Abstract
Infection during pregnancy (i.e., prenatal infection) increases the risk of psychiatric illnesses such as schizophrenia and autism in the adult offspring. The present experiments examined the effects of prenatal immune challenge on behavior in three paradigms relevant to these disorders: pre-pulse inhibition (PPI) of the acoustic startle response, locomotor responses to an unfamiliar environment and the N-methyl-D-aspartate antagonist MK-801, and three forms of recognition memory. Pregnant Long–Evans rats were exposed to the viral mimetic polyinosinic-polycytidylic acid (PolyI:C; 4 mg/kg, i.v.) on gestational day 15. Offspring were tested for PPI and locomotor activity before puberty (postnatal days (PNDs)35 and 36) and during young adulthood (PNDs 56 and 57). Four prepulse-pulse intervals (30, 50, 80, and 140 ms) were employed in the PPI test. Recognition memory testing was performed using three different spontaneous novelty recognition tests (object, object location, and object-in-place recognition) after PND 60. Regardless of sex, offspring of PolyI:C-treated dams showed disrupted PPI at 50-, 80-, and 140-ms prepulse-pulse intervals. In the prepubescent rats, we observed prepulse facilitation for the 30-ms prepulse-pulse interval trials that was selectively retained in the adult PolyI:C-treated offspring. Locomotor responses to MK-801 were significantly reduced before puberty, whereas responses to an unfamiliar environment were increased in young adulthood. Both male and female PolyI:C-treated offspring showed intact object and object location recognition memory, whereas male PolyI:C-treated offspring displayed significantly impaired object-in-place recognition memory. Females were unable to perform the object-in-place test. The present results demonstrate that prenatal immune challenge during mid/late gestation disrupts PPI and locomotor behavior. In addition, the selective impairment of object-in-place recognition memory suggests tasks that depend on prefrontal cortex may be particularly vulnerable following prenatal immune challenge.
Keywords: startle, MK-801, schizophrenia, autism, prefrontal cortex, PolyI:C
Adverse events early in life, such as exposure to infection or inflammation (i.e., prenatal infection), during gestation impart significant risk for the development of psychiatric illnesses such as schizophrenia and autism in the offspring (Pearce, 2001; Patterson, 2009; Brown and Derkits, 2010; Meyer and Feldon, 2010; Brown and Patterson, 2011). These epidemiological studies suggest that prenatal infection alters normal development of the nervous system resulting in cognitive and behavioral abnormalities. Therefore, a detailed understanding of the consequences of prenatal infection for cognition and behavior may aid in the development of therapies or prevention strategies for patients with neurodevelopmental psychiatric disorders. The use of rodents to study the effects of prenatal infection is one strategy that will contribute to this goal (Floresco et al., 2005; Young et al., 2009).
Previous research has shown prenatal immune challenge of rodents with a variety of compounds, including the viral mimetic polyinosinic-polycytidylic acid (PolyI:C), causes significant long-term behavioral alterations in the offspring relevant to neurodevelopmental psychiatric disorders (Pearce, 2001; Meyer et al., 2009; Patterson, 2009; Meyer and Feldon, 2010; Piontkewitz et al., in press). Two such commonly used behavioral assays are prepulse inhibition (PPI) of the acoustic startle response (Koch, 1999; Geyer et al., 2001; Swerdlow et al., 2008) and locomotor responses to an unfamiliar environment or pharmacological challenge (Al-Amin et al., 2001; Le Pen et al., 2011). Adult male and female mice, but not juveniles, born to dams treated with PolyI:C show reduced PPI (Shi et al., 2003; Ozawa et al., 2006; Meyer et al., 2008c; Vuillermot et al., 2010; Hsiao and Patterson, 2011). In rats, disrupted PPI has been reported in juvenile and adult male animals from PolyI:C-treated dams in some studies (Wolff and Bilkey, 2008, 2010; Dickerson et al., 2010) but not others (Fortier et al., 2007). Altered locomotor activity in unfamiliar environments and following pharmacological challenge with MK-801, amphetamine, or methamphetamine has been reported in mice (Ozawa et al., 2006; Meyer et al., 2008b,c; Meyer and Feldon, 2010) and adult rats (Zuckerman et al., 2003; Zuckerman and Weiner, 2005; Piontkewitz et al., 2009, 2010; Bronson et al., 2011; Richtand et al., 2011) born to dams treated with PolyI:C during pregnancy. While these studies address some changes seen in patients with schizophrenia and autism, they do not encapsulate many of the cognitive changes observed in neurodevelopmental psychiatric disorders.
Recently, the importance of cognitive symptoms in psychiatric disorders, such as schizophrenia, has been emphasized given the early emergence of these symptoms, their ubiquitous presentation, and their predictive value for patient outcomes (Elvevag and Goldberg, 2000; Keefe and Fenton, 2007; Lewis and Gonzalez-Burgos, 2008). Importantly, schizophrenia patients with confirmed exposure to influenza in utero are more impaired on tests related to prefrontal cortex function than patients not exposed to prenatal infection (Brown et al., 2009). While these data suggest a salient link between prenatal infection and cognitive disruption in adulthood, our current understanding of this potential link is limited. Consistent with the cognitive deficits in patients, prenatal PolyI:C treatment alters cognition in tasks such as latent inhibition, working memory, set shifting, and reversal learning (Zuckerman et al., 2003; Zuckerman and Weiner, 2003, 2005; Meyer et al., 2005, 2006, 2008b; Piontkewitz et al., 2009, 2010; Bitanihirwe et al., 2010b; Zhang et al., in press). Among the core cognitive deficits observed in patients with schizophrenia are disruptions of short- and long-term recognition memory (Calkins et al., 2005). Pervasive deficits in both Cambridge Neuropsychological Test Automated Battery paired associates learning and in tests of object location binding exist in patients with schizophrenia (Wood et al., 2002; Burglen et al., 2004; Barnett et al., 2005; Bartok et al., 2005; Salame et al., 2006; Chouinard et al., 2007), with the degree of deficit on these tests correlating highly with daily functioning (Aubin et al., 2009). Deficits in object recognition memory (Ozawa et al., 2006; Ibi et al., 2009; Bitanihirwe et al., 2010b) have been found in mice exposed to PolyI:C during gestation or the early neonatal period (but see also Ito et al., 2010). To date, no studies of object memory have been conducted using rats exposed to prenatal immune challenge. In particular, the influence of prenatal immune challenge on object location recognition or associations between objects and location has not been examined despite reports of deficits in these tasks in patients.
The present experiments were designed with two primary aims: (1) test the effects of prenatal infection on PPI and locomotor activity before and after puberty and (2) examine the influence of prenatal infection on both simple recognition memory (object and object location tests; Ennaceur et al., 1997; Howland and Cazakoff, 2010) and associative recognition memory (object-in-place test; Bussey et al., 2000; Barker et al., 2007; Barker and Warburton, 2008). All tests were conducted on male and female offspring of Long–Evans rat dams exposed to PolyI:C on gestational day (GD) 15 (Zuckerman et al., 2003). The interval between the prepulse and pulse was varied as this parameter influences PPI (Mansbach and Geyer, 1991; Reijmers and Peeters, 1994b; Koch, 1999; Brosda et al., 2011) and has not been examined following prenatal infection. Spontaneous locomotor activity and locomotor activity in response to systemic injection with MK-801 were assessed given their relevance to some symptoms of schizophrenia (Al-Amin et al., 2001; Coyle and Tsai, 2004; Bronson et al., 2011; Le Pen et al., 2011).
EXPERIMENTAL PROCEDURES
Subjects
Timed pregnant Long–Evans dams (GD 7; Charles River Laboratories, Quebec, Canada) were singly housed in transparent plastic cages in a temperature-controlled (21 °C) colony room on a 12/12-h light/dark cycle with food (Purina Rat Chow) and water available ad libitum. The offspring of four separate squads of dams purchased between January 2010 and January 2011 were used in the present experiments. Some animals were siblings of those reported in Zhang et al. (in press). Maternal observations were made on some litters as described by Zhang et al. (in press); however, given that the treatment groups did not differ, those data are not included in the present manuscript. All experiments were conducted during the light phase (lights on at 0700 h). Experimenters were blind to the treatment of the dams and pups during the course of all experiments. All experiments were performed in accordance with the Canadian Council on Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board. Efforts were made to minimize the number of animals used and their suffering.
Gestational and neonatal treatment
On GD 15, dams were individually transported to a room where weight and rectal temperature (Homeothermic Blanket System, Harvard Instruments, MA, USA) were measured. Dams were then anesthetized with isoflurane (5% induction and 2.5% maintenance) and injected intravenously with a single dose of either saline (n=18) or PolyI:C (4.0 mg/kg, high molecular weight; InvivoGen, San Diego, CA, USA; n=14) via the tail vein. This procedure took an average of 10 min/animal, and care was taken to ensure the saline-treated dams were anesthetized for the same length of time as the PolyI:C-treated dams. Weight and temperature were measured again 8, 24, and 48 h after the injection. One dam showed evidence of bloody discharge from its vagina 24 h after PolyI:C injection. It went on to successfully give birth to five pups. Dams were otherwise left undisturbed until the day after parturition. The day of parturition was designated postnatal day (PND) 0. On PND 1, litters were weighed and culled to a maximum of 10 pups/litter (six males and four females where possible). Other than maternal observations and routine husbandry (including taking litter weights on PND 8 and 14), litters were left undisturbed until weaning on PND 21. Weaned pups from the same litter were housed in same-sex cages of 2– 4 animals with males and females housed in separate colony rooms. Care was taken to ensure that offspring from several litters (typically 8 –12 litters; specific numbers in the figure captions) were included in each group to reduce the influence of litter effects. Corrections were not made for the inclusion of multiple animals from a single litter because the number of animals taken from each litter was not consistent (see Holson et al., 2008; Meyer et al., 2009; Zorilla, 1997 for a discussion of practical, ethical, and statistical issues related to this design).
Behavioral testing
Rats were handled for 5 min/day in small groups at least three times before behavioral testing. Most animals were tested on PPI and locomotor activity before puberty (PND 35 and 36) and in young adulthood (PND 56 and 57). Recognition memory testing was conducted between PND 60 and 90. A subset of animals were tested only on locomotor activity at PND 57 (n=23) or object-in-place recognition memory after PND 60 (n=22).
Determination of estrous cycle
Vaginal smears were taken daily between the hours of 08:00 and 10:00 for 7 days before behavioral testing and continued during test days. To collect a sample, a pipette tip containing 20 μl of 0.9% saline was inserted 2.5–5 mm into the vaginal cavity. The saline was ejected, immediately reloaded, and then expelled onto a clean glass slide. Wet samples were viewed under a light microscope, and estrous cycle phase was determined using established cytological methods (Marcondes et al., 2002; Goldman et al., 2007). Following determination of phase, samples were preserved with Cytoprep (Fisher Scientific Co., Pittsburgh, PA, USA) spray. All rats displayed normal alterations in estrous cycle.
PPI
The PPI testing procedure was similar to published protocols (Howland et al., 2004a,b, 2007). Briefly, testing was conducted in two standard SR-LAB startle boxes (San Diego Instruments, San Diego, CA, USA). Each PPI test session began with a 5-min acclimatization period during which a 70-dB background noise level was presented that remained constant for the entire test session. Following acclimatization, six pulse-alone trials (120 dB, 40 ms) were presented to achieve a relatively stable level of startle amplitude before presentation of the prepulse+pulse trials. The data from these pulse-alone trials were not considered in the analysis of PPI. Immediately following the six initial pulse-alone trials, a total of 84 trials of three different types were presented in a pseudorandom order: pulse alone (6 trials; 120 dB, 40 ms), prepulse+pulse (6 trials×3 prepulse intensities×4 prepulse-pulse time intervals—discussed below), or no stimulus (6 trials). Prepulse+pulse trials consisted of the presentation of a 20-ms prepulse of 3, 6, or 12 dB above background. Prepulse-pulse time intervals were 30, 50, 80, or 140 ms between the onset of the prepulse and onset of the pulse. Each session ended with six pulse-alone trials. The intertrial interval varied randomly from 3 to 14 s (average 7.5 s). The boxes were washed with 20% ethanol (EtOH) between animals. Two measures were calculated for each rat. The first measure, startle amplitude, represents the mean amplitude of the startle response elicited by each of the six pulse-alone trials that were presented after the six habituation trials. PPI was calculated by averaging the startle amplitudes for each trial type, and the percent PPI for each prepulse intensity was calculated using the formula: [100−(100×startle amplitude on prepulse+pulse trials)÷(startle amplitude on pulse alone trials)]. The average startle amplitudes elicited during three blocks of six pulse alone trials (before, during, and after PPI trials) were compared to measure habituation.
Locomotor activity
All testing took place in 40×40×60-cm open-field arenas constructed of white corrugated plastic. An overhead camera recorded the activity of individual subjects in four separate arenas while data were also tracked using EthoVision software (Noldus Information Technology, Wageningen, The Netherlands). Spontaneous activity was recorded for 30 min following which MK-801 was administered (i.p.; 0.2 mg/kg for all animals before puberty, 0.2 mg/kg for males, and 0.06 mg/kg for females after puberty). Doses were based on pilot experiments in our laboratory and those of previous studies (Al-Amin et al., 2001; Le Pen et al., 2011; Bronson et al., 2011). Following MK-801 administration, activity was recorded for a subsequent 120 min. Distance moved (cm) was calculated for 10-min bins.
Recognition memory
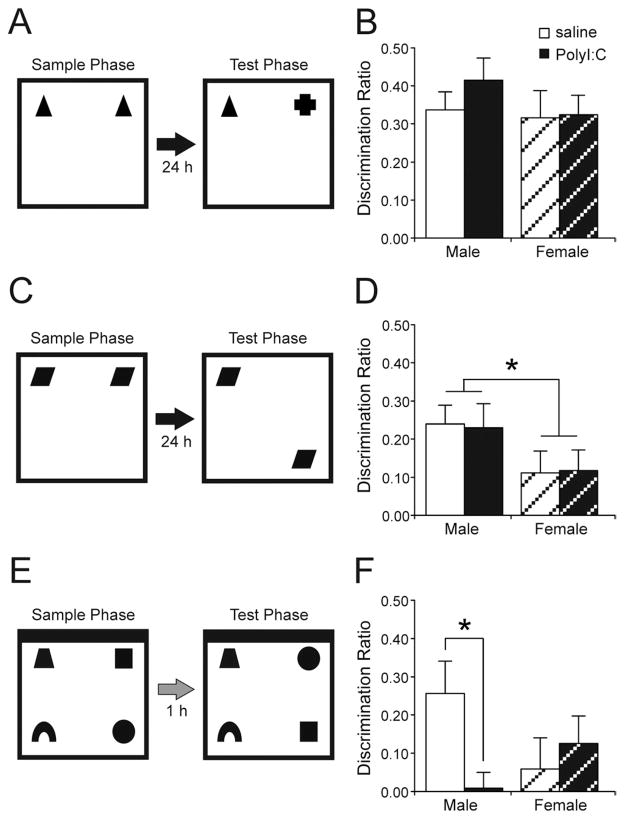
The recognition memory test procedure was similar to established protocols (Fig 5; (Howland and Cazakoff, 2010). Behavioral testing took place in a white rectangular room containing an empty plastic water maze. Four floor lamps provided background illumination. Recognition memory testing was conducted in a square open-field arena (60×60×60 cm) constructed of white corrugated plastic. Between each trial, the floors and walls of the arena were wiped with 40% EtOH and a damp sponge. For the object-in-place test, an additional black wall was inserted along the north wall of the arena (Fig. 5E).
Subjects received three habituation sessions before the first recognition test (the novel object recognition test). During the first two habituations, subjects were brought in pairs into the testing room and placed individually in separate arenas for 10 min. On the last day, subjects were brought individually into the room and again spent 10 min in the arena. Following habituation, subjects were returned to the colony room. The last habituation occurred 24 – 48 h before the first testing session. For subsequent tests (object location and object-in-place tests), subjects received only one habituation session, in pairs, 24 – 48 h before the new test. Objects in all tests were constructed of glass, plastic, or porcelain and were all similar in size (~ 10 cm in height and length). Unique objects with duplicate copies were used for all tests. Location of the novel object(s) was counterbalanced to eliminate the effect of any side preference in all tests. For both the sample and test phases, objects were located in the corners of the arena 10 cm from each of the nearest walls, while subjects were placed in the arena facing the wall opposite the objects. Between each rat, the objects and arena were wiped with 40% EtOH.
Object recognition
During the sample phase, subjects explored two identical objects (A1 and A2) for 4 min (Fig. 5A). Following the 4 min of exploration, rats were then returned to the colony room. Twenty-four hours following the sample phase, memory for the previously encountered objects was tested during the 4-min test phase in which subjects explored a copy of the sample object (A3) and a novel object (B1).
Object location recognition
One week following the novel object recognition test, subjects were tested in the object location paradigm. The sample phase was identical to the novel object recognition sample phase in which subjects explored two identical objects (C1 and C2); Fig 5C. Twenty-four hours later, subjects received a test phase in which they explored two identical copies of the sample objects (C3 and C4), but with one object moved to a corner location at the front of the box and the other object in the original sample phase location.
Object-in-place recognition
One week following object location testing, subjects were tested in the object-in-place paradigm. During the sample phase, subjects explored four different objects (D1, E1, F1, G1) for 5 min. Objects were located in the four corners of the arena 10 cm from each of the nearest walls (Fig. 5E). Following a 1-h delay (spent in colony room home cage), subjects were placed back in the arena and exposed to four additional copies of the objects (D2, E2, F2, G2). However, during the test phase, the positions of two of the objects were switched (Fig. 5E). Only the two objects on the left side of the arena or the right side of the arena were switched (i.e. front object becomes the back object and vice versa). Object-in-place memory is inferred when rats spend more time exploring the pair of objects that switch locations than the objects that remain in their sample positions.
Data analysis
Statistical tests were conducted using SPSS version 18 for Windows (IBM). For all repeated measures analysis of variance (ANOVA), corrections were made for violations of sphericity (Mauchly’s Test). Post hoc analyses were performed separately using the Neuman–Keuls test. Statistical tests were considered significant when P<0.05. Error bars on all figures represent the standard error of the mean (SEM). Acute effects of PolyI:C on maternal weight, temperature, and litter size were analyzed with repeated measures ANOVA (Time and Treatment as factors) and t-tests.
PPI
Strong prepulse facilitation was observed for the 30-ms interval; in contrast, PPI was observed for the 50-, 80-, and 140-ms intervals. Therefore, the data for the 30-ms interval were analyzed separately. Startle data were analyzed with two-way between-within ANOVAs (Sex and Treatment as between-subjects factors; Pulse Block as a repeated measures factor) for each age. Three-way between-within ANOVAs (Prepulse Intensity and Prepulse-pulse Interval as repeated measures factors; Treatment as the between-subjects factor) were performed on the PPI data for each age.
Locomotor activity
Data (distance travelled in cm) for the spontaneous activity and MK-801-evoked activity experiments were analyzed separately at each age using repeated measures ANOVA with Treatment and Sex as between-subject factors and Time (10-min bins) as repeated measures factors.
Recognition memory
Data were scored by an individual blind to the treatment status of the rat according to measures previously described (Howland and Cazakoff, 2010). A rat was judged to be actively exploring an object when its nose was directed within 2 cm of an object and either its head or vibrissae were moving but not when it was standing on top of an object and not directing attention towards it. Previous experiments in our laboratory have shown that rats must explore both objects for at least 15 s in both the sample and test phases to show reliable memory. As a result, rats with less than 15 s of exploration on either phase were eliminated from the study. Given that spontaneous recognition memory tests are most sensitive in the first 1–2 min of the test trial (Dix and Aggleton, 1999; Clark et al., 2000; Barker and Warburton, 2008), the discrimination ratio (DR) was calculated from the first 2 min of the test phase in all tests. Both total exploration times of the objects on a given trial and the DR (time exploring the novel object or location–time exploring the familiar object or location)/total time exploring both objects) were quantified for each rat. Rats whose DR was greater than two standard deviations above or below the group mean were removed. Total exploration times for each test were analyzed using a two-way ANOVA with Treatment and Sex as between-subject factors. For test-phase data, the DR for each group was analyzed using one-sample t-tests (with a comparison value of 0 indicating equal exploration of the two objects or chance performance; Howland and Cazakoff, 2010), whereas between-group comparisons were performed using two-way ANOVA with Treatment and Sex as between-subjects factors.
RESULTS
Effects of PolyI:C treatment on dams and pups
Weight and rectal temperature of all dams were taken 0, 8, 24, and 48 h following PolyI:C injection. Human error resulted in the loss of weight data for one dam and temperature data for five dams. Statistical analysis of dam weights revealed significant main effects of Time (F(3,87)=45.72, P<0.001), Treatment (F(1,29)=5.11, P=0.031), and a significant Treatment by Time interaction (F(3,87)=17.28, P<0.001). Inspection of the data revealed that dam weights were significantly lower for the saline than PolyI:C-treated group (at 0 h: 345.72±8 g vs. 387.38±10 g). Further analysis of the weight data revealed that while dams in both groups lost weight in response to being anesthetized, PolyI:C-treated animals lost more weight and gained significantly less weight than the saline-treated animals over the subsequent 48 h relative to initial weight at 0 h (Fig. 1A; main effect of Treatment: F(1,29)=68.73, P<0.001; Treatment by Time interaction: F(2,58)=5.11, P=0.009). At 8, 24, and 48 h after treatment, saline animals weighed −6.0±1.6, +4.72±1.4, and +15.83±1.3 g compared with their weights at 0 h. PolyI:C-treated dams lost 17.92±2.1, 15.77±2.0, and 3.69±4.1 g relative to their initial weight 8, 24 and 48 h later. To assess the possibility that initial weight of the dam influenced the amount of weight lost in response to treatment, partial correlations were performed on the weight lost (g) and percentage change in weight 8 h after treatment. Both correlations failed to reach significance (weight lost: r=0.30, P=0.104; percentage change: r=−0.15, P=0.432), suggesting that the initial weight of the dams did not influence the acute effects of PolyI:C. Analysis of temperature data (Fig. 1B) with ANOVA showed a significant main effect of Time (F(3,75)=10.24, P<0.001) without significant main effects of Treatment (F(1,25)=0.49, P=0.49) or Time by Treatment interaction (F(3,75)=1.71, P=0.17). Post hoc analyses indicated that both treatment groups showed a significant increase in temperature at 8 h relative to all other time points. An average of 13.06±0.89 (6.86±0.15 g/pup) and 12.21±0.92 (6.62±0.07 g/pup) pups were born to the saline-treated and PolyI:C-treated dams, respectively. No significant effect was noted for prenatal treatment regarding the number (t(30)=0.65, P=0.521) or weight of the pups on PND 1, 8, 14, or 21 (data not shown).
Fig. 1.
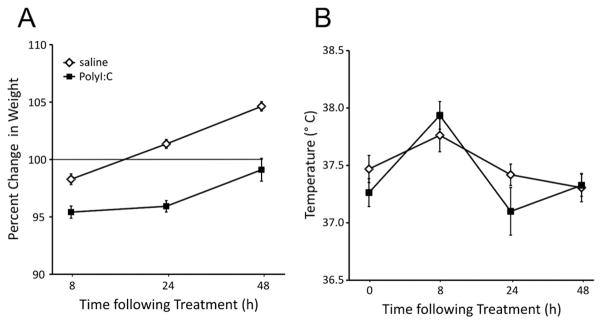
The effects of PolyI:C treatment on maternal weight (A) and rectal temperature (B). Weight change is expressed as a percentage of baseline weight at 0 h immediately before treatment. Dams treated with PolyI:C (4 mg/kg, i.v.; n=14) gained significantly less weight than those treated with saline (n=18) at all time points.
PolyI:C treatment disrupts PPI before and after puberty in male and female offspring
Pre puberty
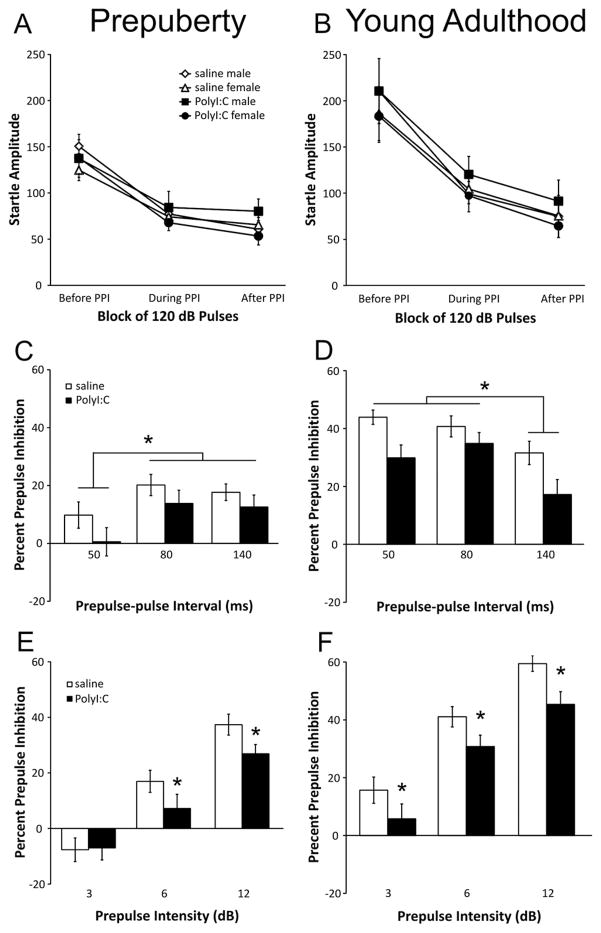
As depicted in Fig 2A, rats in both treatment groups displayed an expected pattern of startle amplitudes with higher responses (≈140 arbitrary startle units (ASU)) during the initial pulse-alone trials. Startle amplitudes were consistent for the rest of the test session (≈70 ASU). Analysis of the data with ANOVA confirmed these impressions with significant main effects of Pulse Block (F(2,114)=149.94, P<0.001) with no significant effects of Treatment (F(1,57)=0.01, P=0.911) or Sex (F(2,57)=0.89, P=0.350). A significant Pulse Block by Treatment by Sex interaction was observed (F(2,114)=5.40, P=0.009) without other significant interaction terms. Movement during the trials in which no stimulus was presented differed by sex (male: 6.91±0.77 ASU; female: 4.69±0.78 ASU; F(1,57)=6.46, P=0.014) but not by treatment (saline: 6.25±0.78 ASU; PolyI:C: 5.41±0.78 ASU; F(1,57)=0.69, P=0.408).
Fig. 2.
Effects of PolyI:C treatment on startle amplitude and prepulse inhibition (PPI) in prepubescent and young adult rats. Prepulse-pulse intervals of 50, 80, and 140 ms are shown. (A) Startle amplitudes (arbitrary units) of rats before puberty for the pulse-alone trials before, during, and after the PPI trials (saline-treated: males n=14 from 11 litters, females n=13 from 11 litters; PolyI:C-treated: males n=16 from 10 litters, females n=18 from 11 litters). (B) Startle amplitudes of young adult rats for the pulse-alone trials before, during, and after the PPI trials (saline-treated: males n=14 from 11 litters, females n=13 from 11 litters; PolyI:C-treated: males n=15 from 10 litters, females n=18 from 11 litters). Panels C and D show percent PPI for each prepulse-pulse interval (50, 80, and 140 ms) for the prepubescent and young adult rats, respectively. Panels E (before puberty) and F (young adulthood) depict percent PPI for each prepulse intensity (3, 6, 12 dB above background) averaged for trials with 50-, 80-, 140-ms prepulse-pulse intervals. Asterisks indicate a significant difference (P<0.05).
The average percent PPI by prepulse-pulse interval (50, 80, and 140 ms) and by prepulse intensity (3, 6, 12 dB) is shown in Fig 2C, E, respectively, for pups on PND 35. Male and female pups were combined in these analyses, as there was no main effect of Sex (F(1,57)=0.70, P=0.41) or interaction of Sex with any other factor (statistics not shown). Rats from the PolyI:C- (n=27) and saline-treated (n=34) dams had varying levels of PPI that depended on both prepulse-pulse interval (F(2,114)=10.42, P<0.001) and prepulse intensity (F(2,114)=122.42, P<0.001). Whereas there was no significant main effect of Treatment (F(1,57)=1.67, P=0.201), the Treatment by Prepulse interaction term approached significance (F(2,114)=3.02, P=0.053). Rats displayed greater PPI for the 80 and 140-ms intervals than the 50-ms interval (Fig. 2C; post hoc P<0.05). As depicted in Fig 2E, rats displayed similar levels of PPI for trials with a 3-dB prepulse intensity. In contrast, PolyI:C-treated rats had lower PPI for trials with 6- and 12-dB prepulses. When trials from the 30-ms interval were considered (Fig. 3A), a significant main effect of prepulse was observed (F(2,114)=19.67, P<0.001) but no other main effects or interactions were significant (statistics not shown). Pre-pulses of 3 and 6 dB, but not 12 dB, caused significantly enhanced startle amplitudes as indicated by negative PPI scores in both the saline and PolyI:C treated pups.
Fig. 3.
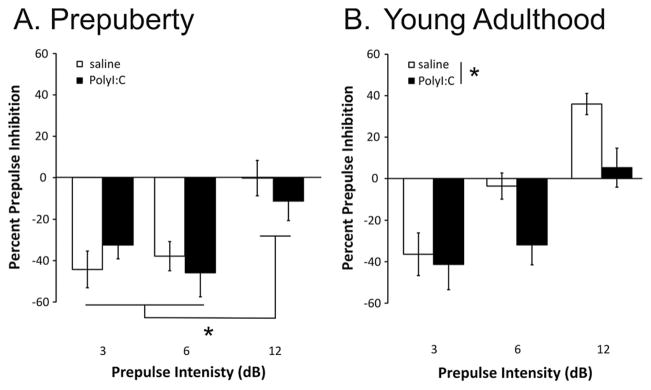
Effects of PolyI:C treatment on PPI for trials with a 30-ms prepulse-pulse interval for rats before puberty (A) and in young adulthood (B). Note that a negative PPI score reflects an increase in startle responding on the trials with a prepulse (3, 6, or 12 dB above background). Groups are as described in Fig 2. Asterisks indicate a significant difference (P<0.05).
Young adulthood
Fig. 2B shows the startle amplitudes for the adult rats. A typical pattern was observed whereby the animals show significantly higher startle responses (≈200 ASU) in response to the first six pulse-alone trials compared with the 12 pulse-alone trials presented afterward (≈75–100ASU). Repeated measures ANOVA revealed a significant main effect of Pulse Block (F(2,112)=67.07, P<0.001) with no significant main effects for either Treatment (F(1,56)=0.03, P=0.872) or Sex (F(1,56)=0.85, P=0.361). No significant interaction terms were found (statistics not shown). Movement during the trials in which no stimulus was presented differed by Sex (male: 6.81±0.78 ASU; female: 3.23±0.33 ASU; F(1,56)=20.05, P<0.001) but not by Treatment (saline: 4.30±0.69 ASU; PolyI:C: 5.49±0.64 ASU; F(1,56)=3.00, P=0.089).
Percent PPI levels are shown in Fig 2D, F according to prepulse-pulse interval and prepulse intensity for the 50-, 80-, 140-ms intervals. The results from male and female rats were combined as no significant effect of Sex was observed (F(1,56)=0.57, P=0.453). In saline-treated rats, robust PPI was observed for all prepulse intensities. The rats from PolyI:C-treated dams had reduced PPI for all prepulse intensities when compared with the saline-treated offspring as reflected by a significant main effect of Treatment (F(1,56)=4.76, P=0.033). Expected significant main effects of prepulse-pulse interval (F(2,112)=25.19, P<0.001) and prepulse (F(2,112)=156.09, P<0.001) were also observed. PPI was significantly greater for the 50- and 80-ms intervals than the 140-ms interval, regardless of sex or treatment (Fig. 2D; post hoc P<0.05). Fig. 3B depicts the percent PPI for the trials with a 30-ms prepulse-pulse interval. Inspection of the data reveals a complex pattern of responding in pups from saline-treated dams, which demonstrated pre-pulse facilitation during trials with 3-dB prepulses, no change in startle for trials with 6-dB prepulses, and strong PPI for trials with 12-dB prepulses. PolyI:C offspring showed a pattern of responding similar to both PND35 groups with strong facilitation of startle for trials with 3- and 6-dB prepulses and no change in startle for trials with 12-dB prepulses. Statistical analyses revealed significant main effects of Treatment (F(1,56)=4.35, P<0.041) and Prepulse Intensity (F(2,112)=36.10, P<0.001) with no other significant terms. Similar to previous findings (Wolff and Bilkey, 2010), PPI scores were not correlated with maternal weight loss at 24 h for either the PolyI:C- or saline-treated groups (data not shown).
Locomotor activity is altered before and after puberty in PolyI:C-treated offspring
Pre puberty
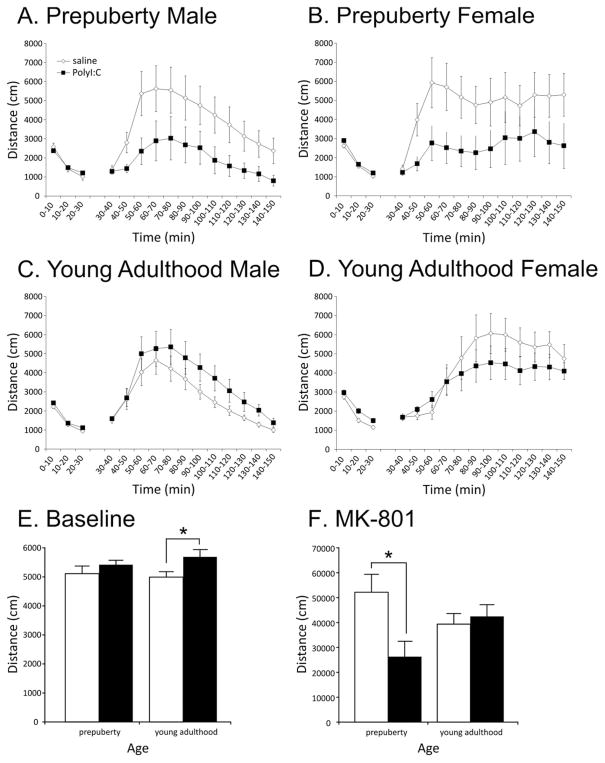
Locomotor activity in response to the novel open-field chambers is depicted for male and female rats in Fig 4A, B, respectively. The rats displayed a typical pattern of more vigorous exploration during the initial 10-min bin followed by a reduction in activity in the subsequent two bins. A repeated measures ANOVA confirmed this impression with a significant main effect of Time (F(2,84)=348.57, P<0.001). Neither the sex of the pups (F(1,42)=2.14, P=0.15) nor PolyI:C treatment (F(1,42)=0.92, P=0.34) significantly affected locomotor activity during this phase of the test (Fig. 4E). No significant interactions were observed (statistics not shown).
Fig. 4.
Locomotor responses of the offspring of dams exposed to PolyI:C or saline on gestational day 15. Locomotor responses to an unfamiliar environment (30 min) and MK-801 (120 min) are depicted in panels A–D in 10-min bins. MK-801 was administered at 30 min (before puberty: all animals 0.2 mg/kg; after puberty: males 0.2 mg/kg, females 0.06 mg/kg, i.p.). Panels E and F depict the total locomotor activity of the animals for during exposure to the unfamiliar environment (E) and after MK-801 administration (F). Groups are composed of the following numbers of subjects: before puberty: spontaneous, saline-treated: males n=12 from 10 litters, females n=11 from nine litters; PolyI:C-treated: males n=11 from seven litters, females n=12 from eight litters; MK-801, saline-treated: males n=12 from 10 litters, females n=12 from 10 litters; PolyI:C-treated: males n=11 from seven litters, females n=11 from seven litters. Young adult: saline-treated: males n=15 from 12 litters, females n=20 from 14 litters; PolyI:C-treated: males n=15 from 10 litters, females n=15 from eight litters; MK-801, saline-treated: males n=15 from 11 litters, females n=8 from four litters; PolyI:C-treated males n=16 from 10 litters, females n=8 from three litters. Asterisks indicate a significant difference (P<0.05).
After 30 min, all rats were injected with MK-801 (0.2 mg/kg, i.p.), and locomotor activity was monitored for an additional 120 min (Fig. 4A, B). Both male and female offspring prenatally treated with saline demonstrated increased locomotor activity in response to MK-801, which peaked approximately 30 min after injection (main effect of Time: F(11,440)=12.10, P<0.001). Most importantly, PolyI:C treatment significantly reduced the stimulatory effect of MK-801 on locomotor activity (Fig. 4F) as confirmed by a significant main effect of Treatment (F(1,40)=5.78, P<0.021). In addition, a sex difference was observed whereby the vigor of the male offspring’s locomotor activity decreased after the initial 30-min increase, whereas the female offspring sustained a high level of activity until the end of the experiment (Time by Sex interaction F(11,440)=4.64, P=0.004). Post hoc analyses indicated that the distance travelled by the male animals was significantly less than the females for all bins from 80 to 120 min (P<0.05). Other interaction terms were not significant (statistics not shown).
Young adulthood
Locomotor responses during baseline exposure to the open field in young adult rats are depicted in Fig 4C, D. Similar to that observed before puberty, rats habituated to the chambers over 30 min (significant main effect of Time: F(2,122)=606.91, P<0.001). In contrast to the results before puberty, prenatal PolyI:C treatment significantly increased locomotor activity for the duration of the 30-min exposure (Fig. 4E, F(1,61)=8.37, P=0.005). Sex of the pups was also a critical determinant of locomotor responses (main effect of Sex: F(1,61)=22.83, P<0.001; Time by Sex interaction: F(2,122)=4.01, P=0.021) with females displaying significantly higher levels of locomotor activity than males. All other interaction terms failed to reach significance (statistics not shown).
Given previously reported sex differences in locomotor response to MK-801, all male offspring were treated with the commonly used dose of 0.2 mg/kg, whereas female offspring were treated with 0.06 mg/kg. As expected, MK-801 treatment increased locomotor activity in all animals (F(11,473)=26.15, P<0.001). PolyI:C treatment did not significantly affect locomotor response to MK-801 (Fig. 4F; F(1,43)=0.003, P=0.96). However, the pattern of responses differed subtly by sex (Time by Sex interaction: F(11,471)=25.19, P<0.001; main effect of Sex: F(1,43)=3.17, P=0.08). Male offspring responded similarly to animals tested before puberty with locomotor responses peaking approximately 30 min after injection and gradually decreasing over the subsequent 90 min (Fig. 4C). In contrast, adult females demonstrated an increase in locomotor activity with peak responses occurring approximately 60 min after injection that were maintained for the duration of the experiment (Fig. 4D). Post hoc analyses indicated that the male rats displayed significantly higher locomotor activity during 20 to 40 min and significantly lower locomotor activity from 50 to 120 min (P<0.05). All other terms failed to reach significance (statistics not shown). Locomotor responses failed to correlate with maternal weight loss at 24 h as has been recently reported (statistics not shown; Bronson et al., 2011).
Object-in-place recognition memory is selectively disrupted in the male PolyI:C-treated offspring
Object recognition
Total exploration times are summarized in Table 1. Analysis of the sample phase exploration with a two-way ANOVA revealed a significant main effect of Sex (F(1,56)=20.39, P<0.001), with no effect of Treatment (F(1,56)=2.55, P=0.12) or Sex by Treatment interaction (F(1,56)=0.62, P=0.44). Post hoc analysis revealed that, during the sample phase, females explored the objects more (88.91±2.49 s) than the males (65.94±2.49 s) regardless of treatment. Analysis of exploration during the 2-min test phase revealed both a significant main effect of Sex (F(1,56)=12.93, P<0.001) and Treatment (F(1,56)=9.50, P=0.003); however, there was no significant Sex by Treatment interaction (F(1,56)=0.45, P=0.51). Post hoc analysis revealed that females (46.26±2.05 s) explored objects for a greater amount of time than males (37.39±1.58 s) regardless of Treatment, whereas PolyI:C-treated rats (45.51±1.73 s) explored the objects for a greater amount of time than the saline-treated rats (37.88±2.03 s) regardless of Sex.
Table 1.
Object recognition, object location, and object-in-place exploration times for saline- and PolyI:C-treated male and female rats. Total exploration time of objects (seconds±SEM) during the sample and test phases of the object recognition, object location, and object-in-place paradigms. The total time for the sample phase is presented, whereas the time for the first 2 min is presented for the test phases.
| Treatment | Object recognition
|
Object location
|
Object-in-place
|
|||
|---|---|---|---|---|---|---|
| Sample phase | Test phase | Sample phase | Test phase | Sample phase | Test phase | |
| Male saline | 63.90±3.5 | 34.51±2.2 | 52.56±5.6 | 31.99±2.6 | 64.70±3.4 | 26.13±1.7 |
| Male PolyI:C | 67.98±3.6 | 40.26±2.0 | 66.34±3.5 | 35.33±2.5 | 83.28±7.8 | 41.40±4.3 |
| Female saline | 82.57±5.1 | 41.49±3.3 | 60.65±5.2 | 35.38±4.0 | 74.90±5.5 | 36.31±3.5 |
| Female PolyI:C | 94.46±7.1 | 50.43±2.2 | 58.18±4.5 | 36.89±2.4 | 81.70±6.1 | 40.81±3.1 |
PolyI:C treatment failed to produce any significant effect on memory for previously viewed objects (Fig. 5B). Both male and female PolyI:C-treated rats performed at levels comparable with their saline-treated counterparts. All groups showed memory that was significantly different from zero (male saline t(14)=7.37, P<0.001; male PolyI:C t(14)=7.38, P<0.001; female saline t(13)=4.82, P<0.001; female PolyI:C t(15)=6.63, P<0.001). A two-way ANOVA revealed no effect of Treatment (F(1,56)=0.65, P=0.42), Sex (F(1,56)=1.02, P=0.32), and no Sex by Treatment interaction (F(1,56)=0.43, P=0.52).
Fig. 5.
Summary of the recognition memory tests and the effects of PolyI:C treatment on each type of memory for male and female subjects. (A) Object recognition. Rats explored two identical objects during a sample phase followed 24 h later by a test phase in which rats explored one copy of the sample object and one novel object. (B) Effect of PolyI:C on object recognition memory (saline-treated: male n=15 from 11 litters, female n=14 from 11 litters; PolyI:C-treated: male n=15 from 10 litters, female: n=16 from nine litters). (C) Object location recognition. Rats explored two identical objects during the sample phase. Following a 24-h delay, they explored two objects identical to the sample phase objects but with one object in a new location. (D) Prenatal PolyI:C treatment did not influence object location recognition memory, although female rats in both groups performed poorer than male rats (saline-treated: male n=15 from 12 litters, female n=13 from 10 litters; PolyI:C-treated: male n=16 from nine litters, female: n=17 from 11 litters). (E) Object-in-place recognition. During the sample phase, rats explored four different objects located in the corners of the box. One hour after the sample phase, rats again explored copies of the same four sample objects, but with two objects in displaced positions. Note the location of the black wall. (F) Effect of PolyI:C on object-in-place recognition memory (saline-treated: male n=17 from 10 litters, female n=17 from 10 litters; PolyI:C-treated: male n=14 from seven litters, female: n=16 from eight litters). Prenatal PolyI:C treatment significantly disrupted object-in-place memory in male rats. Female rats failed to show reliable memory. Asterisks indicate a significant difference (P<0.05).
Object location recognition
A summary of exploration times is displayed in Table 1. In the sample phase, rats across all groups explored the objects for similar amounts of time. ANOVA revealed no main effect of Sex (F(1,57)=0.00, P=0.99), Treatment (F(1,57)=1.49, P=0.23), or a Sex by Treatment interaction (F(1,57)=3.07, P=0.09). During the test phase, rats showed no significant differences in the time spent exploring the objects. Analysis of the first 2 min of exploration revealed no significant effects of Sex (F(1,57)=0.77, P=0.38) or Treatment (F(1,57)=0.74, P=0.39) and no significant Sex by Treatment interaction (F(1,57)=0.11, P=0.75).
Similar to object recognition memory, both PolyI:C-treated male and female rats showed significant object location memory that was similar to that of male and female saline-treated rats (Fig. 5D). One sample t-tests revealed that all treatment groups showed memory that was significantly different from zero (male saline t(14)=5.05, P<0.001; male PolyI:C t(15)=3.72, P=0.002; female saline t(12)=2.01, P=0.055; female PolyI:C t(16)=2.20, P=0.04). Analysis with a two-way ANOVA revealed a significant main effect of Sex (F(1,57)=4.77, P=0.03), no effect of Treatment (F(1,57)=0.00, P=0.98), and no significant Sex by Treatment interaction (F(1,57)=0.03, P=0.87). Inspection of the data revealed that females showed a significantly reduced preference for the object that was moved (DR=0.11±0.04) than males (DR=0.23±0.04) regardless of treatment.
Object-in-place memory
Table 1 displays the mean exploration times. During the sample phase, analysis with two-way ANOVA showed no significant effect of Sex (F(1,59)=0.67, P=0.42) and no significant Sex by Treatment interaction (F(1,59)=0.78, P=0.38). However, there was a significant main effect of Treatment (F(1,59)=5.16, P=0.03). Post hoc analysis revealed that rats in the PolyI:C-treated group displayed more exploration during the sample phase. Analysis of the test phase revealed a significant main effect of Sex (F(1,59)=15.29, P<0.001) but no significant effect of Treatment (F(1,59)=2.23, P=0.14) and no significant Sex by Treatment interaction (F(1,59)=0.17, P=0.68). Inspection of the data revealed that PolyI:C-treated females spent more time exploring the objects than saline-treated males.
During the object-in-place recognition memory task, significant memory is inferred when rats spend more time exploring the objects that switched locations as opposed to the objects that remain in the same location as the sample phase (Fig. 5E). Analysis of the DR during the object-in-place test phase revealed a significant difference between the PolyI:C-treated and saline-treated male rats (Fig. 5F). Whereas saline-treated male rats showed intact object-in-place memory (t(15)=3.14, P<0.01), PolyI:C-treated rats failed to show memory for the object-in-place conjunction (t(13)=0.23, P=0.83). In comparison, female rats in neither the saline nor the PolyI:C treatment group showed intact object-in-place memory (saline-treated: t(16)=0.76, P=0.46; PolyI:C-treated: t(15)=1.82, P=0.09). A two-way ANOVA revealed no significant effect of Sex (F(1,59)=0.31, P=0.58) or Treatment (F(1,59)=1.59, P=0.21). However, there was a significant Sex by Treatment interaction (F(1,59)=4.73, P=0.03). Post hoc analysis revealed that the PolyI:C-treated males differed significantly from the saline-treated males.
DISCUSSION
The present study examined the influence of prenatal immune challenge with PolyI:C on PPI of the acoustic startle response, locomotor activity, and recognition memory in Long Evans rats. Consistent with previous reports, PolyI:C treatment caused significant weight loss in the dams and a subtle increase in temperature (Fig. 1). With prepulse-pulse intervals of 50, 80, and 140 ms, PolyI:C treatment disrupted PPI before puberty and in young adulthood (Fig. 2). Enhanced prepulse facilitation was observed during trials with a 30-ms prepulse-pulse interval in PolyI:C-treated animals in young adulthood but not before puberty (Fig. 3). These effects occurred in both male and female offspring. Locomotor responses to MK-801 were significantly reduced before puberty, whereas responses to an unfamiliar environment were increased in young adulthood (Fig. 4). The duration of the stimulatory effects of MK-801 was longer in female rats of both ages regardless of prenatal treatment (Fig. 4). Although recognition memory was not disrupted in tests of novel object recognition or object location recognition, PolyI:C-treated male rats displayed significantly poorer performance in a test of associative object-in-place memory (Fig. 5).
The regulation of startle and locomotor responses following prenatal infection
We observed disrupted PPI (50-, 80-, and 140-ms pre-pulse-pulse intervals) without significant changes in startle amplitude in PolyI:C-treated Long–Evans rats before and after puberty (Fig. 2). Levels of startle and PPI were similar to previous results obtained in the Long–Evans strain before puberty (Howland et al., 2004a) and in young adulthood (Howland et al., 2004a,b, 2007) using an 80-ms prepulse-pulse interval. The present results obtained with a range of prepulse-pulse intervals (50, 80, and 140 ms) are consistent with previous reports following treatment of Sprague–Dawley rats with PolyI:C in some studies (100-ms interval; Wolff and Bilkey, 2008; Dickerson et al., 2010; Wolff and Bilkey, 2010) but not another (70-ms interval; Fortier et al., 2007).
Surprisingly, prepubescent saline- and PolyI:C-treated rats showed prepulse facilitation for 3- and 6-dB prepulses with a 30-ms prepulse-pulse interval and no change in startle following 12-dB prepulses (Fig. 3A). To our knowledge, this is the first report of prepulse facilitation in pre-pubescent animals using short prepulse-pulse intervals. Young adult animals exposed to PolyI:C displayed a similar pattern of prepulse facilitation, whereas saline-treated adult animals displayed prepulse facilitation to a 3-dB pre-pulse, no change in startle with a 6-dB prepulse, and PPI with a 12-dB prepulse (Fig. 3B). While prepulse facilitation is commonly reported with prepulse-pulse intervals of greater than 500 ms in rodents (Reijmers and Peeters, 1994a) and humans (Wynn et al., 2004; Hsieh et al., 2006), fewer studies have reported prepulse facilitation to short prepulse-pulse intervals. In adult male Sprague–Dawley rats, 10-dB prepulses presented using a 30-ms prepulse-pulse interval did not significantly affect startle, but pre-pulse facilitation was observed for these trials following acute ketamine administration (Mansbach and Geyer, 1991). In another study, PPI was reported for trials with an 18-dB prepulse presented 25 ms before the pulse in adult male Wistar rats, an effect disrupted by MK-801 (Brosda et al., 2011). These findings, following ketamine and MK801 administration, are similar to those of our PolyI:C-treated adult animals for 6- and 12-dB trials whereby prepulse facilitation was observed for trials with a 6-dB prepulse and disrupted PPI was observed for the 12-dB prepulse trials (Fig. 3B). Thus, the responses of PolyI:C-treated adult rats resemble those of rats acutely treated with NMDA antagonists in adulthood and raise the possibility that increased prepulse facilitation to short-interval trials with relatively low-intensity prepulses (<10 dB) may be observed in patients with psychiatric disorders. Short-interval prepulses of high intensity (16 dB) have been found to induce PPI in adults with autism (Perry et al., 2007) and attention deficit hyperactivity disorder (Feifel et al., 2009), although the effects of short-interval prepulses have not been examined in schizophrenia.
Results from the locomotor activity experiments demonstrate that locomotor responses were altered in both prepubescent and adult rats exposed to PolyI:C on GD 15. For the first time in rats, we show that male and female prepubescent offspring of PolyI:C-treated dams are less sensitive to the stimulatory effects of MK-801 (Fig. 4A, B, F). Previous reports indicate that PolyI:C treatment on GD 9 does not significantly affect MK-801-induced locomotion (0.15 mg/kg) in mice before puberty, although none of the animals responded to the drug at this age (Meyer et al., 2008a). Previous work has shown prepubescent rats respond with increased locomotor activity to 0.2 mg/kg of MK-801, an effect exaggerated by neonatal ventral hippocampal lesions (Al-Amin et al., 2001). In early adulthood, male and female PolyI:C-treated rats demonstrated significantly increased locomotor responses to an unfamiliar open field without changes in locomotor response to MK-801 treatment. Elevated (Zuckerman and Weiner, 2005; Meyer et al., 2008a,b) and reduced (Bronson et al., 2011) locomotor respondings to MK-801 have been reported previously in young adult animals prenatally exposed to PolyI:C. Given the variability in these effects, a thorough investigation of the effects of prenatal infection on locomotor activity induced by different doses of MK-801 is warranted (Al-Amin et al., 2001; Le Pen et al., 2011).
While the specific causes of the discrepancies in the PPI and locomotor studies are not clear, the precise timing of injection and route of viral mimetic administration differs between the studies. In mice, timing of injection is critical for determining the effects of PolyI:C treatment of PPI and locomotor responses (Meyer et al., 2006, 2007, 2008b); however, the influence of timing of acute PolyI:C treatment has not been directly tested in rats. In rats, PolyI:C has been most frequently administered once on GD 15 (Zuckerman et al., 2003; Zuckerman and Weiner, 2003, 2005; Wolff and Bilkey, 2008, 2010; Piontkewitz et al., 2009, 2010; Dickerson et al., 2010; Zhang et al., in press; Bronson et al., 2011; Richtand et al., 2011; Roenker et al., 2011), although Fortier and colleagues (2007) administered PolyI:C on GD 10 and GD 11. The route of administration and dose of PolyI:C differs between studies with intravenous administration under anesthesia (the present experiments; Zuckerman et al., 2003; Zuckerman and Weiner, 2003, 2005; Wolff and Bilkey, 2008, 2010; Piontkewitz et al., 2009, 2010; Dickerson et al., 2010; Zhang et al., in press) and intraperitoneal injection without anesthesia (Fortier et al., 2007; Bronson et al., 2011; Richtand et al., 2011; Roenker et al., 2011) being used.
The acute effects of PolyI:C administration are most easily measured using maternal characteristics such as weight and temperature, and these measures have been related to the long-term behavioral effects on the offspring. For example, pups from dams that lost weight 24 h following PolyI:C administration had significantly reduced locomotor responses to MK-801 and amphetamine in early adulthood, whereas pups from dams that did not lose weight did not show altered locomotor activity (Bronson et al., 2011). In contrast, maternal weight loss following PolyI:C treatment was shown not to correlate with PPI scores (Wolff and Bilkey, 2010). In our laboratory (Fig. 1; Zhang et al., in press), we observed increased temperature and reduced maternal weight gain following PolyI:C treatment without effects on pup weight at birth. The average weight loss reported by other groups (Bronson et al., 2011: 0.71 g following 8 mg/kg PolyI:C, i.p.; Wolff and Bilkey, 2010: ≈3–4 g following 4 mg/kg PolyI:C, i.v.) 24 h after PolyI:C treatment is considerably less than we observed (15.83 g). While we cannot be certain why PolyI:C treatment resulted in considerably greater weight loss in the present experiments than previous reports, strain differences (Long–Evans vs. Sprague–Dawley rats) or differences in the supplier of PolyI:C (InvivoGen, high molecular weight vs. Sigma) may have contributed to the weight loss. To our knowledge, systematic studies of these factors on the effects of PolyI:C have not been conducted.
A specific deficit in associative recognition memory following prenatal infection
These data provide the first demonstration of disrupted object-in-place associations as a result of prenatal immune challenge (Fig. 5). The apparent lack of memory was not likely due to alterations in exploratory behavior or attentional processing because memory was normal in the simple recognition tests, and total exploration did not differ between animals during the test phase (Table 1). During the sample phase, exploration times for PolyI:C-treated animals were significantly higher than the saline-treated animals. Although this could reflect impaired encoding of the objects, such an interpretation is unlikely, given the similarities in exploration for the other two tests. The effects of PolyI:C treatment on recognition memory observed in the present study were restricted to male rats. Sex differences in the effects of prenatal infection for cognition have been reported for locomotion, strategy set shifts, and fear conditioning in rodents (Schwendener et al., 2009; Bitanihirwe et al., 2010a; Zhang et al., in press). Regardless of treatment, female rats displayed reduced discrimination compared with the males in the object location and object-in-place tests. Previous reports suggest that phase of the estrous cycle influences novel object location recognition in rodents with higher levels of estrogen and progesterone inhibiting performance (Frye and Sturgis, 1995; Sutcliffe et al., 2007); however, no differences were observed in memory performance for different estrous cycle phases in the present data set.
Some studies have demonstrated novel object recognition deficits in mice prenatally exposed to PolyI:C and LPS (Ozawa et al., 2006; Coyle et al., 2009), whereas another demonstrated enhanced object recognition (Ito et al., 2010). Consistent with our results, Ito and colleagues (2010) showed intact object location memory following prenatal treatment of mice with PolyI:C. The discrepancies between the present results and those of others may reflect differences in the tests and specific prenatal treatment. In previous studies, deficits have been observed with delays of 15 min, 1 h, and 4 h between the sample and test phase (Ozawa et al., 2006; Coyle et al., 2009; Ibi et al., 2009), whereas in the present study, no deficit was observed in the novel object recognition test using a 24-h delay. The lack of effect seen in both the object and object location tasks suggests that processes in the medial temporal lobe regions necessary for long-term recognition memory are not profoundly disturbed by prenatal immune challenge. In contrast, the object-in-place recognition task relies upon processing in both the medial temporal lobe and prefrontal cortex with the integration of objects and locations purported to depend substantially on prefrontal cortex (Barker et al., 2007; Barker and Warburton, 2008, 2009). Thus, these results support the hypothesis that prenatal infection disrupts processing within prefrontal cortex or between prefrontal cortex and interconnected regions (Meyer and Feldon, 2009; Dickerson et al., 2010; Bitanihirwe et al., 2010b; Zhang et al., in press; Roenker et al., 2011; Piontkewitz et al., in press) consistent with findings regarding the pathophysiology of schizophrenia and autism (Lewis and Gonzalez-Burgos, 2008; Brown et al., 2009).
Whether the alterations in PPI and locomotor activity reported in the present manuscript are also caused by disrupted processing in circuits including the medial prefrontal cortex remains to be determined (Meyer and Feldon, 2009). Although the prefrontal cortex is involved in the three tasks examined in the present study, the distributed changes caused by prenatal immune activation also involve striatal and limbic areas (Meyer and Feldon, 2009; Piontkewitz et al., in press). Changes in neurotransmitter systems, including glutamate, GABA, serotonin, and dopamine within this circuitry, have been proposed to underlie the behavioral changes following prenatal immune activation (Meyer and Feldon, 2009). For example, dynamic changes in the dopaminergic system in striatal and midbrain areas following prenatal immune challenge on GD 9 are related to behavioral alterations in locomotor activity and PPI before and after puberty in mice (Vuillermot et al., 2010). Thus, further research will be necessary to specify the commonalities and distinctions between the neurobiological mechanisms underlying the cognitive and behavioral consequences of prenatal immune activation.
CONCLUSION
The present experiments demonstrate that prenatal immune challenge results in cognitive and behavioral disruptions in rats similar to those reported in patients with neurodevelopmental psychiatric disorders such as schizophrenia. The specificity of the recognition memory deficit to the object-in-place test suggests that cognitive functions associated with the prefrontal cortex may be particularly susceptible to alteration following prenatal infection. The relative ease of conducting these tasks make them useful for testing new therapeutics designed to ameliorate the cognitive symptoms of psychiatric disorders.
Acknowledgments
The present work was supported by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, Saskatchewan Health Research Foundation New Investigator Award, and University of Saskatchewan Molstad Trust Intramural Research Award to J.G.H. B.N.C. is the recipient of a National Sciences and Engineering Research Council of Canada Graduate Scholarship. We would like to thank Chester Thai and Quentin Greba for technical assistance with these experiments.
Abbreviations
- ANOVA
analysis of variance
- ASU
arbitrary startle unit
- DR
discrimination ratio
- EtOH
ethanol
- GD
gestational day
- PolyI:C
polyinosinic-polycytidylic acid
- PND
postnatal day
- PPI
pre-pulse inhibition
References
- Al-Amin HA, Shannon Weickert C, Weinberger DR, Lipska BK. Delayed onset of enhanced MK-801-induced motor hyperactivity after neonatal lesions of the rat ventral hippocampus. Biol Psychiatry. 2001;49:528–539. doi: 10.1016/s0006-3223(00)00968-9. [DOI] [PubMed] [Google Scholar]
- Aubin G, Stip E, Gélinas I, Rainville C, Chapparo C. Daily functioning and information-processing skills among persons with schizophrenia. Psychiatr Serv. 2009;60:817–822. doi: 10.1176/ps.2009.60.6.817. [DOI] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. J Neurosci. 2008;28:2837–2844. doi: 10.1523/JNEUROSCI.4447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. Critical role of the cholinergic system for object-in-place associative recognition memory. Learn Mem. 2009;16:8–11. doi: 10.1101/lm.1121309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Sahakian BJ, Werners U, Hill KE, Brazil R, Gallagher O, Bullmore ET, Jones PB. Visuospatial learning and executive function are independently impaired in first-episode psychosis. Psychol Med. 2005;35:1031–1041. doi: 10.1017/s0033291704004301. [DOI] [PubMed] [Google Scholar]
- Bartok E, Berecz R, Glaub T, Degrell I. Cognitive functions in prepsychotic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:621–625. doi: 10.1016/j.pnpbp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010a;35:2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol. 2010b;13:981–996. doi: 10.1017/S1461145710000192. [DOI] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosda J, Hayn L, Klein C, Koch M, Meyer C, Schallhorn R, Wegener N. Pharmacological and parametrical investigation of pre-pulse inhibition of startle and prepulse elicited reactions in Wistar rats. Pharmacol Biochem Behav. 2011;99:22–28. doi: 10.1016/j.pbb.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166:683–690. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burglen F, Marczewski P, Mitchell KJ, van der Linden M, Johnson MK, Danion JM, Salame P. Impaired performance in a working memory binding task in patients with schizophrenia. Psychiatry Res. 2004;125:247–255. doi: 10.1016/j.psychres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Duck J, Muir JL, Aggleton JP. Distinct patterns of behavioural impairments resulting from fornix transection or neurotoxic lesions of the perirhinal and postrhinal cortices in the rat. Behav Brain Res. 2000;111:187–202. doi: 10.1016/s0166-4328(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Gur RC, Ragland JD, Gur RE. Face recognition memory deficits and visual object memory performance in patients with schizophrenia and their relatives. Am J Psychiatry. 2005;162:1963–1966. doi: 10.1176/appi.ajp.162.10.1963. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Stip E, Poulin J, Melun JP, Godbout R, Guillem F, Cohen H. Rivastigmine treatment as an add-on to antipsychotics in patients with schizophrenia and cognitive deficits. Curr Med Res Opin. 2007;23:575–583. doi: 10.1185/030079906X167372. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Tsai G. NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. Int Rev Neurobiol. 2004;59:491–515. doi: 10.1016/S0074-7742(04)59019-0. [DOI] [PubMed] [Google Scholar]
- Coyle P, Tran N, Fung JN, Summers BL, Rofe AM. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res. 2009;197:210–218. doi: 10.1016/j.bbr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30:12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minassian A, Perry W. Prepulse inhibition of startle in adults with ADHD. J Psychiatr Res. 2009;43:484–489. doi: 10.1016/j.jpsychires.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Geyer MA, Gold LH, Grace AA. Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr Bull. 2005;31:888–894. doi: 10.1093/schbul/sbi041. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sturgis JD. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiol Learn Mem. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Holson RR, Freshwater L, Maurissen JP, Moser VC, Phang W. Statistical issues and techniques appropriate for developmental neurotoxicity testing: a report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotoxicol Teratol. 2008;30:326–348. doi: 10.1016/j.ntt.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Howland JG, Cazakoff BN. Effects of acute stress and GluN2B-containing NMDA receptor antagonism on object and object-place recognition memory. Neurobiol Learn Mem. 2010;93:261–267. doi: 10.1016/j.nlm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Howland JG, Hannesson DK, Barnes SJ, Phillips AG. Kindling of basolateral amygdala but not ventral hippocampus or perirhinal cortex disrupts sensorimotor gating in rats. Behav Brain Res. 2007;177:30–36. doi: 10.1016/j.bbr.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Howland JG, Hannesson DK, Phillips AG. Delayed onset of prepulse inhibition deficits following kainic acid treatment on postnatal day 7 in rats. Eur J Neurosci. 2004a;20:2639–2648. doi: 10.1111/j.1460-9568.2004.03731.x. [DOI] [PubMed] [Google Scholar]
- Howland JG, MacKenzie EM, Yim TT, Taepavarapruk P, Phillips AG. Electrical stimulation of the hippocampus disrupts pre-pulse inhibition in rats: frequency- and site-dependent effects. Behav Brain Res. 2004b;152:187–197. doi: 10.1016/j.bbr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh MH, Swerdlow NR, Braff DL. Effects of background and prepulse characteristics on prepulse inhibition and facilitation: implications for neuropsychiatric research. Biol Psychiatry. 2006;59:555–559. doi: 10.1016/j.biopsych.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Kitahara Y, Mizoguchi H, Koike H, Shiraki A, Takuma K, Kamei H, Noda Y, Nitta A, Nabeshima T, Yoneda Y, Yamada K. Neonatal polyI:C treatment in mice results in schizophrenia-like behavioral and neurochemical abnormalities in adulthood. Neurosci Res. 2009;64:297–305. doi: 10.1016/j.neures.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33:912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Jay TM, Krebs MO. Effect of antipsychotics on spontaneous hyperactivity and hypersensitivity to MK-801-induced hyperactivity in rats prenatally exposed to methylazoxymethanol. J Psychopharmacol. 2011;25:822–835. doi: 10.1177/0269881110387839. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. Parametric determinants in pre-stimulus modification of acoustic startle: interaction with ketamine. Psychopharmacology (Berl) 1991;105:162–168. doi: 10.1007/BF02244303. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204:322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology. 2008a;33:441–456. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008b;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2008c;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to do-paminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61:482–486. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull. 2010;37:1257–1269. doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology. doi: 10.1016/j.neuropharm.2011.04.019. (in press) [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Peeters BW. Acoustic prepulses can facilitate the startle reflex in rats: discrepancy between rat and human data resolved. Brain Res Bull. 1994a;35:337–338. doi: 10.1016/0361-9230(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Reijmers LG, Peeters BW. Effects of acoustic prepulses on the startle reflex in rats: a parametric analysis. Brain Res. 1994b;661:174–180. doi: 10.1016/0006-8993(94)91204-1. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand R, Horn P, Stanford K, Bronson SL, McNamara RK. Effects of risperidone and paliperidone pre-treatment on locomotor response following prenatal immune activation. J Psychiatr Res. 2011;45:1194–201. doi: 10.1016/j.jpsychires.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenker NL, Gudelsky G, Ahlbrand R, Bronson SL, Kern JR, Waterman H, Richtand NM. Effect of paliperidone and risperidone on extracellular glutamate in the prefrontal cortex of rats exposed to prenatal immune activation or MK-801. Neurosci Lett. 2011;500:167–171. doi: 10.1016/j.neulet.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamé P, Burglen F, Danion JM. Differential disruptions of working memory components in schizophrenia in an object-location binding task using the suppression paradigm. J Int Neuropsychol Soc. 2006;12:510–518. doi: 10.1017/s1355617706060668. [DOI] [PubMed] [Google Scholar]
- Schwendener S, Meyer U, Feldon J. Deficient maternal care resulting from immunological stress during pregnancy is associated with a sex-dependent enhancement of conditioned fear in the offspring. J Neurodev Disord. 2009;1:15–32. doi: 10.1007/s11689-008-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Marshall KM, Neill JC. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav Brain Res. 2007;177:117–125. doi: 10.1016/j.bbr.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci. 2010;30:1270–1287. doi: 10.1523/JNEUROSCI.5408-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav Brain Res. 2010;213:323–327. doi: 10.1016/j.bbr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Proffitt T, Mahony K, Smith DJ, Buchanan JA, Brewer W, Stuart GW, Velakoulis D, McGorry PD, Pantelis C. Visuospatial memory and learning in first-episode schizophreniform psychosis and established schizophrenia: a functional correlate of hippocampal pathology? Psychol Med. 2002;32:429–438. doi: 10.1017/s0033291702005275. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Dawson ME, Schell AM, McGee M, Salveson D, Green MF. Prepulse facilitation and prepulse inhibition in schizophrenia patients and their unaffected siblings. Biol Psychiatry. 2004;55:518–523. doi: 10.1016/j.biopsych.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cazakoff BN, Thai CA, Howland JG. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. doi: 10.1016/j.neuropharm.2011.02.022. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]