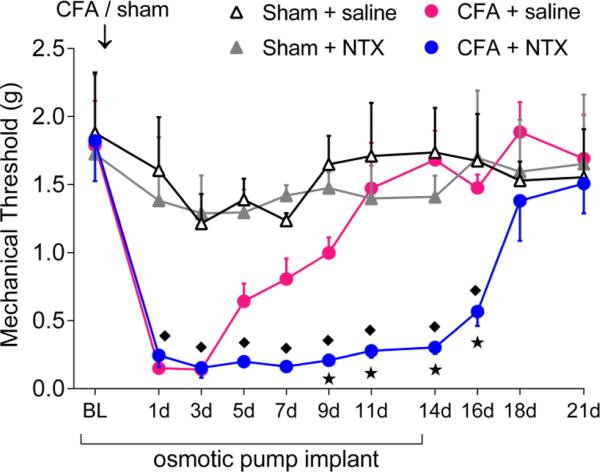
LS is important because it primes nociceptive systems such that, when inhibitory systems fail, a pain episode ensues (Le Roy et al., 2011, Corder et al., 2013). Thus, LS is a form of long-lasting pain vulnerability that develops after traumatic injury or stress, by which the organism may demonstrate greater susceptibility to a potentiated pain response upon subsequent injury or stressor (Aley et al., 2000, Rivat et al., 2002, Parada et al., 2003, Rivat et al., 2007, Summer et al., 2007, Cabanero et al., 2009, Reichling and Levine, 2009, Le Roy et al., 2011). For example, Price and colleagues recently reported that either intraplantar injection of interleukin-6 or plantar paw incision produced a transient acute hypersensitivity that was followed by a long-lasting sensitization of spinal nociceptive pathways (Asiedu et al., 2011). And as described in further detail below, the lasting pain vulnerability promoted by LS can be manifested with pharmacological blockade of pain inhibitory systems (Campillo et al., 2011, Corder et al., 2013). For example, Figure 2 illustrates that minimpump infusion of naltrexone (NTX) prevents the resolution of inflammatory pain. These and the following data indicate that NTX unmasks LS in the spinal cord that persists beyond the resolution of pain and inflammation, reflective of hyperalgesic priming at the peripheral nervous system (Asiedu et al., 2011). In summary, LS represents a predisposition to relapse that may explain the episodic nature and vulnerability to stress that accompanies chronic pain syndromes in humans (Le Roy et al., 2011, Corder et al., 2013), and thus may reflect a critical mechanism responsible for the transition from acute to chronic pain (Rivat et al., 2007). However, LS has not been reported in humans (Pereira et al., 2013), and our understanding of mechanisms of CNS synaptic plasticity in LS is limited to an involvement of NMDAR. Although the cellular mechanisms underlying LS are now emerging (see below and Corder et al), its synaptic basis remains poorly understood and so this is the focus of intense ongoing investigations.
Figure 2. Minipump infusion of NTX increases the duration of mechanical hyperalgesia, indicating that endogenous opioid receptor activity hastens the resolution of inflammatory pain.
Changes in mechanical pain resolution after continual minipump infusion of saline or NTX (10 mg / kg /d) for 14d in Sham and CFA mice (n = 4 - 7 per group). * P < 0.05 compared to CFA + saline group, ◆ P < 0.05 compared to Sham + NTX group. Adapted from Corder et al, 2013.
5. Opioid receptor-masked sensitization
5.1. Endogenous analgesia maintain LS in a state of remission
Until recently, the question as to whether opioid receptor activity persisted for long enough periods to dampen chronic pain remained unanswered. As noted above, opioid receptor density remained elevated for almost 3 months in a CFA model of rheumatoid polyarthritis, long after pain scores had recovered to baseline (Calza et al., 2000).
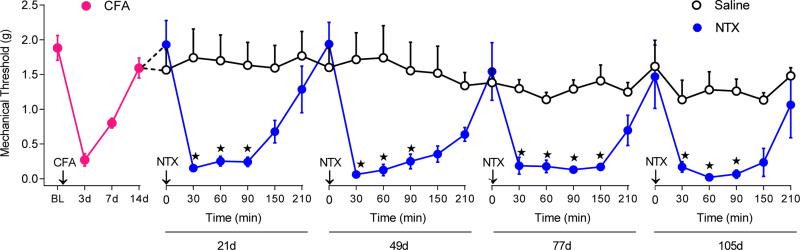
As predicted by long-lasting increases in opioid receptor density and signaling, endogenous opioid inhibition of acute nociception persists even after the initial signs of hyperalgesia have subsided (Yu et al., 1994, Guan et al., 2010, Joseph and Levine, 2010, Campillo et al., 2011). This is demonstrated by the ability of opioid receptor antagonists to precipitate allodynia in the post-hyperalgesic state following tissue or nerve injury states (Yu et al., 1994, Back et al., 2006, Sevcik et al., 2006, Guan et al., 2010, Campillo et al., 2011). In other words, LS outlasts the duration of the injury, and can be revealed with opioid receptor antagonists or inverse agonists that “rekindle” or “reinstate” hyperalgesia (Cabanero et al., 2009, Lian et al., 2010, Le Roy et al., 2011, Corder et al., 2013). For example, our laboratory produced cutaneous inflammation of one paw of the mouse, and then allowed pain behavior to naturally subside over several days to weeks. While the animals were in this post-hyperalgesia state, we disrupted intrinsic opioid receptor activity with inverse agonists. Based on the hypothesis that opioid receptors continue to suppress pain long after an injury, we predicted that inverse agonism would reinstate pain-like behaviors. In addition to pain-like behaviors, we honed in on the spinal cord with a battery of physiological, biochemical, and molecular assays to determine effects on neurons that relay pain signals to the brain. As predicted, we found that inverse agonists reinstated pain-like behaviors when administered months after the induction of inflammation (Figure 3). This suggests that endogenous opioid analgesia silently continues long after an injury has healed and thus promotes the natural recovery of acute inflammatory pain. Thus, pain remission is maintained in part by opioid receptor activity that masks the pronociceptive components of LS.
Figure 3. NTX reinstates inflammatory pain even when administered 6 months after induction of inflammation.
After baseline (BL) measurement of von Frey threshold, mechanical hypersensitivity was allowed to recover (red circles). Effect of repeated subcutaneous saline (open circles) or NTX (3 mg /kg, blue circles) injections on mechanical thresholds at 21, 49, 77, and 105d after CFA (n = 5 – 7). Adapted from Corder et al, 2013.
5.2. Animal models of opioid receptor-masked sensitization
Multiple MOR inverse agonists ((NTX, naloxone, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), β-funaltrexamine (β-FNA)) reinstate hyperalgesia (Corder et al., 2013). These findings are consistent across pain models [CFA, plantar incision (Campillo et al., 2011, Corder et al., 2013), species [rats, mice], and multiple mouse strains [C57Bl/6, CD1 (Campillo et al., 2011, Le Roy et al., 2011, Corder et al., 2013)], and behavioral endpoints [mechanical hyperalgesia, thermal hyperalgesia conditioned place preference (CPP) measure of affective pain (Corder et al.), heat hyperalgesia, nocifensive behavior, mouse grimace scale of spontaneous pain (Corder et al., 2013)], and both sexes (Corder et al., 2013). Our core pain reinstatement model has already been replicated in several laboratories including C. Fairbanks (University of Minnesota), Z. Wang (University of Illinois), J. Mogil (McGill University), and J. Marvizon (University of California, Los Angeles). Thus, opioid receptor-masked LS is long-lasting, powerful, repeatable, and is likely relevant to a broad range of chronic pain subjects.
5.3. Multiple opioid receptors can mask LS
All three opioid receptors, MOR, DOR and KOR have been extensively investigated as potential therapeutic targets for disorders ranging from depression to severe pain (Lutz and Kieffer, 2013). We focused on the MOR, because the μ-selective inverse agonists CTOP and β-FNA reinstated hyperalgesia and spinal pain transmission in CFA 21d mice (Corder et al., 2013). These results do not, however, rule out DOR or KOR. Indeed, KORs might also maintain LS in a state of remission (Schepers et al., 2008b, Campillo et al., 2011). For example, the KOR-selective antagonist nor-binaltorphamine (norBNI) exacerbated CFA mechanical hyperalgesia at the injured paw and unmasked hyperalgesia at the contralateral paw. Also, norBNI reinstated mechanical hyperalgesia when injected weeks after plantar incision performed under remifentanil analgesia (Campillo et al., 2011). Alternatively, DOR may contribute to MOR or KOR function through heterodimerization (Gomes et al., 2000, Law et al., 2005, Yoo et al., 2014), so future studies should evaluate the effect of DOR antagonists as well.
5.4. Multiple neurobiological systems can mask LS
By and large, most of what we know about intrinsic masking of LS comes from interventions that disrupt endogenous opioid receptor systems. However, numerous receptor systems besides MOR likely maintain LS in remission, LS is masked not only by opioid receptor activity, but also by endocannabinoids (Alkaitis et al., 2010, Ji et al., 2011), alpha-2 adrenergic receptors (De Felice et al., 2011), and neuropeptide Y (NPY) receptor activity (Solway et al., 2011). For example, our lab recently used conditional NPY knockout mice and NPY Y1 and Y2 receptor antagonists to demonstrate that the endogenous NPY system in the DH is involved in repressing the LS induced by inflammation or nerve damage (Solway et al., 2011). So while this section focuses on opioid receptors, it should be kept in mind that they might work in concert with endocannabinoid, resolvin, neuropeptide Y, or other pain inhibitory systems to prevent the induction or hasten the resolution of chronic pain.
5.5. Long-lasting endogenous analgesia requires a sensitized state
As indicated above, opioid mechanisms are thought to be recruited in response to intense, sustained nociceptive signaling. On the other hand, in wild-type uninjured mice, transient pain is not modulated by endogenous opioid tone, as opioid receptor antagonists such as NTX, naloxone, CTOP and β-FNA do not change thermal or mechanical thresholds (Grevert and Goldstein, 1978, Kern et al., 2008, Corder et al., 2013). This is consistent with observations in normal MOR knock-out mice that do not show changes in acute nociceptive processing (Fuchs et al., 1999). Similar studies in normal human subjects also report no modulation of pain perception upon opioid antagonist treatment, suggesting little to no tonically active endogenous opioid signaling in the absence of tissue or nerve damage (El-Sobky et al., 1976, Schoell et al., 2010). Even in the setting of acute ongoing nociception, as is the case after the intraplantar injection of dilute formalin, naloxone has no effect (Taylor et al., 1997).
5.6. LS develops bilaterally
Mechanical sensitivity develops not only on the side of the body that receives tissue injury, but also on the contralateral side. This bilateral hyperalgesia is observed in numerous pain models (inflammation (Chillingworth et al., 2006), nerve injuries (Seltzer et al., 1990, Kim and Chung, 1992), formalin (Aloisi et al., 1993), arthritis (Rees et al., 1996), sciatic inflammatory neuritis (Chacur et al., 2001), and muscle pain (Sluka et al., 2001, Ainsworth et al., 2006). Persistent inflammation increases the effectiveness of exogenously applied opioid agonists to inhibit contralateral nociceptive sensitivity in the rostroventral medulla (Hurley and Hammond, 2000, 2001, Schepers et al., 2008a) and the spinal cord (Stanfa et al., 1992, Stanfa and Dickenson, 1995). We now know that this extends to LS, because opioid inverse agonists produce behavioral signs of pain and spinal neuron sensitization (stimulus-evoked expression of phosphorylated extracellular signal-regulated kinase, pERK) when delivered in the post-hyperalgesia state (Corder et al., 2013). Since connections between the two sides of the spinal cord are sparse at best, bilateral hyperalgesia suggests that the brain is involved in LS. Indeed, leading explanations for bilateral hyperalgesia include top-down facilitation involving descending facilitatory fibers from the brainstem (Koltzenburg et al., 1999, Tillu et al., 2008, Chai et al., 2012). Another possible mechanism involves communication of reactive spinal astrocytes through gap-junction networks across large distances (Hatashita et al., 2008, Gao and Ji, 2010b). However the lack of NTX-induced pERK in microglia and astrocytes suggests that glia are not activated upon loss of tonic MOR signaling (Corder et al., 2013).
Tissue injury produces long-lasting contralateral increases in MOR-G-protein coupling and β-funaltrexamine-induced decreases in basal MOR-G-protein coupling (Corder et al., 2013). Thus, MOR is intricately involved in the regulation of contralateral LS. The presence of contralateral spinal MORCA and neural sensitization illustrates the spread of LS to areas of the CNS beyond those directly innervated by the injured tissue. If true, then loss of MORCA antinociception (e.g. during stress) could lead to the emergence of rampant chronic pain (Rivat et al., 2007, De Felice et al., 2011). Thus MORCA might tonically repress wide-spread hyperalgesia.
5.7. Latent sensitization develops across the pain neuraxis
LS is maintained within the periphery (Stein and Lang, 2009, Guan et al., 2010), local spinal circuitry (Ruscheweyh et al., 2011) and by descending facilitatory signals from brainstem nuclei (Porreca et al., 2002, De Felice et al., 2011). NTX is lipophilic and therefore readily crosses the blood-brain barrier (BBB), antagonizing opioid receptors at supraspinal, spinal, and peripheral sites. To address the site of action of endogenous opioid receptors, investigators have: 1) evaluated local administration of low doses of NTX at the site of injury, at the level of the spinal cord using the intrathecal route, or into the brain; 2) used peripherally-restricted receptor antagonists; or 3) evaluated neuronal activation at CNS sites of pain modulation.
5.7.1. Periphery
Peripheral mechanisms contribute to LS. For example, Levine and colleagues describe a large body of literature describing a long-lasting latent hyperresponsiveness within primary afferent nociceptors following local administration of the proinflammatory cytokine prostaglandin E2, for which they have coined the term “hyperalgesic primary” (Hucho and Levine, 2007, Reichling and Levine, 2009, Ferrari et al., 2010, Joseph and Levine, 2010, Green et al., 2011). In a partial L5 spinal nerve ligation model of neuropathic pain, Guan et al waited 7-8 wk for mechanical allodynia to subside, and then injected either naloxone or naloxone methodide (a peripherally acting opioid receptor antagonist) (Guan et al., 2010). Naloxone methiodide reinstated mechanical allodynia, suggesting a contribution of peripheral opioid receptors to LS in the setting of neuropathic pain. In an intraplantar CFA model of inflammatory pain, Stein and Lang waited 4-6 days, and found that local injection of naloxone increased hyperalgesia (Stein and Lang, 2009). In a similar model (using of lower amount of CFA), however, we reported that naltrexone methobromide, another opioid receptor antagonist with a quaternary amine that restricts BBB permeability, did not alter mechanical thresholds when injected 21 days after inflammation. Indeed, we found that tissue edema gradually resolved within 77 d after induction of inflammation (Corder et al., 2013). Beyond that time, we found that NTX reinstated hyperalgesia, suggesting that endogenous opioid signalling persists beyond tissue healing and the resolution of primary hyperalgesia. Thus, peripheral mechanisms may contribute to LS after nerve injury, but might be restricted to early timepoints after non-neural tissue injury. This has prompted a focus on CNS sites of action (Corder et al., 2013).
5.7.2. Spinal cord dorsal horn
Opioids inhibit LTP in the DH (Terman et al., 2001, Benrath et al., 2004), and so we targeted the spinal cord with intrathecal administration of opioid receptor antagonists and inverse agonists. Either intrathecal NTX or NTX methobromide robustly reinstated mechanical hyperalgesia (Corder et al., 2013). This behavioral result was recapitulated with three molecular and neurophysiological markers of spinal nociceptive neuron activation to further localize opioid signaling and LS to the lumbar spinal cord: 1) Within the DH nociceptive circuitry, we visualized pERK. pERK is implicated in the spinal transduction of nociceptive signals and serves as a marker of central sensitization during pathological conditions (Ji et al., 1999, Ji et al., 2009). pERK is typically observed following high-threshold (Aδ- or C-fiber) or noxious stimulation (Ji et al., 1999), however, during the maintenance phase of chronic pain (> 2 days post CFA) a low-threshold (Aβ-fiber) stimulation (Matsumoto et al., 2008) or light-touch (Gao and Ji, 2010a) can induce spinal pERK expression, suggestive of injury-induced central sensitization (Ji et al., 2003). We found that after pain resolution (21d), light-touch was unable to effectively activate superficial DH neurons unless LS was disinhibited by NTX (Corder et al., 2013); 2) Within spinal cord slices, we visualized intracellular Ca2+ concentrations ([Ca2+]i) in populations of DH neurons using Fura-2 labeling and wide-field fluorescence microscopy (Doolen et al., 2012). NTX administration to slices taken from post-hyperalgesia animals precipitated ↑ [Ca2+]I, indicating that LS survives in spinal nociceptive neurons within our slice preparation, and that opioid receptor activity tonically suppresses it (Corder et al., 2013); 3) NTX produced cAMP superactivation in the DH of post-hyperalgesia mice (Corder et al., 2013). Taken together, these data suggest that MOR actively represses signal amplification, similar to spinal GABAergic signaling (Baba et al., 2003, Torsney and MacDermott, 2006, Torsney, 2011). Our results reveal the presence of sensitized spinal nociceptive neurons that outlast the resolution of hyperalgesia and remain under the control of endogenous opioid receptor inhibitory mechanisms (Corder et al., 2013).
6. Mu Opioid Receptor Constitutive Activity (MORCA) inhibits LS
As alluded to above, spinal MORs become tonically active after injury and continue to provide endogenous analgesia beyond wound healing (Corder et al., 2013). Receptor mechanisms driving tonic MOR signaling may involve either continuous opioid agonist stimulation or a unique, agonist-independent form of signaling termed constitutive activity (Costa and Herz, 1989, Kenakin, 2001, Seifert and Wenzel-Seifert, 2002, Kenakin, 2004). Using a multidisciplinary approach involving physiological, genetic, and biochemical strategies, we recently demonstrated that the injury-induced tonic signaling state is facilitated by acquisition of constitutive activity. This MOR constitutive activity, which we term MORCA, continues to quiet nociceptive cells in the spinal cord, even in the absence of signals from opioid peptides. Here we review the concept of constitutive activity and inverse agonism, and then describe the evidence supporting the development of MORCA after repeated drug administration or cutaneous inflammation.
6.1. Classification of opioid receptor antagonists
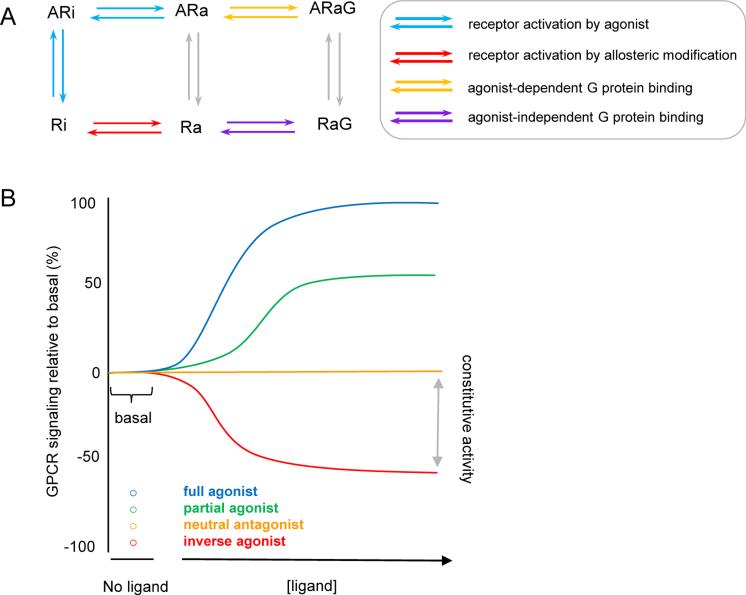
Morphine exerts actions such as pain reduction by binding to the extracellular binding domain of MORs, causing a conformational change in the receptor that then allows for G-protein coupling and intracellular signal transduction. This positive efficacy of morphine broadly classifies this ligand as an agonist. A simple two-state model expounds that the receptor rests in an inactive conformation (Ri) and with the addition of an agonist (A), the receptor becomes active (Ra) and subsequently binds to a G-protein (ARaG) (Figure 4A). The actions of agonists can be blocked by addition of antagonists which prevent binding of the agonist to the receptor. This can occur through competition for the binding pocket or at an allosteric site. Regardless of mechanism, the definition of a true antagonist (i.e. a neutral antagonist) describes its action in terms of blocking an agonist without effecting receptor conformations that might alter signaling (i.e. antagonists do not have efficacy at a given receptor for either the active or inactive state). Thus, a neutral antagonist will not exert effects by itself, and so will not alter MORCA; rather, a neutral antagonist will block an agonist-induced ARaG state (Figure 4B).
Figure 4. Constitutive activity and inverse agonism.
(a) The Extended Ternary Complex model of GPCR signaling which predicts that modulating the allosteric constant (red arrows) produces a spontaneously active receptor (Ra). Ra can then bind to G-proteins (G) independent of agonist (A) facilitation (pink arrows). (b) Effect of various ligands on GPCR activity.
The term inverse agonist is used to describe ligands that display negative efficacy, a concept that requires a 3-state model (Figure 4B). Negative efficacy is the idea that, upon binding to its receptor, a ligand can repress the spontaneous activity of the receptor (RaG) and promote the inactive state (Ri). Thus, inverse agonists characteristically reduce constitutive G-protein coupling to receptors.
6.1.1. Naltrexone as an inverse agonist
A growing body of evidence indicates that opioid receptor ligands like NTX and CTOP have inverse agonist properties that increase following tissue injury (Corder et al., 2013) or chronic opioid exposure (Sadee et al., 2005). As illustrated in Table 2, cells obtained from whole animal models of opioid dependence, e.g. animals treated for several days with morphine, exhibit key characteristics of constitutive opioid receptor activity upon challenge with NTX (Wang et al., 2001a, Wang et al., 2004, Raehal et al., 2005, Sirohi et al., 2009, Lam et al., 2011, Navani et al., 2011). These results seem to outweigh the more discrepant results obtained from studies in cell culture lines treated in vitro with opioid receptor agonists for a decidedly shorter treatment (overnight); these studies yield evidence both for (Sally et al., 2010) and against (Divin et al., 2009) intrinsic activity of NTX.
Table 2.
In vivo studies in rodents subjected to chronic opioids or tissue injury which directly compare the ability of naltrexone and 6β-naltrexol to exert intrinsic activity and precipitate physical withdrawal. All of these studies consistently indicate that naltrexone and 6β-naltrexol behave as an inverse agonist and a neutral antagonist, respectively. Thus, both chronic opioids and tissue injury generate constitutive activity at CNS opioid receptors.
| In vivo mouse model | Robust Physical withdrawal? | Effect on basal GTPγS binding | Effect on cAMP | Comment | Reference | |
|---|---|---|---|---|---|---|
| Naltrexone | Morphine dependence | Yes | ↓ | ↓ | Wang et al 2001 | |
| Morphine dependence | Yes | ↓ | ↑ | Wang et al 2004 | ||
| Morphine dependence | Yes | ↓ | Raehal et al 2005 | |||
| Morphine dependence | Yes | Sirohi et al 2009 | ||||
| Morphine dependence | Yes | Navani et al 2011 | ||||
| Latent pain sensitization | Yes | ↓ | ↑ | Hyperalgesia, neuron activation and cAMP | Corder et al 2013 | |
| 6β-naltrexol | Morphine dependence | No | Wang et al 2001 | |||
| Morphine dependence | No | No effect | No effect | Wang et al 2004 | ||
| Morphine dependence | No | No effect | ↓ NTX-induced decrease in basal GTPγS binding | Raehal et al 2005 | ||
| Morphine dependence | No | ↓ NTX-induced physical withdrawal | Sirohi et al 2009 | |||
| Morphine dependence | No | ↓ NTX-induced physical withdrawal | Navani et al 2011 | |||
| Latent pain sensitization | ↓ NTX-induced hyperalgesia, neuron activation, and cAMP | Corder et al 2013 |
6.1.2. 6β-naltrexol is a neutral antagonist
A large body of evidence indicates that 6β-naltrexol, a metabolite of NTX, exhibits key characteristics of a neutral antagonist when administered to cells obtained from opioid-dependent animals, e.g. little or no intrinsic activity (i.e. no effect) when administered by itself (Table 2). Furthermore, as expected for an opioid receptor neutral antagonist, 6β-naltrexol blocks the effects of inverse agonists (Wang et al., 1994, Bilsky et al., 1996, Kenakin, 2001, Wang et al., 2004, Raehal et al., 2005, Wang et al., 2007, Sirohi et al., 2009, Sally et al., 2010, Lam et al., 2011, Navani et al., 2011) (Table 2). As described in more detail below, recent data in models of LS describes similar actions of 6β-naltrexol in vivo, when administered to the whole animal (Corder et al., 2013).
6.2. Proposed mechanisms of constitutive activity
As noted above, MOR signaling persists for several months after tissue damage. What mechanisms might maintain tonic signal transduction? Receptor signaling is initiated by either ligand binding or spontaneous acquisition of the active conformation. The former scenario is susceptible to antagonists that outcompete for the receptor binding pocket, while the latter case is blocked by inverse agonists with preferential affinity for the inactive state of the receptor and, therefore, reverse basal responses attributed to constitutive activity (Kenakin, 2007). The mechanisms that initiate and maintain constitutive signaling after inflammation are unknown, but essential ideas come from the Extended Ternary Complex model of GPCR signaling (Kenakin, 2004). GPCRs exist in equilibrium of spontaneous Ra and Ri states, known as an allosteric constant. This constant represents a distinctive energy barrier for a given GPCR in a specific cellular environment which dictates the formation of spontaneous Ra states. Thus, lowering the allosteric constant (e.g. receptor point mutations, removal of Na+ ions, loss of negative regulatory, internalizing, desensitizing proteins) increases the formation of Ra. In this paradigm, constitutive activity can be produced upon lowering of the energy barrier to spontaneously form the active state of the receptor. While the biophysical mechanisms of constitutive activity have yet to be fully understood, approximately 30 different studies using site-directed point-mutations in the oprm1 gene encoding MOR have found numerous amino acids that affect ligand binding, G-protein coupling efficiency, and receptor phosphorylation and internalization; all of which increase MORCA (Chavkin et al., 2001). Importantly, genetic mutations are not necessary for constitutive activity to occur. Liu and Prather demonstrated that prolonged exposure of morphine can induce constitutive activity that persists after agonist washout (Liu and Prather, 2001), suggesting that post-translational modifications to MOR or decreased interactions with negative regulatory proteins may induce constitutive activity. This might be achieved through post-translation modification of MOR (e.g. hyper-phosphorylation) (Wang et al., 1994/#128) or a reduction in the association of the receptor with a negative regulatory protein, such as β-arrestin2 (Lam et al., 2011/#119).
6.3. Repeated opiate administration increases MORCA in the brain
An intriguing hypothesis of drug addiction suggests that chronic opiates increase MOR constitutive activity to preserve physical and psychological dependence (Wang et al., 1994, Liu and Prather, 2001, Wang et al., 2004, Shoblock and Maidment, 2006, Meye et al., 2012), which is enhanced by the action of endogenously released enkephalins in the brain (Shoblock and Maidment, 2007). Similarly, prolonged administration of exogenous opioid agonists, such as morphine or DAMGO, have been shown to cause an increase in the number of constitutively active opioid receptors during the period of opioid dependence (Wang et al., 2001a, Sadee et al., 2005, Walker and Sterious, 2005, Xu et al., 2007, Sally et al., 2010). Constitutive activity is experimentally observable as increased basal GTPγS35 binding, increased Bmax of the agonist-responsive high-affinity GTPγS35 binding site and suppressed forskolin-stimulated cAMP accumulation. While constitutive activity is usually difficult to detect outside of in vitro heterologous cell expression systems, as described above (Costa and Herz, 1989, Liu and Prather, 2001, Wang et al., 2007), MORCA has been observed in tissue obtained from the striatum (Wang et al., 2004, Shoblock and Maidment, 2006) and the ventral tegmental area (Meye et al., 2012) of morphine-dependent mice. Inverse agonists decreased basal GTPγS35 binding, produced cAMP superactivation and disinhibited GABAergic signaling, indicative of acquired state-dependent activity following chronic receptor stimulation. Although it remains unclear whether MORCA is important in disease states other than drug addiction, these studies provided the framework for experiments to determine whether MORCA operates in the setting of LS and chronic pain.
6.4. Tissue injury establishes MORCA in the spinal cord
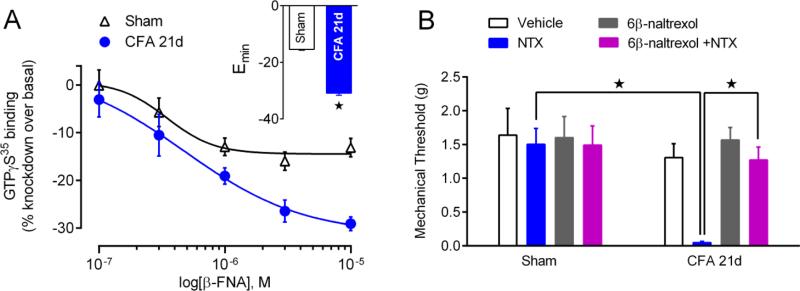
The above suggests that, if constitutive GPCR signaling is responsible for anti-hyperalgesia, then an opioid inverse agonist should produce hyperalgesia. We recently provided two major pieces of evidence indicating that injury establishes MORCA signaling in DH neurons, and that MORCA analgesia maintains LS in a state of pain remission (Corder et al., 2013). First, GTPγS35 binding studies are essential to prove CA, and β-FNA, a putative inverse agonist at MOR (Liu et al., 2001), reduced constitutive GDP/GTPγS35 exchange in DH sections during the pain remission phase of LS in CFA-injured mice (Figure 5A). Second, the MOR neutral antagonist 6-β-naltrexol prevented reinstatement of pain and [Ca2+]i mobilization in spinal slices (Corder et al., 2013) induced by the MOR inverse agonist NTX (Figure 5B). This is consistent with the expected blockade of the effect of an inverse agonist by a neutral antagonist. As noted above, a neutral antagonist is not expected to exert effects by itself and so as predicted, 6β-naltrexol failed to precipitate hyperalgesia or ↑[Ca2+]i. Thus, Corder et al (Corder et al.) demonstrated in vivo acquisition of constitutive MOR signaling that naturally develops after injury, without chronic drug dosing (Sadee et al., 2005) or genetic manipulations (Chavkin et al., 2001).
Figure 5. Spinal μ-opioid receptors acquire constitutive activity (MORCA) after injury.
(A) Dose-response effects of β-FNA intrathecal β-funaltrexamine (β-FNA) on basal GTPγS35 binding in lumbar dorsal horns of Sham or CFA-21d mice; inset: group binding Emax (n = 7 - 9). (B) Effects of intrathecal administration of NTX (1 ug), 6β-naltrexol (10 ug), or co-administration of 6β-naltrexol+NTX in sham and post-hyperalgesia mice on mechanical hyperalgesia. * P<0.05. Adapted from Corder et al, 2013.
One potential limitation of GDP/GTPγS35 exchange studies is the potential presence of trace endogenous opioid peptides. If true, then NTX, CTOP, and β-FNA might have behaved as antagonists to block the actions of opioid agonists. We concede the possibility of continual opioid release in the ex vivo spinal cord slice preparation following dissection. However, all spinal cord sections from injured and non-injured mice were treated identically. Therefore, any residual opioid content (as well as other conditions) were identical between the sections used for basal determinations or those incubated with or without β-funaltrexamine. We believe that the extensive buffer washing and incubation protocols were sufficient to remove any opioid peptides or other agonists from the extracellular space before incubation with GTPγS35 (Corder et al., 2013).
CA GPCRs are, like agonist-activated GPCRs, subject to internalization, phosphorylation, desensitization and down-regulation (Leurs et al., 1998). Interestingly in DRG neurons, CA MORs recycle more rapidly through a caveolin-mediated mechanism, as compared to typical MORs which internalize through a clathrin-mediated mechanism (Walwyn et al., 2007). Whether CFA produces lasting analgesia by increasing the recycling CA MORs in DH, however, is difficult to determine because internalized CA MORs are too close to the membrane to detect by standard confocal microscopy (Walwyn et al., 2007). An alternative approach is to use flow cytometry to quantify surface receptor density.
The CA state of MOR is thought to depend on receptor modifications, including receptor interactions with accessory proteins or phosphorylation at numerous sites on MOR (Sadee et al., 2005, Connor and Traynor, 2010, Mann et al., 2014), including constitutive phosphorylation at S363 in vivo (Illing et al., 2014). With the emergence of phosphosite-specific MOR antibodies and other pharmacological tools (Mann et al., 2014), future studies could determine whether CFA increases MOR phosphorylation or dephosphorylation that then contributes to MORCA.
6.5. Ligand-dependent tonic activation of opioid receptors
Tissue injury produces long-lasting endogenous opioid inhibition of LS that is mediated by ligand-independent MORCA signaling. MORCA is maintained for sufficient duration to hasten the resolution of acute pain, oppose the development chronic pain, and/or temper the severity of chronic pain (Corder, 2013). However, inverse agonists can block not only MORCA, but also MORs that are tonically activated by endogenous opioids. Thus, an emerging controversy is unfolding regarding whether MORCA operates alone or in concert with tonically-activated MOR to generate long-lasting endogenous opioid analgesia after injury that then masks LS. Regardless, either mechanism can promote pain recovery, but can also lead to pathological consequences (i.e. dependence as discussed later) which can create a lasting susceptibility to develop chronic pain.
In support of a ligand-dependent mechanism, noxious input or tissue injury produces rapid increases in extracellular endogenous opioids within DH (Basbaum and Fields, 1984, Noguchi et al., 1992, Song and Marvizon, 2003b, Trafton and Basbaum, 2004) including enkephalins (Yaksh and Elde, 1981, Iadarola et al., 1986, Cesselin et al., 1989) and constitutive increases in prodynorphin products that lead to a modest tonic inhibition of heat nociception or acute inflammatory pain (Iadarola et al., 1988, Wang et al., 2001b, Campillo et al., 2011). Ligand-dependent signaling is an important consideration, because so little is known about the significance of tonically-activated MOR in models of chronic pain. Indeed, plantar incision increases dynorphin levels in the spinal cord that might then tonically inhibit pain (Campillo et al., 2011), and prodynorphin knockout mice exhibit exaggerated hyperalgesia after intraplantar formalin. These data suggest that dynorphin-mediated activation of kappa opioid receptors (KOR) contributes to a (modest) tonic pain inhibition during LS (Wang et al., 2001b). The contribution of ligand-dependent tonic activation of MOR to the silencing of LS, however, remains to be determined, for example with measurements of opioid levels or interventions that disrupt opioid gene expression.
Evidence against a ligand-dependent mechanism is that 6β-naltrexol, acting as a neutral antagonist, failed to precipitate hyperalgesia or ↑[Ca2+]I when administered during the post-hyperalgesia phase after inflammation (Corder et al., 2013). Moreover, blockade of NTX inverse effects by 6β-naltrexol, in all our behavioral and molecular assays, clearly argues that agonist-independent signaling is present in a population of spinal MORs. This is consistent with the expected blockade of the effect of an inverse agonist by a neutral antagonist.
Future studies with opioid peptide knockout mice or siRNA are needed to: 1) ascertain the impact of endogenous opioids on LS; 2) determine whether the pain remission associated with MOR activation is driven by endogenous opioid release; and 3) distinguish hypotheses of MOR constitutive activity with tonic activation of MORs.
7. Molecular mechanisms of latent sensitization
The following addresses the cellular signaling mechanisms by which long-lasting MOR signaling contributes to the persistence of pathological nociceptive plasticity and LS.
7.1. NMDA receptor (NMDAR)
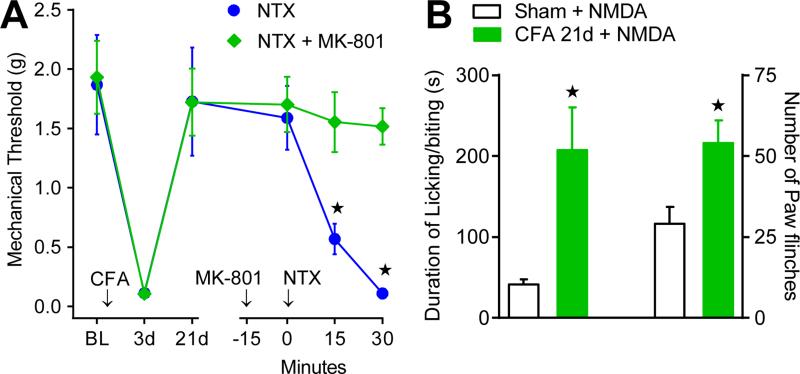
It is well established that both the induction and maintenance phases of central sensitization require N-methyl-D-aspartate receptor (NMDAR)- and Ca2+-dependent mechanisms (Latremoliere and Woolf, 2009). Several studies support the contribution of NMDAR to LS as well. Indeed, either chronic environmental stress (Le Roy et al., 2011), injury (Rivat et al., 2002, Rivat et al., 2007), or exposure to exogenous opioids have been suggested to generate an adaptive sensitization of pronociceptive NMDAR pathways through an unknown cellular mechanism. Similarly, as illustrated in Figure 6, we reported that intrathecal NMDA-induced pain-like behaviors and ↑cAMP were potentiated in CFA-21d mice, indicating that injury increases NMDAR sensitivity (Corder et al., 2013). Furthermore, the NMDAR antagonist MK-801 attenuated NTX-induced reinstatement of spinal pERK activation, glutamate-evoked ↑[Ca2+]I and hyperalgesia (Corder et al., 2013), indicating that NMDAR mechanisms play a critical role in mediating the manifestations of LS that is masked by opioid receptor activity.
Figure 6. NMDAR are required for NTX-induced pain reinstatement and are sensitized during the remission phase of latent pain sensitization.
(A). Effect of intrathecal administration of the NMDAR antagonist, MK-801 (1 ug) on NTX-precipitated mechanical hyperalgesia (n=5-10). (B) Effect of NMDA (i.t.; 3 pmol) on spontaneous nocifensive behaviors (left) and paw flinches (right) over 15 min in Sham (n=5) and CFA (n=5) mice. *p<0.05. Adapted from Corder et al, 2013.
The induction phase of CFA-hyperalgesia (<24 hrs after injury) is dependent on spinal NMDAR signaling, whereas the maintenance phase of hyperalgesia has NMDAR-independent components [Corder et al, 2013]. We reported that NTX does not increase hyperalgesia or glutamate-evoked spinal [Ca2+]intracellular 24 hours after CFA, suggesting that the endogenous opioidergic system does not impinge on the induction of CFA-hyperalgesia. In contrast, continuous infusion of NTX over the first 14d after CFA eliminated endogenous opioid analgesia, suggesting that the opioidergic system opposes the maintenance of CFA-hyperalgesia. Taken together, our data suggest that the cellular mechanisms of the induction and maintenance of LS in our model are different.
NMDARs form heteromers of GluN1 (obligatory), GluN2A-D, and GluN3A-B subunits (Paoletti et al., 2013), with GluN1 and GluN2A-B shown to be critical to central sensitization and inflammatory hyperalgesia (Woolf and Costigan, 1999, Woolf and Salter, 2000, Guo et al., 2002, Abe et al., 2005, Gabra et al., 2007, Luo et al., 2008, Latremoliere and Woolf, 2009). Future studies are needed to determine which of the key GluN2 subunits in DH are essential to LS.
7.2. NMDAR mediated Ca2+ signaling
NMDAR-derived Ca2+ flux is necessary for ERK activation (Krapivinsky et al., 2003, Lever et al., 2003, Sindreu et al., 2007), specifically under pathological conditions or noxious stimulation (Ji, 2004, Matsumoto et al., 2008). Furthermore, it has been demonstrated that withdrawal from exogenous opioids results in synaptic LTP in spinal neurons and relies on post-synaptic NMDAR Ca2+ mobilization to mediate the hyperalgesia associated with physical withdrawal (Drdla et al., 2009). We found that pre-treatment with intrathecal MK-801 resulted not only in prevention of NTX-induced hyperalgesia but also in loss of bilateral increases in dorsal horn pERK levels (Corder et al., 2013). Together these results reveal that loss of tonic MOR signaling initiates an NMDAR—Ca2+—pERK pathway to generate the reinstatement of pain.
7.3. NMDAR-cAMP linkage
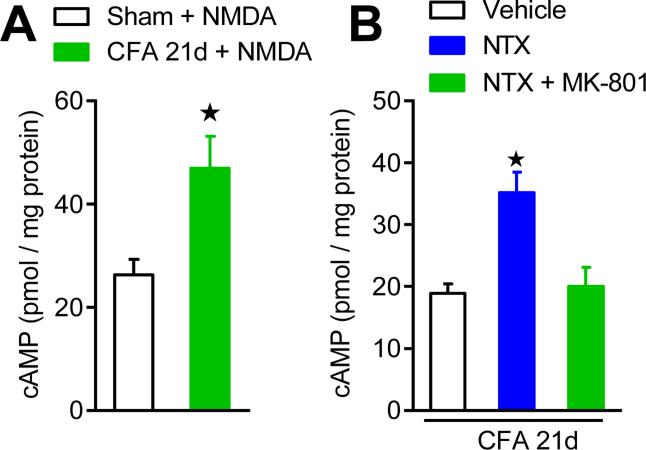
NMDAR-derived Ca2+ increases are linked to cAMP signaling pathways (Chetkovich and Sweatt, 1993, Wong et al., 1999) necessary for LTP initiation (Wang and Zhuo, 2002), opiate physical dependence (Zachariou et al., 2008) and spinal synaptic facilitation (Wei et al., 2006);(Wang et al., 2011). Consistent with previous studies in brain showing that the Ca2+ stimulated isoforms of adenylyl cyclase are activated by NMDARs (Chetkovich and Sweatt, 1993), we reported and Figure 7 illustrates that pretreatment with intrathecal MK-801 abolished the NTX-precipitated increases in cAMP (Corder et al., 2013). Moreover, direct activation of spinal NMDARs with intrathecal NMDA increased spontaneous nocifensive behaviors and spinal cAMP levels. Similarly, direct activation of the spinal AC system with intrathecal forskolin produced enhanced spontaneous nocifensive behaviors and intracellular cAMP. Thus, NMDAR signaling is directly linked to downstream cAMP signaling during opioid receptor inverse agonism.
Figure 7. Endogenous opioid withdrawal initiates NMDAR-dependent AC superactivation.
(A) Effect of intrathecal administration of NMDA (i.t.; 3 pmol) on cAMP levels in Sham (n=5) and CFA (n=5) mice, indicating a latent up-regulation of an NMDAR-AC pathway during the remission phase of LS. (B) Effect of intrathecal administration of MK-801 (3 pmol) pretreatment on NTX-induced cAMP overshoot, indicating that NMDAR signaling contributes to AC superactivation during NTX-precipitated endogenous opioid withdrawal (n=5 per group). *p<0.05. Adapted from Corder et al, 2013.
7.4. Adenylyl cyclase 1 (AC1)
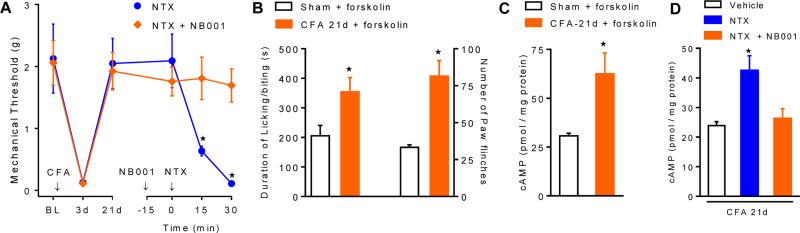
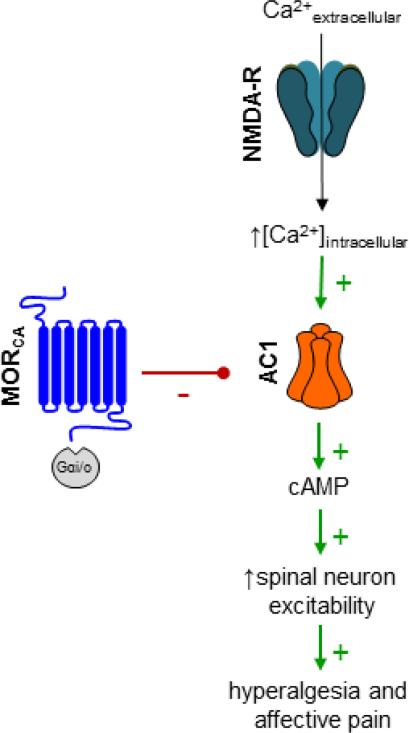
Opioids produce their acute analgesic effects in part through inhibition of ACs. Of the 9 known isoforms, AC1 and AC8 are of particular interest, as they both couple NMDAR-induced cytosolic Ca2+ increases to cAMP signaling in the CNS (Wei et al., 2002). AC1 is expressed in the post-synaptic density of CNS neurons (Xia et al., 1991, Conti et al., 2007), in the brain and the superficial dorsal horn (Wei et al., 2002), and is subject to negative regulation by Gαi/o-coupled receptors in vivo (Nielsen et al., 1996). AC1 promotes phosphorylation and trafficking of AMPA receptors via protein kinase A (PKA) and/or protein kinase C zeta (PKMζ) (Lu et al., 2003, Li et al., 2010, Asiedu et al., 2011, Zhuo, 2012), phosphorylates NMDARs via PKA—Src kinase (Xu et al., 2008, Qiu et al., 2013) and contributes to activity-dependent LTP in the spinal cord (Wang et al., 2011), and plays a prominent role in chronic pain (Wei et al., 2002). For example, as illustrated in Figure 8, our recent data suggest that prolonged endogenous-mediated MOR signaling drives the upregulation of the spinal AC system in an manner that is analogous to opiate cellular dependence, thereby promoting the maintenance of LS (Corder et al., 2013). Thus, MOR blockade during the remission phase of LS with inverse agonists precipitates spinal cAMP overshoot (AC superactivation) and hyperalgesia (Corder et al., 2013). Furthermore, NTX reinstatement of hyperalgesia was lost in AC1-/- mice (Corder et al., 2013)), and NB001, a selective AC1 inhibitor (Wang et al., 2011), prevented NTX-induced reinstatement of affective pain as well as hyperalgesia and cAMP overshoot (Corder et al., 2013)). Also, pain-like behaviors and spinal cAMP elicited by the AC activator, forskolin, were potentiated (Corder et al., 2013), suggesting AC supersensitivity silently strengthens during the remission phase of LS. As illustrated schematically in Figure 9, these data indicate that MORCA silences persistent AC1-mediated LS, and point to AC1 as a central component of LS that can be unleashed by MOR blockade. Future studies are needed to determine whether AC1 and AC8 expression itself is upregulated, and then which of the key effectors for cAMP, such as PKA or exchange proteins activated by cAMP (Epac), are essential to LS. Future studies are also needed to determine whether AC1 is a key pharmacological target for new analgesic drugs such as NB001, an AC1 blocker originally developed by Min Zhuo (Wang et al., 2011).
Figure 8. Superactivation of spinal AC1 faciltates pain reinstatement and cellular opioid withdrawal.
Effect of intrathecal administration of the AC1 antagonist, NB001 (1.5 ug), on NTX-precipitated mechanical hyperalgesia (A) and spinal cAMP content (D). Effect of the AC activator, forskolin (intrathecal, 1.5 ug( on spontaneous nocifensive behaviors (B) and paw flinches (C) over 15 min in sham and CFA mice, indicating AC1 superactivation during the remission phase of LS. n=6-9. *p<0.05. Adapted from Corder et al, 2013.
Figure 9. Cellular signaling pathways underlying opioid receptor-masked latent sensitization.
Spinal cord MOR signaling, through inhibitory Gai/o proteins, tonically represses AC1 production of cAMP, thereby reducing nociceptive signal transduction. Blockade of MOR results in the disinhibition of AC1, allowing for NMDAR-derived, Ca2+-mediated activation and downstream increases in signal transduction, neuron excitability, and ultimately, pain. Adapted from Corder et al, 2013.
8. Cellular dependence on MORCA
As detailed above, administration of NTX during the post-hyperalgesia period of the CFA model activates not only spinal nociceptive transmission and pain, but AC1. Chronic opiate exposure increases the expression of Ca2+-sensitive ACs (Nestler and Aghajanian, 1997, Christie, 2008), particularly AC1 and AC8 (Lane-Ladd et al., 1997). During opioid withdrawal or opioid receptor antagonism, this upregulated AC1 population is disinhibited, producing a cAMP overshoot, or superactivation response {Zachariou, 2008 #4652;Mazei-Robison, 2012 #4628;Li, 2006 #4621.
8.1. Tissue injury triggers cellular dependence on MORCA
As noted above, during NTX-precipitated pain reinstatement, NMDAR–derived Ca2+ mobilization was potentiated, as was downstream activation of AC (reflected by enhanced responsiveness to forskolin) and associated cAMP overshoot in the spinal cord (Corder et al., 2013). This AC supersensitivity following disruption of MOR is remarkably similar to that observed during withdrawal from opiate drugs. Further linking pain to withdrawal is the enhanced pronociceptive synaptic strength (Lu et al., 2003, Xu et al., 2008) that is observed following NMDAR-dependent spinal LTP at C-fiber synapses during withdrawal from exogenous opiates (Drdla et al., 2009).Thus, we propose a novel mechanism that determines the transition from acute to chronic pain: 1) Long-term MORCA inhibition of AC1-mediated central sensitization drives a counter-adaptive, homeostatic increase in pronociceptive AC1 signaling cascades (Wei et al., 2002, Zhuo, 2012), thereby paradoxically promoting the maintenance of LS; 2) Injury initiates a dependence to MORCA that tonically prevents withdrawal hyperalgesia. Thus, anything that disrupts MORCA will trigger the transition to spinal cellular withdrawal (NMDA-mediated AC1 superactivation) and signs of chronic pain.
8.2. Cellular opioid withdrawal may reflect de novo NMDAR-dependent LTP
Abrupt withdrawal from MORCA by an inverse agonist and the associated pain reinstatement could result from one of several mechanism: 1) disinhibition of tonically active primary afferent terminals: 2) disinhibition of AC1 superactivation in dorsal horn neurons; 3) potentiation of descending facilitatory signals from the brainstem; and/or 4) induction of de novo spinal NMDAR-AC1-dependent withdrawal. For several reasons, we favor the latter as a promising area of future investigation. First, exogenous opiate withdrawal in the spinal cord initiates de novo NMDAR-dependent LTP {Drdla, 2009 #4604;Zhou, 2010 #5167}. Second, NMDAR signaling and post-synaptic spinal neuron Ca2+ rise are required in the induction, rather than maintenance, of spinal LTP and hyperalgesia (Weyerbacher et al., 2010, Sandkuhler and Gruber-Schoffnegger, 2012). Third, AC1 is activated by NMDAR-derived Ca2+ and is necessary for LTP induction (Liauw et al., 2005, Wei et al., 2006). Fourth, intrathecal NMDA increased pain behaviors and spinal cAMP levels during the post-hyperalgesia state (Corder, 2013), which suggests that spinal NMDAR–adenylyl cyclase signaling pathways are not occluded (saturated). Fifth, spinal NMDARs are not active prior to NTX-induced withdrawal, since intrathecal MK-801 alone neither increased mechanical threshold, reduced basal spinal intracellular calcium levels, decreased basal pERK expression, nor decreased basal cAMP levels when administered during the post-hyperalgesia state (Corder, 2013).
9. Physical and psychological dependence on endogenous opioid receptor activity
9.1. Endogenous opioid dependence
Humans and other mammals develop tolerance and physical dependence to exogenous opiate drugs, which can be termed exogenous opiate dependence. An emerging literature is suggesting that stress or dietary factors trigger the release of endorphins or enkephalins that then induce physical manifestations of endogenous opioid dependence. Physical (somatic) or psychological dependence can be revealed upon challenge with an opioid receptor antagonist. In the rodent, somatic indices of opiate withdrawal are similar to those encountered by an opiate drug addict experiencing withdrawal (Koob et al., 1992, Kest et al., 2002). For example, following repeated stress for 10 days in rats, naloxone produces whole body tremors (“wet-dog” shakes) (Morley and Levine, 1980). Also, following a stressful chronic schedule of warm water swimming, naloxone precipitates not only body tremors, but also piloerection and ptosis, suggesting that the body develops dependence to stress-induced activation of opioid receptors (Christie and Chesher, 1982). Following excessive ingestion of sugar, naloxone triggers teeth chattering, forepaw flutters, head shaking, and anxiety, suggesting that endogenous dependence develops to sweet foods (Colantuoni et al., 2002). Following the chronic delivery of RB101, an enkephalinase inhibitor, naloxone produced forepaw tremor and decreased body weight, albeit to a mild degree, suggesting the elevated levels of endogenous enkephalin initiates endogenous dependence (Noble et al., 1994). Opioid blockade also elicits withdrawal signs in mice following heavy exposure to UV light (Fell et al., 2014). This is consistent with anecdotal evidence in humans that individuals who seek frequent UV light exposure develop dependence to reinforcing opioids, as indicated by the finding that 50% of subjects (but 0% of controls) exhibited withdrawal-like symptoms including nausea and jitteriness following administration of 5-25 mg of naltrexone (Kaur et al., 2006). Whether the mechanism of this endogenous opioid analgesia involves light-induced elevations of skin β-endorphin, as proposed (Fell et al., 2014), is uncertain and controversial. First, as expected for large molecules, evidence for the ability of β-endorphin to cross the blood-brain-barrier, and thus reach the CNS, is sparse at best (Banks and Kastin, 1990). Second, the chronic UV light conditions used by Fell et al (Fell et al., 2014) were sufficient to trigger signs of tissue injury such as increases in local prostaglandin production. And as we describe next, tissue injury triggers the development of a powerful and well-characterized endogenous opioid dependence (Corder, 2013).
9.2. Tissue injury triggers physical dependence
When administered during the post-hyperalgesia period after inflammatory insult, NTX produced numerous behaviors that reflected opioid withdrawal (Corder, 2013). This is the first set of experiments to describe a physical dependence to endogenous opioid activity in the setting of persistent inflammation. Following NTX administration, mice exhibited jumping behavior (analogous to panic attacks and involuntary movement of the legs and arms observed in the addict when they stop taking opiate drugs), paw tremors, wet-dog shakes, increased locomotion, and importantly, pain-like behaviors (Corder, 2013).
Endogenous opioid dependence persists long after tissue healing, and even increases with the passing of months following the induction of inflammation (Corder et al., 2013). This indicates that physical dependence is not maintained by injury, but rather results from intensifying compensatory opioidergic neuroadaptations within the brain that could be either ligand-dependent or ligand-independent.
9.3. Tissue injury triggers psychological dependence
Pain comprises not only somatic and sensory (hyperalgesia), but also affective (aversiveness) components; the latter can be identified by changes in the rewarding property of analgesics and associated motivational behavior. In a CPP paradigm (Sufka, 1994, King et al., 2009), the negative reinforcing capacity of intrathecal lidocaine (motivation to seek pain relief) demonstrates the presence of aversive pain 1d after inflammatory insult (He et al., 2012). This aversive component, as was the case with the numerous escape and somato-motor opioid withdrawal behaviors, was absent during the post-hyperalgesia period (21d after inflammatory insult), but could be triggered by naloxone (Corder et al., 2013).
9.4. Cellular, physical, and psychological dependence contribute to LS
In summary, endogenous activation of MOR initiates a compensatory process that can lead to a rebound effect when an antagonist / inverse agonist is given. This fits a common definition of “dependence”, and dependence can indeed be transient. Overt evidence of dependence may be masked by the normal agonist effect of endogenous MOR activity. Pain-like behaviors following administration of inverse agonists reflect not only withdrawal from cellular dependence on MORCA in the spinal cord as described above, but also withdrawal from physical and psychological dependence on tonic MOR activity in the brain.
10. Conceptual Model, Conclusions, and Clinical Significance
10.1. Conceptual model of the delicate balance between LS, dependence, and MORCA analgesia
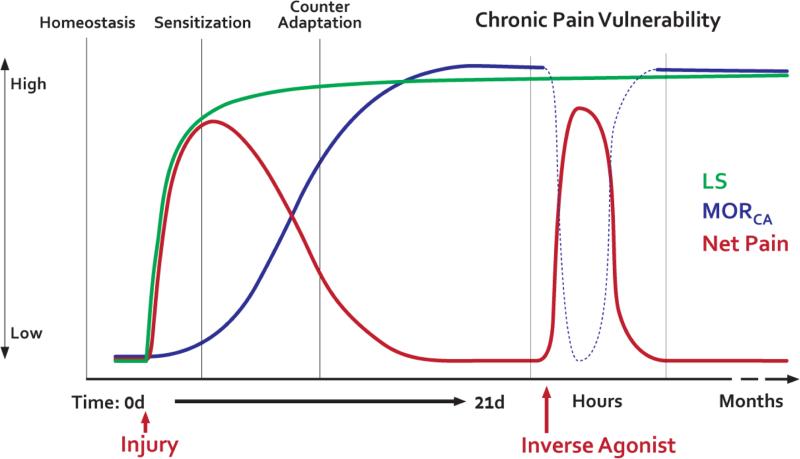
Based on our results, we suggest that injury produces a dynamic relationship between the systems that regulate analgesia and pain. As illustrated in Figure 10, injury first activates pain pathways. Second, the spinal cord establishes MORCA as it attempts to control pain. Third, over time, the body becomes dependent on MORCA, which paradoxically sensitizes pain pathways. Pain begets MORCA begets pain vulnerability in a vicious cycle. The final result is a silent insidious state characterized by the escalation of two opposing excitatory and inhibitory influences on pain transmission: silent pain mediated by AC1 which keeps the foot on the accelerator, and pain inhibition mediated by MORCA (which keeps the foot on the brake). Indeed, stress (Rivat et al., 2007) or injury (Rivat et al., 2002) escalates opposing inhibitory and excitatory influences on nociceptive processing, as a pathological consequence of increased endogenous opioid tone. This raises the prospect that opposing homeostatic interactions between MORCA analgesia and latent NMDAR–AC1-mediated pain sensitization create a lasting vulnerability to develop chronic pain.
Figure 10. Schematic summarizing the sequence of events that occur after injury, leading to a dynamic relationship between the systems that regulate analgesia and pain.
Under naïve conditions nociceptive and opioidergic systems have little to no activity, resting at a basal set point (homeostasis). Several changes occur following injury or strong nociceptive input. First, Severe tissue injury triggers the development of an opioid receptor-masked LS that is driven by NMDA NR2 subunit and AC1/Epac1-mediated signaling (Green line). Second, a compensatory, counter-adaptation -- constitutively active μ-opioid receptor analgesia (Blue line, MORCA) -- enables the resolution of pain (red line) and thus maintains LS in a state of remission. The intensity and degree of sensory information transmitted through the nociceptive system returns to pre-injury levels while the opponent processes are potentiated but functionally cancel out one another. Third, if this balance is perturbed, e.g. upon inverse agonism of MORCA (dotted line), then blockade of tonic MOR signaling produces a superactivation of the NMDAR—AC1 pathways and LS can be visualized as pain reinstatement. Thus, pain is only detectable when endogenous analgesia is removed, leaving LS unchecked. Fourth, the body gradually becomes dependent on MORCA, which paradoxically sensitizes pain pathways. Pain begets MORCA begets pain in a vicious cycle. As a result, the nociceptive system becomes dependent on the counterbalancing signaling of tonic MOR. A recurring failure of MORCA (ie during stress) may reflect the transition from acute pain to a chronic state of pain vulnerability.
It is important to realize that the resolution of pain after an injury does not necessarily reflect a pathology-free condition. Instead, opposing interactions between MORCA analgesia and silent AC1-medicated pain create a lasting susceptibility to develop chronic pain. Indeed, a few clinical chronic pain studies have suggested that a transient up-regulation of endogenous opioid analgesia, followed by opioid dysfunction, is associated with the onset of chronic pain (Bruehl et al., 2004, Bruehl et al., 2010). Using a motor vehicle analogy, the fact that the “pain accelerator” (LS) and the “pain brake” (MORCA) are simultaneously pressed for long periods of time has several implications for vehicles (e.g. patients) where the brake pedal is stuck (e.g. severe trauma or major surgery). To keep the car moving, the accelerator pedal is always on, and the car depends on a constant brake, or else it will speed out of control (e.g. chronic pain). Future studies are needed to better understand the long-term consequences of simultaneous pressing the accelerator and brake on pain. How can we prevent the brake pads from wearing out? Or can we replace them when they do?
10.2. Chronic pain vulnerability
We conclude that tissue injury promotes two opposing processes at pain modulatory synapses in DH: 1) tonic analgesic GPCR signaling such as MORCA that masks LS; and 2) a pathological consequence of long-lasting tonic MORCA signaling that involves the up-regulation in expression and function of NMDA receptor subunits and intracellular cAMP signaling. This drives a LS that persists in the post-hyperalgesia state and not only remains under the inhibitory control of MORCA but is also dependent on MORCA. Indeed, because LS and MORCA pathology spread beyond the injury site, a loss of MORCA analgesia could lead to the emergence of widespread, rampant chronic pain. This raises the prospect that the opposing interactions between MORCA and latent NMDAR–AC1 sensitization create a lasting susceptibility for the emergence of LS as a silent, insidious form of chronic pain. If true, then anything that can disrupt MORCA inhibitory systems would unleash the pain of endogenous opioid withdrawal, causing a pain episode to ensue. This has been modeled with inverse agonist challenge to MORCA, by which an ensuing reinstatement of pain reflects a process of spinal cellular withdrawal and AC1 superactivation to enhance neuronal excitability and pronociceptive synaptic strength. Indeed, we propose that a long-term goal for future clinical studies is the development of a clinical test (e.g. naloxone challenge) for pain vulnerability. If LS exists, then naloxone should reveal this risk factor for pain relapse by reinstating secondary hyperalgesia.
10.3. Translational relevance of LS and MORCA
The latent predisposition to relapse may explain the episodic nature and vulnerability to stressors that accompany chronic pain states in humans (Schweinhardt et al., 2008, Woolf, 2011). But does injury trigger LS and long-term opioid analgesia in humans? Superficial cutaneous heat injury is a well-characterized and validated inflammatory model of human peripheral nociceptor sensitization, primary hyperalgesia (PHA) and SHA. It has been used extensively for pharmacodynamic research (Brennum et al., 2001, Werner et al., 2001, Dirks et al., 2002a, Dirks et al., 2002b, Werner et al., 2002a, Werner et al., 2002b, Dirks et al., 2003, Ravn et al., 2012, Ravn et al., 2013). Sensitization is transient, reversible and replicable (Werner et al., 2013). We recently reported that low dose naloxone (0.021 mg/kg, i.v.), delivered following resolution of hyperalgesia after cutaneous heat injury, did not reinstate heat hyperalgesia (Pereira et al., 2013). Rather than limited tissue injury of the model or species differences, this negative result was likely due to insufficient blockade of endogenous opioid receptors, as higher doses of naloxone have been used to demonstrate reinstatement in humans (M.P. Pereira, J.B. Dahl, B.K. Taylor, M.U. Werner, unpublished results).
10.4. A new approach to prevent the transition from acute to chronic pain
Based on the emerging fields of endogenous opioid receptor analgesia and LS in the setting of injury, the overarching long-term therapeutic goal of future research is to alleviate chronic pain by either: a) facilitating endogenous opioid analgesia, thus restricting LS within a state of remission; or b) extinguishing LS altogether. If LS is responsible for priming individuals to develop chronic pain, then clinically efficacious and novel treatments may stem from preventing its formation. Specifically, targeting the upregulation and/or signaling components of spinal AC1 may prove most beneficial. Treatments should be targeted to knockdown (RNAi or other gene silencing technologies) or pharmacologically inhibit AC1 in order to erase persistent sensitization, while retaining the benefits of endogenous constitutive opioid signaling.
Acknowledgements
The authors thank Charles Anderson for construction of Figure 10 in Illustrator. This work was supported by NIH grants F31DA032496 (G.C.), K02DA19656 (B.K.T.), and R21DA38248 (B.K.T.)
REFERENCES
- Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Punnoose A, Goldberg A. [micro]-Opioid receptors often colocalize with the substance P receptor (NK1) in the trigeminal dorsal horn. Journal of Neuroscience. 2000;20:4345–4354. doi: 10.1523/JNEUROSCI.20-11-04345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth L, Budelier K, Clinesmith M, Fiedler A, Landstrom R, Leeper BJ, Moeller L, Mutch S, O'Dell K, Ross J, Radhakrishnan R, Sluka KA. Transcutaneous electrical nerve stimulation (TENS) reduces chronic hyperalgesia induced by muscle inflammation. Pain. 2006;120:182–187. doi: 10.1016/j.pain.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkaitis MS, Solorzano C, Landry RP, Piomelli D, DeLeo JA, Romero-Sandoval EA. Evidence for a role of endocannabinoids, astrocytes and p38 phosphorylation in the resolution of postoperative pain. PloS one. 2010;5:e10891. doi: 10.1371/journal.pone.0010891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi AM, Lupo C, Carli G. Effects of formalin-induced pain on exploratory behaviour in rabbits. Neuroreport. 1993;4:739–742. doi: 10.1097/00001756-199306000-00035. [DOI] [PubMed] [Google Scholar]
- Anderson WS, Sheth RN, Bencherif B, Frost JJ, Campbell JN. Naloxone increases pain induced by topical capsaicin in healthy human volunteers. Pain. 2002;99:207–216. doi: 10.1016/s0304-3959(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Molecular and cellular neurosciences. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Back SK, Lee J, Hong SK, Na HS. Loss of spinal mu-opioid receptor is associated with mechanical allodynia in a rat model of peripheral neuropathy. Pain. 2006;123:117–126. doi: 10.1016/j.pain.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Peptide transport systems for opiates across the blood-brain barrier. The American journal of physiology. 1990;259:E1–10. doi: 10.1152/ajpendo.1990.259.1.E1. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Tawfik VL, Wang D, Francois A, Solorzano C, Shuster SA, Choudhury P, Betelli C, Cassidy C, Smith K, de Nooij JC, Mennicken F, O'Donnell D, Kieffer BL, Woodbury CJ, Basbaum AI, MacDermott AB, Scherrer G. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron. 2014;81:1312–1327. doi: 10.1016/j.neuron.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Benrath J, Brechtel C, Martin E, Sandkuhler J. Low doses of fentanyl block central sensitization in the rat spinal cord in vivo. Anesthesiology. 2004;100:1545–1551. doi: 10.1097/00000542-200406000-00030. [DOI] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain research. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Bernstein RN, Wang Z, Sadee W, Porreca F. Effects of naloxone and D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2 and the protein kinase inhibitors H7 and H8 on acute morphine dependence and antinociceptive tolerance in mice. J Pharmacol Exp Ther. 1996;277:484–490. [PubMed] [Google Scholar]
- Brennum J, Kaiser F, Dahl JB. Effect of naloxone on primary and secondary hyperalgesia induced by the human burn injury model. Acta AnaesthesiolScand. 2001;45:954–960. doi: 10.1034/j.1399-6576.2001.450806.x. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Chont M. Chronic pain-related changes in endogenous opioid analgesia: a case report. Pain. 2010;148:167–171. doi: 10.1016/j.pain.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Ward P, Johnson B. Endogenous opioids and chronic pain intensity: interactions with level of disability. The Clinical journal of pain. 2004;20:283–292. doi: 10.1097/00002508-200409000-00002. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Davis GC, Bunney WE. Naloxone alters pain perception and somatosensory evoked potentials in humans. Nature (London) 1977;270:620. doi: 10.1038/270620a0. [DOI] [PubMed] [Google Scholar]
- Cabanero D, Campillo A, Celerier E, Romero A, Puig MM. Pronociceptive Effects of Remifentanil in a Mouse Model of Postsurgical Pain Effect of a Second Surgery. Anesthesiology. 2009;111:1334–1345. doi: 10.1097/ALN.0b013e3181bfab61. [DOI] [PubMed] [Google Scholar]
- Calza L, Pozza M, Arletti R, Manzini E, Hokfelt T. Long-lasting regulation of galanin, opioid, and other peptides in dorsal root ganglia and spinal cord during experimental polyarthritis. Exp Neurol. 2000;164:333–343. doi: 10.1006/exnr.2000.7442. [DOI] [PubMed] [Google Scholar]
- Campillo A, Cabanero D, Romero A, Garcia-Nogales P, Puig MM. Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur J Pharmacol. 2011;657:89–96. doi: 10.1016/j.ejphar.2011.01.059. [DOI] [PubMed] [Google Scholar]
- Cesselin F, Bourgoin S, Clot AM, Hamon M, Le Bars D. Segmental release of Met-enkephalin-like material from the spinal cord of rats, elicited by noxious thermal stimuli. Brain research. 1989;484:71–77. doi: 10.1016/0006-8993(89)90349-1. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Chai B, Guo W, Wei F, Dubner R, Ren K. Trigeminal-rostral ventromedial medulla circuitry is involved in orofacial hyperalgesia contralateral to tissue injury. Mol Pain. 2012;8:78. doi: 10.1186/1744-8069-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HM, Berde CB, Holz GGt, Steward GF, Kream RM. Sufentanil, morphine, met-enkephalin, and kappa-agonist (U-50,488H) inhibit substance P release from primary sensory neurons: a model for presynaptic spinal opioid actions. Anesthesiology. 1989;70:672–677. doi: 10.1097/00000542-198904000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, McLaughlin JP, Celver JP. Regulation of opioid receptor function by chronic agonist exposure: constitutive activity and desensitization. Molecular pharmacology. 2001;60:20–25. doi: 10.1124/mol.60.1.20. [DOI] [PubMed] [Google Scholar]
- Chetkovich DM, Sweatt JD. nMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem. 1993;61:1933–1942. doi: 10.1111/j.1471-4159.1993.tb09836.x. [DOI] [PubMed] [Google Scholar]
- Chillingworth NL, Morham SG, Donaldson LF. Sex differences in inflammation and inflammatory pain in cyclooxygenase-deficient mice. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R327–334. doi: 10.1152/ajpregu.00901.2005. [DOI] [PubMed] [Google Scholar]
- Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. British journal of pharmacology. 2008;154:384–396. doi: 10.1038/bjp.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, Chesher GB. Physical dependence on physiologically released endogenous opiates. Life Sci. 1982;30:1173–1177. doi: 10.1016/0024-3205(82)90659-2. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Connor M, Traynor J. Constitutively active mu-opioid receptors. Methods in enzymology. 2010;484:445–469. doi: 10.1016/B978-0-12-381298-8.00022-8. [DOI] [PubMed] [Google Scholar]
- Conti AC, Maas JW, Jr., Muglia LM, Dave BA, Vogt SK, Tran TT, Rayhel EJ, Muglia LJ. Distinct regional and subcellular localization of adenylyl cyclases type 1 and 8 in mouse brain. Neuroscience. 2007;146:713–729. doi: 10.1016/j.neuroscience.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science. 2013;341:1394–1399. doi: 10.1126/science.1239403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder GF. Dissertation. University of Kentucky; 2013. Injury establishes constitutive mu-opioid receptor activity leading to lasting endogenous analgesia and dependence. [Google Scholar]
- Costa T, Herz A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci U S A. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011;152:2701–2709. doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks J, Fredensborg BB, Christensen D, Fomsgaard JS, Flyger H, Dahl JB. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology. 2002a;97:560–564. doi: 10.1097/00000542-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Dirks J, Petersen KL, Dahl JB. The heat/capsaicin sensitization model: a methodologic study. JPain. 2003;4:122–128. doi: 10.1054/jpai.2003.10. [DOI] [PubMed] [Google Scholar]
- Dirks J, Petersen KL, Rowbotham MC, Dahl JB. Gabapentin suppresses cutaneous hyperalgesia following heat-capsaicin sensitization. Anesthesiology. 2002b;97:102–107. doi: 10.1097/00000542-200207000-00015. [DOI] [PubMed] [Google Scholar]
- Divin MF, Bradbury FA, Carroll FI, Traynor JR. Neutral antagonist activity of naltrexone and 6beta-naltrexol in naive and opioid-dependent C6 cells expressing a mu-opioid receptor. British journal of pharmacology. 2009;156:1044–1053. doi: 10.1111/j.1476-5381.2008.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolen S, Blake CB, Smith BN, Taylor BK. Peripheral nerve injury increases glutamate-evoked calcium mobilization in adult spinal cord neurons. Mol Pain. 2012;8:56. doi: 10.1186/1744-8069-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drdla R, Gassner M, Gingl E, Sandkuhler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- Duggan AW, North RA. Electrophysiology of opioids. Pharmacological reviews. 1983;35:219–281. [PubMed] [Google Scholar]
- El-Sobky A, Dostrovsky JO, Wall PD. Lack of effect of naloxone on pain perception in humans. Nature. 1976;263:783–784. doi: 10.1038/263783a0. [DOI] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, Koutsourakis M, Vasseur L, Veinante P, Kieffer BL, Massotte D. A mu-delta opioid receptor brain atlas reveals neuronal co occurrence in subcortical networks. Brain structure & function. 2014 doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin beta-Endorphin Mediates Addiction to UV Light. Cell. 2014;157:1527–1534. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165:896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid M, Singer G, Oei T, Rana C. Reactions to ischemic pain: interactions between individual, situational and naloxone effects. Psychopharmacology. 1981;73:116–119. doi: 10.1007/BF00429200. [DOI] [PubMed] [Google Scholar]
- Fuchs PN, Roza C, Sora I, Uhl G, Raja SN. Characterization of mechanical withdrawal responses and effects of mu-, delta- and kappa-opioid agonists in normal and mu-opioid receptor knockout mice. Brain research. 1999;821:480–486. doi: 10.1016/s0006-8993(99)01060-4. [DOI] [PubMed] [Google Scholar]
- Gabra BH, Kessler FK, Ritter JK, Dewey WL, Smith FL. Decrease in N-methyl-D-aspartic acid receptor-NR2B subunit levels by intrathecal short-hairpin RNA blocks group I metabotropic glutamate receptor-mediated hyperalgesia. J Pharmacol Exp Ther. 2007;322:186–194. doi: 10.1124/jpet.107.120071. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem. 2010a;115:505–514. doi: 10.1111/j.1471-4159.2010.06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010b;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:4965–4971. doi: 10.1523/JNEUROSCI.14-08-04965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff JR, Burkey AR, Goff DJ, Jasmin L. Reorganization of the spinal dorsal horn in models of chronic pain: correlation with behaviour. Neuroscience. 1998;82:559–574. doi: 10.1016/s0306-4522(97)00298-4. [DOI] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152:2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevert P, Goldstein A. Endorphins: naloxone fails fails to alter experimental pain or mood in humans. Science. 1978;199:1093. doi: 10.1126/science.343250. [DOI] [PubMed] [Google Scholar]
- Guan Y, Yuan F, Carteret AF, Raja SN. A partial L5 spinal nerve ligation induces a limited prolongation of mechanical allodynia in rats: an efficient model for studying mechanisms of neuropathic pain. Neuroscience letters. 2010;471:43–47. doi: 10.1016/j.neulet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6208–6217. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatashita S, Sekiguchi M, Kobayashi H, Konno S, Kikuchi S. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine. 2008;33:1344–1351. doi: 10.1097/BRS.0b013e3181733188. [DOI] [PubMed] [Google Scholar]
- He Y, Tian X, Hu X, Porreca F, Wang ZJ. Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. J Pain. 2012;13:598–607. doi: 10.1016/j.jpain.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinke B, Gingl E, Sandkuhler J. Multiple targets of mu-opioid receptor-mediated presynaptic inhibition at primary afferent Adelta- and C-fibers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1313–1322. doi: 10.1523/JNEUROSCI.4060-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A, Millan MJ. Endogenous opioid peptides in the descending control of nociceptive responses of spinal dorsal horn neurons. Progress in brain research. 1988;77:263–273. doi: 10.1016/s0079-6123(08)62794-6. [DOI] [PubMed] [Google Scholar]
- Hori Y, Endo K, Takahashi T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn of rat spinal cord. J Physiol. 1992;450:673–685. doi: 10.1113/jphysiol.1992.sp019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. Journal of Neuroscience. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. Contribution of endogenous enkephalins to the enhanced analgesic effects of supraspinal mu opioid receptor agonists after inflammatory injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:2536–2545. doi: 10.1523/JNEUROSCI.21-07-02536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Tang J, Costa E, Yang HY. Analgesic activity and release of [MET5]enkephalin-Arg6-Gly7-Leu8 from rat spinal cord in vivo. Eur J Pharmacol. 1986;121:39–48. doi: 10.1016/0014-2999(86)90390-0. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Illing S, Mann A, Schulz S. Heterologous regulation of agonist-independent mu-opioid receptor phosphorylation by protein kinase C. British journal of pharmacology. 2014;171:1330–1340. doi: 10.1111/bph.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija S. Enkephalins modulate excitatory synaptic transmission in the superficial dorsal horn by acting at mu opioid receptor sites. Brain research. 1988;460:260–268. doi: 10.1016/0006-8993(88)90371-x. [DOI] [PubMed] [Google Scholar]
- Jessell TM, Iversen LL. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977;268:549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Current Drug Targets-Inflammation and Allergy. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau IV RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Research Reviews. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends in neurosciences. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends in neurosciences. 2011;34:599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungkunz G, Engel RR, King UG, Kuss HJ. Endogenous opiates increase pain tolerance after stress in humans. Psychiatry research. 1983;8:13–18. doi: 10.1016/0165-1781(83)90133-6. [DOI] [PubMed] [Google Scholar]
- Kaur M, Liguori A, Lang W, Rapp SR, Fleischer AB, Jr., Feldman SR. Induction of withdrawal-like symptoms in a small randomized, controlled trial of opioid blockade in frequent tanners. Journal of the American Academy of Dermatology. 2006;54:709–711. doi: 10.1016/j.jaad.2005.11.1059. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Principles: receptor theory in pharmacology. Trends in pharmacological sciences. 2004;25:186–192. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Functional selectivity through protean and biased agonism: who steers the ship? Molecular pharmacology. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- Kern D, Plantevin F, Bouhassira D. Effects of morphine on the experimental illusion of pain produced by a thermal grill. Pain. 2008;139:653–659. doi: 10.1016/j.pain.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J Physiol. 1999;518(Pt 3):803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? [see comments]. Trends in neurosciences. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends in neurosciences. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Lam H, Maga M, Pradhan A, Evans CJ, Maidment NT, Hales TG, Walwyn W. Analgesic tone conferred by constitutively active mu opioid receptors in mice lacking beta-arrestin 2. Mol Pain. 2011;7:24. doi: 10.1186/1744-8069-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ. CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological, and behavioral evidence for a role in opiate dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:7890–7901. doi: 10.1523/JNEUROSCI.17-20-07890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. The Journal of biological chemistry. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin JP, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. J Pain. 2011;12:1069–1079. doi: 10.1016/j.jpain.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Leurs R, Smit MJ, Alewijnse AE, Timmerman H. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends in biochemical sciences. 1998;23:418–422. doi: 10.1016/s0968-0004(98)01287-0. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Pezet S, McMahon SB, Malcangio M. The signaling components of sensory fiber transmission involved in the activation of ERK MAP kinase in the mouse dorsal horn. Molecular and cellular neurosciences. 2003;24:259–270. doi: 10.1016/s1044-7431(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL. Naloxone dose dependently produces analgesia and hyperalgesia in postoperative pain. Nature. 1979;278:740–741. doi: 10.1038/278740a0. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Jones RT, Fields HL. The narcotic antagonist naloxone enhances clinical pain. Nature. 1978;272:826–827. doi: 10.1038/272826a0. [DOI] [PubMed] [Google Scholar]
- Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–1404. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- Lian B, Vera-Portocarrero L, King T, Ossipov MH, Porreca F. Brain research. 2010;Opioid-induced latent sensitization in a model of non-inflammatory viscerosomatic hypersensitivity.1358:64–70. doi: 10.1016/j.brainres.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liauw J, Wu LJ, Zhuo M. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. Journal of neurophysiology. 2005;94:878–882. doi: 10.1152/jn.01205.2004. [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. Chronic exposure to mu-opioid agonists produces constitutive activation of mu-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Molecular pharmacology. 2001;60:53–62. doi: 10.1124/mol.60.1.53. [DOI] [PubMed] [Google Scholar]
- Liu JG, Ruckle MB, Prather PL. Constitutively active mu-opioid receptors inhibit adenylyl cyclase activity in intact cells and activate G-proteins differently than the agonist [D-Ala2,N-MePhe4,Gly-ol5]enkephalin. The Journal of biological chemistry. 2001;276:37779–37786. doi: 10.1074/jbc.M106104200. [DOI] [PubMed] [Google Scholar]
- Lu HC, She WC, Plas DT, Neumann PE, Janz R, Crair MC. Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical 'barrel' map development. Nat Neurosci. 2003;6:939–947. doi: 10.1038/nn1106. [DOI] [PubMed] [Google Scholar]
- Luo C, Seeburg PH, Sprengel R, Kuner R. Activity-dependent potentiation of calcium signals in spinal sensory networks in inflammatory pain states. Pain. 2008;140:358–367. doi: 10.1016/j.pain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends in neurosciences. 2013;36:195–206. doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann A, Illing S, Miess E, Schulz S. Different Mechanisms of Homologous and Heterologous mu-Opioid Receptor Phosphorylation. British journal of pharmacology. 2014 doi: 10.1111/bph.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends in neurosciences. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Marker CL, Lujan R, Loh HH, Wickman K. Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta-but not kappa-opioids. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3551–3559. doi: 10.1523/JNEUROSCI.4899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Xie W, Ma L, Ueda H. Pharmacological switch in Abeta-fiber stimulation-induced spinal transmission in mice with partial sciatic nerve injury. Mol Pain. 2008;4:25. doi: 10.1186/1744-8069-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, van Zessen R, Smidt MP, Adan RA, Ramakers GM. Morphine withdrawal enhances constitutive mu-opioid receptor activity in the ventral tegmental area. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:16120–16128. doi: 10.1523/JNEUROSCI.1572-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Morris BJ, Colpaert FC, Herz A. A model of chronic pain in the rat: high-resolution neuroanatomical approach identifies alterations in multiple opioid systems in the periaqueductal grey. Brain research. 1987;416:349–353. doi: 10.1016/0006-8993(87)90917-6. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS. Stress-Induced Eating Is Mediated through Endogenous Opiates. Science. 1980;209:1259–1261. doi: 10.1126/science.6250222. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Machelska H, Schafer M, Stein C. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. Journal of neuroimmunology. 2002;126:5–15. doi: 10.1016/s0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- Navani DM, Sirohi S, Madia PA, Yoburn BC. The role of opioid antagonist efficacy and constitutive opioid receptor activity in the opioid withdrawal syndrome in mice. Pharmacology, biochemistry, and behavior. 2011;99:671–675. doi: 10.1016/j.pbb.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nielsen MD, Chan GC, Poser SW, Storm DR. Differential regulation of type I and type VIII Ca2+-stimulated adenylyl cyclases by Gi-coupled receptors in vivo. The Journal of biological chemistry. 1996;271:33308–33316. doi: 10.1074/jbc.271.52.33308. [DOI] [PubMed] [Google Scholar]
- Noble F, Coric P, Turcaud S, Fournie-Zaluski MC, Roques BP. Assessment of physical dependence after continuous perfusion into the rat jugular vein of the mixed inhibitor of enkephalin-degrading enzymes, RB 101. Eur J Pharmacol. 1994;253:283–287. doi: 10.1016/0014-2999(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Dubner R, Ruda MA. Preproenkephalin mRNA in spinal dorsal horn neurons is induced by peripheral inflammation and is co-localized with Fos and Fos-related proteins. Neuroscience. 1992;46:561–570. doi: 10.1016/0306-4522(92)90144-q. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. The Journal of clinical investigation. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature reviews Neuroscience. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Parada CA, Vivancos GG, Tambeli CH, Cunha FQ, Ferreira SH. Activation of presynaptic NMDA receptors coupled to NaV1.8-resistant sodium channel C-fibers causes retrograde mechanical nociceptor sensitization. Proc Natl Acad Sci U S A. 2003;100:2923–2928. doi: 10.1073/pnas.252777799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira MP, Werner MU, Ringsted TK, Rowboth MC, Taylor BK, Dahl JB. Does Naloxone Reinstate Secondary Hyperalgesia in Humans after Resolution of a Burn Injury? A Placebo-Controlled, Double-Blind, Randomized, Cross-over Study. PloS one. 2013 doi: 10.1371/journal.pone.0064608. (accepted 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends in neurosciences. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Qiu S, Chen T, Koga K, Guo YY, Xu H, Song Q, Wang JJ, Descalzi G, Kaang BK, Luo JH, Zhuo M, Zhao MG. An Increase in Synaptic NMDA Receptors in the Insular Cortex Contributes to Neuropathic Pain. Science signaling. 2013;6:ra34. doi: 10.1126/scisignal.2003778. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadee W, Bilsky EJ. In vivo characterization of 6beta-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther. 2005;313:1150–1162. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Ravn P, Frederiksen R, Skovsen AP, Christrup LL, Werner MU. Prediction of pain sensitivity in healthy volunteers. J Pain Res. 2012;5:313–326. doi: 10.2147/JPR.S33925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravn P, Secher EL, Skram U, Therkildsen T, Christrup LL, Werner MU. Morphine- and buprenorphine-induced analgesia and antihyperalgesia in a human inflammatory pain model: a double-blind, randomized, placebo-controlled, five-arm crossover study. J Pain Res. 2013;6:23–38. doi: 10.2147/JPR.S36827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees H, Sluka KA, Lu Y, Westlund KN, Willis WD. Dorsal root reflexes in articular afferents occur bilaterally in a chronic model of arthritis in rats. Journal of neurophysiology. 1996;76:4190–4193. doi: 10.1152/jn.1996.76.6.4190. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends in neurosciences. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivat C, Laboureyras E, Laulin JP, Le Roy C, Richebe P, Simonnet G. Non-Nociceptive Environmental Stress Induces Hyperalgesia, Not Analgesia, in Pain and Opioid-Experienced Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- Rivat C, Laulin JP, Corcuff JB, Celerier E, Pain L, Simonnet G. Fentanyl enhancement of carrageenan-induced long-lasting hyperalgesia in rats: prevention by the N-methyl-D-aspartate receptor antagonist ketamine. Anesthesiology. 2002;96:381–391. doi: 10.1097/00000542-200202000-00025. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadee W, Wang D, Bilsky EJ. Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life sciences. 2005;76:1427–1437. doi: 10.1016/j.lfs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Sally EJ, Xu H, Dersch CM, Hsin LW, Chang LT, Prisinzano TE, Simpson DS, Giuvelis D, Rice KC, Jacobson AE, Cheng K, Bilsky EJ, Rothman RB. Identification of a novel “almost neutral” micro-opioid receptor antagonist in CHO cells expressing the cloned human mu-opioid receptor. Synapse. 2010;64:280–288. doi: 10.1002/syn.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J, Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Current opinion in pharmacology. 2012;12:18–27. doi: 10.1016/j.coph.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Gehrke BJ, Shippenberg TS. Endogenous kappa-opioid receptor systems inhibit hyperalgesia associated with localized peripheral inflammation. Pain. 2008a;138:423–439. doi: 10.1016/j.pain.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Shippenberg TS. Inflammation-induced changes in rostral ventromedial medulla mu and kappa opioid receptor mediated antinociception. Pain. 2008b;136:320–330. doi: 10.1016/j.pain.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SP, Eckert WA, 3rd, Light AR. Opioid-activated postsynaptic, inward rectifying potassium currents in whole cell recordings in substantia gelatinosa neurons. Journal of neurophysiology. 1998;80:2954–2962. doi: 10.1152/jn.1998.80.6.2954. [DOI] [PubMed] [Google Scholar]
- Schoell ED, Bingel U, Eippert F, Yacubian J, Christiansen K, Andresen H, May A, Buechel C. The effect of opioid receptor blockade on the neural processing of thermal stimuli. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P, Sauro KM, Bushnell MC. Fibromyalgia: a disorder of the brain? The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2008;14:415–421. doi: 10.1177/1073858407312521. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn-Schmiedeberg's archives of pharmacology. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sevcik MA, Jonas BM, Lindsay TH, Halvorson KG, Ghilardi JR, Kuskowski MA, Mukherjee P, Maggio JE, Mantyh PW. Endogenous opioids inhibit early-stage pancreatic pain in a mouse model of pancreatic cancer. Gastroenterology. 2006;131:900–910. doi: 10.1053/j.gastro.2006.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT. Constitutively active micro opioid receptors mediate the enhanced conditioned aversive effect of naloxone in morphine-dependent mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:171–177. doi: 10.1038/sj.npp.1300782. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maidment NT. Enkephalin release promotes homeostatic increases in constitutively active mu opioid receptors during morphine withdrawal. Neuroscience. 2007;149:642–649. doi: 10.1016/j.neuroscience.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca2+ -stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Madia PA, Yoburn BC. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther. 2009;330:513–519. doi: 10.1124/jpet.109.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A. 2011;108:7224–7229. doi: 10.1073/pnas.1017719108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JC. Dorsal horn neurons firing at high frequency, but not primary afferents, release opioid peptides that produce micro-opioid receptor internalization in the rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003a;23:9171–9184. doi: 10.1523/JNEUROSCI.23-27-09171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Marvizon JC. Peptidases prevent mu-opioid receptor internalization in dorsal horn neurons by endogenously released opioids. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003b;23:1847–1858. doi: 10.1523/JNEUROSCI.23-05-01847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike RC, Puskar Z, Sakamoto H, Stewart W, Watt C, Todd AJ. MOR-1-immunoreactive neurons in the dorsal horn of the rat spinal cord: evidence for nonsynaptic innervation by substance P-containing primary afferents and for selective activation by noxious thermal stimuli. Eur J Neurosci. 2002;15:1306–1316. doi: 10.1046/j.1460-9568.2002.01969.x. [DOI] [PubMed] [Google Scholar]
- Stanfa L, Dickenson A. Spinal opioid systems in inflammation. Inflammation research : official journal of the European Histamine Research Society [et al] 1995;44:231–241. doi: 10.1007/BF01782974. [DOI] [PubMed] [Google Scholar]
- Stanfa LC, Sullivan AF, Dickenson AH. Alterations in neuronal excitability and the potency of spinal mu, delta and kappa opioids after carrageenan-induced inflammation. Pain. 1992;50:345–354. doi: 10.1016/0304-3959(92)90040-I. [DOI] [PubMed] [Google Scholar]
- Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Current opinion in pharmacology. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Suarez-Roca H, Maixner W. Morphine produces a multiphasic effect on the release of substance P from rat trigeminal nucleus slices by activating different opioid receptor subtypes. Brain research. 1992;579:195–203. doi: 10.1016/0006-8993(92)90051-a. [DOI] [PubMed] [Google Scholar]
- Sufka KJ. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58:355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Puntillo KA, Miaskowski C, Green PG, Levine JD. Burn injury pain: the continuing challenge. J Pain. 2007;8:533–548. doi: 10.1016/j.jpain.2007.02.426. [DOI] [PubMed] [Google Scholar]
- Tambeli CH, Levine JD, Gear RW. Centralization of noxious stimulus-induced analgesia (NSIA) is related to activity at inhibitory synapses in the spinal cord. Pain. 2009;143:228–232. doi: 10.1016/j.pain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Peterson MA, Basbaum AI. Continuous intravenous infusion of naloxone does not change behavioral, cardiovascular, or inflammatory responses to subcutaneous formalin in the rat. Pain. 1997;69:171–177. doi: 10.1016/s0304-3959(96)03265-4. [DOI] [PubMed] [Google Scholar]
- Terman GW, Eastman CL, Chavkin C. Mu opiates inhibit long-term potentiation induction in the spinal cord slice. Journal of neurophysiology. 2001;85:485–494. doi: 10.1152/jn.2001.85.2.485. [DOI] [PubMed] [Google Scholar]
- Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136:331–339. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C. Inflammatory pain unmasks heterosynaptic facilitation in lamina I neurokinin 1 receptor-expressing neurons in rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5158–5168. doi: 10.1523/JNEUROSCI.6241-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafton JA, Basbaum AI. [d-Ala2,N-MePhe4,Gly-ol5]enkephalin-induced internalization of the micro opioid receptor in the spinal cord of morphine tolerant rats. Neuroscience. 2004;125:541–543. doi: 10.1016/j.neuroscience.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Walker EA, Sterious SN. Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence. British journal of pharmacology. 2005;145:975–983. doi: 10.1038/sj.bjp.0706247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadee W. Inverse agonists and neutral antagonists at mu opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem. 2001a;77:1590–1600. doi: 10.1046/j.1471-4159.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, Sadee W. Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. J Pharmacol Exp Ther. 2004;308:512–520. doi: 10.1124/jpet.103.054049. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun X, Sadee W. Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. J Pharmacol Exp Ther. 2007;321:544–552. doi: 10.1124/jpet.106.118810. [DOI] [PubMed] [Google Scholar]
- Wang GD, Zhuo M. Synergistic enhancement of glutamate-mediated responses by serotonin and forskolin in adult mouse spinal dorsal horn neurons. Journal of neurophysiology. 2002;87:732–739. doi: 10.1152/jn.00423.2001. [DOI] [PubMed] [Google Scholar]
- Wang H, Xu H, Wu LJ, Kim SS, Chen T, Koga K, Descalzi G, Gong B, Vadakkan KI, Zhang X, Kaang BK, Zhuo M. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Science translational medicine. 2011;3:65ra63. doi: 10.1126/scitranslmed.3001269. [DOI] [PubMed] [Google Scholar]
- Wang Z, Bilsky EJ, Porreca F, Sadee W. Constitutive mu opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life sciences. 1994;54:PL339–350. doi: 10.1016/0024-3205(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr., Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001b;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, Xu HM, Chen ZF, Storm DR, Muglia LJ, Zhuo M. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36:713–726. doi: 10.1016/s0896-6273(02)01019-x. [DOI] [PubMed] [Google Scholar]
- Wei F, Vadakkan KI, Toyoda H, Wu LJ, Zhao MG, Xu H, Shum FW, Jia YH, Zhuo M. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:851–861. doi: 10.1523/JNEUROSCI.3292-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner MU, Lassen B, Kehlet H. Analgesic effects of dexamethasone in burn injury. Reg AnesthPain Med. 2002a;27:254–260. doi: 10.1053/rapm.2002.30664. [DOI] [PubMed] [Google Scholar]
- Werner MU, Lassen B, Pedersen JL, Kehlet H. Local cooling does not prevent hyperalgesia following burn injury in humans. Pain. 2002b;98:297–303. doi: 10.1016/S0304-3959(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Werner MU, Perkins F, Holte K, Pedersen JL, Kehlet H. Effects of gabapentin in acute inflammatory pain in humans. 2001. [DOI] [PubMed]
- Werner MU, Petersen KL, Rowbotham MC, Dahl JB. Healthy volunteers can be phenotyped using cutaneous sensitization pain models. PloS one. 2013;8:e62733. doi: 10.1371/journal.pone.0062733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyerbacher AR, Xu Q, Tamasdan C, Shin SJ, Inturrisi CE. N-Methyl-D-aspartate receptor (NMDAR) independent maintenance of inflammatory pain. Pain. 2010;148:237–246. doi: 10.1016/j.pain.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcockson WS, Chung JM, Hori Y, Lee KH, Willis WD. Effects of iontophoretically released peptides on primate spinothalamic tract cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1984;4:741–750. doi: 10.1523/JNEUROSCI.04-03-00741.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Xia ZG, Refsdal CD, Merchant KM, Dorsa DM, Storm DR. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron. 1991;6:431–443. doi: 10.1016/0896-6273(91)90251-t. [DOI] [PubMed] [Google Scholar]
- Xu H, Partilla JS, Wang X, Rutherford JM, Tidgewell K, Prisinzano TE, Bohn LM, Rothman RB. A comparison of noninternalizing (herkinorin) and internalizing (DAMGO) mu-opioid agonists on cellular markers related to opioid tolerance and dependence. Synapse. 2007;61:166–175. doi: 10.1002/syn.20356. [DOI] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7445–7453. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL. Opioid receptor systems and the endorphins: a review of their spinal organization. Journal of neurosurgery. 1987;67:157–176. doi: 10.3171/jns.1987.67.2.0157. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Al-Rodhan NR, Jensen TS. Sites of action of opiates in production of analgesia. Progress in brain research. 1988;77:371–394. doi: 10.1016/s0079-6123(08)62803-4. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Elde RP. Factors governing release of methionine enkephalin-like immunoreactivity from mesencephalon and spinal cord of the cat in vivo. Journal of neurophysiology. 1981;46:1056–1075. doi: 10.1152/jn.1981.46.5.1056. [DOI] [PubMed] [Google Scholar]
- Yoo JH, Bailey A, Borsodi A, Toth G, Matifas A, Kieffer BL, Kitchen I. Knockout subtraction autoradiography: A novel ex vivo method to detect heteromers finds sparse KOP receptor/DOP receptor heterodimerization in the brain. Eur J Pharmacol. 2014;731C:1–7. doi: 10.1016/j.ejphar.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, North RA. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983;305:529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]
- Yu XM, Sessle BJ, Vernon H, Hu JW. Administration of opiate antagonist naloxone induces recurrence of increased jaw muscle activities related to inflammatory irritant application to rat temporomandibular joint region. Journal of neurophysiology. 1994;72:1430–1433. doi: 10.1152/jn.1994.72.3.1430. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Liu R, LaPlant Q, Xiao G, Renthal W, Chan GC, Storm DR, Aghajanian G, Nestler EJ. Distinct roles of adenylyl cyclases 1 and 8 in opiate dependence: behavioral, electrophysiological, and molecular studies. Biological psychiatry. 2008;63:1013–1021. doi: 10.1016/j.biopsych.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M. Targeting neuronal adenylyl cyclase for the treatment of chronic pain. Drug discovery today. 2012;17:573–582. doi: 10.1016/j.drudis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]